- 1Nepal Health Research Council, Kathmandu, Nepal
- 2Department of Pediatrics, Kanti Children’s Hospital, Kathmandu, Nepal
- 3Shi-Gan International College of Science and Technology, Kathmandu, Nepal
- 4Provincial Hospital, Malangwa, Nepal
- 5Department of Pediatric Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal
Background: Typhoid fever, an infective bacterial disease, is capable of causing fatal systemic infection in humans, and in an era of antimicrobial resistance, it has become of public health importance. This study aimed to investigate the laboratory diagnosis of Salmonella bloodstream infection, its serotype, antimicrobial resistance pattern, and seasonal variation at a tertiary care children’s hospital.
Methods: We undertook a retrospective, cross-sectional study by reviewing hospital-based laboratory records of patients whose blood culture samples were submitted from the outpatient department to the laboratory of a tertiary care children’s hospital in Kathmandu, Nepal, from January 2017 to January 2019.
Results: Among the total blood culture samples obtained (n = 39,771), bacterial isolates (n = 1,055, 2.65%) belonged either to the Genus Enterobacteriaceae or Genus Acinetobacter. Altogether (n = 91, 8.63%), isolates were positive for Salmonella spp., which were further identified as Salmonella enterica subsp. enterica ser. Typhi (n = 79, 7.49%), Salmonella enterica subsp. enterica ser. Paratyphi A (n = 11, 1.04%), and Salmonella enterica subsp. enterica ser. Paratyphi B (n = 1, 0.1%). The median age of patients was 6 years (IQR: 4–9), with male and female patients constituting (n = 53, 58.24%; OR, 1.0; 95% CI, 0.60–1.67) and (n = 38, 41.76%; OR, 0.98; 95% CI, 0.49–2.05) cases, respectively. The disease was observed throughout the year, with a high prevalence toward the spring season (March–May). An antibiogram showed resistance more toward nalidixic acid with S. Typhi, comprising half the isolates (n = 52, 65.82%; p = 0.11). Resistance toward β-lactams with β-lactamase inhibitors (amoxicillin/clavulanate; 1.27%) was seen in a single isolate of S. Typhi. The multidrug resistance pattern was not pronounced. The multiple antibiotic resistance (MAR) index was in the range between 0.14 and 0.22 in S. Typhi and 0.22 and 0.23 in S. Paratyphi.
Conclusion: Salmonella Typhi was the predominant ser. Infection was common among children between 1 and 5 years of age, showing male predominance and with the spring season contributing to a fairly higher number of cases. Antimicrobial susceptibility testing of S. Typhi showed more resistance toward nalidixic acid, with only a single isolate resistant to β-lactamase inhibitors (amoxicillin/clavulanate). Alarming multidrug resistance patterns were not observed. The MAR index in this study indicates the importance of the judicious use of antimicrobials and hospital infection prevention and control practices.
1. Introduction
Typhoid fever is a life-threatening systemic infection affecting 11–20 million people and with almost 128,000 to 161,000 people dying from it each year in numerous growing parts of the WHO African, Eastern Mediterranean, South-East Asia, and Western Pacific Regions (WHO, 2018). This disease is associated with the bacterium Salmonella enterica serotype Typhi, a member of the family Enterobacteriaceae, which shows seropositivity for a range of both capsular and flagellar antigens that include lipopolysaccharide antigens O9 and O12, protein flagellar antigen Hd, and polysaccharide capsular antigen Vi (Parry et al., 2002). Typhoid fever is a disease of significant importance in overcrowded and unsanitary conditions, as seen in many developing countries and, infrequently, in developed nations, where only sporadic cases are reported among travelers returning from endemic areas (Osler, 1912; Ackers et al., 2000). Several factors come into play for the transmission of this infection, some of which include food consumption from street vendors such as ice cream or flavored iced drinks (Black et al., 1985; Luby et al., 1998), substandard housing conditions, and personal hygiene, as well as the recent use of antimicrobial drugs (Luby et al., 1998).
The symptoms of the disease include protracted fever, lassitude, headache, nausea, abdominal pain, constipation or diarrhea, and an occasional rash, with death in severe and complicated cases. Chloramphenicol, once regarded as a game changer in the management of severe, incapacitating, and frequently lethal disease into a treatable illness, saw a shift in its treatment guidelines, including management with the fluoroquinolone group of drugs, paving the path for newer-generation cephalosporins and azithromycin in affected areas (Woodward et al., 2004; WHO, 2014).
Nonetheless, among the preventive measures aimed at reducing the risk of typhoid fever, vaccination also serves as a good strategy for S. Typhi prevention.
The first identification of typhoid infection in Nepal was in a British-Nepalese soldier in 1984, followed by an infant in 1989 (Klonin et al., 1989). Since then, the disease has been reported in all regions of Nepal and has affected individuals of all ages, with a disproportionately high number of cases occurring among children and young adults (Gupta et al., 2021). Moreover, the growing concern about the rise of antimicrobial resistance in all infectious diseases has made S. Typhi an important bacterium of concern for treatment in endemic regions such as Nepal. Therefore, the primary objective of this study was to determine the presence of typhoidal Salmonella in the blood culture of pediatric patients attending the outpatient department of a tertiary care children’s hospital. The study also aimed to identify the serotype of Salmonella, analyze its antimicrobial resistance pattern, and assess any seasonal variations over 2 years.
2. Methods
2.1. Study design and patients
A retrospective, cross-sectional study was conducted by retrieving documented paper-based hospital laboratory records from the Department of Microbiology at Kanti Children’s Hospital, Kathmandu, Nepal. The main catchment area for the hospital is the Kathmandu Valley (population ~3,025,386; Central Bureau of Statistics, 2021), but as the only government-run children’s hospital in Nepal, it also caters to children from all other regions of the country. Blood culture samples collected from pediatric patients visiting the outpatient department of the hospital were reviewed. The demographic data of the patients, isolated organisms, and their respective antibiograms within 2 years duration (January 2017 to January 2019) were included. Any incomplete data were excluded from the study. The study was approved by the Institutional Review Committee (IRC) of Kanti Children’s Hospital, Maharajgunj, Kathmandu, Nepal (IRC No: 969). Laboratory tests were performed as a part of routine diagnostic procedures based on the clinician’s need. Hence, patient-informed consent was not applicable.
2.2. Laboratory procedure
Approximately 3 mL of blood sample was inoculated in Bactalert culture bottles. Once growth was indicated by the Bactalert system, biochemical tests using commercially available media preparations (Hi-Media Laboratories, Mumbai, India) were prepared in-house using standard methods and techniques (Collee et al., 1996) for bacterial identification. A slide agglutination test using commercially available antisera was performed for Salmonella spp. following the manufacturer’s instruction (Denka Seiken Co., Ltd., Chuo-Ku, Tokyo, Japan), and the Genus Salmonella and its serogroups were identified using the antigenic classification of the Kauffmann–White Scheme. An antimicrobial susceptibility test by the Kirby–Bauer disc diffusion method was performed using a Muller Hinton agar (Hi-Media Laboratories, Mumbai, India) following the 29th edition CLSI guidelines (Weinstein et al., 2019). Antibiotic discs included in this study were Ampicillin (10 μg), Amoxicillin (10 μg), Cefixime (5 μg), Cefotaxime (30 μg), Cefpodoxime (30 μg), Ceftriaxone (30 μg), Ceftazidime (30 μg), Cefepime (30 μg), Ciprofloxacin (5 μg), Ofloxacin (5 μg), Trimethoprim/sulfamethoxazole (Cotrimoxazole; 1.25/23.75 μg), Chloramphenicol (30 μg), Nalidixic acid (30 μg), Amoxicillin/clavulanate (20/10 μg), Ampicillin/sulbactam (10/10 μg), and Imipenem (10 μg; Hi-Media Laboratories, Mumbai, India). An antimicrobial susceptibility pattern was determined as sensitive, intermediate, and resistant according to the 29th edition CLSI guidelines (Weinstein et al., 2019). Furthermore, the multiple antibiotic resistance (MAR) index was calculated as the ratio of the number of resistant antibiotics to which the organism is resistant to the total number of antibiotics the organism is exposed to. MAR index values more than 0.2 specify high-risk sources of contamination where antibiotics are frequently used (Krumperman, 1983).
2.3. Sample workflow
See Figure 1.
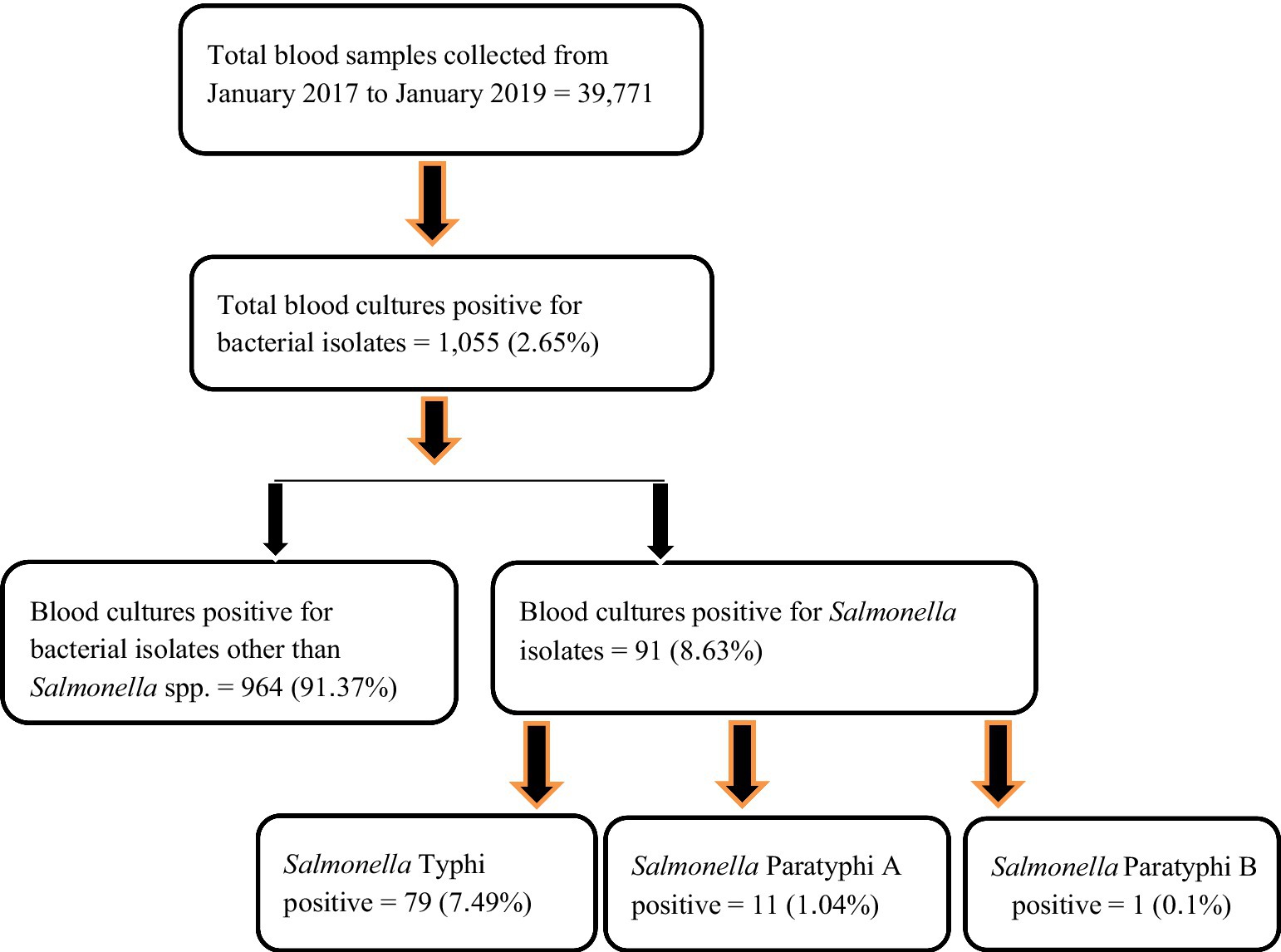
Figure 1. Sample workflow of Salmonella isolates from blood samples of pediatric patients’ (n = 91).
2.4. Statistical analysis
All the data were entered into a Microsoft Excel 2007 spreadsheet from the paper-based hospital records and further analyzed by SPSS software version 17. All the descriptive and inferential data were calculated using SPSS software. The Chi-square test was used to analyze the categorical data.
3. Results
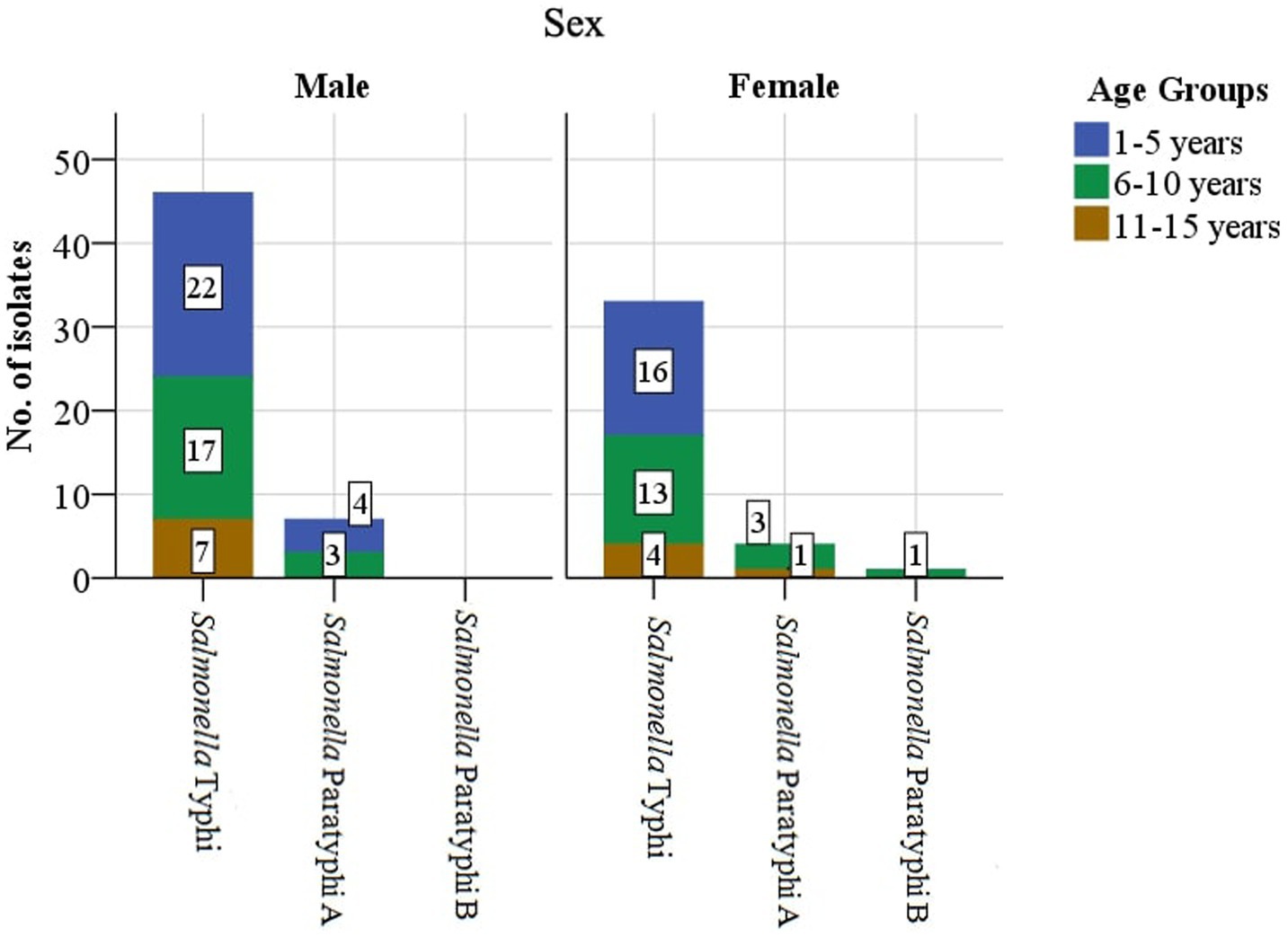
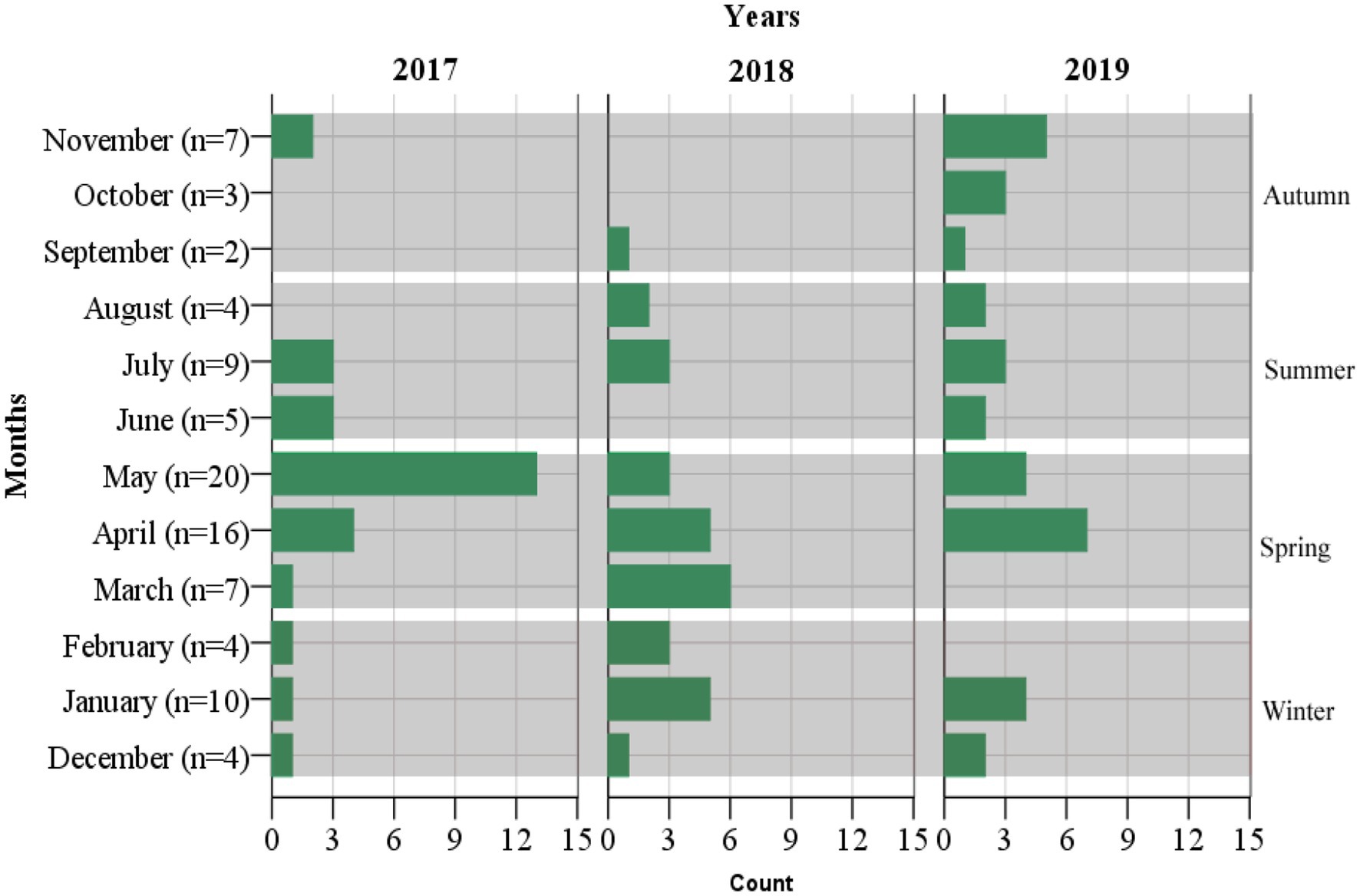
Enteric fever is common in Nepal, and Kathmandu Valley is an endemic region for this infection due to its known risk of substandard water quality at the community level and its poor sanitation and hygiene, jeopardizing the health of individuals and increasing their chances of acquiring infection (Karkey et al., 2013). Our hospital is the only tertiary-level, government-run children’s hospital, located in Maharajgunj, Kathmandu, and it caters to hundreds of children from different socio-economic statuses coming to receive quality treatment. Among the total blood culture samples (n = 39,771) collected, (n = 1,055, 2.65%) samples tested positive for bacteria belonging either to the Genus Enterobacteriaceae or Genus Acinetobacter. The culture-positive isolates (n = 1,055, 2.65%) were further distributed into two groups. The first group constituted of the Genus Enterobacteriaceae (excluding Salmonella spp.) and Genus Acinetobacter (n = 964, 91.37%), with the second group constituting of the Genus Enterobacteriaceae comprising exclusively of Salmonella spp. (n = 91, 8.63%). The Salmonella spp. (n = 91, 8.63%) were further identified as Salmonella ser. Typhi (n = 79, 7.49%), Paratyphi A (n = 11, 1.04%), and Paratyphi B (n = 1, 0.1%). The affected age group was 1–5 years of age, followed by 6–10 years, with the least being 11–15 years. The median age of the patients was 6 years, with an interquartile range of (IQR: 4–9) years (Figure 2). Sex-wise distribution showed the prevalence of Salmonella infection among both male (n = 53, 58.24%; OR, 1.0; 95% CI, 0.60–1.67), and female patients (n = 38, 41.76%; OR, 0.98; 95% CI, 0.49–2.05; Figure 2). Between 2017 and 2019, the highest number of cases was seen during the spring season (March–May; OR, 1.84; 95% CI, 0.46–7.33) and the least during autumn (September to November; OR, 0.51; 95% CI, 0.60–4.30; Figure 3).
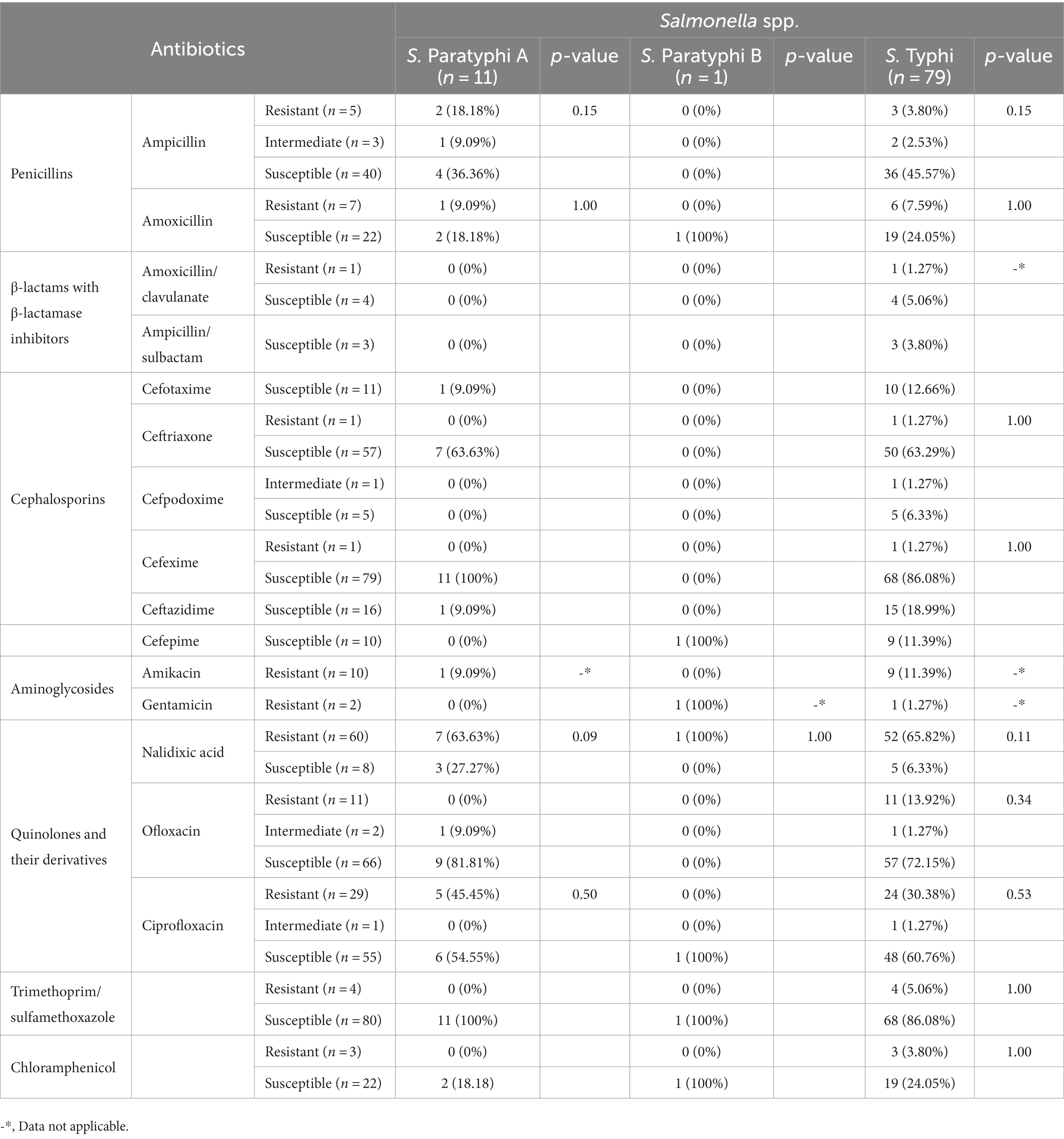
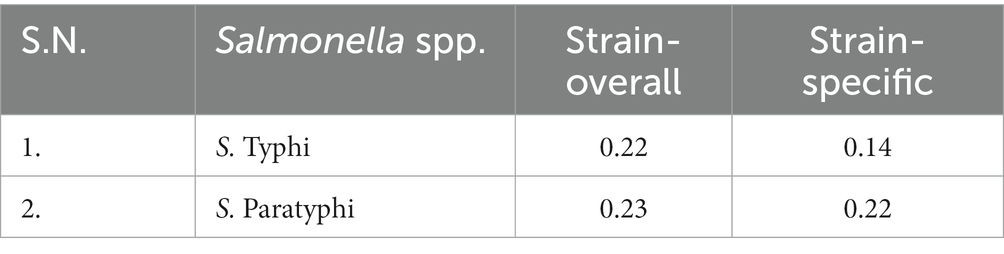
Among the tested antimicrobials, resistance was observed in quinolones and their derivatives (nalidixic acid), showing (n = 60, 88.23%) resistance to all the Salmonella isolates, followed by fluoroquinolone resistance (ciprofloxacin; n = 29, 34.11%) and ofloxacin (n = 11, 13.92%). Moreover, the penicillin group of antimicrobials was second in line with the overall resistance pattern observed, which was more among amoxicillin (n = 7, 24.13%) and ampicillin (n = 5, 10.41%). Among the combination drugs, β-lactams with β-lactamase inhibitors (amoxicillin/clavulanate) showed (n = 1, 20%) resistant isolate (Table 1). Among the total Salmonella ser.isolated, ser. Typhi (n = 79, 86.81%) exhibited (n = 52, 65.82%) resistance to quinolones and their derivatives (nalidixic acid) and resistance to fluoroquinolones such as ciprofloxacin (n = 24, 30.38%). Similarly, the bacterium also showed resistance toward the penicillin group of drugs, such as amoxicillin (n = 6, 7.59%) and ampicillin (n = 3, 3.8%). Furthermore, β-lactams with β-lactamase inhibitors had a single isolate of S. Typhi resistant to amoxicillin/clavulanate (n = 1, 1.27%; Table 1). The median multiple antibiotic resistance index or the MAR index values for S. Typhi were in the range between 0.14 and 0.22. Likewise, for S. Paratyphi, 0.22–0.23 was the mean MAR index (Table 2). There were no clinical outcomes in the form of hospital admission for any of the known complications of typhoid fever among the outpatients visiting the hospital, who were clinically diagnosed with enteric fever in the 2-year study duration.
4. Discussion
Enteric fever is considered one of the leading causes of febrile bacterial illness among adults and children in both developing and developed nations (Sánchez-Vargas et al., 2011). The genetic constitution of Salmonella spp. enhances their adaptability in both mammalian and non-mammalian hosts, including non-animated reservoirs, thereby challenging their eradication by conventional methods. In an era of antimicrobial resistance, Salmonella strains face a similar fate to any other microorganisms exhibiting resistance to multiple drugs, making treatment an uphill task (Sánchez-Vargas et al., 2011). Our study found that Salmonella enterica ser. Typhi is the most common isolate (n = 79, 7.49%), followed by Salmonella enterica ser. Paratyphi A (n = 11, 1.04%) and Salmonella enterica ser. Paratyphi B in a single (n = 1, 0.1%) case. Historically, a high proportion of Salmonella Typhi infections have been reported in Nepal, with a relatively lower proportion of Salmonella Paratyphi (Karkey et al., 2010; Shrestha et al., 2014; Thompson et al., 2017), but the trends have been changing over the past two decades, with an increase in Salmonella Paratyphi A in some parts of Asia (Karkey et al., 2010; Zellweger et al., 2017).
Among the Salmonella isolates, a lower proportion (n = 79, 7.49%) of ser. Typhi in our study was comparable to those conducted in various parts of Nepal, contributing to 5.1% and 5.4% of cases (Khanal et al., 2007; Pokharel et al., 2009), but was in contrast (higher, 12.3%, 55.7%, 77.7,% and 85%) to studies published in Pakistan, Nepal, and India, along with population-based surveillance, respectively (Siddiqui et al., 2006; Petersiel et al., 2018; Biswas et al., 2022; Garrett et al., 2022). The findings from our study could be attributed to prior antimicrobial treatment received by the affected age group (1–5 years) before obtaining a blood sample for culture and sensitivity (Britto et al., 2018). Additionally, only 1.04% of Paratyphi A cases according to our estimates are comparable to a publication from Pakistan (Siddiqui et al., 2006) but discordant (higher, 17% and 12%) to few studies from Nepal (Prajapati et al., 2008; Budhathoki et al., 2020) and another population-based enteric fever surveillance (higher, >99%), respectively (Garrett et al., 2022). The variation in Paratyphi A cases in our findings could be attributed to the asymptomatic infections manifested by this ser. (Sood et al., 1999), with relatively young male adults becoming infected (Karkey et al., 2010), which differed from our study population. Only 3% of Salmonella ser. Paratyphi B infection cases among the Nepalese population have been reported, rendering it an uncommon bloodstream infection (Pokhrel et al., 2009; Karkey et al., 2010; Zellweger et al., 2017; Garrett et al., 2022), which is quite similar (n = 1, 0.1%) to our study observation but differs (higher, 10%) from another scientific publication in Nepal (Budhathoki et al., 2020). The higher proportion of ser. Paratyphi B infection in the latter study reflects the greater predisposition to this infection among older children (11–15 years), who comparatively have a higher exposure to the external environment and outdoor activities than younger ones (1–5 years).
Children in the age group between 1 and 5 years were the most commonly infected (n = 42, 46.1%), with the least among those aged between 11 and 15 years (n = 12, 13.2%), similarly to the findings in India (Das et al., 2016), Pakistan (Rafiq et al., 2009; Britto et al., 2017), and other studies in the series (Mahle and Levine, 1993; Pang et al., 1995). However, this was in contrast (lower, 26.5%, 21.3%, and 14%,) to other publications in Pakistan, Nepal, and India for the age group between 1 and 5 years, respectively (Siddiqui et al., 2006; Budhathoki et al., 2020; Behera et al., 2021). The age-related variation in our findings highlights the possibility of reduced documentation of the disease, poor clinical suspicion, prior antimicrobial treatment before blood culture, and difficulty in withdrawing blood resulting in poor laboratory and clinical outcomes (Britto et al., 2018), along with immunological reasons such as immature and unstable gut microbiome and gut immune function in children between 1 and 5 years of age, easily exposing them to bacterial infections such as S. Typhi in comparison to older ones (Nuriel-Ohayon et al., 2016).
Enteric fever was more common in the male population, constituting more than half (n = 53, 58.24%) of the cases, with similar observations made in countries such as India, Nepal, and African countries, as well as in a population-based enteric fever surveillance (Ramaswamy et al., 2010; Rabasa et al., 2012; Singh et al., 2012; Garrett et al., 2022). The variation in sex proportion in our results could be attributed to factors such as prioritizing a male child over a female for treatment in our context and more outdoor activities seen among male children exposing them to the root of infection.
The wet season in Nepal begins from May to November and the dry season from December to February (Sharma et al., 2021). The frequency of typhoid infections in our study is seen throughout the year, but the incidence was high toward the spring season (end of dry season and beginning of wet season) throughout the two-year duration (March 2017 to May 2019). Our findings were concordant with studies conducted in Nepal (Petersiel et al., 2018), India (Ramaswamy et al., 2010), and Vietnam (Lin et al., 2000) but discordant with other studies conducted in Nepal (Karkey et al., 2010), Bangladesh (Dewan et al., 2013), Pakistan (Siddiqui et al., 2006), and Africa (Rabasa et al., 2012), where cases were seen throughout the year with increased frequency during the peak of the wet months (July–October). The isolation of the bacteria throughout the year in our study with comparatively higher prevalence during spring could be subjected to the microbial contamination of drinking water above the recommended levels in Nepal, thereby impacting the health of Nepalese people and specifically children (Farooqui et al., 1991; Parry et al., 2011; UNICEF Nepal, 2018) via various waterborne diseases (Butler et al., 1991; MR and Nair, 2010).
The treatment for enteric fever over the years has become challenging due to multidrug resistance, with the choice of the drug depending on local patterns of antimicrobial resistance, the severity of the disease, availability, and cost of antimicrobials (JA and Mintz, 2010; WHO, 2014). Our results displayed the occurrence of nalidixic acid-resistant S. Typhi (n = 52, 65.82%; p = 0.11), which was low in comparison to other studies in Nepal (Singh et al., 2011; Petersiel et al., 2018) and India (Walia et al., 2006) but high compared to other similar studies within the nation (Singh et al., 2012) and India (Ramaswamy et al., 2010; Bhumbla et al., 2022). Population-based enteric fever surveillance revealed nalidixic acid resistance in 59% of isolates from Pakistan, 57% from India, 44% from Vietnam, and none from Chinese or Indonesian sites in 2008 (Ochiai et al., 2008). Susceptibility to nalidixic acid is thought to be the best interpreter of clinical response to fluoroquinolones (Parry, 2004), and there have been pleas to adjust the fluoroquinolone breakpoints for all Salmonella spp. (Aarestrup et al., 2003). The resistance to nalidixic acid in our results indicates reduced susceptibility and poor clinical response to older-generation fluoroquinolones, which is still considered a first-line treatment for enteric fever in Nepal (Maskey et al., 2008), with our study revealing S. Typhi (n = 24, 30.38%; p = 0.53) being resistant to ciprofloxacin. Systematic reviews on antimicrobial resistance in S. Typhi conducted worldwide have witnessed 15% resistance to ciprofloxacin, which is lower than our study estimates but analogous to fluoroquinolones resistance observed within the vicinity (Pham et al., 2016). Resistance toward older drugs such as Amoxicillin (n = 6, 7.59%; p = 1.00), Ampicillin (n = 3, 3.80%; p = 0.13), Trimethoprim/sulfamethoxazole (n = 4, 5.06%; p = 1.00), and Chloramphenicol (n = 3, 3.80%; p = 1.00) was more toward S. Typhi, but none of these were statistically significant. Fairly low resistance toward these antimicrobials has been reported in India and Nepal too (Walia et al., 2006; Petersiel et al., 2018). A systematic review on antimicrobial resistance globally among S. Typhi reported 25.9%, 37.9%, and 38.8% resistance toward chloramphenicol, cotrimoxazole, and ampicillin and higher resistance (61.2%) toward amoxicillin (Marchello et al., 2020). The lower level of resistance toward chloramphenicol from our findings could also be due to the lower usage of this drug among the pediatric population due to its known adverse events. Evidence showing more sensitivity toward first-line drugs has created a dilemma in the re-usage and recycling concept of the first-line therapy for enteric fever (Pham et al., 2016), with our study results agreeing with this concept. Cephalosporins are the current drug of choice for the treatment of enteric fever in Nepal (Britto et al., 2018), with our laboratory findings showing resistance to only one isolate each of Ceftriaxone (n = 1, 1.27%; p = 1.00) and Cefixime (n = 1, 1.27%; p = 1.0) among ser. Typhi, resembling the findings of Prajapati et al. (2008) and Marchello et al. (2020). Minimal resistance was observed in beta-lactam with beta-lactamase inhibitors (amoxicillin/clavulanate; n = 1, 1.27%) and was parallel to the results from India (Bhumbla et al., 2022; Biswas et al., 2022) and the findings of a systematic review exhibiting 8.0% resistance toward amoxicillin/clavulanate (Marchello et al., 2020). Among the ser. Paratyphi A isolates, resistance was seen toward Ampicillin (n = 2, 18.18%; p = 0.13), Amoxicillin (n = 1, 9.09%; p = 1.00), Nalidixic acid (n = 2, 63.63%; p = 0.09), and Ciprofloxacin (n = 5, 45.45%; p = 0.50), with only a single isolate of ser. Paratyphi B resistant only to Nalidixic acid (n = 1, 100%; p = 1.00). These findings were in line with studies from India and Nepal (Petersiel et al., 2018; Biswas et al., 2022) but were not statistically significant for both the bacteria.
MAR analysis is a risk evaluation tool that differentiates low- and high-risk regions of antibiotic overuse. The MAR index was in the range between 0.14 and 0.22 in S. Typhi and 0.22 and 0.23 in S. Paratyphi. The MAR index of >0.2 in S. Paratyphi and about 0.2 in S. Typhi indicates the presence of a high-risk source of contamination from the environment where several antimicrobials are used (Osundiya et al., 2013; Davis and Brown, 2016; Ayandele et al., 2020). The findings from our observations could be attributed to high antibiotic use and high selective pressure in the given environment and insufficient infection prevention and control practices, followed by poor surveillance of antimicrobial susceptibility patterns. Bacterial strains resistant to most classes of antimicrobials are emerging from time to time, hinting at various problems such as the injudicious use of antimicrobials and the lack of rigorous training and workshops on infection prevention and control practices (Osundiya et al., 2013); these need to be acknowledged, and measures to reduce these problems should be addressed. Our data limit the clinical characteristics of the patients, MIC data, and antimicrobial-resistant genes, specifically fluoroquinolones. Moreover, genotyping of the isolates would have enhanced the genetic understanding of the antimicrobials and also compared the lineage drift over the years, specifically in the pediatric population, as reported by other studies within the country.
5. Conclusion
In conclusion, this study reveals the prevalence of enteric fever predominantly in children between 1 and 5 years of age, with S. Typhi being the most common causative pathogen, the majority of which are nalidixic acid resistant (NARST). Moreover, the multidrug resistance pattern toward Salmonella isolates was not apparent, but a comparatively acceptable susceptibility was seen toward the cephalosporin and beta-lactamase inhibitor classification of drugs. Therefore, as far as antimicrobial resistance is concerned, the antimicrobial susceptibility situation does not look alarming. The existence of this bacterium in children raises a general concern regarding hand and food hygiene, along with clean and safe drinking water. It also focuses on the need for public health intervention to raise awareness among children, adults, and food vendors about the disease. The inclusion of typhoid vaccines under the routine immunization program in Nepal for children from 15 months to 15 years of age since 8 April 2022 (UNICEF Nepal, 2022) is a great initiative taken toward controlling the disease, and hopefully, in years to come, we can witness a significantly lesser number of cases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Committee (IRC) of Kanti Children’s Hospital, Maharajgunj, Kathmandu, Nepal (IRC No: 969). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
NP: conceptualization, literature search, data curation, and writing—original draft and editing. RC: conceptualization, reviewing, and editing. CT: validation, reviewing, and editing. AB: data analysis, reviewing, and editing. IA: reviewing and editing. RS: reviewing and editing. RG: reviewing and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Anita K. C. for helping us with the data collection. Moreover, the support of Saroj Sharma, Head of Clinical Laboratory, Kanti Children’s Hospital, Maharajgunj, Kathmandu, Nepal, without whom this endeavor would not have been possible, cannot be disregarded. Lastly, we thank laboratory technician, laboratory assistant, and laboratory helper Gyani Singh, Parbati Shrestha, and Ishwari Manandhar for their roles in the sample collection and processing, bacterial isolation, and preparation of the culture media, respectively, which were some of the noteworthy activities carried out at Kanti Children’s Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarestrup, F. M., Wiuff, C., Mølbak, K., and Threlfall, E. J. (2003). Is it time to change fluoroquinolone breakpoints for Salmonella spp.? Antimicrob. Agents Chemother. 47, 827–829. doi: 10.1128/AAC.47.2.827-829.2003
Ackers, M. L., Puhr, N. D., Tauxe, R. V., and Mintz, E. D. (2000). Laboratory-based surveillance of Salmonella serotype typhi infections in the United States: antimicrobial resistance on the rise. JAMA 283, 2668–2673. doi: 10.1001/jama.283.20.2668
Ayandele, A., Oladipo, E. K., Oyebisi, O., and Kaka, M. O. (2020). Prevalence of multi-antibiotic resistant Escherichia coli and Klebsiella species obtained from a tertiary medical institution in Oyo state Nigeria. Qatar Med J 2020, 1–6. doi: 10.5339/qmj.2020.9
Behera, J. R., Rup, A. R., Dash, A. K., Sahu, S. K., Gaurav, A., and Gupta, A. (2021). Clinical and laboratory profile of enteric fever in children from a tertiary Care Centre in Odisha, eastern India. Cureus. 13:e12826. doi: 10.7759/cureus.12826
Bhumbla, U., Chaturvedi, P., and Jain, S. (2022). Prevalence of Salmonella typhi in among febrile patients in a tertiary care hospital of south West Rajasthan. J. Fam. Med. Prim. Care 11, 2852–2855. doi: 10.4103/jfmpc.jfmpc_1976_21
Biswas, M., Biswas, S., Gupta, B., Mascellino, M. T., Rakshit, A., and Chakraborty, B. (2022). Changing paradigms in antibiotic resistance in Salmonella species with focus on fluoroquinolone resistance: a 5-year retrospective study of enteric fever in a tertiary Care Hospital in Kolkata, India. Antibiotics 11:1308. doi: 10.3390/antibiotics11101308
Black, R. E., Cisneros, L., Levine, M. M., Banfi, A., Lobos, H., and Rodriguez, H. (1985). Case-control study to identify risk factors for paediatric endemic typhoid fever in Santiago, Chile. Bull. World Health Organ. 63, 899–904.
Britto, C. D., Dyson, Z. A., Duchene, S., Carter, M. J., Gurung, M., Kelly, D. F., et al. (2018). Laboratory and molecular surveillance of paediatric typhoidal Salmonella in Nepal: antimicrobial resistance and implications for vaccine policy. PLoS Negl. Trop. Dis. 12:e0006408. doi: 10.1371/journal.pntd.0006408
Britto, C., Pollard, A. J., Voysey, M., and Blohmke, C. J. (2017). An appraisal of the clinical features of Pediatric enteric fever: systematic review and Meta-analysis of the age-stratified disease occurrence. Clin. Infect. Dis. 64, 1604–1611. doi: 10.1093/cid/cix229
Budhathoki, S., Rimal, S., Lama, L., Shrestha, S., Sanjel, S., and Amgain, K. (2020). Clinical profile of enteric fever in children of a tertiary Care Centre in Kathmandu, Nepal. J. Karnali Acad. Heal. Sci. 3, 122–127. doi: 10.3126/jkahs.v3i2.31327
Butler, T., Islam, A., Kabir, I., and Jones, P. (1991). Patterns of morbidity and mortality in typhoid fever dependent on age and gender: review of 552 hospitalized patients with diarrhea. Rev. Infect. Dis. 13, 85–90. doi: 10.1093/clinids/13.1.85
Central Bureau of Statistics (2021). Population|National Population and housing census 2021 results. Central Bureau of Statistics. Available at: https://censusnepal.cbs.gov.np/results.
Collee, J. G., Mackie, T. J., and McCartney, J. E. (1996) in Mackie & Mccartney practical medical microbiology. ed. J. G. Collee. 14th ed (New York: Churchill Livingstone)
Das, S., Ray, U., Akhter, I., Chattopadhyay, A., Paul, D. K., and Dutta, S. (2016). Evaluation of fliC-d based direct blood PCR assays for typhoid diagnosis. BMC Microbiol. 16:108. doi: 10.1186/s12866-016-0723-6
Dewan, A. M., Corner, R., Hashizume, M., and Ongee, E. T. (2013). Typhoid fever and its association with environmental factors in the Dhaka metropolitan area of Bangladesh: a spatial and time-series approach. PLoS Negl. Trop. Dis. 7:e1998. doi: 10.1371/journal.pntd.0001998
Farooqui, B. J., Khurshid, M., Ashfaq, M. K., and Ata Khan, M. (1991). Comparative yield of Salmonella typhi from blood and bone marrow cultures in patients with fever of unknown origin. J. Clin. Pathol. 44, 258–259. doi: 10.1136/jcp.44.3.258
Garrett, D. O., Longley, A. T., Aiemjoy, K., Yousafzai, M. T., Hemlock, C., Yu, A. T., et al. (2022). Incidence of typhoid and paratyphoid fever in Bangladesh, Nepal, and Pakistan: results of the surveillance for enteric fever in Asia project. Lancet Glob. Health 10, e978–e988. doi: 10.1016/S2214-109X(22)00119-X
Gupta, B. P., Saluja, T., and Sahastrabuddhe, S. (2021). Epidemiology of typhoid in Nepal: review of literature to identify high burden area for potential use of typhoid vaccine. Pediatr. Infect. Dis. 3, 51–56. doi: 10.5005/jp-journals-10081-1297
Ja, C., and Mintz, E. (2010). Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis. 50, 241–246. doi: 10.1086/649541
Karkey, A., Arjyal, A., Anders, K. L., Boni, M. F., Dongol, S., Koirala, S., et al. (2010). The burden and characteristics of enteric fever at a healthcare facility in a densely populated area of Kathmandu. PloS One 5:e13988. doi: 10.1371/journal.pone.0013988
Karkey, A., Thompson, C. N., Tran Vu Thieu, N., Dongol, S., Le Thi Phuong, T., Voong Vinh, P., et al. (2013). Differential epidemiology of Salmonella typhi and Paratyphi a in Kathmandu, Nepal: a matched case control investigation in a highly endemic enteric fever setting. PLoS Negl. Trop. Dis. 7:e2391. doi: 10.1371/journal.pntd.0002391
Khanal, B., Sharma, S. K., Bhattacharya, S. K., Bhattarai, N. R., Deb, M., and Kanungo, R. (2007). Antimicrobial susceptibility patterns of Salmonella enterica serotype typhi in eastern Nepal. J. Health Popul. Nutr. 25, 82–87.
Klonin, H., Minelli, E., and Adhikari, N. (1989). Three unusual cases of Salmonella infection in infants. Ann. Trop. Paediatr. 9, 240–242. doi: 10.1080/02724936.1989.11748639
Krumperman, P. H. (1983). Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 46, 165–170. doi: 10.1128/aem.46.1.165-170.1983
Lin, F., Vo, A., Phan, V., TT, N., D, B., CT, T., et al. (2000). The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am. J. Trop. Med. Hyg. 62, 644–648. doi: 10.4269/ajtmh.2000.62.644
Luby, S. P., Faizan, M. K., Fisher-Hoch, S. P., Syed, A., Mintz, E. D., Bhutta, Z. A., et al. (1998). Risk factors for typhoid fever in an endemic setting, Karachi, Pakistan. Epidemiol. Infect. 120, 129–138. doi: 10.1017/S0950268897008558
Mahle, W. T., and Levine, M. M. (1993). Salmonella typhi infection in children younger than five years of age. Pediatr. Infect. Dis. J. 12, 627–631. doi: 10.1097/00006454-199308000-00001
Marchello, C. S., Carr, S. D., and Crump, J. A. (2020). A systematic review on antimicrobial resistance among salmonella typhi worldwide. Am. J. Trop. Med. Hyg. 103, 2518–2527. doi: 10.4269/ajtmh.20-0258
Maskey, A. P., Basnyat, B., Thwaites, G. E., Campbell, J. I., Farrar, J. J., and Zimmerman, M. D. (2008). Emerging trends in enteric fever in Nepal: 9124 cases confirmed by blood culture 1993-2003. Trans. R. Soc. Trop. Med. Hyg. 102, 91–95. doi: 10.1016/j.trstmh.2007.10.003
MR, C., and Nair, D. (2010). Quinolone and cephalosporin resistance in enteric fever. J. Glob. Infect. 2, 258–262. doi: 10.4103/0974-777X.68529
Nuriel-Ohayon, M., Neuman, H., and Koren, O. (2016). Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 14:1031. doi: 10.3389/fmicb.2016.01031
Ochiai, R. L., Acosta, C. J., Danovaro-Holliday, M. C., Baiqing, D., Bhattacharya, S. K., Agtini, M. D., et al. (2008). A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull. World Health Organ. 86, 260–268. doi: 10.2471/BLT.06.039818
Osler, W. (1912). The principles and practice of medicine: Designed for the use of practitioners and students of medicine. 8th Edn. New York: D. Appleton.
Osundiya, O., Oladele, R., and Oduyebo, O. (2013). Multiple antibiotic resistance (MAR) indices of Pseudomonas and Klebsiella species isolates in Lagos university teaching hospital. African J. Clin. Exp. Microbiol. 14, 164–168. doi: 10.4314/ajcem.v14i3.8
Pang, T., Bhutta, Z., Finlay, B., and Altwegg, M. (1995). Typhoid fever and other salmonellosis: a continuing challenge. Trends Microbiol. 3, 253–255. doi: 10.1016/s0966-842x(00)88937-4
Parry, C. M. (2004). Typhoid fever. Curr. Infect. Dis. Rep. 6, 27–33. doi: 10.1007/s11908-004-0021-6
Parry, C. M., Hien, T. T., Dougan, G., White, N. J., and Farrar, J. J. (2002). Typhoid fever. N. Engl. J. Med. 347, 1770–1782. doi: 10.1056/NEJMra020201
Parry, C. M., Wijedoru, L., Arjyal, A., and Baker, S. (2011). The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev. Anti Infect. Ther. 9, 711–725. doi: 10.1586/eri.11.47
Petersiel, N., Shresta, S., Tamrakar, R., Koju, R., Madhup, S., Shresta, A., et al. (2018). The epidemiology of typhoid fever in the Dhulikhel area, Nepal: a prospective cohort study. PloS One 13:e0204479. doi: 10.1371/journal.pone.0204479
Pham, T. D., Karkey, A., Dongol, S., Ho, T. N., Thompson, C., Rabaa, M., et al. (2016). A novel ciprofloxacin-resistant subclade of H58 Salmonella Typhi is associated with fluoroquinolone treatment failure. Elife 5:e14003. doi: 10.7554/eLife.14003
Pokharel, P., Rai, S. K., Karki, G., Katuwal, A., Vitrakoti, R., and Shrestha, S. K. (2009). Study of enteric fever and antibiogram of Salmonella isolates at a teaching hospital in Kathmandu Valley. Nepal Med. Coll. J. 11, 176–178.
Pokhrel, B. M., Karmacharya, R., Mishra, S. K., and Koirala, J. (2009). Distribution of antibody titer against Salmonella enterica among healthy individuals in Nepal. Ann. Clin. Microbiol. Antimicrob. 8:1. doi: 10.1186/1476-0711-8-1
Prajapati, B., Rai, G. K., Rai, S. K., Upreti, H. C., Thapa, M., Singh, G., et al. (2008). Prevalence of Salmonella typhi and paratyphi infection in children: a hospital based study. Nepal Med. Coll. J. 10, 238–241.
Davis, R., and Brown, P. (2016). Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 65, 261–271. doi: 10.1099/jmm.0.000229
Rabasa, A., Mava, Y., Pius, S., Timothy, S., and Baba, U. (2012). Typhoid fever in children: clinical presentation and risk factors. Niger. J. Paediatr. 40:11. doi: 10.4314/njp.v40i1.11
Rafiq, H., Zia, R., and Naeem, S. (2009). Typhoid fever—continues as a major threat in children. Signs 25, 1–2.
Ramaswamy, G., Janakiraman, L., Thiruvengadam, V., and Sathiyasekeran, M. (2010). Profile of typhoid fever in children from a tertiary care hospital in Chennai-South India. Indian J. Pediatr. 77, 1089–1092. doi: 10.1007/s12098-010-0196-9
Sánchez-Vargas, F. M., Abu-El-Haija, M. A., and Gómez-Duarte, O. G. (2011). Salmonella infections: an update on epidemiology, management, and prevention. Travel Med. Infect. Dis. 9, 263–277. doi: 10.1016/j.tmaid.2011.11.001
Sharma, S., Khadka, N., Nepal, B., Ghimire, S. K., Luintel, N., and Hamal, K. (2021). Elevation dependency of precipitation over southern slope of central Himalaya. Jalawaayu 1, 1–14. doi: 10.3126/jalawaayu.v1i1.36446
Shrestha, S., Amatya, R., Shrestha, R. K., and Shrestha, R. (2014). Frequency of blood culture isolates and their antibiogram in a teaching hospital. J. Nepal Med. Assoc. 52, 692–696. doi: 10.31729/jnma.2295
Siddiqui, F. J., Rabbani, F., Hasan, R., Nizami, S. Q., and Bhutta, Z. A. (2006). Typhoid fever in children: some epidemiological considerations from Karachi, Pakistan. Int. J. Infect. Dis. 10, 215–222. doi: 10.1016/j.ijid.2005.03.010
Singh, U. K., Neopane, A. K., Thapa, M., Aryal, N., and Agrawal, K. (2011). Salmonella typhi infections and effect of fluroquinolones and third generation cephalosporins in clinical outcome. doi: 10.3126/jnps.v31i3.5361
Singh, D. S., Shrestha, S., Shrestha, N., and Manandhar, S. (2012). Enteric fever in children at Dhulikhel hospital. J. Nepal Paediatr. Soc. 32, 216–220. doi: 10.3126/jnps.v32i3.6682
Sood, S., Kapil, A., Dash, N., Das, B. K., Goel, V., and Seth, P. (1999). Paratyphoid fever in India: an emerging problem [3]. Emerg. Infect. Dis. 5, 483–485. doi: 10.3201/eid0503.990329
Thompson, C. N., Karkey, A., Dongol, S., Arjyal, A., Wolbers, M., Darton, T., et al. (2017). Treatment response in enteric fever in an era of increasing antimicrobial resistance: an individual patient data analysis of 2092 participants enrolled into 4 randomized, controlled trials in Nepal. Clin. Infect. Dis. 64, 1522–1531. doi: 10.1093/cid/cix185
UNICEF Nepal (2018). Water and sanitation (WASH). UNICEF Nepal. Available at: https://www.unicef.org/nepal/water-and-sanitation-wash (Accessed 2 April 2023).
UNICEF Nepal (2022). Nepal introduces typhoid vaccine into routine immunisation across the country. UNICEF Nepal. Available at: https://www.unicef.org/nepal/press-releases/nepal-introduces-typhoid-vaccine-routine-immunisation-across-country#:~:text=Thethree-weekcampaign%2Cwhich,theriseofantimicrobialresistance.
Walia, M., Gaind, R., Paul, P., Mehta, R., Aggarwal, P., and Kalaivani, M. (2006). Age-related clinical and microbiological characteristics of enteric fever in India. Trans. R. Soc. Trop. Med. Hyg. 100, 942–948. doi: 10.1016/j.trstmh.2006.02.015
Weinstein, M., Patel, J., Bobenchik, A., Campeau, S., Cullen, S., Galas, M., et al. (2019). Performance standards for antimicrobial susceptibility testing performance standards for antimicrobial susceptibility testing. CLSI M100, 1–25.
WHO (2014). Background doc: The diagnosis, treatment and prevention of typhoid fever 2014. Geneva, Switzerland, 1–38. Available at: https://www.glowm.com/pdf/WHO-diagnosis%20treatment%20prevention%20of%20typhoid%20fever-2003-CustomLicense.pdf.
WHO (2018). Typhoid. WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/typhoid?
Woodward, T. E., Smadel, J. E., Ley, H. L., Green, R., and Mankikar, D. S. (2004). Preliminary report on the beneficial effect of chloromycetin in the treatment of typhoid fever. 1948. Wilderness Environ. Med. 15, 218–220. doi: 10.1580/1080-6032(2004)15[218,protbe]2.0.co;2
Keywords: antibiogram, blood culture, enteric fever, pediatric population, serotypes, salmonella infection
Citation: Pokhrel N, Chapagain R, Thakur CK, Basnet A, Amatya I, Singh R and Ghimire R (2023) Salmonella infection among the pediatric population at a tertiary care children’s hospital in central Nepal: a retrospective study. Front. Microbiol. 14:1218864. doi: 10.3389/fmicb.2023.1218864
Edited by:
Maurizio Sanguinetti, Catholic University of the Sacred Heart, ItalyReviewed by:
Danilo Buonsenso, Catholic University of the Sacred Heart, ItalyMoataz Abd El Ghany, The University of Sydney, Australia
Lok Bahadur Shrestha, University of New South Wales, Australia
Copyright © 2023 Pokhrel, Chapagain, Thakur, Basnet, Amatya, Singh and Ghimire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nayanum Pokhrel, bmF5YW51bXByQGdtYWlsLmNvbQ==
 Nayanum Pokhrel
Nayanum Pokhrel Ramhari Chapagain
Ramhari Chapagain Chandan Kumar Thakur
Chandan Kumar Thakur Ajaya Basnet3
Ajaya Basnet3 Raghav Ghimire
Raghav Ghimire