- 1Department of Pharmacy, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Department of Hematology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
As a primary cause of death in patients with hematological malignancies and transplant recipients, invasive aspergillosis (IA) is a condition that warrants attention. IA infections have been increasing, which remains a significant cause of morbidity and mortality in immunocompromised patients. During the past decade, antifungal drug resistance has emerged, which is especially concerning for management given the limited options for treating azole-resistant infections and the possibility of failure of prophylaxis in those high-risk patients. Histone posttranslational modifications (HPTMs), mainly including acetylation, methylation, ubiquitination and phosphorylation, are crucial epigenetic mechanisms regulating various biological events, which could modify the conformation of histone and influence chromatin-associated nuclear processes to regulate development, cellular responsiveness, and biological phenotype without affecting the underlying genetic sequence. In recent years, fungi have become important model organisms for studying epigenetic regulation. HPTMs involves in growth and development, secondary metabolite biosynthesis and virulence in Aspergillus. This review mainly aims at summarizing the acetylation, deacetylation, methylation, demethylation, and sumoylation of histones in IA and connect this knowledge to possible HPTMs-based antifungal drugs. We hope this research could provide a reference for exploring new drug targets and developing low-toxic and high-efficiency antifungal strategies.
Introduction
The incidence of invasive fungal disease (IFD) has increased dramatically in recent decades, which is related to the clinical application of broad-spectrum antibiotics, immunosuppressive drugs or antitumor drugs, the development of bone marrow transplantation or other organ transplantation, and the application of long-term and high-dose glucocorticoids, seriously threatens the life of high-risk patients (Enoch et al., 2017; von Lilienfeld-Toal et al., 2019). Aspergillosis invasive (IA) is the second most prevalent invasive fungal illness, and its prevalence continues to rise. IA is a deep fungal infection caused by Aspergillus infection, which mostly occurs in immunocompromised patients and results in a high case-fatality rate. The pathogenic Aspergillus mainly include A. fumigatus, A. flavus, A. niger, A. terreus and A. nidulans. A. fumigatus is the primary cause of aspergillosis, accounting for 80–90% of IA which has a significant death rate (>60%) in immunocompromised patients without proper treatment (Sugui et al., 2014; Cadena et al., 2021). A. flavus, which also causes aspergillosis disorders or liver cancer in humans and animals brought on by eating tainted food, is the second most common Aspergillus pathogen in immunocompromised patients (Lohmar et al., 2019). A. nidulans is a common cause of IA in patients with chronic granulomatous disease (CGD), who have a higher mortality rate, although it seldom causes IA in immunocompromised individuals (Gresnigt et al., 2018). At present, there are few types of low-toxic and high-efficiency antifungal drugs available in clinical practice. The emergence of drug-resistant strains, especially the increasing number of deep drug-resistant fungal infections, has become a worldwide problem that urgently needs to be solved. The pharmaceutical and academic sectors have made the development of novel antifungal medicines capable of addressing this issue a key priority, which possesses a potentially broad spectrum of activity and minimum toxicity (Mani Chandrika and Sharma, 2020).
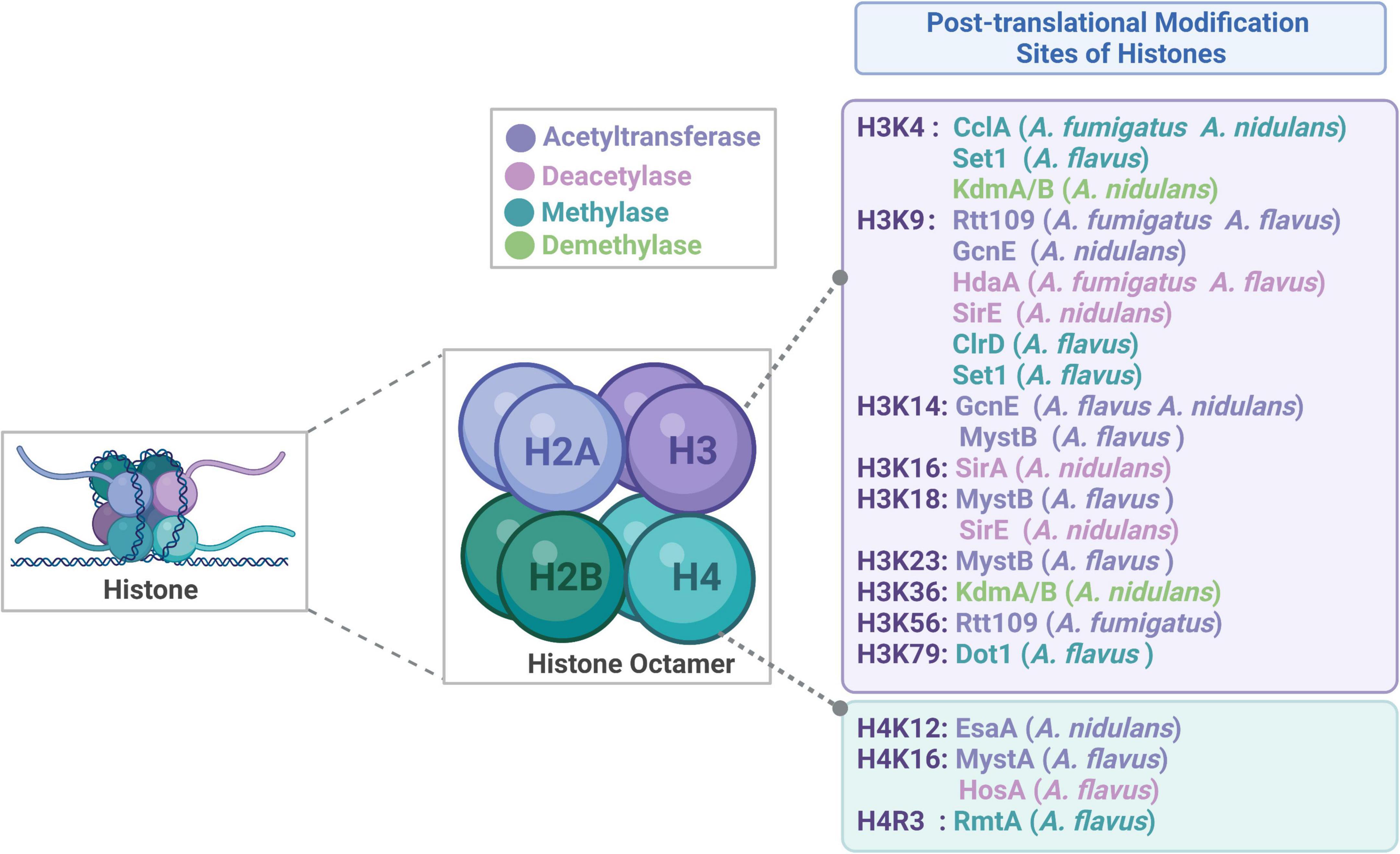
DNA is bound up with chromatin in the nuclei of eukaryotic cells. The fundamental functional unit of chromatin, the nucleosome, is made up of a histone octamer composed of one H2A-H2B tetramer and two H3-H4 dimers, which is surrounded by 146–147 base pairs of DNA (Figure 1; Jenuwein and Allis, 2001; Zhang et al., 2021b; Lin et al., 2022). The amino residues at the N-terminal and C-terminal tails of histones could be post-translationally modified, such as acetylation, methylation, phosphorylation and ubiquitination, which could alter the electronic charge and structure of these histone tails, thereby altering the chromatin state and subsequent gene expression. It is generally known that histone post-translational modifications (HPTMs) in eukaryotic cells provide a crucial link between biological processes and chromatin-based regulation (Nie et al., 2016; Saavedra et al., 2018). HPTMs could not only control the silencing, activation, expression of various genes but also modulate DNA repairment (Garzon-Porras et al., 2022). The changes of histone modification in tumor cells have attracted a lot of attention, and the related drugs have been used in the clinical treatment of tumors with higher curative effects and lower side effects (Xu et al., 2021; Lopez et al., 2022). Research on HPTMs is currently concentrated on metabolism, tumor treatment, and other areas, there is currently relatively limited research on microbial HPTMs, particularly with regard to microbial pathogenicity. Recent advances in pathogenic microorganisms indicate that fungal pathogens could modify their histone modification status to control their growth, development, virulence features, and drug resistance. Recent studies revealed that histone acetyltransferases (HATs) and histone deacetylases (HDACs) play crucial roles in the development, virulence, drug resistance and stress responses of Candida albicans (C. albicans) (Kim et al., 2015). In addition, HPTMs may work with well-known transcription factors in the signaling pathways to contribute to the morphological change in C. albicans (Kim et al., 2015; Kim J. et al., 2018; Su et al., 2020a). Studies have shown that HPTMs are involved in multiple physiological processes in Aspergillus, which can be developed as potential targets for novel antifungal drugs in the future.
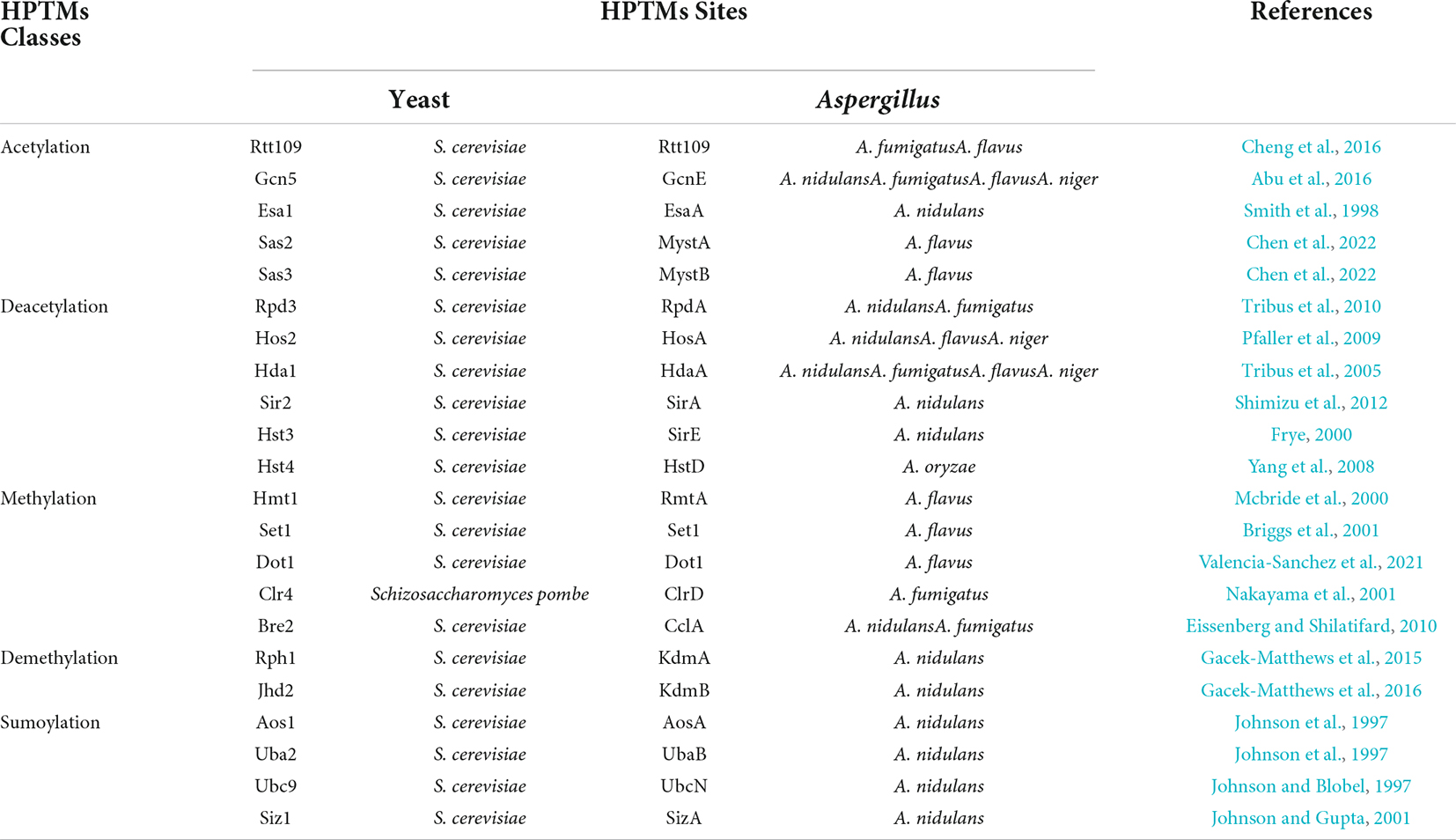
In this review, we discussed the composition of histones and widespread distribution of HPTMs in Aspergillus (Figure 1), especially in the A. fumigatus, A. flavus, A. nidulans, A. niger, and A. oryzae. Then, we highlighted recent examples to illustrate the role of HPTMs, mainly including HATs, HDACs, histone methylases (HMTs), histone demethylases (HDMTs) and sumoylation enzymes in Aspergillus (Figure 2). Finally, we reviewed the HPTMs in yeast and Aspergillus (Table 1). In addition, we summarized the drugs or compounds which possess anti-Aspergillus activity targeting HPTMs, mainly based on HDACs (Table 2), in order to provide alternative targets for new antifungal drug development and provide a potential resource for sensible antifungal usage of antifungals in clinical practice.
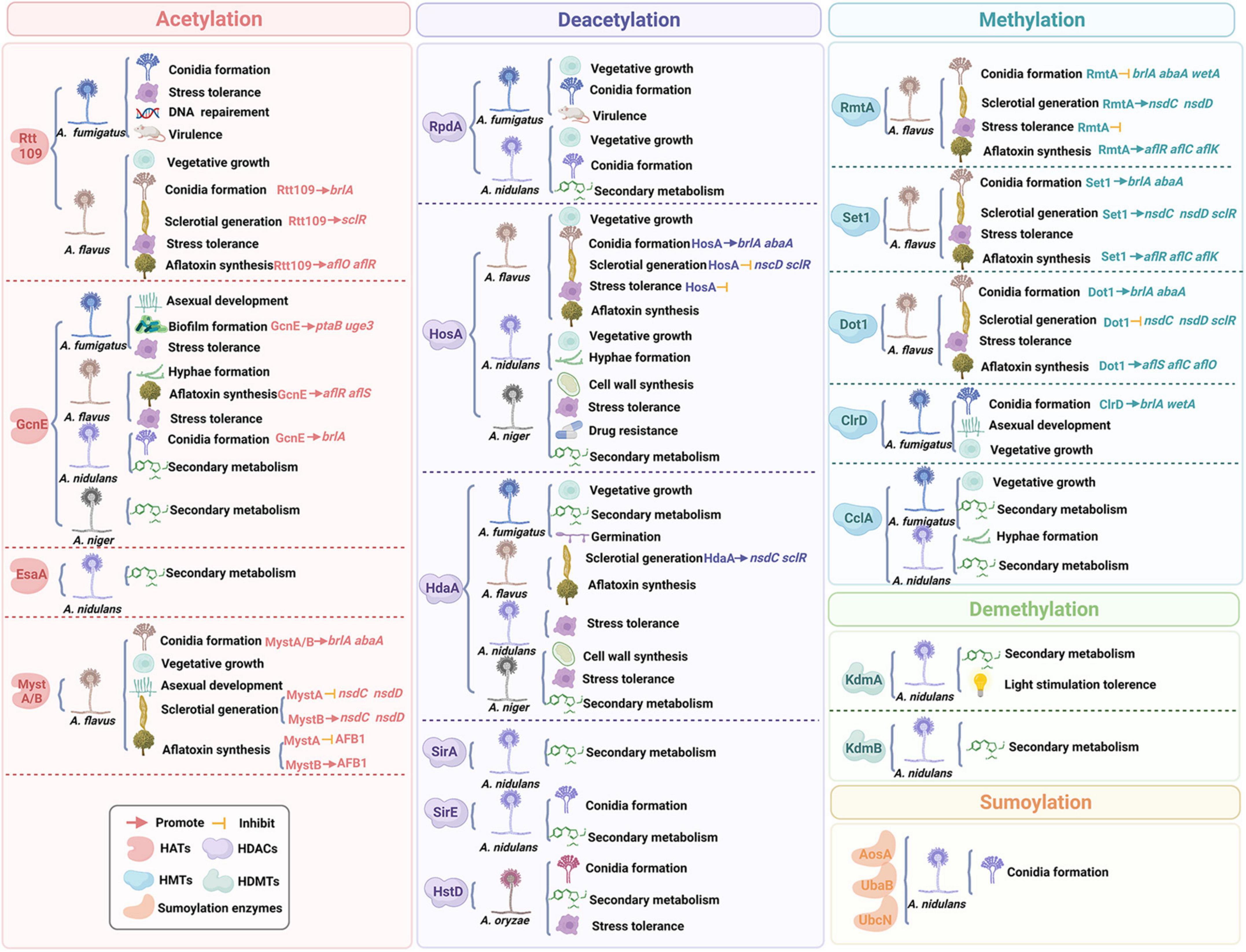
Figure 2. A schematic representation of the relationship between histone post-translational modifications and fungal growth and virulence.
Histone acetyltransferases
The post-translational modification of histone acetylation was the first known chromatin modification mechanism and has been extensively reported in recent years (Buuh et al., 2017; Chen et al., 2022). The acetylation of histones is a dynamic and reversible post-translational alteration of proteins, which is involved in the regulation of gene transcription, protein stability and cellular metabolism (Zhang et al., 2018). Histone acetylation modification was coordinated by HATs and HDACs on the N-terminal of histone lysine residues (Zhang et al., 2021a; Chen et al., 2022). HAT could covalently attach an acetyl group (usually provided by acetyl-CoA) to the ε-amino side chain of lysine. The positively charged lysine could be neutralized by the negatively charged acetyl group, thereby reducing the interaction between histones and DNA, forming a looser chromatin structure named euchromatin, which is conducive to gene transcription (Lennartsson and Ekwall, 2009). Recent research has demonstrated that HATs regulate Aspergillus growth, response to environmental stress, and pathogenicity (Zhang et al., 2021a).
Rtt109
Regulators of Ty1 translocation109 (Rtt109) is a unique HAT in fungi and the sequence is conservative in most filamentous fungi (Lopes da Rosa et al., 2013). Rtt109’s core catalytic domain has a similar three-dimensional structure to that of the mammalian HAT p300/CBP, but their catalytic mechanisms are distinct (Zhang et al., 2021a). Rtt109 is reported to acetylate histone H3 on K9, K27, and K56 (Zhang et al., 2021a). Histone molecular chaperone Asf1 integrates with H3, and then H3K56 is acetylated by Rtt109. Vps75 is a member belongs to the family of the nucleosome assembly proteins (NAP-1). It is another molecular chaperone related to Rtt109 which was first found in Saccharomyces cerevisiae (S. cerevisiae). Vps75 and Rtt109 could interact to produce the Rtt109-Vps75 complex with a special functional domain, which affects the acetylation level of H3K56 (Tang et al., 2008). The involvement of Rtt109 in pathogenic fungi has drawn growing attention due to its implications in the development of novel antifungals. It is hypothesized that Rtt109 acetylation may have a role in maintaining genomic stability, transcriptional control, and DNA damage repair(Zhang et al., 2021a).
In A. fumigatus, Rtt109-Asf1-H3-H4 complex functions as an integral substrate unit which is essential for H3K9 and H3K56 acetylation (Zhang et al., 2018). Rtt109 has histone acetyltransferase activity both in vitro and in vivo (Zhang et al., 2021a). Recent research has confirmed that Rtt109 controls the regulation of conidiation, oxidative stress, cellular metabolism and DNA damage repair in A. fumigatus (Zhang et al., 2021a). Comparing the mutant strain to the wild-type and complemented strains, the Δrtt109 mutant drastically reduced conidia production and colony growth (Yang G. et al., 2019). Gene Ontology (GO) enrichment analysis showed that in Δrtt109 mutant most genes were upregulated and the majority of upregulated genes were involved in oxidation-reduction processes, transmembrane transport, carbohydrate metabolism and amino acid metabolism, while the expression of DNA damage genes was downregulated in the Δrtt109 mutant (Kwon et al., 2018). In addition, Rtt109 is a crucial part of genotoxic stress tolerance. Rtt109 is critical for the virulence of A. fumigatus, survival analysis showed that Δrtt109 mutant exhibited a significantly reduced mortality rate.
In A. flavus, H3K9 is the acetylation target of Rtt109, which played important role in its full functions. Recent studies have shown that Rtt109 contributes to A. flavus’s vegetative growth, conidia formation, sclerotia production, aflatoxin synthesis, and response to environmental stress (Sun et al., 2021). In the Δrtt109 mutant, the vegetative growth, conidial formation and sclerotial generation were significantly suppressed in A. flavus, and the expression levels of the related gene brlA and sclR were lower compared to the wild type (WT) and Δrtt109 complementary strains. A. flavus is the primary producer of aflatoxins, which have been determined to be one of the most harmful and cancer-causing secondary metabolites (SM) (Yang K. et al., 2019). A persistent exposure to aflatoxins may impair the immune response and result in malnutrition, hepatic lesions and even hepatomas (Yang et al., 2018; Yang K. et al., 2019). In Δrtt109 mutant, the production of aflatoxin decreased significantly, the expression levels of aflatoxin-specific regulatory genes aflO and aflR had quite low levels of expression. Moreover, Rtt109 is critical in responding to cell wall stress, oxidative damage, osmotic stress and particularly genotoxic stress in A. flavus. The research results of Rtt109 provide evidence for the role of acetylation in the regulation of the life process of A. flavus and facilitate the prevention and control of A. flavus.
GcnE
General control non-derepressible E (GcnE) (Gcn5) is a kind of HATs that could release histones from chromatin to initiate transcriptional activation. The SAGA (Spt-Ada-GcnE) complex consists of the acetyltransferase GcnE, two transcriptional adapters (Ada2 and Ada3), and proteins such as Spt3, which is conserved in eukaryotic cells and could participate in the regulation of a variety of gene transcription (Koutelou et al., 2010). GcnE has been linked to the growth, development, stress tolerance and virulence in Candida albicans, Candida glabrata, Cryptococcus neoformans and other fungal species (O”Meara et al., 2010; Peng et al., 2015). In Aspergillus, research have shown that GcnE is essential for fungal growth and SM synthesis.
In A. fumigatus, GcnE has been shown in studies to play a role in the regulation of asexual development, stress response and biofilm formation (Nossmann et al., 2018; Lin et al., 2020). The ΔgcnE mutant was defected in asexual development with abnormal phialide formation. GcnE deficiency increased sensitivity to the cell wall perturbing agents (Congo Red, SDS) and oxidative stress inducing agents (H2O2, menadione), whereas the ΔgcnE mutant showed enhanced tolerance to osmotic stress inducing agents (LiCl) in comparison to the wild-type and complemented strains. Moreover, GcnE could control conidiogenesis, germination and biofilm formation of A. fumigatus and the biofilm formation was regulated by controlling the expression of ptaB and uge3 (Nossmann et al., 2018; Lin et al., 2020). GcnE does not seem to control virulence in an invasive A. fumigatus which is neutropenic.
In A. flavus, the acetylation target of GcnE is H3K14. GcnE plays an indispensable role in growth rate, sclerotial formation, morphological development, aflatoxin biosynthesis, stress responses and pathogenicity (Lan et al., 2016; Cai et al., 2018). The ΔgcnE mutant produced less mycelia and fewer branches at the mycelial tips than the WT and complementing strains according to microscopic analyses. Moreover, the cell surface hydrophobicity of the ΔgcnE mutant was decreased, which may be related to the inhibited production of aerial hyphae. ΔGcnE mutant was unable to produce the aflatoxin B1 (AFB1) and AFB2, the aflatoxin-specific regulatory genes (aflR, aflS) were lower than in the other two strains. In addition, GcnE is involved in responses to cell wall (calcofluor white, Congo red) and genotoxic stresses (methyl methanesulfonate), but not to hyperosmotic stresses (NaCl, KCl) or oxidative stresses (H2O2). GcnE may be a potential target for preventing A. flavus infections.
In A. nidulans, GcnE function was found to be required for the acetylation of histone H3K9/K14 (Qi et al., 2018). Conidiophore development was not observed in the ΔgcnE mutant, but it was restored when the gcnE deletion was complementated (Cánovas et al., 2014). Transcriptome analysis revealed that GcnE was involved in the regulation of conidiation and secondary metabolism genes, analysis of the gene expression revealed that the lack of conidiation originated in a complete absence of brlA expression in the ΔgcnE strain (Cánovas et al., 2014). Deletion of the gcnE gene decreased the production of selenic acid, sterigmatocystin, penicillin and terrequinone (Nuetzmann et al., 2011).
In A. niger, GcnE is associated with the production of multiple polyketide metabolites production. Studies have reported that 12 polyketide secondary metabolites were produced when the epigenetic regulator gene gcnE was deleted in A. niger (Wang et al., 2018; Li X. et al., 2021).
MYSTs
The MYST (Moz, YBF2, Sas2p, Tip) proteins are involved in a variety of biological processes, including gene regulation, DNA repair, cell-cycle regulation, and development. They are largely conserved from yeast to humans (Su et al., 2020a). MYST proteins constitute the largest HATs family and are named for the founding members MOZ, Ybf2/Sas3, Sas2, and TIP60 (Luan et al., 2016). In S. cerevisiae, Mysts contain three members including Esa1, Sas2, and Sas3, which function in DNA replication regulation and transcriptional silencing (Sapountzi and Côté, 2011).
EsaA (Esa1, Essential SAS family acetyltransferase) is the human homolog of Tip60, which mainly acetylates histone H4 lysine 5, 8, 12, 14. In A. nidulans, esaA serves a new involvement in the activation of four SM gene clusters via histone 4 lysine 12 (H4K12) acetylation (sterigmatocystin, penicillin, terrequinone, and orsellinic acid). Acetylation of SM gene promoters is increased by esaA overexpression. H4K12 concentrations might serve as a special indicator of relative production potential, particularly for SMs (Smith et al., 1998; Soukup et al., 2012).
In A. flavus, MystA (Sas2 orthologs) and MystB (Sas3 orthologs) are two traditional non-essential Myst enzymes found in nucleus and cytoplasm (Chen et al., 2022). MystA could acetylate H4K16, while MystB acetylates H3K14, H3K18, and H3K23. In the aspects of conidia formation, the regulatory genes of conidia formation (brlA and abaA) were significantly decreased in ΔMystB and ΔMystA/MystB. In A. flavus, MystA and MystB are necessary for vegetative development and the formation of asexual spores, and MysB has a significant function (Chen et al., 2022). In respect of sclerotia formation, MystA and MystB play opposite roles in the sclerotia regulation. Sclerotia is a dormant body interwoven with hyphae, which can resist adverse environments when fungi encounter environmental stress. When the environment is suitable, the sclerotia could germinate to produce new hyphae or form new propagules. Two essential regulatory genes (nsdC and nsdD), required for the production of sclerotia, were both markedly downregulated in the ΔMystB and ΔMystA/MystB but significantly increased in the ΔMystA (Chen et al., 2022). The research indicated that MystA performed a detrimental function in sclerotium development, but MystB was essential in A. flavus (Chen et al., 2022). In the aspect of aflatoxins biosynthesis, MystA and MystB played distinct but significant roles in the biosynthesis of AFB1, and MystB played an essential role in the regulation of aflatoxin (Chen et al., 2022).
Histone deacetylases
Histone deacetylation is the most widely studied among fungal epigenetic modifications (Bauer and Graessle, 2021). HDACs are important in eukaryotic cells which play an essential role in gene expression, transcription and post-transcriptional protein modification by reducing the acetylation level of histones. HDACs are currently divided into three classes in Aspergillus: (1) class I enzymes RpdA and HosA, two enzymes of RPD3-type; (2) class II HDACs HdaA and HosB, two enzymes of HDA1-type; (3) several members of the sirtuins class (Brosch et al., 2008; Tribus et al., 2010).
Histone deacetylases are important targets in tumor therapy, interfering with the acetylation pattern may provide a promising tool to attack diseases (Kim J. et al., 2018). The overexpression of HDACs in cancer cells breaks the acetylation balance of the original gene in transcription and expression, leading to tumorigenesis. HDAC inhibitor (HDACi) can inhibit the activity of HDACs in tumor cells, increase the degree of histone acetylation in tumor cells, re-activate the inhibited tumor suppressor genes, and then induce tumor cell differentiation and apoptosis. In recent years, the design and development of new HDACi has become a hot topic in the field of antitumor drugs, which is of great significance for the treatment of tumor diseases. Several HDAC inhibitors have been approved for clinical use. Non-selctive HDACi vorinostat has been approved to treat the cutaneous T-cell lymphoma, panobinostat could repress the metastasis in triple-negative breast cancer (Li and Seto, 2016; Luan et al., 2019).
Fungal HDACs are crucial in the stress signaling pathway of fungal drug resistance through epigenetic regulation, various virulence factors such as adhesion, hyphal formation and biofilm production are also regulated by fungal HDACs (Patrick et al., 2003; Su et al., 2020a). In C. albicans, HDAC is related to fungal pathogenicity and virulence, especially in the aspect of drug resistance. Studies have shown that HDAC inhibitors in combination with azoles against drug-resistant C. albicans showed synergistic effects in vitro, and histone deacetylation modification may be one of the upstream resistant mechanisms of C. albicans (Su et al., 2020b). The study of modification in Aspergillus will improve our understanding of the pathogenic fungus Aspergillus and provide a theoretical foundation for the prevention and management of fungi.
RpdA
RpdA is an RPD3-type HDAC of Aspergillus, it has become more and more evident that RpdA affects numerous biological processes in Aspergillus. A conserved fungal-specific C-terminal motif found in RpdA protein is necessary for nucleus localization, enzymatic activity, and viability during axenic development. RpdA is a viable target for new, fungus-focused inhibitors due to its fungal-specific motif.
In A. fumigatus, studies have shown that RpdA was related to vegetative growth and sporulation. The HDAC inhibitor Trichostatin A (TSA) decreased the growth of A. fumigatus. Earlier studies showed that single or even combined deletion of the three remaining HDACs (HdaA, HosA, and HosB) did not result in comparable defects in germination, growth or development (Shwab et al., 2007), it is reasonable to speculate that the main effect of TSA therapy is the suppression of RpdA activity. Furthermore, researches have demonstrated that A. fumigatus became avirulent in a mouse model of pulmonary aspergillosis due to downregulation of RpdA (Bauer et al., 2016, 2019).
In A. nidulans, RpdA is in charge of deacetylating H3 and H4. RpdA played an essential role in fungal development, the suppression of rpdA resulted in the loss of viability, growth and sporulation of A. nidulans (Tribus et al., 2010). In addition, RpdA could participate in SM biosynthesis. A large-scale metabolomics study of an A. nidulansΔrpdA mutant showed that RpdA was involved in positively and negatively regulating various products, downregulation of several known metabolites, primarily sterigmatocystin and the emericellamides, was caused by RpdA disturbance (Albright et al., 2015).
HosA
In A. flavus, H4K16 is the deacetylated target of HosA. Through functional analysis, it was found that the class I deacetylase HosA is involved in the growth, spore formation, sclerotium production, oxidative stress response and toxin production of A. flavus. When compared to WT and complementing ΔhosA strains, the ΔhosA mutant grew more slowly and produced much less conidia. HosA is necessary for the fungal vegetative growth and asexual development. In comparison to the WT and complimentary strains, the brlA and abaA essential regulatory genes, which are necessary for the activation of conidiation, were both dramatically downregulated in the ΔhosA mutant. Alternative reproduction and survival mechanisms known as sclerotia are used to adapt to unfavorable environmental conditions, while HosA played a negative role in sclerotial formation. NscD and sclR are transcription regulators encoding sclerotia formation, both gene expressions were increased substantially in the ΔhosA strains. In addition, HosA has detrimental effects on the oxidative stress response, and ΔhosA strain exhibited increased tolerance to medicines that generate free radicals. HosA is also involved in aflatoxin biosynthesis, in comparison to the WT strain, the ΔhosA strain produced less AFB1(Lan et al., 2019).
In A. nidulans, HosA is important for the growth and sporulation of colonies, deletion of HosA resulted in reduced growth and discoloration of hyphae and medium. In addition, deletion of HosA has no effect on A. nidulans’s susceptibility to the selected antifungal medications (voriconazole, fluconazole, amphotericin B, and caspofungin) (Pidroni et al., 2018).
In A. niger, HosA is associated with phenotypic change, stress resistance and SM biosynthesis. HosA is essential for stress tolerance and drug resistance, the ΔhosA strains showed significant defects in response to heat (42°C), oxidative stress (H2O2), osmotic stress (NaCl) and cell wall stress (calcofluor white). In addition, HosA could affect cell wall synthesis, the expression of genes related to cell wall synthesis was downregulated in ΔhosA strains. In the aspect of SM biosynthesis, the synthesis of fumonisin B1 and B2 was reduced (fold change ≥ 2) compared to that of the WT strains in the ΔhosA mutant (Li et al., 2019a).
HdaA
In A. fumigatus, HdaA deacetylates H3K9. HdaA is necessary for germination and vegetative growth and is involved in the regulation of SM production. Studies have shown that ΔhdaA strains were aberrant in SM production and the virulence factor gliotoxin was repressed. Furthermore, HdaA functioned as a positive regulator of germination and radial growth. Nonetheless, HdaA was to be independent of virulence properties and oxidative stress (Lee et al., 2009).
In A. flavus, H3K9 might be the deacetylation target of HdaA. Studies revealed that sclerotia production in the ΔhdaA mutant strain reduced dramatically, whereas, the complemented ΔhdaA.C strain restored the deficiency. The expression of sclerotia formation-related genes nsdC and sclR were decreased substantially in the ΔhdaA strain. In addition, HdaA was involved in aflatoxin biosynthesis, and the ΔhdaA strain produced significantly less AFB1 than the WT strain (Lan et al., 2019).
In A. nidulans, HdaA is involved in the control of enzymes that are crucial for the cellular antioxidant response, ΔhdaA strains showed a noteble reduction of growth under various oxidative stress settings (Tribus et al., 2005).
In A. niger, HdaA exhibited a modest influence on stress tolerance, the ΔhdaA strain was slightly sensitive to H2O2 and heat. Eight genes involved in the formation of cell walls showed downregulated expression in the ΔhdaA strain. HdaA played a role in SM biosynthesis. In ΔhdaA mutants, the well-known secondary metabolites fumonisin and kojic acid were reduced in the production. Consequently, HDACs could offer new strategies for the regulation of SM synthesis (Li et al., 2019a).
Sirtuins
Sirtuins (Silent information regulator 2 protein) are Class III HDACs, requiring NAD+ for histone deacetylation and participating in multiple biological regulation processes (Imai and Armstrong, 2000; Landry et al., 2000).
Sirtuin A (SirA) is a widely distributed NAD+-dependent HDAC with sequence homology to the silent information regulator (Sir2) protein from S. cerevisiae, which could convert euchromatin into heterochromatin and silence gene expression (Brosch et al., 2008). In A. nidulans, SirA could deacetylate the acetylated 16th lysine residue on histone H4. SirA is a transcriptional repressor of SM synthesis, which could suppress the production of austinol, dehydroaustinol, and sterigmatocystin (Itoh et al., 2017b).
Sirtuin E (SirE) is the Class Ic sirtuin of A. nidulans and its deacetylation sites including H3K9, H3K18, and H3K56. Studies have revealed that SirE was involved in autolysis, conidia development and fungal metabolism in A. nidulans. The ΔsirE strain showed reduced mycelial autolysis and defective conidia development. In addition, the expressions of corresponding regulatory genes were also decreased. Moreover, SirE was the global primary and secondary metabolic regulator during the cell growth cycle (Itoh et al., 2017a).
HstD, the homolog of SirE in A. nidulans, is a Class Ic sirtuin in A. oryzae. LaeA is a global regulator of secondary metabolism and cell development of filamentous fungi. HstD is the upstream of LeaA which could involve in the regulation of conidial information, SM biosynthesis and stress tolerance, the ΔhstD strain was sensitive to the osmotic stress (Kawauchi et al., 2013; Kawauchi and Iwashita, 2014).
Histone methylases
Histone methylation, which adds methyl groups to the lysine (K) and arginine (R) residues of histone H3 and H4, is one of the most essential post-transcriptional modifications (Gong and Miller, 2017; Li Y. et al., 2021). HMTs are mainly responsible for the methylation of the basic amino acid lysine on histones, adding one to three methyl groups to ε-amine groups to form monomethyl, dimethyl, or trimethyl lysine to act as the active or repressive marks of gene expression (Freitag, 2017; Zhang et al., 2021b). Histone methylation is inextricably linked to gene expression control, epigenetic disorders are an effective target for fungal treatment interventions.
RmtA
Protein arginine methyltransferases (PRMTs) are capable of catalyzing posttranslational arginine methylation. RmtA is the homolog of human PRMT1, which has intrinsic histone methyltransferase activity and methylates histone H4 at arginine 3 (H4R3) (Li et al., 2017). In A. flavus, studies have shown that RmtA was a positive regulator of sclerotia formation and aflatoxin development (Satterlee et al., 2019). In ΔrmtA mutants, there were fewer sclerotia formed compared to WT, the expression of related genes, nsdC and nsdD, were downregulated. RmtA gene played an important role in AFB1 biosynthesis, when compared to WT, ΔrmtA mutants displayed lower expression levels of the AFB1 synthesis-regulating gene aflR and the AFB1 biosynthesis genes aflC, and aflK (Li et al., 2017). Nevertheless, RmtA was a negative regulator of conidia development and oxidative stress tolerance. The expression levels of conidia development regulatory genes brlA, abaA, and wetA were significantly improved in the ΔrmtA mutants (Li et al., 2017), the ΔrmtA mutants presented an increased tolerance to oxidative stress compared to WT (Satterlee et al., 2016; Li et al., 2017).
Set1
In A. flavus, Set1 controls H3K4 and H3K9 dimethylation, and H3K4 trimethylation, which provides substantial evidence for identifying the biological activities of histone methyltransferase in pathogenic fungi. Studies revealed that Set1 was involved in the regulation of growth and pathogenicity of A. flavus. Set1 positively regulates colony formation and increases conidiation via an abaA and brlA mediated mechanism. Furthermore, Set1 controls the production of sclerotia via conventional sclerotia formation regulators, the expression levels of the major sclerotia formation regulators (nsdC, nsdD and sclR) were significantly reduced in the ΔSet1 strain. In the respect of aflatoxin synthesis, Set1 positively participates in the biological synthesis of AFB1. In the traditional aflatoxin production pathway, the expression levels of major transcriptional factor genes (aflR and aflS) as well as the enzyme genes (aflC, aflD, aflK, and aflO) were substantially down-regulated in ΔSet1 strain compared to WT. In addition, Set1 is also involved in environmental stress regulation including osmotic stress (H2O2), oxidative stress (NaCl, KCl) and plasma membrane stress (SDS). This might offer a genetic target for combating the detrimental effects of the activity of A. flavus (Li et al., 2017; Liu et al., 2020).
Dot1
Dot1 histone methyltransferase, which targets H3K79, is essential for meiotic checkpoint function. The positions and paralogs of Dot1 were notably conserved among Aspergillus genomes in terms of their biological roles. In A. flavus, Dot1 is 33% identical to yeast Dot1 generally (Ontoso et al., 2013). Studies have indicated that Dot1 was related to aflatoxin production, fungal pathogenicity, and fungal development. Disruption of the H3K79 methyltransferase gene dot1 reduced A. flavus radial growth and conidiation by down-regulating the transcriptional levels of the key transcription factors brlA and abaA. Dot1 contributes to aflatoxin biosynthesis, in the Δdot1 mutant, the AF-regulated gene (aflS) and the biosynthetic genes (aflC, aflO) were transcriptionally repressed. Moreover, AF production was drastically reduced. In addition, Dot1 has a potential role in response to genotoxicity stress, cell wall stress and oxidative stress. However, Dot1 plays a negative role in sclerotial reproduction, the transcription of the associated genes (nsdC, nsdD, sclR) was all up-regulated in the Δdot1 mutant (Liang et al., 2017). These studies may present a potential target for new strategies to control A. flavus.
ClrD
In A. fumigatus, ClrD was the homolog of Clr4, which is the major enzyme responsible for histone H3K9 methylation in Schizosaccharomyces pombe. ClrD is associated with the mono- and trimethylation of H3K9 and participates in normal growth and brlA-mediated conidiophore development (Palmer et al., 2008).
CclA
Bre2 is a crucial component of COMPASS in the SPRY domain protein in S. cerevisiae. Analysis of the A. nidulans genome revealed the existence of a possible ortholog of Bre2 called CclA, which is responsible for H3K4 dimethylation and trimethylation (Bok et al., 2009).
In A. fumigatus, ΔcclA strain grew weakly, as seen by diminished radial development. In particular, CclA is responsible for SM suppression. In comparison to WT, the ΔcclA mutant produced more than four times as much gliotoxin. A significant rise in the transcription of gliZ, the C6 transcription factor necessary for the production of gli, was linked to a high level of gliotoxin (Palmer et al., 2013).
In A. nidulans, CclA is critical in the regulation of phenotypic change and SMs synthesis. Studies have shown that ΔcclA led to a decrease in colony diameter and a defect in hyphal morphology (Giles et al., 2011).
Other histone posttranslational modifications
In addition to histone acetylation, deacetylation and methylation, there are also many histones post-translational modifications in Aspergillus species, such as demethylation, sumoylation, ubiquitination and phosphorylation, which could be involved in the regulation of cell growth, transcriptional regulation and secondary metabolism. In this section, we discussed the effects of histone demethylation and sumoylation on Aspergillus growth and pathogenicity.
Histone demethylases
In A. nidulans, KdmA (homolog of yeast Rph1) and KdmB (homolog of yeast Jhd2) are two histone demethylases that function as H3K36me3 demethylase and H3K4me3 demethylase respectively (Gacek-Matthews et al., 2015, 2016). KdmA is essential for the tolerance of detrimental light stimulation, which is associated with cell growth and development. Moreover, KdmA could positively regulate the genes transcribed during SM while negatively regulate the genes involving in energy metabolism and protein production and stability. KdmB plays an important role in transcription, especially in SM biosynthesis. Deletion of KdmB caused the dysregulation of genes involved in SM production (about 50% genes).
Sumoylation enzymes
Small ubiquitin-like modifier (SUMO) is mainly distributed in the nucleus. Sumoylation is an important post-translational modification which participates in the transcriptional regulation, DNA damage repairment and genome integrity maintenance. Sumoylation is a dynamic and reversible process. SUMO could conjugate to lysine residues in target proteins through an isopeptide linkage with the participation of activating enzyme E1 (AosA/UbaB), conjugating enzyme E2 (UbcN) and ligating enzyme E3 (SizA) (Chang and Yeh, 2020). In A. nidulans, potential sumoylation targets including histone modifiers (as the COMPASS complex), metabolic and stress response enzymes. Sumoylation was required for both sexual reproduction and asexual development. Deletion of aosA, ubaB, and ubcN resulted in the decreased asexual spore production and defected ascospores formation. In contrast to E1 and E2, E3 might play a special role in cell development, the sizA knockout strain displayed WT-like conidiospore formation and sexual development (Harting et al., 2013).
Potential antifungal compounds related to histone posttranslational modifications
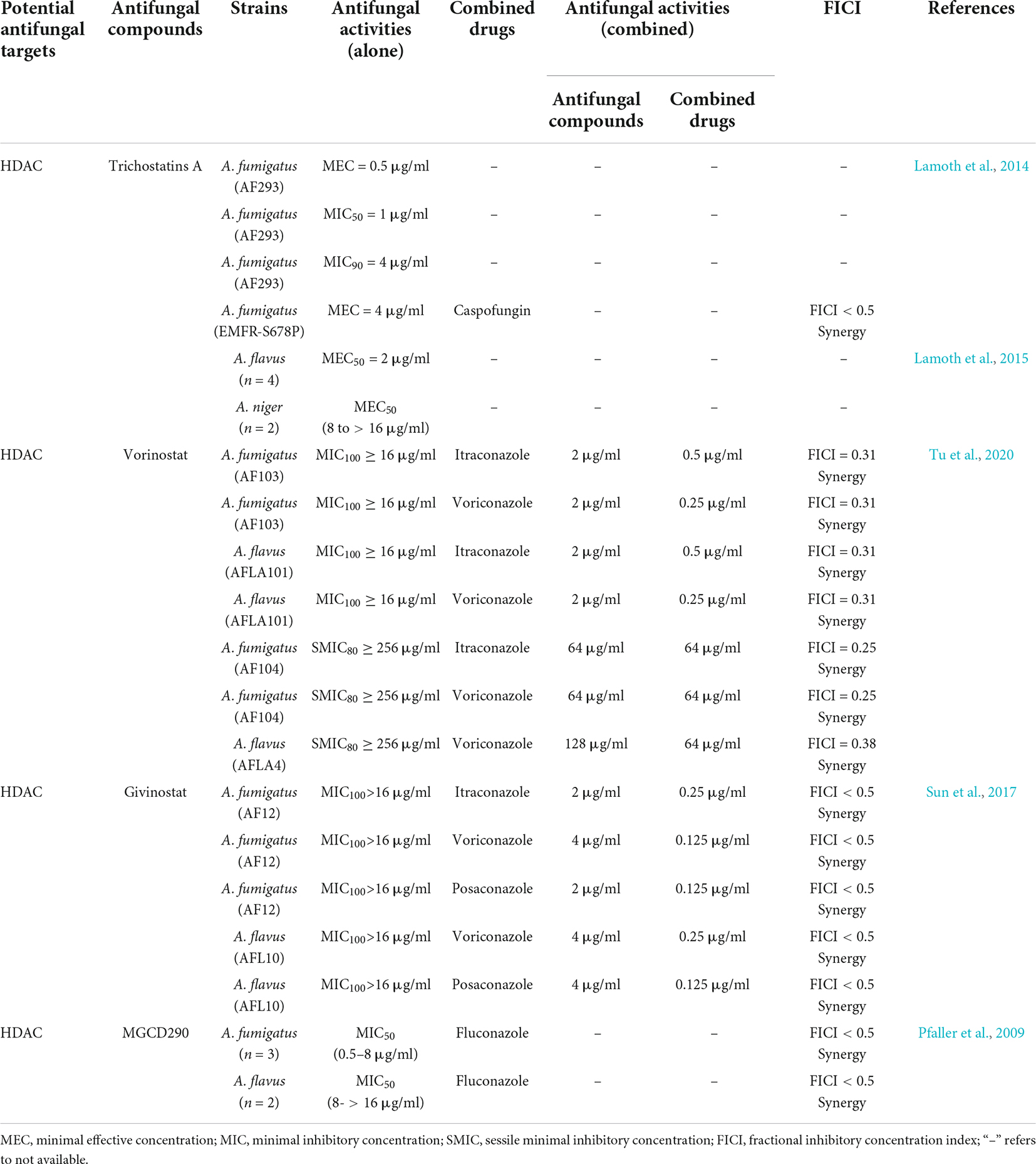
At present, some HPTM inhibitors are being developed as drugs for the treatment of various human diseases. Some HDAC inhibitors for the treatment of cancer, including vorinostat, romidepsin, belinostat, and panobinostat, have received Food and Drug Administration (FDA) approval (Campbell and Thomas, 2017; Sivaraj et al., 2017; Kim Y. H. et al., 2018; Athira et al., 2021; Shi et al., 2021). Although it has not been widely used in the treatment of fungal infections, the use of existing HPTM inhibitors or the use of these proteins as drug targets for the development of new antifungal compounds with great potential. Although these inhibitors may have side effects and toxicity, they are still effective. HPTM inhibitors can not only be applied to the existing approved inhibitors of “drug reuse,” but also be used for large-scale screening methods to determine new compounds for further application in the treatment of fungal infections (Li et al., 2019b). The antifungal activities of compounds targeted HPTMs were summarized in Table 2, and we mainly summarized the compounds targeted HDAC as below.
Trichostatins A (TSA) is a non-selective HDAC inhibitor that displays different degree of in vitro antifungal activity against Aspergillus. Lamoth et al. (2015) reported that the in vitro activity of TSA against A. fumigatus was active with a MEC50, MIC50 and MIC90 were achieved at concentrations of 0.5, 1, and 4 μg/ml, respectively. Moreover, the combination of TSA with caspofungin was synergistic against A. fumigatus. TSA had little activity against A. flavus isolates (MEC50 > 16 μg/ml), but it had better activity against A. niger, A. terreus, A. versicolor, and A. ustus (MEC50 = 2 μg/ml for 90% of these isolates) (Lamoth et al., 2014, 2015).
Vorinostat (SAHA) is a novel HDAC inhibitor which is the analog of TSA that has a prolonged half-life and enhanced oral bioavailability. Tu et al. (2020) reported that the ranges of the MICs (100% inhibition) of SAHA against Aspergillus spp. were ≥ 16 μg/ml, when SAHA was combined with itraconazole (ITR), voriconazole (VRC) and posaconazole (POC), the synergistic effects were observed in A. fumigatus strains, A. flavus strains and A. terreus strains. The sessile minimal inhibitory concentrations (sMIC80) were defined as concentrations that reduced the biofilm’s metabolic activity by 80%. SAHA alone was ineffective against the biofilm, and sMIC80 was greater than 128 μg/ml. For the combination of SAHA and triazoles, the fractional inhibitory concentration index (FICI) revealed synergistic effects against Aspergillus spp. (Tu et al., 2020).
Givinostat is a class I and class II HDAC inhibitor that can be taken orally and has anti-inflammatory characteristics (Sun et al., 2017). All Aspergillus isolates examined by givinostat alone showed minimal antifungal efficacy. Favorable synergistic effects between triazoles and givinostat were shown against A. fumigatus and A. flavus (Sun et al., 2017).
MGCD290 is a novel Hos2 histone inhibitor. Pfaller et al. (2009) revealed that MGCD290 demonstrated synergy against Aspergillus spp. when it was combined with fluconazole, voriconazole and voriconazole. The triazoles changed to a more susceptible category with 70% (7 of 10) Aspergillus isolates when in combination with MGCD290 (Pfaller et al., 2009).
Discussion and conclusion
Histone post-translational modifications play an important role in various cellular progress. In the past decade, advances in mass spectrometry-based proteomics provides new insights into the regulatory scope of post-translational modifications of non-histone proteins, which links to the regulation of pivotal pathways related to cellular response and protein functions (Buuh et al., 2017; Narita et al., 2019; Yang et al., 2022). SntB is a kind of epigenetic reader protein that assists in the recognition of histone tails. In A. nidulans, SntB was functioned as a transcriptional regulator that participated in the regulation of sterigmatocysin biosynthetic gene cluster (BGC). In A. flavus, SntB regulated H3K9 and H3K14 acetylation, aflatoxin synthesis, sclerotia formation and SM biosynthesis (Pfannenstiel et al., 2018). Cytological observations initially divided chromatin into two categories: euchromatin and heterochromatin (van Steensel, 2011). Heterochromatin protein 1 (HP1) can be involved in maintaining heterochromatin stability and transcriptional silencing, regulating gene expression, DNA replication repairmen (Leopold et al., 2019). In A. nidulans, hpeA is the homolog of HP1. Deletion of hepA resulted in the upregulation of SM biosynthesis genes, the genes involved in isopenicillin A production (ipnA) and in terraquinone A biosynthesis (tdiB) were significantly overexpressed (Reyes-Dominguez et al., 2010). In A. oryzae, SppA (signal peptide peptidase) is the homolog of S. cerevisiae SPP1. As a member of the COMPASS complex associated with Set1, SppA was involved in the SMs production including astellolides by affecting the methylation status of H3K4 (Shinohara et al., 2016).
In recent years, with the continuous development of medical treatments such as organ transplantation, immunosuppressants application, radiotherapy and chemotherapy, the incidence of invasive aspergillosis has increased. In addition, it has been reported that the resistance rate has increased. The increased morbidity and the limitation of therapeutic drugs prompted us to explore the mechanisms about the pathogenesis of Aspergillus. The discovery of novel antifungal targets and new antifungal agents is urgently needed. There are abundant histone posttranslational modifications in Aspergillus, possessing an important impact on protein structure, protein activity, stability, localization and protein-protein interactions, thereby regulating fungal growth, virulence, stress response and antifungal drug sensitivity in Aspergillus. In this article, we focused on several major Aspergillus (A. fumigatus, A. flavus, A. niger, A. terreus, A. nidulans, A. oryzae) and primary histone post-translational modifications including the process of histone acetylation, deacetylation, methylation, demethylation, and sumoylation (Zhao et al., 2011). More critically, we highlighted the essential role of HPTMs in fungal growth, pathogenicity and drug resistance, the potential anti-Aspergillus compounds targeting HPTMs were also summarized. This review will help us to further understand the relationship between HPTMs and pathogenicity in Aspergillus. Understanding the functions of HPTMs would contribute us to develop the fungal-specific targets, therefore probably reducing the side effects to the host. These findings will also contribute to the field of epigenetic regulation involved in the Aspergillus development and virulence. We believe that by learning more details between HPTMs and Aspergillus, it will make a lot of sense for developing new antifungal targets against fungal infections, as well as providing a theoretical foundation for fungus prevention and management.
Author contributions
YL wrote the review and created the figures. All authors contributed significantly and intellectually to the work, and they all gave their consent for it to be published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu, U. D., Alexandra, T., and Anna, R. (2016). Structure and functional diversity of GCN5-Related N-Acetyltransferases (GNAT). Int. J. Mol. Sci. 17:1018. doi: 10.3390/ijms17071018
Albright, J. C., Henke, M. T., Soukup, A. A., Mcclure, R. A., Thomson, R. J., Keller, N. P., et al. (2015). Large-scale metabolomics reveals A complex response of aspergillus nidulans to epigenetic perturbation. ACS Chem. Biol. 10, 1535–1541. doi: 10.1021/acschembio.5b00025
Athira, K. V., Sadanandan, P., and Chakravarty, S. (2021). Repurposing vorinostat for the treatment of disorders affecting brain. Neuromolecular Med. 23, 449–465. doi: 10.1007/s12017-021-08660-4
Bauer, I., and Graessle, S. (2021). Fungal lysine deacetylases in virulence, resistance, and production of small bioactive compounds. Genes 12:1470. doi: 10.3390/genes12101470
Bauer, I., Misslinger, M., Shadkchan, Y., Dietl, A. M., Petzer, V., Orasch, T., et al. (2019). The lysine deacetylase rpda is essential for virulence in aspergillus fumigatus. Front. Microbiol. 10:2773. doi: 10.3389/fmicb.2019.02773
Bauer, I., Varadarajan, D., Pidroni, A., Gross, S., Vergeiner, S., Faber, B., et al. (2016). A class 1 histone deacetylase with potential as an antifungal target. mBio 7:e00831-16. doi: 10.1128/mBio.00831-16
Bok, J. W., Chiang, Y. M., Szewczyk, E., Reyes-Dominguez, Y., Davidson, A. D., Sanchez, J. F., et al. (2009). Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol. 5, 462–464. doi: 10.1038/nchembio.177
Briggs, S. D., Bryk, M., Strahl, B. D., Cheung, W. L., Davie, J. K., Dent, S. Y., et al. (2001). Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15, 3286–3295. doi: 10.1101/gad.940201
Brosch, G., Loidl, P., and Graessle, S. (2008). Histone modifications and chromatin dynamics: A focus on filamentous fungi. FEMS Microbiol. Rev. 32, 409–439. doi: 10.1111/j.1574-6976.2007.00100.x
Buuh, Z. Y., Lyu, Z., and Wang, R. E. (2017). Interrogating the roles of post-translational modifications of non-histone proteins. J. Med. Chem. 61, 3239–3252.
Cadena, J., Thompson, G. R. III, and Patterson, T. F. (2021). Aspergillosis: Epidemiology, diagnosis, and treatment. Infect. Dis. Clin. North Am. 35, 415–434. doi: 10.1016/j.idc.2021.03.008
Cai, Q., Wang, J. J., Fu, B., Ying, S. H., and Feng, M. G. (2018). Gcn5-dependent histone H3 acetylation and gene activity is required for the asexual development and virulence of Beauveria bassiana. Environ. Microbiol. 20, 1484–1497. doi: 10.1111/1462-2920.14066
Campbell, P., and Thomas, C. M. (2017). Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma. J. Oncol. Pharm. Pract. 23, 143–147. doi: 10.1177/1078155216634178
Cánovas, D., Marcos, A. T., Gacek, A., Ramos, M. S., and Strauss, J. (2014). The Histone Acetyltransferase GcnE (GCN5) plays a central role in the regulation of Aspergillus asexual development. Genetics 197, 1175–1189. doi: 10.1534/genetics.114.165688
Chang, H. M., and Yeh, E. T. H. (2020). SUMO: From Bench to Bedside. Physiol. Rev. 100, 1599–1619. doi: 10.1152/physrev.00025.2019
Chen, X., Wu, L., Lan, H., Sun, R., Wen, M., Ruan, D., et al. (2022). Histone acetyltransferases MystA and MystB contribute to morphogenesis and aflatoxin biosynthesis by regulating acetylation in fungus Aspergillus flavus. Environ. Microbiol. 24, 1340–1361. doi: 10.1111/1462-2920.15856
Cheng, C., Zhao, X., Zhang, M., and Bai, F. (2016). Absence of Rtt109p, a fungal-specific histone acetyltransferase, results in improved acetic acid tolerance of Saccharomyces cerevisiae. FEMS Yeast Res. 16:fow010. doi: 10.1093/femsyr/fow010
Eissenberg, J. C., and Shilatifard, A. (2010). Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev. Biol. 339, 240–249. doi: 10.1016/j.ydbio.2009.08.017
Enoch, D. A., Yang, H., Aliyu, S. H., and Micallef, C. (2017). The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 1508, 17–65. doi: 10.1007/978-1-4939-6515-1_2
Freitag, M. (2017). Histone methylation by SET domain proteins in fungi. Annu. Rev. Microbiol. 71, 413–439. doi: 10.1146/annurev-micro-102215-095757
Frye, R. A. (2000). Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 273, 793–798. doi: 10.1006/bbrc.2000.3000
Gacek-Matthews, A., Berger, H., Sasaki, T., Wittstein, K., Gruber, C., Lewis, Z. A., et al. (2016). KdmB, a jumonji histone H3 demethylase, regulates genome-wide H3K4 trimethylation and is required for normal induction of secondary metabolism in Aspergillus nidulans. PLoS Genet. 12:e1006222. doi: 10.1371/journal.pgen.1006222
Gacek-Matthews, A., Noble, L. M., Gruber, C., Berger, H., Sulyok, M., Marcos, A. T., et al. (2015). KdmA, a histone H3 demethylase with bipartite function, differentially regulates primary and secondary metabolism in Aspergillus nidulans. Mol. Microbiol. 96, 839–860. doi: 10.1111/mmi.12977
Garzon-Porras, A. M., Chory, E., and Gryder, B. E. (2022). Dynamic opposition of histone modifications. ACS Chem. Biol. [Epub ahead of print]. doi: 10.1021/acschembio.1c01000
Giles, S. S., Soukup, A. A., Lauer, C., Shaaban, M., Lin, A., Oakley, B. R., et al. (2011). Cryptic Aspergillus nidulans antimicrobials. Appl. Environ. Microbiol. 77, 3669–3675. doi: 10.1128/AEM.02000-10
Gong, F., and Miller, K. M. (2017). Histone methylation and the DNA damage response. Mutat. Res. Rev. Mutat. Res. 780, 37–47.
Gresnigt, M. S., Becker, K. L., Leenders, F., Alonso, M. F., Wang, X., Meis, J. F., et al. (2018). Differential kinetics of Aspergillus nidulans and Aspergillus fumigatus phagocytosis. J. Innate Immun. 10, 145–160. doi: 10.1159/000484562
Harting, R., Bayram, O., Laubinger, K., Valerius, O., and Braus, G. H. (2013). Interplay of the fungal sumoylation network for control of multicellular development. Mol. Microbiol. 90, 1125–1145. doi: 10.1111/mmi.12421
Imai, S. I., and Armstrong, C. M. (2000). Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800.
Itoh, E., Shigemoto, R., Oinuma, K. I., Shimizu, M., Masuo, S., and Takaya, N. (2017b). Sirtuin A regulates secondary metabolite production by Aspergillus nidulans. J. Gen. Appl. Microbiol. 63, 228–235. doi: 10.2323/jgam.2016.11.002
Itoh, E., Odakura, R., Oinuma, K. I., Shimizu, M., Masuo, S., and Takaya, N. (2017a). Sirtuin E is a fungal global transcriptional regulator that determines the transition from the primary growth to the stationary phase. J. Biol. Chem. 292, 11043–11054. doi: 10.1074/jbc.M116.753772
Jenuwein, T., and Allis, C. D. (2001). Translating the histone code. Science 293, 1074–1080. doi: 10.1126/science.1063127
Johnson, E. S., Schwienhorst, I., Dohmen, R. J., and Blobel, G. (1997). The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519.
Johnson, E. S., and Blobel, G. (1997). Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J. Biol. Chem. 272, 26799–26802.
Johnson, E. S., and Gupta, A. A. (2001). An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106, 735–744. doi: 10.1016/s0092-8674(01)00491-3
Kawauchi, M., and Iwashita, K. (2014). Functional analysis of histone deacetylase and its role in stress response, drug resistance and solid-state cultivation in Aspergillus oryzae. J. Biosci. Bioeng. 118, 172–176. doi: 10.1016/j.jbiosc.2014.02.004
Kawauchi, M., Nishiura, M., and Iwashita, K. (2013). Fungus-specific sirtuin HstD coordinates secondary metabolism and development through control of LaeA. Eukaryot. Cell 12, 1087–1096. doi: 10.1128/EC.00003-13
Kim, J., Lee, J. E., and Lee, J. S. (2015). Histone deacetylase-mediated morphological transition in Candida albicans. J. Microbiol. 53, 805–811. doi: 10.1007/s12275-015-5488-3
Kim, J., Park, S., and Lee, J. S. (2018). Epigenetic control of oxidative stresses by histone acetyltransferases in Candida albicans. J. Microbiol. Biotechnol. 28, 181–189. doi: 10.4014/jmb.1707.07029
Kim, Y. H., Bagot, M., Pinter-Brown, L., Rook, A. H., Porcu, P., Horwitz, S. M., et al. (2018). Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 19, 1192–1204. doi: 10.1016/s1470-2045(18)30379-6
Koutelou, E., Hirsch, C. L., and Dent, S. Y. (2010). Multiple faces of the SAGA complex. Curr. Opin. Cell Biol. 22, 374–382. doi: 10.1016/j.ceb.2010.03.005
Kwon, S., Lee, J., Jeon, J., Kim, S., Park, S. Y., Jeon, J., et al. (2018). Role of the histone acetyltransferase Rtt109 in development and pathogenicity of the rice Blast fungus. Mol. Plant Microbe Interact. 31, 1200–1210. doi: 10.1094/MPMI-01-18-0015-R
Lamoth, F., Alexander, B. D., Juvvadi, P. R., and Steinbach, W. J. (2015). Antifungal activity of compounds targeting the Hsp90-calcineurin pathway against various mould species. J. Antimicrob. Chemother. 70, 1408–1411. doi: 10.1093/jac/dku549
Lamoth, F., Juvvadi, P. R., Soderblom, E. J., Moseley, M. A., Asfaw, Y. G., and Steinbach, W. J. (2014). Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 58, 1889–1896. doi: 10.1128/AAC.02286-13
Lan, H., Sun, R., Fan, K., Yang, K., Zhang, F., Nie, X. Y., et al. (2016). The Aspergillus flavus Histone Acetyltransferase AflGcnE regulates morphogenesis, aflatoxin biosynthesis, and pathogenicity. Front. Microbiol. 7:1324. doi: 10.3389/fmicb.2016.01324
Lan, H., Wu, L., Sun, R., Keller, N. P., Yang, K., Ye, L., et al. (2019). The HosA histone deacetylase regulates aflatoxin biosynthesis through direct regulation of aflatoxin Cluster Genes. Mol. Plant Microbe Interact. 32, 1210–1228. doi: 10.1094/MPMI-01-19-0033-R
Landry, J., Sutton, A., Tafrov, S. T., Heller, R. C., Stebbins, J., Pillus, L., et al. (2000). The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. U.S.A. 97, 5807–5811.
Lee, I., Oh, J.-H., Keats Shwab, E., Dagenais, T. R. T., Andes, D., and Keller, N. P. (2009). HdaA, a class 2 histone deacetylase of Aspergillus fumigatus, affects germination and secondary metabolite production. Fungal Genet. Biol. 46, 782–790. doi: 10.1016/j.fgb.2009.06.007
Lennartsson, A., and Ekwall, K. (2009). Histone modification patterns and epigenetic codes. Biochim. Biophys. Acta 1790, 863–868.
Leopold, K., Stirpe, A., and Schalch, T. (2019). Transcriptional gene silencing requires dedicated interaction between HP1 protein Chp2 and chromatin remodeler Mit1. Genes Dev. 33, 565–577. doi: 10.1101/gad.320440.118
Li, X., Huang, L., Pan, L., Wang, B., and Pan, L. (2021). CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite. Microbiol. Res. 245:126694. doi: 10.1016/j.micres.2020.126694
Li, Y., Chen, X., and Lu, C. (2021). The interplay between DNA and histone methylation: Molecular mechanisms and disease implications. EMBO Rep. 22:e51803.
Li, X., Yuan, M., Yin, R., Liu, X., Zhang, Y., Sun, S., et al. (2019b). Histone deacetylase inhibitor attenuates experimental fungal keratitis in mice. Sci. Rep. 9:9859. doi: 10.1038/s41598-019-46361-y
Li, X., Pan, L., Wang, B., and Pan, L. (2019a). The histone deacetylases HosA and HdaA affect the phenotype and transcriptomic and metabolic profiles of Aspergillus niger. Toxins 11:520. doi: 10.3390/toxins11090520
Li, Y., He, Y., Li, X., Fasoyin, O. E., Hu, Y., Liu, Y., et al. (2017). Histone Methyltransferase aflrmtA gene is involved in the morphogenesis, mycotoxin biosynthesis, and pathogenicity of Aspergillus flavus. Toxicon 127, 112–121. doi: 10.1016/j.toxicon.2017.01.013
Li, Y., and Seto, E. (2016). HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 6:a026831. doi: 10.1101/cshperspect.a026831
Liang, L., Liu, Y., Yang, K., Lin, G., Xu, Z., Lan, H., et al. (2017). The putative histone methyltransferase DOT1 regulates aflatoxin and pathogenicity attributes in Aspergillus flavus. Toxins 9:232. doi: 10.3390/toxins9070232
Lin, C. J., Hou, Y. H., and Chen, Y. L. (2020). The histone acetyltransferase GcnE regulates conidiation and biofilm formation in Aspergillus fumigatus. Med. Mycol. 58, 248–259. doi: 10.1093/mmy/myz043
Lin, Y., Qiu, T., Wei, G., Que, Y., Wang, W., Kong, Y., et al. (2022). Role of histone post-translational modifications in inflammatory diseases. Front. Immunol. 13:852272. doi: 10.3389/fimmu.2022.852272
Liu, Y., Zhang, M., Xie, R., Zhang, F., Wang, S., Pan, X., et al. (2020). The methyltransferase AflSet1 is involved in fungal morphogenesis, AFB1 biosynthesis, and virulence of Aspergillus flavus. Front. Microbiol. 11:234. doi: 10.3389/fmicb.2020.00234
Lohmar, J. M., Puel, O., Cary, J. W., and Calvo, A. M. (2019). The Aspergillus flavus rtfA Gene regulates plant and animal pathogenesis and secondary metabolism. Appl. Environ. Microbiol. 85:e02446-18. doi: 10.1128/AEM.02446-18
Lopes da Rosa, J., Bajaj, V., Spoonamore, J., and Kaufman, P. (2013). A small molecule inhibitor of fungal histone acetyltransferase Rtt109. Bioorg. Med. Chem. Lett. 23, 2853–2859. doi: 10.1016/j.bmcl.2013.03.112
Lopez, C., Barnon, M. T., Beacon, T. H., Nardocci, G., and Davie, J. R. (2022). The key role of differential broad H3K4me3 and H3K4ac domains in breast cancer. Gene 826:146463. doi: 10.1016/j.gene.2022.146463
Luan, Y., Li, J., Bernatchez, J., and Li, R. (2019). Kinase and histone deacetylase hybrid inhibitors for cancer therapy. J. Med. Chem. 62.
Luan, Y., Wu, J., and Zhang, X. (2016). Tip60: Main functions and its inhibitors. Mini Rev. Med. Chem. 17, 675–682.
Mani Chandrika, K. V. S., and Sharma, S. (2020). Promising antifungal agents: A minireview. Bioorg. Med. Chem. 28:115398. doi: 10.1016/j.bmc.2020.115398
Mcbride, A. E., Weiss, V. H., Kim, H. K., Hogle, J. M., and Silver, P. A. (2000). Analysis of the yeast arginine methyltransferase Hmt1p/Rmt1p and its in vivo function COFACTOR BINDING AND SUBSTRATE INTERACTIONS. J. Biol. Chem. 275, 3128–3136.
Nakayama, J., Rice, J. C., Strahl, B. D., Allis, C. D., and Grewal, S. I. (2001). Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113. doi: 10.1126/science.1060118
Narita, T., Weinert, B. T., and Choudhary, C. (2019). Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 20, 156–174. doi: 10.1038/s41580-018-0081-3
Nie, X., Yu, S., Qiu, M., Wang, X., Wang, Y., Bai, Y., et al. (2016). Aspergillus flavus SUMO contributes to fungal virulence and toxin attributes. J. Agric. Food Chem. 64, 6772–6782. doi: 10.1021/acs.jafc.6b02199
Nossmann, M., Boysen, J. M., Krüger, T., König, C. C., Hillmann, F., Munder, T., et al. (2018). Yeast two-hybrid screening reveals a dual function for the histone acetyltransferase GcnE by controlling glutamine synthesis and development in Aspergillus fumigatus. Curr. Genet. 65, 523–538. doi: 10.1007/s00294-018-0891-z
Nuetzmann, H. W., Reyes-Dominguez, Y., Scherlach, K., Schroeckh, V., Horn, F., Gacek, A., et al. (2011). Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. U.S.A. 108, 14282–14287. doi: 10.1073/pnas.1103523108
O”Meara, T. R., Hay, C., Price, M. S., Giles, S., and Alspaugh, J. A. (2010). Cryptococcus neoformans histone acetyltransferase Gcn5 regulates fungal adaptation to the host. Eukaryot. Cell 9, 1193–1202. doi: 10.1128/EC.00098-10
Ontoso, D., Acosta, I., van Leeuwen, F., Freire, R., and San-Segundo, P. A. (2013). Dot1-dependent histone H3K79 methylation promotes activation of the Mek1 meiotic checkpoint effector kinase by regulating the Hop1 adaptor. PLoS Genet. 9:e1003262. doi: 10.1371/journal.pgen.1003262
Palmer, J. M., Bok, J. W., Lee, S., Dagenais, T. R. T., Andes, D. R., Kontoyiannis, D. P., et al. (2013). Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 1:e4. doi: 10.7717/peerj.4
Palmer, J. M., Perrin, R. M., Dagenais, T. R., and Keller, N. P. (2008). H3K9 methylation regulates growth and development in Aspergillus fumigatus. Eukaryot. Cell 7, 2052–2060. doi: 10.1128/EC.00224-08
Patrick, T., Brandtner, E. M., Gerald, B., Peter, L., Johannes, G., Roland, L., et al. (2003). Histone deacetylases in fungi: Novel members, new facts. Nucleic Acids Res. 31, 3971–3981. doi: 10.1093/nar/gkg473
Peng, C., Fan, X., and Chen, J. (2015). Function and subcellular localization of Gcn5, a histone acetyltransferase in Candida albicans. Fungal Genet. Biol. 81, 132–141. doi: 10.1016/j.fgb.2015.01.011
Pfaller, M. A., Messer, S. A., Georgopapadakou, N., Martell, L. A., Besterman, J. M., and Diekema, D. J. (2009). Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J. Clin. Microbiol. 47, 3797–3804. doi: 10.1128/JCM.00618-09
Pfannenstiel, B. T., Greco, C., Sukowaty, A. T., and Keller, N. P. (2018). The epigenetic reader SntB regulates secondary metabolism, development and global histone modifications in Aspergillus flavus. Fungal Genet. Biol. 120, 9–18. doi: 10.1016/j.fgb.2018.08.004
Pidroni, A., Faber, B., Brosch, G., Bauer, I., and Graessle, S. (2018). A Class 1 Histone deacetylase as major regulator of secondary metabolite production in Aspergillus nidulans. Front. Microbiol. 9:2212. doi: 10.3389/fmicb.2018.02212
Qi, S., He, L., Zhang, Q., Dong, Q., Wang, Y., Yang, Q., et al. (2018). Cross-pathway control gene CPC1/GCN4 coordinates with histone acetyltransferase GCN5 to regulate catalase-3 expression under oxidative stress in Neurospora crassa. Free Radic. Biol. Med. 117, 218–227. doi: 10.1016/j.freeradbiomed.2018.02.003
Reyes-Dominguez, Y., Bok, J. W., Berger, H., Shwab, E. K., Basheer, A., Gallmetzer, A., et al. (2010). Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol. Microbiol. 76, 1376–1386. doi: 10.1111/j.1365-2958.2010.07051.x
Saavedra, F., Marty-Lombardi, S., and Loyola, A. (2018). Characterization of posttranslational modifications on histone variants. Methods Mol. Biol. 1832, 21–49. doi: 10.1007/978-1-4939-8663-7_2
Sapountzi, V., and Côté, J. (2011). MYST-family histone acetyltransferases: Beyond chromatin. Cell. Mol. Life Sci. 68, 1147–1156.
Satterlee, T., Cary, J. W., and Calvo, A. M. (2016). RmtA, a putative arginine methyltransferase, regulates secondary metabolism and development in Aspergillus flavus. PLoS One 11:e0155575. doi: 10.1371/journal.pone.0155575
Satterlee, T., Entwistle, S., Yin, Y., Cary, J. W., Lebar, M., Losada, L., et al. (2019). rmtA-dependent transcriptome and its role in secondary metabolism, environmental stress, and virulence in Aspergillus flavus. G3 9, 4087–4096. doi: 10.1534/g3.119.400777
Shi, Y., Fu, Y., Zhang, X., Zhao, G., Yao, Y., Guo, Y., et al. (2021). Romidepsin (FK228) regulates the expression of the immune checkpoint ligand PD-L1 and suppresses cellular immune functions in colon cancer. Cancer Immunol. Immunother. 70, 61–73. doi: 10.1007/s00262-020-02653-1
Shimizu, M., Masuo, S., Fujita, T., Doi, Y., Kamimura, Y., and Takaya, N. (2012). Hydrolase controls cellular NAD, sirtuin, and secondary metabolites. Mol. Cell. Biol. 32, 3743–3755. doi: 10.1128/MCB.00032-12
Shinohara, Y., Kawatani, M., Futamura, Y., Osada, H., and Koyama, Y. (2016). An overproduction of astellolides induced by genetic disruption of chromatin-remodeling factors in Aspergillus oryzae. J. Antibiot. 69, 4–8. doi: 10.1038/ja.2015.73
Shwab, E. K., Bok, J. W., Tribus, M., Galehr, J., Graessle, S., and Keller, N. P. (2007). Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell 6, 1656–1664.
Sivaraj, D., Green, M. M., and Gasparetto, C. (2017). Panobinostat for the management of multiple myeloma. Future Oncol. 13, 477–488.
Smith, E. R., Eisen, A., Gu, W., Sattah, M., Pannuti, A., Zhou, J., et al. (1998). ESA1 is a histone acetyltransferase that is essential for growth in yeast. Proc. Natl. Acad. Sci. U.S.A. 95, 3561–3565.
Soukup, A. A., Chiang, Y. M., Bok, J. W., Reyes-Dominguez, Y., Oakley, B. R., Wang, C. C., et al. (2012). Overexpression of the Aspergillus nidulans histone 4 acetyltransferase EsaA increases activation of secondary metabolite production. Mol. Microbiol. 86, 314–330. doi: 10.1111/j.1365-2958.2012.08195.x
Su, S., Li, X., Yang, X., Li, Y., and Jia, S. (2020a). Histone acetylation/deacetylation in Candida albicans and their potential as antifungal targets. Future Microbiol. 15, 1075–1090. doi: 10.2217/fmb-2019-0343
Su, S., Shi, X., Xu, W., Li, Y., Chen, X., Jia, S., et al. (2020b). Antifungal activity and potential mechanism of panobinostat in combination with fluconazole against Candida albicans. Front. Microbiol. 11:1584. doi: 10.3389/fmicb.2020.01584
Sugui, J. A., Kwon-Chung, K. J., Juvvadi, P. R., Latge, J. P., and Steinbach, W. J. (2014). Aspergillus fumigatus and related species. Cold Spring Harb. Perspect. Med. 5:a019786. doi: 10.1101/cshperspect.a019786
Sun, R., Wen, M., Wu, L., Lan, H., Yuan, J., and Wang, S. (2021). The Fungi-specific histone Acetyltransferase Rtt109 mediates morphogenesis, Aflatoxin synthesis and pathogenicity in Aspergillus flavus by acetylating H3K9. IMA Fungus 12:9. doi: 10.1186/s43008-021-00060-4
Sun, Y., Gao, L., He, C., Wu, Q., Li, M., and Zeng, T. (2017). Givinostat exhibits in vitro synergy with posaconazole against Aspergillus spp. Med. Mycol. 55, 798–802. doi: 10.1093/mmy/myw131
Tang, Y., Meeth, K., Jiang, E., Luo, C., and Marmorstein, R. (2008). Structure of Vps75 and implications for histone chaperone function. Proc. Natl. Acad. Sci. U.S.A. 105, 12206–12211. doi: 10.1073/pnas.0802393105
Tribus, M., Bauer, I., Galehr, J., Rieser, G., Trojer, P., Brosch, G., et al. (2010). A novel motif in fungal class 1 histone deacetylases is essential for growth and development of Aspergillus. Mol. Biol. Cell. 21, 345–353. doi: 10.1091/mbc.E09-08-0750
Tribus, M., Galehr, J., Trojer, P., Brosch, G., Loidl, P., Marx, F., et al. (2005). HdaA, a major class 2 histone deacetylase of Aspergillus nidulans, affects growth under conditions of oxidative stress. Eukaryot. Cell 4, 1736–1745. doi: 10.1128/EC.4.10.1736-1745.2005
Tu, B., Yin, G., and Li, H. (2020). Synergistic effects of vorinostat (SAHA) and azoles against Aspergillus species and their biofilms. BMC Microbiol. 20:28. doi: 10.1186/s12866-020-1718-x
Valencia-Sanchez, M. I., De Ioannes, P., Wang, M., Truong, D. M., Lee, R., Armache, J. P., et al. (2021). Regulation of the Dot1 histone H3K79 methyltransferase by histone H4K16 acetylation. Science 371:eabc6663. doi: 10.1126/science.abc6663
van Steensel, B. (2011). Chromatin: Constructing the big picture. EMBO J. 30, 1885–1895. doi: 10.1038/emboj.2011.135
von Lilienfeld-Toal, M., Wagener, J., Einsele, H., Cornely, O. A., and Kurzai, O. (2019). Invasive Fungal Infection. Dtsch. Arztebl. Int. 116, 271–278. doi: 10.3238/arztebl.2019.0271
Wang, B., Li, X., Yu, D., Chen, X., Tabudravu, J., Deng, H., et al. (2018). Deletion of the epigenetic regulator GcnE in Aspergillus niger FGSC A1279 activates the production of multiple polyketide metabolites. Microbiol. Res. 217, 101–107. doi: 10.1016/j.micres.2018.10.004
Xu, H., Wu, M., Ma, X., Huang, W., and Xu, Y. (2021). Function and mechanism of novel histone posttranslational modifications in health and disease. Biomed. Res. Int. 2021:6635225. doi: 10.1155/2021/6635225
Yang, B., Miller, A., and Kirchmaier, A. L. (2008). HST3/HST4-dependent deacetylation of lysine 56 of histone H3 in silent chromatin. Mol. Biol. Cell 19, 4993–5005. doi: 10.1091/mbc.E08-05-0524
Yang, G., Hu, Y., Fasoyin, O., Yue, Y., Chen, L., Qiu, Y., et al. (2018). The Aspergillus flavus phosphatase CDC14 regulates development, aflatoxin biosynthesis and pathogenicity. Front. Cell. Infect. Microbiol. 8:141. doi: 10.3389/fcimb.2018.00141
Yang, K., Liu, Y., Wang, S., Wu, L., Xie, R., Lan, H., et al. (2019). Cyclase-associated protein cap with multiple domains contributes to mycotoxin biosynthesis and fungal virulence in Aspergillus flavus. J. Agric. Food Chem. 67, 4200–4213. doi: 10.1021/acs.jafc.8b07115
Yang, G., Yue, Y., Ren, S., Yang, M., Zhang, Y., Cao, X., et al. (2019). Lysine acetylation contributes to development, aflatoxin biosynthesis and pathogenicity in Aspergillus flavus. Environ. Microbiol. 21, 4792–4807. doi: 10.1111/1462-2920.14825
Yang, K., Tian, J., and Keller, N. P. (2022). Post-translational modifications drive secondary metabolite biosynthesis in Aspergillus: A review. Environ. Microbiol. 24, 2857–2881. doi: 10.1111/1462-2920.16034
Zhang, L., Serra-Cardona, A., Zhou, H., Wang, M., Yang, N., Zhang, Z., et al. (2018). Multisite Substrate Recognition in Asf1-Dependent Acetylation of Histone H3 K56 by Rtt109. Cell 174, 818–830.e11. doi: 10.1016/j.cell.2018.07.005
Zhang, Y., Sun, Z., Jia, J., Du, T., Zhang, N., Tang, Y., et al. (2021b). Overview of Histone Modification. Adv. Exp. Med. Biol. 1283, 1–16. doi: 10.1007/978-981-15-8104-5_1
Zhang, Y., Fan, J., Ye, J., and Lu, L. (2021a). The fungal-specific histone acetyltransferase Rtt109 regulates development, DNA damage response, and virulence in Aspergillus fumigatus. Mol. Microbiol. 115, 1191–1206. doi: 10.1111/mmi.14665
Keywords: invasive aspergillosis, histone post-translational modifications, histone acetylation, histone deacetylation, histone methylation
Citation: Li Y, Song Z, Wang E, Dong L, Bai J, Wang D, Zhu J and Zhang C (2022) Potential antifungal targets based on histones post-translational modifications against invasive aspergillosis. Front. Microbiol. 13:980615. doi: 10.3389/fmicb.2022.980615
Received: 28 June 2022; Accepted: 27 July 2022;
Published: 09 August 2022.
Edited by:
Shigeki Nakamura, Tokyo Medical University, JapanReviewed by:
Takashi Umeyama, National Institute of Infectious Diseases (NIID), JapanShihua Wang, Fujian Agriculture and Forestry University, China
Kunlong Yang, Jiangsu Normal University, China
Copyright © 2022 Li, Song, Wang, Dong, Bai, Wang, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Zhang, bGF1cmFsLnpoYW5nQHlhaG9vLmNvbQ==
 Yiman Li1
Yiman Li1 Zhihui Song
Zhihui Song Ente Wang
Ente Wang Chao Zhang
Chao Zhang