Abstract
In underdeveloped and developing countries, due to poverty, fermentation is one of the most widely used preservation methods. It not only allows extending the shelf life of food, but also brings other benefits, including inhibiting the growth of pathogenic microorganisms, improving the organoleptic properties and product digestibility, and can be a valuable source of functional microorganisms. Today, there is a great interest in functional strains, which, in addition to typical probiotic strains, can participate in the treatment of numerous diseases, disorders of the digestive system, but also mental diseases, or stimulate our immune system. Hence, fermented foods and beverages are not only a part of the traditional diet, e.g., in Africa but also play a role in the nutrition of people around the world. The fermentation process for some products occurs spontaneously, without the use of well-defined starter cultures, under poorly controlled or uncontrolled conditions. Therefore, while this affordable technology has many advantages, it can also pose a potential health risk. The use of poor-quality ingredients, inadequate hygiene conditions in the manufacturing processes, the lack of standards for safety and hygiene controls lead to the failure food safety systems implementation, especially in low- and middle-income countries or for small-scale products (at household level, in villages and scale cottage industries). This can result in the presence of pathogenic microorganisms or their toxins in the food contributing to cases of illness or even outbreaks. Also, improper processing and storage, as by well as the conditions of sale affect the food safety. Foodborne diseases through the consumption of traditional fermented foods are not reported frequently, but this may be related, among other things, to a low percentage of people entering healthcare care or weaknesses in foodborne disease surveillance systems. In many parts of the world, especially in Africa and Asia, pathogens such as enterotoxigenic and enterohemorrhagic Escherichia coli, Shigella spp., Salmonella spp., enterotoxigenic Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus have been detected in fermented foods. Therefore, this review, in addition to the positive aspects, presents the potential risk associated with the consumption of this type of products.
Introduction
Fermentation is one of the oldest processes that allows the preservation of food stability with the participation of microorganisms. The term itself comes from the Latin word fervere, which means “to cook.” The action of microorganisms is based on the breakdown of complex compounds (carbohydrates and other macromolecules) into simple ones, which is accompanied by the formation of various types of beneficial catabolites, such as B vitamins, minerals, or Omega-3 fatty acids (Sivamaruthi et al., 2018). In most fermented products, lactic acid bacteria (LAB) play a major role in production. Also, several dozen types of bacteria, yeast, and filamentous fungi participate in the food fermentation (Laranjo et al., 2017; Rezac et al., 2018; Dimidi et al., 2019; van Reckem et al., 2019; Vilela et al., 2020; Zang et al., 2020; García-Díez and Saraiva, 2021; Xu et al., 2021).
A variety of single or mixed raw materials of plant origin (including cereals), meat and fish, and dairy products can be fermented. Such food can be eaten as a main course, drink, or snack.
Fermented foods can include processed foods on a small scale (household, craft industry) and large scale (industrially processed foods). A relevant role in traditionally fermented food play available plant or animal raw materials but also the customs, culture, and religion of indigenous peoples. Techniques of the fermentation process in some geographic areas are passed only orally, from generation to generation, and therefore are known to communities living close to each other (Anyogu et al., 2021).
Since ancient times, fermented foods have been produced by a process of natural (wild, spontaneous) fermentation, carried out by indigenous microorganisms naturally present in the raw material or processing environment (Campbell-Platt, 1987). The dominance of fermenting microorganisms, their metabolites and the changing pH of the raw material inhibit the growth of pathogenic microorganisms. Natural fermentation occurs also when a component containing a large number of microorganisms that initiate the fermentation process is added to the raw material. In both cases, the microorganisms involved in fermentation and the microclimate impact a product quality. The backsloping method, involving the use of a previously fermented product to inoculate a new batch, has also been used. This approach increases the chances of the desired microorganisms domination and competition with microorganisms that responsible for the product spoilage or disease. These traditional fermentation methods are still used today, primarily in home-based, local food production, or small-scale production. However, in the twentieth century, the development of microbiology, including food microbiology, has led to starter cultures introduction, which initiate the fermentation process and at the same time ensure greater product standardization. Such method results in products with constant organoleptic properties. Fermentation with well-defined cultures has found application, especially in the case of products obtained on an industrial scale. The process conducted under controlled conditions, it allows increasing the pace of the process and its throughput. The predominance of native microbiota allows limiting the growth of undesirable strains or species of microorganisms, as well as to reducing the toxic compounds they produce, ensuring the food safety. In developed countries, fermentation with the use of starter cultures also aims to achieve new health goals (Hesseltine and Wang, 1980; Holzapfel, 1997; Tamang et al., 2016, 2020; Voidarou et al., 2020; Mannaa et al., 2021). Research aimed at improving the starter cultures properties, carried out using the innovative CRISPR/Cas9 technology (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9) which allows modification of the genome of any microorganism, may play an essential role here (Wu et al., 2020; Vilela, 2021). On the other hand, due to the current trend toward organic and biodynamic production, and the “flat” taste of products made with the participation of bacterial and fungal starter cultures, the strategy of traditional, spontaneous fermentation, and artisanal returns. This, however, increases the risk of the dangerous microorganisms presence in food (Capozzi et al., 2017).
Benefits
The health-promoting effect of fermented products is due to the presence of functional microorganisms in them. Microorganisms can occur naturally in various products (e.g., genera Lactobacillus, Lacticaseibacillus, Levilactobacillus) or, having GRAS (Generally Recognized As Safe) status, can be added to them (e.g., bacteria of the genus Bifidobacterium). The beneficial effect of microorganisms present in fermented products can be multidirectional (Figure 1). Since the consumption of functional foods can play a positive role in gut dysfunction, research is being conducted to determine their use in intestinal diseases. A study by Zhang et al. (2016), has shown that microorganisms present in fermented foods can transiently affect the gut microbiome. This allows for its modification and modulation of intestinal function, improving the health or reducing the risk of diseases associated with dysbiosis. Food can be a vehicle for probiotics, prebiotics, or synbiotics (Milani et al., 2019). The beneficial effects of probiotic strains include normalization of the gastrointestinal microbiota, antagonistic effects against pathogens, protection against pathogens’ colonization, short-chain fatty acid production, or metabolism of bile acid salts. Such properties make probiotics useful in intestinal diseases treatment (including Clostridioides difficile etiology), in the treatment and prevention of obesity, lactose intolerance, diabetes, osteoporosis, and cardiovascular diseases. An example of the positive effects of fermented foods on the intestinal al microbiota is alleviation of symptoms of irritable bowel syndrome resulting from the consumption of fermented probiotic milk containing Bifidobacterium lactis CNCM I-2494 (Marteau et al., 2013). Studies have also confirmed an improvement in gastrointestinal passage and a decrease in common complaints in the human population, such as bloating and flatulence. This may be related to changes in the expression of bacterial genes that encode enzymes involved, among others, in carbohydrate metabolism (Agrawal et al., 2009; McNulty et al., 2011). The importance of probiotics in enhancing non-specific and specific immunity (modulation of the host immune response) is also highlighted. Probiotic bacteria stimulate the mucosa-associated lymphoid tissue (MALT) immune system, formed, among others, by gut-associated lymphoid tissue (GALT) immune elements. Due to the production of chemokines, cytokines, growth factors, or immunoglobulins, MALT acts as a microbial fighter. Furthermore, probiotics influence the balance of the gut microbiome composition, reducing the risk of disease gut (Tokarz-Deptuła and Deptuła, 2017; Azad et al., 2018; Li et al., 2019; Uusitupa et al., 2020; Zhang et al., 2021). As microbes are able to produce neurochemicals, as well as respond to them, they can play a crucial role in the treatment of depressive and anxiety disorders (Romijn et al., 2017). Furthermore, the consumption of fermented products has a positive impact on the oral microbiota. The functional bacteria in the food reduce tooth decay, gum disease, and oral inflammation by lowering pH and producing antioxidants that inhibit plaque growth. They are also used in the treatment of halitosis, as they metabolize volatile sulfur compounds the source of unpleasant mouth odor (Gungor et al., 2015; Voidarou et al., 2020).
FIGURE 1
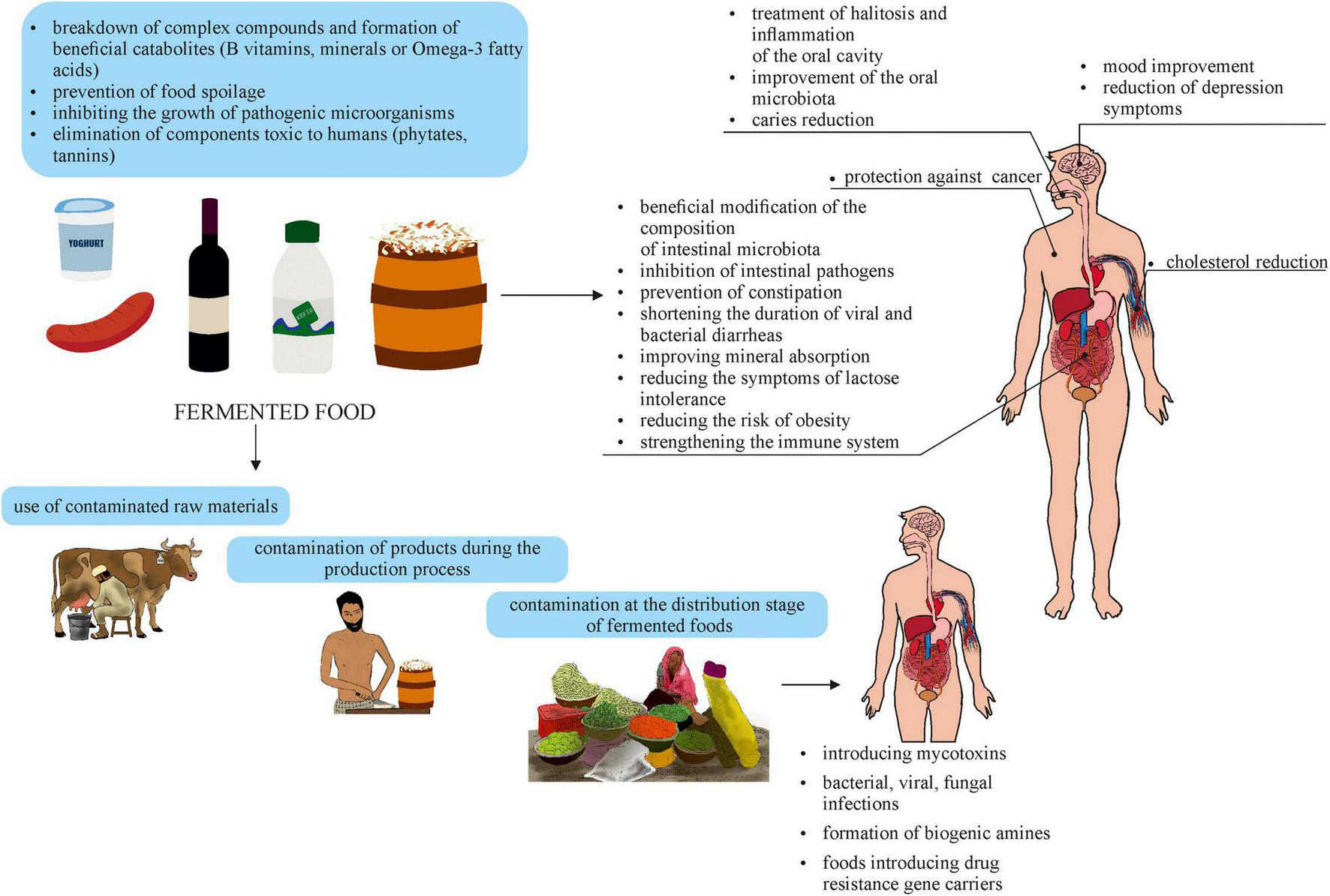
Effects of consuming fermented foods.
The microorganisms present in fermented products with high titers can interact with microorganisms that inhabit the digestive tract and colonize it temporarily or permanently (Davoren et al., 2019; Nemska et al., 2019; Roselli et al., 2021). Currently, due to the high degree of the variability of studies (heterogeneity of study design and methods used), and interindividual variability in the composition of the gastrointestinal microbiota, there is insufficient evidence for permanent colonization of the human gut by food microorganisms. However, the transient colonization shown in some cases indicates the need for permanent introduction of fermented products into the diet to maintain the positive effects of strains on the human body (Roselli et al., 2021).
The fermentation process is generally carried out to obtain a nutrient-enhanced product. However, in some cases, the overriding purpose is to prevent food spoilage. The metabolites produced by microorganisms (lactic acid, acetic acid, hydrogen peroxide, ethanol, compounds with antagonistic properties to other microorganisms) inhibit the growth of pathogenic microorganisms or those responsible for food spoilage. Fermented food is of great importance in low- and middle-income countries (subregions of Africa and Southeast Asia), which typically lack access to refrigeration facilities. In countries where the interplay of the dry season and the growing season results in a lack of availability of fresh food, preservation is a necessary solution to protect the population from starvation. The advantage of fermentation is also the ability to eliminate various types of toxic components present in raw materials, such as polyphenols (e.g., pachyrrhizine, rotenone, catechin derivatives), phytates, and tannins (Montagnac et al., 2009; Lautié et al., 2013). An example is the reduction of up to 95% of lectins and other toxic components in tempe produced from soybeans (Uzogara et al., 1990; Evans et al., 2013). It is possible to eat fermented products that would not be suitable for consumption without proper preparation (e.g., cassava, due to its cyanogen content) (Agbor-Egbe and Mbome, 2006). The increased digestibility of vegetable protein, by its partial breakdown, decreases the risk of food allergies and gastrointestinal disorders. An additional advantage of fermented products is their reduced mass, compared to the initial raw material, resulting from the processes (e.g., grating, soaking, squeezing) it undergoes before fermentation. This facilitates the transport of products which, especially in developing countries, is at a low level. In addition, the heat treatment time relative to the cooking time of the raw substrate is reduced (Uzogara et al., 1990; Nkhata et al., 2018).
Fermentation as a food processing technique can influence the level of mycotoxins in food. Mycotoxins pose a serious threat to human health due to their demonstrated carcinogenic, mutagenic, nephrogenic, hepato-, cytotoxic, neurotoxic, and teratogenic effects, and induction of immunosuppression. Toxins detected in foods produced by genera such as Aspergillus, Penicillium, and Fusarium include, among others, ochratoxin A, aflatoxins, zearalenone, and trichothecenes (Raiola et al., 2015; Ostry et al., 2017; Pei et al., 2021). Many strains of LAB producing antifungal metabolites (lactic acid, phenyllactic acid, hydroxyphenyllactic acid, indole, bioactive peptides) can reduce both fungal growth and mycotoxin synthesis. In addition to the inhibitory effect of bacterial organic compounds, antifungal activity may also be related to competition for the occupied niche and nutrients needed for growth. Modification of the external environment is also important here, as well as the binding of mycotoxins by components of the cell wall (polysaccharides, peptidoglycans) of bacteria. The species for which such properties have been demonstrated include strains of the genus Lactobacillus (e.g., L. rossiae, L. fermentum L. sanfranciscensis), as well as other bacterial genera such as Bifidobacterium, Lactococcus, Pediococcus (Valerio et al., 2009; Adedokun et al., 2016; Luz et al., 2017; Aarti et al., 2018; Guimarães et al., 2018; Khattab et al., 2018; Sivamaruthi et al., 2018; Sadiq et al., 2019).
Consumption of fermented foods also alleviate the severity of symptoms of COVID-19. This is due to the lactobacilli that are potent activators of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a major regulator of the cellular oxidative stress response (Bousquet et al., 2021). However, more this correlation merits further studies.
Problems
External and internal factors affect the growth capacity of pathogenic microorganisms in fermented foods (Figure 1). The risk of obtaining a contaminated fermented product increases when low-quality ingredients are used for its production, initially containing a sufficiently high number of bacteria, fungi, or toxins produced by them. An example is the pork used to make nem chua, a traditional raw sausage eaten in Vietnam after a short spontaneous fermentation process. Research by Le et al. (2012) showed repeatedly exceeded the level of microbiological contamination in meat intended for nem chua production. The presence of Escherichia coli and Staphylococcus aureus detected in the raw material did not meet the requirements for hygiene and safety. In countries with high poverty, raw materials of better quality are used mainly for export, as they are the primary source of income. On the contrary, secondary crops are used in household or small-scale food production, resulting in products of inappropriate microbiological standards.
The water used in the dilution stage or in the fermentation itself should also be free from microbiological contamination. Unfortunately, limited access to water in some regions, especially in rural areas and the use of potentially contaminated water from streams or rivers for production, increases the risk E. coli and Salmonella spp. presence in food (Anyogu et al., 2021).
In developing countries, the lack of Good Manufacturing Practices (GMPs), has a major impact on the safety of traditional, home-made, or cottage-made food (Oguntoyinbo, 2014). Their sale under unsanitary conditions without the use of protective coverings, such as gloves, is also a public health risk. Moreover, in developing countries, due to poverty and low consumer awareness, fermented foods sold locally are usually packaged in non-sterile utensils, used jute bags, or paper (e.g., newspaper), as well as gourds, or leaves. The inability to buy adequate packaging to limit microbial spoilage, even with a properly executed production process, poses a significant additional risk of food contamination (Oguntoyinbo, 2014).
Despite the positive impact of fermented products on human health, there is a risk that their consumption can introduce into the body carriers of antibiotic and chemotherapeutic resistance genes, leading to the selection of multidrug resistant strains responsible for infections that are difficult to treat. This selection also occurs as a result of the overuse of antibiotics in agriculture and livestock farming. Bacterial resistance mechanisms can be generated by the presence of drugs or their residues in food products at concentrations below the minimum inhibitory concentration (MIC). Due to horizontal gene transfer (transformation, conjugation, or transduction), there is a risk of spreading drug resistance among gastrointestinal microorganisms or foodborne pathogens (Figure 2; Tóth et al., 2020, 2021; Miranda et al., 2021). Therefore, in the case of fermented food production, in addition to the prudent use of antibiotics by breeders and farmers, it is important to use strains that do not contain drug resistance genes in their genetic material or to monitor the content of these genes in starter cultures and manufactured products.
FIGURE 2
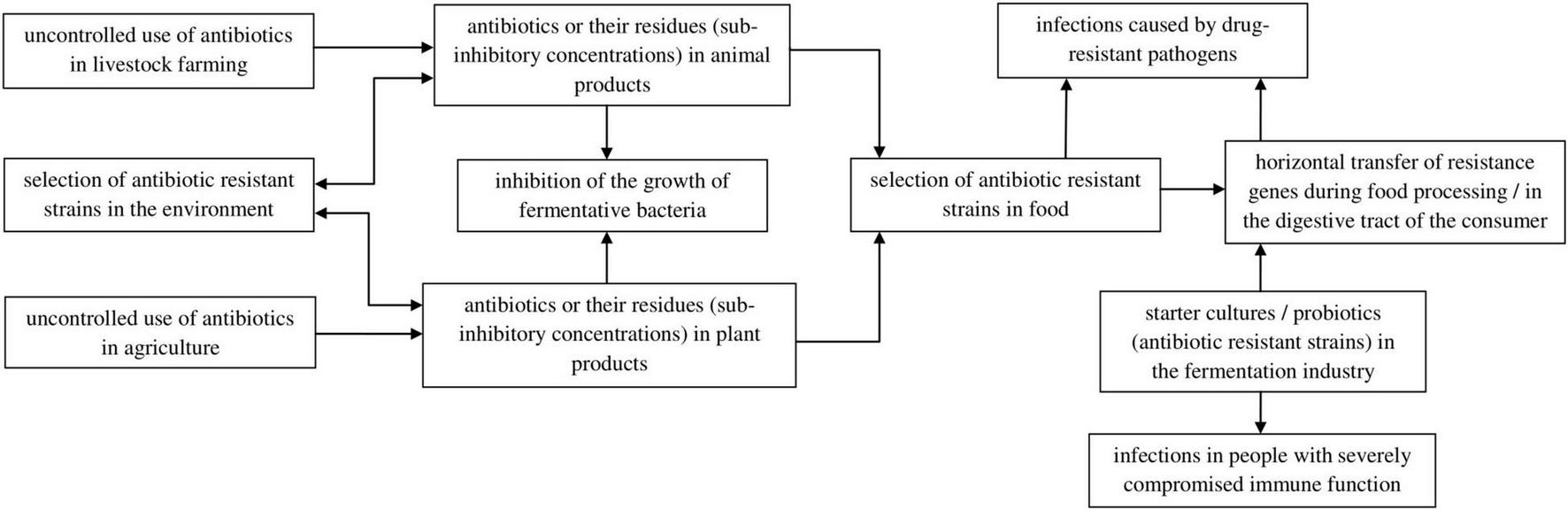
Factors responsible for strain resistance to antibiotics in food and its effect.
The negative aspects of fermented foods also include biogenic amines (BAs) (Figure 3), which are formed during fermentation with both microorganisms naturally present in the raw material and with the participation of starter cultures. BAs are a product of amino acid decarboxylation, amination of aldehydes or ketones, or their transamination, and therefore their formation is influenced not only by the type of microorganism that performs these processes, but also by the composition of the raw material (e.g., free amino acid content), the fermentation time, and the conditions under which food processing takes place. Due to the higher accumulation of BAs in products that do not meet microbiological standards, it is believed that the amounts of these compounds may reflect the degree of spoilage (Doeun et al., 2017; Xiang et al., 2019). In order to reduce the BA content, it is necessary to use microorganisms or processes leading to the degradation of these potentially toxic compounds in food production. However, the main activities are to control the quality of the raw materials that undergo fermentation and its conditions, but they are not always carried out.
FIGURE 3
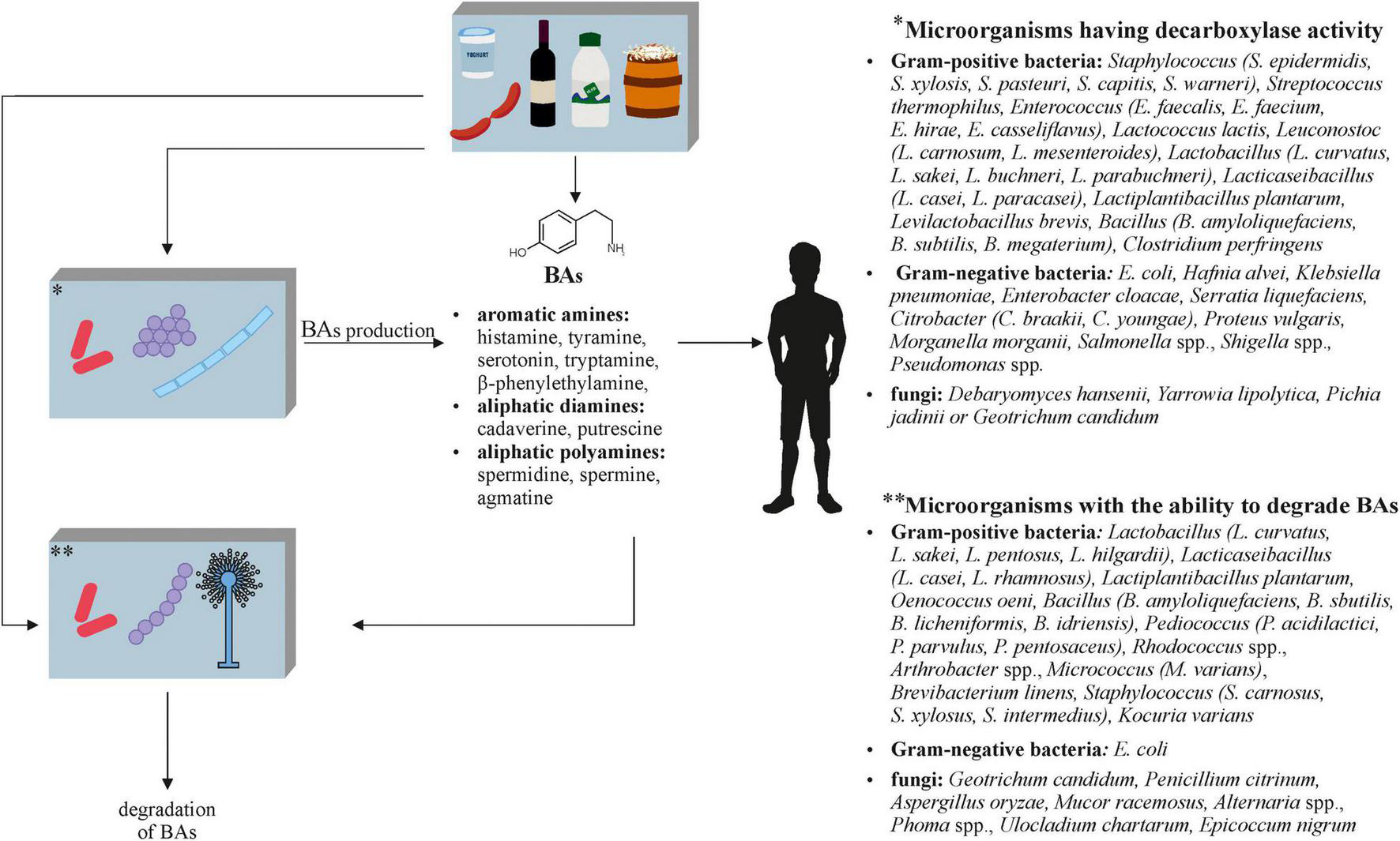
The role of microorganisms in the metabolism of biogenic amines (BAs) in fermented foods (Smith, 1980; Latorre-Moratalla et al., 2010; Lorenzo et al., 2010; Spano et al., 2010; Cueva et al., 2012; Alvarez and Moreno-Arribas, 2014; De Mey et al., 2014; Zaman et al., 2014; Ladero et al., 2015; Ordóñez et al., 2016; Sun et al., 2016; Pugin et al., 2017; Ekici and Omer, 2018; Li et al., 2018; Barbieri et al., 2019; Mah et al., 2019; Park et al., 2019; Ruiz-Capillas and Herrero, 2019).
Adverse health effects associated with the consumption of fermented foods can include isolated illnesses, outbreaks, and even deaths. Among other things, due to the mycotoxins present in some products, long-term consequences are also possible in the form of diseases of the gastrointestinal tract, kidney stones, or cancer (Singh et al., 1986; Phukan et al., 2006; Gajamer and Tiwari, 2014; Keisam et al., 2019). The actual number of infections and outbreaks resulting from the consumption of fermented foods is probably underestimated. In developed countries, difficulties in detecting outbreaks that may originate from a variety of food products are associated with incomplete epidemiological data (mild cases of infection are not reported or documented) and the frequent lack of information exchange between diagnostic laboratories. This leads to delays in investigations and the inability to confirm hypotheses about a potential source of infection.
The composition of some fermented products may also cause some minor health disadvantages. An example of a product of this type can be kombucha made from tea and sugar. Its consumption can lead to excess sugar and calorie intake, which may also lead to bloating and gas.
Dairy Products
Many food products, such as yogurts, kefirs, and cheese, are obtained from milk, usually cow’s milk, as a result of the fermentation process. Due to the diversity of the microbiome, various fermentation routes can be carried out, which leads to the production of thousands of final products (such as cheeses), differing in consistency, taste, and aroma (Voidarou et al., 2020).
Raw and fermented camel milk, which has high nutritional value, is consumed by the populations of Asian and African countries. It is considered a product with therapeutic properties because of the immunoglobulins, lysozyme, and lactoferrin content, which have antimicrobial activity. In some regions, it is used to treat diseases such as chronic hepatitis (Saltanat et al., 2009; Sharma and Singh, 2014). Suusac, consumed by the people of the arid and semi-arid areas of Kenya, is obtained from the spontaneous fermentation of fresh unpasteurized milk in cleaned smoke-treated gourds (Lore et al., 2005). A study by Maitha et al. (2019) on milk samples collected from different areas of northeastern Kenya found that suusac consumption also poses health risks to consumers. Researchers found E. coli in all samples analyzed, while Shigella spp. and Klebsiella spp. were present in 88.1 and 77.4% of the samples, respectively. Furthermore, bacteria of the species S. aureus, which are often responsible for animal udder infections, including camels, were detected in more than half of the samples. Another study reported on the detection of Mycobacterium strains other than tuberculosis (MOTT) (Mycobacterium avium, M. intracellulare, M. kansasii, M. malmoense) in suusac (8.2% of positive samples) (Mwangi et al., 2017). The authors of the above study also highlighted the problem related to the widespread distribution of Brucella spp. rods in dairy products in African countries. They showed that the risk of these microorganisms increases from 14 to 26% in suusac production. The ability of some Brucella species to survive at 4°C and at pH below 4.0 indicates the need for vaccination of livestock against brucellosis (Zúñiga Estrada et al., 2005; Dadar et al., 2019). Pasteurization of milk before suusac production or heat treatment before consumption of the finished product is also crucial to consumer health. Also, Iran by Yam et al. (2014) conducted a research aimed at determining the microbiological quality of fermented camel milk, chalout in (2014). Chal is obtained, like suusac, by spontaneous fermentation, carried out in leather bags or bottles. In samples collected in the province of Golestan, Staphylococcus spp. and rods from the Enterobacterales order were identified, while no bacteria of the genus Salmonella and Shigella were detected.
In addition to mastitis, poor hygiene of the milking personnel, the milking environment (milking is usually conducted in the open areas without prior washing and disinfection of the teats), and the fermentation tanks also contribute to milk contamination. The quality of the water used for washing is important, as it should be drinking water quality. Greater risk of infection poses a cheap product, kept in poor sales conditions. Fermented milk can be a particularly significant source of infection, as it is often consumed without heat treatment.
Among the microorganisms present in fermented milk are strains of E. coli. A study by Yakubu et al. (2018) showed the presence of the most pathogenic serotype E. coli O157: H7 in samples of this product from 100 points of sale in the area of Nigeria. The main source of E. coli strains, including those with a high pathogenic potential, is the gastrointestinal tract of cattle (animals do not show clinical signs of infection). These microorganisms, excreted in the feces, can contaminate milk, especially if premilking hygiene is not practiced (Leedom, 2006).
Many other studies have confirmed the contamination of products obtained by cow’s milk fermentation. An example is roub, a drink obtained by inoculating milk with starter culture from the previous day’s fermentation. It is drunk when diluted with water or added to make a soup to be eaten with pudding. In studies conducted on traditionally prepared roub samples collected in three regions of Sudan, a high level of contamination and the presence of S. aureus and coliform bacteria were demonstrated (Abdalla and Hussain, 2010). This confirms previously reported results (Abdalla and El Zubeir, 2006). Dehkordi et al. (2014) assessing the presence of shiga toxin-producing E. coli strains in traditionally produced dairy products (yogurt, doogh, and kashk) sold in supermarkets or retailers in Iran. The characteristics of the strains isolated from more than 8% of the products showed diversity in terms of serogroups, among which the serogroup reported the most frequently was O157 (26%) and O26 (12%). The relatively high frequency of strains responsible for various forms of infection, including bloody and non-bloody diarrhea or hemolytic uremic syndrome (HUS), may indicate failure to maintain the appropriate temperature or time parameters at the dairy production stage. It may also result from the use of contaminated water by the local population, who do not have access to water that meets microbiological requirements.
Nahidul-Islam et al. (2018) analyzed traditional hand-made fermented dairy products (dahi, chanar-misti, paneer, and borhani), produced in India but also popular in other Middle East to South East Asian countries. Their research with the use of pyrosequencing showed the presence in food, in addition to the dominant LAB (Lactobacillus spp. and Streptococcus spp.), differently numerically (depending on the product) bacteria, including Acinetobacter spp. and Pseudomonas spp., rods from Enterobacterales, as well as fungi of the genus Aspergillus. The probable sources of these microorganisms are skin, bovine intestine, and mastitis.
Ras and karish cheese showed significant contamination with E. coli strains (8–21.7% and 74.5%, respectively) (Ombarak et al., 2016; Hegab et al., 2020). These cheeses are consumed in Egypt, a country where fresh milk and products thereof are important components of the daily diet. The cheeses are usually obtained in artisanal rural areas from raw cow’s milk or a mixture of cow’s and buffalo milk. Fermentation and ripening, which take 3–8 months, usually involve only microorganisms that are native to the milk microbiota. E. coli strains isolated from cheese possessed different pathogenic potential. Some strains, were responsible for gastroenteritis. The most common source of these microorganisms in raw milk and dairy products derived from it is feces that contaminates the milk at the milking stage. Hegab et al. (2020) also noted S. aureus strains in 26% of ras cheese samples, 15% of which were enterotoxigenic (demonstrated presence of seb and sed genes). Abdel-Hameid Ahmed et al. (2019) found an even higher percentage (50%) of enterotoxin producing S. aureus strains isolated from this type of cheese. The above results may indicate poor hygiene of people involved in the production of cheese.
In developing countries, pathogens that have been largely eliminated in other parts of the world may also be present in fermented dairy products. Examples include the previously mentioned Brucella spp., or MOTT, found in milk. Based on a study by Michel et al. (2015), Mycobacterium bovis, an etiological agent of bovine tuberculosis, can survive in souring cow’s milk. This species is isolated in Africa from unpasteurized milk, but data on the incidence of the disease in humans are insufficient to conclude how high the risk of consuming contaminated products with M. bovis is. Inappropriate veterinary control of livestock in some countries is certainly a significant cause of approximately 70,000 cases of zoonotic tuberculosis reported annually in Africa (Olea-Popelka et al., 2017; Owusu-Kwarteng et al., 2020).
Despite the presence of many viruses in food, infections of this etiology caused by the consumption of contaminated fermented products are recorded less frequently than bacterial infections. This is due to the fact that viruses present in food are primarily bacteriophages and yeast-infecting viruses (Pringsulaka et al., 2011; Kleppen et al., 2012). The low number of these microorganisms in food products and the greater difficulty of detecting them may also be a reason. Foodborne viruses include hepatitis A and E virus, noroviruses, and rotaviruses (Maske et al., 2021). In 2016, two cases of tick-borne encephalitis were reported in Germany after consumption of unpasteurized goat cheese (Brockmann et al., 2018). Infections caused by tick-borne encephalitis virus transmitted by this type of cheese, although rarely, have also been found in other countries of Central and Eastern Europe (Croatia, Czech Republic, Austria) (Holzmann et al., 2009; Kríz et al., 2009; Markovinović et al., 2016). The work of Rehfeld et al. (2017) suggests that in Brazil, where cow’s milk contamination with the Vaccinia virus (VACV) is noted, consumption of artisanal cheese from unpasteurized milk may also lead to illnesses of this etiology. The route of transmission of SARS-CoV-2 through food consumption has not been confirmed, but is considered unlikely (Center for Disease Control and Infection, 2020).
Products of Plant Origin
Various substrates of plant origin, such as cereals, oil seeds, nuts, roots, tubers, and plant juice, are fermented. Some of them are an important and inexpensive source of protein, which provides energy for the body. Additionally, the breakdown of proteins into amino acids during the fermentation process increases the digestibility of the product.
Sorghum is the second most commonly grown cereal in sub-Saharan Africa, thanks to its tolerance to drought, its ability to grow under harsh conditions, and its nutritional value (high starch content, among other things). Due to its benefits, it is an ingredient in the main meals consumed by the inhabitants of Africa. In addition, it is an important component of the diet of people with gluten intolerance. However, a fermentation process is required to convert the plant into an edible form (Odunmbaku et al., 2017; Adebo, 2020). The most common fermentation process in sorghum is lactic fermentation, in which primarily LAB participate, although certain fermented products involves also fungi. One of the traditionally produced sorghum-based products (or millet) is obushera. Due to its widespread use (in weaning, as a thirst-quenching drink and as a source of energy), its production is a source of income for households, but it is also being commercialized. However, obushera sold on the market does not always meet microbiological requirements. In the study by Byakika et al. (2019), despite the absence of Salmonella spp. rods in the samples tested, most did not meet the standards for coliforms and Staphylococcus spp. Microbiological contamination could result from the use of poor quality raw materials in production, but also by the lack of pasteurization processes.
Adedeji et al. (2017) and Ademola et al. (2018) conducted studies on iru and ogiri, traditional food condiments used in Nigeria and some parts of West Africa. They are popular due to the aromas and flavors, resulting from the primary and secondary metabolites of microorganisms produced during fermentation, and their high protein content, which is of a particular importance in the poor regions of the country. The first of these condiments is obtained from carob seeds [African locust bean (Parkia biglobosa) seeds], while the raw materials for ogiri may be melon seeds or castor bean seeds. The production of condiments is preceded by spontaneous fermentation of seeds, usually carried out at the household level. Previously conducted analysis of these products, using classical methods of bacterial identification, based on their biochemical characteristics, showed the presence of potentially pathogenic bacteria, such as Bacillus cereus, Bacillus subtilis, S. aureus, E. coli, Proteus spp., Pseudomonas spp. (Falegan, 2011; Ajayi, 2014). Adedeji et al. (2017) and Ademola et al. (2018) extended the research to include genotyping methods, confirming the participation of various microorganisms in condiments, representing both actual fermenters and undesirable species introduced at different stages of production. Ademola et al. (2018), analyzing the dynamics of the bacterial population during the production of iru and ogiri, showed that species belonging to the genera Bacillus (B. encimensis and B. safensis), Enterococcus (E. dispar), and Lysinibacillus are present at almost every stage of spice processing. Therefore, they can be potential starters in the fermentation process. Other microorganisms, including potential pathogens, detected only at certain stages of processing are evidence of poor hygiene practices leading to contamination of foods that play an important role in the diets of rural populations in poor countries.
Douchi is a Chinese condiment obtained from black beans. In its production, strains of Aspergillus spp., Mucor spp. or B. subtilis are used, carrying out the first fermentation process, while the second process is an anaerobic spontaneous fermentation, with the participation of bacteria and fungi (Zhang and Liu, 2000). Douchi was the cause of an outbreak of food poisoning that occurred in Kunming, China (Zhou et al., 2014). The symptoms of food poisoning that occurred in 139 people who consumed a dish containing douchi were caused by cereulide or Nhe enterotoxin produced by strains of B. cereus. However, this was not the first case of douchi-related food poisoning reported in China. A two other previously reported outbreaks, B. cereus strains were also the etiological agent (Shen et al., 2005; Liu et al., 2006).
Species such as B. cereus, Clostridium botulinum, Proteus mirabilis, and E. coli have been detected in fermented soy products. Keisam et al. (2019) carried out analysis of such products sold in the northeastern region of India was using the next generation sequencing technique (MiSeq) combined with qPCR and immunoassays. The first two species mentioned were found in all samples taken for testing in the amount of > 107 cells/g. In addition, diarrheal or emetic toxins (hemolysin BL (HBL) and non-hemolytic enterotoxin (NHE) were detected in all isolated strains of B. cereus, while cereulide (an emetic toxin) was detected in less than half. In turn, the strains of P. mirabilis produced hemolysins, urease, and also showed multidrug resistance. The common presence of these intestinal rods in fermented foods could explain the high percentage of cases of urolithiasis in India.
The contamination of fermented soybean products with B. cereus strains is also a significant problem in Korea, where these products are common in the daily diet. Doenjang, kochujang, meju, or cho-kochujang are obtained by natural fermentation, either at home or with factory-made starter cultures. Its pro-health properties, such as its anticancer effect, has led to an increase in interest and frequency of its consumption not only among Koreans, but also among people around the world (Jung et al., 2006; Lee N. et al., 2017). However, numerous studies have confirmed the presence of strains of B. cereus in such products (Kim et al., 2015; Yim et al., 2015; Park et al., 2016; Lee N. et al., 2017). Although the detection rate of this bacilli species, as well as the level of product contamination, were varied (not always exceeding the acceptable standards of the Korean Food and Drug Administration), the NHE and/or HBL toxins detected in the strains indicate the need to monitor the level of B. cereus contamination in soybean products.
In South Korea, two outbreaks of gastroenteritis caused by enterotoxigenic E. coli (ETEC) O6 strains were reported in 2013–2014 (Shin et al., 2016). The first included 167 children attending a middle school in the Jeollanam-do province, and the other involved 1,022 cases in 10 schools in the Incheon Provence. Kimchi was suspected to be a carrier of pathogenic strains. This traditional Korean dish is prepared by fermenting various types of vegetables, the most popular of which is cabbage. The product is consumed about 1 week to several months after its preparation and contains beneficial bacteria that carry out the fermentation process, mainly from the genera Leuconostoc, Lactobacillus, and Weissella. Epidemiological and laboratory investigations reported ETEC O6 strain in kimchi prepared from cabbage in the school canteen and young radish prepared by a food company. A potential carrier of the bacteria could be poor quality water used for food preparation. Both, too short fermentation process and established environmental conditions may allow pathogens to grow of intestinal pathogen. Furthermore, excessive pH does not inhibit the secretion of the heat-labile (LT) toxin responsible for diarrhea by ETEC strains, as shown in a study by Gonzales et al. (2013). Kimchi prepared from cabbage or radish for the canteens of 7 schools in Incheon, Korea, was also likely the cause of a previous large outbreak of 1,642 cases of enteritis (Cho et al., 2014). Retrospective cohort studies carried out allowed the isolation of ETEC O169 strains indistinguishable in pulsed field gel electrophoresis (PFGE) from 230 students and kimchi produced by one food company. However, the presence of these pathogens was not found in raw vegetables or other food products. The reported outbreaks are evidence that not only homemade fermented foods, but also foods produced by food companies, may pose a risk of infection for the consumer. Hence, there is a need for continuous monitoring of food safety and the need to define precise criteria for the production process in food establishments. This is especially important in the case of a wide distribution of products due to the risk of causing a large outbreak in a relatively short period of time.
The concentration of mycotoxins depends on their initial content in the raw material. Therefore, in low-income countries, due to the frequent use of low-quality cereals, mycotoxins are found in products obtained from fermented crops. The presence of fungi producing toxic metabolites is influenced by the timely failure to clean crops from the soil, as the well as long-term and improper (at high humidity) storage of grains, especially crops without hulls. Also, crops damage during harvesting or storage increases the risk of mycotoxins in raw material. Favorable environmental conditions, such as temperature or water activity, are important factors that contribute to mycotoxins production (Milani and Maleki, 2014; Viaro et al., 2017). The risk of consuming fermented products prepared from raw material contaminated with mycotoxins is high, even with previous heat treatment, due to the stability of these toxins at processing and cooking temperatures.
People’s awareness, mainly in developing countries, of the food contamination risk with fungi and mycotoxins is low (Siegrist and Cvetkovich, 2001; Ezekiel et al., 2013; Matumba et al., 2016; Adekoya et al., 2017). Therefore, few activities, such as sorting moldy seeds or proper drying, are undertaken to reduce the contamination of raw materials used in food production, as well as the contamination that can occur during its preparation or storage. Ignorance or neglect by sellers, in the turn, results in mixing products that meet microbiological requirements with moldy food. Mycotoxins are often found in various types of products typically consumed in African countries, produced based on spontaneous fermentation or small-scale rural processing. In a study by Adekoya et al. (2017), more than 80% of the samples analyzed of foods produced in Nigeria and obtained by fermentation of raw materials such as melon seed (Citrullus colocynthis), oil bean seed (Pentaclethra macrophylla), maize or sorghum contained single toxins or combinations thereof. Some of them (fumonisin, aflatoxin, ochratoxin A, and zearalenone) exceeded the limit specified by the European Commission.
In China, fermented pastes, made from soybeans, broad beans, flour, and chili, are of great interest. Contamination with fungi and the formation of mycotoxins often occur during the cultivation stage if the species of fungi are compatible with the crop. However, studies have shown that mycotoxin production in this type of food can also occur during a long fermentation process (Shukla et al., 2014). Aflatoxins (present in soybean pods and seeds) and ochratoxins (present in wheat and soybeans) are frequently detected in fermented pastes (Zhao et al., 2020). The presence of aflatoxins was also found in soybean sauces obtained with the participation of Aspergillus orizae (Vu and Nguyen, 2016).
In Korea in 2013, kimchi produced by a food company was the source of acute gastroenteritis outbreaks caused by the GI.4 human norovirus (HNoV) genotype (Park et al., 2015). The analysis showed that the kimchi was probably contaminated with groundwater used by the company during the production stage. Spreading the product before the completion of the fermentation process may not have lowered the pH sufficiently, with the result that HNoV, which is resistant to pH > 5, was able to survive in kimchi. Research by Lee H. M. et al. (2017) also showed that despite the reduction of the HNoV titer in experimentally contaminated cabbage kimchi, the fermentation conditions (acidity, salinity, organic acid content) may be an insufficient factor to eliminate this virus.
Meat Products
Due to the high likelihood of pathogens in raw meat, the risk of infection after consumption of fermented meats not heat treated prior to consumption is high. The addition of nitrite to meat helps reduce the growth of microorganisms such as C. botulinum, S. aureus, L. monocytogenes, and Salmonella spp. (Hospital et al., 2016; Majou and Christieans, 2018). However, since these compounds are an important risk factor for colorectal cancer, there is a trend toward eliminating them from the meat industry and introducing alternative preservation methods. This, however, may lead to a lower microbiological safety of the product (Christieans et al., 2018; Gonzalez-Fandos et al., 2021).
One pathogen most frequently reported responsible for foodborne disease outbreaks associated with meat products is Salmonella spp. (Patarata et al., 2020). In Italy, an outbreak involving 79 cases occurred in 2009–2010, caused by the Goldcoast Salmonella enterica serotype (Scavia et al., 2013). It is believed that its source could have been salami, dry, fermented sausages. Nonetheless, a delayed investigation, carried out after the peak of the outbreak, did not allow to confirm this suspicion. Stool samples collected from the patients for diagnostic testing were too small. It was also difficult to take samples of suspect food products and examine the trace-back activity of food, especially in the case of salami, produced from the meat of various species of animals. Furthermore, various typing methods were used in different laboratories. Despite low pH and water activity, as well as high salinity, Salmonella may remain in salami due to the too short fermentation period. The salami production process reduces significantly Salmonella spp. levels but the scale of reduction with high primary meat contamination may be insufficient. Cases of increased infections (60 cases of diarrhea, abdominal cramps, or fever) of Salmonella Goldcoast etiology were reported at a similar time in Hungary, where contaminated pork was the likely source (Horváth et al., 2013). In turn, raw pork and fermented raw pork sausage, Zwiebelmettwurst, were the likely vehicle for the transmission of Salmonella enterica serovar Bovismorbificans during the German outbreak (Gilsdorf et al., 2005). At that time, 525 cases of gastroenteritis were reported, with one death.
Dry fermented sausages are highly diversified, influenced, among others, by the degree of grinding meat and fat, its acidity, or the presence of mold on the surface (Meloni, 2015). Although listeriosis outbreaks associated with the consumption of these types of meat are rarely reported (Meloni, 2019), L. monocytogenes is relatively frequently detected in final products, demonstrating the ability of these bacteria to overcome barriers at the production stage. The source of L. monocytogenes in fermented sausages can be raw meat, the slaughterhouse environment, or people in contact with the raw material unprocessed or after processing. Resistance to disinfectants and the ability to form biofilms on various types of surfaces increase the risk of contamination of ready-to-eat (RTE) products (Martin et al., 2011; Meloni et al., 2014; Meloni, 2015).
Díez and Patarata (2013) studied the pathogen’s survival during the production of chouriço, a dry fermented sausage. This product, which is produced both on a farm scale (using natural fermentation) and on an industrial scale (fermentation carried out under controlled conditions, using starter cultures), differs not only in the spices added, but also in the smoking, drying, and maturation times, which last between 1 and 4 weeks. An important role in meat preservation plays the amount of salt, which reduction for health reasons in recent years simultaneously decreases the microbiological safety of the product. The provocation tests carried out by Díez and Patarata (2013) showed that although the levels of L. monocytogenes, S. aureus, and Salmonella spp. were reduced in the early stages of drying, all pathogens were undetectable in chouriço after a longer period (30 days) of this process. In addition to salt concentration, a sufficiently high glucose content, the addition of antimicrobial compounds, or a low pH affect the survival of microorganisms (Piras et al., 2019). The conditions under which fermentation occurs are also important. The fermentation temperature and postproduction processing, including storage temperature and time, as well as product freezing and thawing processes, are crucial in reducing the content of toxigenic strains of E. coli (Heir et al., 2013; McLeod et al., 2016; Shane et al., 2018). Therefore, to ensure the microbiological safety of fermented meat, while maintaining its taste, it is necessary to optimize the ingredients added to it and the process parameters.
Lee H. S. et al. (2017) have suggested that feeding animals with feed contaminated with mycotoxins can contaminate meat products. The research was carried out in Vietnam, where the tropical climate (high temperature and humidity) is particularly conducive to fungal growth in a variety of agricultural products, including pig feed products. Evaluation of exposure of pigs to aflatoxins has shown their presence in the urine of animals, suggesting a risk of toxins’ presence in pork meat. However, the authors did not conduct any research in this regard.
Fermented meat products can also be a source of hepatitis E virus (HEV) infection, which is transmitted through meat from infected pigs or wild boar. In fact, a study by Wolff et al. (2020b) showed that despite the inhibitory effect of an acidic environment on many microorganisms, HEV shows a minimal decrease in infectivity at pH 2. Other studies by Wolff et al. (2020a) indicate that high salt concentrations that are usually applied for fermented raw sausages are also not sufficient to limit HEV survival in food products. Furthermore, an assessment of the prevalence of HEV genotype 3 in RTE products containing raw meat and originating from the Swiss retail market showed the presence of genetic material of these viruses in approximately 6% of samples (Moor et al., 2018). The RNA of HEV genotype 3 was also confirmed in a study of raw sausage samples collected from retail stores in the Netherlands (14.6% of samples) (Boxman et al., 2020). The greatest risk of infection with HEV etiology occurs when consumed raw pork products contain the liver of a contaminated animal (Pavio et al., 2014). This exemplified the hepatitis E outbreak reported in France associated with the consumption of figatelli, raw pig liver sausages (Renou et al., 2014). Since there is a high risk of pork meat contamination with the virus, it would be advisable to consider the HEV test, and the obligation to report cases of hepatitis E, which is currently not mandatory in many countries. In some European countries, raw sausages do not contain a pork liver (Vignolo et al., 2010). Another option is to inform people about the risk of consuming such foods without heat treatment.
Fish Products
Fermented fish products, due to their protein content, are an important part of the diet in some countries such as Thailand, the Philippines, Cambodia and Indonesia. Salt and sometimes sun-drying are used for preservation, while microorganisms play a primarily role in developing the fish’s characteristic flavor and aroma. In Norway, rakfisk, a traditionally produced fermented fish product, is very popular. Its raw material is freshwater salmonid fish, which are stored in brine at 3–8°C for 3–12 months, during which the fermentation process takes place. The finished product does not require heat treatment before consumption and therefore microorganisms that exhibit tolerance to increased salt concentrations can pose a risk to the consumer (Skåra et al., 2015; Bjerke et al., 2019). An example of such microorganisms is L. monocytogenes, which adapts to environments with high salinity and low temperatures in which it can multiply. A study by Axelsson et al. (2020) showed that temperature and salt may not sufficiently reduce the growth of L. monocytogenes in rakfisk. Therefore, additional strategies in the production of fermented fish are necessary, such as the use of the P100 bacteriophage, which reduces the pathogen’s number and has GRAS status (generally recognized as safe) (Allende et al., 2016).
Raw oysters are one of the foods that transmit noroviruses and are responsible for several foodborne outbreaks of gastroenteritis (Le Guyader et al., 2006). The virus accumulates in the shellfish bodies during water filtration and is able to survive for a long time in oyster tissues. In South Korea and other Asian countries, raw oysters can be fermented before consumption. The process is carried out at room temperature for about 2 weeks in the presence of 5–10% salt. The HNoV outbreak, which affected 8 students at a high school in Gyeonggi Province (Cho et al., 2016), indicates that although the viral load decreases significantly during oyster fermentation, this reduction may not be sufficient to eliminate the risk of gastroenteritis, as confirmed by research by Seo et al. (2014).
Hepatitis A outbreaks that occurred in South Korea in 2019 have contributed to the research on the causal source of this disease. Jeong et al. (2021) showed that hepatitis A virus (HAV) strains were present in yogaejot, a traditional fermented food from that country that contains raw clams. Phylogenetic analysis confirmed that they are closely related to the strains prevalent in East Asia. The results obtained indicate the need for proper hygiene practices in the production stage. Furthermore, since an important source of HAV infection is fecally contaminated coastal waters where bivalve mollusks are harvested, it is necessary to improve regulations protecting against this activity.
Alcoholic Beverages
Alcoholic beverages containing more than 0.5% v/v of alcohol are made from raw materials such as cereals, vegetables and fruits, palm juice or honey (Voidarou et al., 2020).
Bacteria can develop tolerance to the acid and alcohol contained in various fermented products, as exemplified by the results obtained by Gómez-Aldapa et al. (2012). These researchers evaluated the behavior of E. coli O157: H7 during the fermentation from nectar of maguey agave plants used to make pulque, a traditional Mexican alcoholic beverage. The presence of E. coli O157: H7 strains in the pulque, although not proven, is highly probable. Although some companies industrialized the production of this beverage, the most commonly consumed is pulque made by artisans, also involved in cattle and sheep farming. These animals constitute an important reservoir of pathogenic E. coli strains and, often grazed on agave plantations, can contaminate the plant (Moxley, 2004; Varela-Hernández et al., 2007). Also importantly, E. coli O157: H7 strains can develop an adaptive response to acidic environmental conditions and survive the fermentation process (Bachrouri et al., 2006). Moreover, Gómez-Aldapa et al. (2012) showed that these bacteria can exhibit tolerance to alcohol at low pH, and thus can be present in finished pulque posing a high potential risk to the consumers’ health.
Pombe is an alcoholic beverage obtained from a variety of raw materials such as corn, millet, bananas, and pineapples (Kubo, 2016). In 2015, there was an outbreak in a village in Mazambique associated with the consumption of pombe, prepared from maize flour (Falconer et al., 2017; Gudo et al., 2018). Of the more than 230 people who developed symptoms of food poisoning and respiratory problems, 75 people died. The analysis revealed the presence of potentially lethal levels of bongkrekic acid, a potent toxin produced by Burkholderia gladioli pv. cocovenenans strains, in the collected beverage samples. This highly unsaturated tricarboxylic fatty acid can block the mitochondrial adenine nucleotide translocator (ANT) and prevent respiratory chain phosphorylation. The severity of symptoms caused by bongkrekic acid depends primarily on the amount of toxin-containing product consumed (Peng et al., 2021). Although B. cocovenenans shows sensitivity to high temperatures, the toxin itself is thermostable contributing to high mortality rate. Therefore, cooking foods containing bacteria does not protect against the effects of bongkrekic acid. Furthermore, Falconer et al. (2017) concluded from their research that the presence of Rhizopus oryzae in the raw material could enhance the toxin synthesis. Mass illnesses associated with the consumption of fermented foods containing bongkrekic acid had been reported many years earlier, in Indonesia (following the consumption of coconut-based tempeh) (van Veen, 1967) and in China (following the consumption of homemade fermented corn flour products) (Meng et al., 1988). However, many more similar outbreaks could not have been detected due to the smaller scale or the lack of research. Also in China, homemade sour soup prepared from fermented corn flour caused the death of all nine people consuming it (Yuan et al., 2020). Inadequate storage and processing conditions of raw materials, especially those rich in oleic acid, which create a good environment for the production of toxins by these bacteria may enable growth of B. cocovenenans.
A study by Scussel et al. (2013) demonstrated the presence of various types of toxins in wines randomly collected from a retail market in the Netherlands. Ochratoxin A and penicillinic acid produced by Aspergillus spp. and Penicillium spp. as well as alternariol and alternariol methyl produced by the genus Alternaria were detected in the samples tested by liquid chromatography based on tandem mass spectrometry. Primarily the region in which the grapes are grown and the associated climatic conditions affect the presence of mycotoxins in wine. The type of wine is also important because, unlike white wine, fermentation in red wines begins with the peel, which may contain mycotoxins. Therefore, the level of toxins detected by the researchers varied and depended on the type of sample tested and the country from which the wine originated. Hence, even low contamination of wine, with regular or frequent consumption, characteristic for some countries (e.g., France, Italy), due to the carcinogenic properties of mycotoxins, can pose a significant threat to human health. Also cork can be a source of toxins in wine. Treatment of corks with fungicides can reduce the risk of toxigenic fungi, but contamination can also occur after processing. A consequence of this is the transfer of mycotoxins from the cork to the wine (Centeno and Calvo, 2002).
Drinking tari, the fermented sap of the date palm, may contribute to the transmission of Nipah virus. Research in Bangladesh (Islam et al., 2016) found that this product could have been responsible for three outbreaks that occurred between 2011 and 2014 in the country. As sap collection usually consists of hanging clay vessels on the palm, into which sap drips, it can easily become contaminated with excrements and bat secretions, which are the source of the virus. Despite the sensitivity of the Nipah virus to alcohol solutions, its content (5–8%) in the sap after fermentation is too low to eliminate this microorganism from the beverage. It is important to remember that the lack of strategies to prevent sap contamination during harvest by bat droppings and secretions can also lead to the development of other diseases caused by viruses for which these animals are an important reservoir. The use of protective bamboo covers as a barrier for animals can be an effective solution.
Conclusion
The benefits of consuming fermented foods may be particularly important to people in developing countries where there is no access to probiotic. Such foods can be a source of good microbes, help reduce diarrhea, and stimulate the immune system to fight other microbes. However, despite many advantages that result from the fermentation processes with the participation of various microorganisms, especially functional ones, the lack of good production practices creates the risk of microbiological contamination of food products. This phenomenon is particularly visible in developing countries, where food processing is highly dispersed and individual, or there is no system and institutions supervising the processes of food production, including fermented ones. In this case, the risk of consuming fermented foods should be considered, especially if they are contaminated with pathogens, including viruses. The purchase and consumption of such foods by tourists poses a real risk of spreading infection around the world. Therefore, it is advisable to inform food handlers of the risks associated with the consumption of food contaminated with fungi, bacteria, the toxins they produce, and viruses. This will make individual producers want to pay more attention to the sanitary safety of the food they produce. Concerns about the quality of the raw material should also be recommended. A contaminated raw material will not produce a safe product, especially a fermented product that is made without heat treatment. Hence, it is necessary to ensure appropriate harvesting dates, pay attention to the weather during harvesting, reject batches of raw material, which visual quality deviates from the expected (serious mechanical damage, mold, discoloration, etc.), and avoid obtaining milk from animals manifesting symptoms of disease. Equally important is the concern for personal hygiene of those who work in harvesting/collecting, processing, packaging, and distributing food. For this purpose, it is necessary to increase access to clean water sources or to enable the use of portable sources. It is also crucial to ensure optimal storage conditions, especially the cleanliness of storage rooms, and to optimize its temperature. These relatively simple treatments will significantly reduce the spread of foodborne pathogens. Also, in developed countries, it is possible to improve certain procedures by strictly adhering to the HACCP system, good manufacturing and hygiene practices, and the appropriate design of food processing plants. It is also worth trying to introduce various types of innovative solutions, mainly in food packaging, but not only. Reducing the contamination of food products with mycotoxins is possible by using adsorption materials in animal husbandry or toxin-degrading microbial catalysts. Following these rules will allow producing microbiologically safe food and enjoying the benefits associated with the consumption of fermented products.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
KS, AB, and KG-B: conceptualization and supervision. AB, EW-Z, and NW-K: writing—original draft preparation. KS, KG-B, MA, EW-Z, and EG-K: writing—review and editing. KS and AB: visualization. EG-K: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This article was financed with a contest fund in the “Excellence Initiative Research University” program at Nicolaus Copernicus University in Toruń.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Aarti C. Khusro A. Varghese R. Arasu M. V. Agastian P. Al-Dhabi N. A. et al (2018). In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1.Arch. Oral Biol.8999–106. 10.1016/j.archoralbio.2018.02.014
2
Abdalla M. O. Hussain S. K. (2010). Enumeration and identification of microflora in Roub, a Sudanese traditional fermented dairy product.Br. J. Dairy Sci.130–33.
3
Abdalla W. M. El Zubeir I. E. M. (2006). Microbial hazards associated with fermented milk (Roub and Mish) processing in Sudan.Int. J. Dairy Sci.121–26.
4
Abdel-Hameid Ahmed A. Saad Maharik N. M. Valero A. Kamal S. M. (2019). Incidence of enterotoxigenic Staphylococcus aureus in milk and Egyptian artisanal dairy products.Food Control10420–27.
5
Adebo O. A. (2020). African sorghum-based fermented foods: past, current and future prospects.Nutrients12:1111. 10.3390/nu12041111
6
Adedeji B. S. Ezeokoli O. T. Ezekiel C. N. Obadina A. O. Somorin Y. M. Sulyok M. et al (2017). Bacterial species and mycotoxin contamination associated with locust bean, melon and their fermented products in south-western Nigeria.Int. J. Food Microb.25873–80. 10.1016/j.ijfoodmicro.2017.07.014
7
Adedokun E. O. Rather I. A. Bajpai V. K. Park Y. H. (2016). Biocontrol efficacy of Lactobacillus fermentum YML014 against food spoilage moulds using the tomato puree model.Front. Life Sci.964–68. 10.1080/21553769.2015.1084951
8
Adekoya I. Njobeh P. Obadina A. Chilaka C. Okoth S. De Boevre M. et al (2017). Awareness and prevalence of mycotoxin contamination in selected Nigerian fermented foods.Toxins9:363. 10.3390/toxins9110363
9
Ademola O. M. Adeyemi T. E. Ezeokoli O. T. Ayeni K. I. Obadina A. O. Somorin Y. M. et al (2018). Phylogenetic analyses of bacteria associated with the processing of iru and ogiri condiments.Lett. Appl. Microbiol.67354–362. 10.1111/lam.13040
10
Agbor-Egbe T. Mbome I. (2006). The effects of processing techniques in reducing cyanogen levels during the production of some Cameroonian cassava foods.J. Food Compost. Anal.19354–363. 10.1016/j.jfca.2005.02.004
11
Agrawal A. Houghton L. A. Morris J. Reilly B. Guyonnet D. Goupil Feuillerat N. et al (2009). Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation.Aliment. Pharmacol. Ther.29104–114. 10.1111/j.1365-2036.2008.03853.x
12
Ajayi O. A. (2014). Bacteriology and qualitative study of African locust bean (Parkia biglobosa).Open J. Soc. Sci.273–78. 10.4236/jss.2014.211010
13
Allende A. Bolton D. Chemaly M. Davies R. Escamez P. S. F. Girones R. et al (2016). Evaluation of the safety and efficacy of Listex (TM) P100 for reduction of pathogens on different ready-to-eat (RTE) food products.EFSA J.14:e04565. 10.2903/j.efsa.2016.4565
14
Alvarez M. A. Moreno-Arribas M. V. (2014). The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution.Trends Food Sci. Technol.39146–155. 10.1016/j.tifs.2014.07.007
15
Anyogu A. Olukorede A. Anumudu C. Onyeaka H. Areo E. Adewale O. et al (2021). Microorganisms and food safety risks associated with indigenous fermented foods from Africa.Food Control129:108227. 10.1016/j.foodcont.2021.108227
16
Axelsson L. Bjerke G. A. McLeod A. Berget I. Holck A. L. (2020). Growth behavior of Listeria monocytogenes in a traditional Norwegian fermented fish product (Rakfisk), and its inhibition through bacteriophage addition.Foods9:119. 10.3390/foods9020119
17
Azad M. Sarker M. Li T. Yin J. (2018). Probiotic species in the modulation of gut microbiota: an overview.Biomed Res. Int.2018:9478630. 10.1155/2018/9478630
18
Bachrouri M. Quinto E. J. Mora M. T. (2006). Kinetic parameters of Escherichia coli 0157:H7 survival during fermentation of milk and home-made yogurt.Int. Dairy J.16474–481.
19
Barbieri F. Montanari C. Gardini F. Tabanelli G. (2019). Biogenic amine production by lactic acid bacteria: a review.Foods8:17. 10.3390/foods8010017
20
Bjerke G. A. Rudi K. Avershina E. Moen B. Blom H. Axelsson L. (2019). Exploring the brine microbiota of a traditional Norwegian fermented fish product (Rakfisk) from six different producers during two consecutive seasonal productions.Foods8:72. 10.3390/foods8020072
21
Bousquet J. Anto J. M. Czarlewski W. Haahtela T. Fonseca S. C. Iaccarino G. et al (2021). Cabbage and fermented vegetables: from death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19.Allergy76735–750. 10.1111/all.14549
22
Boxman I. L. A. Jansen C. C. C. Zwartkruis-Nahuis A. J. T. Hägele G. Sosef N. P. Dirks R. A. M. (2020). Detection and quantification of hepatitis E virus RNA in ready to eat raw pork sausages in The Netherlands.Int. J. Food Microbiol.333:108791. 10.1016/j.ijfoodmicro.2020.108791
23
Brockmann S. O. Oehme R. Buckenmaier T. Beer M. Jeffery-Smith A. Spannenkrebs M. et al (2018). A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016.Euro Surveill.23:17-00336. 10.2807/1560-7917.ES.2018.23.15.17-00336
24
Byakika S. Mukisa I. M. Byaruhanga Y. B. Male D. Muyanja C. (2019). Influence of food safety knowledge, attitudes and practices of processors on microbiological quality of commercially produced traditional fermented cereal beverages, a case of Obushera in Kampala.Food Control100212–219. 10.1016/j.foodcont.2019.01.024
25
Campbell-Platt G. (1987). Fermented Foods of the World: A Dictionary and Guide.London: Butterworths.
26
Capozzi V. Fragasso M. Romaniello R. Berbegal C. Russo P. Spano G. (2017). Spontaneous food fermentations and potential risks for human health.Fermentation3:49. 10.3390/fermentation3040049
27
Centeno S. Calvo M. (2002). Mycotoxins produced by fungi isolated from wine cork stoppers.Pak. J. Nutr.1267–269. 10.3923/pjn.2002.267.269
28
Center for Disease Control and Infection (2020). Food and Coronavirus Disease 2019 (COVID-19). Available Online at: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/food-and-COVID-19.html [accessed February 4, 2022].
29
Cho H. G. Lee S. G. Lee M. Y. Hur E. S. Lee J. S. Park P. H. et al (2016). An outbreak of norovirus infection associated with fermented oyster consumption in South Korea, 2013.Epidemiol. Infect.1442759–2764. 10.1017/S0950268816000170
30
Cho S. H. Kim J. Oh K. H. Hu J. K. Seo J. Oh S. S. et al (2014). Outbreak of enterotoxigenic Escherichia coli O169 enteritis in schoolchildren associated with consumption of Kimchi, Republic of Korea, 2012.Epidemiol. Infect.142616–623. 10.1017/S0950268813001477
31
Christieans S. Picgirard L. Parafita E. Lebert A. Gregori T. (2018). Impact of reducing nitrate/nitrite levels on the behavior of Salmonella Typhimurium and Listeria monocytogenes in French dry fermented sausages.Meat Sci.137160–167. 10.1016/j.meatsci.2017.11.028
32
Cueva C. García-Ruiz A. González-Rompinelli E. Bartolome B. Martín-Álvarez P. J. Salazar O. et al (2012). Degradation of biogenic amines by vineyard ecosystem fungi. Potential use in winemaking.J. Appl. Microbiol.112672–682. 10.1111/j.1365-2672.2012.05243.x
33
Dadar M. Shahali Y. Whatmore A. M. (2019). Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention.Int. J. Food Microbiol.29239–47. 10.1016/j.ijfoodmicro.2018.12.009
34
Davoren M. J. Liu J. Castellanos J. Rodríguez-Malavé N. I. Schiestl R. H. (2019). A novel probiotic, Lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period.Gut Microbes10458–480. 10.1080/19490976.2018.1547612
35
De Mey E. De Klerck K. De Maere H. Dewulf L. Derdelinckx G. Peeters M. C. et al (2014). The occurrence of N-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation.Meat Sci.96(2 Pt A), 821–828. 10.1016/j.meatsci.2013.09.010
36
Dehkordi F. S. Yazdani F. Mozafari J. Valizadeh Y. (2014). Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products.BMC Res. Notes7:217. 10.1186/1756-0500-7-217
37
Díez J. G. Patarata L. (2013). Behavior of Salmonella spp., Listeria monocytogenes, and Staphylococcus aureus in Chouriço de Vinho, a dry fermented sausage made from wine-marinated meat.J. Food Prot.76588–594. 10.4315/0362-028X.JFP-12-212
38
Dimidi E. Cox S. R. Rossi M. Whelan K. (2019). Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease.Nutrients11:1806. 10.3390/nu11081806
39
Doeun D. Davaatseren M. Chung M. S. (2017). Biogenic amines in foods.Food Sci. Biotechnol.261463–1474. 10.1007/s10068-017-0239-3
40
Ekici K. Omer A. K. (2018). The determination of some biogenic amines in Turkish fermented sausages consumed in Van.Toxicol. Rep.5639–643. 10.1016/j.toxrep.2018.05.008
41
Evans E. Musa A. Abubakar Y. Mainuna B. (2013). “Nigerian indigenous fermented foods: processes and prospects,” in Mycotoxin and Food Safety in Developing Countries, ed.MakunH. A. (Nigeria: Intech), 153–180. 10.5772/52877
42
Ezekiel C. N. Sulyok M. Babalola D. A. Warth B. Ezekiel V. C. Krska R. (2013). Incidence and consumer awareness of toxigenic Aspergillus section Flavi and Aflatoxin B1 in peanut cake from Nigeria.Food Control30596–601. 10.1016/j.foodcont.2012.07.048
43
Falconer T. M. Kern S. E. Brzezinski J. L. Turner J. A. Boyd B. L. Litzau J. J. (2017). Identification of the potent toxin bongkrekic acid in a traditional African beverage linked to a fatal outbreak.Forensic Sci. Int.270e5–e11. 10.1016/j.forsciint.2016.10.015
44
Falegan C. (2011). Microbiology profile and biochemical characteristics of commercial “ogiri” samples from South Western Nigeria.J. Microbiol. Biotechnol. Food Sci.1187–203.
45
Gajamer V. R. Tiwari H. K. (2014). Prevalence of gastrointestinal disease and its associated risk factors in Sikkim and Darjeeling Districts.J. Community Health.39767–774.
46
García-Díez J. Saraiva C. (2021). Use of starter cultures in foods from animal origin to improve their safety.Int. J. Environ. Res. Public Health18:2544. 10.3390/ijerph18052544
47
Gilsdorf A. Jansen A. Alpers K. Dieckmann H. van Treeck U. Hauri A. M. et al (2005). A nationwide outbreak of Salmonella bovismorbificans PT24, Germany, December 2004-March 2005.Euro Surveill.10:E050324.1.
48
Gómez-Aldapa C. A. Díaz-Cruz C. A. Villarruel-López A. Del Refugio Torres-Vitela M. Rangel-Vargas E. Castro-Rosas J. (2012). Acid and alcohol tolerance of Escherichia coli O157:H7 in pulque, a typical Mexican beverage.Int. J. Food Micrbiol.15479–84. 10.1016/j.ijfoodmicro.2011.12.027
49
Gonzales L. Ali Z. B. Nygren E. Wang Z. Karlsson S. Zhu B. et al (2013). Alkaline pH Is a signal for optimal production and secretion of the heat labile toxin, LT in enterotoxigenic Escherichia coli (ETEC).PLoS One8:e74069. 10.1371/journal.pone.0074069
50
Gonzalez-Fandos E. Vazquez de Castro M. Martinez-Laorden A. (2021). Behaviour of Listeria monocytogenes and natural microflora during the manufacture of Riojano chorizo (Spanish dry cured sausage).Microorganisms9:1963. 10.3390/microorganisms9091963
51
Gudo E. S. Cook K. Kasper A. M. Vergara A. Salomão C. Oliveira F. et al (2018). Description of a mass poisoning in a rural district in Mozambique: the first documented bongkrekic acid poisoning in Africa.Clin. Infect. Dis.661400–1406. 10.1093/cid/cix1005
52
Guimarães A. Santiago A. Teixeira J. A. Venâncio A. Abrunhosa L. (2018). Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum.Int. J. Food Microbiol.26431–38. 10.1016/j.ijfoodmicro.2017.10.025
53
Gungor O. E. Kirzioglu Z. Kivanc M. (2015). Probiotics: can they be used to improve oral health?Benef. Microbes6647–656. 10.3920/BM2014.0167
54
Hegab O. W. Abdel-Latif E. F. Moawad A. A. (2020). Isolation of enterotoxigenic Staphylococcus aureus harboring seb gene and enteropathogenic Escherichia coli (serogroups O18, O114, and O125) from soft and hard artisanal cheeses in Egypt.Open Vet. J.10297–307. 10.4314/ovj.v10i3.8
55
Heir E. Holck A. L. Omer M. K. Alvseike O. Måge I. Høy M. et al (2013). Effects of post-processing treatments on sensory quality and Shiga toxigenic Escherichia coli reductions in dry-fermented sausages.Meat Sci.9447–54. 10.1016/j.meatsci.2012.12.020
56
Hesseltine C. W. Wang H. L. (1980). The importance of traditional fermented foods.Bioscience30402–404. 10.2307/1308003
57
Holzapfel W. H. (1997). Use of starter cultures in fermentation on a household scale.Food Control8241–258.
58
Holzmann H. Aberle S. W. Stiasny K. Werner P. Mischak A. Zainer B. et al (2009). Tick-borne encephalitis from eating goat cheese in a mountain region of Austria.Emerg. Infect. Dis.151671–1673. 10.3201/eid1510.090743
59
Horváth J. K. Mengel M. Krisztalovics K. Nogrady N. Pászti J. Lenglet A. et al (2013). Investigation into an unusual increase of human cases of Salmonella Goldcoast infection in Hungary in 2009.Euro Surveill.18:20422. 10.2807/ese.18.11.20422-en
60
Hospital X. F. Hierro E. Stringer S. Fernández M. (2016). A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite-reduced dry fermented sausages.Int. J. Food Microbiol.218I66–I70. 10.1016/j.ijfoodmicro.2015.11.009
61
Islam M. S. Sazzad H. M. Satter S. M. Sultana S. Hossain M. J. Hasan M. et al (2016). Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011-2014.Emerg. Infect. Dis.22664–670. 10.3201/eid2204.151747
62
Jeong H. W. Kim M. K. Yi H. J. Kim D. M. Jeon S. J. Lee H. K. et al (2021). Hepatitis A virus strains identified in jogaejeot associated with outbreaks in Seoul, South Korea.Lett. Appl. Microbiol.73107–112. 10.1111/lam.13482
63
Jung K. O. Park S. Y. Park K. Y. (2006). Longer aging time increases the anticancer and antimetastatic properties of doenjang.Nutrition22539–545. 10.1016/j.nut.2005.11.007
64
Keisam S. Tuikhar N. Ahmed G. Jeyaram K. (2019). Toxigenic and pathogenic potential of enteric bacterial pathogens prevalent in the traditional fermented foods marketed in the Northeast region of India.Int. J. Food Microbiol.29621–30. 10.1016/j.ijfoodmicro.2019.02.012
65
Khattab A. A. Ibrahim M. I. M. El-Kady A. A. (2018). Ochratoxin A biosorption onto genetically improved of Lactobacillus delbrueckii mutants.Int. Food Res. J.25515–522.
66
Kim C. W. Cho S. H. Kang S. H. Park Y. B. Yoon M. H. Lee J. B. et al (2015). Prevalence, genetic diversity, and antibiotic resistance of Bacillus cereus isolated from Korean fermented soybean products.J. Food Sci.80M123–M128. 10.1111/1750-3841.12720
67
Kleppen H. P. Holo H. Jeon S. R. Nes I. F. Yoon S. S. (2012). Novel Podoviridae family bacteriophage infecting Weissella cibaria isolated from Kimchi.Appl. Environ. Microbiol.787299–7308. 10.1128/AEM.00031-12
68
Kríz B. Benes C. Daniel M. (2009). Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997-2008).Epidemiol. Mikrobiol. Imunol.5898–103.
69
Kubo R. (2016). The reason for the preferential use of finger millet (Eleusine coracana) in Eastern African Brewing.J. Inst. Brew.122175–180.
70
Ladero V. Martín M. C. Redruello B. Mayo B. Flórez A. B. Fernández M. et al (2015). Genetic and functional analysis of biogenic amine production capacity among starter and non-starter lactic acid bacteria isolated from artisanal cheeses.Eur. Food Res. Technol.241377–383. 10.1007/s00217-015-2469-z
71
Laranjo M. Elias M. Fraqueza M. J. (2017). The use of starter cultures in traditional meat products.J. Food Qual.2017:9546026. 10.1155/2017/9546026
72
Latorre-Moratalla M. L. Bover-Cid S. Talon R. Aymerich T. Garriga M. Zanardi E. et al (2010). Distribution of aminogenic activity among potential autochthonous starter cultures for dry fermented sausages.J. Food Prot.73524–528. 10.4315/0362-028X-73.3.524
73
Lautié E. Rozet E. Hubert P. Vandelaer N. Billard F. Felde T. Z. et al (2013). Fast method for the simultaneous quantification of toxic polyphenols applied to the selection of genotypes of yam bean (Pachyrhizus sp.) seeds.Talanta11794–101. 10.1016/j.talanta.2013.08.038
74
Le T. M. Do T. N. Le M. H. Tran T. M. K. Van V. Q. (2012). Evaluation of hygiene and safety status of nem chua production in Hanoi.Vietnam J. Sci. Technol.50231–237.
75
Le Guyader F. S. Bon F. DeMedici D. Parnaudeau S. Bertone A. Crudeli S. et al (2006). Detection of multiple noroviruses associated with an international gastroenteritis outbreak linked to oyster consumption.J. Clin. Microbiol.443878–3882. 10.1128/JCM.01327-06
76
Lee H. M. Lee J. H. Kim S. H. Yoon S. R. Lee J. Y. Ha J. H. (2017). Correlation between changes in microbial/physicochemical properties and persistence of human norovirus during cabbage Kimchi fermentation.J. Microbiol. Biotechnol.272019–2027. 10.4014/jmb.1707.07041
77
Lee H. S. Lindahl J. Nguyen-Viet H. Khong N. V. Nghia V. B. Xuan H. N. et al (2017). An investigation into aflatoxin M1in slaughtered fattening pigs and awareness of aflatoxins in Vietnam.BMC Vet. Res.13:363. 10.1186/s12917-017-1297-8
78
Lee N. Kim M. D. Chang H. J. Choi S. W. Chun H. S. (2017). Genetic diversity, antimicrobial resistance, toxin gene profiles, and toxin production ability of Bacillus cereus isolates from doenjang, a Korean fermented soybean paste.J. Food Saf.37:e12363. 10.1111/jfs.12363
79
Leedom J. M. (2006). Milk of nonhuman origin and infectious diseases in humans.Clin. Infect. Dis.43610–615. 10.1086/507035
80
Li L. Wen X. Wen Z. Chen S. Wang L. Wei X. (2018). Evaluation of the biogenic amines formation and degradation abilities of Lactobacillus curvatus from Chinese Bacon.Front. Microbiol.9:1015. 10.3389/fmicb.2018.01015
81
Li R. Zheng X. Yang J. Shen X. Jiao L. Yan Z. et al (2019). Relation between gut microbiota composition and traditional spontaneous fermented dairy foods among Kazakh nomads in Xinjiang, China.J. Food Sci.843804–3814. 10.1111/1750-3841.14912
82
Liu H. Qiu H. Che Z. Xu Q. Shu H. (2006). Investigation on a food poisoning case caused by Bacillus cereus.Dis. Surveill.21:652.
83
Lore T. A. Mbugua S. K. Wangoh J. (2005). Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product.LWT Food Sci. Technol.38125–130.
84
Lorenzo J. M. Cachaldora A. Fonseca S. Gómez M. Franco I. Carballo J. (2010). Production of biogenic amines “in vitro” in relation to the growth phase by Enterobacteriaceae species isolated from traditional sausages.Meat Sci.86684–691. 10.1016/j.meatsci.2010.06.005
85
Luz C. Saladino F. Luciano F. B. Mañes J. Meca G. (2017). In vitro antifungal activity of bioactive peptides produced by Lactobacillus plantarum against Aspergillus parasiticus and Penicillium expansum.LWT Food Sci. Technol.81128–135.
86
Mah J. H. Park Y. K. Jin Y. H. Lee J. H. Hwang H. J. (2019). Bacterial production and control of biogenic amines in Asian fermented soybean foods.Foods8:85. 10.3390/foods8020085
87
Maitha I. M. Kaindi D. W. M. Wangoh J. Mbugua S. (2019). Microbial quality and safety of traditional fermented camel milk product suusac sampled from different regions in North Eastern, Kenya.Asian Food Sci. J.81–9. 10.9734/afsj/2019/v8i229986
88
Majou D. Christieans S. (2018). Mechanisms of the bactericidal effects of nitrate and nitrite in cured meats.Meat Sci.145273–284. 10.1016/j.meatsci.2018.06.013
89
Mannaa M. Han G. Seo Y. S. Park I. (2021). Evolution of food fermentation processes and the use of multi-omics in deciphering the roles of the microbiota.Foods10:2861. 10.3390/foods10112861
90
Markovinović L. Kosanović Ličina M. L. Tešić V. Vojvodić D. Vladušić Lucić I. Kniewald T. et al (2016). An outbreak of tick-borne encephalitis associated with raw goat milk and cheese consumption, Croatia, 2015.Infection44661–665. 10.1007/s15010-016-0917-8
91
Marteau P. Guyonnet D. Lafaye de Micheaux P. Gelu S. (2013). A randomized, double-blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I-2494 in healthy women reporting minor digestive symptoms.Neurogastroenterol. Motil.25331–e252. 10.1111/nmo.12078
92
Martin B. Garriga M. Aymerich T. (2011). Prevalence of Salmonella spp. and Listeria monocytogenes at small-scale Spanish factories producing traditional fermented sausages.J. Food Prot.74812–815. 10.4315/0362-028X.JFP-10-437
93
Maske B. L. de Melo Pereira G. V. da Silva Vale A. Souza D. S. M. De Dea Lindner J. Soccol C. R. (2021). Viruses in fermented foods: are they good or bad? Two sides of the same coin.Food Microbiol.98:103794. 10.1016/j.fm.2021.103794
94
Matumba L. Monjerezi M. Kankwamba H. Njoroge S. M. Ndilowe P. Kabuli H. et al (2016). Knowledge, attitude, and practices concerning presence of molds in foods among members of the general public in Malawi.Mycotoxin Res.3227–36. 10.1007/s12550-015-0237-3
95
McLeod A. Måge I. Heir E. Axelsson L. Holck A. L. (2016). Effect of relevant environmental stresses on survival of enterohemorrhagic Escherichia coli in dry-fermented sausage.Int. J. Food Microbiol.22915–23. 10.1016/j.ijfoodmicro.2016.04.005
96
McNulty N. P. Yatsunenko T. Hsiao A. Faith J. J. Muegge B. D. Goodman A. L. et al (2011). The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins.Sci. Transl. Med.3:106ra106. 10.1126/scitranslmed.3002701
97
Meloni D. (2015). Presence of Listeria monocytogenes in Mediterranean-style dry fermented sausages.Foods434–50. 10.3390/foods4010034
98
Meloni D. (2019). High-Hydrostatic-Pressure (HHP) processing technology as a novel control method for Listeria monocytogenes occurrence in Mediterranean-style dry-fermented sausages.Foods8:672. 10.3390/foods8120672
99
Meloni D. Consolati S. G. Mazza R. Mureddu A. Fois F. Piras F. et al (2014). Presence and molecular characterization of the major serovars of Listeria monocytogenes in ten Sardinian fermented sausage processing plants.Meat Sci.97443–450. 10.1016/j.meatsci.2014.02.012
100
Meng Z. Li Z. Jin J. Zhang Y. Liu X. Yiang X. et al (1988). Studies on fermented corn flour poisoning in rural areas of China. I. Epidemiology, clinical manifestations, and pathology.Biomed. Environ. Sci.1101–104.
101
Michel A. L. Geoghegan C. Hlokwe T. Raseleka K. Getz W. M. Marcotty T. (2015). Longevity of Mycobacterium bovis in raw and traditional souring milk as a function of storage temperature and dose.PLoS One10:e0129926. 10.1371/journal.pone.0129926
102
Milani C. Duranti S. Napoli S. Alessandri G. Mancabelli L. Anzalone R. et al (2019). Colonization of the human gut by bovine bacteria present in Parmesan cheese.Nat. Commun.10:1286. 10.1038/s41467-019-09303-w
103
Milani J. Maleki G. (2014). Effects of processing on mycotoxin stability in cereals.J. Sci. Food Agric.942372–2375. 10.1002/jsfa.6600
104
Miranda C. Contente D. Igrejas G. Câmara S. Dapkevicius M. Poeta P. (2021). Role of exposure to lactic acid bacteria from foods of animal origin in human health.Foods10:2092. 10.3390/foods10092092
105
Montagnac J. A. Davis C. R. Tanumihardjo S. A. (2009). Processing techniques to reduce toxicity and antinutrients of cassava for use as a staple food.Compr. Rev. Food Sci. Food Saf.817–27. 10.1111/j.1541-4337.2008.00064.x
106
Moor D. Liniger M. Baumgartner A. Felleisen R. (2018). Screening of ready-to-eat meat products for hepatitis E virus in Switzerland.Food Environ. Virol.10263–271. 10.1007/s12560-018-9340-x
107
Moxley R. A. (2004). Escherichia coli 0157:H7: an update on intestinal colonization and virulence mechanisms.Anim. Health Res. Rev.515–33. 10.1079/ahr200463
108
Mwangi L. Matofari J. W. Muliro P. Bebe B. (2017). Occurrence of Brucella and Mycobacteria species in raw and fermented camel milk along the value Chain.Asian J. Agric. Food Sci.4212–218.
109
Nahidul-Islam S. M. Kuda T. Takahashi H. Kimura B. (2018). Bacterial and fungal microbiota in traditional Bangladeshi fermented milk products analysed by culture-dependent and culture-independent methods.Food Res. Int.111431–437. 10.1016/j.foodres.2018.05.048
110
Nemska V. Logar P. Rasheva T. Sholeva Z. Georgieva N. Danova S. (2019). Functional characteristics of lactobacilli from traditional Bulgarian fermented milk products.Turk. J. Biol.43148–153. 10.3906/biy-1808-34
111
Nkhata S. G. Ayua E. Kamau E. H. Shingiro J. B. (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes.Food Sci. Nutr.62446–2458. 10.1002/fsn3.846
112
Odunmbaku L. A. Sobowale S. S. Adenekan M. K. Oloyede T. Adebiyi J. A. Adebo O. A. (2017). Influence of steeping duration, drying temperature, and duration on the chemical composition of sorghum starch.Food Sci. Nutr.6348–355. 10.1002/fsn3.562
113
Oguntoyinbo F. A. (2014). Safety challenges associated with traditional foods of West Africa.Food Rev. Int.30338–358. 10.1080/87559129.2014.940086
114
Olea-Popelka F. Muwonge A. Perera A. Dean A. S. Mumford E. Erlacher-Vindel E. et al (2017). Zoonotic tuberculosis in human beings caused by Mycobacterium bovis-a call for action.Lancet Infect. Dis.17e21–e25. 10.1016/S1473-3099(16)30139-6
115
Ombarak R. A. Hinenoya A. Awasthi S. P. Iguchi A. Shima A. Elbagory A. M. et al (2016). Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt.Int. J. Food Microbiol.22169–76. 10.1016/j.ijfoodmicro.2016.01.009
116
Ordóñez J. L. Troncoso A. M. García-Parrilla M. Callejón R. M. (2016). Recent trends in the determination of biogenic amines in fermented beverages - a review.Anal. Chim. Acta93910–25. 10.1016/j.aca.2016.07.045
117
Ostry V. Malir F. Toman J. Grosse Y. (2017). Mycotoxins as human carcinogens-the IARC monographs classification.Mycotoxin Res.3365–73. 10.1007/s12550-016-0265-7
118
Owusu-Kwarteng J. Akabanda F. Agyei D. Jespersen L. (2020). Microbial safety of milk production and fermented dairy products in Africa.Microorganisms8:752. 10.3390/microorganisms8050752
119
Park J. H. Jung S. Shin J. Lee J. S. Joo I. S. Lee D. Y. (2015). Three gastroenteritis outbreaks in South Korea caused by the consumption of Kimchi tainted by norovirus GI.4.Foodborne Pathog. Dis.12221–227. 10.1089/fpd.2014.1879
120
Park K. M. Kim H. J. Jeong M. C. Koo M. (2016). Occurrence of toxigenic Bacillus cereus and Bacillus thuringiensis in Doenjang, a Korean Fermented Soybean Paste.J. Food Prot.79605–612. 10.4315/0362-028X.JFP-15-416
121
Park Y. K. Lee J. H. Mah J. H. (2019). Occurrence and reduction of biogenic amines in Kimchi and Korean Fermented seafood products.Foods8:547. 10.3390/foods8110547
122
Patarata L. Novais M. Fraqueza M. J. Silva J. A. (2020). Influence of meat spoilage microbiota initial load on the growth and survival of three pathogens on a naturally fermented sausage.Foods9:676. 10.3390/foods9050676
123
Pavio N. Merbah T. Thébault A. (2014). Frequent hepatitis E virus contamination in food containing raw pork liver, France.Emerg. Infect. Dis.201925–1927. 10.3201/eid2011.140891
124
Pei X. Zhang W. Jiang H. Liu D. Liu X. Li L. et al (2021). Food-origin mycotoxin-induced neurotoxicity: intend to break the rules of neuroglia cells.Oxid. Med. Cell. Longev.2021:9967334. 10.1155/2021/9967334
125
Peng Z. Dottorini T. Hu Y. Li M. Yan S. Fanning S. et al (2021). Comparative genomic analysis of the foodborne pathogen Burkholderia gladioli pv. cocovenenans harboring a bongkrekic acid biosynthesis gene cluster.Front. Microbiol.12:628538. 10.3389/fmicb.2021.628538
126
Phukan R. K. Narain K. Zomawia E. Hazarika N. C. Mahanta J. (2006). Dietary habits and stomach cancer in Mizoram, India.J. Gastroenterol.41418–424.
127
Piras F. Spanu C. Mocci A. M. Demontis M. Santis E. Scarano C. (2019). Occurrence and traceability of Salmonella spp. in five Sardinian fermented sausage facilities.Ital. J. Food Saf.8:8011. 10.4081/ijfs.2019.8011
128
Pringsulaka O. Patarasinpaiboon N. Suwannasai N. Atthakor W. Rangsiruji A. (2011). Isolation and characterisation of a novel Podoviridae-phage infecting Weissella cibaria N 22 from Nham, a Thai fermented pork sausage.Food Microbiol.28518–525. 10.1016/j.fm.2010.10.011
129
Pugin B. Barcik W. Westermann P. Heider A. Wawrzyniak M. Hellings P. et al (2017). A wide diversity of bacteria from the human gut produces and degrades biogenic amines.Microb. Ecol. Health Dis.28:1353881. 10.1080/16512235.2017.1353881
130
Raiola A. Tenore G. C. Manyes L. Meca G. Ritieni A. (2015). Risk analysis of main mycotoxins occurring in food for children: an overview.Food Chem. Toxicol.84169–180.
131
Rehfeld I. S. Fraiha A. L. S. Matos A. C. D. Guedes M. I. M. C. Costa E. A. de Souza M. R. et al (2017). Short communication: survival of Vaccinia virus in inoculated cheeses during 60-day ripening.J. Dairy Sci.1007051–7054. 10.3168/jds.2016-12560
132
Renou C. Roque-Afonso A. M. Pavio N. (2014). Foodborne transmission of hepatitis E virus from raw pork liver sausage, France.Emerg. Infect. Dis.201945–1947. 10.3201/eid2011.140791
133
Rezac S. Kok C. R. Heermann M. Hutkins R. (2018). Fermented foods as a dietary source of live organisms.Front. Microbiol.9:1785. 10.3389/fmicb.2018.01785
134
Romijn A. R. Rucklidge J. J. Kuijer R. G. Frampton C. (2017). A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression.Aust. N. Z. J. Psychiatry51810–821.
135
Roselli M. Natella F. Zinno P. Guantario B. Canali R. Schifano E. et al (2021). Colonization ability and impact on human gut microbiota of foodborne microbes from traditional or probiotic-added fermented foods: a systematic review.Front. Nutr.8:689084. 10.3389/fnut.2021.689084
136
Ruiz-Capillas C. Herrero A. M. (2019). Impact of biogenic amines on food quality and safety.Foods8:62. 10.3390/foods8020062
137
Sadiq F. A. Yan B. W. Tian F. W. Zhao J. X. Zhang H. Chen W. (2019). Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: a comprehensive review.Compr. Rev. Food. Sci. Food Saf.101403–1436. 10.1111/1541-4337.12481
138
Saltanat H. Li H. Xu Y. Wang J. Liu F. Geng X. H. (2009). The influences of camel milk on the immune response of chronic hepatitis B patients.Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi25431–433.
139
Scavia G. Ciaravino G. Luzzi I. Lenglet A. Ricci A. Barco L. et al (2013). A multistate epidemic outbreak of Salmonella Goldcoast infection in humans, June 2009 to March 2010: the investigation in Italy.Euro Surveill.18:20424. 10.2807/ese.18.11.20424-en
140
Scussel V. M. Scholten J. M. Rensen P. M. Spanjer M. C. Giordano B. N. E. Savi G. D. (2013). Multitoxin evaluation in fermented beverages and cork stoppers by liquid chromatography-tandem mass spectrometry.Int. J. Food Sci. Technol.4896–102. 10.1111/j.1365-2621.2012.03163.x
141
Seo D. J. Lee M. H. Seo J. Ha S. D. Choi C. (2014). Inactivation of murine norovirus and feline calicivirus during oyster fermentation.Food Microbiol.4481–86. 10.1016/j.fm.2014.05.016
142
Shane L. E. Porto-Fett A. Shoyer B. A. Phebus R. K. Thippareddi H. Hallowell A. et al (2018). Evaluation of post-fermentation heating times and temperatures for controlling Shiga toxin-producing Escherichia coli cells in a non-dried, pepperoni-type sausage.Ital. J. Food Saf.7:7250. 10.4081/ijfs.2018.7250
143
Sharma C. Singh C. (2014). Therapeutic value of camel milk – a review.Adv. J. Pharm. Life Sci. Res.27–13.
144
Shen Q. Yang Q. Li Y. (2005). Investigation of one Bacillus cereus food poisoning case.Pract. Prevent. Med.12:713.
145
Shin J. Yoon K. B. Jeon D. Y. Oh S. S. Oh K. H. Chung G. T. et al (2016). Consecutive outbreaks of enterotoxigenic Escherichia coli O6 in schools in South Korea caused by contamination of fermented vegetable Kimchi.Foodborne Pathog. Dis.13535–543. 10.1089/fpd.2016.2147
146
Shukla S. Park H. K. Lee J. S. Kim J. K. Kim M. (2014). Reduction of biogenic amines and aflatoxins in Doenjang samples fermented with various Meju as starter cultures.Food Control42181–187. 10.1016/j.foodcont.2014.02.006
147
Siegrist M. Cvetkovich G. (2001). Better negative than positive? Evidence of a bias for negative information about possible health dangers.Risk Anal.21199–206. 10.1111/0272-4332.211102
148
Singh P. P. Rathore V. Singh L. B. (1986). A possible role of fish preparation ‘Hentak’ in urolithiasis in Manipur–an experimental study.Indian J. Exp. Biol.2488–90.
149
Sivamaruthi B. S. Kesika P. Chaiyasut C. (2018). Toxins in fermented foods: prevalence and preventions-a mini review.Toxins11:4. 10.3390/toxins11010004
150
Skåra T. Axelsson L. Stefánsson G. Ekstrand B. Hagen H. (2015). Fermented and ripened fish products in the northern European countries.J. Ethnic Foods218–24.
151
Smith T. A. (1980). Amines in food.Food Chem.6169–200. 10.1016/0308-8146(81)90008-X
152
Spano G. Russo P. Lonvaud-Funel A. Lucas P. Alexandre H. Grandvalet C. et al (2010). Biogenic amines in fermented foods.Eur. J. Clin. Nutr.64(Suppl. 3), S95–S100. 10.1038/ejcn.2010.218
153
Sun Q. Chen Q. Li F. Zheng D. Kong B. (2016). Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum.Food Control68358–366. 10.1016/j.foodcont.2016.04.021
154
Tamang J. P. Cotter P. D. Endo A. Han N. S. Kort R. Liu S. Q. et al (2020). Fermented foods in a global age: east meets West.Compr. Rev. Food Sci. Food Saf.19184–217. 10.1111/1541-4337.12520
155
Tamang J. P. Holzapfel W. H. Watanabe K. (2016). Diversity of microorganisms in global fermented foods and beverages.Front. Microbiol.7:377. 10.3389/fmicb.2016.00377
156
Tokarz-Deptuła B. Deptuła W. (2017). Probiotics and mammalian gastrointestinal immune system.Postêpy Mikrobiol.56157–162. 10.21307/PM-2017.56.2.157
157
Tóth A. G. Csabai I. Judge M. F. Maróti G. Becsei Á. Spisák S. et al (2021). Mobile antimicrobial resistance genes in probiotics.Antibiotics10:1287. 10.3390/antibiotics10111287
158
Tóth A. G. Csabai I. Maróti G. Jerzsele Á. Dubecz A. Patai Á. V. et al (2020). A glimpse of antimicrobial resistance gene diversity in kefir and yoghurt.Sci. Rep.10:22458. 10.1038/s41598-020-80444-5
159
Uusitupa H. M. Rasinkangas P. Lehtinen M. J. Mäkelä S. M. Airaksinen K. Anglenius H. et al (2020). Bifidobacterium animalis subsp. lactis 420 for metabolic health: review of the research.Nutrients12:892. 10.3390/nu12040892
160
Uzogara S. G. Agu L. N. Uzogara E. O. (1990). A review of traditional fermented foods, condiments and beverages in Nigeria: their benefits and possible problems.Ecol. Food Nutr.24267–288. 10.1080/03670244.1990.9991145
161
Valerio F. Favilla M. De Bellis P. Sisto A. de Candia S. Lavermicocca P. (2009). Antifungal activity of strains of lactic acid bacteria isolated from a semolina ecosystem against Penicillium roqueforti, Aspergillus niger and Endomyces fibuliger contaminating bakery products.Syst. Appl. Microbiol.32438–448. 10.1016/j.syapm.2009.01.004
162
van Reckem E. Geeraerts W. Charmpi C. Van der Veken D. De Vuyst L. Leroy F. (2019). Exploring the link between the geographical origin of European fermented foods and the diversity of their bacterial communities: the case of fermented meats.Front. Microbiol.10:2302. 10.3389/fmicb.2019.02302
163
van Veen A. G. (1967). “The bongkrek toxins,” in Biochemistry of Some Foodborne Microbial Toxins, edsMatelesE. I.WoganG. N. (Cambridge, MA: MIT Press), 43–50.
164
Varela-Hernández J. J. Cabrera-Díaz E. Cardona-López M. A. Ibarra-Velázquez L. M. Rangel-Villalobos H. Castillo A. et al (2007). Isolation and characterization of Shiga toxin-producing Escherichia coli O157:H7 and non-O157 from beef carcasses at a slaughter plant in Mexico.Int. J. Food Microbiol.113237–241.
165
Viaro H. P. da Silva J. J. de Souza Ferranti L. Bordini J. G. Massi F. P. Fungaro M. (2017). The first report of A. novoparasiticus, A. arachidicola and A. pseudocaelatus in Brazilian corn kernels.Int. J. Food Microbiol.2146–51. 10.1016/j.ijfoodmicro.2016.12.002
166
Vignolo G. Fontana C. Fadda S. (2010). “Semidry and dry fermented sausages,” in Handbook of Meat Processing, ed.ToldráF. (Ames, IA: Blackwell Publishing), 379–398.
167
Vilela A. (2021). An overview of CRISPR-based technologies in wine yeasts to improve wine flavor and safety.Fermentation.7:5. 10.3390/fermentation7010005
168
Vilela A. Cosme F. Inês A. (2020). Wine and non-dairy fermented beverages: a novel source of pro- and prebiotics.Fermentation6:113. 10.3390/fermentation6040113
169
Voidarou C. Antoniadou M. Rozos G. Tzora A. Skoufos I. Varzakas T. et al (2020). Fermentative foods: microbiology, biochemistry, potential human health benefits and public health issues.Foods10:69. 10.3390/foods10010069
170
Vu N. T. Nguyen T. V. A. (2016). “Ethnic fermented foods and beverages of Vietnam,” in Ethnic Fermented Foods and Alcoholic Beverages of Asia, ed.TamangJ. P. (New Delhi: Springer India), 383–409.
171
Wolff A. Günther T. Albert T. Johne R. (2020a). Effect of sodium chloride, sodium nitrite and sodium nitrate on the infectivity of hepatitis E virus.Food Environ. Virol.12350–354. 10.1007/s12560-020-09440-2
172
Wolff A. Günther T. Albert T. Schilling-Loeffler K. Gadicherla A. K. Johne R. (2020b). Stability of hepatitis E virus at different pH values.Int. J. Food Microbiol.325:108625. 10.1016/j.ijfoodmicro.2020.108625
173
Wu D. Xie W. Li X. Cai G. Lu J. (2020). Metabolic engineering of Saccharomyces cerevisiae using the CRISPR/Cas9 system to minimize ethyl carbamate accumulation during Chinese rice wine fermentation.Appl. Microbiol. Biotechnol.1044435–4444. 10.1007/s00253-020-10549-4
174
Xiang H. Sun-Waterhouse D. Waterhouse G. I. N. Cui C. Ruan Z. (2019). Fermentation-enabled wellness foods: a fresh perspective.Food Sci. Hum. Wellness8203–243. 10.1016/j.fshw.2019.08.003
175
Xu Y. Zang J. Regenstein J. M. Xia W. (2021). Technological roles of microorganisms in fish fermentation: a review.Crit. Rev. Food Sci. Nutr.611000–1012. 10.1080/10408398.2020.1750342
176
Yam B. A. Z. Khomeiri M. Mahounak A. S. Jafari S. M. (2014). Hygienic quality of camel milk and fermented camel milk (Chal) in Golestan Province, Iran.J. Microbiol. Res.498–103.
177
Yakubu Y. Shuaibu A. B. Ibrahim A. M. Hassan U. L. Nwachukwu R. J. (2018). Risk of shiga toxigenic Escherichia coli O157:H7 infection from raw and fermented milk in sokoto metropolis, Nigeria.J. Pathog2018:8938597. 10.1155/2018/8938597
178
Yim J. H. Kim K. Y. Chon J. W. Kim D. H. Kim H. S. Choi D. S. et al (2015). Incidence, antibiotic susceptibility, and toxin profiles of Bacillus cereus sensu lato isolated from Korean fermented soybean products.J. Food Sci.80M1266–M1270. 10.1111/1750-3841.12872
179
Yuan Y. Gao R. Liang Q. Song L. Huang J. Lang N. et al (2020). A foodborne bongkrekic acid poisoning incident - Heilongjiang Province, 2020.China CDC Wkly.2975–978. 10.46234/ccdcw2020.264
180
Zaman M. Z. Bakar F. A. Selamat J. Bakar J. Ang S. S. Chong C. Y. (2014). Degradation of histamine by the halotolerant Staphylococcus carnosus FS19 isolate obtained from fish sauce.Food Control4058–63. 10.1016/j.foodcont.2013.11.031
181
Zang J. Xu Y. Xia W. Regenstein J. M. (2020). Quality, functionality, and microbiology of fermented fish: a review.Crit. Rev. Food Sci. Nutr.601228–1242. 10.1080/10408398.2019.1565491
182
Zhang C. Derrien M. Levenez F. Brazeilles R. Ballal S. A. Kim J. et al (2016). Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes.ISME J.102235–2245. 10.1038/ismej.2016.13
183
Zhang S. H. Liu Y. (2000). Prepare Technology of Seasoning.Guangzhou: South China University of Technology Press, 85–95.
184
Zhang Z. Jin M. Wang K. Zhang N. Zhang Q. Tao X. et al (2021). Short-term intake of Lactiplantibacillus plantarum ZDY2013 fermented milk promotes homoeostasis of gut microbiota under enterotoxigenic Bacillus cereus challenge.Food Funct.125118–5129. 10.1039/d1fo00162k
185
Zhao G. Wang Y. F. Chen J. Yao Y. (2020). Predominant mycotoxins, pathogenesis, control measures, and detection methods in fermented pastes.Toxins12:78. 10.3390/toxins12020078
186
Zhou G. Bester K. Liao B. Yang Z. Jiang R. Hendriksen N. B. (2014). Characterization of three Bacillus cereus strains involved in a major outbreak of food poisoning after consumption of fermented black beans (Douchi) in Yunan, China.Foodborne Pathog. Dis.11769–774. 10.1089/fpd.2014.1768
187
Zúñiga Estrada A. Mota de la Garza L. Sánchez Mendoza M. Santos López E. M. Filardo Kerstupp S. López Merino A. (2005). Survival of Brucella abortus in milk fermented with a yoghurt starter culture.Rev. Latinoam. Microbiol.4788–91.
Summary
Keywords
contaminated fermented products, fermented foods, food safety, foodborne, outbreaks
Citation
Skowron K, Budzyńska A, Grudlewska-Buda K, Wiktorczyk-Kapischke N, Andrzejewska M, Wałecka-Zacharska E and Gospodarek-Komkowska E (2022) Two Faces of Fermented Foods—The Benefits and Threats of Its Consumption. Front. Microbiol. 13:845166. doi: 10.3389/fmicb.2022.845166
Received
29 December 2021
Accepted
15 February 2022
Published
07 March 2022
Volume
13 - 2022
Edited by
Laurent Dufossé, Université de la Réunion, France
Reviewed by
Folarin Anthony Oguntoyinbo, Appalachian State University, United States; Anna Ligocka, UTP University of Science and Technology, Poland; Giau Van Vo, Gachon University, South Korea
Updates
Copyright
© 2022 Skowron, Budzyńska, Grudlewska-Buda, Wiktorczyk-Kapischke, Andrzejewska, Wałecka-Zacharska and Gospodarek-Komkowska.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krzysztof Skowron, skowron238@wp.pl
This article was submitted to Food Microbiology, a section of the journal Frontiers in Microbiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.