- 1Department of Developmental Cell Biology, Key Laboratory of Cell Biology, Ministry of Public Health, Key Laboratory of Medical Cell Biology, Ministry of Education, China Medical University, Shenyang, China
- 2Department of Surgical Oncology and General Surgery, Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Ministry of Education, The First Affiliated Hospital of China Medical University, Shenyang, China
Neonatal bacterial meningitis remains a life-threatening and causative sequelae disease in newborns, despite the effective usage of antibiotics and improved critical medical care. Polymorphonuclear leukocyte (PMN) transendothelial migration across the blood-brain barrier, one of the three hallmarks of bacterial meningitis, now is considered as a “double-edge sword”. When participating in host immune system defending against virulent pathogens, it results in tissue inflammation and following severe damage of central nervous system at the same time, which contributes to a disastrous consequence. Recently, several researches have focused on this multi-step process and the mechanism of how the virulent factors of different pathogens influence PMN migration. The great progression they made has enlightened a new research hotspot and a novel therapeutic strategy. This mini review outlines the determinants and progression of PMN transmigration in neonatal meningitis caused by different predominant pathogens.
Introduction
Neonatal bacterial meningitis is regarded as a lethal and causative sequelae disease among newborns. Despite the process of the rapid diagnosis of pathogens and availability of effective bactericidal antibiotics, neonatal meningitis maintains a high neonatal mortality and morbidity worldwide (Huang et al., 2000; Lawrence et al., 2015). The epidemiology for neonatal bacterial meningitis varies from country to country (Zaidi et al., 2009; Khalessi and Afsharkhas, 2014). In general, in developed countries, the incidence is estimated at 0.3 per 1000 live births and mortality ranges 10–15%. Meanwhile, the major pathogens are group B Streptococcus (GBS), Escherichia coli (E. coli), and Listeria monocytogenes (L. monocytogenes). However, the incidence remains much higher at 0.8–6.1 per 1000 live births and 40–58% of neonates dies from it in developing countries where the important microorganisms are GBS, other Gram negatives (excluding E. coli), L. monocytogenes and Gram-positive organisms, respectively (Zaidi et al., 2009; Furyk et al., 2011; Khalessi and Afsharkhas, 2014; Lawrence et al., 2015). The successful meningitic pathogens must enter the peripheral blood to form the bacteremia and cross the blood-brain barrier (BBB) which anatomically have a characteristic tight junction of brain microvascular endothelial cells (BMEC) (Bowman et al., 1983; Rubin and Staddon, 1999; Huang et al., 2000). Different bacteria use different mechanisms to cross the BBB. In the past several decades, microbiologists focused more on identified virulence factors in bacteria that taking part in their traversal of the BBB in bacterial meningitis. However, pathogens crossing the BBB is only the primitive step during the progression of bacterial meningitis. In the subsequent process of the bacterial meningitis following the entry of bacteria into the cerebrospinal fluid, polymorphonuclear leukocyte (PMN) transendothelial migration across the BBB is another substantial feature of bacterial meningitis. The penetration of PMN across BBB is regard as a “double-edge sword”. On one hand, PMN can help the host defense fight against pathogens; oppositely, PMN may also cause significant tissue damage to the central nervous system (CNS), leading to the serious neurologic sequelae (Flier et al., 2003; Wang et al., 2016). In bacterial meningitis, PMN transendothelial migration is a multi-step process involving pathogen, neutrophil, as well as BMEC. This mini review outlines the determinants and progression of PMN transmigration in neonatal meningitis caused by different predominant pathogens.
Bacterial Meningitis Caused by Escherichia Coli
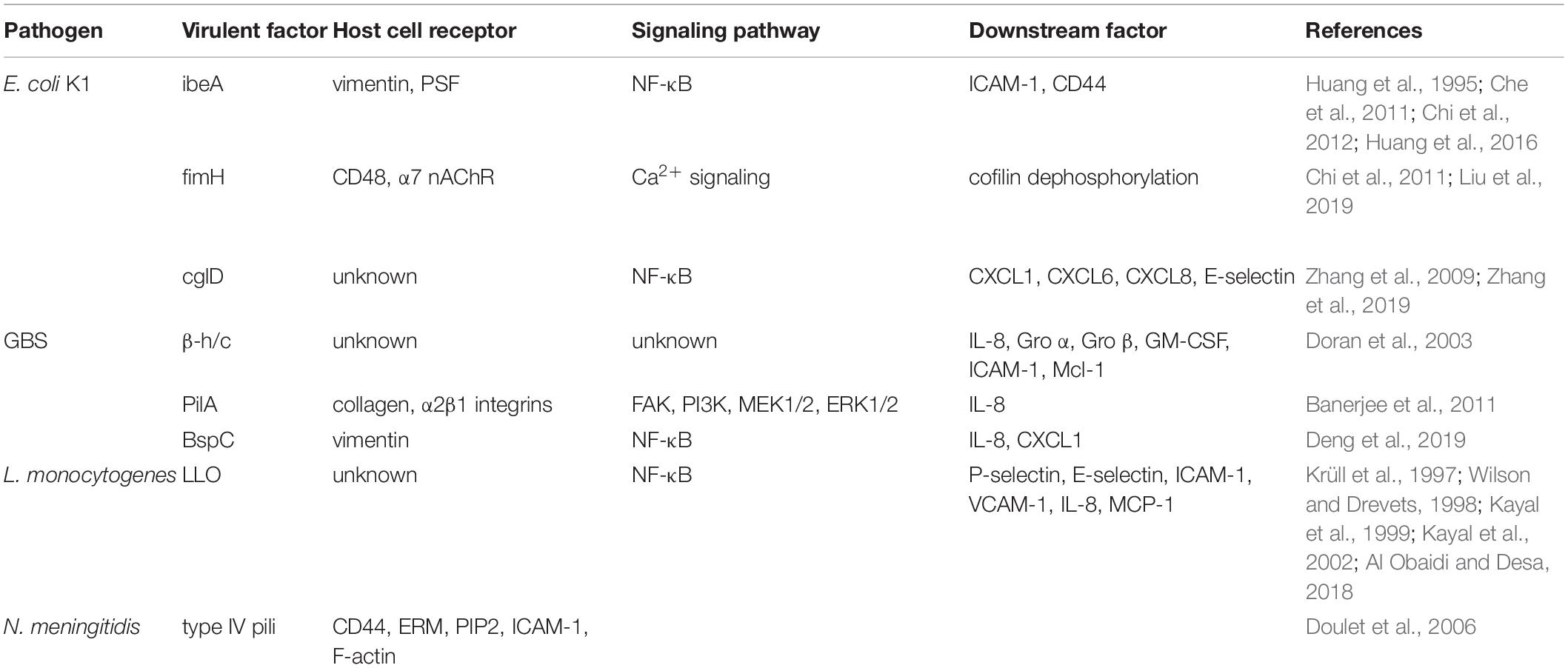
Escherichia coli is the most common Gram-negative bacterium causing neonatal meningitis, which is also the main pathogen in developing countries (Khalessi and Afsharkhas, 2014). The bacteria with K1 capsule is the dominant (∼80%) serotype in E. coli meningitis (Huang et al., 2000). IbeA (invasion brain endothelial protein A) was firstly validated as a vital determinant of E. coli K1 to promote bacterial penetration across the BBB (Huang et al., 1995). IbeA also played an important role in facilitating PMN transmigration. Not only ibeA+ E. coli K1, purified ibeA protein can induce PMN transmigration independently (Che et al., 2011; Chi et al., 2012). IbeA, presenting on the outer membrane of E. coli K1, can interact with its primary receptor vimentin together with its co-receptor PTB-associated splicing factor (PSF) on BMEC to activate NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling, which consequently accelerates the recruitment the PMN to BMEC (Chi et al., 2012; Huang et al., 2016). Meantime, ibeA+ E. coli K1 and purified ibeA protein triggered the upregulation of adhesion molecules containing intercellular adhesion molecule-1 (ICAM-1) and CD44, which were involved in the PMN movement across the BMEC monolayer through the enhanced adhesion of PMN to BMEC (Che et al., 2011). FimH (type 1 fimbrial tip adhesin), expressed on E. coli K1, made the bacteria have a mannose-sensitive binding ability, which is another important determinant participating not only in bacterial adhesion and invasion, but also in PMN transmigration (Liu et al., 2019). The fimH knock-out mutant in E. coli K1 presented inefficiency in promoting PMN transmigration compared with wild-type E. coli K1 (Chi et al., 2011). FimH in E. coli K1 can mediate PMN transmigration across BMEC through binding to the protein complex composed of CD48 (the receptor of fimH) and alpha 7 nicotinic acetylcholine receptor (α7 nAChR) on the lipid raft of BMEC (Chi et al., 2011). Previous study showed that α7 nAChR plays a critical role in modulation of pathogen invasion and PMN recruitment in E. coli meningitis as an independent factor. In addition, fimH interacted with CD48/α7 nAChR complex to active Ca2+ signaling and induce cofilin dephosphorylation, which may be the probable molecular mechanism for PMN transmigration mediated by fimH (Chi et al., 2011; Liu et al., 2019).
Recently, we found E. coli K1 virulence factor cglD promote PMN transendothelial migration (Zhang et al., 2009, 2019). CglD and ibeA genes are located at the same pathogenicity island named GimA (genetic island of meningitic E. coli containing ibeA) (Huang et al., 2001). The former has a 1083-bp open reading frame (ORF) and the product of cglD gene expression has the activity of glycerol dehydrogenase (Zhang et al., 2009). Different from other virulence factors, cglD did not participate in the invasion of the BMEC (Zhang et al., 2009). Meanwhile, cglD in E. coli K1 may bear a part in the activation of the NF-κB signaling pathway in BMEC. This results in the release of some inflammation-related cytokines, including CXCL1 (chemokine (C-X-C motif) ligand 1), CXCL6 and CXCL8, which enhanced the attraction of PMN to BMEC. Meantime, with the increase of E-selectin expression in BMEC, the adhesion of PMN to BMEC are strengthened, which ultimately promotes transendothelial migration across the BBB into the brain (Zhang et al., 2019). Although there is a crosstalk between E. coli and BMEC, the molecular and cellular mechanisms for PMN transmigration remain to be defined. Further detailed study is needed to discover the novel therapeutic targets which can be modulated to make the PMN playing more positive roles in bacterial meningitis progression.
Bacterial Meningitis Caused by Group B Streptococcus
Group B Streptococcus is the most frequent Gram-positive bacterium and also a leading cause of meningitis in newborn infants in developed country such as United States (Khalessi and Afsharkhas, 2014). GBS capsular serotypes III strain is commonly associated with bacteremia and GBS meningitis develops as a consequence when pathogens move across the BBB (Nizet et al., 1997). BMEC challenged with GBS produces some functional genes expression products including IL-8 (interleukin 8), Gro α (growth-related gene product α)/CXCL1, Gro β/CXCL2, IL-6, GM-CSF (granulocyte-macrophage colony-stimulating factor), myeloid cell leukemia sequence-1 (Mcl-1), and ICAM-1 (Doran et al., 2003). Among them, IL-8, Gro α, and Gro β are responsible for the recruitment of PMN; GM-CSF is contributed to stimulating PMN produced from bone marrow; ICAM-1 and Mcl-1 are responsible for the adhesion of PMN to BMEC and prevention of PMN apoptosis, respectively (Doran et al., 2003). These specific expressions of genes are mainly mediated by β-hemolysin/cytolysin toxin (β-h/c) of GBS (Doran et al., 2003). Deletion of β-h/c results in a significantly reduction in expression of these genes when the BMEC infected with β-h/c deletion mutant strain, consequently causes decreased PMN transmigration (Doran et al., 2003). Therefore, β-h/c plays an important role in neutrophil across the BMEC, furthermore, Doran et al. (2003) showed that capsular polysaccharide in GBS is not essential for the recruitment of PMN transmigration. Pili, cell surface appendage, was recently discovered in GBS, which is contributed to the adhesion of GBS to brain microvascular endothelium (Maisey et al., 2007). The gene pilA was identified to participate in assembling the pili and the expression of gene pilA has a positive effect on the adhesion of GBS (Nobbs et al., 2008). With further study, PilA–GST proteins can induce a significant release of IL-8 by BMEC; accordingly, PilA-deficient mutant caused a downregulation of IL-8 in BMEC, which results in a reduced PMN recruitment to BMEC (Banerjee et al., 2011). PilA has the capacity to interact with collagen, which typically binds to α2β1 integrins to initiate the activation of FAK (focal adhesion kinase) and subsequent PI3K (phosphoinositide 3-kinases) and MEK1/2 (MAPK/ERK kinases)-ERK1/2 (extracellular signal regulated kinase) signaling pathway in BMEC. These events lead to the release of IL-8 and neutrophil recruitment by BMEC and ultimately enhance the PMN transmigration (Banerjee et al., 2011). Lately, surface antigen I/II protein BspC was verified in GBS, which was studied as a multifunctional adhesins in other Streptococci. Beside the traditional adherent and invasive function, BspC also takes part in the PMN transmigration through stimulating the activation of NF-κB signaling pathway and expression of IL-8 and CXCL1. During this process, cytoskeletal component vimentin in BMEC made a great contribution through interacting with BspC in BMEC (Deng et al., 2019). It is worth noting that vimentin expressed by BMEC has a cross talk with a variety of bacteria, which indicated that vimentin is a potential target to regulate the PMN transendothelial migration (Tim and Holger, 2016).
Bacterial Meningitis Caused by Other Bacteria
In addition to E. coli and GBS, L. monocytogenes is the third common reported bacteria which can cause the neonatal meningitis (Disson and Lecuit, 2012; Khalessi and Afsharkhas, 2014). L. monocytogenes is a Gram-positive bacterium widely spread in soil, animals and human. Although reported as a rare type of meningitis, it is always life-threatening, because L. monocytogenes is nearly tenfold more efficient in invading the CNS than other Gram-positive bacteria, including GBS (Schuchat et al., 1997). At present, there is no exact study on PMN transendothelial migration in L. monocytogenes meningitis. But L. monocytogenes can stimulate the expression of P-selectin, E-selectin, ICAM-1 and VCAM-1 (vascular cell adhesion molecule 1), as well as IL-8 and MCP-1 (monocyte chemoattractant protein 1) both in BMEC and brain microvessels through activating NF-κB signaling pathway, which enhance the adhesion of neutrophil to BMEC (Krüll et al., 1997; Wilson and Drevets, 1998; Kayal et al., 1999, 2002). During this process, the pore-forming toxin Listeriolysin O (LLO) in L. monocytogenes makes a great contribution in triggering PMN adhesion to BMEC by facilitating and enhancing the expression of these functional proteins (Al Obaidi and Desa, 2018). In Neisseria meningitidis (N. meningitidis) meningitis, the bacteria boost firm adhesion to BMEC, and then PMN transendothelial migration is inhibited ultimately, which is different from other neuroinvasive bacteria (Doulet et al., 2006). Doulet et al. (2006) explained that an “endothelial docking structures” consisted of actin-rich membrane protrusion caused by the adhesion of PMN on BMEC is required for the PMN transmigration. While BMEC are infected with N. meningitidis, BMEC effectively recruit ezrin and moesin (known as ERM), and ezrin binding adhesion molecules, such as ICAM-1, ICAM-2, VCAM-1, and CD44. The segregation of ERM and these adhesion molecules caused by N. meningitidis results in the abolishment of “endothelial docking structures,” which lead to the failure of PMN transmigration (Doulet et al., 2006).
Discussion
Bacterial meningitis usually displays triad hallmark features: pathogen penetration, NF-κB activation and leukocyte transmigration. Although antibiotics and critical medical care have improved, the prognosis is still unsatisfying. Together with the severe “side effect” of CNS inflammation following the pathogens across BBB, the PMN transendothelial migration has been brought to our attention. Several researches about different determinant pathogens have made and the molecular and cellular mechanisms have been revealed. Among these, E. coli and GBS, reported as the leading pathogens, are well studied, respectively. But the detailed mechanism should be defined in the future. For example, the cglD in E. coli K1 is a cytoplasmic protein. The mechanism of how it activates NF-κB signaling pathway in BMEC is needed further investigation. The influence of other common pathogens on PMN migration also is needed to be explored.
Overall, PMN transendothelial migration occurring in neonatal meningitis is driven by interactions between meningitic pathogens and brain microvascular endothelium. The molecular and cellular mechanisms about these interactions in neonatal meningitis have been revealed by basic medical research and crucial bacterial pathogenic determinants and host factors have been explored (Table 1 and Figure 1). In future, discovering whether they share a common strategy to influence neutrophil transmigration, such as the vimentin, will bring us a novel therapeutic strategy. Furthermore, along with more and more attempt to regulate PMN transmigration, prevention of bacterial meningitis progression will make an improvement.
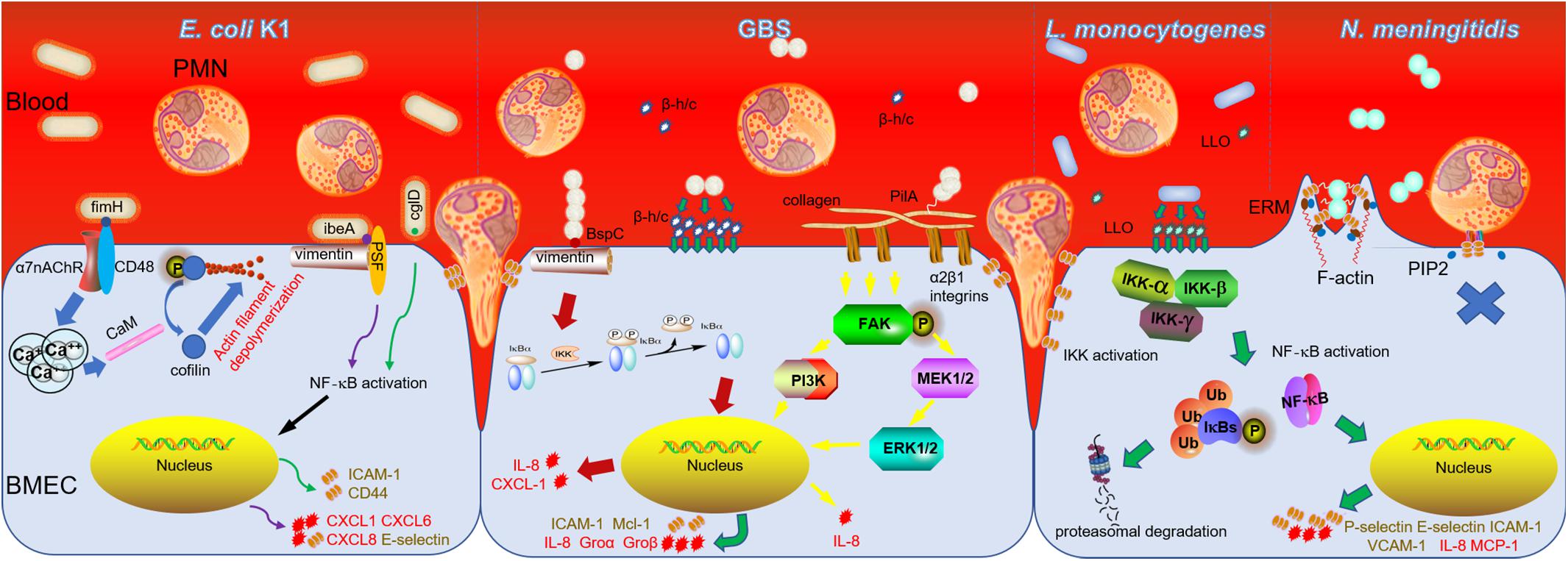
Figure 1. PMN transmigration in bacterial meningitis caused by E. coli K1, GBS, L. monocytogenes, and N. meningitidis. The secreted toxins and other virulent factors presenting either on or in the pathogens mainly interact with receptors of BMEC which transduce signals to nucleus and induce expression of numerous chemoattractive cytokines and adhesion molecules to promote PMN migration across BBB ultimately, while N. meningitidis adheres to BMEC to form a “tight” junction which prevents PMN transendothelial migration subsequently.
Author Contributions
KZ conceived and designed the mini review. ZN and KZ wrote the manuscript. ZN, Y-HC, and KZ corrected the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81571046, 81101225, 31571057, and 31771258).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Al Obaidi, M. M. J., and Desa, M. N. M. (2018). Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial–host interactions facilitate the bacterial pathogen invading the brain. Cell. Mol. Neurobiol. 38, 1349–1368. doi: 10.1007/s10571-018-0609-2
Banerjee, A., Kim, B. J., Carmona, E. M., Cutting, A. S., Gurney, M. A., Carlos, C., et al. (2011). Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat. Commun. 2:462. doi: 10.1038/ncomms1474
Bowman, P. D., Ennis, S. R., Rarey, K. E., Betz, A. L., and Goldstein, G. W. (1983). Brain microvascular endothelial cells in tissue culture: a model for study of blood-brain permeability. Ann. Neurol. 14, 396–402. doi: 10.1002/ana.410140403
Che, X. J., Chi, F., Wang, L., Jong, T. D., Wu, C. H., Wang, X. N., et al. (2011). Involvement of ibeA in meningitic Escherichia coli K1-induced polymorphonuclear leukocyte transmigration across brain endothelial cells. Brain Pathol. 21, 389–404. doi: 10.1111/j.1750-3639.2010.00463.x
Chi, F., Bo, T., Wu, C. H., Jong, A., and Huang, S. H. (2012). Vimentin and PSF act in concert to regulate ibeA+ E. coli K1 induced activation and nuclear translocation of NF-kB in human brain endothelial cells. PLoS One 7:e35863. doi: 10.1371/journal.pone.0035862
Chi, F., Wang, L., Zheng, X. Y., Wu, C. H., Jong, A., Sheard, M. A., et al. (2011). Meningitic Escherichia coli K1 penetration and neutrophil transmigration across the blood–brain barrier are modulated by alpha7 nicotinic receptor. PLoS One 6:e25016. doi: 10.1371/journal.pone.0025016
Deng, L. W., Spencer, B. L., Holmes, J. A., Mu, R., Rego, S., Weston, T. A., et al. (2019). The Group B Streptococcal surface antigen I/II protein, BspC, interacts with host vimentin to promote adherence to brain endothelium and inflammation during the pathogenesis of meningitis. PLoS Pathog. 15:e1007848. doi: 10.1371/journal.ppat.1007848
Disson, O., and Lecuit, M. (2012). Targeting of the central nervous system by Listeria monocytogenes. Virulence 3, 213–221. doi: 10.4161/viru.19586
Doran, K. S., Liu, G. Y., and Nizet, V. (2003). Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Invest. 112, 736–744. doi: 10.1172/JCI17335
Doulet, N., Donnadieu, E., Laran-Chich, M. P., Niedergang, F., Nassif, X., and Couraud, P. O. (2006). Neisseria meningitidis infection of human endothelial cells interferes with leukocyte transmigration by preventing the formation with leukocyte transmigration by preventing the formation of endothelial docking structures. J. Cell. Biol. 173, 627–637. doi: 10.1083/jcb.200507128
Flier, M. V. D., Geelen, S. P. M., Kimpen, J. L. L., Hoepelman, I. M., and Tuomanen, E. I. (2003). Reprogramming the host response in bacterial meningitis: how best to improve outcome? Clin. Microbiol. Rev. 16, 415–429. doi: 10.1128/cmr.16.3.415-429.2003
Furyk, J. S., Swann, O., and Molyneux, E. (2011). Systematic review: neonatal meningitis in the developing world. Trop. Med. Int. Health 16, 672–679. doi: 10.1111/j.1365-3156.2011.02750.x
Huang, S. H., Chen, Y. H., Kong, G., Chen, S. H., Besemer, J., Borodovsky, M., et al. (2001). A novel genetic island of meningitic Escherichia Coli K1 containing the ibeA invasion gene (GimA): functional annotation and carbon-source-regulated invasion of human brain microvascular endothelial cells. Funct. Integr. Genomics. 1, 312–322. doi: 10.1007/s101420100039
Huang, S. H., Chi, F., Peng, L., Bo, T., Zhang, B., Liu, L. Q., et al. (2016). Vimentin, a novel NF-κB regulator, is required for meningitic Escherichia coli K1- induced pathogen invasion and PMN transmigration across the blood-brain barrier. PLoS One 11:e0162641. doi: 10.1371/journal.pone.0162641
Huang, S. H., Stins, M. F., and Kim, K. S. (2000). Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2, 1237–1244. doi: 10.1016/s1286-4579(00)01277-6
Huang, S. H., Wass, C., Fu, Q., Prasadarao, N. V., Stins, M., and Kim, K. S. (1995). Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 63, 4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995
Kayal, S., Lilienbaum, A., Join-Lambert, O., Li, X., Israël, A., and Berche, P. (2002). Listeriolysin O secreted by listeria monocytogenes induces NF-kappaB signalling by activating the I kappa B kinase complex. Mol. Microbiol. 44, 1407–1419. doi: 10.1046/j.1365-2958.2002.02973.x
Kayal, S., Lilienbaum, A., Poyart, C., Memet, S., Lsrael, A., and Berche, P. (1999). Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31, 1709–1722. doi: 10.1046/j.1365-2958.1999.01305.x
Khalessi, N., and Afsharkhas, L. (2014). Neonatal meningitis: risk factors, causes and neurologic complications, Iran. J. Child. Neurol. 8, 46–50.
Krüll, M., Nöst, R., Hippenstiel, S., Domann, E., Chakraborty, T., and Suttorp, N. (1997). Listeria monocytogenes potently induces up-regulation of endothelial adhesion molecules and neutrophil adhesion to cultured human endothelial cells. J. Immunol. 159, 1970–1976.
Lawrence, C. K., Kim, A. B., and Michael, C. W. (2015). Bacterial meningitis in infant. Clin. Perinatol. 42, 29–45. doi: 10.1016/j.clp.2014.10.004
Liu, R., Wu, C., Li, L., Chi, F., Zhang, T. S., Xu, Y. T., et al. (2019). CD48 and α7 nicotinic acetylcholine receptor synergistically regulate fimH-mediated Escherichia coli K1 penetration and neutrophil transmigration across human brain microvascular endothelial cells. Infect. Dis. 219, 470–479. doi: 10.1093/infdis/jiy531
Maisey, H. C., Hensler, M., Nizet, V., and Doran, K. S. (2007). Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 189, 1464–1467. doi: 10.1128/JB.01153-06
Nizet, V., Kim, K. S., Stins, M., Jonas, M., Chi, E. Y., Nguyen, D., et al. (1997). Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65, 5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997
Nobbs, A. H., Rosini, R., Rinaudo, C. D., Maione, D., Grandi, G., and Telford, J. L. (2008). Sortase A utilizes an ancillary protein anchor for efficient cell wall anchoring of pili in Streptococcus agalactiae. Infect. Immun. 76, 3550–3560. doi: 10.1128/IAI.01613-07
Rubin, L. L., and Staddon, J. M. (1999). The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22, 11–28. doi: 10.1146/annurev.neuro.22.1.11
Schuchat, A., Robinson, K., Wenger, J. D., Harrison, L. H., Farley, M., Reingold, A. L., et al. (1997). Bacterial meningitis in the united states in 1995. N. Engl. J. Med. 337, 970–976. doi: 10.1056/NEJM199710023371404
Tim, N. M., and Holger, B. (2016). Vimentin in bacterial infections. Cells 5:18. doi: 10.3390/cells5020018
Wang, S. F., Peng, L., Gai, Z. T., Zhang, L. H., Jong, A., Cao, H., et al. (2016). Pathogenic triad in bacterial meningitis: pathogen invasion, NF-κB activation, and leukocyte transmigration that occur at the blood-brain barrier. Front. Microbiol. 7:148. doi: 10.3389/fmicb.2016.00148
Wilson, S. L., and Drevets, D. A. (1998). Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J. Infect. Dis. 178, 1658–1666. doi: 10.1086/314490
Zaidi, A. K. M., Thaver, D., Ali, S. A., and Khan, T. A. (2009). Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr. Infect. Dis. J. 28, S10–S18. doi: 10.1097/INF.0b013e3181958769
Zhang, K., Shi, M. J., Niu, Z., Chen, X., Wei, J. Y., Miao, Z. W., et al. (2019). Activation of brain endothelium by Escherichia coli K1 virulence factor cglD promotes polymorphonuclear leukocyte transendothelial migration. Med. Microbiol. Immunol. 208, 59–68. doi: 10.1007/s00430-018-0560-3
Zhang, K., Zhao, W. D., Li, Q., Fang, W. G., Zhu, L., Shang, D. S., et al. (2009). Tentative identification of glycerol dehydrogenase as Escherichia coli K1 virulence factor cglD and its involvement in the pathogenesis of experimental neonatal meningitis. Med. Microbiol. Immunol. 198, 195–204. doi: 10.1007/s00430-009-0119-4
Keywords: polymorphonuclear leukocyte, transendothelial migration, blood-brain barrier, brain microvascular endothelium, neonatal meningitis, bacteria, host
Citation: Niu Z, Chen Y-H and Zhang K (2020) Polymorphonuclear Leukocyte Transendothelial Migration Proceeds at Blood-Brain Barrier in Neonatal Meningitis. Front. Microbiol. 11:969. doi: 10.3389/fmicb.2020.00969
Received: 14 March 2020; Accepted: 22 April 2020;
Published: 26 May 2020.
Edited by:
Marina De Bernard, University of Padua, ItalyReviewed by:
Tatiana Barichello, The University of Texas Health Science Center at Houston, United StatesSheng-He Huang, University of Southern California, United States
Copyright © 2020 Niu, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Zhang, a3poYW5nQGNtdS5lZHUuY24=
 Zhuo Niu1,2
Zhuo Niu1,2 Yu-Hua Chen
Yu-Hua Chen Ke Zhang
Ke Zhang