- 1Key Laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), College of Life Sciences/Institute of Agro-Bioengineering, Guizhou University, Guiyang, Guizhou, China
- 2College of Agriculture, Guizhou University, Guiyang, Guizhou, China
- 3College of Agriculture, Guizhou Engineering Research Center for Fruit Crops, Guizhou University, Guiyang, Guizhou, China
Arbuscular mycorrhizal fungi (AMF) can penetrate plant root cortical cells, establish a symbiosis with most land plant species, and form branched structures (known as arbuscules) for nutrient exchange. Plants have evolved a complete plant–AMF symbiosis system to sustain their growth and development under various types of abiotic stress. Here, we highlight recent studies of AM symbiosis and the regulation of symbiosis process. The roles of mycorrhizal symbiosis and host plant interactions in enhancing drought resistance, increasing mineral nutrient uptake, regulating hormone synthesis, improving salt resistance, and alleviating heavy metal stress were also discussed. Overall, studies of AM symbiosis and a variety of abiotic stresses will aid applications of AMF in sustainable agriculture and can improve plant production and environmental safety.
1 Introduction
According to the morphological and anatomical characteristics of mycorrhiza, the species of mycorrhiza fungi and the species of plants, mycorrhiza are usually divided into 7 types (Table 1). Arbuscular mycorrhizas (AMs) are considered to be the most familiar type of mycorrhizae in nature; they are found in approximately 72% of land plants and play important roles in the rhizosphere of plants (Genre et al., 2020). Arbuscular mycorrhizal fungi (AMF) belong to the Glomeromycotina in Mucoromycota (Bonfante and Venice, 2020). AM formation is the result of long-term co-evolution between AMF and plant roots or even leaves (Genre et al., 2020; Rich et al., 2021). The main structures of AMs include hyphae, arbuscules, and spores, and fewer species have vesicles and auxiliary cells. Mycelia can be divided into extraradical and intraradical mycelia. Extraradical mycelia are distributed in the soil, and most of them have a network structure. Extraradical mycelia can penetrate areas that cannot be reached by the plant roots and absorb water and nutrients (Gao et al., 2021; Xie K. et al., 2022). The extrinsic mycelium enters the epidermal cells of the host roots and the cortical tissue of the plants, where it forms a dendritic arbuscular structure through continuous bifurcation. The arbuscules, which are referred to as the heart of the arbuscular mycorrhizas, are considered to be the most critical structure of AMF, as they mediate the mutual exchange of many types’ nutrients between plant cells and fungi (Pimprikar and Gutjahr, 2018).
AMs play a fundamental role in ecosystems and have significant effects on plant development, water utilization, nutrition uptake, and hormone balance from biological and abiotic stresses. One of the most important ecological functions of AMs is to enhance plant biomass accumulation (Chitarra et al., 2016; Chen S. et al., 2017; Huang et al., 2020a; Zhang Q. et al., 2023). After AMF successfully invade host plant root epidermal cells to form symbionts, they can expand the effective absorption range of host plants in soil. Glycoproteins synthesized by mycelia is beneficial to the suitable rhizosphere environment creation, that enhance the host plants growth by uptake and transfer water and mineral nutrients from the outside rhizosphere (Hu et al., 2017). AMF form a direct connection between the soil and roots, which can improve plant nutrition absorption, water uptake, and photosynthetic capacity, and this reduces the negative effects of abiotic stresses such as nutrient deficiency, salt, and drought (Li et al., 2013; Liu et al., 2018; Bahadur et al., 2019; Begum et al., 2022). For example, AMF can translocate polyphosphate to the host through hyphae over a long distance by Rhizophagus clarus aquaporin 3, which is important for nutrient exchange between the host and fungal symbionts (Kikuchi et al., 2016). In Medicago truncatula, the membrane protein MtPT4 in mycorrhizal roots can also absorb the phosphate (Pi) released by AMF (Harrison et al., 2002); this protein is beneficial to plants and necessary for AM symbiosis (Javot et al., 2007). In addition to promoting plant growth and improving plant nutrient absorption capacity, AMF also plays an important role in improving plant heavy metal tolerance (Gómez-Gallego et al., 2019; Chen et al., 2023). Organic acids such as citric acid and malic acid released by AMF can be combined with metals to form complexes, and metal ions can be passivated by the chelation and filtration mechanism of extrinsic mycelium (Huang et al., 2021), so they excellent fungi for bioremediation. Table 2 depicts the studies of AM symbiosis on plant abiotic stress alleviation in different plants.
2 The establishment of AM symbiosis
Arbuscular mycorrhiza symbiosis relies on the formation of arbuscules for efficient nutrient exchange between plants and AM fungi. The process of symbiosis between different plants and AMFs is relatively consistent (Oldroyd, 2013). AMF need to recruit fatty acids to facilitate mycorrhizal colonization, and the R. irregularis is proved to be auxotroph of fatty acid. The transfer of fatty acids between the host plants and the fungus depends on RAM2 (REQUIRED FOR ARBUSCULAR MYCORRHIZATION 2) and the ATP-binding cassette transporter (Jiang et al., 2017). Luginbuehl et al. (2017) proved that the primary organic carbon transferred from host plants to AMF was the lipids. The transcription factor RAM1 is crucial for AM symbiosis, as it regulates the activity of glycerol-3-phosphate acyltransferase RAM2, which regulates the transfer of lipids from plants to AMF. The lipid biosynthetic enzyme FatM and ABC transporter (STR) are also required for symbiosis in the early stage and are uniquely conserved in plants engaged in AM symbiosis (Bravo et al., 2017). The transcriptional activators ERM1 and WRI5a can directly bind to the promoters then activate the expression of FatM, STR, and STR2; these target genes play important roles in fatty acid biosynthesis and transfer. Furthermore, the transcriptional regulatory complex ERM1/WRI5a–ERF12–TOPLESS plays an important regulatory role in arbuscule-containing cells, which help maintain a stable and beneficial symbiosis (Zhang Q. et al., 2023). In addition to obtaining fatty acids from host plants, AMF can also obtain sugars from host plants in the mature stage. The expression of Sugar Will Eventually be Exported Transporter (SWEET) family member MtSWEET1b is highly up-regulated in arbuscule-containing cells, and this gene has a significant effect on mediating the transport of glucose to stabilize the Medicago truncatula AM symbiosis (An et al., 2019).
Many genes are involved in the symbiosis of AMs, in addition to genes related to fatty acid and sugar metabolism. The expression of carotenoid cleavage dioxygenase CCD7 and CCD8 is increased under low phosphorus (P) conditions, which facilitates the synthesis of strigolactones (SLs) and their secretion outside the root system by the transporter PDR (Kretzschmar et al., 2012). Once AMF sense the SLs secreted by plant roots, the metabolism of AMF is stimulated in vivo, and mycelia grow close to the root of plant which will become a host in the future (Besserer et al., 2008). Both the synthesis of SLs and the mutation of the transporter gene directly reduce the infection rate, indicating that these prophase signals are important in the establishment of symbiosis (Figure 1). Another N-acetylglucosamine transporter (NOPE1) has been observed in maize and rice, and previous studies have shown that AMF are unable to infect plant roots after NOPE1 mutation. This suggests that SLs may not be the only important signaling molecule in the pre-contact phase (Nadal et al., 2017). The DELLA protein binds to CCaMK and CYCLOPS to form a complex that activates the downstream GRAS transcription factor RAM1 in Medicago sativa, and this inhibits the arbuscular development of the ram1 mutant (Park et al., 2015; Jin et al., 2016; Pimprikar et al., 2016). The Arbuscule Development Kinase 1 (OsADK1), which is a novel kinase, is necessary for arbuscule development in rice (Guo et al., 2022). In addition, mutations of DIPI, NSP1, NSP2, MIG1, and other transcription factors can affect the development of intracellular AMs, suggesting that these transcription factors also have crucial roles in arbuscular mycorrhizal symbiosis (Maillet et al., 2011; Delaux et al., 2013; Yu et al., 2014; Heck et al., 2016).
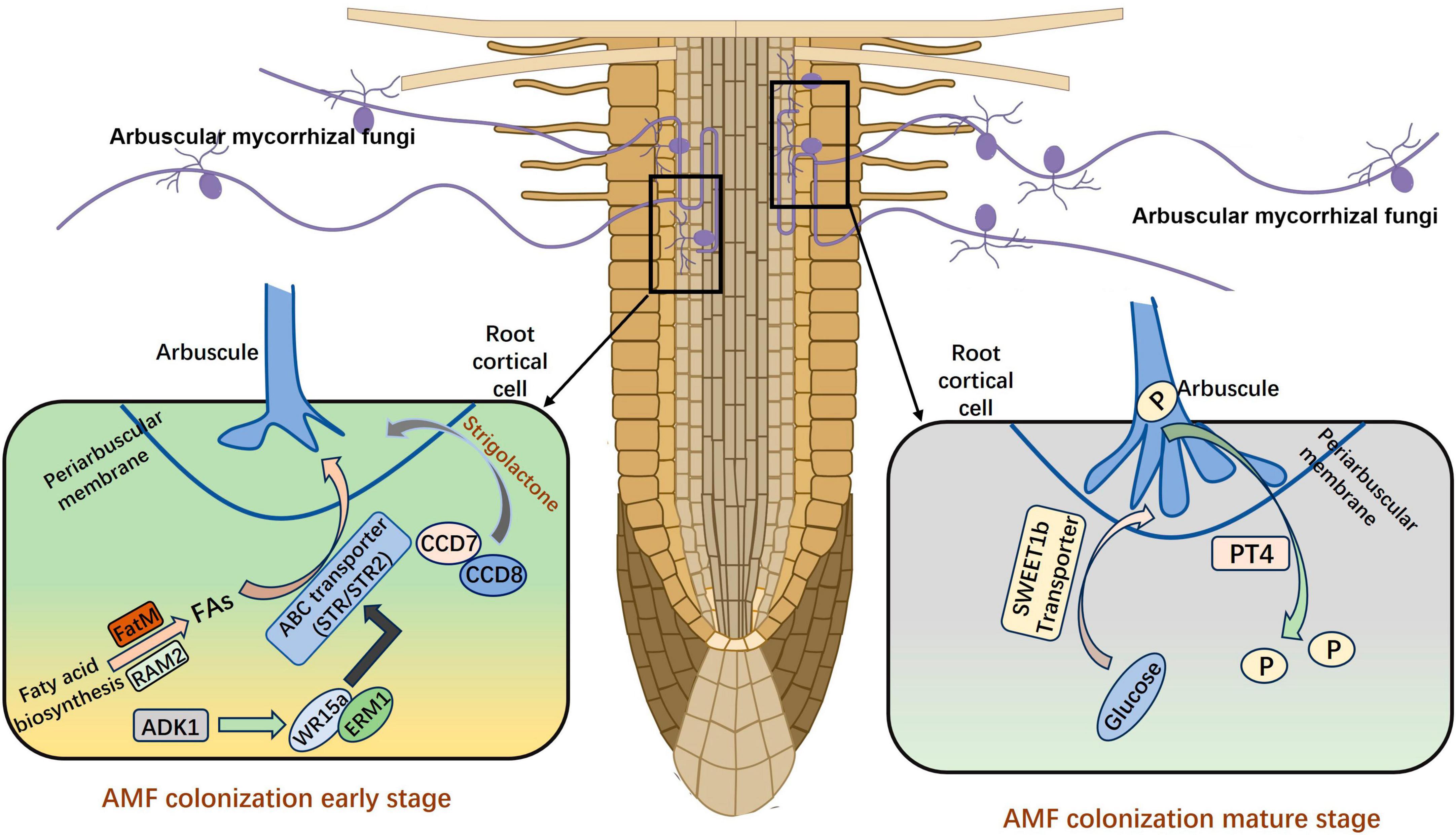
Figure 1. The establishment of AM symbiosis. FAs, fatty acids; P, phosphorus. The figure was created with Figdraw (https://www.figdraw.com/).
Here, we reviewed the effects of AMF as a biological symbiont on plant growth, drought resistance, plant hormone regulation, salt tolerance, mineral nutrient absorption, and heavy metal resistance. We then focused on its potential roles in plants. Finally, future perspectives on the introduction of AM symbiosis to plants are proposed. Some of the details on plant–AMF interactions presented here have implications for research on the regulation of AM symbiosis and host plants and will aid the use of AMF in plants to enhance resistance to abiotic stress.
3 Regulatory roles of AMF in plants
3.1 AMF enhance the drought resistance of host plants
Drought is a mainly adverse abiotic factors stunting plant production and threatening global food security (Zhang et al., 2018). As plants are immobile and fixed organisms, drought stress can have a major effect on their biomass accumulation and development (Aroca et al., 2008). Previous studies have shown that AM symbiosis can promote the adaptation of plants to drought stress (Bahadur et al., 2019). The regulation of plant drought tolerance by AM symbiosis is a complicated process that relates to many metabolites and metabolic pathways. AMF can enhance plants drought stress resistance by (1) improving soil aggregate structures; (2) promoting the absorption of nutrient elements and water by the host plants; (3) increasing water and nutrient use efficiency of the host; (4) enhancing the osmotic adjustment ability; (5) enhancing the antioxidant capacity and alleviating the negative effects due to the accumulation of reactive oxygen species (ROS) to plants; (6) regulating hormone balances in plants; and (7) inducing the expression of stress resistance gene (Figure 2).
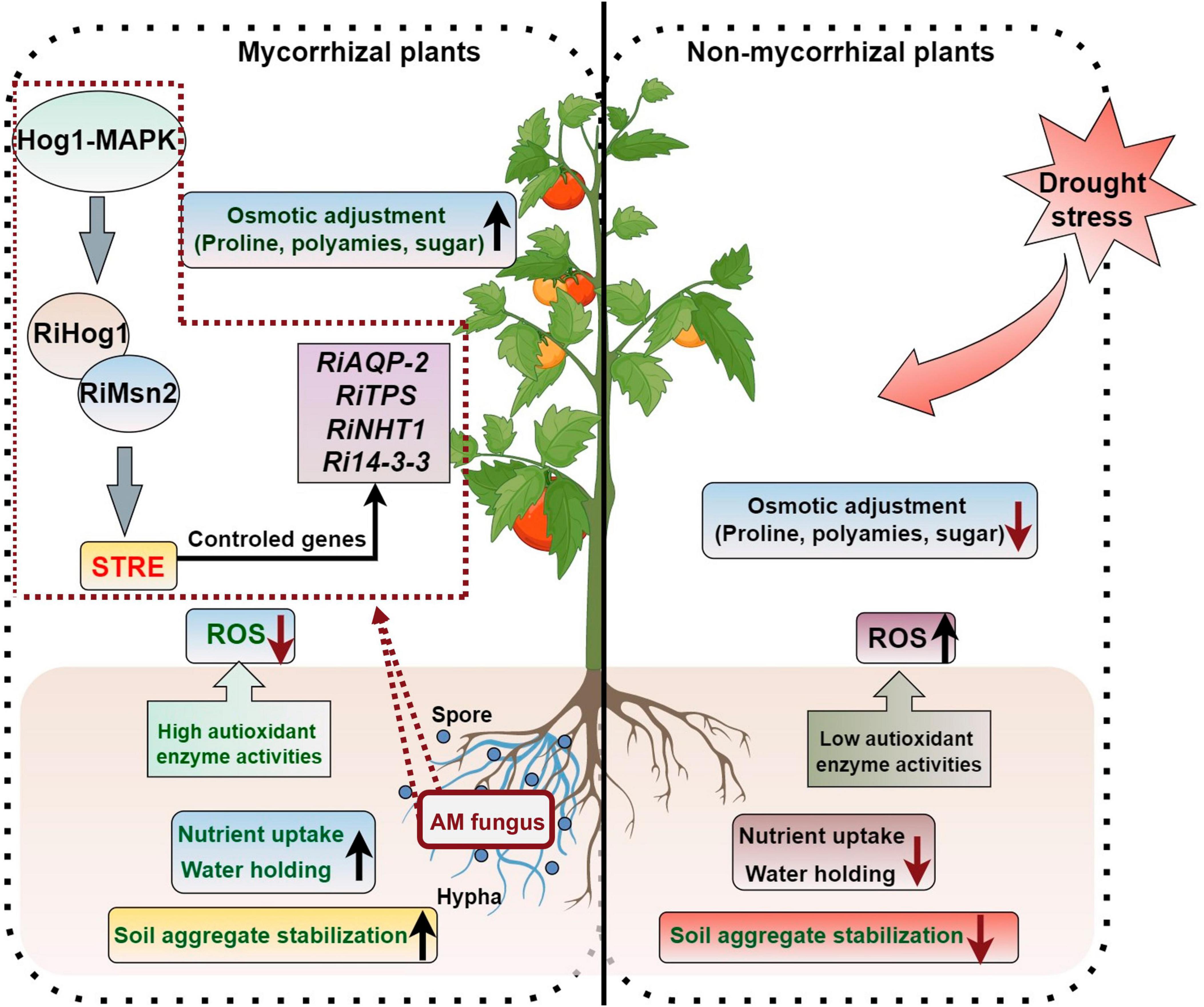
Figure 2. AM symbiosis regulate the response of plants to drought stress. The figure was created with Figdraw (https://www.figdraw.com/).
After the symbiotic relationship is established between AMF and host plants, AMF can expand the root volume of plants, thus increasing the area of water absorption. AMF can also improve water use efficiency and root hydraulic conductance by absorbing nutrients. Aquaporins (AQPs), which regulate water transport across the cell membrane, play an important role in plant water transport, and plasma membrane intrinsic proteins (PIPs) belong to a family of AQPs. Huang et al. (2020a) showed that the water use efficiency of Malus hupehensis seedlings inoculated with R. irregularis has a significantly higher level under drought stress than that of the non-inoculated seedlings, as the expression of PIP1-3 and PIP1-4 in the mycorrhizal seedling roots was significantly up-regulated compared with the untreated plants, and the expression level of Rir-AQP1 and Rir-AQP2 in the AMF was up-regulated under drought. Li et al. (2013) found that the AQP genes GintAQPF1 and GintAQPF2 was significantly up-regulated in extra-root hyphae and infected mycorrhizae under drought stress, which suggests that AMF have directly involvement in the response process to drought stress of plants. D-myo-inositol-3-phosphate synthase (IPS) and 14-3-3-like protein GF14 (14-3GF) are responsible for the signal communication between maize and AMF under drought stress condition, and co-expression of these two genes can enhance maize drought tolerance (Li et al., 2016).
The transcription factor RiMsn2 from R. irregularis is essential for arbuscule formation and can enhance the drought tolerance of plants. RiMsn2 can interact with RiHog1 and regulate the STRE-controlled genes from R. irregularis; the RiHog1-RiMsn2-STREs module regulates the drought stress response genes expression of AM fungal symbionts (Fan et al., 2023). Three Hog1 (High Osmolarity Glycerol 1)-MAPK cascade genes from R. irregularis, named RiSte11, RiPbs2, and RiHog1 have been identified and the silencing of those genes resulted in the reduced expression of drought-resistance genes (RiAQPs, RiTPSs, RiNTH1, and Ri14-3-3), which inhibited the development of arbuscule and reduced the resistance ability of host plants to drought stress (Wang S. et al., 2023). The encoding 14-3-3-like protein genes Ri14-3-3, Fm201, and RiBMH2 have been shown to participate in arbuscule formation and make responses to drought stress in host plants (Sun et al., 2018).
3.2 AMF improve the mineral nutrient absorption of host plants
Mineral nutrient has an important effect on plant abiotic stress resistance, such as drought stress (Shi et al., 2017). Wang et al. (2019) reported an enhancement of antioxidant enzyme activities under normal nitrogen (N) supply conditions compared with a low N treatment, which effectively inhibited the accumulation of ROS and consequently reduced the damage caused by drought stress on plants. After forming a symbiotic relationship with host plants, AMs can promote the absorption of a large number of nutrients, usually P and N (Fu et al., 2023; Shi et al., 2023). This stems from the extra-root hyphae of AMF, which are 1–2 times smaller in diameter than plant roots. These hyphae can thus penetrate deeper into the soil to absorb nutrients and expand the absorption area, which can promote root growth and increase nutrient uptake (Smith et al., 2010). The most important effect of AMF is considered to be promote the uptake of P by host plants (Wang et al., 2024). The R. irregularis Pi transporter RiPT7, containing an SPX (SYG1/Pho81/XPR1) domain, contributes to the inflow and outflow of Pi across to the plasma membrane according to the Pi gradient. RiPT7 silencing impedes R. irregularis mycorrhizal symbiosis and Pi delivery under low-Pi conditions (Xie X. et al., 2022). Shi et al. (2021) proved that the P starvation response (PHR) transcription factors are needed for mycorrhizal symbiosis and the PHR2 can regulate the expression of mycorrhizal symbiosis-related genes by the P1BS motif. Another R. irregularis transcription factor RiPho4, which contains an HLH domain and is significantly induced by P starvation, play a positive role on the downstream phosphate (PHO) pathway genes RiPT1, RiPT2, and RiPT3 in R. irregularis (Zhang S. et al., 2023). The GigmPT, a high-affinity Pi transceptor in Gigaspora margarita, is necessary for AM symbiosis development, which can activate both the Pi signaling pathway and the signaling cascade of protein kinase A (Xie et al., 2016). Kumar et al. (2008) found that P in soil forms a complex with calcium (Ca) and magnesium (Mg), which is usually not conducive to plant absorption. However, high acid phosphatase activity stemming from AMF can promote the release of P from these complexes.
Arbuscular mycorrhizas can effectively increase the N uptake and utilization by host plants, as they not only transfer N between fungi and host plants but also between different plants by hyphal bridges (Gao et al., 2021). AMF has its own N transport system, and three AMT genes (GintAMT1, GintAMT2, and GintAMT3) have been identified from R. irregularis (Kobae et al., 2010). GintAMT1 and GintAMT2 are expressed in both extraneous and intraradicular mycelium, and they are involved in NH4+ absorption of AMF and recovery of NH4+ loss at the symbiotic interface due to fungal metabolism (Pérez-Tienda et al., 2012). GintAMT3 is highly induced only in intraradicular mycelium, and its expression is regulated by the substrate concentration and carbon source (Calabrese et al., 2016). Symbiotic plant N uptake from the peri-arbuscular space depends on plant N transporters that localized in the peri-arbuscular membrane, such as the AMF induced NO3– transporter OsNPF4.5 in O. sativa, the amino acid transporter LjLHT1.2, and the NH4+ transporter LjAMT2;2 in Lotus japonicus roots (Wang et al., 2020, 2022b; Guether et al., 2022; Zhang Y. et al., 2023). In maize (Zea mays) and sorghum (Sorghum bicolor), the expression level of NPF4.5 homologs also up-regulated by the AMF infection, indicating that the NO3– absorption pathways are highly active under AM symbiosis (Wang et al., 2020). Saia et al. (2015) indicated that AM symbiosis significantly increase the expression level of NRT1.1, AMT1;2, and AMT2;1 in durum wheat (Triticum durum Desf.). Another AMF-inducible ammonium transporter ZmAMT3;1, which specifically expressed in cortical cells that were AMF infected, can absorb 68–74% of the N transported from AMF to plants in maize (Hui et al., 2022; Figure 3). In Medicago truncatula, two signaling peptides, C-terminally encoded peptides (CEPs) and CLAVATA3/endosperm surrounding region-related peptides (CLEs), positively and negatively regulate symbiotic nodule development, respectively, in response to NO3– levels (Oldroyd and Leyser, 2020; Luo et al., 2021). Additionally, AM colonization can enhance plant potassium, sulfur, zinc (Zn), iron, Mg, and Ca uptake in various plants (Talaat and Shawky, 2011; Garcia and Zimmermann, 2014; Giovannetti et al., 2014).
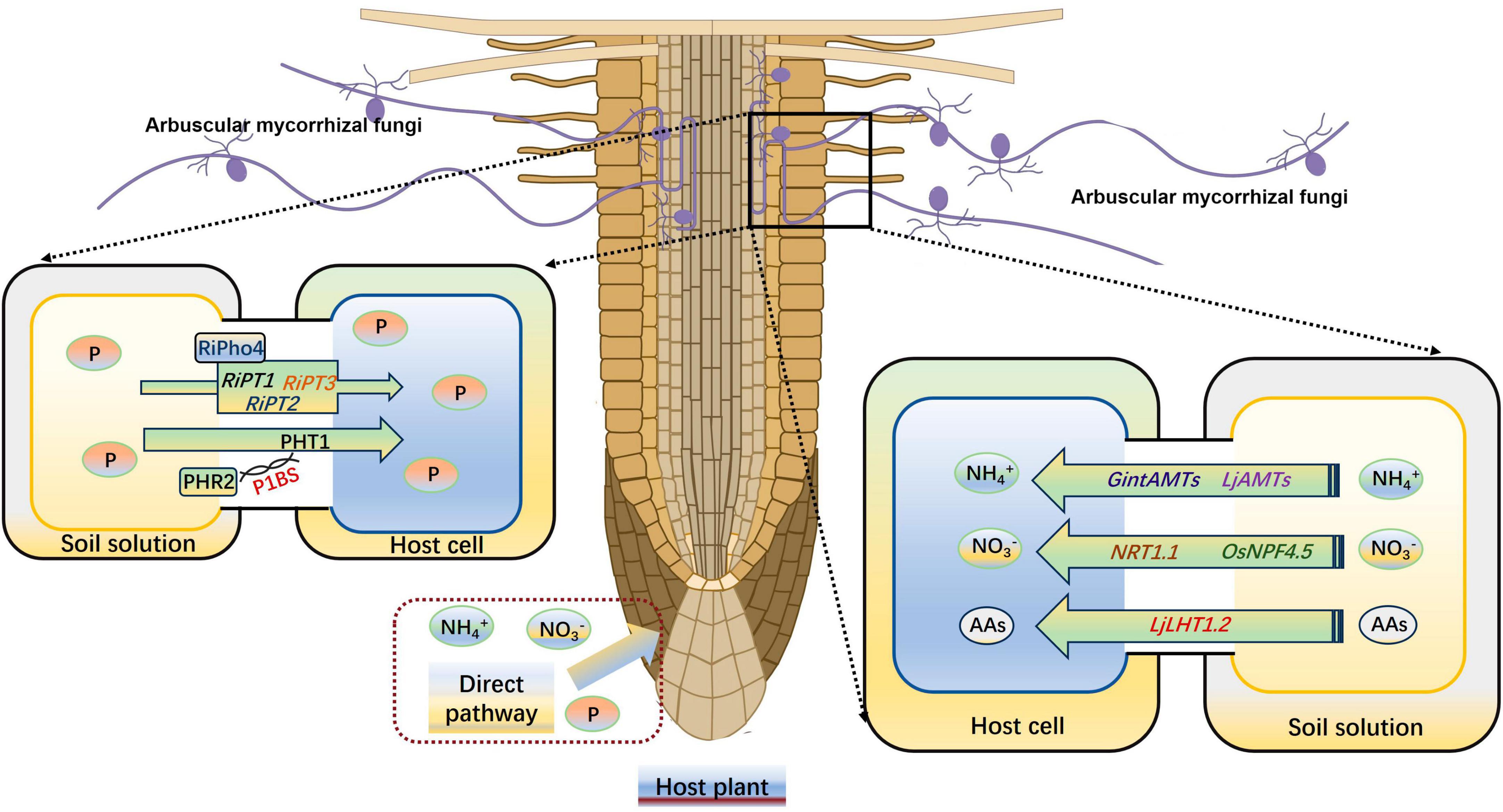
Figure 3. AM symbiosis enhances the uptake of P and N by plants. N, nitrogen; P, phosphorus. AAs, amino acids. The figure was created with Figdraw (https://www.figdraw.com/).
3.3 AMF enhance the salt tolerance of host plants
Salt stress affects plants growth in many regions around the world, especially irrigated land, and limits the healthy development of agricultural industries and the distribution of plants in the world (Cui et al., 2021). Approximately one in five of irrigated land in the world is influenced by salinization, and even worse, this figure is continually expanding. The adverse effects of salt stress on plants mainly stems from the decrease of available water as Na+ accumulates around the roots and the toxic actions of Na+ and Cl– on plants, which disrupt the life processes of plants (Parihar et al., 2015). Plants have developed a series of adaptive strategies to cope with salt stress that relate to signal transduction such as calcium signal, phosphatidylinositol, protein kinases, and phytohormones. These lead to adaptive responses, such as compatible solute production, ion efflux and segregation, ROS homeostasis regulation, and the growth rate change. In addition, plants can establish symbiotic relationships with beneficial rhizosphere microorganisms to deal with the adverse effects of salt stress, such as AMF (Porcel et al., 2015; Evelin et al., 2019).
Zhang X. et al. (2021) conducted transcriptome sequencing on asparagus (Asparagus officinalis L.) seedling roots and found that 391 differentially expressed transcripts under salt stress were regulated by AMF, and these were mainly involved in ROS eliminating, mineral elements and water uptake regulation, and cell wall functional construction. Under salt stress conditions, seedlings of mycorrhizal halophytes have lower sodium ion concentrations and lower soluble sugar concentrations than non-mycorrhizal halophytes. These differences might be related to the carbohydrate and energy metabolism regulation, for example the glyoxylate and dicarboxylic acid metabolic pathways (Diao et al., 2021). Cui et al. (2021) proved that AMF improved the chlorophyll content of leaves, photosynthetic rate, and fluorescence correlation parameters and enhanced the utilization of light energy of Phoebe zhennan, which alleviated the damage induced by salt and promoted the growth of P. zhennan in saline–alkali soil. In addition, the Na+/K+ ratio is changed in AM symbiosis plants to sustain the osmotic equilibrium state when subjected to salt stress (Hdar et al., 2023). AMF colonization can enhance jujube root H+ efflux and K+ influx and fatty acid metabolism during salt stress, and the fatty acid content is increased in leaves and roots of AM symbiosis plants, which confers greater salt stress in wild jujube. The plasma membrane ATPase gene ZjAHA7 is activated by mycorrhizal colonization, which initiates H+ efflux; the expression of ZjHAK2 is also up-regulated, and this promotes K+ accumulation in AM plants to achieve a high K+/Na+ state (Ma et al., 2022).
When subjected to salt stress, the expression of the salt tolerance-related genes OsPRX, Os10g, OsHBP1b, and OsNCX in mycorrhizal plants was higher than that in non-mycorrhizal plants, which increased the ROS scavenging ability and decreased malondialdehyde accumulation in rice (Zhang B. et al., 2023). Wang et al. (2022a) found that the expression of some ion transfer, ROS scavenging, and carbohydrate metabolism related genes, including HAK5, PIP1-2, MYB46, NAC43, GLP10, SKOR, CPER, and WRKY19 was significantly enhanced by R. irregularis, which increased the salt stress tolerance of C. glauca. When AMF is applied to Casuarina glauca, the expression of some CgNHXs and CgCLCs can increase plant biomass, the K+ content, and the compartmentalization of Na+ and Cl– in vacuoles, which improves salt tolerance (Wang Y. et al., 2023). After AM symbiosis, the up-regulation of SOS1/NHX7 in Robinia pseudoacacia roots can enhance saline stress tolerance by promoting the efflux of Na+ from plant roots (Chen J. et al., 2017). The salt overly sensitive (SOS) genes SlSOS1 and SlSOS2 is up-regulated by AMF colonization when subjected to salt stress, which enhances the salt tolerance of tomatoes (Liu et al., 2023). Ren et al. (2018) found that the hub genes in the module related to the response to salt stress in Sesbania cannabina inoculated with AMF included a range of transcription factors, such as WRKY, ERF, MYB, and TCP members. These results provide new bases for further understanding the regulatory mechanisms underlying plant–AMF interactions during salt stress responses (Figure 4).
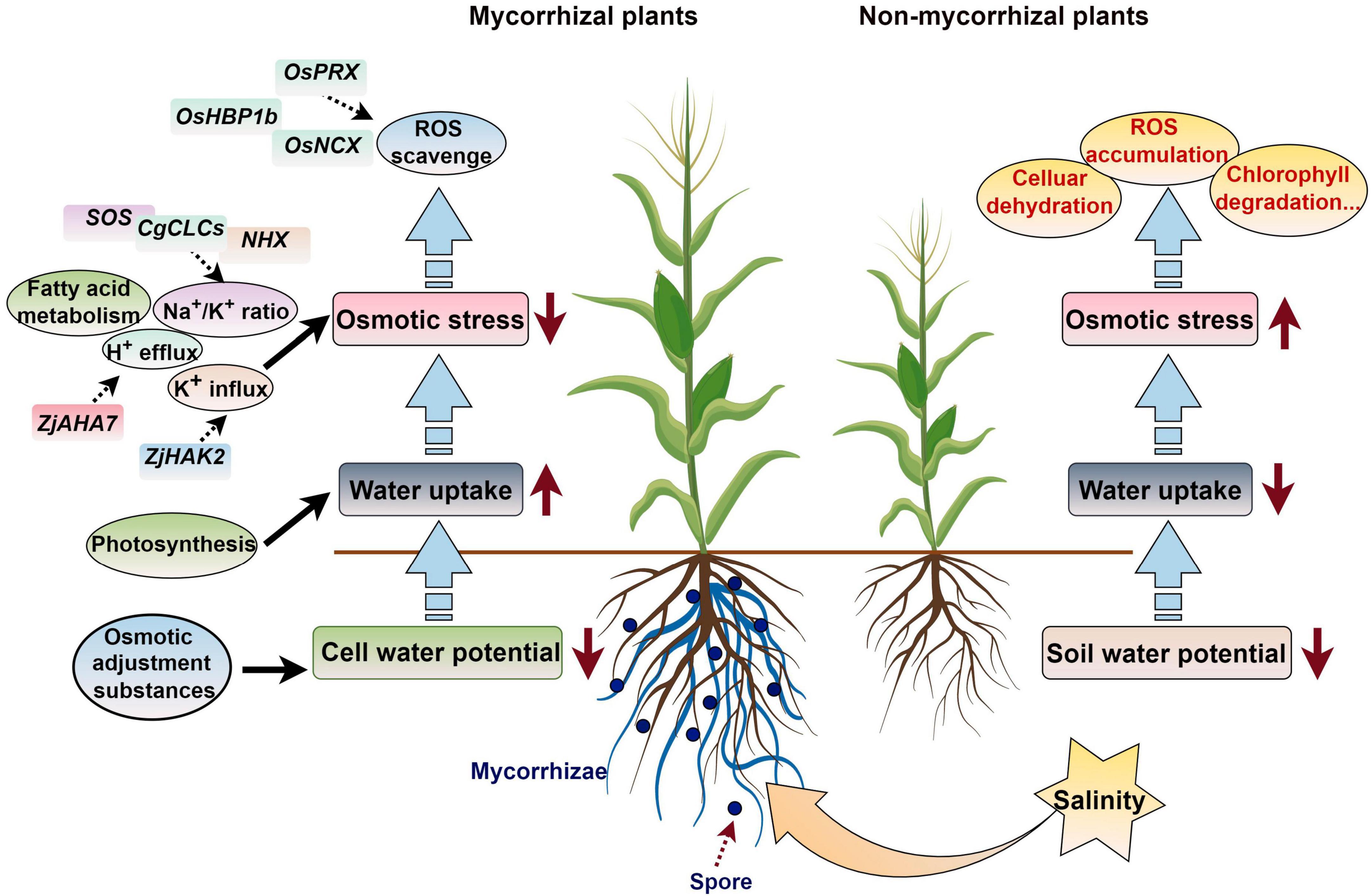
Figure 4. Working model of how AM symbiosis regulates the response of plants to salt stress. ROS, reactive oxygen species. The figure was created with Figdraw (https://www.figdraw.com/).
3.4 AM symbiotic regulation of plant hormones to alleviate abiotic stress
Plant hormones are multifunctional substances that play key roles in plant life cycle, including the plant–AMF interactions process (Foo et al., 2013; Charpentier et al., 2014; Etemadi et al., 2014). Auxin (IAA) is a key endogenous plant hormone that has been identified to play a very important role in arbuscular development process in plants (Etemadi et al., 2014; Wang et al., 2021). Meixner et al. (2005) proved that soybean roots inoculated with AMF had significantly higher IAA concentrations than those in the uninoculated control. AMF can promote the development of roots, with emphasis on the lateral roots, by increasing indole-3-acetic acid (IAA) levels (Arzanesh et al., 2011). Ma et al. (2022) showed that AM symbiosis alters phytohormonal levels and that the IAA and abscisic acid (ABA) content is significantly increased in jujube roots after mycorrhizal formation but reduced by salt stress.
Strigolactones are important for symbiotic fungi, especially AMF (Mitra et al., 2021). Drought stress decreases SL content in both non-AMF-colonized and AMF-colonized plants (Ruiz-Lozano et al., 2016). Huang et al. (2020b) showed that overexpression of the auxin/indole-3-acetic acid gene MdIAA24 can promote the mycorrhizal infection of apple roots by increasing the content of SLs, which results in a higher mycorrhizal infection rate and greater drought resistance of transgenic lines. The SL contents are negative correlation with plant P status and directly regulation the process of AM symbiosis (Yoneyama et al., 2007). Low P in plants induces the genes expression relating to SL biosynthetic, and two GRAS transcription factors (NSP1 and NSP2) are responsible for mediating this response; these two transcription factors also take part in the AM symbiosis signaling pathway (Liu et al., 2011; Luginbuehl and Oldroyd, 2017). Gibberellic acid (GA) is another important hormone involved in regulating changes associated with plant P status and AM symbiosis. The biosynthesis of GA is regulated by P status in plants, and low P stress down-regulates the expression of GA biosynthesis related genes but enhances the transcription of DELLA genes (Devaiah et al., 2009). Floss et al. (2013) showed that the arbuscule development process is affected by DELLA proteins, which act as repressors of GA signaling and stunt plants biomass accumulation and development process. Under AM symbiosis, the expression levels of genes related to the degradation and signaling of GA are altered substantially (Yu et al., 2014).
Arbuscular mycorrhizal fungi play an important role in maintaining hormone balances in plants (Figure 5). Zhang J. et al. (2021) found that AMF maintain hormone balances after roots are damaged by inducing significant increases of IAA and cytokinin levels but decreases in ABA levels in both the roots and leaves, which leads to increases in the ratio of fine roots and promotes root growth. In addition, AMF can respond to drought stress by triggering plant hormone signaling. Increased ABA biosynthesis ability in both AM mycorrhizal and stressed plants can increase the establishment of AM symbiosis and improve drought tolerance (Bahadur et al., 2019). Co-inoculation with AMF and rhizobacteria can improve the ABA and IAA contents in the tobacco shoots when subjected to drought stress (Begum et al., 2022). Inoculation of watermelon with different numbers of AMF spores revealed that the concentrations of ABA, IAA, GA3, and zeatin riboside (ZR) were differentially altered as the number of spores increased. For example, ABA concentrations were highest when 300 spores were inoculated per plant and lower when 600 spores were inoculated per plant (Wu et al., 2021). In Lycium barbarum L. (Goji), the IAA content in both the leaves and roots and ABA content in the leaves are enhanced after inoculation with AMF, and this maintains the osmotic balance and improves salt stress resistance (Liu et al., 2016). Under arsenic (As) stress conditions, AMF-inoculated plants can regulate the concentrations and ratios of phytohormones to alleviate stress, which includes an increasing in IAA and ABA contents but a decreasing in the GA and zeatin riboside contents (Zhang et al., 2020).
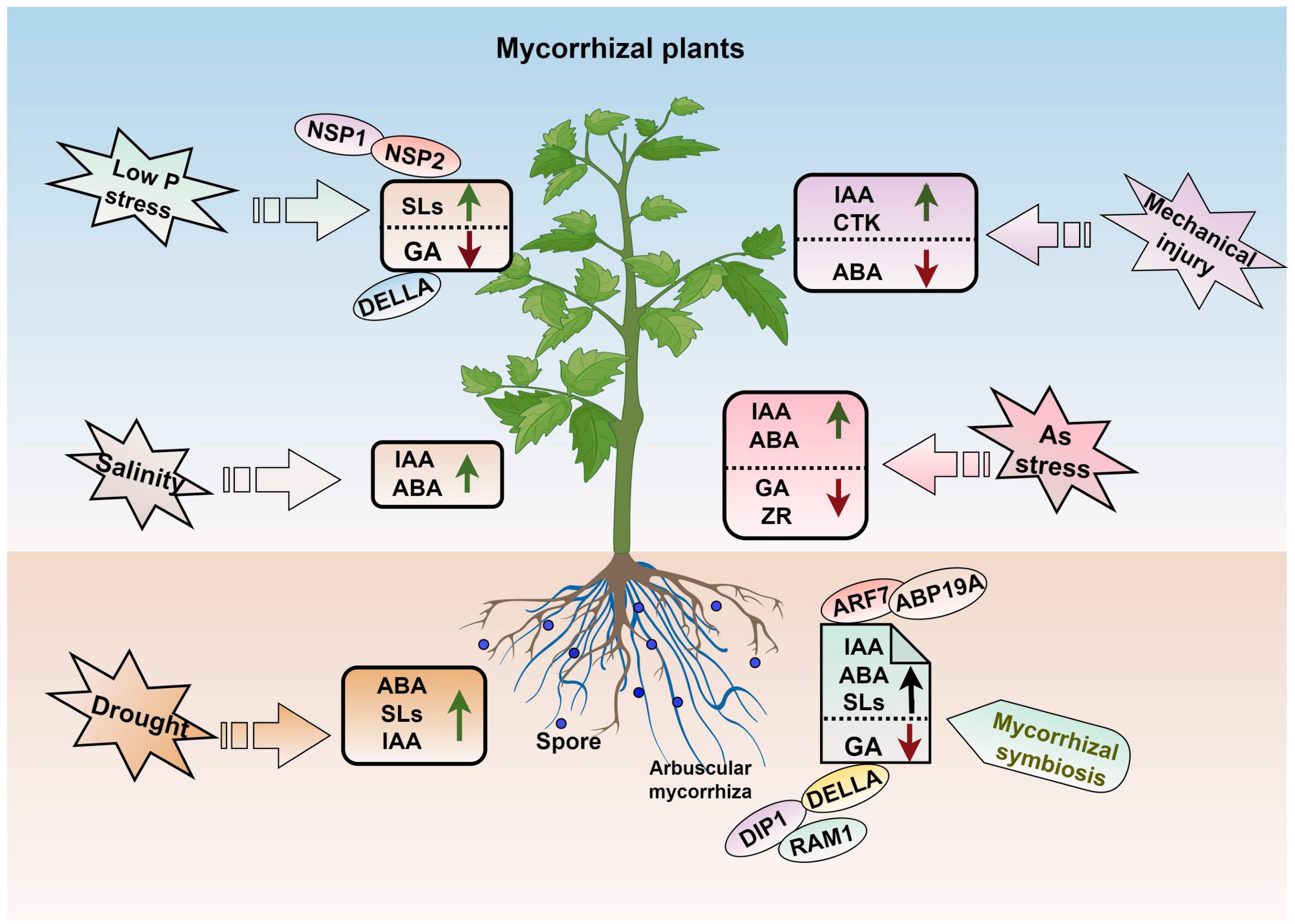
Figure 5. AM symbiosis and hormone regulation in plant abiotic stress. IAA, auxin; ABA, abscisic acid; GA, gibberellic acid; SLs, strigolactone; ZR, zeatin riboside; CTK, cytokinin. The figure was created with Figdraw (https://www.figdraw.com/).
3.5 AMF symbiosis enhances heavy metal resistance in plants
Heavy metal pollution such as cadmium (Cd), mercury (Ag), As, and lead (Pb) has become increasingly severe due to the excessive use of pesticides in agricultural production, large-scale livestock and poultry farming, and industrial discharge (Wu et al., 2018). Heavy metals, take Cd stress as an example, generally cause damage to plants in two biological pathways. First, when Cd are absorbed by plant roots, they disturb the ion balance within the plant, impede normal life activities, and induce metabolic activity disorders. Second, after Cd is absorbed by the plant, it may form complexes with proteins, enzymes, and other organic biological macromolecules to take the place of the necessary elements in the original structure of enzymes and other biological macromolecules, which makes them lose specific functions, become inactivated, and even denatured. Usually, plants undergo various physiological and molecular changes to escape or mitigate the protein inactivation and oxidative damage due to the ROS burst when exposed to Cd stress (Sofo et al., 2013). AM symbioses have been proved to enhance the resistance of plants to soil contaminated with heavy metals, such as Cd (Chen et al., 2004a), Pb (Chen X. et al., 2005), copper (Cu) (Chen et al., 2007), Zn (Chen et al., 2004b), and uranium (Chen X. et al., 2005).
Two common mechanisms have been shown to improve the heavy metal resistance of plants through mycorrhizal symbiosis: the “growth dilution effect” and “mycorrhizal immobilization.” That is, AM symbiosis can increase plant P content, which promotes the biomass accumulation of plants and the dilution of heavy metals in plants, or AMF can restrict heavy metals within the plant roots by precipitating polyphosphate complexes, thereby inhibiting their transfer to the shoots (Wu et al., 2015; Liu et al., 2018). Chen X. et al. (2005) suggest that mycorrhizae under Pb stress can facilitate plant growth via improving P absorb and mitigating Pb stress toxicity through fixing more Pb in the roots. AMF can also colonize plants in natural As-contaminated soils and alleviate As phytotoxicity (Sun et al., 2016). In addition, AM symbiosis can contribute to methylation (Li et al., 2018), which provides a new pathway by which AMF can alleviate heavy metal phytotoxicity. AMF can also enhance the antioxidant enzyme activity of plants by secreting lipids and proteins or changing the expression level of heavy metal stress respond related genes, thereby reducing the serious damage of heavy metal stress to plants (Aloui et al., 2009). Zhang et al. (2019) showed that AM plants might sustain ROS homeostasis under exposure to Pb stress by down-regulating the expression of the respiratory burst oxidase homologs (RBOHs) gene MtRbohC-G, decreasing the H2O2 accumulation in Medicago truncatula. When subjected to heavy metal stress, AMF can induce the hormone synthesis, including IAA, ABA, and GA, in host plants so as to enhance resistance to heavy metal stress. Under Cd stress, AM symbiosis results in an increase in ABA in a high Cd uptake maize cultivar and an increase in IAA in a low Cd uptake maize cultivar (Chen et al., 2023).
Some related genes in plants also take part in the AMF-mediated responses process to heavy metal stress. In maize, the expression of heavy metal ATPase (HMA) ZmHMA3a and ZmHMA4 isoforms was induced by Cu treatment in mycorrhizal plants, suggesting that AM symbiosis enhanced the expression of HMA genes putatively encoding Cu detoxification proteins to alleviate Cu toxicity by AM (Gómez-Gallego et al., 2022). Gómez-Gallego et al. (2019) identified two Cu transporter (CTR) family members (RiCTR1 and RiCTR2) and a CTR-like protein (RiCTR3A) from R. irregularis; these three genes are involved in Cu transport and tolerance. Further analysis showed that RiCTR3A may act as a Cu receptor under Cu stress. Three other genes (RiATOX1, RiSco1, and RiSSC) from R. irregularis have been identified, and these genes encode putative chaperones that separately mediate the transfer of Cu to ATPases, cytochrome C oxidases, and Cu or Zn superoxide dismutase (Ferrol et al., 2016). Huang et al. (2021) showed that AMF inoculation can reduce Cd accumulation in apple, and the MdGH3-2/12 silenced seedlings appeared lower Cd stress resistance for which reduced the AM symbiosis ratio compared to wild type.
4 Conclusion and future perspectives
Arbuscular mycorrhizal fungi assume a crucial role in the plant life cycle, and the generation of AM is regarded as the final results of long-term co-evolution between plants and AMF. Its functions include (1) increasing the absorption of P and other nutrient elements for example Ca, Zn, Cu, and N by host plants; (2) improving the resistance of hosts to drought, cold, and saline–alkali stress, as well as various diseases, including soil-borne diseases; and (3) promoting the generation of soil aggregate’s structure and improving the ability of soil to retain water and fertilizer. The present review mainly highlighted the effects of interactions between AMF and host plants on drought resistance, nutrient uptake, hormone regulation, salt resistance, and heavy metal stress resistance (Figure 6).
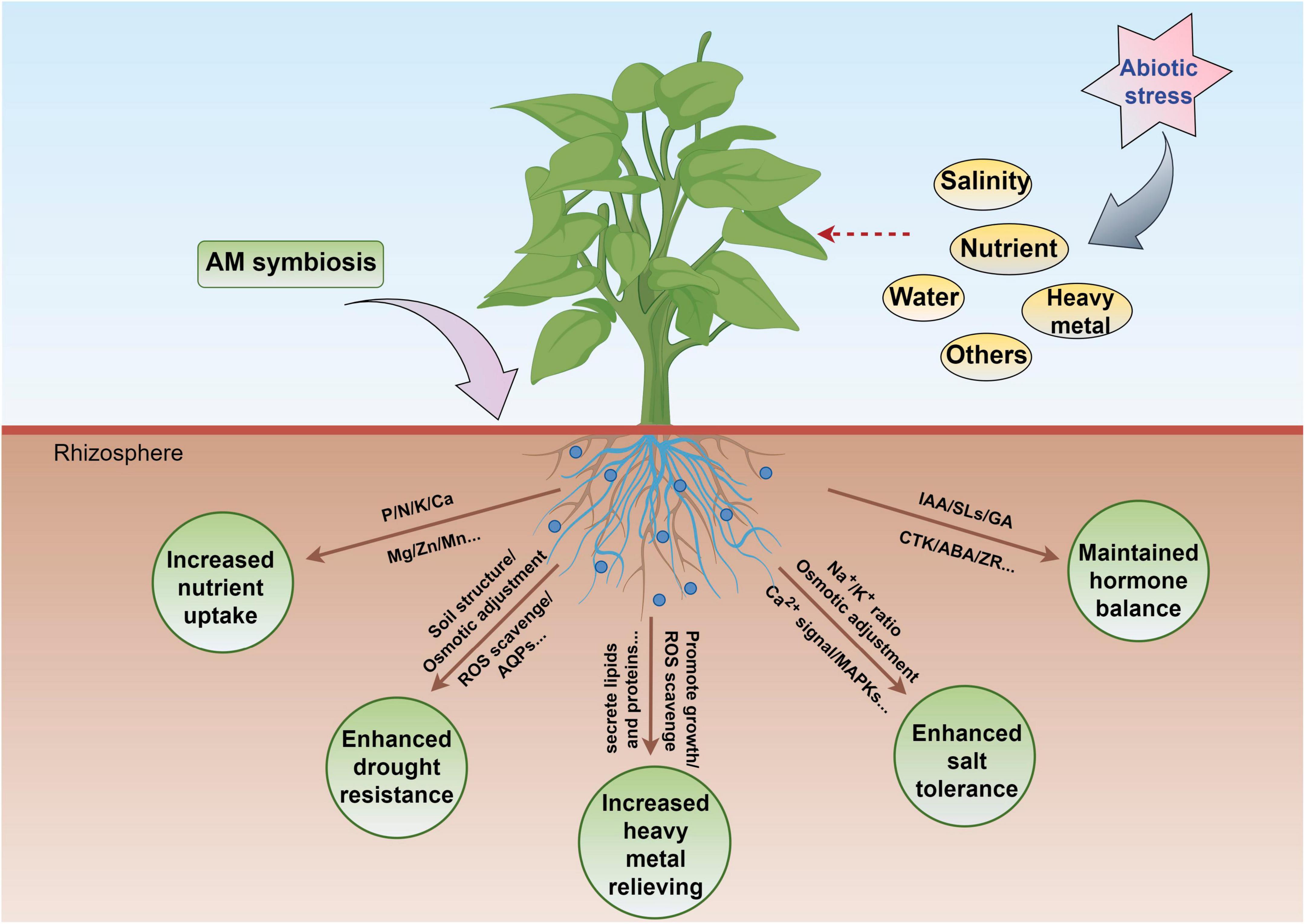
Figure 6. Some potential applications of AMF in plants exposed to abiotic stress. The figure was created with Figdraw (https://www.figdraw.com/).
Though AM fungi has great potential applications in plant production, the successful application of AM fungi in agriculture still has severe key microbial technical challenges. First, it is difficult to produce the inoculum on a large scale, on account of the nature of obligate biotrophy of AMF. Some pure culture approaches have recently been born (Kameoka et al., 2019; Sugiura et al., 2020; Tanaka et al., 2022), which will bring hope to obtain high yield and high-quality AM fungal by asymbiotic mass production. Second, different strains should be selected in different crop systems based on plant and AM fungal genotypes, colonization ability and symbiotic efficiency. Moreover, it urgently needs to establish a quality management framework for AM fungal inoculants in agricultural practices to further optimize the purity and infection rate of AMF inoculants that used in production (Salomon et al., 2022). Strengthening the research on the functional diversity and purification of AMF will help the development of AMF related industries, promote the wide application of AMF in agricultural production, and provide important support for sustainable agricultural development.
Author contributions
QW: Funding acquisition, Investigation, Writing—original draft. DH: Funding acquisition, Supervision, Writing—review and editing. ML: Investigation, Writing—original draft. ZW: Investigation, Writing—original draft. JL: Data curation, Investigation, Writing—original draft. KL: Investigation, Writing—original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Project of Guizhou Province (Grant number: Qiankehe-ZK [2023]103 and 101); the Scientific Research Project of General Undergraduate Universities (Youth Project) of Guizhou (Grant number: Qianjiaoji [2022]117); the Open Project Fund of the Key Laboratory of Plant Resources Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education) (Grant number: Qianjiaoji [2022]431); and the Natural Science Foundation of Guizhou University (Grant number: 600106).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aloui, A., Recorbet, G., Gollotte, A., Robert, F., Valot, B., Gianinazzi-Pearson, V., et al. (2009). On the mechanisms of cadmium stress alleviation in Medicago Truncatula by arbuscular mycorrhizal symbiosis: a root proteomic study. Proteomics 9, 420–433. doi: 10.1002/pmic.200800336
An, J., Zeng, T., Ji, C., Graaf, S., Zheng, Z., Xiao, T., et al. (2019). A Medicago truncatula SWEET transporteshr implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis. New Phytol. 224, 396–408. doi: 10.1111/nph.15975
Aroca, R., Vernieri, P., and Ruiz-Lozano, J. M. (2008). Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 59, 2029–2041. doi: 10.1093/jxb/ern057
Arzanesh, M. H., Alikhani, H. A., Khavazi, K., Rahimian, H. A., and Miransari, M. (2011). Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 27, 197–205. doi: 10.1007/s11274-010-0444-1
Bagheri, V., Shamshiri, M. H., Shirani, H., and Roosta, H. R. (2012). Nutrient uptake and distribution in mycorrhizal pistachio seedlings under drought stress. J. Agric. Sci. Technol. 14, 1591–1604.
Bahadur, A., Batool, A., Nasir, F., Jiang, S. J., Qin, M. S., Zhang, Q., et al. (2019). Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 20:4199. doi: 10.3390/ijms20174199
Begum, N., Wang, L., Ahmad, H., Akhtar, K., Roy, R., Khan, M. I., et al. (2022). Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 83, 971–988. doi: 10.1007/s00248-021-01815-7
Besserer, A., Becard, G., Jauneau, A., Roux, C., and Séjalon-Delmas, N. (2008). GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 148, 402–413. doi: 10.1104/pp.108.121400
Bonfante, P., and Venice, F. (2020). Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 34, 100–113. doi: 10.1016/j.fbr.2019.12.003
Bravo, A., Brand, M., Wewer, V., Dormann, P., and Harrison, M. J. (2017). Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 214, 1631–1645. doi: 10.1111/nph.14533
Calabrese, S., Pérez-Tienda, J., Ellerbeck, M., Arnould, C., Chatagnier, O., Boller, T., et al. (2016). GintAMT3-a low-affinity ammonium transporter of the arbuscular mycorrhizal Rhizophagus irregularis. Front. Plant Sci. 7:679. doi: 10.3389/fpls.2016.00679
Charpentier, M., Sun, J., Wen, J., Mysore, K. S., and Oldroyd, G. E. (2014). Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the PROTEIN PHOSPHATASE 2A complex. Plant Physiol. 166, 2077–2090. doi: 10.1104/pp.114.246371
Chen, B., Liu, Y., Shen, H., Li, X., and Christie, P. (2004a). Uptake of cadmium from an experimentally contaminated calcareous soil by arbuscular mycorrhizal maize (Zea mays L.). Mycorrhiza 14, 347–354. doi: 10.1007/s00572-003-0281-2
Chen, B., Shen, H., Li, X., Feng, G., and Christie, P. (2004b). Effects of EDTA application and arbuscular mycorrhizal colonization on growth and zinc uptake by maize (Zea mays L.) in soil experimentally contaminated with zinc. Plant Soil 261, 219–229. doi: 10.1023/B:PLSO.0000035538.09222.ff
Chen, B., Roos, P., Borggaard, O. K., Zhu, Y., and Jakobsen, I. (2005). Mycorrhiza and root hairs in barley enhance acquisition of phosphorus and uranium from phosphate rock but mycorrhiza decreases root to shoot uranium transfer. New Phytol. 165, 591–598. doi: 10.1111/j.1469-8137.2004.01244.x
Chen, X., Wu, C., Tang, J., and Hu, S. (2005). Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 60, 665–671. doi: 10.1016/j.chemosphere.2005.01.029
Chen, B., Zhu, Y., Duan, J., Xiao, X., and Smith, S. E. (2007). Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ. Pollut. 147, 374–380. doi: 10.1016/j.envpol.2006.04.027
Chen, J., Wang, L., Liang, X., Li, B., He, Y., and Zhan, F. (2023). An arbuscular mycorrhizal fungus differentially regulates root traits and cadmium uptake in two maize varieties. Ecotoxicol. Environ. Saf. 264:115458. doi: 10.1016/j.ecoenv.2023.115458
Chen, J., Zhang, H., Zhang, X., and Tang, M. (2017). Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 8:1739. doi: 10.3389/fpls.2017.01739
Chen, S., Zhao, H., Zou, C., Li, Y., Chen, Y., Wang, Z., et al. (2017). Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 8:2516. doi: 10.3389/fmicb.2017.02516
Chitarra, W., Pagliarani, C., Maserti, B., Lumini, E., Siciliano, I., Cascone, P., et al. (2016). Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 171, 1009–1023. doi: 10.1104/pp.16.00307
Cui, L., Liu, Y., Lin, J., and Shi, K. (2021). Effects of AMF on photosynthetic characteristics of Phoebe zhennan under salt stress. J. Nanjing For. Univ. 45, 101–106.
Delaux, P. M., Bécard, G., and Combier, J. P. (2013). NSP1 is a component of the Myc signaling pathway. New Phytol. 199, 59–65. doi: 10.1111/nph.12340
Devaiah, B. N., Madhuvanthi, R., Karthikeyan, A. S., and Raghothama, K. G. (2009). Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant 2, 43–58. doi: 10.1093/mp/ssn081
Diao, F., Dang, Z., Cui, X., Xu, J., Jia, B., Ding, S., et al. (2021). Transcriptomic analysis revealed distinctive modulations of arbuscular mycorrhizal fungi inoculation in halophyte Suaeda salsa under moderate salt conditions. Environ. Exp. Bot. 183:104337. doi: 10.1016/j.envexpbot.2020.104337
Etemadi, M., Gutjahr, C., Couzigou, J., Zouine, M., Lauressergues, D., Timmers, A., et al. (2014). Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 166, 281–292. doi: 10.1104/pp.114.246595
Evelin, H., Devi, T. S., Gupta, S., and Kapoor, R. (2019). Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front. Plant Sci. 10:470. doi: 10.3389/fpls.2019.00470
Fan, X., Xie, H., Huang, X., Zhang, S., Nie, Y., Chen, H., et al. (2023). A module centered on the transcription factor Msn2 from arbuscular mycorrhizal fungus Rhizophagus irregularis regulates drought stress tolerance in the host plant. New Phytol. 240, 1497–1518. doi: 10.1111/nph.19077
Ferrol, N., Tamayo, E., and Vargas, P. (2016). The heavy metal paradox in arbuscular mycorrhizas: from mechanisms to biotechnological applications. J. Exp. Bot. 67, 6253–6565. doi: 10.1093/jxb/erw403
Floss, D. S., Levy, J. G., Levesque-Tremblay, V., Pumplin, N., and Harrison, M. J. (2013). DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 110, 5025–5034. doi: 10.1073/pnas.1308973110
Foo, E., Ross, J. J., Jones, W. T., and Reid, J. B. (2013). Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann. Bot. 111, 769–779. doi: 10.1093/aob/mct041
Fu, W., Yan, M., Zhao, L., Zeng, X., Cai, B., Qu, S., et al. (2023). Inoculation with arbuscular mycorrhizal fungi increase calcium uptake in Malus robusta. Sci. Hortic. 321:112295. doi: 10.1016/j.scienta.2023.112295
Gao, W., Zaynur, T., Sang, Y., and Ma, X. (2021). Effect of arbuscular mycorrhizal fungi on nitrogen absorption of plants: a review. Chin. Agric. Sci. Bull. 37, 53–58.
Garcia, K., and Zimmermann, S. D. (2014). The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 5:337. doi: 10.3389/fpls.2014.00337
Genre, A., Lanfranco, L., Perotto, S., and Bonfante, P. (2020). Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18, 649–660. doi: 10.1038/s41579-020-0402-3
Giovannetti, M., Tolosano, M., Volpe, V., Kopriva, S., and Bonfante, P. (2014). Identification and functional characterization of a sulfate transporter induced by both sulfur starvation and mycorrhiza formation in Lotus japonicus. New Phytol. 204, 609–619. doi: 10.1111/nph.12949
Gómez-Gallego, T., Benabdellah, K., Merlo, M. A., Jiménez-Jiméne, A. M., Alcon, C., Berthomieu, P., et al. (2019). The Rhizophagus irregularis genome encodes two CTR copper transporters that mediate Cu import into the cytosol and a CTR-Like protein likely involved in copper tolerance. Front. Plant Sci. 10:604. doi: 10.3389/fpls.2019.00604
Gómez-Gallego, T., Valderas, A. A., Tuinen, D., and Ferrol, N. (2022). Impact of arbuscular mycorrhiza on maize P1B-ATPases gene expression and ionome in copper-contaminated soils. Ecotoxicol. Environ. Saf. 234:113390. doi: 10.1016/j.ecoenv.2022.113390
Guether, M., Volpe, V., Balestrini, R., Requena, N., Wipf, D., and Bonfante, P. (2022). LjLHT1.2-a mycorrhiza-inducible plant amino acid transporter from Lotus japonicus. Biol. Fertil. Soils 47, 925–936. doi: 10.1007/s00374-011-0596-7
Guo, R., Wu, Y., Liu, C., Liu, Y., Tian, L., Cheng, J., et al. (2022). OsADK1, a novel kinase regulating arbuscular mycorrhizal symbiosis in rice. New Phytol. 234, 256–268. doi: 10.1111/nph.17979
Harrison, M. J., Dewbre, G. R., and Liu, J. (2002). A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14, 2413–2429. doi: 10.1105/tpc.004861
Hdar, M., Mrazvi, S., Singh, N., Mushtaq, A., Dar, S., and Hussain, S. (2023). Arbuscular mycorrhizal fungi for salinity stress: anti-stress role and mechanisms. Pedosphere 33, 212–224. doi: 10.1016/j.pedsph.2022.06.027
Heck, C., Kuhn, H., Heidt, S., Walter, S., Rieger, N., and Requena, N. (2016). Symbiotic fungi control plant root cortex development through the novel GRAS transcription factor MIG1. Curr. Biol. 26, 2770–2778. doi: 10.1016/j.cub.2016.07.059
Hu, W., Zhang, H., Chen, H., and Tang, M. (2017). Arbuscular mycorrhizas influence Lycium barbarum tolerance of water stress in a hot environment. Mycorrhiza 27, 451–463. doi: 10.1007/s00572-017-0765-0
Huang, D., Ma, M., Wang, Q., Zhang, M., Jing, G., Li, C., et al. (2020a). Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 149, 245–255. doi: 10.1016/j.plaphy.2020.02.020
Huang, D., Wang, Q., Jing, G., Ma, M., Ma, F., and Li, C. (2020b). Overexpression of MdIAA24 improves apple drought resistance by positively regulating strigolactone biosynthesis and mycorrhization. Tree Physiol. 41, 134–146. doi: 10.1093/treephys/tpaa109
Huang, D., Wang, Q., Zou, Y., Ma, M., Jing, G., Ma, F., et al. (2021). Silencing MdGH3-2/12 in apple reduces cadmium resistance via the regulation of AM colonization. Chemosphere 269:129407. doi: 10.1016/j.chemosphere.2020.129407
Hui, J., An, X., Li, Z., Neuhäuser, B., Ludewig, U., Wu, N., et al. (2022). The mycorrhiza-specific ammonium transporter ZmAMT3;1 mediates mycorrhiza-dependent nitrogen uptake in maize roots. Plant Cell 34, 4066–4087. doi: 10.1093/plcell/koac225
Javot, H., Penmetsa, R. V., Terzaghi, N., Cook, D. R., and Harrison, M. J. (2007). A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 104, 1720–1725. doi: 10.1073/pnas.0608136104
Jiang, Y., Wang, W., Xie, Q., Liu, N., Liu, L., Wang, D., et al. (2017). Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356, 1172–1175. doi: 10.1126/science.aam9970
Jin, Y., Liu, H., Luo, D., Yu, N., Dong, W., Wang, C., et al. (2016). DELLA proteins are common components of symbiotic rhizobial and mycorrhizal signalling pathways. Nat. Commun. 7:12433. doi: 10.1038/ncomms12433
Kameoka, H., Maeda, T., Okuma, N., and Kawaguchi, M. (2019). Structure-specific regulation of nutrient transport and metabolism in arbuscular mycorrhizal fungi. Plant Cell Physiol. 60, 2272–2281. doi: 10.1093/pcp/pcz122
Kikuchi, Y., Hijikata, N., Ohtomo, R., Handa, Y., Kawaguchi, M., Saito, K., et al. (2016). Aquaporin-mediated long-distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: application of virus-induced gene silencing. New Phytol. 211, 1202–1208. doi: 10.1111/nph.14016
Kobae, Y., Tamura, Y., Takai, S., Banba, M., and Hata, S. (2010). Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol. 51, 1411–1415. doi: 10.1093/pcp/pcq099
Kretzschmar, T., Kohlen, W., Sasse, J., Borghi, L., Schlegel, M., Bachelier, J. B., et al. (2012). A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483, 341–344. doi: 10.1038/nature10873
Kumar, K. V., Singh, N., and Behl, H. M. (2008). Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 72, 678–683. doi: 10.1016/j.chemosphere.2008.03.025
Li, J., Sun, Y., Zhang, X., Hu, Y., Li, T., Zhang, X., et al. (2018). A methyltransferase gene from arbuscular mycorrhizal fungi involved in arsenic methylation and volatilization. Chemosphere 209, 392–400. doi: 10.1016/j.chemosphere.2018.06.092
Li, T., Hu, Y., Hao, Z., Li, H., Wang, Y., and Chen, B. (2013). First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 197, 617–630. doi: 10.1111/nph.12011
Li, T., Sun, Y., Ruan, Y., Xu, L., Hu, Y., Hao, Z., et al. (2016). Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 26, 879–893. doi: 10.1007/s00572-016-0723-2
Liu, H., Wang, Y., Hart, M., Chen, H., and Tang, M. (2016). Arbuscular mycorrhizal symbiosis regulates hormone and osmotic equilibrium of Lycium barbarum L. under salt stress. Mycosphere 7, 828–843. doi: 10.5943/mycosphere/7/6/14
Liu, L., Li, J., Yue, F., Yan, X., Wang, F., Blosizes, S., et al. (2018). Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194, 495–503. doi: 10.1016/j.chemosphere.2017.12.025
Liu, M. Y., Li, Q. S., Ding, W. Y., Dong, L. W., Deng, M., et al. (2023). Arbuscular mycorrhizal fungi inoculation impacts expression of aquaporins and salt overly sensitive genes and enhances tolerance of salt stress in tomato. Chem. Biol. Technol. Agric. 10:5. doi: 10.1186/s40538-022-00368-2
Liu, W., Kohlen, W., Lillo, A., Op, den Camp, R., Ivanov, S., et al. (2011). Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23, 3853–3865. doi: 10.1105/tpc.111.089771
Luginbuehl, L. H., Menard, G. N., Kurup, S., Erp, H. V., Radhakrishnan, G. V., and Breakspear, A. (2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356, 1175–1178. doi: 10.1126/science.aan0081
Luginbuehl, L. H., and Oldroyd, G. E. D. (2017). Understanding the arbuscule at the heart of endomycorrhizal symbioses in plants. Curr. Biol. 27, R952–R963. doi: 10.1016/j.cub.2017.06.042
Luo, Z., Lin, J., Zhu, Y., Fu, M., Li, X., and Xie, F. (2021). NLP1 reciprocally regulates nitrate inhibition of nodulation through SUNN–CRA2 signaling in Medicago truncatula. Plant Commun. 2:100183. doi: 10.1016/j.xplc.2021.100183
Ma, Z., Zhao, X., He, A., Cao, Y., Han, Q., Lu, Y., et al. (2022). Mycorrhizal symbiosis reprograms ion fluxes and fatty acid metabolism in wild jujube during salt stress. Plant Physiol. 189, 2481–2499. doi: 10.1093/plphys/kiac239
Maillet, F., Poinsot, V., André, O., Puech-Pagès, V., Haouy, A., Gueunier, M., et al. (2011). Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhizal. Nature 469, 58–63. doi: 10.1038/nature09622
Meixner, C., Ludwig-Müller, J., Miersch, O., Gresshoff, P., Staehelin, C., and Vierheilig, H. (2005). Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 222, 709–715. doi: 10.1007/s00425-005-0003-4
Mitra, D., Rad, K. V., Chaudhary, P., Ruparelia, J., Sagarika, M. S., Boutaj, H., et al. (2021). Involvement of strigolactone hormone in root development, influence and interaction with mycorrhizal fungi in plant: mini-review. Curr. Res. Microb. Sci. 2:100026. doi: 10.1016/j.crmicr.2021.100026
Nadal, M., Sawers, R., Naseem, S., Bassin, B., Kulicke, C., Sharman, A., et al. (2017). An N-acetylglucosamine transporter required for arbuscular mycorrhizal symbioses in rice and maize. Nat. Plants 3:17073. doi: 10.1038/nplants.2017.73
Oldroyd, G. E. D. (2013). Speak, friend and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 11, 252–263. doi: 10.1038/nrmicro2990
Oldroyd, G. E. D., and Leyser, O. (2020). A plant’s diet, surviving in a variable nutrient environment. Science 368:eaba0196. doi: 10.1126/science.aba0196
Parihar, P., Singh, S., Singh, R., Singh, V. P., and Prasad, S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: a review. Environ. Sci. Pollut. Res. 22, 4056–4075. doi: 10.1007/s11356-014-3739-1
Park, H. J., Floss, D. S., Levesque-Tremblay, V., Bravo, A., and Harrison, M. J. (2015). Hyphal branching during arbuscule development requires reduced arbuscular mycorrhiza. Plant Physiol. 169, 2774–2788. doi: 10.1104/pp.15.01155
Pérez-Tienda, J., Valderas, A., Camañes, G., García-Agustín, P., and Ferrol, N. (2012). Kinetics of NH4+ uptake by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mycorrhiza 22, 485–491. doi: 10.1007/s00572-012-0452-0
Pimprikar, P., Carbonnel, S., Paries, M., Katzer, K., Klingl, V., Bohmer, M. J., et al. (2016). A CCaMK-CYCLOPS-DELLA complex activates transcription of RAM1 to regulate arbuscule branching. Curr. Biol. 26, 987–998. doi: 10.1016/j.cub.2016.01.069
Pimprikar, P., and Gutjahr, C. (2018). Transcriptional regulation of arbuscular mycorrhiza development. Plant Cell Physiol. 59, 673–690. doi: 10.1093/pcp/pcy024
Porcel, R., Redondo-Gómez, S., Mateos-Naranjo, E., Aroca, R., Garcia, R., and Ruiz-Lozano, J. M. (2015). Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 185, 75–83. doi: 10.1016/j.jplph.2015.07.006
Ren, C., Kong, C., and Xie, Z. (2018). Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 18:74. doi: 10.1186/s12870-018-1292-7
Rich, M. K., Vigneron, N., Libourel, C., Keller, J., Xue, L., Hajheidari, M., et al. (2021). Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 372, 864–868. doi: 10.1126/science.abg0929
Ruiz-Lozano, J. M., Aroca, R., Zamarreño, A. M., Molina, S., Andreo-Jiménez, B., Porcel, R., et al. (2016). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 39, 441–452. doi: 10.1111/pce.12631
Saia, S., Rappa, V., Ruisi, P., Abenavoli, M. R., Sunseri, F., Giambalvo, D., et al. (2015). Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 6:815. doi: 10.3389/fpls.2015.00815
Salomon, M. J., Watts-Williams, S. J., McLaughlin, M. J., Bücking, H., Singh, B. K., Hutter, I., et al. (2022). Establishing a quality management framework for commercial inoculants containing arbuscular mycorrhizal fungi. iScience 25:104636. doi: 10.1016/j.isci.2022.104636
Shi, H., Ma, W., Song, J., Lu, M., Rahman, S., Bui, T. T. X., et al. (2017). Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient- and deficient-nitrogen conditions. Tree Physiol. 247, 1–12. doi: 10.1093/treephys/tpx090
Shi, J., Wang, X., and Wang, E. (2023). Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annu. Rev. Plant Biol. 74, 569–607. doi: 10.1146/annurev-arplant-061722-090342
Shi, J., Zhao, B., Zheng, S., Zhang, X., Wang, L., Dong, W., et al. (2021). A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 184, 5527–5540. doi: 10.1016/j.cell.2021.09.030
Smith, S. E., Facelli, E., Pope, S., and Smith, F. A. (2010). Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326, 3–20. doi: 10.1007/s11104-009-9981-5
Sofo, A., Vitti, A., Nuzzaci, M., Tataranni, G., Scopa, A., Vangronsveld, J., et al. (2013). Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana, seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiol. Plantarum 149, 487–498. doi: 10.1111/ppl.12050
Sugiura, Y., Akiyama, R., Tanaka, S., Yano, K., Kameoka, H., Marui, S., et al. (2020). Myristate can be used as a carbon and energy source for the asymbiotic growth of arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. U.S.A. 117, 25779–25788. doi: 10.1073/pnas.2006948117
Sun, Y., Zhang, X., Wu, Z., Hu, Y., Wu, S., and Chen, B. (2016). The molecular diversity of arbuscular mycorrhizal fungi in the arsenic mining impacted sites in Hunan Province of China. J. Environ. Sci. 39, 110–118. doi: 10.1016/j.jes.2015.10.005
Sun, Z., Song, J., Xin, X., Xie, X., and Zhao, B. (2018). Arbuscular mycorrhizal fungal 14-3-3 proteins are involved in arbuscule formation and responses to abiotic stresses during AM symbiosis. Front. Microbiol. 9:91. doi: 10.3389/fmicb.2018.00091
Talaat, N. B., and Shawky, B. T. (2011). Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J. Plant Nutr. Soil Sci. 174, 283–291. doi: 10.1002/jpln.201000051
Tanaka, S., Hashimoto, K., Kobayashi, Y., Yano, K., Maeda, T., Kameoka, H., et al. (2022). Asymbiotic mass production of the arbuscular mycorrhizal fungus Rhizophagus clarus. Commun. Biol. 5:43. doi: 10.1038/s42003-021-02967-5
Wang, B., Xiao, Q., Geng, X., Lin, K., Li, Z., Li, Y., et al. (2024). Arbuscular mycorrhizal fungi alter rhizosphere bacterial diversity, network stability and function of lettuce in barren soil. Sci. Hortic. 323:112533. doi: 10.1016/j.scienta.2023.112533
Wang, Q., Liu, C., Huang, D., Dong, Q., Li, P., and Ma, F. (2019). High-efficient utilization and uptake of N contribute to higher NUE of ‘Qinguan’ apple under drought and N-deficient conditions compared with ‘Honeycrisp’. Tree Physiol. 39, 1880–1895. doi: 10.1093/treephys/tpz093
Wang, S., Chen, A., Xie, K., Yang, X., Luo, Z., Chen, J., et al. (2020). Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. U.S.A. 117, 16649–16659. doi: 10.1073/pnas.2000926117
Wang, S., Xie, X., Che, X., Lai, W., Ren, Y., Fan, X., et al. (2023). Host- and virus-induced gene silencing of HOG1-MAPK cascade genes in Rhizophagus irregularis inhibit arbuscule development and reduce resistance of plants to drought stress. Plant Biotechnol. J. 21, 866–883. doi: 10.1111/pbi.14006
Wang, Y., Dong, F., Chen, H., Xu, T., and Tang, M. (2023). Effects of arbuscular mycorrhizal fungus on sodium and chloride ion channels of Casuarina glauca under salt stress. Int. J. Mol. Sci. 24:3680. doi: 10.3390/ijms24043680
Wang, Y., Dong, F., and Tang, M. (2022a). Transcriptome analysis of arbuscular mycorrhizal Casuarina glauca in damage mitigation of roots on NaCl stress. Microorganism 10:15. doi: 10.3390/microorganisms10010015
Wang, Y., Zhou, W., Wu, J., Xie, K., and Li, X. (2022b). LjAMT2;2 promotes ammonium nitrogen transport during arbuscular mycorrhizal fungi symbiosis in Lotus japonicus. Int. J. Mol. Sci. 23:9522. doi: 10.3390/ijms23179522
Wang, Y., Zhang, W., Liu, W., Ahammed, G., Wen, W., Guo, S., et al. (2021). Auxin is involved in arbuscular mycorrhizal fungi-promoted tomato growth and NADP-malic enzymes expression in continuous cropping substrates. BMC Plant Biol. 21:48. doi: 10.1186/s12870-020-02817-2
Wu, M., Yan, Y., Wang, Y., Mao, Q., Fu, Y., Peng, X., et al. (2021). Arbuscular mycorrhizal fungi for vegetable (VT) enhance resistance to Rhizoctonia solani in watermelon by alleviating oxidative stress. Biol. Control 152:104433. doi: 10.1016/j.biocontrol.2020.104433
Wu, S., Liu, H., Liu, S., Wang, Y., Gu, B., Jin, S., et al. (2018). Review of current situation of agricultural non-point source pollution and its prevention and control technologies. Strateg. Stud. Chin. Acad. Eng. 20, 23–30. doi: 10.15302/J-SSCAE-2018.05.004
Wu, S., Zhang, X., Sun, Y., Wu, Z., Li, T., Hu, Y., et al. (2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environ. Sci. Technol. 49, 14036–14047. doi: 10.1021/acs.est.5b03659
Xie, K., Ren, Y., Chen, A., Yang, C., Zheng, Q., Chen, J., et al. (2022). Plant nitrogen nutrition: the roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 269:153591. doi: 10.1016/j.jplph.2021.153591
Xie, X., Lai, W., Che, X., Wang, S., Ren, Y., Hu, W., et al. (2022). A SPX domain-containing phosphate transporter from Rhizophagus irregularis handles phosphate homeostasis at symbiotic interface of arbuscular mycorrhizas. New Phytol. 234, 650–671. doi: 10.1111/nph.17973
Xie, X., Lin, H., Peng, X., Xu, C., Sun, Z., Jiang, K., et al. (2016). Arbuscular mycorrhizal symbiosis requires a phosphate transceptor in the Gigaspora margarita fungal symbiont. Mol. Plant 9, 1583–1608. doi: 10.1016/j.molp.2016.08.011
Yoneyama, K., Xie, X., Kusumoto, D., Sekimoto, H., Sugimoto, Y., Takeuchi, Y., et al. (2007). Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227, 125–132. doi: 10.1007/s00425-007-0600-5
Yu, N., Luo, D., Zhang, X., Liu, J., Wang, W., Jin, Y., et al. (2014). A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Res. 24, 130–133. doi: 10.1038/cr.2013.167
Zhang, B., Shi, F., Zheng, X., Pan, H., Wen, Y., and Song, F. (2023). Effects of AMF compound inoculants on growth, ion homeostasis, and salt tolerance-related gene expression in Oryza sativa L. under salt treatments. Rice 16:18. doi: 10.1186/s12284-023-00635-2
Zhang, Q., Wang, S., Xie, Q., Xia, Q., Lu, L., Wang, M., et al. (2023). Control of arbuscule development by a transcriptional negative feedback loop in Medicago. Nat. Commun. 14:5743. doi: 10.1038/s41467-023-41493-2
Zhang, S., Nie, Y., Fan, X., Wei, W., Chen, H., Xie, X., et al. (2023). A transcriptional activator from Rhizophagus irregularis regulates phosphate uptake and homeostasis in AM symbiosis during phosphorous starvation. Front. Microbiol. 13:1114089. doi: 10.3389/fmicb.2022.1114089
Zhang, Y., Feng, H., Druzhinina, I. S., Xie, X., Wang, E., Martin, F., et al. (2023). Phosphorus/nitrogen sensing and signaling in diverse root-fungus symbioses. Trends Microbiol. Online ahead of print. doi: 10.1016/j.tim.2023.08.005
Zhang, F., Zou, Y., and Wu, Q. (2018). Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 229, 132–136. doi: 10.1016/j.scienta.2017.10.038
Zhang, J., Bi, Y., Song, Z., Xiao, L., and Christie, P. (2021). Arbuscular mycorrhizal fungi alter root and foliar responses to fissure-induced root damage stress. Ecol. Indic. 127:107800. doi: 10.1016/j.ecolind.2021.107800
Zhang, X., Gao, H., Liang, Y., and Cao, Y. (2021). Full-length transcriptome analysis of asparagus roots reveals the molecular mechanism of salt tolerance induced by arbuscular mycorrhizal fungi. Environ. Exp. Bot. 185:104402. doi: 10.1016/j.envexpbot.2021.104402
Zhang, Q., Gong, M., Liu, K., Chen, Y., Yuan, J., and Chang, Q. (2020). Rhizoglomus intraradices improves plant growth, root morphology and phytohormone balance of Robinia pseudoacacia in arsenic-contaminated soils. Front. Microbiol. 11:1428. doi: 10.3389/fmicb.2020.01428
Keywords: arbuscular mycorrhizal fungi (AMF), drought, salt stress, mineral nutrient, heavy metal stress, hormone
Citation: Wang Q, Liu M, Wang Z, Li J, Liu K and Huang D (2024) The role of arbuscular mycorrhizal symbiosis in plant abiotic stress. Front. Microbiol. 14:1323881. doi: 10.3389/fmicb.2023.1323881
Received: 18 October 2023; Accepted: 29 December 2023;
Published: 18 January 2024.
Edited by:
Decai Jin, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Xianan Xie, South China Agricultural University, ChinaEva Nouri, Université de Fribourg, Switzerland
Renuka Ramalingam Manoharan, Palacký University Olomouc, Czechia
Wenjin Zhang, Ningxia Medical University, China
Copyright © 2024 Wang, Liu, Wang, Li, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Huang, bXJoYW9kZWVAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qian Wang
Qian Wang Mengmeng Liu2†
Mengmeng Liu2† Dong Huang
Dong Huang