- 1International Institute of Tropical Agriculture, Ibadan, Nigeria
- 2International Institute of Tropical Agriculture, Abuja, Nigeria
- 3Agricultural Research Service, United States Department of Agriculture, Tucson, AZ, United States
In sub-Saharan Africa (SSA), diverse fungi belonging to Aspergillus section Flavi frequently contaminate staple crops with aflatoxins. Aflatoxins negatively impact health, income, trade, food security, and development sectors. Aspergillus flavus is the most common causal agent of contamination. However, certain A. flavus genotypes do not produce aflatoxins (i.e., are atoxigenic). An aflatoxin biocontrol technology employing atoxigenic genotypes to limit crop contamination was developed in the United States. The technology was adapted and improved for use in maize and groundnut in SSA under the trademark Aflasafe. Nigeria was the first African nation for which an aflatoxin biocontrol product was developed. The current study includes tests to assess biocontrol performance across Nigeria over the past decade. The presented data on efficacy spans years in which a relatively small number of maize and groundnut fields (8–51 per year) were treated through use on circa 36,000 ha in commercially-produced maize in 2018. During the testing phase (2009–2012), fields treated during one year were not treated in the other years while during commercial usage (2013–2019), many fields were treated in multiple years. This is the first report of a large-scale, long-term efficacy study of any biocontrol product developed to date for a field crop. Most (>95%) of 213,406 tons of maize grains harvested from treated fields contained <20 ppb total aflatoxins, and a significant proportion (>90%) contained <4 ppb total aflatoxins. Grains from treated plots had preponderantly >80% less aflatoxin content than untreated crops. The frequency of the biocontrol active ingredient atoxigenic genotypes in grains from treated fields was significantly higher than in grains from control fields. A higher proportion of grains from treated fields met various aflatoxin standards compared to grains from untreated fields. Results indicate that efficacy of the biocontrol product in limiting aflatoxin contamination is stable regardless of environment and cropping system. In summary, the biocontrol technology allows farmers across Nigeria to produce safer crops for consumption and increases potential for access to premium markets that require aflatoxin-compliant crops.
Introduction
Throughout sub-Saharan Africa (SSA), certain Aspergillus species frequently contaminate with aflatoxins several staple crops, including maize and groundnut (Shephard, 2008; Udomkun et al., 2017). In SSA, human and animal aflatoxin exposure is high (JECFA, 2018; Sirma et al., 2018; Blankson et al., 2019). Consumption of highly contaminated food can result in acute health effects such as liver diseases and death (Gieseker, 2004; Probst et al., 2010; Kamala et al., 2018). Chronic, sub-lethal exposure may cause child stunting, immunosuppression, impaired food conversion, and cancer (Coursaget et al., 1993; Gong et al., 2008; JECFA, 2018; Leroy et al., 2018; Voth-Gaeddert et al., 2018). Trade sectors also become affected. High aflatoxin content restricts farmers’ access to local and international premium markets. This results in reduced income for farmers but also aggregators, processors, and exporters (Williams, 2008; Wu, 2015). Because of the challenges posed by aflatoxins, substantial efforts have been made to both understand the contamination process and design management programs to reduce food safety risks (James et al., 2007; Hell et al., 2008; Bandyopadhyay et al., 2016; Seetha et al., 2017).
Aflatoxin-producing Aspergillus species of agricultural importance belong to section Flavi (Frisvad et al., 2019). Some species produce only B aflatoxins while others produce both B and G aflatoxins. There are four major aflatoxins (B1, B2, G1, G2) with aflatoxin B1 both the most toxic and prevalent and classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC, 2002; JECFA, 2018). The major causal agent of contamination is A. flavus, which produces only B aflatoxins (Amaike and Keller, 2011). A. flavus is composed of the L and S morphotypes, which differ in morphological, physiological, and genetic criteria (Cotty, 1989). Each morphotype can be further subdivided in numerous vegetative compatibility groups (VCGs) (Bayman and Cotty, 1991; Ortega-Beltran and Cotty, 2018). Genetic variation within members of a VCG is low because they descend from a single clonal lineage but members of different VCGs vary in several characters (Leslie, 1993; Grubisha and Cotty, 2010, 2015). Most L morphotype fungi produce aflatoxins (at varying levels) while others produce no aflatoxins (i.e., are atoxigenic) due to lesions in the aflatoxin biosynthesis gene cluster (Adhikari et al., 2016). Furthermore, there are L morphotype VCGs composed exclusively of atoxigenic members (Mehl et al., 2012; Grubisha and Cotty, 2015; Atehnkeng et al., 2016).
Species other than A. flavus may be important etiologic agents of aflatoxin contamination. In West Africa, fungi resembling the A. flavus S morphotype but that produce both B and G aflatoxins are associated with several crops, including maize and groundnut (Cotty and Cardwell, 1999; Atehnkeng et al., 2008a; Donner et al., 2009; Diedhiou et al., 2011; Agbetiameh et al., 2018; Ezekiel et al., 2019). These fungi were previously known as unnamed taxon SBG. Fungi in this group may be any of recently described species that include A. aflatoxiformans, A. austwickii, A. cerealis, A. minisclerotigenes, and unnamed taxa (Probst et al., 2014; Frisvad et al., 2019; Singh and Cotty, 2019). As in a previous study (Ezekiel et al., 2019), in the current study we use the term SBG strains for all fungi with S morphotype producing both B and G aflatoxins. Even though SBG strains are found at low levels in some years/crops, the extremely high aflatoxin-producing potential of the group warrants special consideration as a major contributor to aflatoxin contamination events in West Africa.
Aflatoxin management strategies have been sought for more than 40 years. Most pre- and post-harvest strategies may reduce incidences and severities of aflatoxin contamination (Bruns, 2003; Cotty et al., 2008; Hell et al., 2008; Waliyar et al., 2015; Seetha et al., 2017; Mahuku et al., 2019). However, the use of a single management strategy in isolation may not prevent initiation of aflatoxin contamination and be insufficient to reduce aflatoxin contamination to acceptable levels [i.e., at least below 20 parts per billion (ppb)] (Bandyopadhyay et al., 2016). Therefore, aflatoxin management strategies must address the contamination process throughout crop production and until crops are consumed using holistic interventions (Ayalew et al., 2017; Logrieco et al., 2018).
The most promising strategy to control aflatoxins is the use of atoxigenic A. flavus strains to competitively displace aflatoxin producers (Amaike and Keller, 2011). This strategy favors the prevalence of atoxigenic strains in the treated fields and throughout the environment. When less aflatoxin producers are associated with a crop, less aflatoxins accumulate (Mehl et al., 2012). This aflatoxin management strategy protects crops from the field, throughout storage, and until consumption. The US Department of Agriculture – Agricultural Research Service (USDA-ARS) developed the first atoxigenic biocontrol product, Aspergillus flavus AF36, and initially registered it with the US Environmental Protection Agency (USEPA) for use in cotton (USEPA, 2003, 2004). Together with Afla-Guard®, a second atoxigenic biocontrol product, the biocontrol technology has been used commercially for >15 years in the US in several crops (Cotty et al., 2007; Dorner, 2009; Doster et al., 2014; Ortega-Beltran and Bandyopadhyay, 2019).
In 2003, the International Institute of Tropical Agriculture (IITA) started a collaboration with USDA-ARS to adapt and improve the biocontrol technology for use in Nigeria and subsequently in other SSA nations (Bandyopadhyay et al., 2016). Initial efforts in Nigeria included examining fungal communities associated with maize and field soils and assessing aflatoxin-producing potentials of the fungi (Atehnkeng et al., 2008a; Donner et al., 2009). Distribution and frequencies of atoxigenic genotypes across target environments in Nigeria were used as criteria to select those atoxigenic African Aspergillus flavus VCGs (AAVs) with superiority in adaptation, fitness, and competitiveness (Atehnkeng et al., 2016). In parallel, studies to determine genetic lesions causing atoxigenicity in atoxigenic AAVs were conducted (Donner et al., 2010). Moreover, representative isolates of selected atoxigenic AAVs were evaluated under laboratory conditions (Atehnkeng et al., 2008b), and in farmers’ fields across Nigeria to further select competitive and widely adapted atoxigenic AAVs (Atehnkeng et al., 2014). Four superior isolates, each representing a unique AAV composed only of atoxigenic members, are the active ingredient fungi of the biocontrol product Aflasafe® (hitherto called ‘biocontrol product’). In 2014, Nigeria’s National Agency for Food and Drug Administration and Control (NAFDAC) approved the full registration of the biocontrol product for unrestricted use in both maize and groundnut across Nigeria (Bandyopadhyay et al., 2016).
Several technologies aiming to improve crop productivity and/or quality—including technologies to reduce crop aflatoxin content—are often evaluated in experimental stations, or on farmer fields managed by researchers, and typically in a few fields (Alaniz Zanon et al., 2013; Weaver et al., 2015; Accinelli et al., 2018; Molo et al., 2019; Weaver and Abbas, 2019). Evaluating technologies under farmer field conditions, in multiple fields of multiple agro-ecological zones (AEZ), and in multiple years, under real-life situations that farmers face can aid in drawing unbiased conclusions about a technology’s efficacy (Ortega-Beltran and Bandyopadhyay, 2019). Multi-year, multi-location evaluations are needed to determine if a technology is valuable to farmers. Indeed, this important biocontrol technology has been often questioned and one of the main concerns is that multi-year, multi-area studies are needed to clarify whether aflatoxin management using atoxigenic fungi is a valuable tool, particularly in African contexts (Ehrlich et al., 2015; Njoroge, 2018; Stepman, 2018; Pitt, 2019; Kagot et al., 2019).
The current study reports the longest-term efficacy study of any aflatoxin biocontrol product developed to date. We present results of biocontrol product efficacy across Nigeria at first in (i) field trials in maize and groundnut farmers’ fields (2009–2012), and then in (ii) commercially-produced maize (2013–2018). Post-harvest benefits of using pre-harvest biocontrol are discussed as well. The results obtained during these 10 years demonstrate that biocontrol provides stable aflatoxin reductions and is a valuable aflatoxin mitigation tool used by maize and groundnut farmers across Nigeria regardless of their farming practices, crop varieties, or environmental challenges. Biocontrol allows farmers in Nigeria to produce maize and groundnut with aflatoxin concentrations safe for consumption and trade.
Materials and Methods
The Active Ingredient Fungi of the Biocontrol Product
The biocontrol product contains four atoxigenic A. flavus L morphotype isolates (Ka16127, La3304, La3279, and Og0222) that belong to atoxigenic AAVs native and relatively common in the major maize and groundnut producing areas of Nigeria (Atehnkeng et al., 2014, 2016). Ka16127 was recovered from maize produced in Saminaka, Kaduna state; La3304 and La3279 were recovered from maize cropped in Lafia, Nasarawa state; Og0222 was recovered from maize cropped in Ogbomosho, Oyo state (Atehnkeng et al., 2016). The four strains are maintained in the fungal collection of both IITA and USDA-ARS as sporulating cultures in water vials and silica grain vials for short- and long-term storage, respectively.
The Treated Fields
The biocontrol product was applied in maize and groundnut farmer field trials (2009–2012) and in commercial maize (maize produced for commercial markets) fields (2013–2018) (Table 1). All fields belonged to smallholder farmers. Cropping systems, climatic conditions, maize and groundnut cultivars used by farmers, disease and pest pressure, among other factors were variable across the different areas of Nigeria where the biocontrol product was used. Results from field efficacy trials conducted in 2009 and 2010 were used to prepare a dossier for registration of the biocontrol product with NAFDAC (Bandyopadhyay et al., 2016). Trials conducted in 2011 and 2012 were used to demonstrate to regulators efficacy of the product under additional conditions and to create market-linkages with poultry and food industries seeking aflatoxin-compliant crops. Commercial maize fields treated with the biocontrol product during 2013–2018 were part of the AgResults Nigeria AflasafeTM Challenge Project, hitherto called as the ‘AgResults Project’ (Bandyopadhyay et al., 2016; AgResults, 2019; Schreurs et al., 2019).
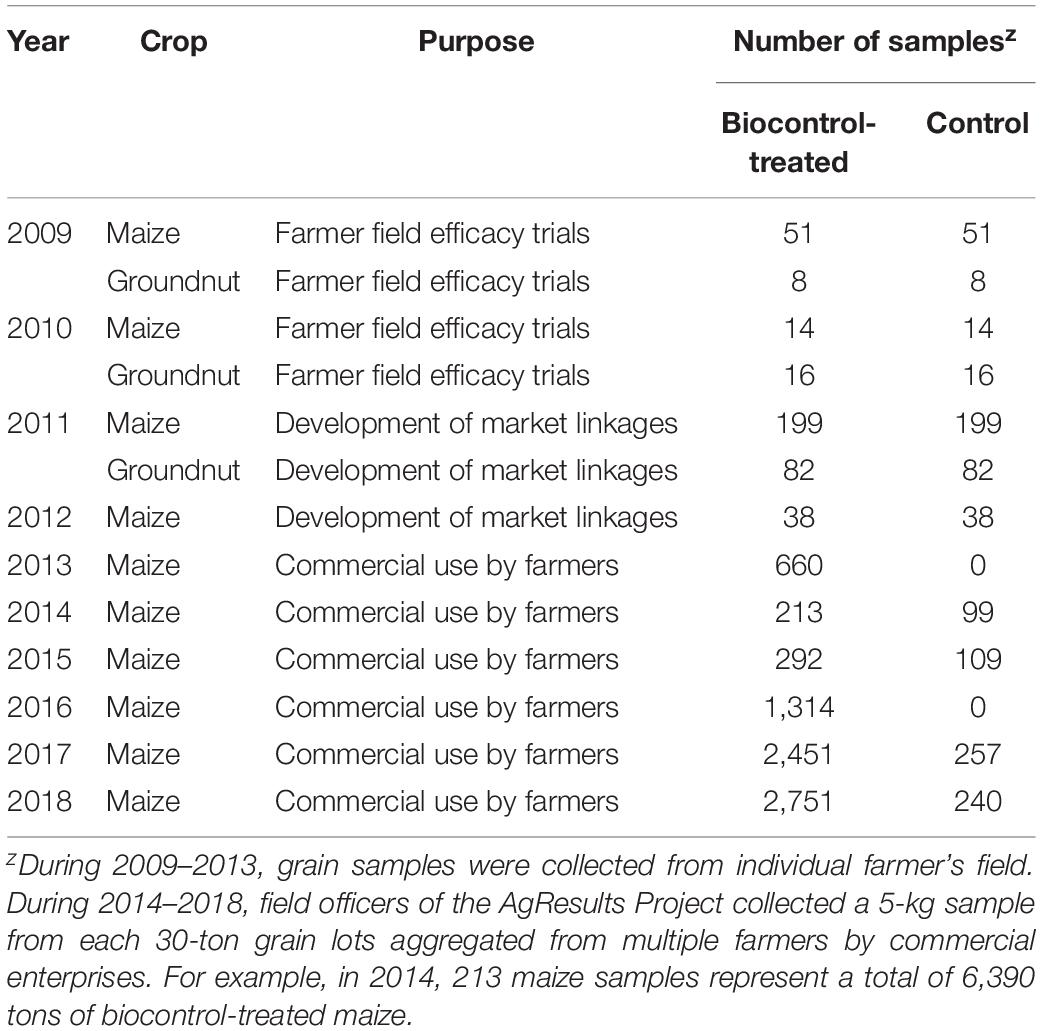
Table 1. Number of maize and groundnut samples from fields treated with an aflatoxin biocontrol product and accompanying untreated fields (field efficacy trials, 2009–2012) and samples taken from commercially treated and control maize (2013–2018).
Biocontrol Product Manufacturing
For the field trials (2009–2012), the biocontrol product was produced using a laboratory-scale method described earlier (Cotty et al., 2007; Atehnkeng et al., 2014). Briefly, spore suspensions of each of the four atoxigenic isolates were harvested from 5-day-old cultures on 5-2 agar [5% V8 juice (Campbell Soup Company, Camden, NJ, United States), 2% Bacto-agar (Difco Laboratories Inc., Detroit, MI, United States), pH 5.2 (Cotty, 1994)] using 0.1% TWEEN® 80. Suspensions were adjusted to a concentration of 106 spores ml–1 using a hemocytometer. White sorghum grains were soaked in water for 2 h, drained, and autoclaved for 45 min in polyethylene bags (45 cm × 20 cm). Batches of 1-kg of grain were seeded independently with 100 ml of spore suspensions of each atoxigenic active ingredient isolate, mixed to spread the suspension uniformly, and incubated for 18 h at 31°C. Then, grains were dried at 55°C for 4 days to stop fungal growth. Several batches were prepared for each active ingredient isolate. The final product was formulated by combining batches of 2.5 kg of each isolate into a polyethylene bag and mixed by hand shaking. Product in the polyethylene bags were placed within 10-kg-capacity plastic containers, sealed, and stored at room temperature until use.
The biocontrol product applied in commercial maize fields (2013–2018) was produced in the Aflasafe Manufacturing Plant in IITA-Ibadan (Bandyopadhyay et al., 2016). Briefly, spores of the four atoxigenic isolates were obtained as mentioned above for field trials, and each isolate was multiplied separately in glass bottles containing sterilized sorghum grains that were pre-conditioned before in sterile 1-l plastic bottles. The pre-conditioning process raised the moisture content of sorghum grain to 30% by adding sterile distilled water to the bottles, which were subsequently rolled for 4 h on a 240 Vac Benchtop Roller (Wheaton, Millville, NJ, United States). Pre-conditioned grain (30 g) were added to 250-ml glass bottles along with two Teflon balls (1/2′′ dia) and autoclaved (20 min, 121°C). Each cooled bottle containing sorghum was independently inoculated with 4 ml of spore suspension of each atoxigenic isolate. After incubation (7 days, 31°C), 125 ml sterile 0.1% TWEEN® 80 was added to each bottle to harvest spores. Bottles were placed on a Roto-Shake Genie reciprocal shaker (Scientific Industries, Bohemia, NY, United States) at 200 rpm for 20 min. The Teflon balls helped to dislodge spores from sorghum grains. For each atoxigenic isolate, a suspension was adjusted to 4 × 107 spores ml–1 using an Orbeco-Helling digital direct reading turbidimeter (Orbeco Analytical Systems Inc., Farmingdale, NY, United States) and a nephelometric turbidity unit (NTU) vs. CFU (colony-forming units) standard curve (y = 49,937x; x = NTU; y = spores ml–1). To prepare 100 kg of the product, a spore suspension (1 l, 4 × 107 spores ml–1) of the constituent atoxigenic isolates were individually combined with 150 ml of a polymer (SentryTM, Precision Laboratories, Waukegan, IL, United States) and 200 ml of a blue non-toxic dye (PrismTM, Milliken and Company, Spartanburg, SC, United States), and coated on roasted, sterile sorghum grain with a seed treater (Bandyopadhyay et al., 2016).
Quality of the Biocontrol Product
Samples from biocontrol product batches produced either in the laboratory or the manufacturing plant were examined. For each sample, 100 sorghum grains were plated onto two plates each of 5-2 agar, Nutrient Agar (Lab M, United Kingdom; 28 g l–1, 20 g l–1glucose), and Violet Red Bile Agar (VRBA; Difco Laboratories, 41.5 g l–1, pH 7.4). After incubation (7 days, 31°C) plates were examined to count numbers of grains colonized by A. flavus and to detect presence or absence of any other microorganism, including fecal coliforms on VRBA. Spore yield was evaluated by placing 24 grains from each batch in individual wells of 24-well cell culture plates, in triplicate, and incubated as above. After incubation, three replicates of two randomly selected grains were rinsed three times with 10 ml 100% ethanol. The resulting wash from each replicate was mixed with 10 ml distilled water and transferred into a turbidimeter vial. Spore yield was quantified by turbidity as above.
Frequencies of the atoxigenic AAVs to which the four active ingredient isolates belong to were determined in all biocontrol product batches. Microbial isolations were done following protocols previously described (Atehnkeng et al., 2008a; Agbetiameh et al., 2018). Aspergillus isolates were characterized and saved as previously described (Agbetiameh et al., 2018; Ezekiel et al., 2019). Mutants of A. flavus isolates (nit) were generated and saved as previously described (Atehnkeng et al., 2014, 2016). Assignment of isolates to one of the four atoxigenic AAV ingredients of the biocontrol product was conducted by performing vegetative compatibility analyses (VCA). In VCA, fungal suspensions of each atoxigenic AAV tester pair and a nit mutant being evaluated were individually seeded into wells, spaced by 1 cm in a triangular pattern, in starch media (36 g l–1 dextrose, 20 g l–1 soluble starch, 3 g l–1 NaNO3, 20 g l–1 Bacto-agar, pH 6.0) (Cotty and Taylor, 2003). Complementary nit mutants produce regions of prototrophy indicating restoration of a functional nitrate reductase enzyme (Leslie, 1993). Complementation occurs only between nit mutants isogenic at loci governing vegetative incompatibility. Nit mutants complementing a tester pair were assigned to the atoxigenic AAV defined by that tester pair.
Farmers’ Fields
Efficacy Trials
During 2009–2012, efficacy of the biocontrol product was tested by smallholder farmers who voluntarily consented to participate in the experiments in Enugu North, Isi-Uzo, Oji River, and Uzo-Uwani (Enugu state); Birnin-Gwari, Lere, Maigana, Giwa, Soba, and Ikara (Kaduna state); Gwarzo, Tudun Wada, Tsanyanwa, Danbata, Rinin Gado, Rano, Dawakin, Tofa, Albasu, Doguwa, and Warawa (Kano state); and Ogbomosho (Oyo state) (Figure 1). All participating farmers along with agricultural extension agents and trainers were made aware of aflatoxins and their impact (e.g., what aflatoxins are, occurrence in crops, impact on health and trade) and trained on use of biocontrol and other management practices. The on-farm efficacy of the biocontrol product was evaluated using paired plot experimental design (Supplementary Table 1). For each treated maize and groundnut field, there was an accompanying untreated control field of the same crop separated by at least 500 m to avoid interference from movement of biocontrol isolates from treated to control fields (Bock et al., 2004). To avoid carryover influence of inoculum from 1 year to the next, no treated field used in any year was used in subsequent years. Willingness of farmers to participate in the efficacy trials and the crops grown by them also determined the site of the treated and untreated fields. The size of fields ranged from 0.25 to 5.0 ha. Due to interplot interference from movement of inoculum from treated area to control area in small plot settings (Weaver and Abbas, 2019), it was not possible to replicate treatments several times within the small-sized farmer’s fields. Instead, an entire farmer’s field was considered as an experimental unit (replicate) and was either treated or untreated. The number of replicates varied from year to year and are given in Table 1. Farmers grew their crops following their own agronomic practices. In general, farmers’ fields were weeded, earthed-up (i.e., piling up soil around the base of the plants), and top-dressed with urea prior to application of the biocontrol product. Within 15 days post biocontrol product application, no other agronomic intervention was made to avoid burying the product. All fields were rain-fed. The number of treated and control fields for each year is given in Table 1. The biocontrol product was uniformly tossed across the field by hand, at a rate of 10 kg ha–1. Prior to application, areas of fields were measured using Garmin eTrex GPS units (Garmin Ltd., Olathe, KS, United States) to determine the quantity of product required to treat individual fields. Extension agents, trainers, and/or IITA staff supervised biocontrol product application.
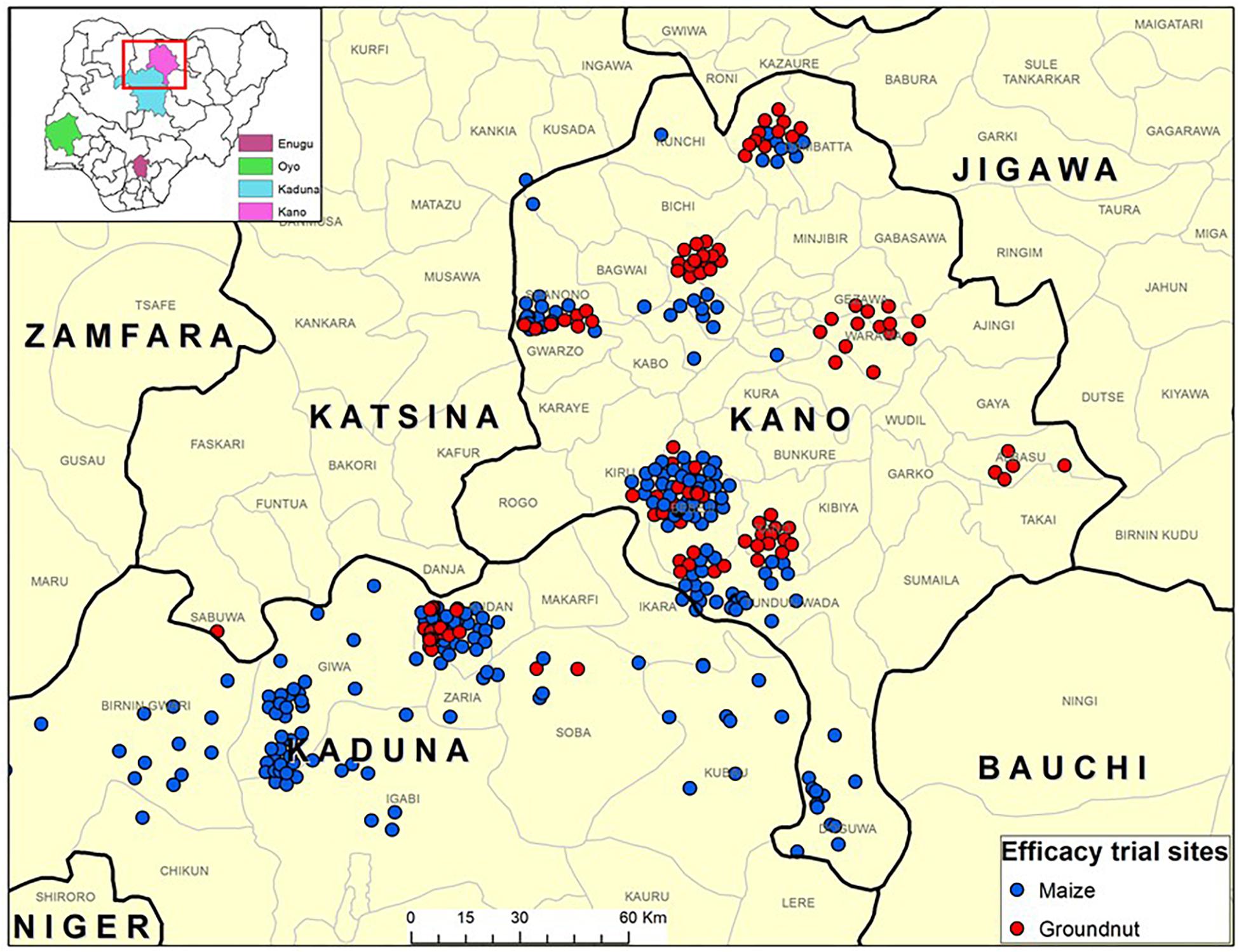
Figure 1. Map of various states of Nigeria (inset) where efficacy trials of an atoxigenic biocontrol product were conducted by maize and groundnut farmers for aflatoxin mitigation during 2009 to 2012. Majority of the trials were conducted in Kano and Kaduna states while a few were conducted in Enugu and Oyo states (inset).
Commercial Use
During 2013–2018, biocontrol was used only in commercial maize fields by farmers and implementers of the AgResults Project (Bandyopadhyay et al., 2016; Schreurs et al., 2019). Briefly, implementers were private (mostly) or public sector enterprises that worked with groups of smallholder farmers and enabled them to produce biocontrol-treated maize. Implementers purchased and distributed the biocontrol product to farmers and had mutually agreed cost-recovery and profit-sharing arrangements. Implementers were selected by the AgResults Project based on several criteria: (i) ability to organize and coordinate smallholder farmers throughout the cropping season, (ii) ability to provide extension services and access to farm inputs, (iii) possess downstream market linkages to efficiently aggregate and sell quality crops at a premium, and (iv) committed to maximizing transparency, disclosing records, and document-sharing of premium payments or other benefits to their participating farmers, among others. Implementers were trained on improved maize production practices and both pre- and post-harvest aflatoxin mitigation strategies, including use of biocontrol. Once trained, the implementers passed the acquired knowledge to their farmers. During the project, in any given year, some implementers worked with less than 100 farmers while others worked with over 9,000 farmers (Figure 2). The biocontrol product was applied in thousands of fields in the states of Benue, Edo, Ekiti, Enugu, Gombe, Kaduna, Kano, Kogi, Ogun, Osun, Oyo, Plateau, Taraba, Zamfara, and the Federal Capital Territory (FCT) (Figure 3). It is likely that several farmers applied the product in the same field for more than 1 year leading to an unknown extent of carryover effect of biocontrol inoculum from 1 year to the next. As much as possible, samples from non-treated maize were collected from fields about 500 m away from those of participating farmers to enable comparison of paired treated and non-treated samples. In contrast to the 2009–2012 period, the number of control samples was lower than the number of treated samples in 2014, 2015, 2017, and 2018 (Table 1). Control samples were not collected in 2013 and 2016.
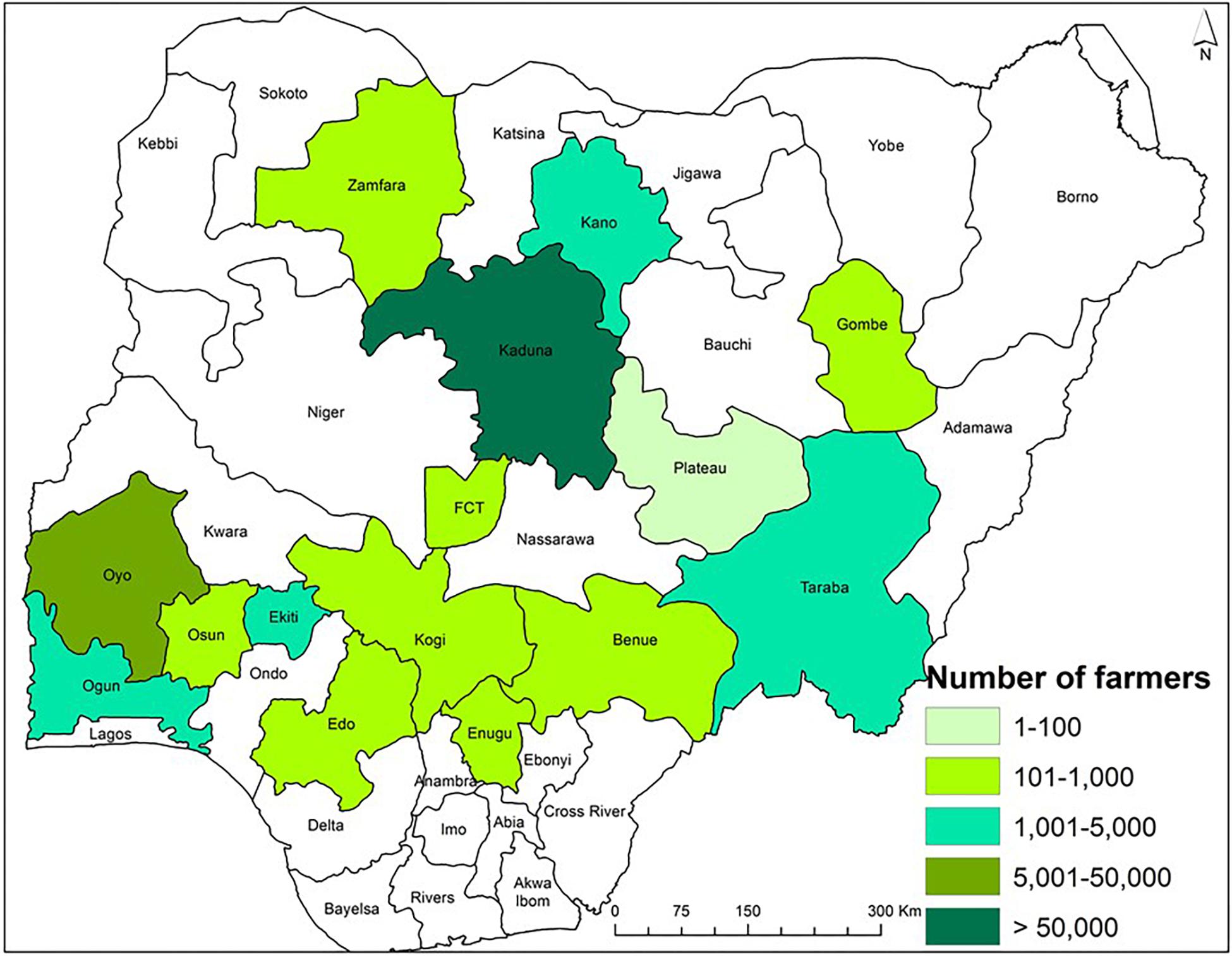
Figure 2. Map of Nigeria showing the number of farmers that participated in the AgResults Project and applied an atoxigenic biocontrol product in maize in various states during 2013 to 2018.
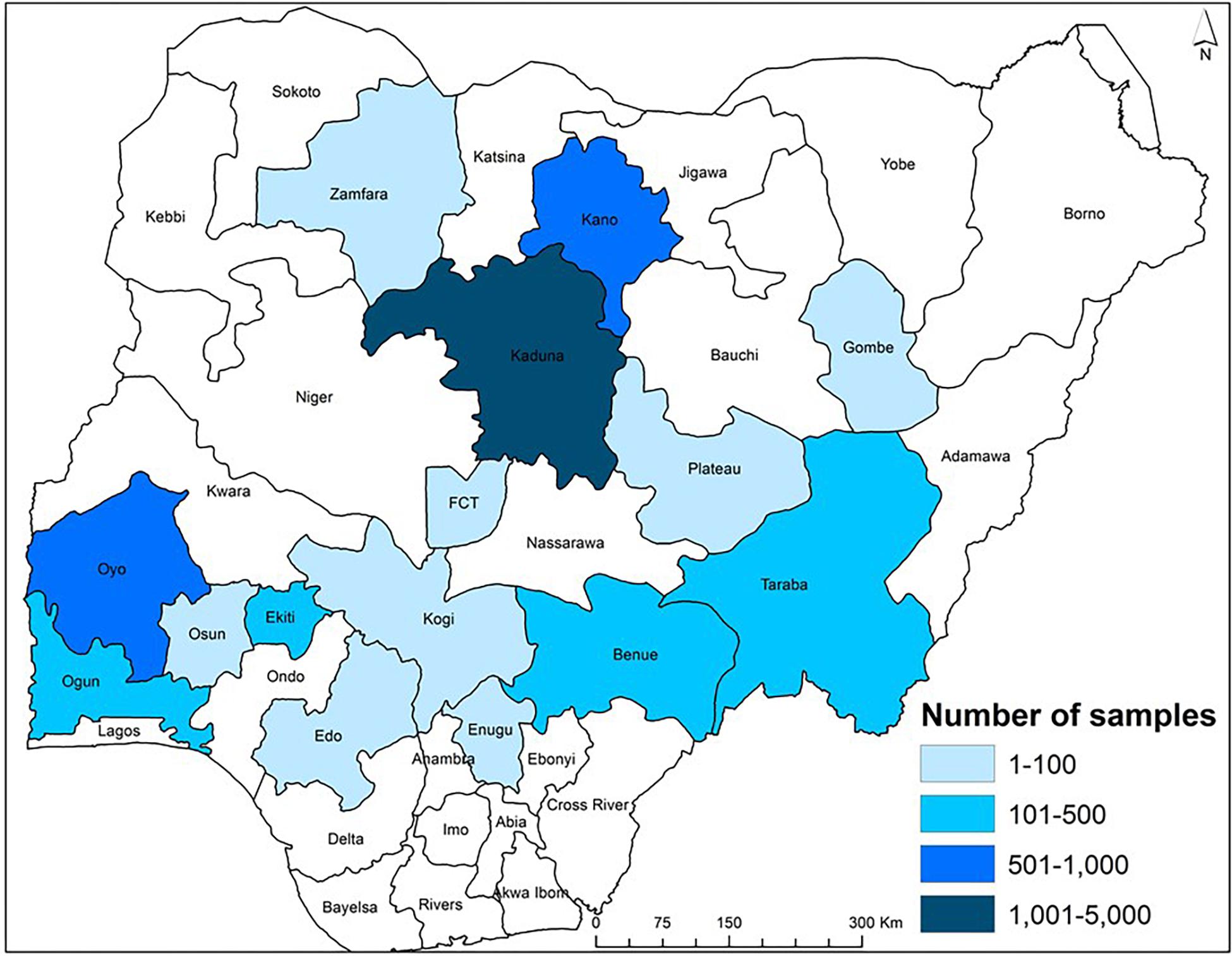
Figure 3. Map of Nigeria showing the range in the number of maize samples collected from aggregation points in various states during 2013 to 2018. Each sample represents approximately a 30-ton grain lot aggregated from farmers who used an atoxigenic biocontrol product on a commercial basis.
Sampling and Sample Treatment
In both 2009 and 2010, soils of all treated and control fields were collected prior to biocontrol application. Soil from the top 2-cm layer were collected by sub-sampling (∼50 times) from three random locations within each field (Cotty, 1997). Composite samples were placed in cotton bags inside sealed plastic bags and transported to IITA-Ibadan Pathology laboratory. Samples were dried at 48°C for 48 h. Following elimination of soil clods in sterile mortars, samples were manually homogenized within the bags, and then stored at 4°C until subjected to further analyses.
During 2009–2012, 25 maize ears were randomly harvested by hand from treated and control fields. The ears were placed in paper bags and transported to the laboratory. Aflatoxins were quantified on two sets of grain: (i) grain immediately after harvest (15 ears) and (ii) grain subjected to simulated poor storage where 10 ears with husks were wetted for 4 h and allowed to dry slowly over a 10-day period, followed by drying at 48°C for 48 h. Ears from each set were husked and shelled for aflatoxin analysis (Atehnkeng et al., 2014). Sub-samples (500 g) were ground using a blender (Waring Commercial, Springfield, MO, United States) for 1 min in a 250 ml stainless steel blending jar (MC-2), which was decontaminated between samples with 80% ethanol to avoid cross-contamination. Blended and unblended grain samples were kept at 4°C until analyzed. During 2009–2011, groundnut was randomly harvested by hand from treated and control fields. Five kg of unshelled groundnut were placed in paper bags and transported to the laboratory. Aflatoxins were quantified on two sets of grains: (i) grain immediately after harvest (4 kg, 2009–2011) and (ii) grain (1 kg, 2010–2011) subjected to the simulated poor storage conditions, as described for maize. Pods from each set were manually shelled inside a biological safety cabinet and dried as above. Milling and storage of samples was done as above.
For maize from commercial fields (2013–2018), farmers harvested the ears and the implementers aggregated the maize in their stores. Some implementers assisted farmers with threshing, bagging, labeling, and transporting the maize to aggregation stores. At the aggregation point, maize bags (containing around 100 kg maize) were tagged with farmers’ details and arranged according to the quantity supplied by each farmer. Since it was not possible to quantify aflatoxin concentration in individual fields of the >90,000 farmers, grain samples for each 30 tons of treated maize were examined, except for 2013 when samples were collected from individual farmers’ fields. Field officers of the AgResults Project collected a 5-kg sample for each 30 tons of aggregated maize by randomly sampling 50 g of maize from each of 100 bags. The samples were transported to IITA-Ibadan, where they were homogenized, milled, and stored as above.
Densities of Aflatoxin-Producing Fungi in Soil Before Biocontrol Product Application and Grain at Harvest
These analyses were conducted for 2009 and 2010 biocontrol field efficacy trials. Aspergillus section Flavi fungi were obtained from soil and grain by dilution plate technique on Modified Rose Bengal Agar (MRBA) (Cotty, 1994). Briefly, 1 g sample was suspended in 10 ml sterile distilled water contained in 40 ml glass vials and vortexed for 1 min. Appropriate dilutions were plated on MRBA and plates were incubated for 3 days (31°C, dark). Incidence of Aspergillus species was calculated as CFU g–1 of sample. Isolates from plates with less than 10 putative Aspergillus section Flavi colonies were transferred onto 5-2 agar and incubated (5 days, 31°C, dark). Aspergillus isolates were assigned to their corresponding species based on colony morphology, spore ornamentation, and aflatoxin production profile (Cotty, 1989; Cotty and Cardwell, 1999; Frisvad et al., 2019). Isolates were saved as agar plugs (3-mm dia) of sporulating cultures in 4 ml vials containing 2 ml sterile distilled water and maintained at room temperature until further characterization.
Vegetative Compatibility Analyses
Frequencies of the AAVs to which the four active ingredient isolates of the biocontrol product belong to were determined in both soils before application and grains at harvest during 2009 and 2010. Mutants of isolates (nit) were generated as previously described (Atehnkeng et al., 2014, 2016). Assignment of isolates to one of the four atoxigenic AAVs was conducted by performing VCA as above.
Quantifying Aflatoxin Concentration in Crops at Harvest and After Poor Storage
For crops examined during 2009–2012 (at harvest and after simulated poor storage), aflatoxins were extracted from sub-samples (20 g ground sample) by adding 100 ml 70% methanol (Atehnkeng et al., 2008a) for maize and 100 ml 80% methanol for groundnut (Cole and Dorner, 1993). Aflatoxin extraction and quantification was conducted as previously described (Atehnkeng et al., 2008a).
For maize from commercial fields (2013–2018), aflatoxins were quantified using GIPSA-approved Neogen Reveal® Q+ for Aflatoxin kit (Neogen Corp., Lansing, MI, United States). Briefly, a 20-g sub-sample was transferred into a 500 ml media bottle and 100 ml 65% ethanol was added. The mixture was shaken for 3 min using an orbital shaker at 200 rpm, allowed to settle for 3 min, and then filtered through Whatman No. 1 filter paper (Whatman International Ltd., Maidstone, United Kingdom) into a Tri-Pour® beaker. Thereafter, 500 μl of sample diluent was transferred to a sample cup and 100 μl of sample filtrate was added. A 100-μl aliquot of diluted sample was transferred into a new cup and mixed thoroughly and mixed by aspiration. A new test lateral flow strip was placed in the sample cup for 6 min ensuring that the strip touched the mixture. Then the strip was removed and read either on a Neogene AccuScan® Pro or Gold Reader.
Data Analysis
Data on CFU g–1, Aspergillus species distribution, incidence of atoxigenic AAVs, and aflatoxin concentration (response variable, x) were transformed using the equation y = log10(1 + x) to stabilize the variance prior to analysis. Depending on the comparisons, means were separated using paired t-tests (PROC T TEST, α = 0.05) or protected Fisher’s least significant difference test (LSD, α = 0.05) using SAS software (version 9.2, SAS Institute Inc., Cary, NC, United States). Untransformed data are presented in summary tables and graphs in this paper.
Results
Quality of the Biocontrol Products
During the 10-year study, 382 batches of the biocontrol product were produced and examined for quality control parameters. Batches include the biocontrol formulation produced using both the laboratory- and industrial-scale processes. The quantity of the biocontrol product produced per year varied (range = 1.54 tons in 2009 to 779.42 tons in 2017). In all examined batches, 100% of carrier grains were colonized by A. flavus. Other microorganisms were not found in any of the examined carrier grains of any of the 382 batches. VCA revealed that the recovered fungi belonged solely to the four atoxigenic AAV active ingredients of the biocontrol product. In general, each of the four atoxigenic AAVs was found on 25 ± 8% of the carrier grains. On an average, there were 3,500 ± 500 CFU g–1 of product.
Fungal Densities in Field Efficacy Trials: 2009 and 2010
Across treated and control fields, Aspergillus section Flavi densities ranged from 304 CFU g–1 to 1,050 CFU g–1 of soil (Table 2). In all cases, population densities in soil prior to biocontrol application were similar (P > 0.05) in treated and control fields. At harvest, maize and groundnut grains from treated fields contained Aspergillus section Flavi densities ranging from 185 CFU g–1 to 4,117 CFU g–1 compared to 72 CFU g–1 to 4,839 CFU g–1 in grains from control fields (Table 2). There were no significant (P > 0.05) differences in CFU g–1 between grains from treated and control fields.
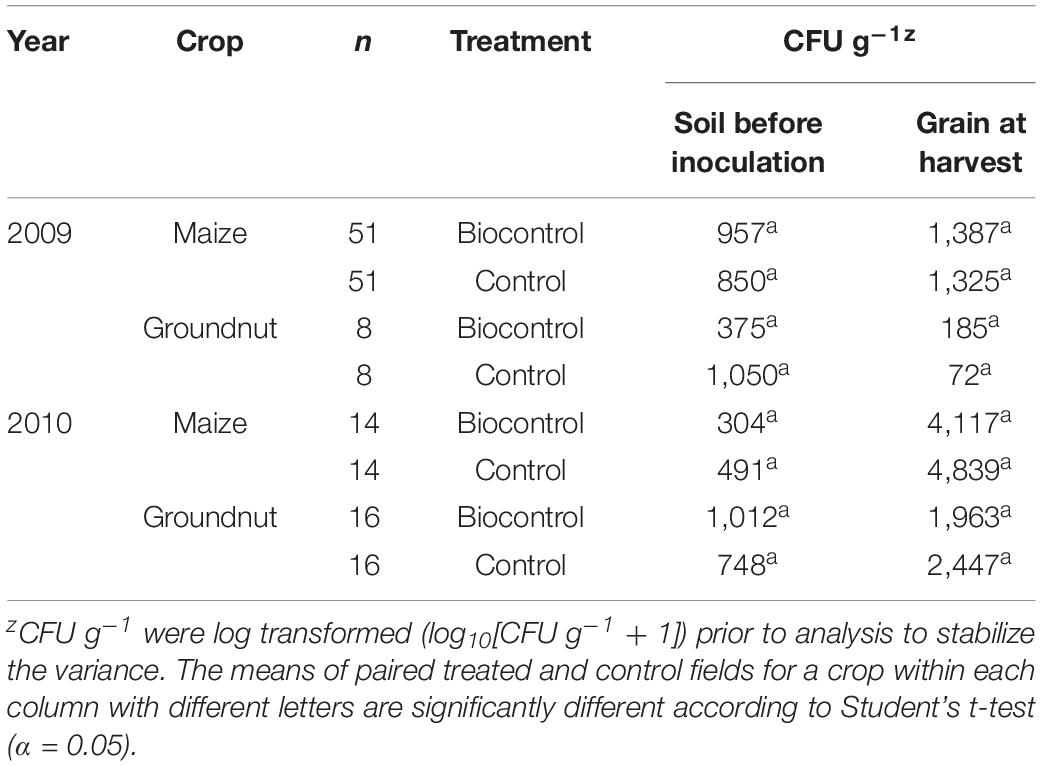
Table 2. Colony-forming units (CFU) g–1 of Aspergillus section Flavi fungi in soil before biocontrol application and in maize and groundnut grains harvested from farmers’ fields that were either treated or not treated during 2009 and 2010.
Distribution of Aspergillus Section Flavi in Field Efficacy Trials: 2009 and 2010
The native Aspergillus fungi found in soil were potentially high aflatoxin producers in all farmers’ fields where efficacy trials were conducted. In 2009, groundnut fields had high levels of SBG strains while in 2010, maize fields had high levels of both SBG strains and A. parasiticus before treatment (Table 3). In treated and control maize and groundnut fields, the A. flavus L morphotype dominated both soils before inoculation and grains at harvest. In the corresponding treated and control fields, frequencies of each fungal type were similar (P > 0.05) except for both the L morphotype and SBG strains in treated groundnut soil during 2009 (Table 3) and treated maize grain during 2010 (Table 3). When comparing fungal types within treated or control samples, in all cases, the L morphotype had significantly (P > 0.05) higher frequencies than the other types (Table 3). Members of the SBG strain, which produce large concentrations of B and G aflatoxins, were rarely detected in treated maize and A. parasiticus was never detected in treated maize or groundnut fields (Table 3). These aflatoxin producers were almost completely replaced by A. flavus L morphotype fungi, to which the four atoxigenic active ingredient isolates of the biocontrol product belong (Table 3).
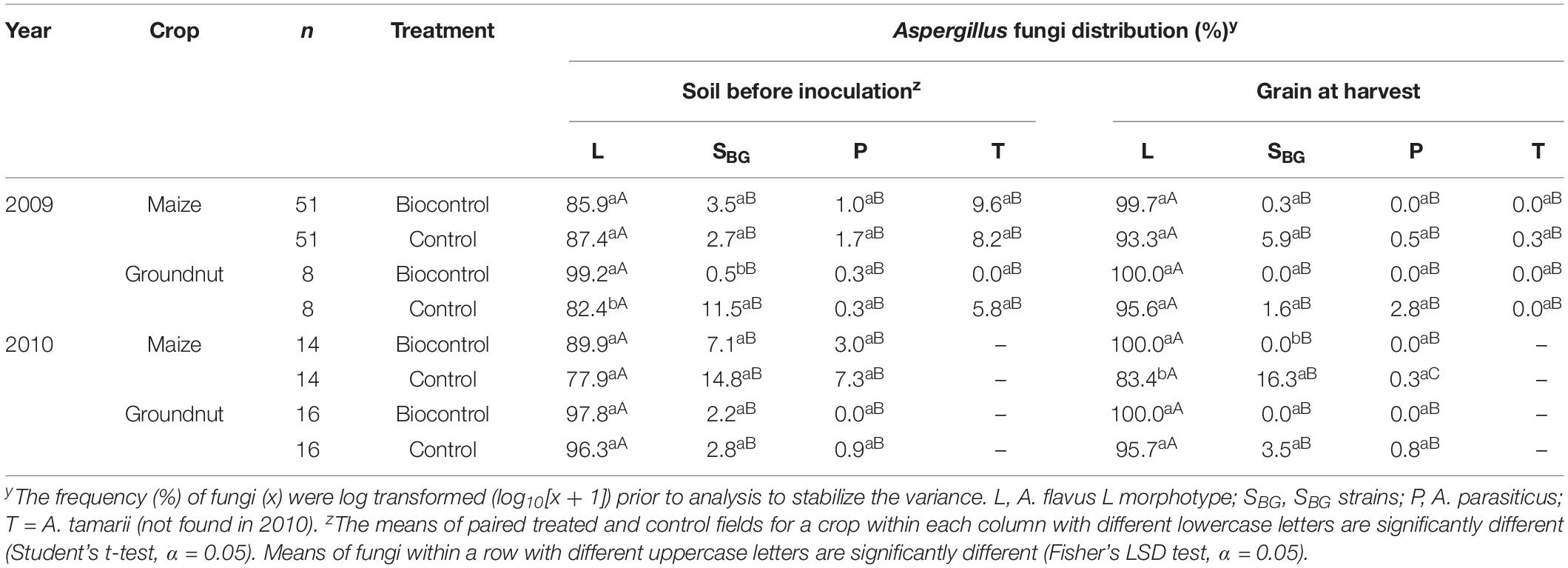
Table 3. Frequencies of Aspergillus section Flavi fungi in soil before biocontrol application, and in maize and groundnut grains from biocontrol -treated and control fields during 2009 and 2010.
Vegetative Compatibility Analyses in Samples From Field Efficacy Trials: 2009 and 2010
In both 2009 and 2010, frequencies of the biocontrol active ingredient AAVs in soils before inoculation were not significantly (P > 0.05) different between fields to be treated (range = 1.1–6.5%) and their respective control fields (range = 0.7–7.8%) (Table 4). The observed frequencies prior to product application demonstrate that the biocontrol active ingredient AAVs are relatively common across the major maize and groundnut producing regions of Nigeria.
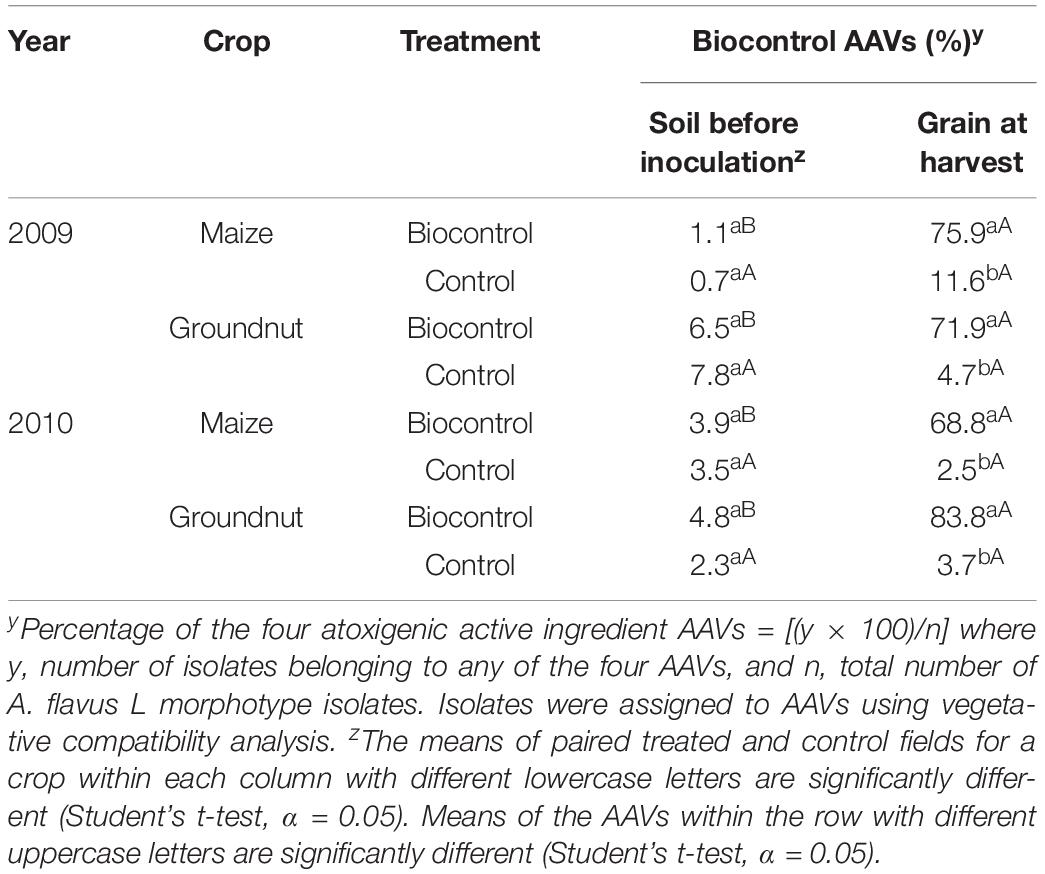
Table 4. Incidence of atoxigenic African Aspergillus flavus vegetative compatibility groups (AAVs) active ingredients of a biocontrol product in soil before its application, and maize and groundnut grains from treated fields and control fields during 2009 and 2010.
Frequencies of the biocontrol product active ingredient AAVs were always higher (P < 0.05) in treated crops (range = 68.8–83.8%) than in control crops (range = 2.5–11.6%). In treated fields, the frequencies of atoxigenic AAVs in soil before inoculation were always lower (P < 0.05) than in grains at harvest (Table 4). In contrast, in all control fields, frequencies of atoxigenic AAVs were similar (P > 0.05) in soils before inoculation and grains at harvest (Table 4).
Frequencies did not differ significantly among the biocontrol product AAVs in soils before treatment in control plots (Table 5). There were, however, some differences (P < 0.05) among atoxigenic AAV frequencies in maize soil to be treated during both 2009 and 2010 and groundnut soil to be treated during 2010 (Table 5). Ka16127 had higher frequencies than the other active ingredients in maize soil during 2009. Both La3279 and Ka16127 had higher frequencies than the other two atoxigenic AAVs in both maize and groundnut soil during 2010. In grains of treated fields, both La3279 and Ka16127 generally dominated communities of both crops during both years (Table 5). In grain from control fields, La3279 had higher frequencies in maize (2009) and groundnut (2010); Ka16127 and La3279 had similar frequencies in groundnut (2010) (Table 5).
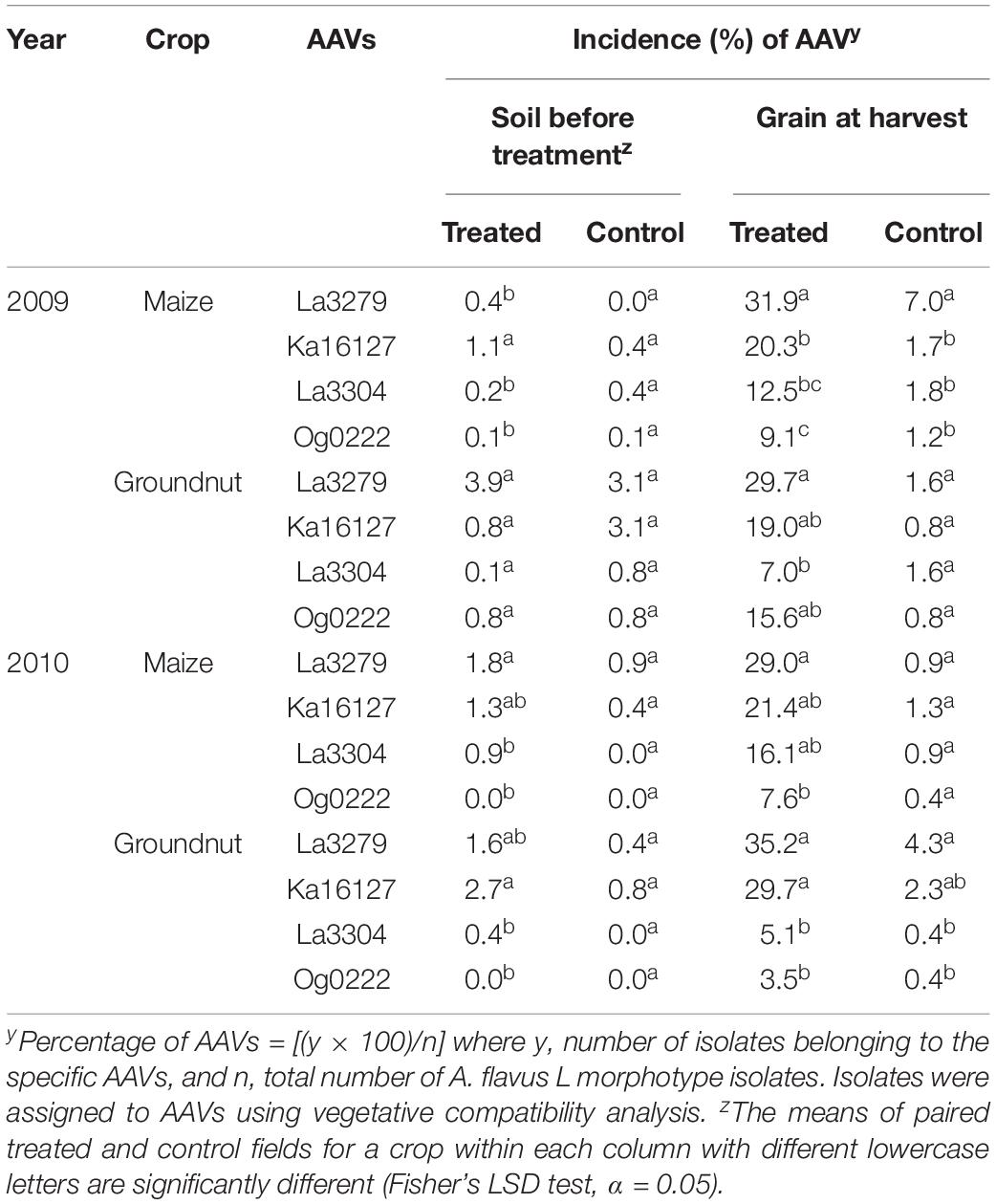
Table 5. Incidence (%) of the four atoxigenic African Aspergillus flavus vegetative compatibility groups (AAVs) constituting an aflatoxin biocontrol product in soil before its application and in maize and groundnut grains from treated and control fields during 2009 and 2010.
Aflatoxin Concentrations in Grain at Harvest and After Poor Storage
At harvest, treated crops from field efficacy trials (2009–2012) generally contained low aflatoxin content and significantly (P < 0.05) less aflatoxins (82–95% less) than untreated crops (Table 6). The only case in which the average of treated crops contained >20 ppb total aflatoxin was maize in 2010 (21 ppb). However, during that year, average aflatoxin content in maize from the 14 examined control fields was 372.4 ppb (Table 6), a high and unsafe level.
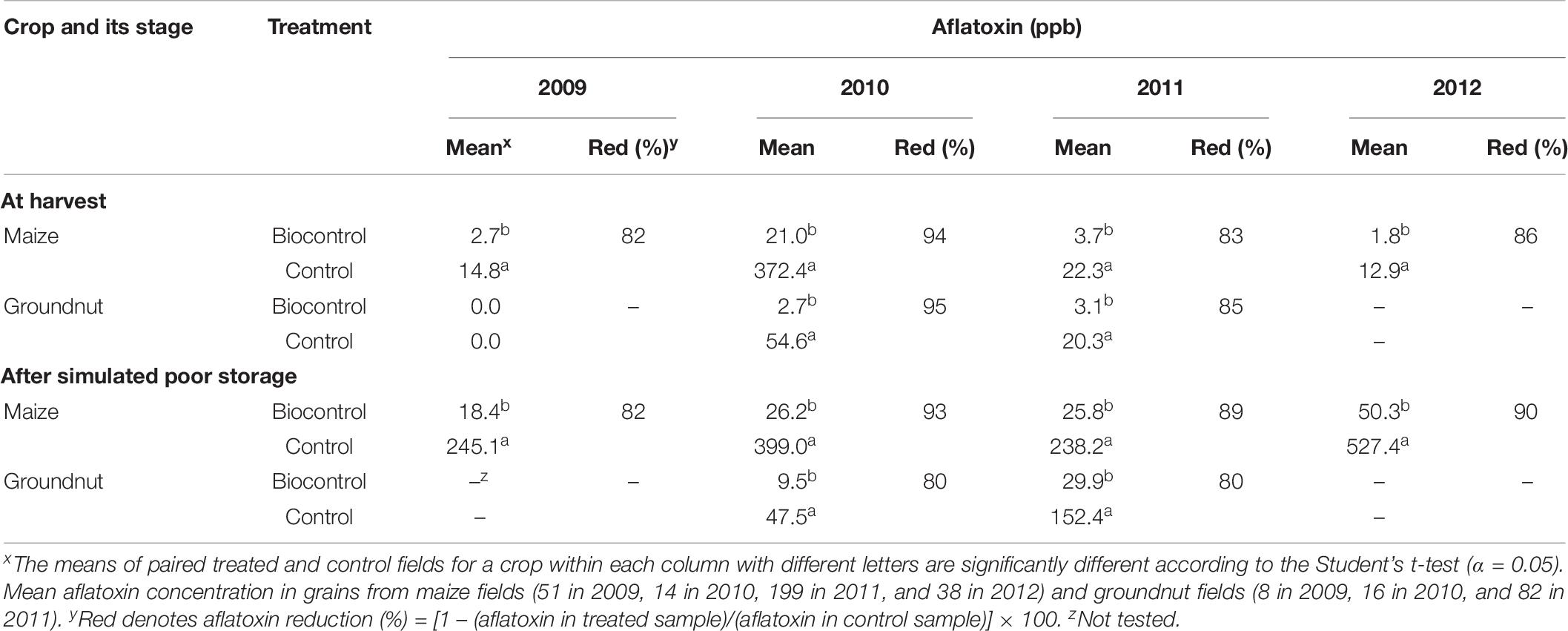
Table 6. Total aflatoxin concentration in freshly harvested and poorly stored maize and groundnut grains from biocontrol-treated and control fields in Nigeria during 2009 to 2012.
When crops were subjected to simulated poor storage conditions, the protection provided by biocontrol continued, with treated crops accumulating 80% to 100% less aflatoxins than untreated crops (Table 6). Aflatoxin content in untreated crops increased dramatically due to simulated poor storage conditions. For example, the total aflatoxin content in maize at harvest from 2009 was 14.8 ppb and after simulated poor storage it increased to 245.1 ppb. Large increases in aflatoxin content occurred in the other poorly stored control grains, with up to 40-fold aflatoxin increase (maize 2012; Table 6).
In commercial maize grain samples, there were 72–94% less aflatoxin in treated maize compared to controls during 2015, 2017, and 2018 (Table 7). During years when aflatoxin levels were <10 ppb in untreated grains (2014 and 2015), aflatoxin reductions provided by the biocontrol were less pronounced (72–76%) compared to years when untreated maize contained high aflatoxin levels (88–94%; Table 7). A few samples with high aflatoxin level were encountered among treated samples in 2016 and 2017 (Table 7). Variance of aflatoxin concentration in grain samples from treated fields was 53% (in 2014) to 99% (in 2018) lower than samples from control fields (Table 7). Although aflatoxin concentration was measured in grains from treated fields in 2013 and 2016, aflatoxin reduction could not be quantified due to lack of samples from control fields.
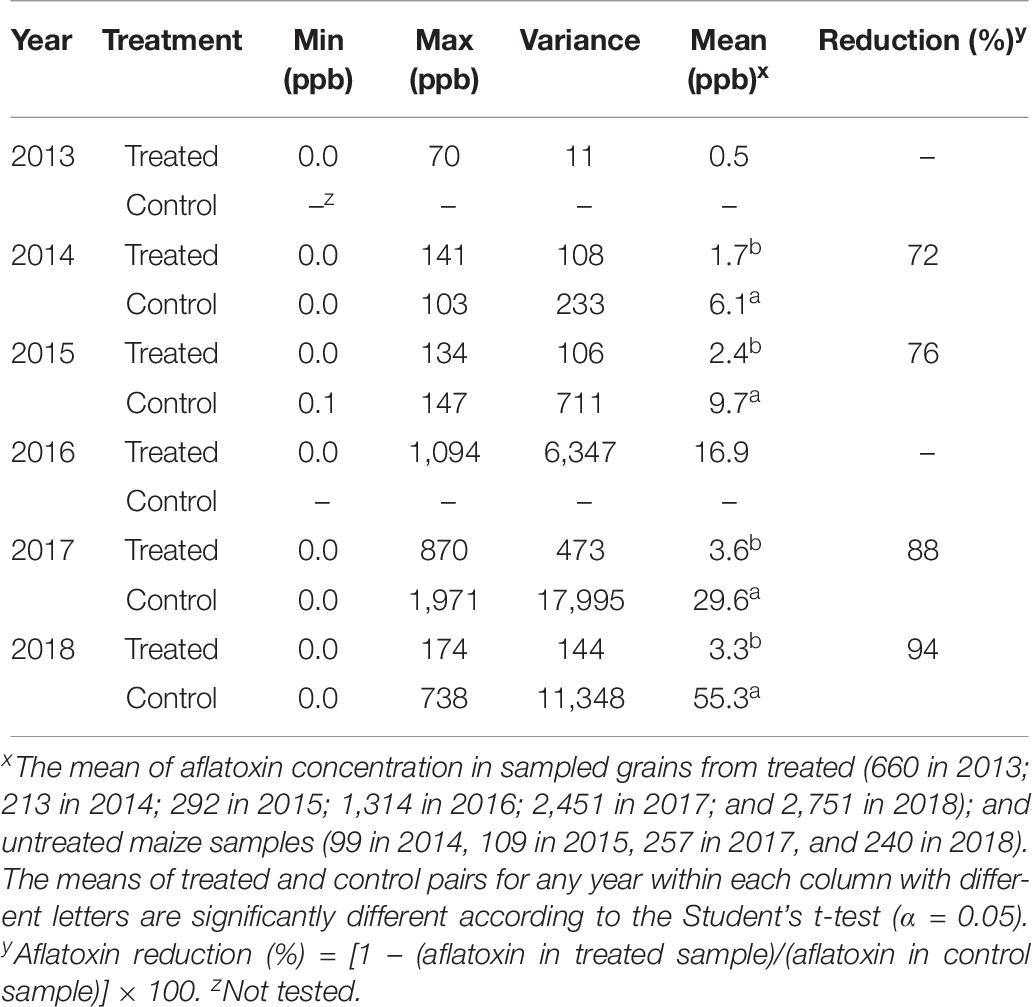
Table 7. Aflatoxin concentration in samples from commercial maize grain aggregated from biocontrol-treated and control fields by commercial enterprises in Nigeria during 2013–2018.
Samples Meeting Standards
Treated crops in efficacy trials had higher proportions of samples below the European Union 4 ppb maximum allowable level for total aflatoxins both at harvest (2009–2012) and after simulated poor storage (2009 and 2010) compared to controls (Table 8). In 2009, 2010, and 2012, none of the treated crops exceeded the US action level of 20 ppb total aflatoxins at harvest. In 2011, only 6.5% of the treated maize and 9.8% of the treated groundnut had >20 ppb total aflatoxins at harvest (Table 8), even though during that year high total aflatoxin content occurred in both control maize (avg. = 372.4 ppb) and control groundnut (avg. = 54.6 ppb) (Table 6). Also see Supplementary Table 2.
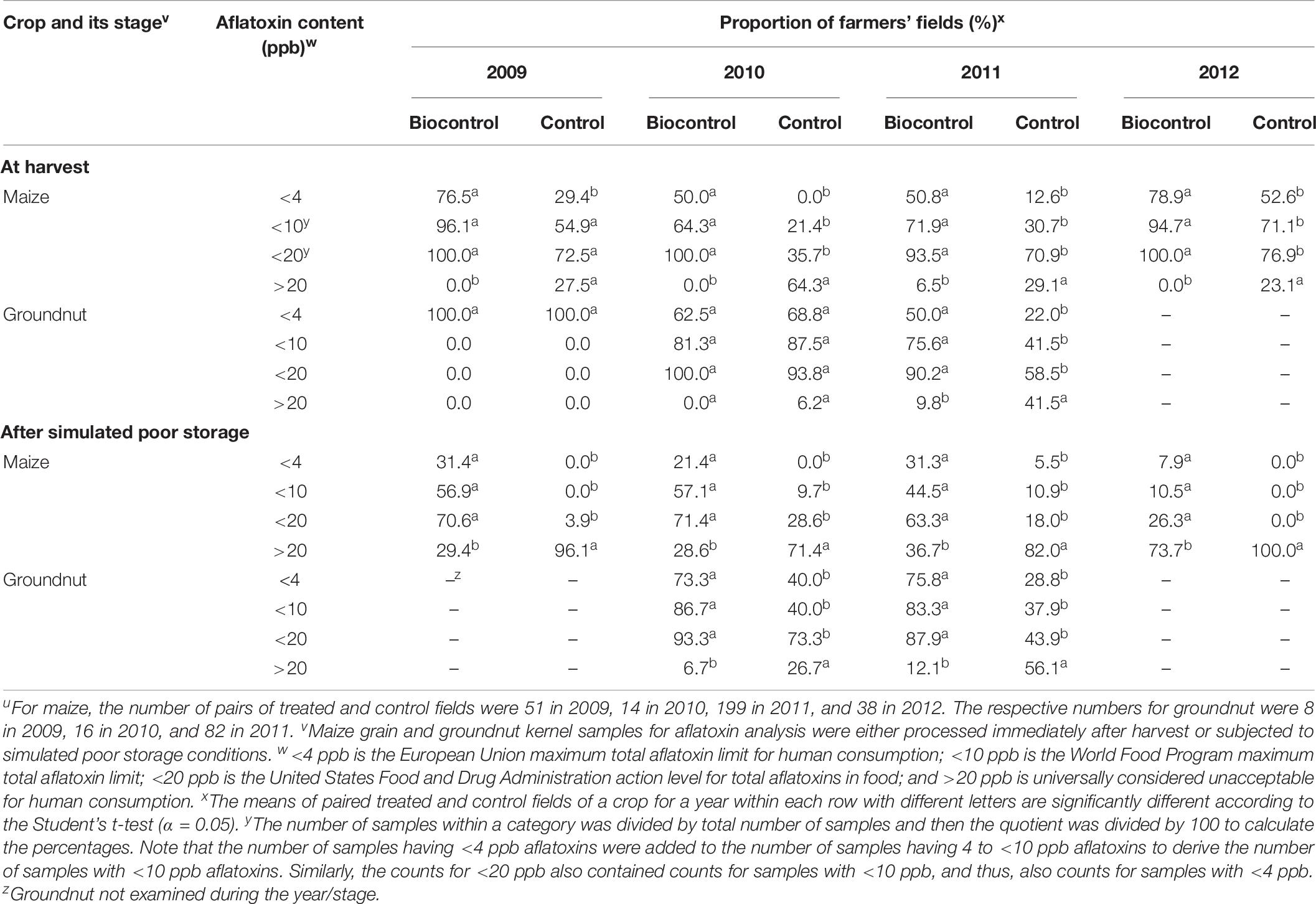
Table 8. Proportion of farmers meeting various total aflatoxin standards in freshly harvested and poorly stored maize and groundnut grains from farmers’ fields that were either treated or not treated (control) with a biocontrol product in Nigeria during 2009 to 2012u.
After simulated poor storage, frequencies of biocontrol-treated crops containing <4 ppb total aflatoxins ranged from 7.9 to 31.4% while for untreated crops the range was 0–5.5% (Table 8). The proportion of crops containing >20 ppb total aflatoxins ranged from 28.6 to 73.7% for treated crops and from 71.4 to 100% for control crops (Table 8).
For commercially produced maize treated with the biocontrol product during the 2013 to 2018 seasons, the proportion of samples containing <4 ppb total aflatoxins ranged from 65.8 to 98.5% (Table 9) with only 0.6–9.7% having >20 ppb total aflatoxins. The proportion of crops from control fields examined in 2014, 2015, 2017, and 2018 containing <4 ppb total aflatoxins were 81, 84, 43, and 25%, respectively. The proportion of control samples containing >20 ppb total aflatoxins in the same years were 9.1, 11.9, 23.0, and 37.1%, respectively (Table 9). Also see Supplementary Table 3.
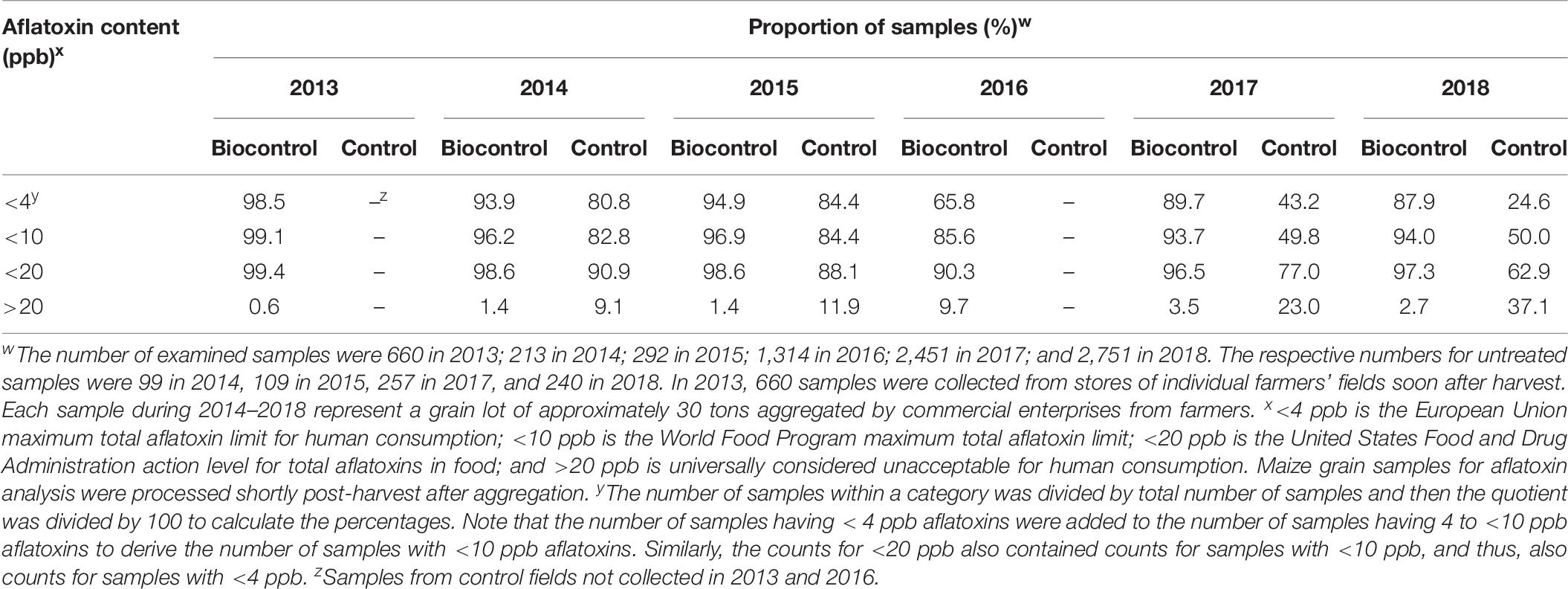
Table 9. Proportion of samples meeting various total aflatoxin standards in freshly harvested maize grain from farmers’ fields that were treated commercially with a biocontrol product in Nigeria during 2013–2018 and from control fields in nearby locations that did not apply the product.
Discussion
The current study provides a decade-long summary of efficacy of the aflatoxin biocontrol product Aflasafe® in maize and groundnut cropped in Nigeria. Aflasafe® is the first aflatoxin biocontrol product registered for use in any African nation and the third worldwide. The 10-year record provides substantial evidence of the stability of the biocontrol product efficacy in limiting aflatoxin content in farmers’ fields across the diverse cropping systems of Nigeria within fluctuating and sometimes challenging environmental conditions. Biocontrol-treated crops became contaminated with significantly less aflatoxins than untreated crops. Most of the treated crops met the stringent tolerance thresholds of local and international food and feed premium markets. A significant proportion (94%) of the 7,681 biocontrol-treated commercial crop samples that were examined had less than the World Food Program maximum limit of 10 ppb total aflatoxins. Initially (2009–2012), biocontrol efficacy was evaluated in a relatively modest number of maize and groundnut fields (14–199 for maize; 8–82 for groundnut; total of 408 treated and 408 control fields). However, the number of fields and the size of each field (∼1 ha) during the 2009–2012 efficacy evaluations were considerably larger than other reported biocontrol efficacy studies that used fewer than five replicates per year with some plots less than 0.01 ha (Alaniz Zanon et al., 2013, 2016; Pitt et al., 2015; Weaver et al., 2015; Molo et al., 2019; Weaver and Abbas, 2019). After the testing and refinement phase, aflatoxin biocontrol usage in Nigeria grew exponentially through the AgResults Project (2013–2018) in which thousands of farmers used biocontrol and other good agricultural practices. Over 210,000 tons of maize with acceptable aflatoxin concentrations (<10 ppb) were produced through the AgResults Project. A large portion of the commercially produced biocontrol-treated maize entered premium markets in Nigeria while the other portion was either consumed by the farmers and their families or sold in local, informal markets (Schreurs et al., 2019). Reduced aflatoxin exposure brought health benefits for maize famers and consumers, and economic benefits for farmers and associated industries (Narayan et al., 2019).
Several crops grown in Nigeria frequently become contaminated with unsafe aflatoxin concentrations (Bandyopadhyay et al., 2007; Singh and Cotty, 2017; Ezekiel et al., 2018, 2019; JECFA, 2018) leading to unacceptable aflatoxin exposure in humans (Ezekiel et al., 2014). This negatively impacts health, income, and trade. One example is lack of access of Nigerian groundnuts to European markets due to high aflatoxin levels. Agricultural, climatic, cultural, infrastructural, and institutional practices and/or conditions all contribute to aflatoxin contamination (Bandyopadhyay et al., 2016). Therefore, aflatoxin management is best with a holistic approach (Ayalew et al., 2017; Logrieco et al., 2018). Intervention during the initial stages of crop infection by aflatoxin producers is a desired component of such strategies and use of atoxigenic A. flavus as biocontrol agents during crop development is effective (Cotty and Mellon, 2006).
Improvement in Biocontrol
The atoxigenic biocontrol technology developed by USDA-ARS uses a single atoxigenic genotype as active ingredient fungus (Cotty et al., 2007). This strategy protects crops from field to plate because after sporulating in the field and becoming associated with the treated crop, the beneficial fungi move with the crops to storage and continue to prevent aflatoxin production should conditions for production again become favorable (Mehl et al., 2012; Atehnkeng et al., 2014; Bandyopadhyay et al., 2016; Senghor et al., 2019). The adaptation and improvement of the technology for use in SSA included use of four atoxigenic AAVs as active ingredient fungi rather than a single isolate (Bandyopadhyay et al., 2016). Use of multiple atoxigenic isolates belonging to diverse AAVs may provide longer-term protection compared to using single genotypes (Probst et al., 2011; Mehl et al., 2012). In the current study, four atoxigenic AAVs native to Nigeria were used in a biocontrol formulation to limit maize and groundnut aflatoxin content.
Testing Under Natural Setting and Product Registration
When IITA and USDA-ARS initiated talks with NAFDAC, the biopesticide regulator in Nigeria, to develop an aflatoxin biocontrol program for Nigeria, it was decided that the work would require applications in multiple fields covering several agro-ecologies over multiple years. Testing the biocontrol product under natural settings without researcher interventions (i.e., farmers used their traditional practices) other than farmer training in application method and provision of the biocontrol product for a single application allowed testing under real-life situations that smallholder Nigerian farmers face. A natural-setting strategy, although in large-scale commercial agriculture, was conducted during efficacy trials to register the biocontrol products AF36 (Cotty, 2006) and Afla-Guard® (Dorner, 2004) in the United States (USEPA, 2003, 2004). The results from 2009 and 2010, used for preparing a dossier for registration, demonstrated that, compared to control, biocontrol application (i) did not increase fungal densities in treated crops (Table 2), (ii) significantly lowered frequencies of aflatoxin producers in treated crops (Table 3), (iii) promoted a higher incidence of atoxigenic AAVs in treated crops (Tables 4, 5), and (iv) lowered aflatoxin content in treated crops both at harvest and after simulated poor storage (Tables 6, 7). Because of these results, in 2014 NAFDAC approved the unrestricted use of Aflasafe® for aflatoxin mitigation in maize and groundnut throughout Nigeria. The registration allowed thousands of smallholder farmers in Nigeria to use biocontrol commercially for producing aflatoxin standard-compliant maize. In addition, the biocontrol product has also been proven to effectively limit aflatoxin contamination of chili peppers although its registration with NAFDAC for unrestricted use in chili peppers is still pending (Ezekiel et al., 2019).
Aspergillus Community Modulation by Biocontrol
In treated crops, A. flavus L morphotype predominated, and most of the L morphotype isolates belonged to the atoxigenic active ingredient AAVs of the biocontrol product (Table 4). The high frequency of atoxigenic AAVs in grains from treated crops illustrate excellent competitiveness for displacing aflatoxin producers. Across substrates and years, AAVs La3279 and Ka16127 were consistently the most commonly recovered genotypes in treated fields (Table 5). La3279 and Ka16127 were also the most commonly recovered genotypes from treated fields of the preliminary experiments (Atehnkeng et al., 2014). Both genotypes appear to be more competitive than the two other atoxigenic AAVs composing the biocontrol product. Some of the factors that affect the competitiveness of biocontrol isolates are differential ability to infect and colonize hosts/debris, produce spores, disperse from soil to crop, cause secondary infection, among others (Mehl et al., 2012). The observed low aflatoxin levels in the treated crops are a direct consequence of the almost complete replacement of high aflatoxin producers by the atoxigenic AAVs composing the biocontrol product. Even when SBG strains and A. parasiticus occur at a relatively low frequency, as in the control crops (range = 0.3–16.3%; Table 3), aflatoxin levels can reach to over 300 ppb (maize in 2010; Table 6). Therefore, the biocontrol application promoted communities with less aflatoxin-producing potential and consequently lower aflatoxin levels were detected in treated crops both at harvest and after the simulated poor storage.
One of the advantages of changing the Aspergillus community structure in favor of the applied atoxigenic AAVs is that the safer Aspergillus community structure translates to not only nearly uniform low aflatoxin levels but also reduced variance in aflatoxin concentration in grains. Relatively recently, it was reported that Aflasafe SN01® usage in Senegal resulted in reduced variance in aflatoxin content of treated crops in efficacy trials in farmers’ fields in groundnut and maize (Senghor et al., 2019). Our studies on maize in commercial fields in Nigeria confirmed the previous observations in Senegal. Low variance in aflatoxin content indicates that sample to sample variations in aflatoxin concentration are low. This reduces the risk that lots measured as low in aflatoxin would actually have unacceptable aflatoxin content and be rejected after receipt in a location with high value markets. Thus, biocontrol treatments might be expected to reduce both aflatoxin-concentration heterogeneity and costs associated with shipment of crops that ultimately become rejected and destroyed or turned away at the destination. High variance in control grain lots suggests significant risk of undetected aflatoxin contamination in the absence of biocontrol treatment. Maximum aflatoxin level in a sample from treated maize reached 1,094 ppb in a grain lot of 2016. Thus, although the sample was considered as treated, the high aflatoxin content suggests that some or few of the farmers that contributed to that 30-ton lot did not apply the biocontrol product at the indicated rate and/or at the mandatory maize growth stage (2-to-3 weeks before flowering).
Aflatoxin Reduction by Biocontrol
Even though use of biocontrol results in excellent aflatoxin reductions at harvest and after storage (Tables 6–9), there were cases in which the protection provided by biocontrol was insufficient to limit aflatoxin content to below tolerance thresholds. For example, treated maize in 2010 had an average total aflatoxin content of 21 ppb (Table 6) and, in 2016, 10% of treated samples contained >20 ppb total aflatoxins (Table 9). In addition, if treated crops were stored poorly, aflatoxin content, although dramatically lower than poorly stored control crops, may increase above tolerance thresholds (Table 6). Therefore, it is critical to use biocontrol along with all other available, practical management practices. Relatively recently, use of biocontrol without other intervention was considered a pitfall (Njoroge, 2018). One of the objectives of the preliminary experiments (Atehnkeng et al., 2008b, 2014) and of the field efficacy trials reported in the current study was to demonstrate the value of biocontrol with no other intervention. This allowed determining the extent of protection by biocontrol alone. Aflatoxin reduction by biocontrol was prominent when aflatoxin pressure was high. In years when biocontrol efficacy resulted in less than 80% reduction, the aflatoxin content in untreated crops was low (<10 ppb total aflatoxin). Therefore, the efficacy of biocontrol in those years was not as obvious as in years where higher aflatoxin concentrations occurred in control crops (Table 6). Our investigations during 2009 to 2012 revealed that the atoxigenic AAVs of the biocontrol product are effective at limiting contamination well over 70% and up to 100% even in the absence of improved agronomic and storage practices. Certainly, if other management strategies were used during both the initial studies (Atehnkeng et al., 2008b, 2014) and the farmer-field efficacy trials (2009–2012) reported in the current study, the aflatoxin concentrations both at harvest and after storage would have been even lower, especially after storage since at harvest most treated crops contained low total aflatoxin levels.
Protection provided by atoxigenic genotypes during post-harvest conditions has been questioned (Villers, 2014; Ehrlich et al., 2015; Gressel and Polturak, 2018). However, treated crops become associated with high frequencies of the applied fungi before harvest (Tables 4, 5). If conditions for fungal infection and aflatoxin formation occur during storage, most Aspergillus fungi growing in stored treated crops would be the applied atoxigenic isolates. Less aflatoxin content occurred in treated crops compared to untreated crops (Table 6) and this is attributed to high frequencies of atoxigenic AAVs composing the biocontrol product in the treated crops (Tables 4, 5). Thus, treating crops with the biocontrol product during crop development provided protection during deliberately poor post-harvest storage (Table 6). Post-harvest benefits when treating groundnut with Aflasafe SN01® in Senegal (Senghor et al., 2019) and maize with an experimental biocontrol formulation in Nigeria (Atehnkeng et al., 2014) have been reported.
Paradigm Shifts in Perception of Biocontrol in Africa
There is the notion that in SSA, and consequently in Nigeria, biocontrol usage has not gone beyond the research stage because smallholder farmers cannot afford biocontrol and there is no incentive to use the technology since aflatoxin contamination is not a factor that determines price competitiveness of grains in market (Njoroge, 2018; Stepman, 2018; Pitt, 2019). In general, these are indeed challenges for scaling up any aflatoxin mitigation technology, including biocontrol, to reach thousands of farmers. Nevertheless, through diverse innovative mechanisms and partnership arrangements with various stakeholders, large-scale use of biocontrol and other aflatoxin management interventions has reached thousands of farmers in Nigeria by implementing strategies for sustainable biocontrol use through the AgResults Project and other initiatives (Bandyopadhyay et al., 2016; AgResults, 2019; Schreurs et al., 2019). The strategies include awareness and sensitization campaigns, use of improved agronomic practices, improved pre-harvest practices, use of the aflatoxin biocontrol product, improved harvest and post-harvest practices, sorting of moldy/diseased grains, proper storage and use of hermetic bags, testing, market development, policies, and any other novel, practical, and available management tool for farmers. These holistic interventions have helped to create markets willing to pay for aflatoxin standard-compliant maize (Johnson et al., 2018, 2019) resulting in farmers’ willingness to pay $11-19/ha for biocontrol (Ayedun et al., 2017). Farm-based agricultural enterprises have enabled thousands of farmers to adopt aflatoxin management strategies, centered in biocontrol, to produce and commercialize more than 200,000 tons of aflatoxin-compliant maize demonstrating that sustainability and scaling of the technology is possible. It is necessary to increase awareness about aflatoxin and create new market opportunities for aflatoxin-compliant crops for farmers to further enhance adoption of aflatoxin-mitigation practices, including biocontrol.
Biocontrol applications of atoxigenic strains have long-term carryover effects and as a result additive benefits accrue as the product is used over multiple years (Cotty et al., 2007). However, because aflatoxins influence human health managing the toxins to the lowest possible level is desired, and it is recommended that the products be applied each season maize or groundnut are produced. Costs associated with applying biocontrol products every crop cycle have been viewed as a negative aspect of the biocontrol technology (Ehrlich et al., 2015; Njoroge, 2018; Molo et al., 2019). However, optimal crop production requires application of inputs (e.g., certified seeds, fertilizers, insecticides) on a yearly basis. All of these critical inputs must be applied each cropping season and it should not be expected that atoxigenic genotypes must replace toxigenic strains with a single application. Farmers producing commercial crops in Nigeria and other SSA nations utilize inputs to increase crop production and many are now using biocontrol as a necessary input to protect crops and access premium markets. This is similar to what has occurred in high-risk portions of Texas where use of aflatoxin biocontrol products is considered a necessary cost of maize production.
Long-term studies on the efficacy and stability of aflatoxin biocontrol agents have been recommended to determine the true value of the technology in SSA (Kagot et al., 2019). It takes over a decade to develop aflatoxin biocontrol programs because it is necessary to sensitize farmers and several key stakeholders, screen for appropriate biocontrol agents, test their efficacy in multiple years, multiple areas, in real-farming conditions, develop delivery methods, register the biocontrol product with national authorities, develop commercialization strategies, establish market entry strategies, create sustainable models for biocontrol adoption in a nation-wide manner, develop infrastructure to manufacture and distribute the biocontrol product en masse, among other actions (Mehl et al., 2012; Bandyopadhyay et al., 2016; Ortega-Beltran and Bandyopadhyay, 2019; Schreurs et al., 2019). We are hopeful that the substantial evidence of the benefits of integrated management strategies centered on biocontrol products presented in this long-term study may change certain ambiguous and sometimes negative perceptions about the effectiveness of biocontrol in African contexts, its adoption, and its sustainability.
Conclusion
Our results demonstrate that an aflatoxin biocontrol product registered for use in maize and groundnut in Nigeria, is a practical, cost-effective, and environmentally safe aflatoxin mitigation tool that enables farmers in Nigeria to produce both crops with little to no aflatoxin content. This work is the most extensive published study of the long-term efficacy of any aflatoxin biocontrol product. The results indicate that biocontrol is a stable, effective aflatoxin management tool regardless of environment or cropping system and is highly effective on crops grown by smallholder farmers. In addition, evidence of large-scale adoption of the biocontrol product in Nigeria is provided. The results suggest that biocontrol is a preferred component of holistic aflatoxin management strategies tackling agricultural, behavioral, institutional, and policy challenges. A holistic maize and groundnut aflatoxin management strategy with biocontrol as the centerpiece is contributing to better health, increased income, and greater trading opportunities. Farmers and consumers of other susceptible crops (e.g., chili peppers, sesame, sorghum) in Nigeria and elsewhere in SSA would benefit if the technology is adapted and registered for its use in those crops.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
RB and PC designed the overall projects from which data are derived. JA, AA, AO-B, PC, and RB contributed to the conception and design of the experiments. JA, AA, AO-B, and TF conducted the experiments and field studies, and collected and analyzed the data. AA, PC, and RB provided guidance. AO-B drafted the original manuscript. JA, TF, PC, and RB edited the manuscript. All authors read, reviewed, and approved the final manuscript. RB secured funds for the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was funded by the Bill & Melinda Gates Foundation (OPP1007117), African Agricultural Technology Foundation, CGIAR Research Program on Agriculture for Nutrition and Health, Commercial Agriculture Development Project of the World Bank, United States Department of Agriculture – Foreign Agricultural Service, and the European Union MycoRed project. The efficacy data from treated commercial fields (2013–2018) were largely derived from work done under the AgResults Nigeria AflasafeTM Challenge Project funded by the Bill and Melinda Gates Foundation, and the Governments of Australia, Canada, the United Kingdom, and the United States. Open Access fees were paid by the Bill & Melinda Gates Foundation.
Acknowledgments
We thank the maize and groundnut farmers in Nigeria for willingly allowing the trials to be conducted in their fields and participating in this study. We also thank the staff of extension agencies of various Nigerian states as well as the implementers of the AgResults Nigeria AflasafeTM Challenge Project for assisting in field selection, training, product application and monitoring, and sample collection. Special thanks to the field and laboratory staff of the AgResults Nigeria AflasafeTM Challenge Project for collecting and processing samples from more than 200,000 tons of maize grains used in this study. The use of trade, firm, or corporation names in these methods is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the USDA Agricultural Research Service (USDA-ARS), of any product or service to the exclusion of others that may be suitable. In addition, USDA-ARS makes no warranties as to the merchantability or fitness of the methodologies described on these pages for any particular purpose, or any other warranties expressed or implied. These methodologies provide a guide and do not replace published work. USDA-ARS is not liable for any damages resulting from the use or misuse of these methodologies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02528/full#supplementary-material
References
Accinelli, C., Abbas, H. K., Little, N. S., Kotowicz, J. K., and Shier, W. T. (2018). Biological control of aflatoxin production in corn using non-aflatoxigenic Aspergillus flavus administered as a bioplastic-based seed coating. Crop Prot. 107, 87–92. doi: 10.1016/j.cropro.2018.02.004
Adhikari, B. N., Bandyopadhyay, R., and Cotty, P. J. (2016). Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 6:62. doi: 10.1186/s13568-016-0228-6
Agbetiameh, D., Ortega-Beltran, A., Awuah, R. T., Atehnkeng, J., Cotty, P. J., and Bandyopadhyay, R. (2018). Prevalence of aflatoxin contamination in maize and groundnut in Ghana: population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 102, 764–772. doi: 10.1094/PDIS-05-17-0749-RE
AgResults, (2019). Nigeria AflasafeTM Challenge Project. Available at: http://agresults.org/en/283/ (accessed July 1, 2019).
Alaniz Zanon, M. S., Barros, G. G., and Chulze, S. N. (2016). Non-aflatoxigenic Aspergillus flavus as potential biocontrol agents to reduce aflatoxin contamination in peanuts harvested in Northern Argentina. Int. J. Food Microbiol. 231, 63–68. doi: 10.1016/j.ijfoodmicro.2016.05.016
Alaniz Zanon, M. S., Chiotta, M. L., Giaj-Merlera, G., Barros, G., and Chulze, S. (2013). Evaluation of potential biocontrol agent for aflatoxin in Argentinean peanuts. Int. J. Food Microbiol. 162, 220–225. doi: 10.1016/j.ijfoodmicro.2013.01.017
Amaike, S., and Keller, N. P. (2011). Aspergillus flavus. Annu. Rev. Phytopathol. 49, 107–133. doi: 10.1146/annurev-phyto-072910-095221
Atehnkeng, J., Donner, M., Ojiambo, P. S., Ikotun, B., Augusto, J., Cotty, P. J., et al. (2016). Environmental distribution and genetic diversity of vegetative compatibility groups determine biocontrol strategies to mitigate aflatoxin contamination of maize by Aspergillus flavus. Microb. Biotechnol. 9, 75–88. doi: 10.1111/1751-7915.12324
Atehnkeng, J., Ojiambo, P. S., Cotty, P. J., and Bandyopadhyay, R. (2014). Field efficacy of a mixture of atoxigenic Aspergillus flavus link: FR vegetative compatibility groups in preventing aflatoxin contamination in maize (Zea mays L.). Biol. Control 72, 62–70. doi: 10.1016/j.biocontrol.2014.02.009
Atehnkeng, J., Ojiambo, P. S., Donner, M., Ikotun, B., Sikora, R. A., Cotty, P. J., et al. (2008a). Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 122, 74–84. doi: 10.1016/j.ijfoodmicro.2007.11.062
Atehnkeng, J., Ojiambo, P. S., Ikotun, B., Sikora, R. A., Cotty, P. J., and Bandyopadhyay, R. (2008b). Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 25, 1264–1271. doi: 10.1080/02652030802112635
Ayalew, A., Kimanya, M., Matumba, L., Bandyopadhyay, R., Menkir, A., and Cotty, P. J. (2017). “Controlling aflatoxins in maize in Africa: strategies, challenges and opportunities for improvement,” in Achieving Sustainable Cultivation of Maize: Cultivation Techniques, Pest and Disease Control, Vol. 2 ed. D. Watson, (Cambridge: Burleigh Dodds Science Publishing), 1–24.
Ayedun, B., Okpachu, G., Manyong, V., Atehnkeng, J., Akinola, A., Abu, G. A., et al. (2017). An assessment of willingness to pay by maize and groundnut farmers for aflatoxin biocontrol product in Northern Nigeria. J. Food Prot. 80, 1451–1460. doi: 10.4315/0362-028X.JFP-16-281
Bandyopadhyay, R., Kumar, M., and Leslie, J. F. (2007). Relative severity of aflatoxin contamination of cereal crops in West Africa. Food Addit. Contam. 24, 1109–1114. doi: 10.1080/02652030701553251
Bandyopadhyay, R., Ortega-Beltran, A., Akande, A., Mutegi, C., Atehnkeng, J., Kaptoge, L., et al. (2016). Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin J. 9, 771–789. doi: 10.3920/WMJ2016.2130
Bayman, P., and Cotty, P. J. (1991). Vegetative compatibility and genetic diversity in the Aspergillus flavus population of a single field. Can. J. Bot. 69, 1707–1711. doi: 10.1139/b91-216
Blankson, G. K., Mills-Robertson, F. C., and Ofosu, I. W. (2019). Survey of occurrence levels of aflatoxins in selected locally processed cereal-based foods for human consumption from Ghana. Food Control 95, 170–175. doi: 10.1016/j.foodcont.2018.08.005
Bock, C. H., Mackey, B., and Cotty, P. J. (2004). Population dynamics of Aspergillus flavus in the air of an intensively cultivated region of south-west Arizona. Plant Pathol. 53, 422–433. doi: 10.1111/j.1365-3059.2004.01015.x
Bruns, H. (2003). Controlling aflatoxin and fumonisin in maize by crop management. J. Toxicol. Toxin Rev. 22, 153–173. doi: 10.1081/TXR-120024090
Cole, R. J., and Dorner, J. W. (1993). Extraction of aflatoxins from naturally contaminated peanuts with different solvents and solvent/peanut ratios. J. AOAC Int. 77, 1509–1511.
Cotty, P. J. (1989). Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 79, 808–814. doi: 10.1094/Phyto-79-808
Cotty, P. J. (1994). Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathologia 125, 157–162. doi: 10.1007/BF01146521
Cotty, P. J. (1997). Aflatoxin producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 101, 698–704.
Cotty, P. J. (2006). “Biocompetitive exclusion of toxigenic fungi,” in The Mycotoxin Factbook, eds D. Barug, D. Bhatnagar, H. P. van Egdmond, J. W. van der Kamp, W. A. van Osenbruggen, and A. Visconti, (Wageningen: Wageningen Academic Publishers), 179–197.
Cotty, P. J., Antilla, L., and Wakelyn, P. J. (2007). “Competitive exclusion of aflatoxin producers: farmer-driven research and development,” in Biological Control: a Global Perspective, eds C. Vincent, N. Goettel, and G. Lazarovits, (Wallingford: CAB International), 242–253.
Cotty, P. J., and Cardwell, K. F. (1999). Divergence of West African and North American communities of Aspergillus section Flavi. Appl. Env. Microbiol. 65, 2264–2266.
Cotty, P. J., and Mellon, J. E. (2006). Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 22, 110–117. doi: 10.1007/BF02956774
Cotty, P. J., Probst, C., and Jaime-Garcia, R. (2008). “Etiology and management of aflatoxin contamination,” in Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade, eds J. F. Leslie, R. Bandyopadhyay, and A. Visconti, (Wallingford: CAB International), 287–299. doi: 10.1079/9781845930820.0287
Cotty, P. J., and Taylor, D. R. (2003). Influence of complementation medium composition on vegetative compatibility analyses of Aspergillus flavus. Phytopathology 93:S18.
Coursaget, P., Depril, N., Chabaud, M., Nandi, R., Mayelo, V., LeCann, P., et al. (1993). High prevalence of mutations at codon 249 of the p53 gene in hepatocellular carcinomas from Senegal. Br. J. Cancer 67, 1395–1397. doi: 10.1038/bjc.1993.258
Diedhiou, P. M., Bandyopadhyay, R., Atehnkeng, J., and Ojiambo, P. S. (2011). Aspergillus colonization and aflatoxin contamination of maize and sesame kernels in two agro-ecological zones in Senegal. J. Phytopathol. 159, 268–275. doi: 10.1111/j.1439-0434.2010.01761.x
Donner, M., Atehnkeng, J., Sikora, R. A., Bandyopadhyay, R., and Cotty, P. J. (2009). Distribution of Aspergillus section Flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol. Biochem. 41, 37–44. doi: 10.1016/j.soilbio.2008.09.013
Donner, M., Atehnkeng, J., Sikora, R. A., Bandyopadhyay, R., and Cotty, P. J. (2010). Molecular characterization of atoxigenic strains for biological control of aflatoxins in Nigeria. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 27, 576–590. doi: 10.1080/19440040903551954
Dorner, J. W. (2004). Biological control of aflatoxin contamination of crops. J. Toxicol. Toxin Rev. 23, 425–450. doi: 10.1081/TXR-200027877
Dorner, J. W. (2009). Development of biocontrol technology to manage aflatoxin contamination in peanuts. Peanut Sci. 36, 60–67. doi: 10.3146/AT07-002.1
Doster, M. A., Cotty, P. J., and Michailides, T. J. (2014). Evaluation of the atoxigenic Aspergillus flavus strain AF36 in pistachio orchards. Plant Dis. 98, 948–956. doi: 10.1094/PDIS-10-13-1053-RE
Ehrlich, K. C., Moore, G. G., Mellon, J. E., and Bhatnagar, D. (2015). Challenges facing the biological control strategy for eliminating aflatoxin contamination. World Mycotoxin J. 8, 225–233. doi: 10.3920/WMJ2014.1696
Ezekiel, C., Ayeni, K., Misihairabgwi, J., Somorin, Y., Chibuzor-Onyema, I., Oyedele, O., et al. (2018). Traditionally processed beverages in Africa: a review of the mycotoxin occurrence patterns and exposure assessment. Compr. Rev. Food Sci. Food Saf. 17, 334–351. doi: 10.1111/1541-4337.12329
Ezekiel, C. N., Ortega-Beltran, A., Oyedeji, E., Atehnkeng, J., Kössler, P., Tairu, F., et al. (2019). Aflatoxin in chili peppers in Nigeria: extent of contamination and control using atoxigenic Aspergillus flavus genotypes as biocontrol agents. Toxins 11:429. doi: 10.3390/toxins11070429
Ezekiel, C. N., Warth, B., Ogara, I. M., Abia, W. A., Ezekiel, V. C., Atehnkeng, J., et al. (2014). Mycotoxin exposure in rural residents in northern Nigeria: a pilot study using multi-urinary biomarkers. Environ. Int. 66, 138–145. doi: 10.1016/j.envint.2014.02.003
Frisvad, J. C., Hubka, V., Ezekiel, C. N., Hong, S.-B., Novakova, A., Chen, A. J., et al. (2019). Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 93, 1–63. doi: 10.1016/j.simyco.2018.06.001
Gieseker, K. E. (2004). Outbreak of Aflatoxin Poisoning — Eastern and Central Provinces, MMWR, Morbidity and Mortality Weekly Report. Kenya: Centers for Disease Control and Prevention.
Gong, Y. Y., Turner, P. C., Hall, A. J., and Wild, C. P. (2008). “Aflatoxin exposure and impaired child growth in West Africa: an unexplored international public health burden,” in Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade, eds J. F. Leslie, R. Bandyopadhyay, and A. Visconti, (Wallingford: CAB International), 53–66.
Gressel, J., and Polturak, G. (2018). Suppressing aflatoxin biosynthesis is not a breakthrough if not useful. Pest Manag. Sci. 74, 17–21. doi: 10.1002/ps.4694
Grubisha, L. C., and Cotty, P. J. (2010). Genetic isolation among sympatric vegetative compatibility groups of the aflatoxin-producing fungus Aspergillus flavus. Mol. Ecol. 19, 269–280. doi: 10.1111/j.1365-294X.2009.04467.x
Grubisha, L. C., and Cotty, P. J. (2015). Genetic analysis of the Aspergillus flavus vegetative compatibility group to which a biological control agent that limits aflatoxin contamination in U.S. crops belongs. Appl. Environ. Microbiol. 81, 5889–5899. doi: 10.1128/AEM.00738-15
Hell, K., Fandohan, P., Bandyopadhyay, R., Kiewnick, S., Sikora, R., and Cotty, P. J. (2008). “Pre- and post-harvest management of aflatoxin in maize: an African perspective,” in Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade, eds J. F. Leslie, R. Bandyopadhyay, and A. Visconti, (Wallingford: CAB International), 219–219.
IARC, (2002). Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphtalene and Styrene. Summary of Data Reported and Evaluation, Vol. 82. Lyon: IARC.
James, B., Adda, C., Cardwell, K. F., Annang, D., Hell, K., Korie, S., et al. (2007). Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Addit. Contam. 24, 1283–1291. doi: 10.1080/02652030701416558
JECFA, (2018). “Safety evaluation of certain contaminants in food,” in Paper Presented by the Eighty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), World Health Organization and Food and Agriculture Organization of the United Nations, Geneva, doi: 10.1016/j.ijfoodmicro.2007.01.001
Johnson, A. M., Abdoulaye, T., Ayedun, B., Fulton, J. R., Widmar, N. J. O., Adebowale, A., et al. (2019). Willingness to pay of Nigerian poultry producers and feed millers for aflatoxin-safe maize. Agribusiness doi: 10.1002/agr.21621
Johnson, A. M., Fulton, J. R., Abdoulaye, T., Ayedun, B., Widmar, N. J. O., Akande, A., et al. (2018). Aflatoxin awareness and Aflasafe adoption potential of Nigerian smallholder maize farmers. World Mycotoxin J. 11, 437–446. doi: 10.3920/WMJ2018.2345
Kagot, V., Okoth, S., De Boevre, M., and De Saeger, S. (2019). Biocontrol of Aspergillus and Fusarium mycotoxins in Africa: benefits and limitations. Toxins 11:109. doi: 10.3390/toxins11020109
Kamala, A., Shirima, C., Jani, B., Bakari, M., Sillo, H., Rusibamayila, N., et al. (2018). Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 11, 311–320. doi: 10.3920/wmj2018.2344
Leroy, J. L., Sununtnasuk, C., García-Guerra, A., and Wang, J. S. (2018). Low level aflatoxin exposure associated with greater linear growth in southern Mexico: a longitudinal study. Matern. Child Nutr. 14, 1–7. doi: 10.1111/mcn.12619
Leslie, J. F. (1993). Fungal vegetative compatibility. Annu. Rev. Phytopathol. 31, 127–150. doi: 10.1146/annurev.py.31.090193.001015
Logrieco, A. F., Miller, J. D., Eskola, M., Krska, R., Ayalew, A., Bandyopadhyay, R., et al. (2018). The Mycotox Charter: increasing awareness of, and concerted action for, minimizing mycotoxin exposure worldwide. Toxins 10:149. doi: 10.3390/toxins10040149
Mahuku, G., Nzioki, H. S., Mutegi, C., Kanampiu, F., Narrod, C., and Makumbi, D. (2019). Pre-harvest management is a critical practice for minimizing aflatoxin contamination of maize. Food Control 96, 219–226. doi: 10.1016/j.foodcont.2018.08.032
Mehl, H. L., Jaime, R., Callicott, K. A., Probst, C., Garber, N. P., Ortega-Beltran, A., et al. (2012). Aspergillus flavus diversity on crops and in the environment can be exploited to reduce aflatoxin exposure and improve health. Ann. N. Y. Acad. Sci. 1273, 7–17. doi: 10.1111/j.1749-6632.2012.06800.x
Molo, M. S., Heiniger, R. W., Boerema, L., and Carbone, I. (2019). Trial summary on the comparison of various non-aflatoxigenic strains of Aspergillus flavus on mycotoxin levels and yield in maize. Agron. J. 111, 942–946. doi: 10.2134/agronj2018.07.0473
Narayan, T., Mainville, D., Geyer, J., Hausdorff, K., and Cooley, D. (2019). AgResults Impact Evaluation Report: Nigeria AflasafeTM Challenge Project. Available at: https://www.abtassociates.com/insights/publications/report/agresults-nigeria-aflasafe-pilot-evaluation (accessed July 1, 2019).
Njoroge, S. M. C. (2018). A critical review of aflatoxin contamination of peanuts in Malawi and Zambia: the past, present, and future. Plant Dis. 102, 2394–2406. doi: 10.1094/pdis-02-18-0266-fe
Ortega-Beltran, A., and Bandyopadhyay, R. (2019). Comments on “Trial summary on the comparison of various non-aflatoxigenic strains of Aspergillus flavus on mycotoxin levels and yield in maize” by M.S. Molo, et al. Agron. J. 111: 942–946 (2019). Agron. J. 111, 2625–2631. doi: 10.2134/agronj2019.04.0281
Ortega-Beltran, A., and Cotty, P. J. (2018). Frequent shifts in Aspergillus flavus populations associated with maize production in Sonora, Mexico. Phytopathology 108, 412–420. doi: 10.1094/PHYTO-08-17-0281-R
Pitt, J. I. (2019). The pros and cons of using biocontrol by competitive exclusion as a means for reducing aflatoxin in maize in Africa. World Mycotoxin J. 12, 103–112. doi: 10.3920/wmj2018.2410
Pitt, J. I., Manthong, C., Siriacha, P., Chotechaunmanirat, S., and Markwell, P. (2015). Studies on the biocontrol of aflatoxin in maize in Thailand. Biocontrol Sci. Technol. 5, 1070–1091. doi: 10.1080/09583157.2015.1028893
Probst, C., Bandyopadhyay, R., and Cotty, P. J. (2014). Diversity of aflatoxin-producing fungi and their impact on food safety in sub-Saharan Africa. Int. J. Food Microbiol. 174, 113–122. doi: 10.1016/j.ijfoodmicro.2013.12.010
Probst, C., Bandyopadhyay, R., Price, L. E., and Cotty, P. J. (2011). Identification of atoxigenic Aspergillus flavus isolates to reduce aflatoxin contamination of maize in Kenya. Plant Dis. 95, 212–218. doi: 10.1094/PDIS-06-10-0438
Probst, C., Schulthess, F., and Cotty, P. J. (2010). Impact of Aspergillus section Flavi community structure on the development of lethal levels of aflatoxins in Kenyan maize (Zea mays). J. Appl. Microbiol. 108, 600–610. doi: 10.1111/j.1365-2672.2009.04458.x
Schreurs, F., Bandyopadhyay, R., Kooyman, C., Ortega-Beltran, A., Akande, A., Konlambigue, M., et al. (2019). “Commercial products promoting plant health in African agriculture,” in Critical Issues in Plant Health: 50 Years of Research in African Agriculture, eds P. Neuenschwander, and M. Tamò, (Cambridge: Burleigh Dodds Science Publishing), 345–363. doi: 10.19103/AS.2018.0043.14
Seetha, A., Munthali, W., Msere, H. W., Swai, E., Muzanila, Y., Sichone, E., et al. (2017). Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 33, 323–331. doi: 10.1007/s12550-017-0286-x
Senghor, L. A., Ortega-Beltran, A., Atehnkeng, J., Callicott, K. A., Cotty, P. J., and Bandyopadhyay, R. (2019). The atoxigenic biocontrol product Aflasafe SN01 is a valuable tool to mitigate aflatoxin contamination of both maize and groundnut cultivated in Senegal. Plant Dis. doi: 10.1094/PDIS-03-19-0575-RE
Shephard, G. S. (2008). Risk assessment of aflatoxins in food in Africa. Food Addit. Contam. 25, 1246–1256. doi: 10.1080/02652030802036222
Singh, P., and Cotty, P. J. (2017). Aflatoxin contamination of dried red chilies: contrasts between the United States and Nigeria, two markets differing in regulation enforcement. Food Control 80, 374–379. doi: 10.1016/j.foodcont.2017.05.014
Singh, P., and Cotty, P. J. (2019). Characterization of Aspergilli from dried red chilies (Capsicum spp.): insights into the etiology of aflatoxin contamination. Int. J. Food Microbiol. 289, 145–153. doi: 10.1016/j.ijfoodmicro.2018.08.025
Sirma, A. J., Lindahl, J. F., Makita, K., Senerwa, D., Mtimet, N., Kang’ethe, E. K., et al. (2018). The impacts of aflatoxin standards on health and nutrition in sub-Saharan Africa: the case of Kenya. Glob. Food Sec. 18, 57–61. doi: 10.1016/j.gfs.2018.08.001
Stepman, F. (2018). Scaling-up the impact of aflatoxin research in Africa. The role of social sciences. Toxins 10:136. doi: 10.3390/toxins10040136
Udomkun, P., Wiredu, A., Nagle, M., Bandyopadhyay, R., Müller, J., and Vanlauwe, B. (2017). Mycotoxins in sub-Saharan Africa: present situation, socio-economic impact, awareness, and outlook. Food Control 72, 110–122. doi: 10.1016/j.foodcont.2016.07.039
USEPA, (2003). Biopesticide Registration Action Document Aspergillus flavus AF36. Available at: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006456_3-Jul-03.pdf (accessed July 1, 2019).
USEPA, (2004). Biopesticide Registration Action Document Aspergillus flavus NRRL 21882. Available at: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006500_24-Mar-04.pdf (accessed July 1, 2019).
Villers, P. (2014). Aflatoxins and safe storage. Front. Microbiol. 5:158. doi: 10.3389/fmicb.2014.00158
Voth-Gaeddert, L. E., Stoker, M., Torres, O., and Oerther, D. B. (2018). Association of aflatoxin exposure and height-for-age among young children in Guatemala. Int. J. Environ. Health Res. 28, 280–292. doi: 10.1080/09603123.2018.1468424
Waliyar, F., Osiru, M., Ntare, B. R., Kumar, K. V. K., Sudini, H., Traore, A., et al. (2015). Post-harvest management of aflatoxin contamination in groundnut. World Mycotoxin J. 8, 245–252. doi: 10.3920/WMJ2014.1766
Weaver, M., and Abbas, H. K. (2019). Field displacement of aflatoxigenic Aspergillus flavus strains through repeated biological control applications. Front. Microbiol. 10:1788. doi: 10.3389/fmicb.2019.01788
Weaver, M. A., Abbas, H. K., Falconer, L. L., Allen, T. W., Pringle, H. C., and Sciumbato, G. L. (2015). Biological control of aflatoxin is effective and economical in Mississippi field trials. Crop Prot. 69, 52–55. doi: 10.1016/j.cropro.2014.12.009
Williams, J. H. (2008). “Institutional stakeholders in mycotoxin issues—past, present and future,” in Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade, eds J. F. Leslie, R. Bandyopadhyay, and A. Visconti, (Oxfordshire: CAB International), 349–358. doi: 10.1079/9781845930820.0349
Keywords: aflatoxin, biocontrol, efficacy trials, long-term efficacy, maize, groundnut, safe food
Citation: Bandyopadhyay R, Atehnkeng J, Ortega-Beltran A, Akande A, Falade TDO and Cotty PJ (2019) “Ground-Truthing” Efficacy of Biological Control for Aflatoxin Mitigation in Farmers’ Fields in Nigeria: From Field Trials to Commercial Usage, a 10-Year Study. Front. Microbiol. 10:2528. doi: 10.3389/fmicb.2019.02528
Received: 07 August 2019; Accepted: 21 October 2019;
Published: 08 November 2019.
Edited by:
Mahendra Kumar Rai, Sant Gadge Baba Amravati University, IndiaReviewed by:
Ebrahim Hadavi, Islamic Azad University of Karaj, IranDimitris Tsaltas, Cyprus University of Technology, Cyprus
Copyright © 2019 Bandyopadhyay, Atehnkeng, Ortega-Beltran, Akande, Falade and Cotty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranajit Bandyopadhyay, ci5iYW5keW9wYWRoeWF5QGNnaWFyLm9yZw==
†Present address: Joseph Atehnkeng, International Institute of Tropical Agriculture, Bukavu, Democratic Republic of Congo; Peter J. Cotty, Independent Researcher, Tucson, AZ, United States
 Ranajit Bandyopadhyay
Ranajit Bandyopadhyay Joseph Atehnkeng
Joseph Atehnkeng Alejandro Ortega-Beltran
Alejandro Ortega-Beltran Adebowale Akande
Adebowale Akande Titilayo D. O. Falade
Titilayo D. O. Falade Peter J. Cotty
Peter J. Cotty