- Laboratory of Microbiology, Department of Biochemistry and Microbiology, Ghent University, Ghent, Belgium
Almost all bacteria secrete spherical membranous nanoparticles, also referred to as membrane vesicles (MVs). A variety of MV types exist, ranging from 20 to 400 nm in diameter, each with their own formation routes. The most well-known vesicles are the outer membrane vesicles (OMVs) which are formed by budding from the outer membrane in Gram-negative bacteria. Recently, other types of MVs have been discovered and described, including outer-inner membrane vesicles (OIMVs) and cytoplasmic membrane vesicles (CMVs). The former are mainly formed by a process termed endolysin-triggered cell lysis in Gram-negative bacteria, the latter are formed by Gram-positive bacteria. MVs carry a wide range of cargo, such as nucleic acids, virulence factors and antibiotic resistance components. Moreover, they are involved in a multitude of biological processes that increase bacterial pathogenicity. In this review, we discuss the functional aspects of MVs secreted by bacteria associated with cystic fibrosis and nosocomial pneumonia. We mainly focus on how MVs are involved in virulence, antibiotic resistance, biofilm development and inflammation that consequently aid these bacterial infections.
Diversity of Membrane Vesicles
Secretion of membrane vesicles (MVs) is a common feature in almost all bacteria. Over the last decade, studies of the MV content and physiological analyses revealed that bacterial MVs are involved in a wide range of biological processes, including virulence, antibiotic resistance, horizontal gene transfer, cell-cell communication, iron scavenging, nutrient acquisition, modulating the host immune system, and protection against phage infections (Manning and Kuehn, 2011; Elhenawy et al., 2014; Haurat et al., 2015; Schwechheimer and Kuehn, 2015). MVs protect their cargo against extracellular degrading enzymes, enable long distance transport, facilitate efficient delivery to target host cells and deliver their cargo at increased concentrations, which makes them perfectly suited for secretion or uptake of nucleic acids, proteins and other biomolecules (Bonnington and Kuehn, 2014).
Early MV research focused on one specific type of MVs, namely the outer membrane vesicles (OMVs). They are formed by pinching of the outer membrane (OM) of Gram-negative bacteria and are enriched for OM proteins and periplasmic components. Several mechanisms of OMV production have already been described (Mashburn-Warren et al., 2008, 2009; Deatherage et al., 2009; Tashiro et al., 2010; Schwechheimer et al., 2014; Haurat et al., 2015; Schwechheimer and Kuehn, 2015; Roier et al., 2016a, b; Florez et al., 2017; Horspool and Schertzer, 2018; Cooke et al., 2019). Up until recently, OMVs were the only known MVs secreted by Gram-negative bacteria. Today, several other types of MVs have been discovered and described, for example outer-inner membrane vesicles (OIMVs) and cytoplasmic membrane vesicles (CMVs). Compelling evidence was provided that OIMV production in Pseudomonas aeruginosa is dependent on the action of a prophage endolysin, resulting in a phenomenon referred to as phage endolysin-triggered cell lysis (Turnbull et al., 2016). Lastly, it was thought that MVs only arose from Gram-negative bacteria, but the observation of vesiculation in several Gram-positive bacteria changed that view (Brown et al., 2015). Since Gram-positive bacteria do not possess an OM, MVs released by these bacteria are termed cytoplasmic membrane vesicles (CMVs). CMV release can be triggered by phage endolysin-triggered cell lysis as well, as was demonstrated in Bacillus subtilis and Staphylococcus aureus (Toyofuku et al., 2017; Andreoni et al., 2019). The different MV types each with their own formation routes were recently reviewed by Toyofuku et al. (2019). It should be recognized that some forms of MVs were only recently described. Previous studies may have ignored them and their results could thus refer to a mixture of different types of MVs instead of pure OMVs. Consequently, in this review, we use the term MVs to cover all types.
Membrane Vesicles from Bacteria Associated with Cystic Fibrosis and Nosocomial Pneumonia
Several bacterial species are associated with airway infections in immunocompromised and cystic fibrosis (CF) patients, including the Gram-negative bacteria P. aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia, Klebsiella pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Burkholderia spp., and the Gram-positive bacteria Streptococcus pneumoniae and S. aureus. They all produce MVs involved in a multitude of biological processes that increase bacterial pathogenicity (Figure 1). Table 1 gives an overview of the different pathogenicity mechanisms associated with MVs secreted by these pathogens.
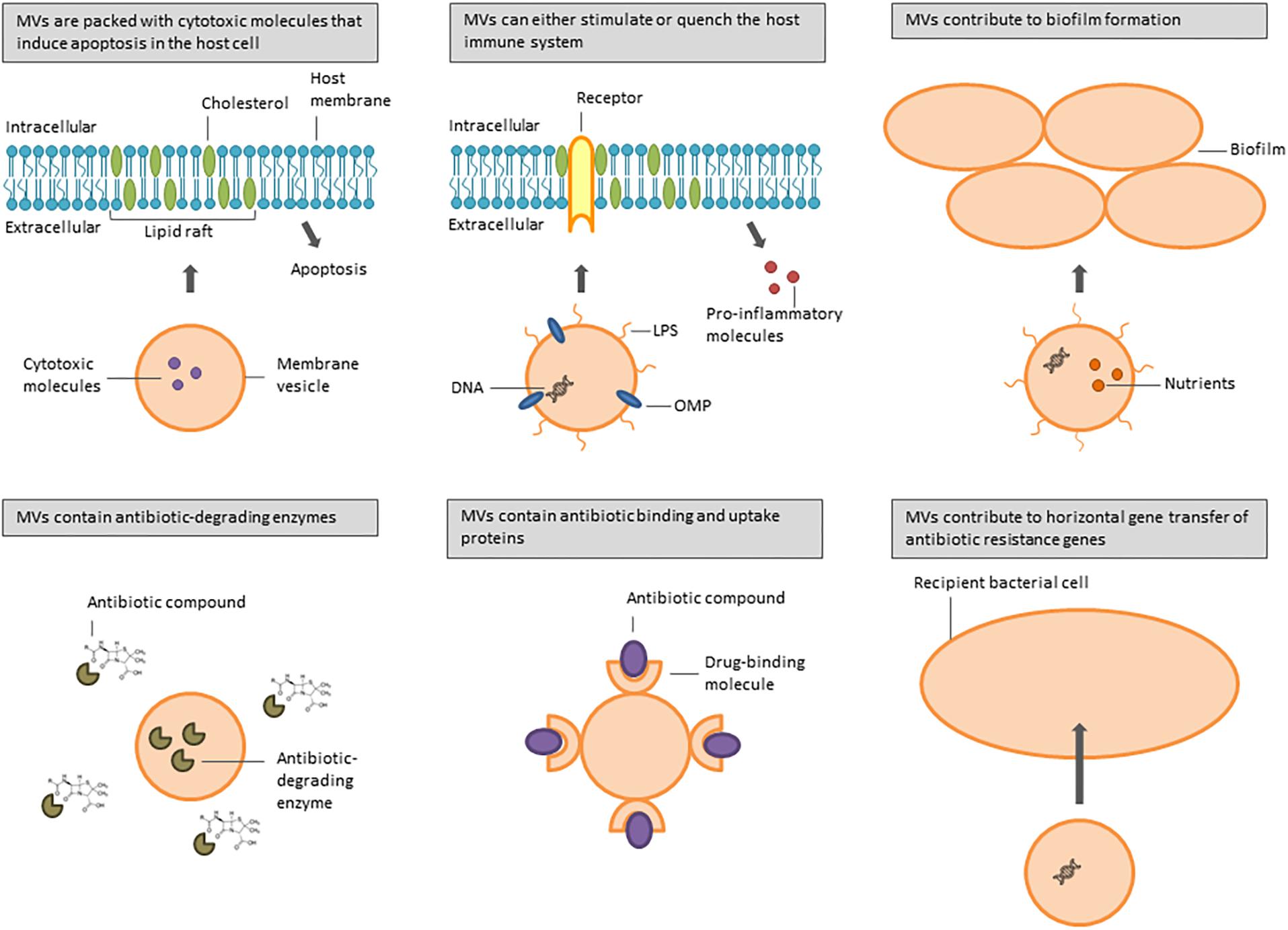
Figure 1. Overview of functions of MVs secreted by bacteria involved in CF and nosocomial lung infections.
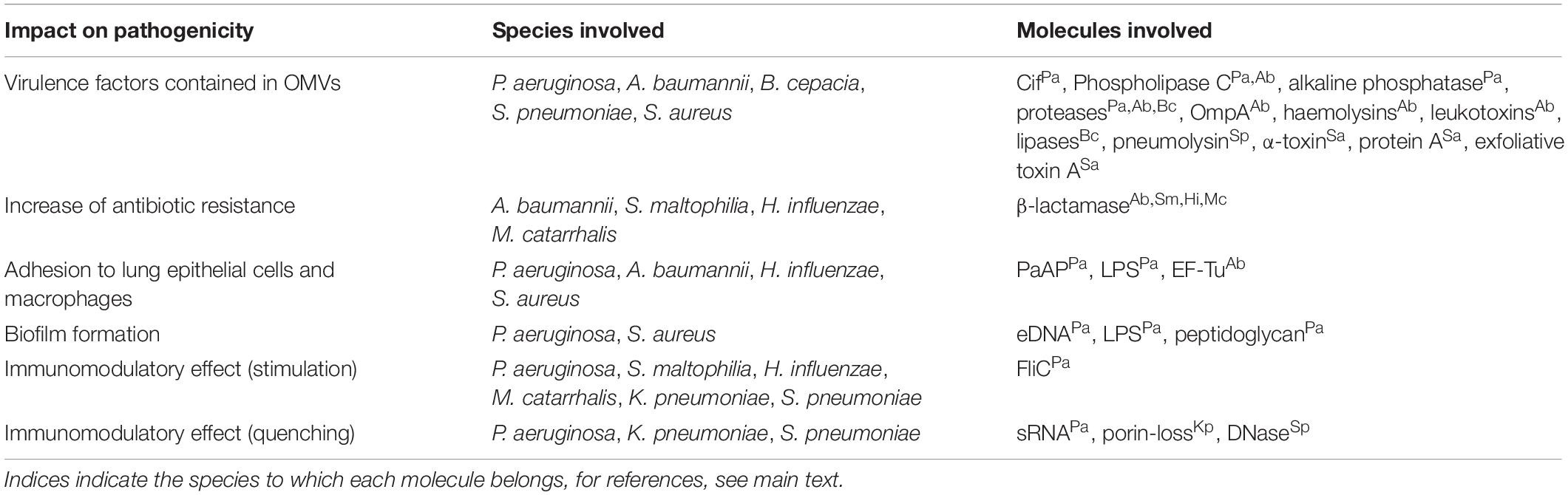
Table 1. Overview of the impact on membrane vesicles produced by species associated with CF and nosocomial pneumonia on pathogenicity, based on factors identified in MVs.
MV Cargo
The cargo of MVs can be very versatile, from nucleic acids to virulence factors and antibiotic resistance components. Depending on the different MV formation routes, different types of cargo are selected. Logically, OMVs tend to be enriched for OM proteins and periplasmic components, while OIMVs and CMVs are enriched for DNA, RNA and cytoplasmic proteins. Extracellular oriented membrane components such as lipopolysaccharides (LPS) might be expected in any of these vesicles.
Pseudomonas aeruginosa MVs released during the exponential growth phase were specifically packed with chromosomal DNA, consisting of specific chromosomal regions encoding proteins involved in stress response, virulence, metabolism and antibiotic resistance. These MVs can transfer DNA or sRNA into cultured lung epithelial or bronchial cells and by doing so, modulate the host cell responses (Koeppen et al., 2016; Bitto et al., 2017). Next to nucleic acids, P. aeruginosa MVs tend to pack several proteinaceous virulence factors, including hemolytic phospholipase C, alkaline phosphatase and the cystic fibrosis transmembrane conductance regulator (CFTR) inhibitory factor Cif. The latter inhibits CFTR-mediated chlorine secretion in the airways resulting in a decreased mucociliary clearance (Kadurugamuwa and Beveridge, 1995; Bomberger et al., 2009). Other proteins identified in P. aeruginosa MVs are involved in proteolysis, antibiotic resistance, and bacteria-host interactions (Choi et al., 2011).
Acinetobacter baumannii MVs are likewise enriched for several virulence factors, such as proteases, phospholipase C, hemolysins and leukotoxins. The MVs seem to interact with and deliver their content to host cells. In addition, the cytotoxic outer membrane protein A (OmpA) was identified in these MVs as well. OmpA, an important virulence factor in A. baumannii, is targeted to the mitochondria in epithelial cells and consequently induces apoptosis in these cells (Kwon et al., 2009; Jha et al., 2017). Moreover, Dallo et al. (2012) discovered that the elongation factor Tu (EF-Tu) is associated with A. baumannii MVs and interacts with macrophages through its binding with fibronectin.
Burkholderia cepacia MVs contain peptidoglycan-degrading enzymes together with a variety of virulence factors, including lipases, phospholipases and proteases (Allan et al., 2003). In S. pneumoniae, MVs are mainly enriched for lipoproteins as well as the cytosolic pore-forming toxin pneumolysin (Olaya-Abril et al., 2014). Further, MVs from clinical S. aureus isolates have a different cytotoxic activity on host cells, depending on their MV proteomes. The exfoliative toxin A (ETA) was specifically enriched in MVs with a high cytotoxic activity (Jeon et al., 2016).
Interaction of MVs With Lung Epithelial Cells and Macrophages
The presence of MVs in the lungs of patients with severe lung infections (Bomberger et al., 2009) and the fact that MVs can carry virulence factors, may point to a role of these MVs in the infection process. A few examples of MV-host cell interactions were already mentioned above. Additionally, Bauman and Kuehn (2009) discovered that MVs from a P. aeruginosa CF isolate interact with human lung cells and were internalized in a time- and dose-dependent manner. These bacteria secrete PaAP, an aminopeptidase mainly present on the surface of MVs and was found to be important for the association of the MVs with the lung cells. Further, MVs secreted by P. aeruginosa interact with cholesterol-rich lipid rafts in the apical membrane of lung epithelial cells. By doing so, P. aeruginosa is able to deliver the virulence factor Cif to the cytoplasm of the host cell and consequently reduce the CFTR chloride secretion (Bomberger et al., 2009, 2011; Ballok et al., 2014; O’Donoghue and Krachler, 2016). Lowering the cholesterol content of CF airway epithelial cells (Phe508del) by cyclodextrin lowers the impact of P. aeruginosa vesicles on Cl– secretion after lumacaftor treatment (Barnaby et al., 2019).
Likewise, purified vesicles secreted by Non-Typeable H. influenzae (NTHi) co-localize with caveolin, a protein involved in endocytosis. This indicates that the uptake of MVs is mediated by caveolae, which are cholesterol-rich lipid rafts. On top of that, the interactions of these MVs with epithelial cells resulted in the release of the immunomodulatory cytokine interleukin-8 (IL-8) and the antimicrobial peptide LL-37 (Sharpe et al., 2011). Further, NTHi released MVs while infecting primary respiratory epithelial cells grown at the air-liquid interface. Transmission electron microscopy (TEM) revealed that these MVs directly interact with the host-cell membranes. However, the role of these vesicles during NTHi infection is yet to be determined (Ren et al., 2012).
Membrane vesicles released by S. aureus also fuse in a cholesterol-dependent manner with the plasma membrane of human cells, resulting in the delivery of α-toxin. This toxin, also known as α-hemolysin (HIa), is a 33 kDa pore-forming protein and a key virulence factor capable of lysing human cells and the induction of apoptosis in T-lymphocytes. Furthermore, this MV-associated protein is involved in HeLa cell cytotoxicity and erythrocyte lysis (Thay et al., 2013). Gurung et al. (2011) discovered that S. aureus MVs interact with the plasma membrane of human cells through a cholesterol-rich micro-domain as well. The MVs subsequently delivered the immunoglobulin G-binding protein (protein A) and induced apoptosis of HEp-2 cells in a dose-dependent manner.
The same mechanism is true for A. baumannii. MVs from A. baumannii ATCC 19606(T) interacted with lipid rafts in the plasma membrane of human cells and induced apoptosis in the host cells. The effect was lost when MVs secreted by the ΔompA mutant strain were studied. Suggesting a role of the MV-associated virulence factor OmpA in host cell-death (Jin et al., 2011).
While most of these studies were performed in in vitro systems, a few studies in mice models also indicate the impact of bacterial membrane vesicles in lungs. Jang et al. (2015) injected Escherichia coli derived vesicles intraperitoneally and showed that these vesicles spread into lungs. Proteins from A. baumannii MVs delivered intranasally in mice were detected in the lungs and provoked an immune response (Marion et al., 2019).
Effect of Antibiotics on MV Secretion and Function
Devos et al. (2015) demonstrated that antibiotic stress leads to an increased secretion of MVs in S. maltophilia 44/98, suggesting that this could have potential implications on the function of these MVs. Indeed, the exposure of S. maltophilia to the β-lactam antibiotic imipenem led to an increased secretion of MVs comprising two chromosomally encoded β-lactamases. These β-lactamase-containing MVs are capable of mediating extracellular β-lactam degradation and consequently enhance the β-lactam tolerance of other CF pathogens, including P. aeruginosa and Burkholderia cenocepacia (Devos et al., 2016). Several other bacteria exposed to β-lactam antibiotics release MVs containing functional β-lactamases as well. MVs secreted by β-lactam-resistant M. catarrhalis and NTHi can hydrolyze amoxicillin and consequently protect co-localized species, such as Group A Streptococci, S. pneumoniae and H. influenzae, from killing by amoxicillin (Schaar et al., 2014). In this regard, imipenem-treatment of A. baumannii resulted in an elevated secretion of MVs, containing β-lactamase OXA-23, with a higher cytotoxicity toward A549 human lung cells (Yun et al., 2018).
Moreover, Allan and Beveridge (2003) discovered that the treatment of P. aeruginosa PAO1 with the aminoglycoside antibiotic gentamicin resulted in the release of MVs containing gentamicin and peptidoglycan hydrolase. By becoming bactericidal, these MVs are capable of killing group IIIa B. cepacia. Also B. subtilis 168 and S. aureus D2C were affected by these type of MVs and Listeria monocytogenes ATCC 19113 was susceptible to a lesser extent (MacDonald and Beveridge, 2002).
On another note, P. aeruginosa infections treated with tobramycin led to a reduced secretion of MV-associated virulence factors, including AprA, which is an alkaline protease that reduces CFTR-mediated chloride secretion. AprA is essential for the survival of P. aeruginosa in the lungs as it inhibits the bacterial clearance (Koeppen et al., 2019).
Interspecies Interactions of MVs
Cell-cell communication between bacteria during lung infections is key to the survival of the infecting species, e.g., in the mixed species biofilm in the lungs of patients with CF. Most interspecies interactions have been studied in vitro, but some advanced three-dimensional lung cell culture models approve some of the findings performed in co-culturing experiments (Rodriguez-Sevilla et al., 2018). Communication via MVs can mediate changes in the expression of biofilm-related genes, or protect other species from antibiotics and host defense mechanisms. Kadurugamuwa and Beveridge (1999) discovered that MVs secreted by P. aeruginosa and Shigella flexneri can be integrated into the membrane of other Gram-negative bacteria. Moreover, the MVs of two carbapenem-resistant clinical strains of A. baumannii harboring the plasmid-borne blaOXA–24 gene, encoding a β-lactamase, were capable of protecting a carbapenem-susceptible A. baumannii strain. The presence of these plasmids in the carbapenem-susceptible strain suggests that A. baumannii releases MVs to mediate horizontal gene transfer of antibiotic-resistance genes (Rumbo et al., 2011). In addition, vesicles released by A. baumannii can mediate gene transfer of the blaNDM–1 and aac(6′)-Ib-cr genes to other A. baumannii and E. coli recipient cells (Chatterjee et al., 2017). Next to this, the MVs of M. catarrhalis mediated protection of H. influenzae against the complement system during infection (Tan et al., 2007).
MVs in Biofilm
Single- or poly-microbial infections in the lungs are mostly paired with biofilm formation. Biofilms are characterized by a thick layer of bacterial cells formed by the co-operation of several virulence factors, including flagella, fimbriae, pili and LPS, and is surrounded by a self-producing extracellular matrix consisting of polysaccharides, proteins, lipids and nucleic acids (O’Toole et al., 2000; Flemming and Wingender, 2010).
Membrane vesicles are very abundant in biofilm related infections (Schooling and Beveridge, 2006; Toyofuku et al., 2012; Grande et al., 2015). Indeed, the proteome analysis of P. aeruginosa biofilms revealed that MV-associated proteins contribute to more or less 20% of the whole matrix proteome. The MV-related proteins identified were OM enzymes and proteins involved in the transport of small molecules, the uptake of iron and antibiotic resistance (Couto et al., 2015). In addition, vesicles purified from late-stage P. aeruginosa biofilms are enriched for drug-binding proteins, which makes the bacterial species inside these biofilms even better protected against antibiotics (Park et al., 2014). It has been suggested further that MVs secreted by P. aeruginosa are under the control of the quorum sensing system and supply the forming biofilm with extracellular DNA (eDNA) and LPS (Nakamura et al., 2008). Moreover, studies in P. aeruginosa biofilms revealed that MVs secreted by one species are able to lyse neighboring bacteria in order to release nutrients as a source for growth and eDNA to build the biofilm (Beveridge et al., 1997). However, following research revealed that the MVs themselves are actually incorporated in the biofilm matrix (Schooling and Beveridge, 2006). This was seen in Francisella biofilms as well (van Hoek, 2013).
He et al. (2017, 2019) discovered that MV secretion in methicillin-resistant S. aureus (MRSA) was correlated with biofilm formation during improper vancomycin chemotherapy. They also demonstrated that the treatment of MRSA with β-lactam antibiotics induces biofilm formation as a consequence of MV secretion with a higher hydrophobicity.
Immunomodulatory Effects of MVs
Several reports indicated that MVs can exert immunomodulatory effects and aid in pathogenesis, a few examples were already mentioned. MVs secreted by P. aeruginosa are able to activate an IL-8 response by lung epithelial cells, as was seen in NTHi as well (see above). In this way, vesicles could contribute to inflammation (Bauman and Kuehn, 2006). It was demonstrated that P. aeruginosa MVs induce the upregulation of pro-inflammatory cytokines in macrophages. The response was even greater compared to the induction of cytokines with purified LPS. This study revealed that MV-associated LPS is required for binding to the macrophages and the internalization is mediated by the protein content of the MVs. Interestingly, they also showed that intensity of IL-8 response is strain dependent and was the highest in a CF isolate (compared to the acute PAO1 strain). In addition, flagellin (FliC) was identified as one of the most abundant proteins in P. aeruginosa MVs and is responsible for the cytokine release in macrophages (Ellis et al., 2010). Moreover, MVs from P. aeruginosa cause pulmonary inflammation in a bacteria-free in vivo setting. The MVs caused a time- and dose-dependent pulmonary inflammation comparable to the response of live bacteria (Park et al., 2013). A. baumannii MVs provoked an inflammatory response in vivo (mouse model) resulting in secretion of cytokines and chemokines, mediated via Toll-like receptors (Marion et al., 2019). Although LPS embedded in MVs might be very important in this inflammatory response, it should be noted that MVs obtained from LPS-free Neisseria meningitidis did not provoke a significant different response than LPS positive MVs, indicating that other components like OMPs can function as complement activators (Bjerre et al., 2002).
Similarly, S. pneumoniae MVs are internalized into A549 lung epithelial cells and human monocyte-derived dendritic cells and result in pro-inflammatory cytokine responses (Codemo et al., 2018). Also M. catarrhalis MVs are internalized by human epithelial cells and induce an inflammatory response. On the other hand, proteomic analyses revealed that these MVs contain factors that aid these bacteria to evade the host defense system as well (Schaar et al., 2011). Further, MVs secreted by respiratory pathogens, including NTHi, M. catarrhalis, and P. aeruginosa, induce a strong pro-inflammatory response by naïve THP-1 macrophages (Volgers et al., 2017b). Regarding to this, N-acetyl-L-cysteine (NAC), a mucolytic that reduces the production of thick mucus, induced the release of pro-inflammatory MVs by these respiratory pathogens, but decreased the release of pro-inflammatory cytokines in macrophages (Volgers et al., 2017a). Moreover, MVs secreted by S. maltophilia ATCC 13637 were cytotoxic to A549 epithelial cells and induced the expression of pro-inflammatory cytokine and chemokine genes in these lung cells (Kim et al., 2016). MVs originated from K. pneumoniae ATCC 13883 likewise induced changes in the expression of immune-related genes in epithelial cells. The expression of genes encoding for IL-8, IL-1b, MIP-1α, HMOX1, HSPA1A, and IL-24, was increased after treatment of these cells with K. pneumoniae MVs (Lee et al., 2012; You et al., 2019). Further, several Gram-negative bacteria bind to epithelial cells through lipid rafts and deliver peptidoglycan-containing MVs to the intracellular sensor NOD1 to promote inflammation (Kaparakis et al., 2010).
Koeppen et al. (2016) discovered a novel mechanism of host-pathogen interaction mediated by MVs secreted by P. aeruginosa. The MVs are packed with sRNA molecules that bind to mRNA inside human lung cells and in this way quench the human immune response. Similarly, an extracellular DNase was identified in MVs from S. pneumoniae that blocks neutrophil activity and helps to evade the host innate immune response (Jhelum et al., 2018). Porin-loss, which is common in antibiotic-resistant strains of K. pneumoniae, impacts the MV composition and the host-inflammatory response. MVs lacking several OM porins were less likely to elicit the secretion of pro-inflammatory cytokines in macrophages. Antibiotic resistance resulting in porin-loss in K. pneumoniae can thus have an impact on the survival of this pathogen (Turner et al., 2015). Further, MVs from Gram-negative bacteria induced vitronectin in mouse lungs and in A549 epithelial cells, which is released into the bronchoalveolar space and mediates protection against complement-mediated clearance (Paulsson et al., 2018).
The immunomodulatory effects of MVs can be useful to protect patients from bacterial infections. A vaccine based on detergent-extracted OMVs originating from the pathogenic bacterium Neisseria meningitides, complemented with recombinant proteins, has recently been approved and used to protect people against meningitis B. Several traits, such as the overexpression of certain antigens or the modification of the LPS reactogenicity, can be altered by genetically engineering the OMV-producing bacteria to yield a vaccine that meets the specific needs (van der Pol et al., 2015). Furthermore, active immunization of mice with P. aeruginosa MVs resulted in mice that were protected from P. aeruginosa infections (Zhang et al., 2018). Also NTHi, K. pneumoniae and S. aureus MVs are potential vaccine candidates, as was demonstrated in several studies (Lee et al., 2015; Roier et al., 2015; Winter and Barenkamp, 2017; Askarian et al., 2018).
Concluding Remarks
Bacteria associated with lung infections are increasingly posing a threat for the public health worldwide. In particular, the impact on CF and immunocompromised patients is concerning. Therefore, it is crucial to shed more light on the mechanisms these bacteria use to increase their pathogenicity. MVs were discussed to play an important role herein. By targeting MV-associated components that are involved in the interaction of these vesicles with human lung cells or macrophages, new therapeutic options to treat these infections could arise. Furthermore, the immunomodulatory effects of MVs could be exploited to produce vaccines leading to the protection of patients against the infecting bacteria. Taken together, it is important to further investigate the role of MVs during bacterial infection and the use of MVs to eventually combat these infections. Importantly, more in vivo studies are required to investigate the real impact of MVs on the progression of disease.
Author Contributions
JV wrote the manuscript. BD was the supervisor of JV and edited and corrected the manuscript together with JV.
Funding
This work was supported by a grant from the FWO-Vlaanderen (G056116N).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allan, N. D., and Beveridge, T. J. (2003). Gentamicin delivery to Burkholderia cepacia group IIIa strains via membrane vesicles from Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 47, 2962–2965. doi: 10.1128/aac.47.9.2962-2965.2003
Allan, N. D., Kooi, C., Sokol, P. A., and Beveridge, T. J. (2003). Putative virulence factors are released in association with membrane vesicles from Burkholderia cepacia. Can. J. Microbiol. 49, 613–624. doi: 10.1139/w03-078
Andreoni, F., Toyofuku, M., Menzi, C., Kalawong, R., Mairpady Shambat, S., François, P., et al. (2019). Antibiotics stimulate formation of vesicles in staphylococcus aureus in both phage-dependent and -independent fashions and via different routes. Antimicrob. Agents Chemother. 63, e1439–e1418.
Askarian, F., Lapek, J. D., Dongre, M., Tsai, C.-M., Kumaraswamy, M., Kousha, A., et al. (2018). Staphylococcus aureus membrane-derived vesicles promote bacterial virulence and confer protective immunity in murine infection models. Front. Microbiol. 9:262. doi: 10.3389/fmicb.2018.02346
Ballok, A. E., Filkins, L. M., Bomberger, J. M., Stanton, B. A., and O’Toole, G. A. (2014). Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles. J. Bacteriol. 196, 3633–3642. doi: 10.1128/jb.01760-14
Barnaby, R., Koeppen, K., and Stanton, B. A. (2019). Cyclodextrins reduce the ability of Pseudomonas aeruginosa outer-membrane vesicles to reduce CFTR Cl- secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 316, L206–L215.
Bauman, S. J., and Kuehn, M. J. (2006). Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8, 2400–2408. doi: 10.1016/j.micinf.2006.05.001
Bauman, S. J., and Kuehn, M. J. (2009). Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 9:26. doi: 10.1186/1471-2180-9-26
Beveridge, T. J., Makin, S. A., Kadurugamuwa, J. L., and Li, Z. (1997). Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20, 291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x
Bitto, N. J., Chapman, R., Pidot, S., Costin, A., Lo, C., Choi, J., et al. (2017). Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep. 7:7072.
Bjerre, A., Brusletto, B., Mollnes, T. E., Fritszonn, E., Rosenqvist, E., Wedege, E., et al. (2002). Complement activation induced by purified Neisseria meningitidis lipopolysaccharide (LPS), outer membrane Vesicles, whole bacteria, and an LPS-free mutant. J. Infect. Dis. 185, 220–228. doi: 10.1086/338269
Bomberger, J. M., Maceachran, D. P., Coutermarsh, B. A., Ye, S., O’Toole, G. A., and Stanton, B. A. (2009). Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. doi: 10.1371/journal.ppat.1000382
Bomberger, J. M., Ye, S., Maceachran, D. P., Koeppen, K., Barnaby, R. L., O’Toole, G. A., et al. (2011). A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 7:e1001325. doi: 10.1371/journal.ppat.1001325
Bonnington, K. E., and Kuehn, M. J. (2014). Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 1843, 1612–1619. doi: 10.1016/j.bbamcr.2013.12.011
Brown, L., Wolf, J. M., Prados-Rosales, R., and Casadevall, A. (2015). Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630. doi: 10.1038/nrmicro3480
Chatterjee, S., Mondal, A., Mitra, S., and Basu, S. (2017). Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J. Antimicrob. Chemother. 72, 2201–2207. doi: 10.1093/jac/dkx131
Choi, D.-S., Kim, D.-K., Choi, S. J., Lee, J., Choi, J.-P., Rho, S., et al. (2011). Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11, 3424–3429. doi: 10.1002/pmic.201000212
Codemo, M., Muschiol, S., Iovino, F., Nannapaneni, P., Plant, L., Wai, S. N., et al. (2018). Immunomodulatory effects of pneumococcal extracellular vesicles on cellular and humoral host defenses. MBio 9:e559-18.
Cooke, A. C., Nello, A. V., Ernst, R. K., and Schertzer, J. W. (2019). Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS ONE 14:e0212275. doi: 10.1371/journal.pone.0212275
Couto, N., Schooling, S. R., Dutcher, J. R., and Barber, J. (2015). Proteome profiles of outer membrane vesicles and extracellular matrix of Pseudomonas aeruginosa biofilms. J. Proteome Res. 14, 4207–4222. doi: 10.1021/acs.jproteome.5b00312
Dallo, S. F., Zhang, B., Denno, J., Hong, S., Tsai, A., Haskins, W., et al. (2012). Association of Acinetobacter baumannii EF-Tu with cell surface, outer membrane vesicles, and fibronectin. Sci. World J. 2012, 1–10. doi: 10.1100/2012/128705
Deatherage, B. L., Lara, J. C., Bergsbaken, T., Barrett, S. L. R., Lara, S., and Cookson, B. T. (2009). Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72:1395. doi: 10.1111/j.1365-2958.2009.06731.x
Devos, S., Stremersch, S., Raemdonck, K., Braeckmans, K., and Devreese, B. (2016). Intra- and interspecies effects of outer membrane vesicles from Stenotrophomonas maltophilia on β-lactam resistance. Antimicrob. Agents Chemother. 60:2516. doi: 10.1128/aac.02171-15
Devos, S., Van Oudenhove, L., Stremersch, S., Van Putte, W., De Rycke, R., Van Driessche, G., et al. (2015). The effect of imipenem and diffusible signaling factors on the secretion of outer membrane vesicles and associated Ax21 proteins in Stenotrophomonas maltophilia. Front. Microbiol. 6:298. doi: 10.3389/fmicb.2015.00298
Elhenawy, W., Debelyy, M. O., and Feldman, M. F. (2014). Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. MBio 5, e909–e914.
Ellis, T. N., Leiman, S. A., and Kuehn, M. J. (2010). Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 78, 3822–3831. doi: 10.1128/iai.00433-10
Florez, C., Raab, J. E., Cooke, A. C., and Schertzer, J. W. (2017). Membrane distribution of the Pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. MBio 8:e1034-17.
Grande, R., Di Marcantonio, M. C., Robuffo, I., Pompilio, A., Celia, C., Di Marzio, L., et al. (2015). Helicobacter pylori ATCC 43629/NCTC 11639 outer membrane vesicles (OMVs) from biofilm and planktonic phase associated with extracellular DNA (eDNA). Front. Microbiol. 6:369. doi: 10.3389/fmicb.2015.01369
Gurung, M., Moon, D. C., Choi, C. W., Lee, J. H., Bae, Y. C., Kim, J., et al. (2011). Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 6:e27958. doi: 10.1371/journal.pone.0027958
Haurat, M. F., Elhenawy, W., and Feldman, M. F. (2015). Prokaryotic membrane vesicles: new insights on biogenesis and biological roles. Biol. Chem. 396, 95–109. doi: 10.1515/hsz-2014-0183
He, X., Li, S., Yin, Y., Xu, J., Gong, W., Li, G., et al. (2019). Membrane vesicles are the dominant structural components of ceftazidime-induced biofilm formation in an oxacillin-sensitive MRSA. Front. Microbiol. 10:571. doi: 10.3389/fmicb.2019.00571
He, X., Yuan, F., Lu, F., Yin, Y., and Cao, J. (2017). Vancomycin-induced biofilm formation by methicillin-resistant Staphylococcus aureus is associated with the secretion of membrane vesicles. Microb. Pathogen. 110, 225–231. doi: 10.1016/j.micpath.2017.07.004
Horspool, A. M., and Schertzer, J. W. (2018). Reciprocal cross-species induction of outer membrane vesicle biogenesis via secreted factors. Sci. Rep. 8:9873.
Jang, S. C., Kim, S. R., Yoon, Y. J., Park, K.-Y., Kim, J. H., Lee, J., et al. (2015). In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small 11, 456–461. doi: 10.1002/smll.201401803
Jeon, H., Oh, M. H., Jun, S. H., Kim, S. I. L., Choi, C. W., Kwon, H. I. L., et al. (2016). Variation among Staphylococcus aureus membrane vesicle proteomes affects cytotoxicity of host cells. Microb. Pathogen. 93, 185–193. doi: 10.1016/j.micpath.2016.02.014
Jha, C., Ghosh, S., Gautam, V., Malhotra, P., and Ray, P. (2017). In vitro study of virulence potential of Acinetobacter baumannii outer membrane vesicles. Microb. Pathogen. 111, 218–224. doi: 10.1016/j.micpath.2017.08.048
Jhelum, H., Sori, H., and Sehgal, D. (2018). A novel extracellular vesicle-associated endodeoxyribonuclease helps Streptococcus pneumoniae evade neutrophil extracellular traps and is required for full virulence. Sci. Rep. 8:7985.
Jin, J. S., Kwon, S.-O., Moon, D. C., Gurung, M., Lee, J. H., Kim, S. I. L., et al. (2011). Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS ONE 6:e17027. doi: 10.1371/journal.pone.0017027
Kadurugamuwa, J. L., and Beveridge, T. J. (1995). Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177, 3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995
Kadurugamuwa, J. L., and Beveridge, T. J. (1999). Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other Gram-negative bacteria. Microbiology 145, 2051–2060. doi: 10.1099/13500872-145-8-2051
Kaparakis, M., Turnbull, L., Carneiro, L., Firth, S., Coleman, H. A., Parkington, H. C., et al. (2010). Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 12, 372–385. doi: 10.1111/j.1462-5822.2009.01404.x
Kim, Y. J., Jeon, H., Na, S. H., Kwon, H. I. L., Selasi, G. N., Nicholas, A., et al. (2016). Stenotrophomonas maltophilia outer membrane vesicles elicit a potent inflammatory response in vitro and in vivo. Pathog. Dis. 74:ftw104.
Koeppen, K., Barnaby, R., Jackson, A. A., Gerber, S. A., Hogan, D. A., and Stanton, B. A. (2019). Tobramycin reduces key virulence determinants in the proteome of Pseudomonas aeruginosa outer membrane vesicles. PLoS ONE 14:e0211290. doi: 10.1371/journal.pone.0211290
Koeppen, K., Hampton, T. H., Jarek, M., Scharfe, M., Gerber, S. A., Mielcarz, D. W., et al. (2016). A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 12:e1005672. doi: 10.1371/journal.ppat.1005672
Kwon, S.-O., Gho, Y. S., Lee, J. C., and Kim, S. I. L. (2009). Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297, 150–156.
Lee, J. C., Lee, E. J., Lee, J. H., Jun, S. H., Choi, C. W., Kim, S. I. L., et al. (2012). Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol. Lett. 331, 17–24. doi: 10.1111/j.1574-6968.2012.02549.x
Lee, W.-H., Choi, H.-I., Hong, S.-W., Kim, K.-S., Gho, Y. S., and Jeon, S. G. (2015). Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 47:e183. doi: 10.1038/emm.2015.59
MacDonald, K. L., and Beveridge, T. J. (2002). Bactericidal effect of gentamicin-induced membrane vesicles derived from Pseudomonas aeruginosa PAO1 on gram-positive bacteria. Can. J. Microbiol. 48, 810–820. doi: 10.1139/w02-077
Manning, A. J., and Kuehn, M. J. (2011). Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 11:258. doi: 10.1186/1471-2180-11-258
Marion, C. R., Lee, J., Sharma, L., Park, K. S., Lee, C., Liu, W., et al. (2019). Role of vitamin A in the immune system. Infect. Immunol. 87:e300243-19.
Mashburn-Warren, L., Howe, J., Brandenburg, K., and Whiteley, M. (2009). Structural requirements of the Pseudomonas quinolone signal for membrane vesicle stimulation. J. Bacteriol. 191, 3411–3414. doi: 10.1128/jb.00052-09
Mashburn-Warren, L., Howe, J., Garidel, P., Richter, W., Steiniger, F., Roessle, M., et al. (2008). Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69, 491–502. doi: 10.1111/j.1365-2958.2008.06302.x
Nakamura, S., Higashiyama, Y., Izumikawa, K., Seki, M., Kakeya, H., Yamamoto, Y., et al. (2008). The roles of the quorum-sensing system in the release of extracellular DNA, lipopolysaccharide, and membrane vesicles from Pseudomonas aeruginosa. Jpn. J. Infect. Dis. 61, 375–378.
O’Donoghue, E. J., and Krachler, A. M. (2016). Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 18, 1508–1517. doi: 10.1111/cmi.12655
O’Toole, G., Kaplan, H. B., and Kolter, R. (2000). Biofilm formation as microbial development. Annu. Rev. Microbiol. 54, 49–79.
Olaya-Abril, A., Prados-Rosales, R., McConnell, M. J., Martín-Peña, R., González-Reyes, J. A., Jiménez-Munguía, I., et al. (2014). Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteomics 106, 46–60. doi: 10.1016/j.jprot.2014.04.023
Park, A. J., Surette, M. D., and Khursigara, C. M. (2014). Antimicrobial targets localize to the extracellular vesicle-associated proteome of Pseudomonas aeruginosa grown in a biofilm. Front. Microbiol. 5:464. doi: 10.3389/fmicb.2014.00464
Park, K.-S., Lee, J., Jang, S. C., Kim, S. R., Jang, M. H., Lötvall, J., et al. (2013). Pulmonary inflammation induced by bacteria-free outer membrane vesicles from Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 49, 637–645. doi: 10.1165/rcmb.2012-0370oc
Paulsson, M., Che, K. F., Ahl, J., Tham, J., Sandblad, L., Smith, M. E., et al. (2018). Bacterial outer membrane vesicles induce vitronectin release into the bronchoalveolar space conferring protection from complement-mediated killing. Front. Microbiol. 9:1559. doi: 10.3389/fmicb.2018.01559
Ren, D., Nelson, K. L., Uchakin, P. N., Smith, A. L., Gu, X.-X., and Daines, D. A. (2012). Characterization of extended co-culture of non-typeable Haemophilus influenzae with primary human respiratory tissues. Exp. Biol. Med. (Maywood, N.J.) 237, 540–547. doi: 10.1258/ebm.2012.011377
Rodriguez-Sevilla, G., Rigauts, C., Vandeplassche, E., Ostyn, L., Mahillo-Fernandez, I., Esteban, J., et al. (2018). Influence of three-dimensional lung epithelial cells and interspecies interactions on antibiotic efficacy against Mycobacterium abscessus and Pseudomonas aeruginosa. Pathog. Dis. 76:fty034.
Roier, S., Blume, T., Klug, L., Wagner, G. E., Elhenawy, W., Zangger, K., et al. (2015). A basis for vaccine development: Comparative characterization of Haemophilus influenzae outer membrane vesicles. Int. J. Med. Microbiol. 305, 298–309. doi: 10.1016/j.ijmm.2014.12.005
Roier, S., Zingl, F. G., Cakar, F., Durakovic, S., Kohl, P., Eichmann, T. O., et al. (2016a). A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 7:105151.
Roier, S., Zingl, F. G., Cakar, F., and Schild, S. (2016b). Bacterial outer membrane vesicle biogenesis: a new mechanism and its implications. Microb. Cell (Graz, Austria) 3, 257–2592.
Rumbo, C., Fernández-Moreira, E., Merino, M., Poza, M., Mendez, J. A., Soares, N. C., et al. (2011). Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55, 3084–3090. doi: 10.1128/aac.00929-10
Tan, T. T., Mörgelin, M., Forsgren, A., and Riesbeck, K. (2007). Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 195, 1661–1670. doi: 10.1086/517611
Tashiro, Y., Ichikawa, S., Nakajima-Kambe, T., Uchiyama, H., and Nomura, N. (2010). Pseudomonas quinolone signal affects membrane vesicle production in not only gram-negative but also gram-positive bacteria. Microbes Environ. 25, 120–125. doi: 10.1264/jsme2.me09182
Thay, B., Wai, S. N., and Oscarsson, J. (2013). Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE 8:e54661. doi: 10.1371/journal.pone.0054661
Toyofuku, M., Cárcamo-Oyarce, G., Yamamoto, T., Eisenstein, F., Hsiao, C.-C., Kurosawa, M., et al. (2017). Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 8:481.
Toyofuku, M., Nomura, N., and Eberl, L. (2019). Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24. doi: 10.1038/s41579-018-0112-2
Toyofuku, M., Roschitzki, B., Riedel, K., and Eberl, L. (2012). Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J. Proteome Res. 11, 4906–4915.
Turnbull, L., Toyofuku, M., Hynen, A. L., Kurosawa, M., Pessi, G., Petty, N. K., et al. (2016). Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7:11220.
Turner, K. L., Cahill, B. K., Dilello, S. K., Gutel, D., Brunson, D. N., Albertí, S., et al. (2015). Porin loss impacts the host inflammatory response to outer membrane vesicles of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 60, 1360–1369. doi: 10.1128/aac.01627-15
Schaar, V., Uddback, I., Nordstrom, T., and Riesbeck, K. (2014). Group A streptococci are protected from amoxicillin-mediated killing by vesicles containing -lactamase derived from Haemophilus influenzae. J. Antimicrob. Chemother. 69, 117–120. doi: 10.1093/jac/dkt307
Schaar, V., de Vries, S. P. W., Perez Vidakovics, M. L. A., Bootsma, H. J., Larsson, L., Hermans, P. W. M., et al. (2011). Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell. Microbiol. 13, 432–449. doi: 10.1111/j.1462-5822.2010.01546.x
Schooling, S. R., and Beveridge, T. J. (2006). Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957. doi: 10.1128/jb.00257-06
Schwechheimer, C., and Kuehn, M. J. (2015). Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619. doi: 10.1038/nrmicro3525
Schwechheimer, C., Kulp, A., and Kuehn, M. J. (2014). Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 14:324. doi: 10.1186/s12866-014-0324-1
Sharpe, S. W., Kuehn, M. J., and Mason, K. M. (2011). Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect. Immun. 79, 4361–4369. doi: 10.1128/iai.05332-11
van der Pol, L., Stork, M., and van der Ley, P. (2015). Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 10, 1689–1706. doi: 10.1002/biot.201400395
van Hoek, M. L. (2013). Biofilms: an advancement in our understanding of Francisella species. Virulence 4, 833–846. doi: 10.4161/viru.27023
Volgers, C., Benedikter, B. J., Grauls, G. E., Hellebrand, P. H. M., Savelkoul, P. H. M., and Stassen, F. R. M. (2017a). Effects of N-acetyl-L-cysteine on the membrane vesicle release and growth of respiratory pathogens. FEMS Microbiol. Lett. 364:1. doi: 10.1155/2015/540271
Volgers, C., Benedikter, B. J., Grauls, G. E., Savelkoul, P. H. M., and Stassen, F. R. M. (2017b). Immunomodulatory role for membrane vesicles released by THP-1 macrophages and respiratory pathogens during macrophage infection. BMC Microbiol. 17:2162. doi: 10.1186/s12866-017-1122-3
Yun, S. H., Park, E. C., Lee, S.-Y., Lee, H., Choi, C.-W., Yi, Y.-S., et al. (2018). Antibiotic treatment modulates protein components of cytotoxic outer membrane vesicles of multidrug-resistant clinical strain, Acinetobacter baumannii DU202. Clin. Proteomics 15:28.
Zhang, X., Yang, F., Zou, J., Wu, W., Jing, H., Gou, Q., et al. (2018). Immunization with Pseudomonas aeruginosa outer membrane vesicles stimulates protective immunity in mice. Vaccine 36, 1047–1054. doi: 10.1016/j.vaccine.2018.01.034
Winter, L. E., and Barenkamp, S. J. (2017). Immunogenicity of nontypeable Haemophilus influenzae outer membrane vesicles and protective ability in the chinchilla model of otitis media. Clin. Vaccine Immunol. 24: e00138-17.
Keywords: bacteria, lung infection, pneumonia, cystic fibrosis, pathogenesis, membrane vesicles, antimicrobial resistance
Citation: Vitse J and Devreese B (2020) The Contribution of Membrane Vesicles to Bacterial Pathogenicity in Cystic Fibrosis Infections and Healthcare Associated Pneumonia. Front. Microbiol. 11:630. doi: 10.3389/fmicb.2020.00630
Received: 31 October 2019; Accepted: 20 March 2020;
Published: 09 April 2020.
Edited by:
Araceli Contreras-Rodriguez, National Polytechnic Institute, MexicoReviewed by:
Yosuke Tashiro, Shizuoka University, JapanTimothy James Wells, The University of Queensland, Australia
Copyright © 2020 Vitse and Devreese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bart Devreese, QmFydC5kZXZyZWVzZUB1Z2VudC5iZQ==
 Jolien Vitse
Jolien Vitse Bart Devreese
Bart Devreese