- 1National Clinical Research Center for Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2The State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 3College of Medicine, Zhejiang University, Hangzhou, China
- 4Department of Radiology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Background: The clinical relevance of single or repeated episodes of Candida spp. in cerebrospinal fluid (CSF) in adult patients is debatable.
Methods: Forty-two patients with positive Candida episodes in CSF were enrolled in this retrospective study.
Results: A total of 42.9% (18/42) were determined to have probable Candida meningitis (PCM). Neurosurgery [odds ratio (OR) (95% confidence interval), OR: 14.4 (1.6–126.1), P = 0.004], lumbar drainage [OR: 5.8 (1.5–23.3), P = 0.009], VP shunt [(OR: 5.6 (1.2–25.8), P = 0.020)], external ventricular drainage [OR: 4.7 (1.3–17.7), P = 0.018], CRP ≥ 10.0 mg/L [OR: 4.9 (1.3–18.1), P = 0.034], and postsurgical broad-spectrum antibiotics [OR: 9.5 (1.8–50.5), P = 0.004] were risk factors associated with PCM. A single CSF Candida episode for the diagnosis of PCM had 7.7% (0.4–37.9%) sensitivity and 20.7% (8.7–40.3%) specificity, whereas repeated episodes of Candida had 66.7% (41.2–85.6%) sensitivity and 95.8% (76.9–99.8%) specificity. No significant difference was found in radiological imaging or CSF profiles between PCM and non-PCM patients. A total of 37.5% (9/24) of patients without PCM received empirical antifungal treatment, and 88.9% (16/18) of patients with PCM received preemptive antifungal treatment. PCM patients had hospitalized mortality rates of 50.0% (9/18). The odds ratio of mortality was 23.0 (2.5–208.6) for PCM patients compared with non-PCM patients (P = 0.001).
Conclusion: Both single and repeated positive CSF samples have low validity for the diagnosis of PCM, suggesting that novel strategies for diagnosis algorithms of PCM are urgently needed. Empirical antifungal treatment should be started immediately for suspicious patients with risk factors.
Introduction
Candida meningitis (C. meningitis) is rare, but severe cases have been encountered in clinical practice. A previous publication reported that C. meningitis mainly presented in neonates with extremely low birth weight due to blood brain barrier immaturity (Marr et al., 1994; Cohen-Wolkowiez et al., 2007; Agarwal et al., 2008; Ancalle et al., 2010). Central nervous system (CNS) Candida infections in adult patients are even rarer in clinical practice and are depicted only in some case reports. Those limited case reports suggest that CNS candidiasis is observed mainly in patients with predisposing conditions, such as hematological malignancy, organ transplant, intravenous drug use, diabetes mellitus, or human immunodeficiency virus (HIV) (Montero et al., 2000; Donnelly et al., 2020; Chaussade et al., 2021). Furthermore, neurosurgery history or foreign intracranial material are also risk factors for the development of CNS Candida infection (Chen et al., 2002; Fadel et al., 2018).
Although CNS Candida infection is a critical event in clinical practice, the proof and criteria of diagnosis for C. meningitis are ambiguous. Currently, the diagnosis of C. meningitis is dependent mainly on the culture of Candida spp. in cerebrospinal fluid (CSF). However, the relative importance of Candida spp. isolated from CSF by different routes remains unclear. For example, a study including 21 neurosurgical patients indicated that Candida species were isolated from multiple CSF samples in 10 cases and only isolated from CSF through indwelling devices in 11 cases. Interestingly, none of the 9 of 11 patients from whom Candida spp. were isolated from indwelling devices and who were not treated with antifungal regimens died of infection (Geers and Gordon, 1999). These data suggested that a single positive CSF sample drawn through an indwelling device is insufficient for a reliable diagnosis in patients receiving neurosurgery, and repeated positive CSF samples from drainage devices might be necessary for diagnosing C. meningitis. Furthermore, Candida cultures from CSF have poor sensitivity and can take a few days to grow. Unlike Cryptococcus infections, there is currently no widely accepted molecular-, antigen-, or nucleic acid-based testing method to expedite identification of the organism; thus, delaying the diagnosis and treatment of C. meningitis is of great importance in clinical practice.
The emerging occurrence of C. meningitis and the ambiguity of CNS Candida spp. diagnosis prompted us to conduct a retrospective study to summarize the utility of the different numbers and compositions of CSF Candida spp., CSF profiles and CT/MRI findings on the diagnosis of C. meningitis in patients with risk factors.
Materials and Methods
Study Cohort and Patient Enrollment
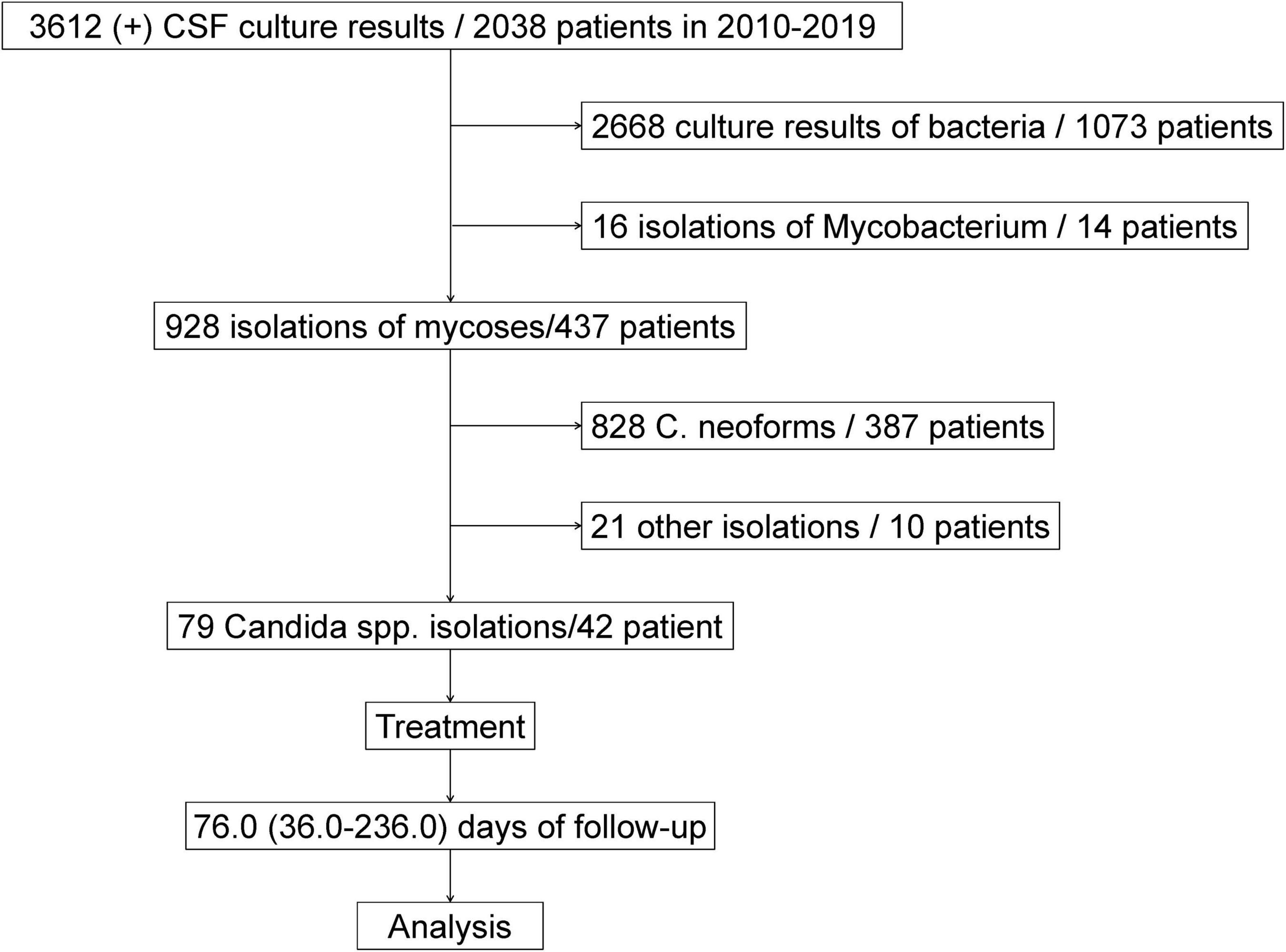
Between January 2010 and December 2019, a total of 3612 CSF-positive culture results were obtained from 2,038 patients from the First Affiliated Hospital, School of Medicine of Zhejiang University, Hangzhou, China. Of those, 2,668 culture results were bacteria from 1,073 patients, 16 isolations were mycobacterium or non-Mycobacterium infection from 14 patients, and 928 isolations were fungal infection from 437 patients. There were 387 patients with 828 Cryptococcus spp. isolations, 42 patients with 79 Candida spp. isolations and 10 patients with 21 other fungal infections in CSF culture (Zhao et al., 2021). Ultimately, the 42 patients from whom Candida spp. were isolated were enrolled in our cohort study. The flowchart of patient selection is briefly described in Figure 1.
Diagnosis Criteria of Probable Candida meningitis
The diagnosis of probable C. meningitis (PCM) was made according to the following criteria: (1) at least one new onset of neurologic symptoms or signs such as fever, headache, vomiting/nausea, mental change, and encephalocele; (2) at least one episode of positive CSF Candida spp.; (3) a CSF profile that supported an infectious process, such as pleocytosis, elevated protein levels, and decreased glucose; and (4) the lack of an adequate treatment response to presumed bacterial or mycobacterial meningitis.
Antifungal Susceptibility Testing
The broth dilution quantitative method was used to determine the minimum inhibitory concentration (MIC) of an antimicrobial agent that inhibited the growth of organisms in vitro. Antifungal analysis was automatically performed by the VITEK® 2 COMPACT system and Etest package insert (Biomérieux, Marcy-l’Etoile, France).
Follow-Up and Collection of Clinical Data
Basic medical information of patients (such as name, sex, BMI, blood test, imaging examination, and treatments) was recorded and saved in the electronic medical records system (EMRS) of hospital. Patients were followed up for 76.0 (36.0–236.0) days.
Statistical Analyses
Continuous normal variables are presented as the mean ± standard deviation, and continuous abnormal variables are presented as the median (interquartile range, IQR). Categorical variables are presented as the number of cases (percentage). Continuous variables were compared by Student’s t-test or the Mann-Whitney U test, whereas categorical variables were compared using χ2 analyses or Fisher’s exact test. A P-value of < 0.05 (two-tailed) was considered significant. Data analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, United States).
Ethical Approval of the Study Protocol
This study protocol was performed in accordance with the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China) (No. 2021-599). All data analyzed were anonymous.
Results
Patient Characteristics and Demographic Details
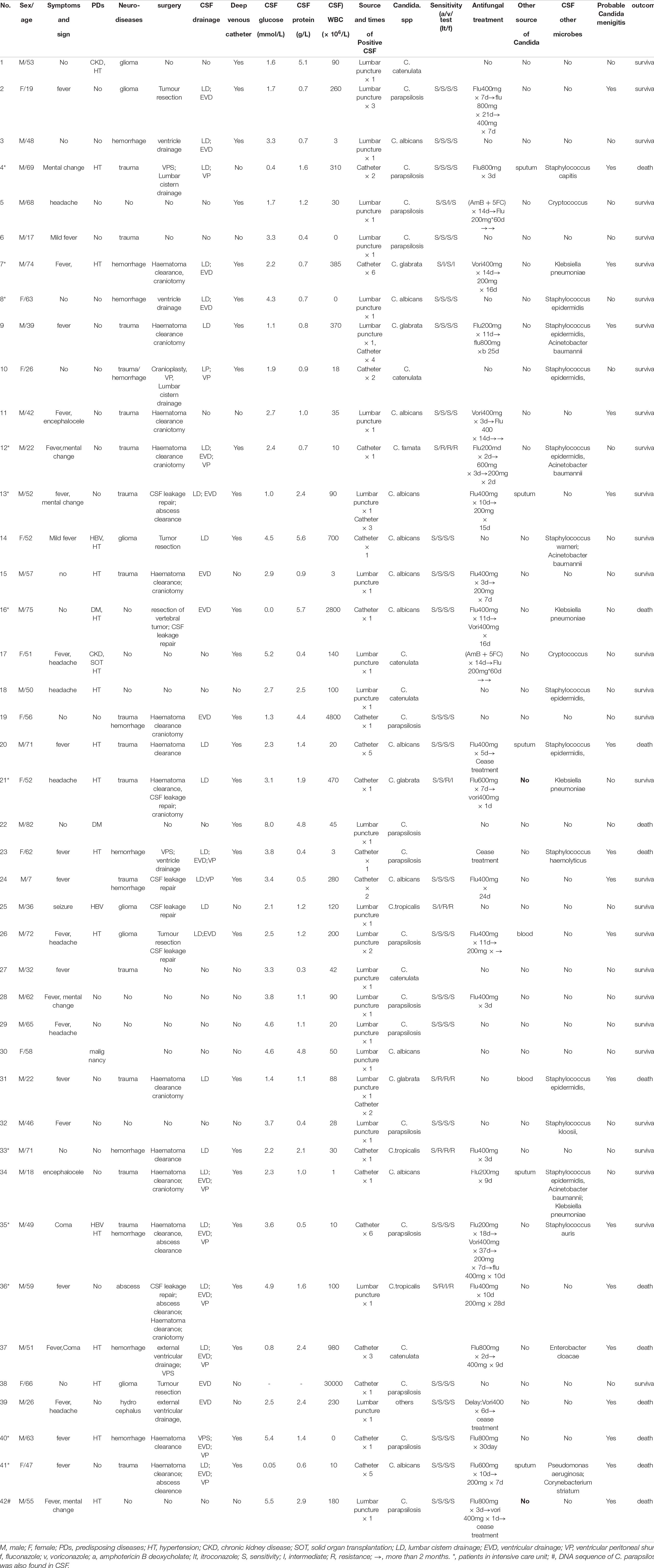
There were 73.8% (31/42) male patients and 26.2% (11/42) female patients. The mean age of patients was 50.1 ± 18.5 years old. A total of 45.2% (19/42) of the patients had predisposing diseases, including 9.5% (4/42) with chronic hematological disease, 4.8% (2/42) with hepatitis B virus (HBV) infection, 4.8% (2/42) with diabetes, 4.8% (2/42) with chronic kidney disease, 2.4% (1/42) with solid organ transplantation, 2.4% (1/42) with malignancy and 40.5% (17/42) with hypertension. In addition, 40.5% (17/42) of the patients had craniocerebral trauma, 26.2% (11/42) had hemorrhage in the CNS, 14.3% (6/42) had glioma and 4.8% (2/42) had craniocerebral abscess at admission. Overall, there were 71.4% (30/42) neurosurgical patients and 28.6% (12/42) non-neurosurgical patients. A total of 28.6% (12/42) of the patients had a history of stay in the intensive care unit (ICU). No difference in predisposing conditions was found between the neurosurgical and non-neurosurgical patients. The characteristics and demographic details are summarized in Table 1.
Isolation of Candida spp. From CSF Samples
Candida albicans (C. albicans) was isolated from 28.6% (12/42) of the patients, Candida parapsilosis (C. parapsilosis) from 35.7% (15/42), Candida glabrata (C. glabrata) from 9.5% (4/42), Candida tropicalis (C. tropicalis) from 7.1% (3/42), and other species from 19.0% (8/42). The percentage of C. albicans cases was 36.7% (11/30) and that of non-C. albicans cases was 63.3% (19/30) among the neurosurgical patients; meanwhile, the percentage of C. albicans cases was 8.3% (1/12) and that of non-C. albicans cases was 91.7% (11/12) in the non-neurosurgical patients (P = 0.128). Twenty-nine patients had 1 episode of Candida spp., 3 patients had 2 episodes of Candida spp., and 10 patients had ≥ 3 Candida spp. isolates. Overall, 56.7% (17/30) of cases had a single episode of Candida spp., and 43.3% (13/30) of cases had repeated episodes of Candida spp. among patients with neurosurgery, whereas 100% (12/12) of cases had a single episode of Candida spp. from CSF among non-neurosurgical patients (P = 0.006). In addition, positive culture and DNA sequencing of C. parapsilosis by next-generation sequencing (NGS) were also found in CSF in one patient (Patient 42).
There were 3 patients (9, 13, and 31) with repeated positive CSF samples both from lumbar puncture and drain devices, 19 with single positive CSF samples from lumbar puncture, 2 (2 and 26) with repeated positive CSF samples from lumbar puncture, 10 with single positive CSF samples from indwelling drain devices and 8 patients (4, 7, 10, 20, 24, 35, 37, and 41) with repeated positive CSF samples from indwelling drain devices.
Patients in the ICU had a higher positive rate of CSF samples from drain devices [83.3% (10/12) vs. 36.7% (11/30), P = 0.019] but a lower positive rate of CSF samples from lumbar puncture [25.0% (3/12) vs. 70.0% (21/30), P = 0.008] than those not in the ICU.
Diagnosis of Probable Candida meningitis
Five patients (4, 13, 20, 34, and 41) had positive sputum cultures, and 2 patients (26 and 31) had positive blood cultures. Eighteen of 42 (42.9%) patients were considered to have PCM, including one non-neurosurgical patient (42) and 17 neurosurgical patients. Of those neurosurgical patients with PCM, 17.6% (3/17) of the patients (9, 13, and 31) had positive CSF samples obtained both from lumbar puncture and indwelling drain devices, 29.4% (5/17) had positive CSF samples obtained from lumbar puncture, and 52.9% (9/17) had positive CSF samples obtained from indwelling drain devices. Furthermore, among those 8 neurosurgical PCM patients with positive lumbar puncture samples, 2/8 (25.0%) patients had repeated positive CSF samples, and 6/8 (75.0%) had a single positive CSF sample. Meanwhile, among the 12 neurosurgical PCM patients with positive CSF samples obtained from indwelling drainage devices, 10/12 (83.3%) patients had repeated positive CSF samples, and 2/12 (16.7%) had a single positive sample.
In total, 24 patients had positive cultures of CSF from lumbar puncture (22 single positive cultures + 2 repeated positive cultures), and 21 patients had positive cultures from indwelling drainage samples (10 single positive cultures + 11 repeated positive cultures). Single positive cultures from lumbar puncture had 77.8% (95% CI: 40.2–96.1%) sensitivity and 0–25.3% specificity, whereas repeated positive samples from lumbar puncture had 22.2% (95% CI: 4.0–59.8%) sensitivity and 100.0% specificity for the diagnosis of PCM. A single positive CSF culture from drainage devices had a sensitivity of 16.7% (95% CI: 2.9–49.1%) and a specificity of 11.1% (95% CI: 0.6–49.3%), whereas a repeated positive culture from drainage devices had a sensitivity of 83.3% (95% CI: 50.9–97.1%) and a specificity of 88.9% (95% CI: 50.6–99.4%). Altogether, single positive CSF cultures had a sensitivity of 7.7% (95% CI: 0.4–37.9%) and a specificity of 20.7% (95% CI: 8.7–40.3%). Repeated CSF samples from the same or different routines had 66.7% (95% CI: 41.2–85.6%) sensitivity and 95.8% (95% CI: 76.9–99.8%) specificity for the diagnosis of PCM.
Furthermore, bacteria were isolated from CSF in 16/30 (53.3%) neurosurgical patients, including Staphylococcus spp. from 12/30 (40/0%) patients, Klebsiella pneumoniae from 4/30 (13.3%) patients, Acinetobacter baumannii from 4/30 (13.3%) patients, Enterobacter cloacae from 1/30 (3.3%) patient, Pseudomonas aeruginosa from 1/30 (3.3%) patient and Corynebacterium striatum from 1/30 (3.3%) patient. The CSF isolation rates of bacteria were 52.9% (9/17) in PCM and 53.8% (7/13) in non-PCM patients among those who underwent neurosurgery (P = 0.961).
We found that neurosurgery [OR: 14.4 (1.6–126.1), P = 0.004], lumbar drainage [OR: 5.8 (1.5–23.3), P = 0.009], VP shunt [(OR: 5.6 (1.2–25.8), P = 0.020)], external ventricular drainage [(OR: 4.7 (1.3–17.7), P = 0.018], CRP ≥ 10.0 mg/L [(OR: 4.9 (1.3–18.1), P = 0.034] and postsurgical broad-spectrum antibiotics [OR: 9.5 (1.8–50.5), P = 0.004] were associated with PCM. Deep vein catheterization [(OR: 2.5 (0.6–9.9), P = 0.186] and ICU stay [OR: 2.4 (0.6–9.5), P = 0.200] were marginally associated with PCM. However, sex, age, predisposing disease, hypoalbumin (< 35 g/L), anemia (< 110 g/L) and cerebral hemorrhage were not associated with PCM.
The CSF protein level of 1.2 (1.0–2.4) g/L for patients with PCM was close to the protein level of 1.1 (0.4–4.8) for patients without PCM (P = 0.636), and the CSF WBC level of 100.0 (61.5–215.0) × 106 cells/L in patients with PCM was slightly higher than that of 42.0 (3.0–100.0) × 106 cells/L for patients without PCM (P = 0.347). CSF glucose was 2.6 ± 1.6 mmol/L in patients with PCM and 3.2 ± 1.6 mmol/L in patients without PCM (P = 0.248). The ICP was 180.0 (105.0–310) mmH2O in PCM patients and 120.0 (80.0–135.0) mmH2O in non-PCM patients (P = 0.073).
CT and/or MRI Image Findings
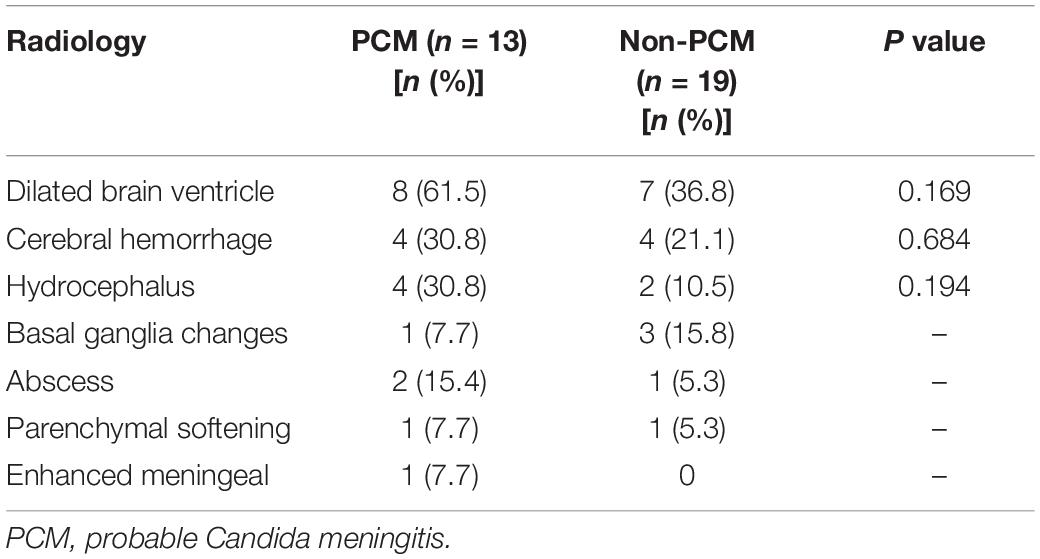
CT and/or MRI images were available in 76.2% (32/42) of patients, including 13 PCM patients and 19 non-PCM patients. Of those 13 patients with PCM, 8/13 (61.5%) had enlarged ventricles, 4/13 (30.8%) had parenchymal hemorrhage, 4/13 (30.8%) had hydrocephalus, 1/13 (7.7%) had abnormal basal ganglia changes, 2/13 (15.4%) had abscess changes, 1/13 (7.7%) had parenchymal softening, and 1/13 (7.7%) had enhanced meningeal ganglia. PCM patients had a trend of a higher incidence of enlarged ventricles than non-PCM patients [61.5% (8/13) vs. 36.8% (7/19), P = 0.169]. No other significant difference was found in CT and/or MRI imaging findings between the groups (Table 2).
Treatment and Outcome
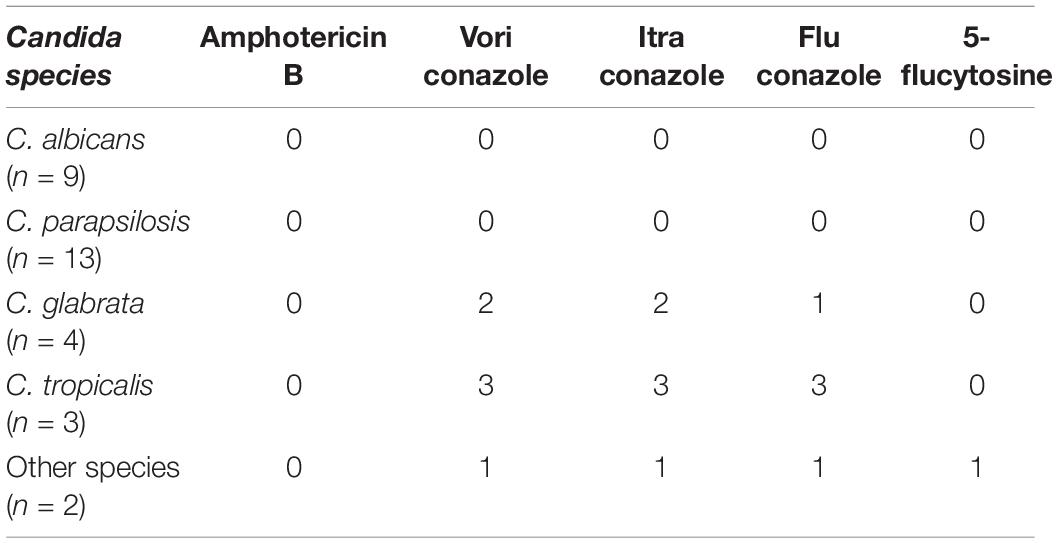
Of the 42 patients, 26 received antifungal treatment, including 2 patients initially treated with AmB + 5FC (0.5–0.7 mg/kg/d), 3 initially treated with voriconazole (200 mg iv bid), 18 initially treated with fluconazole (200–800 mg/d) as induction treatment and 4 initially treated with fluconazole and then switched to voriconazole (Table 1). Two patients (5 and 17) who received antifungal treatment were eventually confirmed to have cryptococcal meningitis, although Candida spp. were initially isolated in the CSF samples. In particular, 16/18 (88.9%) PCM patients (including two patients (20 and 42) who ceased treatment) and 10/24 (41.7%) patients without PCM were treated with antifungal regimens. There were 31 patients with antifungal susceptibility testing data available. Our data showed that non-C. albicans cases exhibited a higher rate of intermediate/resistance to azoles than C. albicans [36.4% (8/22) vs. 0 (0/9), P = 0.068] (Tables 1, 3). Ten patients died during the follow-up. Of those, the hospitalized mortality rates were 50.0% (9/18) in PCM patients and 4.8% (1/24) in non-PCM patients. The odds ratio of mortality was 23.0 (2.5–208.6) for PCM patients compared with non-PCM patients (P = 0.001).
Discussion
Candida meningitis is a rare but emerging problem in clinical practice. A previous study suggested that the occurrence of C. meningitis was associated with low body weight in infants and neurosurgery in adults (Marr et al., 1994; Nguyen and Yu, 1995; Geers and Gordon, 1999). However, the diagnostic criteria and clinical characteristics of C. meningitis remain unclear due to the rarity of the disease. In the present study, we found that (1) non-C. albicans was responsible for nearly 70% of Candida episodes; (2) neurosurgery, drainage devices, high CRP levels and usage of broad-spectrum antibiotics after surgery were associated with PCM; (3) although a single positive CSF sample drawn through an indwelling device was difficult to assess, repeated episodes of positive CSF samples displayed high specificity and low sensitivity for the diagnosis of PCM; and (4) episodes of bacteria in CSF and the hospitalized mortality of PCM patients were both 50.0%.
Some predisposing conditions, such as long-term steroid usage, chemotherapy, and intravenous catheters, are associated with PCM. Neurosurgery is also recognized as a risk factor for Candida meningitis, especially shunt devices, which are closely related to CSF Candida infection (Montero et al., 2000; Bridges et al., 2017; Chen et al., 2020). In the present study, we found that approximately 70% of patients had a history of neurosurgery, and 30% did not. Neurosurgery and drainage devices were associated with PCM. The possible reason for Candida infection caused by neurosurgery and drainage devices might be related to the disruption of the blood-brain barrier. External ventricular and lumbar drainage devices both contribute to ventriculitis (Scheithauer et al., 2010; Citerio et al., 2015). However, the different impacts of external ventricular drainage and lumbar drainage on CNS infection are debatable. One study found evidence that lumbar drainage was associated with a low rate of ventriculitis (Schade et al., 2005), but another study indicated that lumbar drainage was a significant risk factor for ventriculitis (Scheithauer et al., 2010). CSF collected by lumbar puncture had a higher diagnostic accuracy than that collected by ventriculostomy for the diagnosis of CNS infection (Finger et al., 2020). Drainage device infection usually occurs after the surgical procedure and is believed to result from contamination (Lipton et al., 1984). Our study also indicated that VP shunts, lumbar drainage devices, and external ventricular drainage were risk factors for C. meningitis, and we speculated that contamination more easily occurred in drainage devices.
Twelve patients without neurosurgery had episodes of Candida in the CSF sample. Some studies indicated that patients with extracranial factors (such as abdominal surgery, recent broad-spectrum antibiotic therapy, indwelling catheters, malignancy and steroid use) were at high risk for the occurrence of C. meningitis (Bridges et al., 2017). In our study, we found that 50.0% (6/12) of patients without neurosurgery had predisposing diseases, but only one patient without neurosurgery had PCM. These data suggested that PCM in non-neurosurgical patients was rare, and the majority of episodes of Candida in CSF were from contamination during lumbar puncture in non-neurosurgical patients. In addition, we found that neurosurgery and drainage devices, rather than predisposing diseases, were risk factors for the development of PCM, revealing that the risk factors for the development of PCM in neurosurgical patients were different from those in non-neurosurgical patients.
Some studies suggested that broad-spectrum antibiotic therapy was a contributor to PCM, and 50% of patients suffered from antecedent bacterial meningitis (Nguyen and Yu, 1995). In fact, previous studies indicated that infection with Candida spp. was always associated with concurrent bacterial infection (Peres-Bota et al., 2004; Tarumoto et al., 2014). In the present study, 52.9% of PCM patients had CSF bacterial infection, which was close to the incidence of 53.8% in non-PCM patients, indicating that broad-spectrum antibiotics were a concurrent treatment for coinfection rather than a cause of CNS Candida episodes. Thus, it was reasonable that a high CRP level was associated with the incidence of PCM.
Some data indicated diffuse or multiple miliary nodules and hyperintense signals on DWI, while non-significant signal changes in T1WI and T2WI on MRI images were found among preterm infants with C. albicans infection (Mao et al., 2012). Marked hydrocephalus and leptomeningeal enhancement were observed on brain MRI of patients with Candida dubliniensis infection (Yamahiro et al., 2016). Our data suggested that enlarged ventricles, parenchymal hemorrhage and hydrocephalus were the most common findings in PCM patients. However, no significant difference was found in enlarged ventricles (P = 0.169), parenchymal hemorrhage (P = 0.684) or hydrocephalus (P = 0.150) between patients with and without CNS Candida infection (Table 2). It was previously noted that patients with postneurosurgical C. meningitis usually had a recent history of bacterial meningitis, antibiotic therapy and multiple surgeries involving the CNS (Lipton et al., 1984). PCM patients had a trend of higher ventricle involvement in our study (61.5% vs. 36.8%, P = 0.169), which was thought to be associated with CNS infection through indwelling devices (Scheithauer et al., 2010; Citerio et al., 2015; Finger et al., 2020). We hypothesize that the limited sample size, coinfection of bacteria and surgical comorbidities might make it difficult to outline the unique radiological characteristics of PCM patients in our study.
Although all patients had episodes of Candida in the CSF, only 40% of patients were diagnosed with PCM. Furthermore, 41.6% of patients without PCM and 88.9% of patients with PCM were treated with antifungal regimens. A previous study also revealed that all patients with CSF Candida episodes accepted antifungal treatment, although only 50% of patients were considered PCM patients (Geers and Gordon, 1999). Therefore, it is difficult to determine true PCM patients in clinical practice. These data suggest that the diagnostic strategy of PCM should be revised urgently. Although a single episode of Candida spp. in CSF was insufficient for the diagnosis of PCM, repeated episodes of Candida spp. had approximately 66.7% sensitivity and 95.8% specificity for the diagnosis of PCM. These results indicated that the diagnosis of PCM by single or repeated positive CSF samples drawn through lumbar puncture or indwelling devices is insufficient for diagnostic performance. Notably, repeated positive samples from lumbar puncture had only ∼11.7% sensitivity and 100.0% (71.7–100.0%) specificity for the diagnosis of PCM. Another important issue is that most neurosurgical PCM patients in our study did not have host risk factors such as neutropenia, malignancy and immunosuppressive therapy, demonstrating that the risk factors for postneurosurgical patients with C. meningitis are divergent from those for non-neurosurgical patients. Thus, a novel strategy for the diagnosis of CM is needed. Some studies revealed that CSF (1,3)-β-D-glucan was a surrogate marker for PCM, and the persistence of (1,3)-β-D-glucan in CSF was associated with the clinical and microbiological failure of PCM (Lyons et al., 2015; Ceccarelli et al., 2016; Farrugia et al., 2016). Although elevated CSF (1,3)-β-D-glucan was not specifically present in CNS infections of Aspergillus, Cryptococcus and Histoplasma (Rhein et al., 2014; Chen et al., 2017; Myint et al., 2018), CSF (1,3)-β-D-glucan testing may still be a useful surrogate marker of C. meningitis (Davis et al., 2020). Although it seemed that a single episode of CSF Candida was insufficient for PCM diagnosis, a study indicated that normal CSF parameters were found in 43% (3/7) of infants with C. meningitis, and only 37% (7/19) of them had positive blood cultures for Candida (Cohen-Wolkowiez et al., 2007). Some CSF samples of PCM often resemble bacterial profiles, and cultures can be falsely negative (Bridges et al., 2017). Even culture-negative C. meningitis diagnosed by the detection of Candida mannan antigen in CSF was reported (Biesbroek et al., 2013). Thus, CSF culture combined with CSF (1,3)-β-D-glucan assay should be considered in patients who are suspected of infectious meningitis of unknown cause. Alternatively, quantitative Candida colonies were used to assist in the diagnosis of candidiasis (Pfeiffer et al., 2011; Zhou et al., 2017). It is unknown whether quantitative analysis of Candida colonies in CSF obtained from lumbar puncture or indwelling devices is helpful for the diagnosis of PCM.
Previous studies indicated that non-C. albicans species accounted for over two-thirds of isolates from blood in China (Xiao et al., 2020; Zhang et al., 2020). In the present study, we found that the percentage of non-C. albicans exceeded 70%, and non-C. albicans species had higher trends of resistance to azoles than C. albicans. Our data depicted a trend of potential risk for treatment failure of PCM, a high rate of misdiagnosis in patients with CSF episodes of Candida and high mortality in PCM patients. Importantly, the mortality of PCM was 50.0% in our study, which was consistent with previous reports of 42–50% mortality (Geers and Gordon, 1999; Chaussade et al., 2021). These results suggested that antifungal agents should be initiated promptly in patients whose cerebrospinal fluid is positive for Candida and who have one or more of the identified risk factors.
There were some limitations in our study. First, we did not perform a CSF (1,3)-β-D-glucan assay to further support our diagnosis. Second, our data are based on a small sample size, and we were unable to identify the risk factors associated with mortality. Third, our study is a retrospective study, which is prone to certain biases.
In summary, our study showed that neurosurgery, indwelling drainage devices and usage of broad-spectrum antibiotics after neurosurgery led to a high risk for Candida CNS infection. Although it was difficult to diagnose CNS Candida infection from a single episode of Candida spp. in the CSF, repeated episodes of Candida spp. in the CSF had low sensitivity for diagnosis of PCM. CSF (1,3)-β-D-glucan, Candida colony quantitation and next-generation sequencing might be considered helpful for the improved diagnosis of CNS Candida infection.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
This study protocol was performed in accordance with the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China) (No. 2021-599). All data analyzed were anonymous. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
LX designed the study, drafted the manuscript, and analyzed and interpreted the data. HZ performed the study and performed the follow-ups. MZ and GL collected the data. HL reread the radiological images. All authors reviewed and approved the final manuscript.
Funding
This work was supported by Independent Research Funding of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, School of Medicine, Zhejiang University (2020ZZ19).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Yonghong Xiao (First Affiliated Hospital, School of Medicine, Zhejiang University) who provided many suggestions during manuscript preparation.
References
Agarwal, S., Thakur, K., Kanga, A., Singh, G., and Gupta, P. (2008). Catheter-related candidemia caused by Candida lipolytica in a child with tubercular meningitis. Indian J. Pathol. Microbiol. 51, 298–300. doi: 10.4103/0377-4929.41709
Ancalle, I. M., Rivera, J. A., García, I., García, L., and Valcárcel, M. (2010). Candida albicans meningitis and brain abscesses in a neonate: a case report. Bol. Asoc. Med. P. R. 102, 45–48.
Biesbroek, J. M., Verduyn Lunel, F. M., Kragt, J. J., Amelink, G. J., and Frijns, C. J. (2013). Culture-negative Candida meningitis diagnosed by detection of Candida mannan antigen in CSF. Neurology 81, 1555–1556. doi: 10.1212/wnl.0b013e3182a95871
Bridges, K. J., Li, R., Fleseriu, M., and Cetas, J. S. (2017). Candida meningitis after transsphenoidal surgery: a single-institution case series and literature review. World Neurosurg. 108, 41–49. doi: 10.1016/j.wneu.2017.08.115
Ceccarelli, G., Ghezzi, M. C., Raponi, G., Brunetti, G., Marsiglia, C., Fallani, S., et al. (2016). Voriconazole treatment of Candida tropicalis meningitis: persistence of (1,3)-β-D-glucan in the cerebrospinal fluid is a marker of clinical and microbiological failure: a case report. Medicine 95:e4474. doi: 10.1097/md.0000000000004474
Chaussade, H., Cazals, X., Desoubeaux, G., Jouvion, G., Bougnoux, M. E., Lefort, A., et al. (2021). Central nervous system candidiasis beyond neonates: lessons from a nationwide study. Med. Mycol. 59, 266–277. doi: 10.1093/mmy/myaa051
Chen, M., Chen, C., Yang, Q., and Zhan, R. (2020). Candida meningitis in neurosurgical patients: a single-institute study of nine cases over 7 years. Epidemiol. Infect. 148:e148.
Chen, T. K., Groncy, P. K., Javahery, R., Chai, R. Y., Nagpala, P., Finkelman, M., et al. (2017). Successful treatment of Aspergillus ventriculitis through voriconazole adaptive pharmacotherapy, immunomodulation, and therapeutic monitoring of cerebrospinal fluid (1?3)-ß-D-glucan. Med. Mycol. 55, 109–117. doi: 10.1093/mmy/myw118
Chen, T. L., Tsai, C. A., Fung, C. P., Lin, M. Y., Yu, K. W., and Liu, C. Y. (2002). Clinical significance of Candida species isolated from cerebrospinal fluid. J. Microbiol. Immunol. Infect. 35, 249–254.
Citerio, G., Signorini, L., Bronco, A., Vargiolu, A., Rota, M., and Latronico, N. (2015). External ventricular and lumbar drain device infections in ICU patients: a prospective multicenter Italian study. Crit. Care Med. 43, 1630–1637. doi: 10.1097/ccm.0000000000001019
Cohen-Wolkowiez, M., Smith, P. B., Mangum, B., Steinbach, W. J., Alexander, B. D., Cotten, C. M., et al. (2007). Neonatal Candida meningitis: significance of cerebrospinal fluid parameters and blood cultures. J. Perinatol. 27, 97–100. doi: 10.1038/sj.jp.7211628
Davis, C., Wheat, L. J., Myint, T., Boulware, D. R., and Bahr, N. C. (2020). Efficacy of cerebrospinal fluid Beta-d-Glucan diagnostic testing for fungal meningitis: a systematic review. J. Clin. Microbiol. 58:e02094-19.
Donnelly, J. P., Chen, S. C., Kauffman, C. A., Steinbach, W. J., Baddley, J. W., Verweij, P. E., et al. (2020). Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 71, 1367–1376.
Fadel, H., Moon, S. J., Klinger, N. V., Chamiraju, P., Eltahawy, H. A., Moisi, M. D., et al. (2018). Candida parapsilosis Infection of ventriculoperitoneal shunt in adult: case report and literature review. World Neurosurg. 119, 290–293. doi: 10.1016/j.wneu.2018.08.023
Farrugia, M. K., Fogha, E. P., Miah, A. R., Yednock, J., Palmer, H. C., and Guilfoose, J. (2016). Candida meningitis in an immunocompetent patient detected through (1–(3)-beta-d-glucan. Int. J. Infect. Dis. 51, 25–26. doi: 10.1016/j.ijid.2016.08.020
Finger, G., Worm, P. V., Dos Santos, S. C., Do Nascimento, T. L., Gallo, P., and Stefani, M. A. (2020). Cerebrospinal fluid collected by lumbar puncture has a higher diagnostic accuracy than collected by ventriculostomy. World Neurosurg. 138, e683–e689.
Geers, T. A., and Gordon, S. M. (1999). Clinical significance of candida species isolated from cerebrospinal fluid following neurosurgery. Clin. Infect. Dis. 28, 1139–1147. doi: 10.1086/514755
Lipton, S. A., Hickey, W. F., Morris, J. H., and Loscalzo, J. (1984). Candidal infection in the central nervous system. Am. J. Med. 76, 101–108. doi: 10.1016/0002-9343(84)90757-5
Lyons, J. L., Erkkinen, M. G., and Vodopivec, I. (2015). Cerebrospinal fluid (1,3)-β-D-glucan in isolated Candida meningitis. Clin. Infect. Dis. 60, 161–162. doi: 10.1093/cid/ciu737
Mao, J., Li, J., Chen, D., Zhang, J., Du, Y. N., Wang, Y. J., et al. (2012). MRI-DWI improves the early diagnosis of brain abscess induced by Candida albicans in preterm infants. Transl. Pediatr. 1, 76–84.
Marr, B., Gross, S., Cunningham, C., and Weiner, L. (1994). Candidal sepsis and meningitis in a very-low-birth-weight infant successfully treated with fluconazole and flucytosine. Clin. Infect. Dis. 19, 795–796. doi: 10.1093/clinids/19.4.795
Montero, A., Romero, J., Vargas, J. A., Regueiro, C. A., Sánchez-Aloz, G., De Prados, F., et al. (2000). Candida infection of cerebrospinal fluid shunt devices: report of two cases and review of the literature. Acta Neurochir. 142, 67–74. doi: 10.1007/s007010050009
Myint, T., Chow, F. C., Bloch, K. C., Raymond-Guillen, L., Davis, T. E., Wright, P. W., et al. (2018). Detection of (1,3)-β-d-Glucan in Cerebrospinal Fluid in Histoplasma Meningitis. J. Clin. Microbiol. 56:e00663-18.
Nguyen, M. H., and Yu, V. L. (1995). Meningitis caused by Candida species: an emerging problem in neurosurgical patients. Clin. Infect. Dis. 21, 323–327. doi: 10.1093/clinids/21.2.323
Peres-Bota, D., Rodriguez-Villalobos, H., Dimopoulos, G., Melot, C., and Vincent, J. L. (2004). Potential risk factors for infection with Candida spp. in critically ill patients. Clin. Microbiol. Infect. 10, 550–555. doi: 10.1111/j.1469-0691.2004.00873.x
Pfeiffer, C. D., Samsa, G. P., Schell, W. A., Reller, L. B., Perfect, J. R., and Alexander, B. D. (2011). Quantitation of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 49, 2879–2883. doi: 10.1128/jcm.00609-11
Rhein, J., Bahr, N. C., Morawski, B. M., Schutz, C., Zhang, Y., Finkelman, M., et al. (2014). Detection of High Cerebrospinal Fluid Levels of (1→3)-β-d-Glucan in Cryptococcal Meningitis. Open Forum Infect. Dis. 1:ofu105.
Schade, R. P., Schinkel, J., Visser, L. G., Van Dijk, J. M., Voormolen, J. H., and Kuijper, E. J. (2005). Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J. Neurosurg. 102, 229–234. doi: 10.3171/jns.2005.102.2.0229
Scheithauer, S., Bürgel, U., Bickenbach, J., Häfner, H., Haase, G., Waitschies, B., et al. (2010). External ventricular and lumbar drainage-associated meningoventriculitis: prospective analysis of time-dependent infection rates and risk factor analysis. Infection 38, 205–209. doi: 10.1007/s15010-010-0006-3
Tarumoto, N., Kinjo, Y., Kitano, N., Sasai, D., Ueno, K., Okawara, A., et al. (2014). Exacerbation of invasive Candida albicans infection by commensal bacteria or a glycolipid through IFN-γ produced in part by iNKT cells. J. Infect. Dis. 209, 799–810. doi: 10.1093/infdis/jit534
Xiao, M., Chen, S. C., Kong, F., Xu, X. L., Yan, L., Kong, H. S., et al. (2020). Distribution and antifungal susceptibility of candida species causing Candidemia in China: an update from the CHIF-NET Study. J. Infect. Dis. 221, S139–S147.
Yamahiro, A., Lau, K. H., Peaper, D. R., and Villanueva, M. (2016). Meningitis caused by Candida Dubliniensis in a Patient with Cirrhosis: a case report and review of the literature. Mycopathologia 181, 589–593. doi: 10.1007/s11046-016-0006-7
Zhang, W., Song, X., Wu, H., and Zheng, R. (2020). Epidemiology, species distribution, and predictive factors for mortality of candidemia in adult surgical patients. BMC Infect. Dis. 20:506. doi: 10.1186/s12879-020-05238-6
Zhao, H., Zhou, M., Zheng, Q., Zhu, M., Yang, Z., Hu, C., et al. (2021). Clinical features and Outcomes of Cryptococcemia patients with and without HIV infection. Mycoses 64, 656–667. doi: 10.1111/myc.13261
Keywords: Candida meningitis, neurosurgery, cerebrospinal fluid, central nervous system, fungal infection, diagnosis
Citation: Xu L, Zhao H, Zhou M, Lang G and Lou H (2021) Single and Repeated Episodes of Candida Species Isolated From Cerebrospinal Fluid for Diagnosing Probable Candida meningitis. Front. Microbiol. 12:742931. doi: 10.3389/fmicb.2021.742931
Received: 17 July 2021; Accepted: 13 September 2021;
Published: 15 October 2021.
Edited by:
Maurizio Sanguinetti, Catholic University of the Sacred Heart, ItalyReviewed by:
László Majoros, University of Debrecen, HungarySomayeh Dolatabadi, Westerdijk Fungal Biodiversity Institute, Netherlands
Copyright © 2021 Xu, Zhao, Zhou, Lang and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Xu, eHVsaWp1bjE5NzZAemp1LmVkdS5jbg==; Haiyan Lou, bG91bGFuMTk4OEB6anUuZWR1LmNu
 Lijun Xu
Lijun Xu Handan Zhao3
Handan Zhao3 Haiyan Lou
Haiyan Lou