- 1Laboratory of Protozoological Biodiversity and Evolution in Wetland, College of Life Sciences, Shaanxi Normal University, Xi’an, China
- 2Department of Life Sciences, Natural History Museum, London, United Kingdom
The morphology and the regulation of cortical pattern associated with the cell size, division, and phylogenetic position of a new hypotrichous ciliate, Quadristicha subtropica n. sp. collected from a freshwater pond in southern China, were investigated. Quadristicha subtropica n. sp. is characterized as follows: size in vivo 60–115 μm × 25–45 μm; 19–21 adoral membranelles; buccal cirrus near anterior end of endoral and paroral; cirrus IV/3 at about level of buccal vertex; right marginal row begins ahead of buccal vertex; 11–16 right and 12–19 left marginal cirri; and dorsal cilia about 5 μm long. The basic morphogenetic process in Q. subtropica n. sp. is consistent with that of the type species, Quadristicha setigera. Phylogenetic analyses based on small subunit ribosomal DNA sequence data reveal that the systematic position of Q. subtropica n. sp. is rather unstable with low support values across the tree and the genus Quadristicha is not monophyletic.
Introduction
Ciliates are one of the most species-rich groups within Protozoa and live in a variety of habitats, such as soil, freshwater, and seawater (Small and Lynn, 1985; Cheng et al., 2019; Bai et al., 2020; Lian et al., 2020; Wu et al., 2020; Zhao et al., 2020). Hypotrichia Stein, 1859 is considered to have the most complex morphology and morphogenesis within the phylum Ciliophora Doflein, 1901. They are thus increasingly recognized as being of significance to the study of cell biology, genetics, and ecology. Recent studies have revealed numerous new taxa of hypotrichs, suggesting that this group is even more diverse than previously supposed (Berger, 1999; Foissner, 2016; Song and Shao, 2017; Hu et al., 2019; Chen et al., 2020; Dong et al., 2020; Lu et al., 2020; Paiva, 2020; Park et al., 2020; Shao et al., 2020; Wang et al., 2020, 2021a; Xu et al., 2020; Zhang et al., 2020; Li et al., 2021a,b; Vďačný and Foissner, 2021).
Oxytrichidae Ehrenberg, 1830 is a species-rich family within the subclass Hypotrichia (Berger, 1999, 2018; Foissner, 2016). Recently, some new genera have been established for some of the Oxytricha species, namely, Fragmospina Foissner, 2016, Paroxytricha Foissner, 2016, Monomicrocaryon Foissner, 2016, Quadristicha Foissner, 2016, Aponotohymena Foissner, 2016 and Oxytrichella Foissner, 2016. Quadristicha Foissner, 2016 is an oxytrichid genus that is characterized by having a flexible body with 18 frontal-ventral-transverse cirri, two macronuclear nodules with a micronucleus in between, dorsal kineties that do not fragment during ontogenesis, and three caudal cirri. The type species is Quadristicha setigera (Stokes, 1891) Foissner, 2016.
In September 2018, an oxytrichid ciliate was isolated from a freshwater pond in Peninsula Lake Park, Wanning, Hainan Province, China. In the present study, we investigate its morphology, morphogenesis, and the phylogenetic position.
Materials and Methods
Quadristicha subtropica n. sp. was isolated from a freshwater pond in Peninsula Lake Park in Wanning, China (18°41′03″N; 110°24′02″E), on September 12, 2018. Some bark and rotten leaves were taken together with water from the sampling site. Cells were cultured at the room temperature in the laboratory with mineral water (Nongfu Spring), enriched with rice. Although we failed to establish a clonal culture, no other oxytrichid morphotypes were present in the protargol preparations. Therefore, we are confident that the morphological, morphogenetic, and molecular studies reported here deal solely with the same species.
Cells were studied in vivo using a high-power oil immersion objective and differential interference contrast. Protargol (Wilbert, 1975) was used to reveal the infraciliature. Measurements of silvered specimens were performed with the imaging software cellSens Entry (Olympus). Drawings of live specimens are based on photographic records, and those of impregnated cells were made with a camera lucida. For clarity, parental cirri are shown only by outline, whereas new ones are shaded. Terminology follows Berger (1999) and Foissner (2016).
DNA Extraction, PCR Amplification, and Sequencing
The genomic DNA extraction, PCR amplification, and gene sequencing were carried out according to Wang et al. (2021b).
Phylogenetic Analyses
The SSU rDNA sequence of Quadristicha subtropica n. sp. was aligned with sequences of 70 other hypotrich species downloaded from GenBank database for phylogenetic analyses. Euplotid species were used as the outgroup taxa. Phylogenetic analyses were carried out according to Wang et al. (2021b).
Results
ZooBank Registration
Present work: urn:lsid:zoobank.org:pub:4CBB9B60-4158-4F2C-B603-02D50B4F8ABE Quadristicha subtropica n. sp.: urn:lsid:zoobank.org:act:A8FB0E8F-91F1-4768-8DEE-522580AA55B3.
Quadristicha subtropica n. sp.
Diagnosis
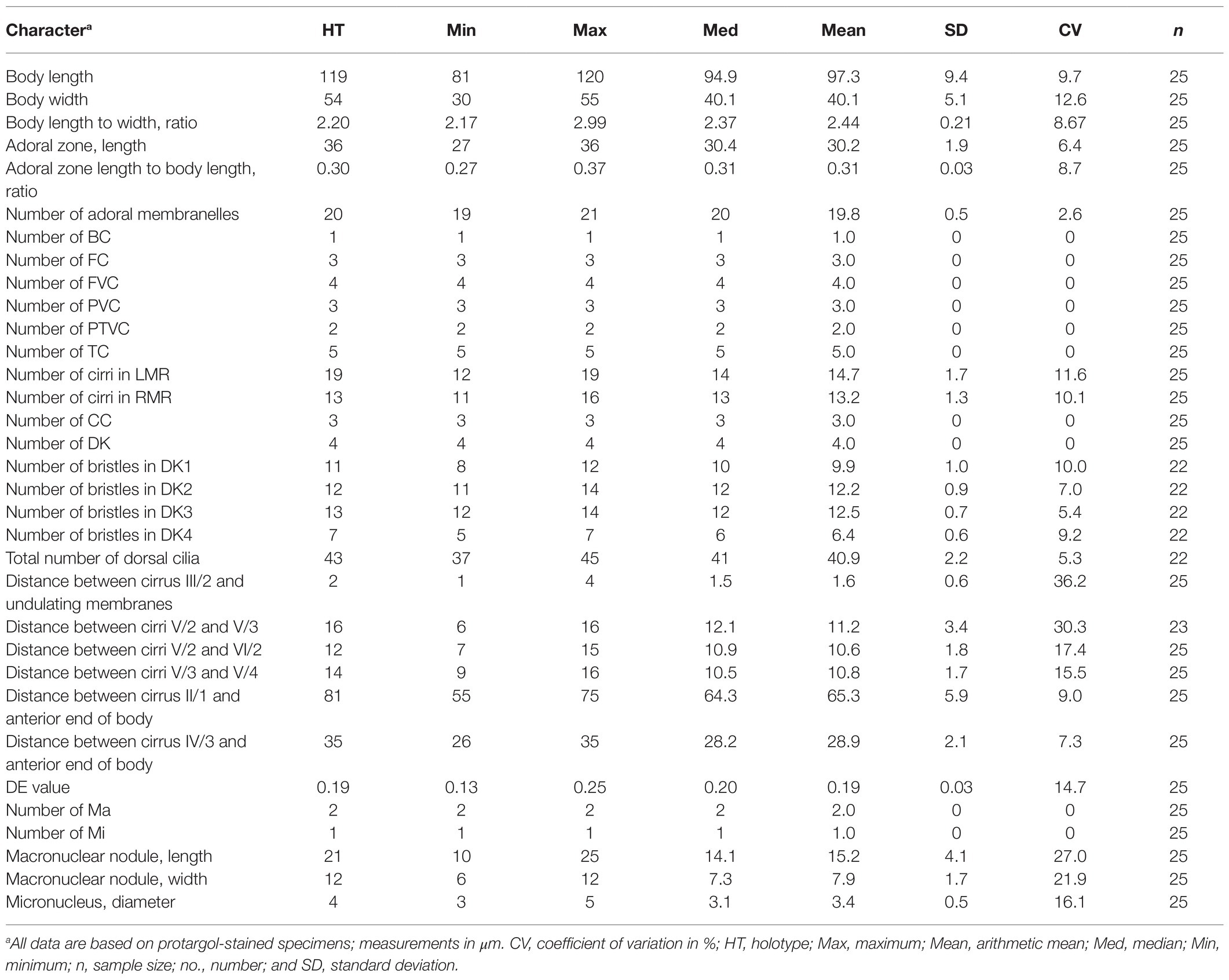
Size in vivo 60–115 μm × 25–45 μm, usually elliptical or elongate ovoid in shape. Cortical granules absent. 19–21 adoral membranelles (Figures 1A–F, 2A–K, 3A–I, 4A–D, 5A–M and Table 1). Buccal cirrus near anterior end of undulating membranes. Cirrus III/2 slightly ahead of level of cirrus VI/3. Cirrus IV/2 anterior to level of cirrus V/4. Distance between cirri V/2 and V/3 slightly longer than distance between cirri V/3 and V/4 or cirri V/2 and VI/2. Transverse cirri subterminal. 11–16 right and 12–19 left marginal cirri. Four dorsal kineties. Three narrowly spaced caudal cirri cilia of which are distinctly long.
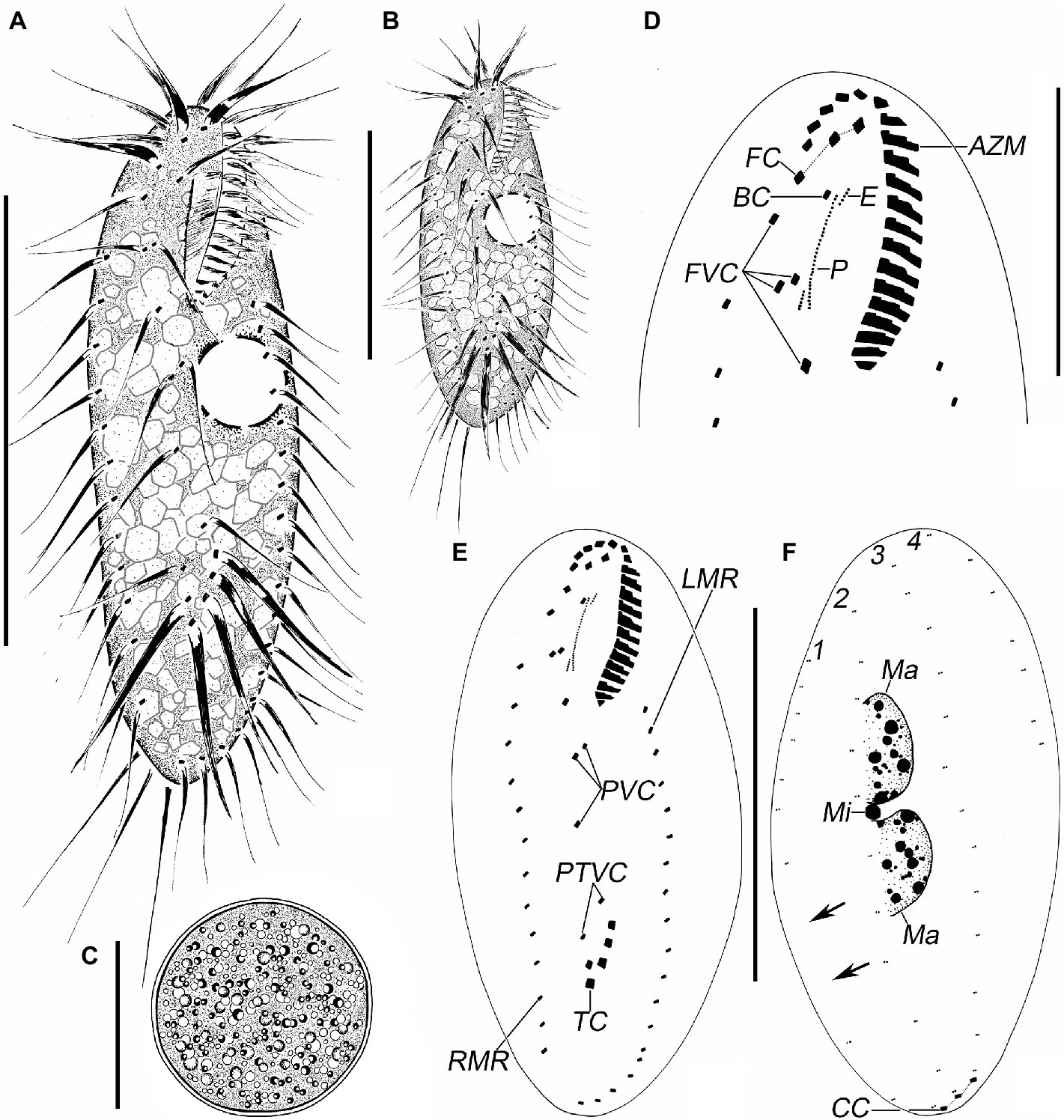
Figure 1. Quadristicha subtropica n. sp., morphology from life (A–C) and after protargol staining (D–F). (A,B) Ventral views, to show different body shapes. (C) Resting cyst. (D) Detailed ventral view of the anterior region. (E,F) Ventral (E) and dorsal (F) view of the holotype specimen to demonstrate the infraciliature; arrows mark the gap in dorsal kinety 1. 1–4, dorsal kineties 1–4. Bars: 50 μm (A,B); 30 μm (C,D); 70 μm (E,F).
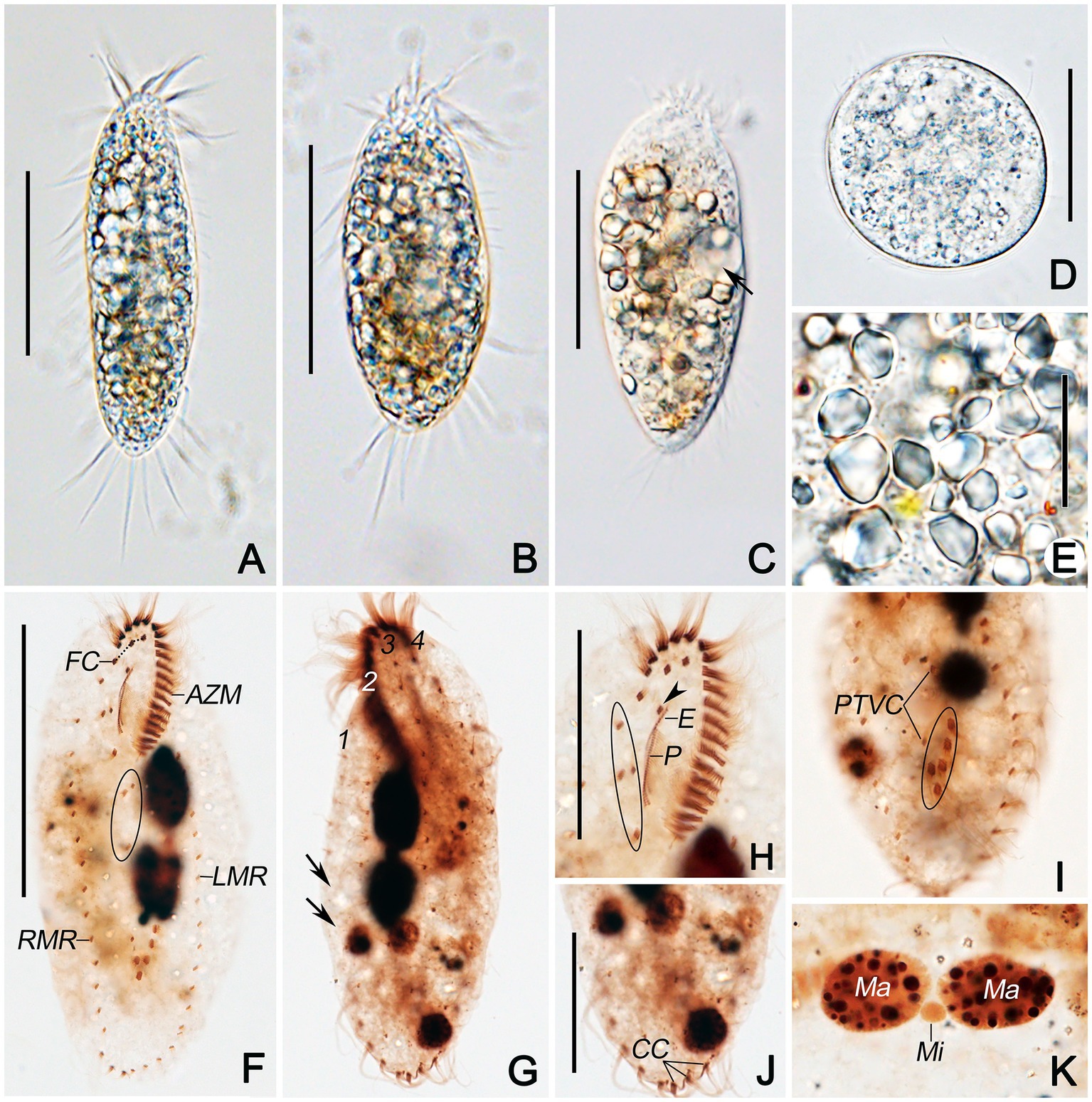
Figure 2. Photomicrographs of Quadristicha subtropica n. sp. from life (A–E) and after protargol staining (F–K). (A,B) Ventral views of representative individuals to show different body shapes. (C) Ventral view, to denote contractile vacuole (arrow). (D) Resting cyst. (E) Crystals. (F,H) Ventral views to demonstrate the ciliature. Arrowhead marks the buccal cirrus; circle in F and H demonstrates the postoral ventral and the frontoventral cirri, respectively. (I) Ventral view of posterior end, to show transverse cirri (circle). (G,J,K) Dorsal views to demonstrate the dorsal kineties, caudal cirri, and nuclear apparatus. Arrows in G show the gap in dorsal kinety 1. 1–4, dorsal kineties 1–4. Bars: 50 μm (A–C); 30 μm (D,H,J); 20 μm (E); 70 μm (F).
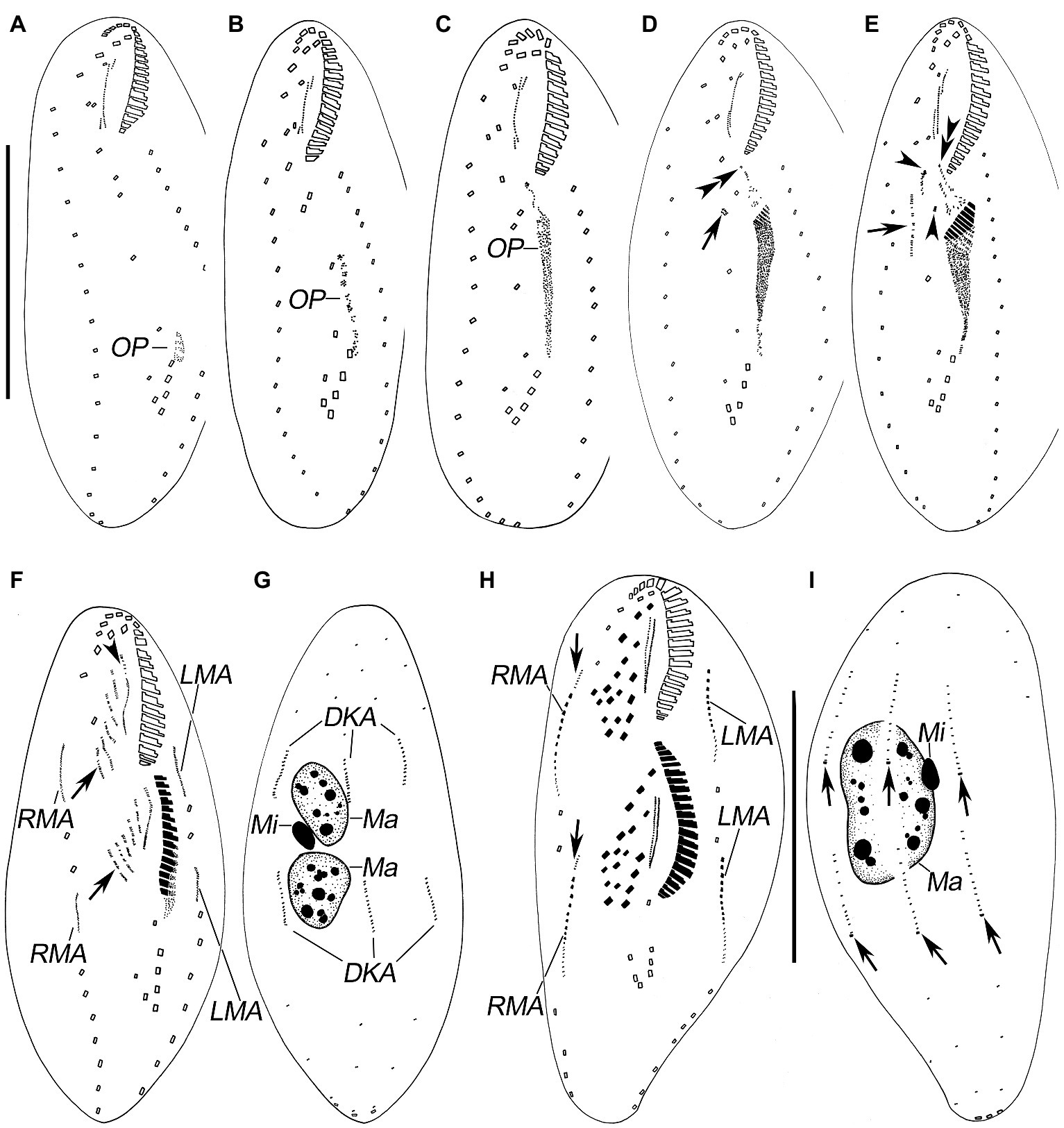
Figure 3. Morphogenesis of Quadristicha subtropica n. sp. after protargol staining. (A–C) Ventral views of very early dividers showing the newly formed oral primordium. (D) Ventral view of an early divider, arrow marks the dedifferentiation of cirrus V/4, and double arrowheads indicate the UM-anlage. (E) Ventral view of a slightly later divider, arrow shows the frontal-ventral-transverse cirral anlagen, arrowheads mark the dedifferentiation of cirri IV/2 and IV/3, and double arrowheads indicate the UM-anlage. (F,G) Ventral and dorsal view of a divider, arrows show frontal-ventral-transverse cirral anlagen and arrowhead denote the frontal cirrus separated from the frontal-ventral-transverse cirral anlage I. (H,I) Ventral and dorsal view of a mid-divider, arrows in H mark the dorsomarginal kineties anlagen and arrows in I demonstrate the newly formed caudal cirri. Bars: 50 μm (A–G); 60 μm (H,I).
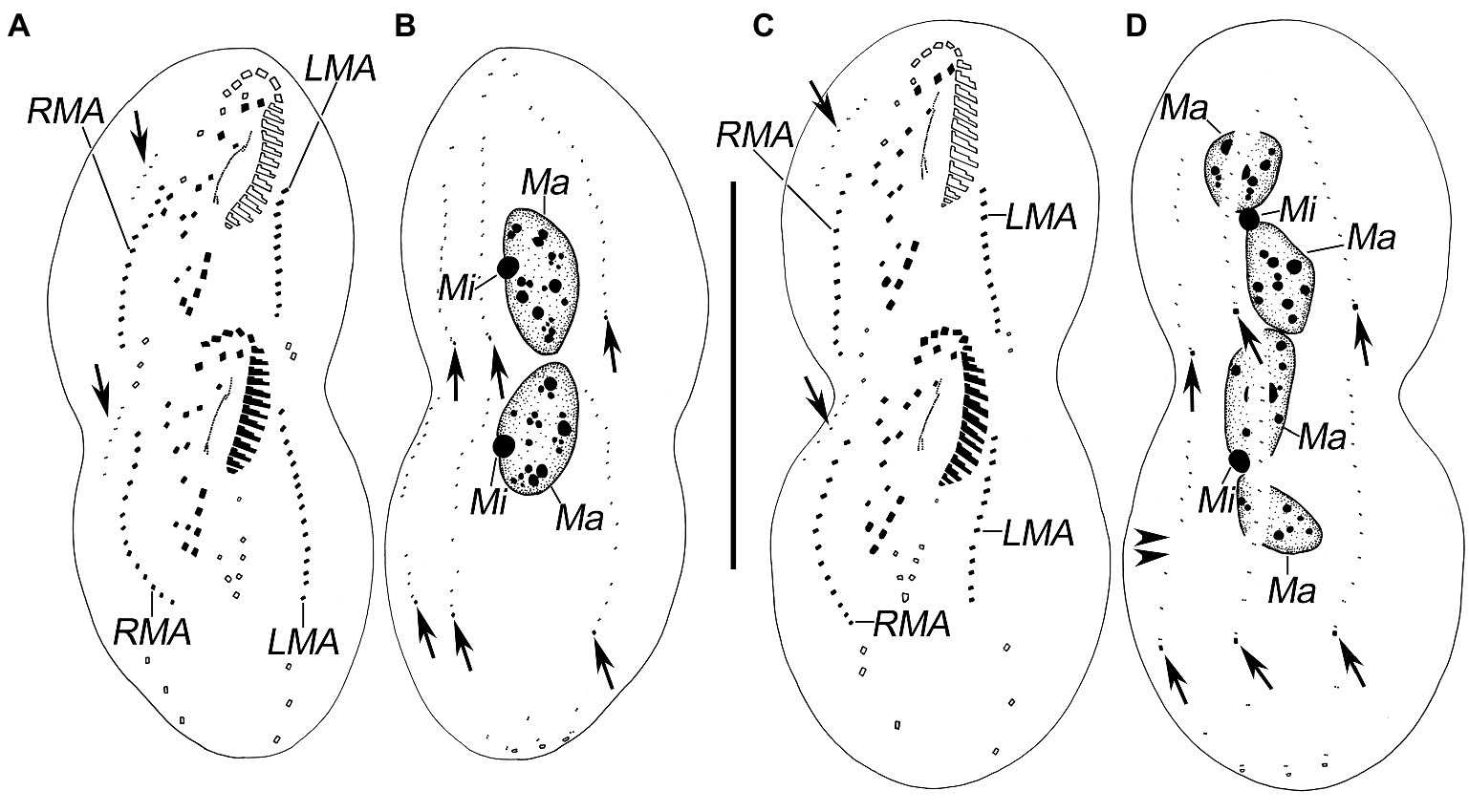
Figure 4. Late stages of morphogenesis in Quadristicha subtropica n. sp. after protargol staining. (A,B) Ventral and dorsal view of a late divider, to show the dorsomarginal kineties anlagen (arrows in A) and the caudal cirri (arrows in B). (C,D) Ventral and dorsal view of a very late divider to demonstrate the dorsomarginal kineties (arrows in C) and caudal cirri (arrows in D). Arrowheads in D show the gap in dorsal kinety 1. Bar: 70 μm.
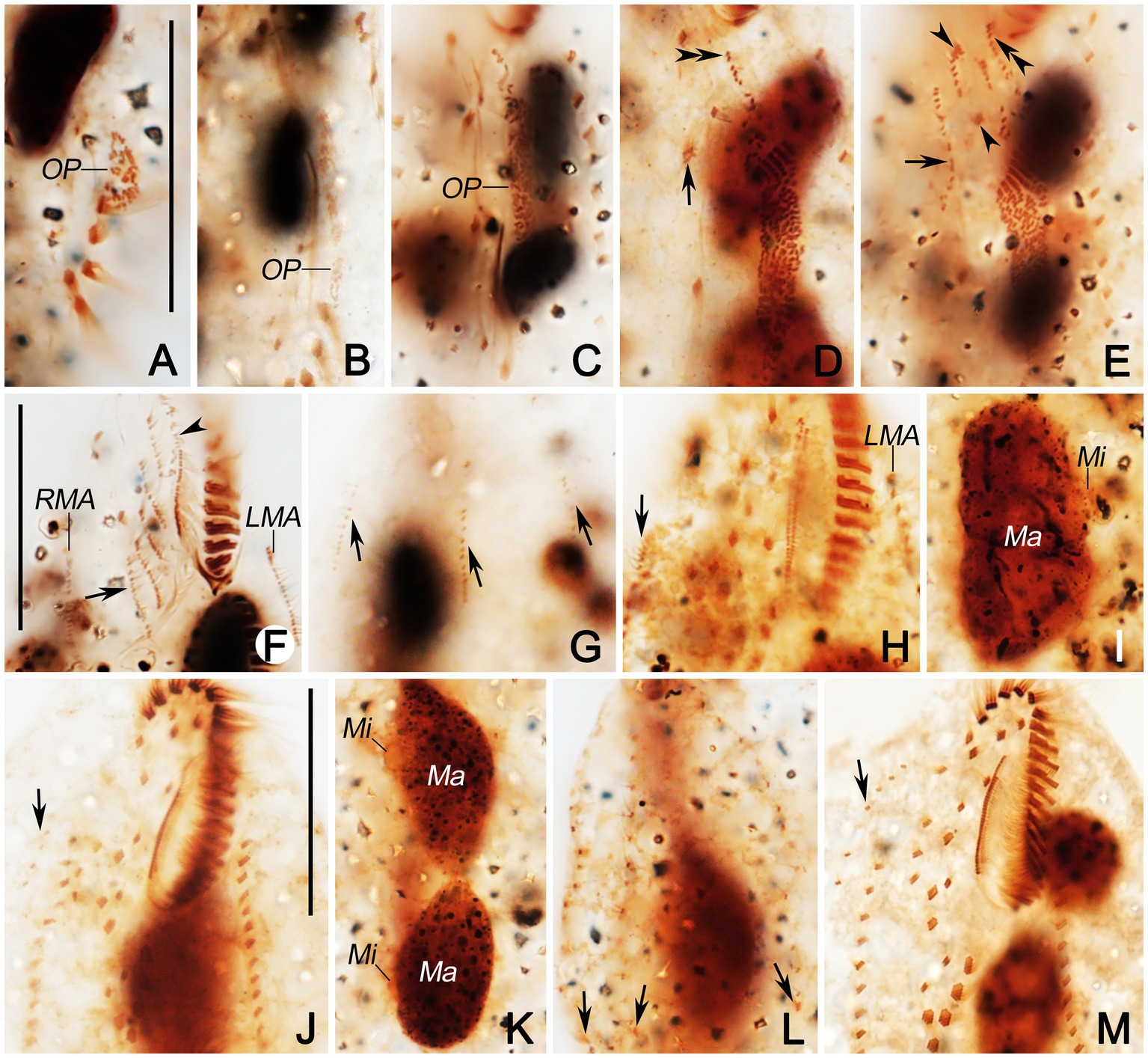
Figure 5. Photomicrographs of Quadristicha subtropica n. sp. during morphogenesis, after protargol staining. (A–C) Early morphogenetical stages in ventral views, showing the newly formed oral primordium. (D) Ventral view of early divider, arrow marks the dedifferentiation of cirrus V/4, and double arrowheads indicate the UM-anlage. (E) Ventral view of early divider, arrow shows the frontal-ventral-transverse cirral anlagen, arrowheads mark the dedifferentiation of cirri IV/2 and IV/3, and double arrowheads indicate the UM-anlage. (F,G) Ventral and dorsal view of a divider, arrow in (F) shows frontal-ventral-transverse cirral anlagen, arrowhead marks undulating membranelles anlage, and arrows in (G) show dorsal kineties anlagen. (H) Ventral view of a mid-divider, arrow marks the dorsomarginal kineties anlage. (I) Dorsal view, to denote the fusion of the macronuclear nodules. (J,M) Ventral views, to show the ciliature. Arrows mark the dorsomarginal kinety anlagen. (K) Dorsal views, to demonstrate the development of the macronuclear nodules. (L) Dorsal view, arrows point to the caudal cirri. Bars: 30 μm.
Type Material
The protargol slide (registry no. GQ2018091201A) with the holotype specimen (Figures 1E,F, 2F) and four paratype slides (registry no. GQ2018091201B–E) were deposited in the Laboratory of Protozoological Biodiversity and Evolution in Wetland, Shaanxi Normal University, China. A paratype slide (registry no. GQ2018091201F) with protargol-stained specimens is deposited in the Laboratory of Protozoology, Ocean University of China.
Type Locality
A freshwater pond in Peninsula Lake Park (18°41′03″N; 110°24′02″E) in Wanning, China.
Etymology
The Latin adjective subtropicus, −a, −um (masc., fem., neut.) recalls the fact that the type material was found in a subtropical area of China.
Morphological Description
Cells in interphase in vivo 60–115 μm × 25–45 μm (n = 12), and after protargol staining 81–120 μm × 30–55 μm. Ratio of length to width after protargol staining about 2.4:1 (Figures 1A–F, 2A–K and Table 1). Body ellipsoid or elongate ovoid, flexible, but not contractile (Figures 1A,B, 2A–C). Two closely spaced ellipsoidal macronuclear nodules (Ma) about 10–25 μm × 6–12 μm in size (after protargol staining), located in mid-body region slightly left of midline. One globular micronucleus (Mi), about 3 μm in diameter (after protargol staining), located between macronuclear nodules (Figures 1F, 2K). Contractile vacuole located ahead of mid-region of body near left margin, about 13 μm across, contracting at intervals of about 12 s (Figures 1A,B, 2C). Cortical granules absent. Cytoplasm grayish, containing a mass of irregular crystals (about 2–10 μm large, and many lipid droplets (1–2 μm dia.), which render cell opaque and dark at low magnifications (Figure 2E). Movement moderately rapid gliding. Resting cyst spherical with smooth surface, about 40 μm in diameter (Figures 1C, 2D).
Infraciliature as shown in Figures 1E,F, 2F,G. Adoral zone about 31% of cell length, 27–36 μm long in protargol preparations, composed of 19–21 (n = 25) membranelles. DE-value ca. 0.2 (n = 25). Paroral (P) and endoral (E) short, intersecting at middle region, and inconspicuously curved (Figures 1D,H and Table 1).
Eighteen frontal-ventral-transverse cirri: three slightly enlarged frontal cirri (FC) near distal portion of adoral zone of membranelles (AZM), cilia about 19 μm long in vivo; buccal cirrus (BC) near anterior end of paroral; four frontoventral cirri, cirrus III/2 slightly ahead of level of cirrus VI/3, cirrus IV/3 at about level of buccal vertex; three postoral ventral cirri located behind buccal vertex, with cirrus IV/2 arranged anterior to level of cirrus V/4, distance between cirri V/3 and V/4 slightly shorter than that between cirri V/3 and V/2; two pretransverse ventral cirri, cirrus VI/2 located between the levels of cirri II/1 and III/1, distance between cirri V/2 and VI/2 slightly shorter than that between cirri V/2 and V/3; five transverse cirri (TC) located about three-quarters down length of body, bases distinctly enlarged, cilia about 23 μm long in vivo and slightly protruding beyond posterior cell margin (Figures 1D,E, 2F,H,I). Marginal cirri are disposed in two rows, on the right and left of the cell, respectively, composed of 11–16 and 12–19 cirri, respectively, in life about 16 μm long; left marginal row commences at level of buccal vertex and terminates subcaudally, while right marginal row (RMR) commences slightly below level of buccal vertex (Figures 1E, 2F).
Invariably in four dorsal kineties (DK) with about 5 μm long cilia composed of 8–12, 11–14, 12–14, and 5–7 dikinetids, respectively. Dorsal kineties 1–3 almost bipolar with kinety 1 always with a wide gap in posterior portion. Dorsal kinety 4 terminates at about mid-body. Three narrowly spaced caudal cirri (CC) located at posterior body margin, one each at posterior end of dorsal kineties 1–3; cilia of caudal cirri conspicuously long, about 25 μm in vivo (Figures 1F, 2G,J).
Divisional Morphogenesis
Stomatogenesis
Opisthe: The earliest cortical morphogenetic event is the apokinetal appearance of a small patch of basal bodies (kinetosomes) in irregular arrangement, the oral primordium (OP; Figures 3A–I, 4A–D, 5A–M). Subsequently, a long and narrow oral primordium is formed (Figures 3B,C, 5B,C). The membranelles of the opisthe’s adoral zone organize in a posterior direction. Simultaneously, the anlage for the undulating membranes (UM-anlage) is formed to the right of the oral primordium as a streak of basal bodies (Figures 3D,E, 5D,E). Later, the membranelles of the opisthe’s adoral zone are organized completed and the anterior end of the newly built adoral zone bends to the right, forming the new oral structure. It is suggested that the leftmost frontal cirrus is generated from the anterior end of the undulating membrane-anlage (= anlage I). Subsequently, the undulating membrane-anlage of both the proter and the opisthe is separated from which the endoral and paroral are formed (Figures 3H, 4A,C, 5H).
Proter: The parental AZM is retained by the proter, so changes to the oral structure are confined to the paroral membranes and endoral membranes. The UM-anlage is formed by the dedifferentiation of the parental undulating membranes. In subsequent stages, the basic development of the UM-anlage follows a similar pattern to that in the opisthe (Figures 3F,H, 4A,C, 5F,H,J,M).
Development of Cortical Ciliature
Along with the organization of the membranelles of the opisthe’s adoral zone, division continues with the formation of the development of the frontoventral-transverse cirral anlagen (FVT-anlagen). We failed to obtain specimens in the stage between those as shown in Figures 3E,F and hence were unable to determine the origin of anlagen II to VI. We speculate that FVT-anlagen I and II in the opisthe develop de novo, and cirri IV/3, IV/2, and V/4 contribute the formation of the FVT-anlagen. Five thread-like anlagen are formed in both proter and opisthe (Figures 3F, 5F). Subsequently, cortical morphogenesis proceeds with the cirral segregation from these streaks. After migration and differentiation, 17 cirri will be formed from each group, whereas the remaining one (the leftmost frontal cirrus) may derive from the undulating membrane-anlage (Figures 3H, 4A,C, 5H,J,M). Finally, the constant 18 cirri are formed within the anlagen I–VI as follows: 1, 3, 3, 3, 4, and 4 cirri.
The anteriormost marginal cirri and some cirri near the prospective division furrow of the marginal rows disaggregate to form the marginal anlagen [left marginal anlagen (LMA) and right marginal anlagen (RMA); Figures 3F, 5F]. The new marginal cirri then develop and replace the old ones (Figures 3H, 4A,C, 5H,J,M).
New dorsal kineties are formed in a typical Urosomoida pattern. Firstly, within dorsal kineties 1, 2, and 3, basal bodies are proliferated to form dorsal kineties anlagen (DKA) at two sites above and below the prospective division furrow (Figures 3G,I, 4B,D, 5G). In the later stage, a gap is always present in posterior portion of kinety 1 (Figure 4D). Subsequently, above the anteriormost portion of the proter’s and opisthe’s right marginal primordia a short streak of paired basal bodies develops, viz. the anlage for the shortened dorsal kinety 4 (Figures 3H, 5H). The posterior ends of the new dorsal kineties 1, 2, and 3 commence with the differentiation of caudal cirri (Figures 3I, 4B,D, 5L).
Division of Nuclear Apparatus
The nuclear apparatus divides in the usual way and hence requires no further comment (Figures 3G,I, 4B,D, 5I,K).
Phylogenetic Analyses Based on SSU rDNA Gene Sequences
The SSU rDNA sequence of Quadristicha subtropica n. sp. was deposited in GenBank with the accession number MZ338339. The length and GC content of the new sequence are 1,627 bp and 45.09%, respectively.
Phylogenetic trees using two different methods (ML and BI) had almost identical topologies; therefore, only the ML tree is presented with support values from both algorithms at the nodes (Figure 6). Quadristicha subtropica n. sp. nests within a poorly supported clade (ML/BI, 15/0.50) that also contains Heterogastrostyla salina Lu et al., 2020, Heterourosomoida lanceolata (Berger, 1999) Singh and Kamra, 2014, Heterourosomoida sinica Wang et al., 2020, Kleinstyla dorsicirrata Singh and Kamra, 2014, and Oxytricha lithofera Foissner, 2016. Quadristicha setigera, the only congener of Q. subtropica n. sp., clusters with Monomicrocaryon euglenivorum euglenivorum (Kahl, 1932) Foissner, 2016 with low support (ML/BI, 32/0.71). The two clades containing species of Quadristicha are sister groups. Given the low support values across the tree, the present phylogeny is far from robust despite the fact that several preliminary phylogenetic analyses were performed using different taxon samples and outgroup species.
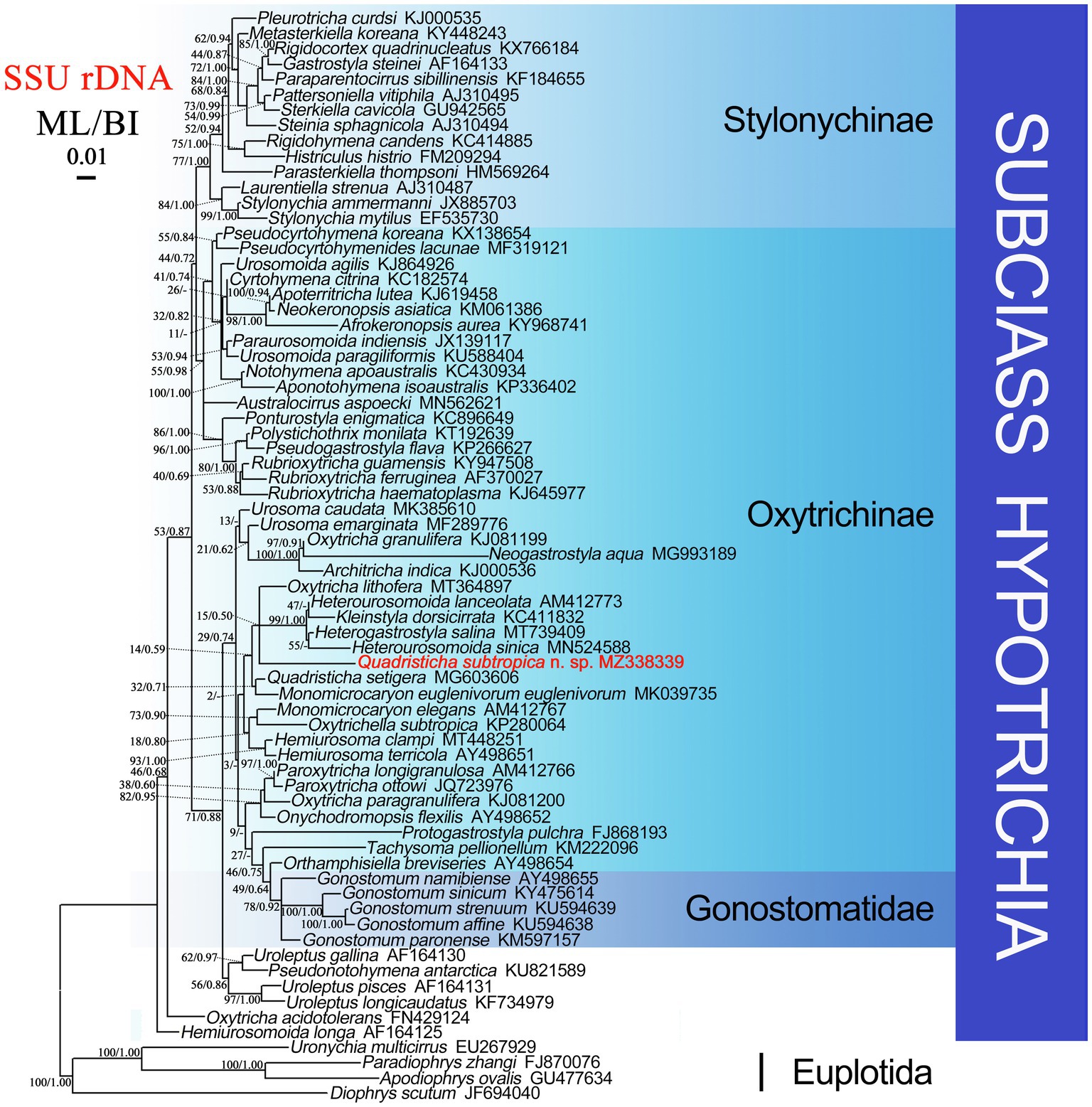
Figure 6. Maximum likelihood (ML) tree based on SSU rDNA sequence data. Quadristicha subtropica n. sp. is indicated in bold. Support values for nodes are for ML and BI (ML/BI), respectively. Clades with a different topology in the BI tree are indicated by “-”. All branches are drawn to scale. The scale bar corresponds to 1 substitution per 100 nucleotide positions.
The similarities of the SSU rDNA sequence of Q. subtropica n. sp. to H. sinica, H. lanceolata, K. dorsicirrata, O. lithofera, H. salina, and Q. setigera are 89.3, 90.3, 90.3, 90.7, 93.8, and 93.8%, respectively.
Discussion
Comparison With Closely Related Species
Quadristicha subtropica n. sp. differs from the type species Q. setigera in its body size in vivo (60–115 μm × 25–45 μm vs. 40–60 μm × 15–21 μm), length of dorsal cilia (about 5 μm vs. 10–15 μm), number of adoral membranelles (19–21 vs. 13–18), number of right (11–16 vs. 3–8) and left (12–19 vs. 6–8) marginal cirri, location of the anterior termination of the RMR (ahead of the level of the buccal vertex vs. behind the level of the buccal vertex), endoral and paroral slightly (vs. strongly) curved, location of the buccal cirrus near the anterior (vs. posterior) end of the paroral, and location of cirrus IV/3 at about (vs. behind) the level of the buccal vertex (Berger, 1999; Kim et al., 2020).
In terms of the features, such as (1) two macronuclear nodules with a micronucleus in between, (2) four dorsal kineties without fragmentation of kinety 3, (3) cortical granules absent, and (4) three prolonged caudal cirri, Q. subtropica n. sp. should be compared with six Monomicrocaryon species, i.e., Monomicrocaryon alfredi (Berger, 1999) Foissner, 2016, Monomicrocaryon crassistilata (Kahl, 1932) Foissner, 2016, Monomicrocaryon halophilum (Kahl, 1932) Foissner, 2016, Monomicrocaryon kahlovatum (Berger, 1999) Foissner, 2016, Monomicrocaryon parahalophilum (Wang and Nie, 1935) Foissner, 2016, and Monomicrocaryon sphagni (Kahl, 1932) Foissner, 2016.
Quadristicha subtropica n. sp. can be separated from M. alfredi by the RMR starting below the level of cirrus VI/3 (vs. ahead of the level of cirrus VI/4), and cilia of caudal cirri protruding rightward (vs. straight or indistinct) (Berger, 1999; Foissner, 2016).
Quadristicha subtropica n. sp. can be distinguished from M. halophilum by the locations of the rightmost frontal cirrus slightly (vs. distinctly) ahead of the level of the buccal cirrus, transverse cirri (subcaudal vs. caudal), cirrus IV/2 (ahead of vs. below) cirrus V/4 and the anterior termination of the RMR (below the level of cirrus VI/3 vs. ahead of the level of cirrus VI/4), and also the number of dikinetids in dorsal kinety 1 (8–12 vs. 18 or 21 in population from Kahl, 1932, data from drawings), cilia of caudal cirri protruding rightward (vs. straight) and the habitat (fresh water vs. saline water) (Berger, 1999; Foissner, 2016).
Quadristicha subtropica n. sp. can be separated from M. parahalophilum by the location of the transverse cirri (subterminal vs. terminal), and the number of dikinetids in dorsal kinety 1 (8–12 vs. 27 in population from Wang and Nie, 1935, data from drawing) (Wang and Nie, 1935; Berger, 1999; Foissner, 2016).
Monomicrocaryon crassistilata resembles Q. subtropica n. sp. reasonably well; however, the former differs from the latter in the length of the dorsal cilia (8–10 μm vs. 5 μm) and the orientation of the caudal cirri (straight vs. projecting rightward) (Berger, 1999; Foissner, 2016).
Quadristicha subtropica n. sp. can be separated from M. sphagni by the length of the dorsal cilia (5 μm vs. about 15 μm), the ratio of body length to width in vivo (about 3:1 vs. about 5:1, data from drawing), the location of the transverse cirri (subcaudal vs. caudal), and the location of cirrus IV/3 (at about the level of the buccal vertex vs. ahead of the level of the buccal vertex) (Berger, 1999; Foissner, 2016).
Monomicrocaryon kahlovatum can be separated from Q. subtropica n. sp. by its oval (vs. ellipsoid or elongate ovoid) body shape and the orientation of the caudal cirri (straight vs. protruding rightward) (Berger, 1999; Foissner, 2016).
Morphogenetic Comparison
Until now, the cortical morphogenesis of Q. subtropica n. sp. is the only detailed study within the genus Quadristicha, which proceeds in a similar way to other members of the family Oxytrichidea (Berger, 1999).
Berger (1999) documented some middle and late stages of morphogenesis in Q. setigera. Based on these and the present data, the mid-to-late stages of the two congeners are consistent (Berger, 1999). The early stages of morphogenesis in Quadristicha are revealed here for the first time.
Phylogenetic Analyses
The genus Quadristicha comprises two species, namely, Q. setigera (type species) and Q. subtropica n. sp. both of which were included in the present phylogenetic analyses. Quadristicha subtropica n. sp. groups with H. salina, H. lanceolata, H. sinica, and K. dorsicirrata. The close relationship among these species is supported by several morphological features including: a flexible pellicle, two macronuclear nodules, one marginal cirral row on each side, two pretransverse cirri and five transverse cirri, and four dorsal kineties (Berger, 1999; Singh and Kamra, 2014; Foissner, 2016; Lu et al., 2020; Wang et al., 2021b).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at https://www.ncbi.nlm.nih.gov/genbank/, MZ338339.
Author Contributions
CS and QG collected the samples and carried out almost all of the experiments (preparations, illustrations, micrographs, etc.). AW was responsible for the language correction. JW did the identification of the species and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (project number: 32070428) and the Natural Science Foundation of Shaanxi province (2021JQ-307).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank reviewers for their thoughtful critiques that helped improve the manuscript.
References
Bai, Y., Wang, R., Song, W., Suzuki, T., and Hu, X. (2020). Redescription of five tintinnine ciliates (Alveolata: Ciliophora: Oligotrichea) from coastal waters of Qingdao. Mar. Life. Sci. Technol. 2, 209–221. doi: 10.1007/s42995-020-00034-2
Berger, H. (1999). Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monogr. Biol. 78, 29–48. doi: 10.1007/978-94-011-4637-1
Berger, H. (2018). “Cyrtohymena Foissner, 1989 and Cyrtohymena muscorum (Kahl, 1932) Foissner, 1989 (original combination Oxytricha (Steinia) muscorum Kahl, 1932) (Ciliophora, Hypotricha): update 1.0 on monographic treatment,” Ser. Mono-graphiae Ciliophorae. Vol. 3, 1–28.
Chen, L., Dong, J., Wu, W., Xin, Y., Warren, A., Ning, Y., et al. (2020). Morphology and molecular phylogeny of a new hypotrich ciliate, Anteholosticha songi nov. spec., and an American population of Holosticha pullaster (Müller, 1773) Foissner et al., 1991 (Ciliophora, Hypotrichia). Eur. J. Protistol. 72:125646. doi: 10.1016/j.ejop.2019.125646
Cheng, T., Wang, Y., Huang, J., Chen, X., Zhao, X., Gao, S., et al. (2019). Our recent progress in epigenetic research using the model ciliate, Tetrahymena thermophila. Mar. Life Sci. Technol. 1, 4–14. doi: 10.1007/s42995-019-00015-0
Dong, J., Li, L., Fan, X., Ma, H., and Warren, A. (2020). Two Urosoma species (Ciliophora, Hypotrichia): a multidisciplinary approach provides new insights into their ultrastructure and systematics. Eur. J. Protistol. 72:125661. doi: 10.1016/j.ejop.2019.125661
Foissner, W. (2016). “Terrestrial and semiterrestrial ciliates (Protozoa, Ciliophora) from Venezuela and Galápagos,” in Denisia Vol. 35, 1–912.
Hu, X., Lin, X., and Song, W. (2019). Ciliate Atlas: Species Found in the South China Sea. Beijing: Science Press.
Kahl, A. (1932). Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria). 3 Spirotricha. Tierwelt Dtl. 25, 399–650.
Kim, J. H., Omar, A., and Jung, J. H. (2020). Brief description of 18 newly recorded ciliate species from soil and inland waters (Protozoa, Ciliophora) in South Korea. J. Spec. Res. 9, 251–268. doi: 10.12651/JSR.2020.9.3.251
Li, J., Li, L., Wang, J., Zhu, E., and Shao, C. (2021a). Morphology, morphogenesis and molecular phylogeny of a novel soil ciliate, Afrokahliella paramacrostoma n. sp. (Ciliophora, Hypotrichia). Eur. J. Protistol. 77:125748. doi: 10.1016/j.ejop.2020.125748
Li, J., Wang, J., Wang, Y., Ma, J., and Shao, C. (2021b). Morphology, ontogenesis and molecular phylogeny of a new saline soil ciliate, Uroleptoides salina nov. spec. (Ciliophora, Hypotrichia). Eur. J. Protistol. 78:125766. doi: 10.1016/j.ejop.2021.125766
Lian, C., Luo, X., Warren, A., Zhao, Y., and Jiang, J. (2020). Morphology and phylogeny of four marine or brackish water spirotrich ciliates (Protozoa, Ciliophora) from China, with descriptions of two new species. Eur. J. Protistol. 72:125663. doi: 10.1016/j.ejop.2019.125663
Lu, X., Wang, Y., Al-Farraj, S. A., El-Serehy, H., Huang, J., and Shao, C. (2020). The insights into the systematic relationship of Gastrostyla-affinitive genera, with report on a new saline soil ciliate genus and new species (Protozoa, Ciliophora). BMC Evol. Biol. 20:92. doi: 10.1186/s12862-020-01659-8
Paiva, T. d. S. (2020). Systematic redefinition of the Hypotricha (Alveolata, Ciliophora) based on combined analyses of morphological and molecular characters. Protist 171:125755. doi: 10.1016/j.protis.2020.125755
Park, K. M., Jung, J. H., Jeong, H. K., Min, G. S., and Kim, S. (2020). Morphology, morphogenesis, and molecular phylogeny of a new freshwater ciliate, Gonostomum jangbogoensis n. sp. (Ciliophora, Hypotricha), from Victoria land, Antarctica. Eur. J. Protistol. 73:125669. doi: 10.1016/j.ejop.2019.125669
Shao, C., Chen, X., and Jiang, J. (2020). Hypotrichous Ciliates in China. Beijing: Science Press, 429.
Singh, J., and Kamra, K. (2014). Molecular phylogeny of an Indian population of Kleinstyla dorsicirrata (Foissner, 1982) Foissner et al., 2002. comb. nov. (Hypotrichia, Oxytrichidae): an oxytrichid with incomplete dorsal kinety fragmentation. J. Eukaryot. Microbiol. 61, 630–636. doi: 10.1111/jeu.12142
Small, E. B., and Lynn, D. H. (1985). “Phylum Ciliophora Doflein, 1901” in An Illustrated Guide to the Protozoa. eds. J. J. Lee, S. H. Hutner, and E. C. Bovee (Lawrence: Society of Protozoologists), 393–575.
Stokes, A. C. (1891). Notes of new infusoria from the fresh waters of the United States. J. R. Microsc. Soc. 11, 697–704. doi: 10.1111/j.1365-2818.1891.tb01611.x
Vďačný, P., and Foissner, W. (2021). Morphology and ontogenesis of two new Hemiholosticha species (Ciliophora, Hypotrichia, Hemiholostichidae nov. fam.). Eur. J. Protistol. 77:125763. doi: 10.1016/j.ejop.2020.125763
Wang, C., and Nie, D. (1935). Report on the rare and new species of freshwater infusoria, part II. Sinensia, Shanghai 6, 399–524.
Wang, J., Li, J., and Shao, C. (2020). Morphology, morphogenesis, and molecular phylogeny of a novel saline soil ciliate, Heterourosomoida sinica n. sp. (Ciliophora, Hypotrichia). Eur. J. Protistol. 73:125666. doi: 10.1016/j.ejop.2019.125666
Wang, J., Zhang, T., Li, F., Warren, A., Li, Y., and Shao, C. (2021a). A new hypotrich ciliate, Oxytricha xianica sp. nov., with notes on the morphology and phylogeny of a Chinese population of Oxytricha auripunctata Blatterer and Foissner, 1988 (Ciliophora, Oxytrichidae). Mar. Life Sci. Technol. doi: 10.1007/s42995-020-00089-1
Wang, J., Zhao, Y., Lu, X., Lyu, Z., Warren, A., and Shao, C. (2021b). Does the Gonostomum-patterned oral apparatus in Hypotrichia carry a phylogenetic signal? Evidence from morphological and molecular data based on extended taxon sampling using three nuclear genes (Ciliophora, Spirotrichea). Sci. China Life Sci. 64, 311–322. doi: 10.1007/s11427-020-1667-3
Wilbert, N. (1975). Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64, 171–179.
Wu, T., Li, Y., Lu, B., Shen, Z., Song, W., and Warren, A. (2020). Morphology, taxonomy and molecular phylogeny of three marine peritrich ciliates, including two new species: Zoothamnium apoarbuscula n. sp. and Z. apohentscheli n. sp. (Protozoa, Ciliophora, Peritrichia). Mar. Life. Sci. Technol. 2, 334–348. doi: 10.1007/s42995-020-00046-y
Xu, W., Wang, Y., Cheng, T., Yu, Y., El-Serehy, H., Al-Farraj, S. A., et al. (2020). Reevaluation of the ‘well-known’ Paraurostyla weissei complex, with notes on the ontogenesis of a new Paraurostyla species (Ciliophora, Hypotrichia). Eur. J. Protistol. 73:125672. doi: 10.1016/j.ejop.2020.125672
Zhang, T., Dong, J., Cheng, T., Duan, L., and Shao, C. (2020). Reconsideration of the taxonomy of the marine ciliate Neobakuella aenigmatica Moon et al., 2019 (Protozoa, Ciliophora, Hypotrichia). Mar. Life Sci. Technol. 2, 97–108. doi: 10.1007/s42995-020-00032-4
Keywords: ciliates, morphology, new species, ontogenesis, phylogeny
Citation: Shao C, Gao Q, Warren A and Wang J (2021) Morphology, Morphogenesis, and Molecular Phylogeny of a New Freshwater Ciliate, Quadristicha subtropica n. sp. (Ciliophora, Hypotrichia). Front. Microbiol. 12:705826. doi: 10.3389/fmicb.2021.705826
Edited by:
Weiwei Liu, Chinese Academy of Sciences, ChinaReviewed by:
Santosh Kumar, Zoological Survey of India, IndiaZheng Wang, Yale University, United States
Copyright © 2021 Shao, Gao, Warren and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyi Wang, d2FuZ2ppbmd5aTYyOUAxNjMuY29t
†These authors have contributed equally to this work
 Chen Shao
Chen Shao Qi Gao1†
Qi Gao1† Jingyi Wang
Jingyi Wang