- 1Clinical Microbiology Laboratory, The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China
- 2China Australia Joint Laboratory for Animal Health Big Data Analytics, College of Animal Science and Technology, Zhejiang Agricultural and Forestry University, Hangzhou, China
- 3Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 4Clinical Laboratory Department, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 5Clinical Laboratory, Lishui People’s Hospital, Lishui, China
- 6Department of Laboratory Medicine, Jinhua People’s Hospital, Jinhua, China
Contezolid is a novel oxazolidinone, which exhibits potent activity against gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and penicillin-resistant Streptococcus pneumoniae (PRSP). In this study, the in vitro activity of contezolid was compared with linezolid (LZD), tigecycline (TGC), teicoplanin (TEC), vancomycin (VA), daptomycin (DAP), and florfenicol (FFC) against MRSA and VRE strains isolated from China. Contezolid revealed considerable activity against MRSA and VRE isolates with MIC90 values of 0.5 and 1.0 μg/mL, respectively. For VRE strains with different resistance genotypes, including vanA- and vanM-type strains, contezolid did not exhibit significantly differential antibacterial activity. Furthermore, the antimicrobial activity of contezolid is similar to or slightly better than that of linezolid against MRSA and VRE strains. Subsequently, the activity of contezolid was tested against strains carrying linezolid resistance genes, including Staphylococcus capitis carrying cfr gene and Enterococcus faecalis carrying optrA gene. The results showed that contezolid exhibited similar antimicrobial efficacy to linezolid against strains with linezolid resistance genes. In general, contezolid may have potential benefits to treat the infections caused by MRSA and VRE pathogens.
Introduction
Increasing resistance to antibiotics in gram-positive cocci is a major concern of health care. In particular, the emergence of multidrug-resistant (MDR) bacteria leads to a decline in the treatment options. The World Health Organization (WHO) published a list of antibiotic-resistant “priority pathogens” in 2017 (Asokan et al., 2019). Among these pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are of particular concern since they are responsible for several severe infections. MRSA exhibits resistance to most available antibiotics, including fluoroquinolones and peptides, aminoglycosides, macrolides, and tetracycline (Osei Sekyere and Mensah, 2020). Therefore, novel antibacterial agents are urgently needed to treat infectious diseases caused by MDR gram-positive pathogens.
Oxazolidinones are a class of synthetic antimicrobial agents that are used to treat serious infections caused by gram-positive pathogens, including MRSA and VRE (Zurenko et al., 2001). Linezolid is the first member of the oxazolidinone antibiotics, which has some adverse effects (Hashemian et al., 2018). Clinical utilization of linezolid is restricted due to its toxicity such as myelosuppression and monoamine oxidase inhibition (MAOI) (Zahedi Bialvaei et al., 2017; Lee and Caffrey, 2018). In addition, the prevalence of linezolid resistance is increasing in many countries (Gu et al., 2013). The presence of optrA and cfr genes is one of the mechanisms mediating resistance to linezolid (Sadowy, 2018; Ruiz-Ripa et al., 2020).
Contezolid is a novel ortho-fluoro dihydropyridone oxazolidinone that replaces the morpholine in linezolid with a piperidinone (Meng et al., 2015). Contezolid inhibits the formation of functional 70S initiation complex by binding to the 23S rRNA region adjacent to the peptidyl transferase center of the 50S ribosomal subunit, thereby interfering with bacterial protein synthesis (Shinabarger, 1999). Contezolid has demonstrated potent antibacterial activity against resistant gram-positive pathogens (Gordeev and Yuan, 2014; Li et al., 2014; Wu et al., 2018). Additionally, contezolid showed antibacterial potential in multiple animal models, generally comparable with or slightly better than that for linezolid (Li et al., 2014), coupled with markedly attenuated human bone marrow cytotoxicity (Gordeev and Yuan, 2014; Huang et al., 2014; Li et al., 2014; Eckburg et al., 2017). In a phase III trial conducted in China (CTR20150855), contezolid was in development to treat complicated skin and soft tissue infections (Bassetti et al., 2020). According to the study, the most common adverse events associated with contezolid were gastrointestinal disorders such as nausea, and the incidence of myelosuppression was significantly lower than linezolid. Furthermore, contezolid displays a low propensity of spontaneous resistance (Gordeev and Yuan, 2014), and low potential to trigger resistance in S. aureus (Huang et al., 2014). Consequently, contezolid has the potential of offering a promising alternative therapy for MDR gram-positive organism infections.
The objective of this study was to evaluate the in vitro activity of contezolid relative to that of other comparator antimicrobial agents against MRSA, VRE, and strains carrying linezolid resistance genes using clinical isolates collected from China.
Materials and Methods
Bacterial Isolates
A total of 450 existing clinical isolates were collected from The Second Affiliated Hospital Zhejiang University School of Medicine, Huashan Hospital Affiliated to Fudan University, Henan Provincial People’s Hospital, and China Agricultural University from 2018 to 2020. The bacterial collection included 321 MRSA and 129 VRE isolates. Identification of strains was performed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF/MS) (Bruker Daltonik, Bremen, Germany).
Kirby-Bauer method was used for MRSA and VRE screening according to the Clinical and Laboratory Standards Institute (CLSI) uniform standards. Isolates resistant to cefoxitin (8 μg/mL) with inhibition zone ≤21 mm were classified as MRSA and then confirmed by polymerase chain reaction (PCR) of mecA gene. Strains resistant to vancomycin with inhibition zone ≤14 mm were classified as VRE and then performed PCR of vanA, vanB, and vanM genes to determine vancomycin resistance genotypes. The vanM gene cluster sequences were determined by Sanger sequencing and BLAST program.
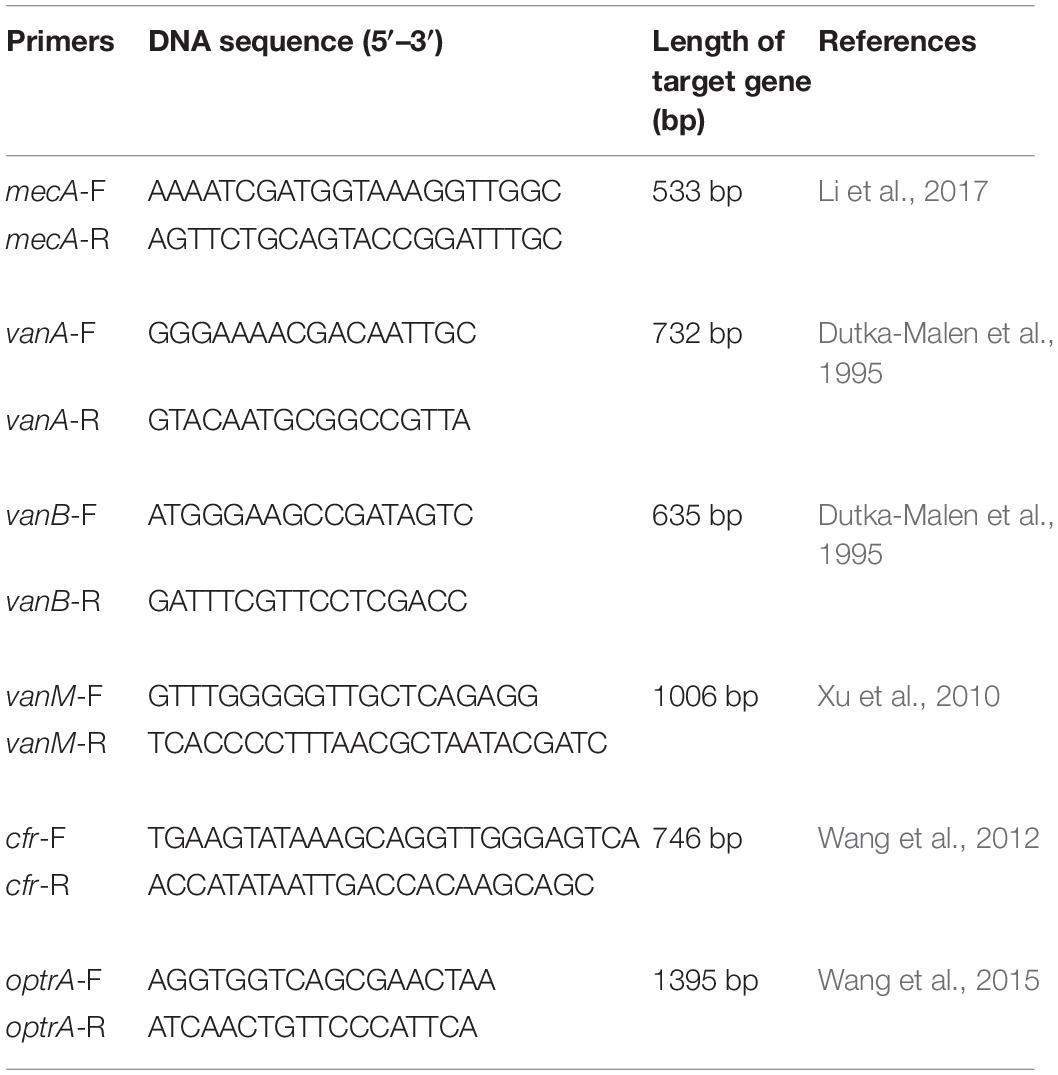
Eighteen previously described strains with linezolid resistance genes, including nine Staphylococcus capitis carrying cfr gene and nine Enterococcus faecalis carrying optrA gene collected from China Agricultural University (Wang et al., 2015) were used in this study. The primers used in this study were summarized in Table 1.
Antimicrobial Agents
Contezolid, linezolid, tigecycline, teicoplanin, vancomycin, daptomycin, cefoxitin, and florfenicol were obtained from National Institutes for Food and Drug Control. Broth microdilution panels were produced by Zhuhai DL Biotech Co., Ltd. The range of concentrations tested was: contezolid (0.125–16 μg/mL), linezolid (0.125–16 μg/mL), tigecycline (0.0625–2 μg/mL), teicoplanin (1–32 μg/mL), vancomycin (1–32 μg/mL), daptomycin (0.25–8 μg/mL), and florfenicol (1–32 μg/mL).
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility tests were performed by reference broth microdilution methods following CLSI procedures (CLSI, 2020a). Minimum inhibitory concentrations (MICs) were interpreted based on CLSI (CLSI, 2020b) and EUCAST.1 Quality control was conducted by using CLSI-recommended strains, including S. aureus ATCC 29213 and E. faecalis ATCC 29212. Statistical significance was calculated using the Chi-squared test via SPSS® 20.0 software and P < 0.05 was considered as statistically significant.
Results
Antimicrobial Activity of Contezolid Against Tested MRSA and VRE Isolates
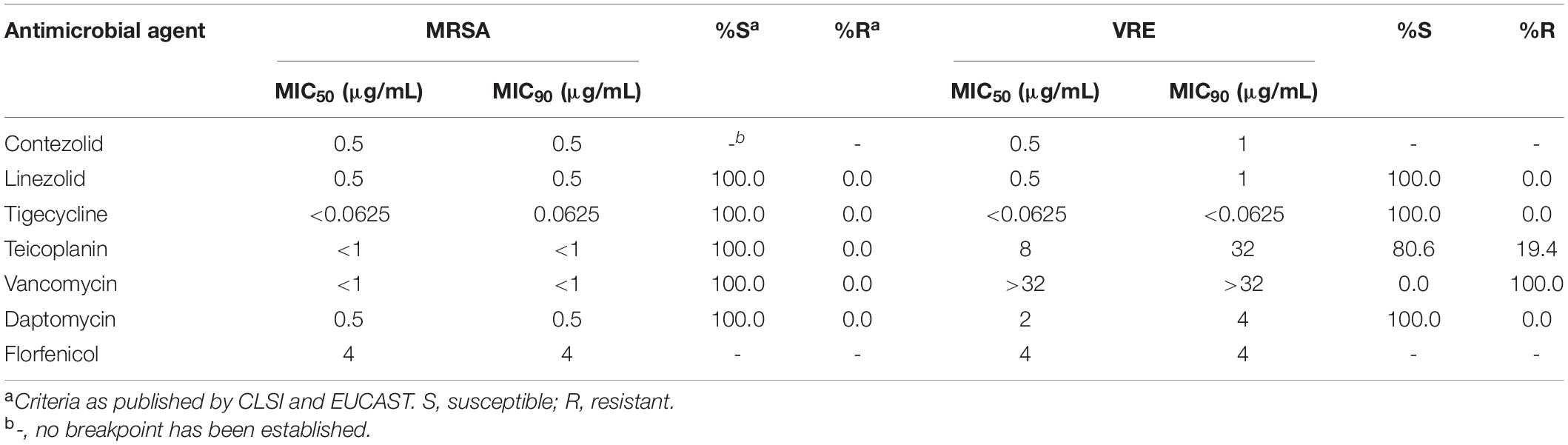
The MIC50 and MIC90 (MICs to inhibit the growth of 50% and 90% of organisms, respectively) of contezolid and comparator agents against MRSA and VRE strains were summarized in Table 2. Overall, contezolid demonstrated potent in vitro activity against MRSA and VRE isolates. All MRSA isolates tested were inhibited at a contezolid MIC value of ≤1 μg/mL (ranged from 0.25 to 1 μg/mL). Contezolid inhibited all VRE isolates at MIC ≤2 μg/mL (ranged from 0.25 to 2 μg/mL). Notably, only one of the VRE isolates showed a MIC at 2 μg/mL. MIC90 of contezolid against MRSA and VRE isolates were both ≤1 μg/mL. Moreover, there were 98.13% (315/321) of MRSA strains with MIC values ≤0.5 μg/mL and 79.84% (103/129) for VRE strains. In addition, for vanA- and vanM-type VRE strains, contezolid displayed similar MIC distributions, regardless of the vancomycin-resistant genotypes.
Antimicrobial Effect of Contezolid Compared With Linezolid
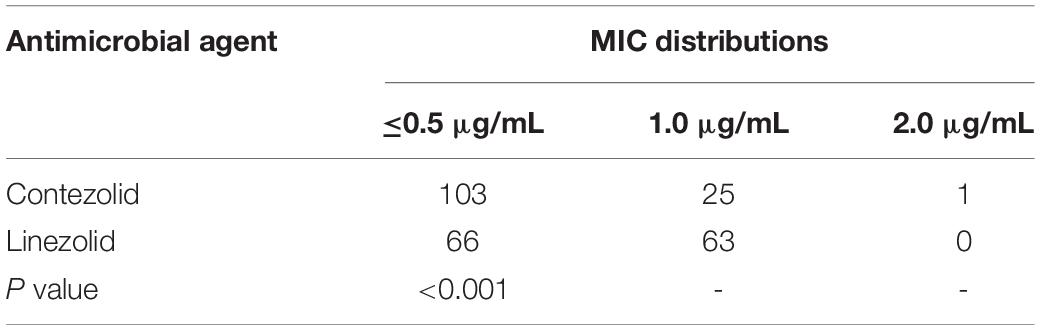
Contezolid and linezolid displayed similar antimicrobial activity against MRSA and VRE isolates, with the same MIC50 and MIC90 values. However, when considering the MIC distributions, the number of strains with linezolid MIC values ≤0.5 μg/mL was less than that of contezolid in both MRSA and VRE isolates. Among the MRSA strains, there were 315 and 309 strains with MIC ≤0.5 μg/mL for contezolid and linezolid, respectively. However, it is worth noting that when it comes to VRE strains, there were 103 and 66 strains with MIC ≤0.5 μg/mL for contezolid and linezolid, respectively, which had statistical significance (P < 0.001) (Table 3).
Subsequently, the antimicrobial activity of contezolid was explored in strains carrying linezolid resistance genes. Both against S. capitis with cfr gene and E. faecalis with optrA gene, contezolid showed similar MIC distributions to linezolid (Table 4). These results demonstrated that contezolid displayed limited activity against strains carrying linezolid resistance genes.
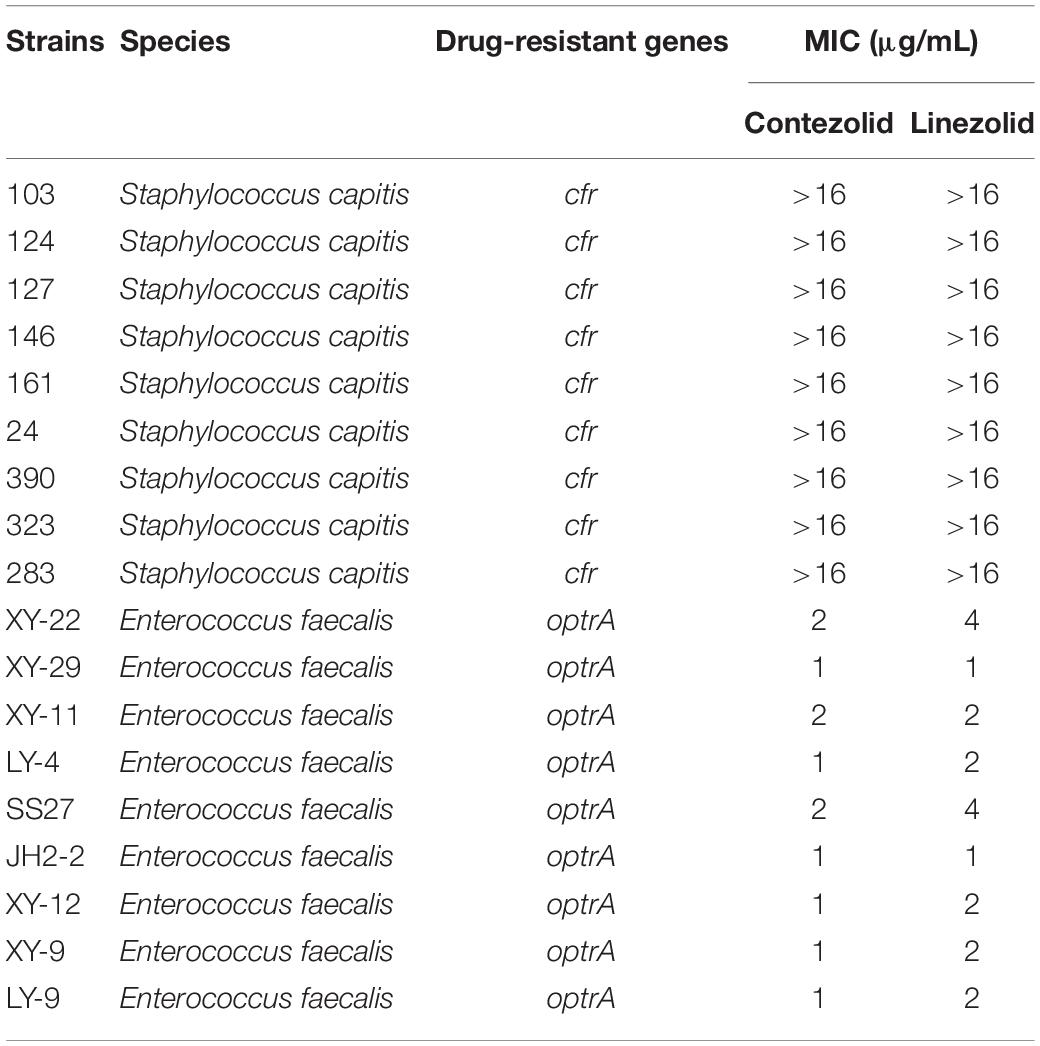
Table 4. In vitro activity of contezolid and linezolid against strains with linezolid resistance genes.
Antimicrobial Effect of Contezolid Compared With Other Comparator Antimicrobial Agents
The MIC50 and MIC90 of contezolid against MRSA and VRE strains were not higher than that of teicoplanin, vancomycin, daptomycin, and florfenicol. However, the MICs of tigecycline were remarkably lower than that of contezolid against both MRSA and VRE strains. Accordingly, the antimicrobial activity of contezolid against MRSA and VRE isolates was similar to or slightly better than that of other comparator agents, except for tigecycline.
Discussion
The antibacterial resistance toward currently available antibiotics is a widespread global health crisis. MDR gram-positive bacteria, accounting for both community-acquired and healthcare-associated infections, create numerous clinical challenges (Stevenson et al., 2005; Hoskins et al., 2018). Among them, MRSA and VRE deserve special attention for their high level of drug resistance. Accordingly, the development of new antibiotics is eagerly required to counter resistance.
Contezolid is a new oxazolidinone antibacterial agent with activity against gram-positive bacteria, including some multi-drug resistant organisms, such as MRSA, VRE, and PRSP (Gordeev and Yuan, 2014). Contezolid markedly reduces the potential for myelosuppression and monoamine oxidase inhibition compared to linezolid (Gordeev and Yuan, 2014), which seems to increase the clinical attractiveness of contezolid. Moreover, contezolid was reported to be not inferior to linezolid for the treatment of complicated skin and soft tissue infections with fewer hematology-associated adverse events in a phase three clinical trial conducted in China (Bassetti et al., 2020), indicating similar therapeutic outcomes between contezolid and linezolid. Contezolid acefosamil is the prodrug of the contezolid. In vivo, the double prodrug structure undergoes metabolic degradation including O-deacetylation and N-dephosphorylation, followed by the release of the active drug, contezolid. The prodrug form, which is water-soluble, could be used for either oral or intravenous administration of contezolid (Wang et al., 2021). Contezolid was approved for clinical use in China on July 2, 2021 for the treatment of complicated skin and soft tissue infections. And contezolid has been granted QIDP designation and Fast Track status by the US FDA.
In the present study, contezolid displayed potent activity against the whole collection of MRSA and VRE isolates. The antimicrobial activity of contezolid is comparable to that of linezolid based on MIC50 and MIC90 values. These results are in accordance with previous studies conducted in the United States and Europe (Carvalhaes et al., 2020). Notably, among VRE isolates, isolates with linezolid MIC values ≤0.5 μg/mL were statistically less than that of contezolid (P < 0.001). This indicated that the MIC distributions of contezolid against VRE are better than that of linezolid. Of concern, cross-resistance between linezolid and tedizolid, which both belong to oxazolidinone agents, was reported in staphylococci previously (Barber et al., 2016). In the current study, contezolid exhibited limited activity against strains with linezolid resistance genes. Consequently, the presence of the cfr and optrA genes may result in resistance to contezolid. This indicates that cross-resistance may also exist between contezolid and linezolid, which may limit the clinical application of contezolid. It also suggests the need to strengthen the clinical monitoring of cross-resistance between contezolid and linezolid. Among all the comparator agents tested, contezolid had relatively lower MIC50 and MIC90 values, indicating that its antimicrobial activity against MRSA and VRE was better than some antibiotics. Therefore, contezolid may offer another option for the clinical treatment of MDR gram-positive bacteria.
In summary, contezolid displayed potent in vitro activity against MRSA and VRE isolates collected from China. The antimicrobial activity of contezolid is similar to or slightly better than that of linezolid against MRSA and VRE isolates. However, cross-resistance may exist between contezolid and linezolid. The in vitro data in the current study imply that contezolid may be a promising candidate to treat MRSA and VRE infections, but may not be helpful for infections caused by linezolid-resistant strains. Further experimental and clinical researches are demanded to promote the progress of contezolid to reach clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
RZ and HZ designed the study. SW, YS, CS, QS, NW, SZ, and JQ did the experiment. CC, RZ, and HZ analyzed and interpreted the data. SW, HZ, and RZ wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81971987 and 31761133004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank Prof. Yang Wang (China Agricultural University) for providing cfr and optrA positive strains.
Footnotes
References
Asokan, G. V., Ramadhan, T., Ahmed, E., and Sanad, H. (2019). WHO global priority pathogens list: a bibliometric analysis of medline-pubmed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 34, 184–193. doi: 10.5001/omj.2019.37
Barber, K. E., Smith, J. R., Raut, A., and Rybak, M. J. (2016). Evaluation of tedizolid against Staphylococcus aureus and enterococci with reduced susceptibility to vancomycin, daptomycin or linezolid. J. Antimicrob. Chemother. 71, 152–155. doi: 10.1093/jac/dkv302
Bassetti, M., Del Puente, F., Magnasco, L., and Giacobbe, D. R. (2020). Innovative therapies for acute bacterial skin and skin-structure infections (ABSSSI) caused by methicillin-resistant Staphylococcus aureus: advances in phase I and II trials. Expert Opin. Investig. Drugs. 29, 495–506. doi: 10.1080/13543784.2020.1750595
Carvalhaes, C. G., Duncan, L. R., Wang, W., and Sader, H. S. (2020). In vitro activity and potency of the novel oxazolidinone contezolid (MRX-I) tested against gram-positive clinical isolates from the United States and Europe. Antimicrob. Agents Chemother. 64, e01195–20. doi: 10.1128/AAC.01195-20
CLSI (2020a). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. CLSI Document M07-A8. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2020b). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Document M100-S19. Wayne, PA: Clinical and Laboratory Standards Institute.
Dutka-Malen, S., Evers, S., and Courvalin, P. (1995). Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33, 24–27. doi: 10.1128/jcm.33.1.24-27.1995
Eckburg, P. B., Ge, Y., and Hafkin, B. (2017). Single- and multiple-dose study to determine the safety, tolerability, pharmacokinetics, and food effect of oral MRX-I versus linezolid in healthy adult subjects. Antimicrob. Agents Chemother. 61, e02181–16. doi: 10.1128/AAC.02181-16
Gordeev, M. F., and Yuan, Z. Y. (2014). New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J. Med. Chem. 57, 4487–4497. doi: 10.1021/jm401931e
Gu, B., Kelesidis, T., Tsiodras, S., Hindler, J., and Humphries, R. M. (2013). The emerging problem of linezolid-resistant Staphylococcus. J. Antimicrob. Chemother. 68, 4–11. doi: 10.1093/jac/dks354
Hashemian, S. M. R., Farhadi, T., and Ganjparvar, M. (2018). Linezolid: a review of its properties, function, and use in critical care. Drug Des. Devel. Ther. 12, 1759–1767. doi: 10.2147/DDDT.S164515
Hoskins, A. J., Worth, L. J., Imam, N., Johnson, S. A., Bull, A. L., Richards, M. J., et al. (2018). Validation of healthcare-associated infection surveillance in smaller Australian hospitals. J. Hosp. Infect. 99, 85–88. doi: 10.1016/j.jhin.2017.10.006
Huang, Y., Xu, Y., Liu, S., Wang, H., Xu, X., Guo, Q., et al. (2014). Selection and characterisation of Staphylococcus aureus mutants with reduced susceptibility to the investigational oxazolidinone MRX-I. Int. J. Antimicrob. Agents. 43, 418–422. doi: 10.1016/j.ijantimicag.2014.02.008
Lee, E. Y., and Caffrey, A. R. (2018). Thrombocytopenia with tedizolid and linezolid. Antimicrob. Agents Chemother. 62, e01453–17. doi: 10.1128/AAC.01453-17
Li, C. R., Zhai, Q. Q., Wang, X. K., Hu, X. X., Li, G. Q., Zhang, W. X., et al. (2014). In vivo antibacterial activity of MRX-I, a new oxazolidinone. Antimicrob. Agents Chemother. 58, 2418–2421. doi: 10.1128/AAC.01526-13
Li, J., Jiang, N., Ke, Y., Feßler, A. T., Wang, Y., Schwarz, S., et al. (2017). Characterization of pig-associated methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 201, 183–187. doi: 10.1016/j.vetmic.2017.01.017
Meng, J., Zhong, D., Li, L., Yuan, Z., Yuan, H., Xie, C., et al. (2015). Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2,3-dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab. Dispos. 43, 646–659. doi: 10.1124/dmd.114.061747
Osei Sekyere, J., and Mensah, E. (2020). Molecular epidemiology and mechanisms of antibiotic resistance in Enterococcus spp., Staphylococcus spp., and Streptococcus spp. in Africa: a systematic review from a one health perspective. Ann. N. Y. Acad. Sci. 1465, 29–58. doi: 10.1111/nyas.14254
Ruiz-Ripa, L., Feßler, A. T., Hanke, D., Eichhorn, I., Azcona-Gutiérrez, J. M., Pérez-Moreno, M. O., et al. (2020). Mechanisms of linezolid resistance among enterococci of clinical origin in Spain-detection of optrA- and cfr(D)-carrying E. faecalis. Microorganisms. 8:1155. doi: 10.3390/microorganisms8081155
Sadowy, E. (2018). Linezolid resistance genes and genetic elements enhancing their dissemination in enterococci and streptococci. Plasmid. 99, 89–98. doi: 10.1016/j.plasmid.2018.09.011
Shinabarger, D. (1999). Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin. Investig. Drugs. 8, 1195–1202. doi: 10.1517/13543784.8.8.1195
Stevenson, K. B., Searle, K., Stoddard, G. J., and Samore, M. (2005). Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in rural communities, western United States. Emerg. Infect. Dis. 11, 895–903. doi: 10.3201/eid1106.050156
Wang, W., Voss, K. M., Liu, J., and Gordeev, M. F. (2021). Nonclinical evaluation of antibacterial oxazolidinones contezolid and contezolid acefosamil with low serotonergic neurotoxicity. Chem. Res. Toxicol. 34, 1348–1354. doi: 10.1021/acs.chemrestox.0c00524
Wang, Y., Lv, Y., Cai, J., Schwarz, S., Cui, L., Hu, Z., et al. (2015). A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 70, 2182–2190. doi: 10.1093/jac/dkv116
Wang, Y., Zhang, W., Wang, J., Wu, C., Shen, Z., Fu, X., et al. (2012). Distribution of the multidrug resistance gene cfr in Staphylococcus species isolates from swine farms in China. Antimicrob. Agents Chemother. 56, 1485–1490. doi: 10.1128/AAC.05827-11
Wu, X., Li, Y., Zhang, J., Zhang, Y., Yu, J., Cao, G., et al. (2018). Short-term safety, tolerability, and pharmacokinetics of MRX-I, an oxazolidinone antibacterial agent, in healthy chinese subjects. Clin. Ther. 40, 322–332. doi: 10.1016/j.clinthera.2017.12.017
Xu, X., Lin, D., Yan, G., Ye, X., Wu, S., Guo, Y., et al. (2010). vanM, a new glycopeptide resistance gene cluster found in Enterococcus faecium. Antimicrob. Agents Chemother. 54, 4643–4647. doi: 10.1128/AAC.01710-09
Zahedi Bialvaei, A., Rahbar, M., Yousefi, M., Asgharzadeh, M., and Samadi Kafil, H. (2017). Linezolid: a promising option in the treatment of Gram-positives. J. Antimicrob. Chemother. 72, 354–364. doi: 10.1093/jac/dkw450
Keywords: contezolid, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, linezolid, antibiotics, antimicrobial activity, multidrug-resistance, gram-positive
Citation: Wang S, Cai C, Shen Y, Sun C, Shi Q, Wu N, Zheng S, Qian J, Zhang R and Zhou H (2021) In vitro Activity of Contezolid Against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus, and Strains With Linezolid Resistance Genes From China. Front. Microbiol. 12:729900. doi: 10.3389/fmicb.2021.729900
Received: 24 June 2021; Accepted: 30 July 2021;
Published: 19 August 2021.
Edited by:
Yvonne Mast, German Collection of Microorganisms and Cell Cultures GmbH (DSMZ), GermanyReviewed by:
Michael Henry Cynamon, Syracuse VA Medical Center, United StatesWerner Solbach, University of Lübeck, Germany
Copyright © 2021 Wang, Cai, Shen, Sun, Shi, Wu, Zheng, Qian, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Zhou, emhvdWhvbmd3ZWlAemp1LmVkdS5jbg==
 Siheng Wang
Siheng Wang Chang Cai
Chang Cai Yingbo Shen3
Yingbo Shen3 Chengtao Sun
Chengtao Sun Ningjun Wu
Ningjun Wu Shufang Zheng
Shufang Zheng Jiao Qian
Jiao Qian Rong Zhang
Rong Zhang Hongwei Zhou
Hongwei Zhou