- 1College of Food Engineering, Jilin Engineering Normal University, Changchun, China
- 2Jilin Province Key Field of Social Sciences (Food Industry) Research Base, Changchun, China
Driven by the good developmental potential and favorable environment at this stage, Ganoderma lucidum is recognized as a precious large fungus with medicinal and nutritional health care values. Among them, polysaccharides, triterpenoids, oligosaccharides, trace elements, etc. are important bioactive components in G. lucidum. These bioactive components will have an impact on gut flora, thus alleviating diseases such as hyperglycemia, hyperlipidemia and obesity caused by gut flora disorder. While numerous studies have demonstrated the ability of G. lucidum and its active components to regulate gut flora, a systematic review of this mechanism is currently lacking. The purpose of this paper is to summarize the regulatory effects of G. lucidum and its active components on gut flora in cardiovascular, gastrointestinal and renal metabolic diseases, and summarize the research progress of G. lucidum active components in improving related diseases by regulating gut flora. Additionally, review delves into the principle by which G. lucidum and its active components can treat or assist treat diseases by regulating gut flora. The research progress of G. lucidum in intestinal tract and its potential in medicine, health food and clinical application were fully explored for researchers.
1 Introduction
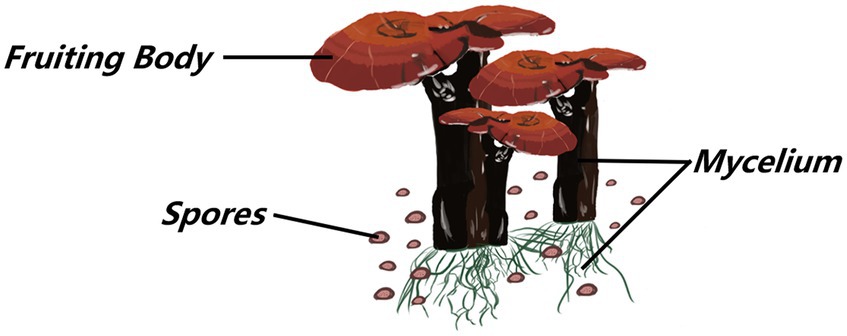
Ganoderma lucidum is a kind of dicotyledonous fungus belonging to the Ganoderma genus of the Ganodermataceae family Polyporales order, and Basidiomycota division, which is widely cultivated in China (Blundell et al., 2023). The beneficial characteristics of G. lucidum are attributed to bioactive compounds found at two stages of its life cycle: the fruiting body and mycelium. The mycelial stage is the most vigorous phase of the G. lucidum life cycle, and spore powder is released for propagation when G. lucidum grows and matures (Nakagawa et al., 2018; Figures 1, 2).
According to modern scientific research, G. lucidum is composed of 90% water and the remaining 10–40% macromolecules, 3–28% fat and 2% others (fibrous substances and crude proteins). G. lucidum also contains vitamins, minerals, metals, inorganic substances, essential fatty acids, amino acids, and other ashes. G. lucidum has a variety of secondary metabolites, such as polysaccharides, triterpenoids, adenosine, sterols, alkaloids, oligosaccharides and so on, which are all effective active components (Longgang et al., 2020; Lepakshi et al., 2023). (The chemical structure of the reviewed components is shown in Table 1). A large number of modern pharmacological studies have shown that G. lucidum is a mushroom with dual functions as a medicine and food, and possesses biological functions such as anticancer (Jin et al., 2016), anti-inflammatory, antitumor, antiviral, anti-infection, anti-oxidative, immunomodulating, nerve-calming, hepatoprotective, liver-detoxifying, antihypertensive, and antidiabetic activities, as well as the prevention and treatment of cardiovascular diseases (Chen et al., 2016; Yiqiong et al., 2020). In recent years, an increasing number of studies have emphasized the health care function of G. lucidum in diseases (Batra et al., 2013), and many researchers have found that the gut flora is closely involved in the biological functions of the secondary metabolites of G. lucidum. This review aims to emphasize the pharmacological (Thuy et al., 2023) effects of G. lucidum and explore the regulatory mechanism of its active components on gut flora in disease treatment (summarized in Table 2). Through this review, we hope that investigators can better understand the progress of research on G. lucidum and its active components in the intestinal field.
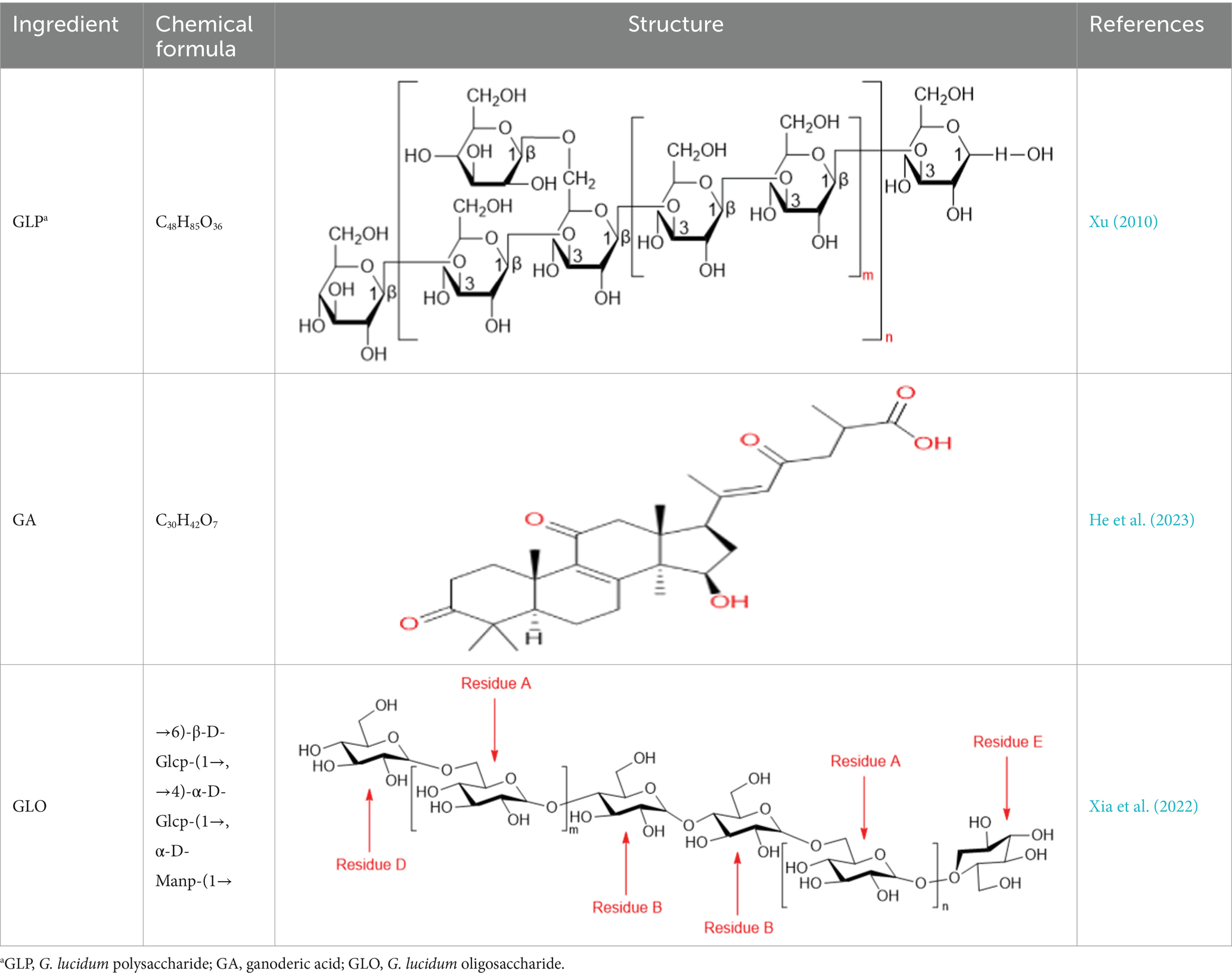
Table 1. Chemical formula and structure of active components of Ganoderma lucidum for regulating gut flora.
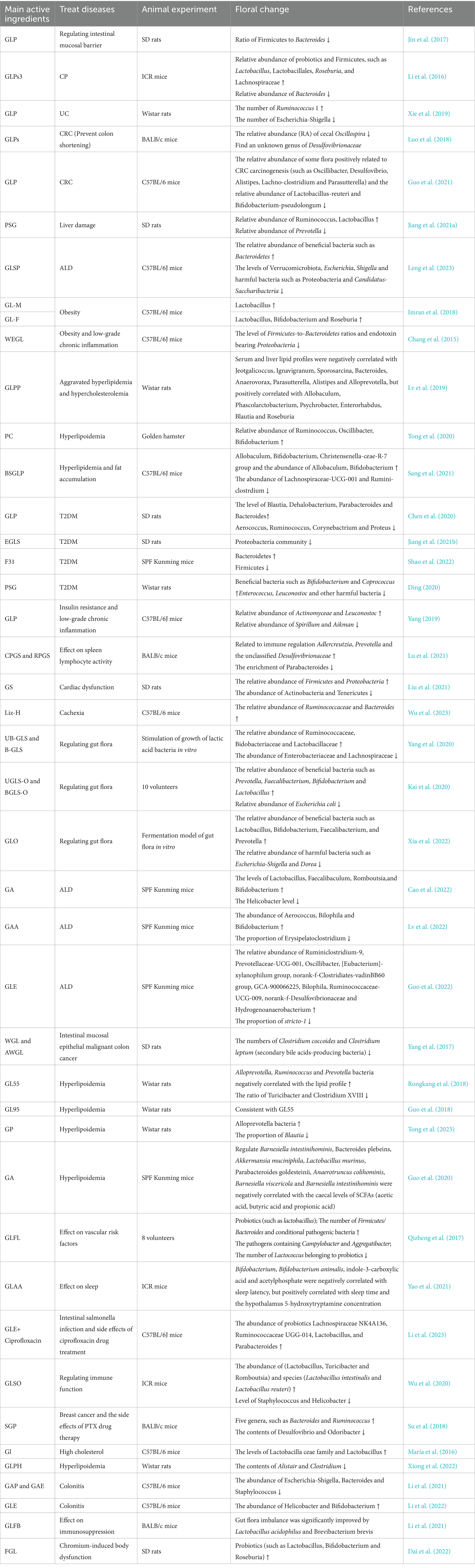
Table 2. Pharmacological action and main active components of G. lucidum and changes of intestinal microbiota.
2 Gut flora
Gut flora are the normal microorganisms in the human intestine. According to their function in the human intestine, Jie et al. (2021) divided them into three categories: probiotics (approximately 25%), neutral bacteria (approximately 50%), and pathogenic bacteria (approximately 25%). The main probiotics include Bifidobacterium and Lactobacillus (Ventura et al., 2012). The main pathogens include Staphylococcus aureus, Salmonella, Proteus, Clostridium, Enterobacter, and Enterococcus. Neutral bacteria include Escherichia coli, Lactobacillus, and Streptococcus (also known as fecal cocci). Studies have shown that the increase of the abundance of Prevotella can improve the function of glucose metabolism and enhance the storage capacity of glycogen (Kovatcheva-Datchary et al., 2015). Faecalibacterium can produce butyric acid and CO2 in colon, which has potential benefits for inflammatory bowel disease (IBD) and obesity suppression (La Fata et al., 2017; Healey et al., 2018) Bifidobacterium and Lactobacillus are typical anaerobic probiotics in intestinal bacteria. Probiotics represented by Bifidobacterium and lactobacillus can reduce cholesterol, resist tumor, delay aging, inhibit the adhesion of pathogenic bacteria, and relaxing bowel (Yang et al., 2013).
Gut flora has many functions such as developing immune system (Daniel and Ramnik, 2023), producing vitamins, maintaining intestinal cells and neutralizing toxins (Liu et al., 2022). Human health is dependent on intestinal microorganisms (Hongtao, 2022). The gut flora regulates intestinal homeostasis through interactions with the host (Xinyi, 2022), Because the ability of gut microbiota (GM) to produce short-chain fatty acids (SCFAs) is related to the host’s metabolic characteristics, which is a causal relationship between SCFAs and improvement of metabolic abnormalities (Tian et al., 2023). Functional components from natural products are widely used to treat a variety of intractable intestinal disorders due to their generally accepted safety profile in the treatment of diseases and efficacy with a long tradition of use (Wang et al., 2023). Gut flora imbalance will reduce beneficial substances, including SCFAs. The characteristics of an imbalanced or diseased gut flora are the decrease in beneficial bacteria and an enrichment of proteolytic flora, which will destroying the intestinal barrier and leading to a disruption in intestinal homeostasis. Gut flora imbalance can cause a wide range of chronic inflammatory diseases, including pancreatitis, enteritis, diabetes, asthma, atherosclerosis, and thrombosis (Sachdev and Pimentel, 2013). Gut flora usually regulates the human intestine by releasing different metabolites. In addition, the gut flora is easily influenced by external factors such as diet and living habits, and this can lead to metabolic diseases such as cardiovascular system (Jie et al., 2017) and digestive system (Li et al., 2017). As mentioned above, G. lucidum is rich in active ingredients such as polysaccharides and triterpenoids, which can be absorbed by the gastrointestinal tract, while macromolecules (polysaccharides) cannot be absorbed by the gastrointestinal tract, so it needs to be decomposed into micromolecules and then absorbed by the intestinal and hepatic circulation to play a pharmacological role.
3 Effects of different components of Ganoderma lucidum on gut flora in related diseases
3.1 Ganoderma lucidum polysaccharide (GLP)
GLP is one of the main active ingredients of G. lucidum and has many properties, including antibacterial, anti-inflammatory, anticancer, antioxidant, and antidiabetic functions (Liu et al., 2023). In addition, GLP can protect cardiovascular health, enhance the immune system (Guo et al., 2022), nourish and protect the liver (Faruque et al., 2023), and slow the progression of age-related health conditions (Wang et al., 2017). It has been reported that G. lucidum polysaccharides with different molecular weights have different protective effects on ethanol-induced acute gastric injury in rats, which proves that GLP can be used as a potential raw material for functional foods and dietary supplements (Tian et al., 2022). But so far, there is no report to summarize the mechanism of GLP in regulating the gut flora of diseases. Therefore, in this section, we will summarize the regulation mechanism of GLP on gut flora in different parts and ways from different angles of three lines of defense.
The intestinal immune system is the first line of defense against external pathogens. Extensive epidemiological investigations have shown that gut floral imbalances lead to chronic inflammation and diseases, such as chronic pancreatitis (CP), IBD, and colorectal cancer (CRC) (O’Keefe, 2016; Wong and Yu, 2019). Recently, although the pharmacological mechanism of food has been widely concerned by researchers, GLP stands out among many foods and has been recognized by many researchers. Jin et al. (2017) isolated and purified GLP from G. lucidum myceliumincreased the richness of microbiota in the cecum of Sprague–Dawley (SD) rats, and the ratio of Firmicutes and Bacteroides in rats in the GLP group was significantly reduced compared with the control group, proving that GLP can serve as a functional factor to regulate the intestinal barrier function. Li et al. (2016) induced ICR mice with diethyldithiocarbamate (DDC) and revealed the potential role of G. lucidum mycelium strain S3 (GLPs3) in the treatment of chronic pancreatitis (CP) mechanism. GLPs3 increased the relative abundance of probiotics and Firmicutes in mice, decreased the relative abundance of Bacteroides, significantly inhibited the inflammatory response of mice and made their intestinal barrier and gut flora tend to be healthy. Therefore, GLP can be used as a natural medicine to treat and prevent intestinal diseases, and provide data support for further study on the regulation of GLP on IBD gut flora in host immune system. Ulcerative colitis (UC) is one of the main types of IBD with chronic recurrent bowel disease. Xie et al. (2019) used edible G. lucidum polysaccharide GLP rich in β-glucan (>90%) to significantly alleviate diarrhea symptoms induced by dextran sulfate sodium (DSS) in Wistar UC rats because GLP The β-glucan in it can break down dietary polysaccharides and promote the growth of lactococci. Metagenomics and transcriptomic analysis revealed that GLP reduced the number of pathogens associated with acute diarrhea and increased the number of Ruminococcus-1 and SCFAs. In addition, SCFAs produced by gut flora fermentation can regulate immune response, alleviate IBD and reduce the risk of colorectal cancer (CRC) (Llewellyn et al., 2018). Two groups of researchers conducted animal experiments on CRC induced by azomethane (AOM)/DSS and found that a variety of intestinal commensal bacteria and their metabolites help promote the occurrence of CRC (Sears and Garrett, 2014), they also found that gut flora imbalance (reduced microbial diversity) is one of the important factors leading to CRC (Dokht et al., 2022). Luo et al. (2018) extracting G. lucidum polysaccharides (GLPs) whose main component is β-1,3-glucan effectively prevents mice colon shortening and significantly reduces the relative abundance (RA) of cecal Oscillospira; Guo et al. (2021) water-soluble GLP extracted from peeled spores of G. lucidum reduced the relative abundance of some bacterial groups that are positively related to CRC carcinogenesis by inhibiting the TLR4/MyD88/NF-κB signaling pathway, and effectively improved the imbalance of gut flora in mice and increase the number of SCFAs. The above studies have shown that GLP can restore intestinal barrier function, reduce inflammatory responses and the risk of acquiring CRC, and enhance intestinal immunity, by reversing the flora that is positively related to the disease or increasing beneficial bacteria. However, the use of GLP as a supplement for patients with intestinal dysfunction or colitis requires further research to determine the most appropriate dosage for humans.
The liver is the largest detoxification organ in the human body and the second line of defense in the intestinal immune system. GLP can promote hepatocyte repair, prevent hepatic fibrosis, and also has pharmacological activity against metabolic diseases. Jiang et al. (2021a) in vitro fermentation of black G. lucidum polysaccharide (PSG) effectively improves the gut flora disorder in SD rats with acrylamide (AA)-induced liver injury, by increasing the relative abundance of Ruminococcus and Lactobacillus and reducing Prevotella It is a relative abundance, thereby achieving the liver protective effect. The edible G. lucidum spore powder (GLSP) produced by Leng et al. (2023) effectively improves alcoholic liver damage (ALD) induced by 50% ethanol. GLSP can not only reduce the levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) increased by ALD, but also regulate aminotransferase to improve ALD and restore intestinal health. The animal experiment of GLP provides more guarantee for the pharmaceutical or clinical use of G. lucidum, and also provides treatment ideas for metabolic diseases such as long-term inflammation caused by obesity. Imran et al. (2018) extracted polysaccharides from G. lucidum fruiting bodies (GL-M) and mycelium (GL-F) for the treatment of high-fat diet (HFD)-induced obesity in mice. Polysaccharides significantly promote the growth of lactic acid-producing bacteria (LAP) that can improve the host digestive system and intestinal mucosa (Vigsnæs et al., 2013), changing the gut flora of obese mice by increasing the number of SCFAs and Lactobacillus. The GL-F group has more beneficial bacteria that can enhance metabolic capacity than GL-M. The enhanced metabolic capacity can achieve the effect of weight loss. Four groups of researchers have proved that different types of GLP and its compositions can improve the gut flora disorder caused by obesity, hyperlipidemia and fat accumulation to varying degrees. The water extract of G. lucidum mycelium (WEGL) of Chang et al. (2015) can inhibit obesity and improve the symptoms of low-grade chronic inflammation by improving the disorder of gut flora and maintaining the integrity of intestinal barrier in mice, it can alleviate dyslipidemia, inhibit obesity and steatosis, and improve the symptoms of low-grade chronic inflammation. Lv et al. (2019) G. lucidum polysaccharide peptide (GLPP) obtained from the fruiting body through alcohol extraction, precipitation, centrifugation, and freeze-drying can also reduce serum triglycerides (TG) and cholesterol (TC) in Wistar rats, free fatty acids (FFA) and low-density lipoprotein cholesterol (LDL-C) levels, inhibiting liver fat accumulation. Metagenomics analysis showed that the relative abundance of gut flora in high-fat rats was significantly changed, among which the number of Bacteroidetes was significantly increased and bile acid metabolism was accelerated, thereby improving lipid metabolism disorders. Tong et al. (2020) gave golden hamsters oral administration of a combination of G. lucidum fruiting body polysaccharide and chitosan (PC), which restored their lipid metabolism and gut flora disorders to normal. PC similar to GLPP, can reduce hyperlipidemia by reducing blood lipid content, and PC also additionally reduces AST content. In addition, PC also increased the relative abundance and SCFAs of beneficial bacteria negatively correlated with blood lipid profile. The (Sang et al., 2021) polysaccharide (BSGLP) extracted from the broken spores of G. lucidum significantly alleviates the upregulation of the TLR4/Myd88/NF-κB signaling pathway in mice adipose tissue and tends to reduce TG and non-esterified fatty acids (NEFA) levels, reversed the potential probiotics that were positively related to anti-obesity and improved hyperlipidemia. Ganoderma polysaccharide can regulate gut flora, which is partly responsible for inhibiting obesity, selectively improves the growth of benign bacteria, and improves metabolic syndrome by regulating gastrointestinal microbiota. I hope our summary can provide valuable data for the potential mechanism of GLP in treating dyslipidemia. Diabetes is a typical chronic metabolic disease in which type II diabetes mellitus (T2DM) accounts for more than 90% of patients who are diabetic (Ehtasham et al., 2022), especially those who are obese. Five groups of researchers obtained that different types of GLP can restore the gut flora of T2DM animals induced by HFD and STZ to normal level. Chen et al. (2020) and Jiang et al. (2021b) both used SD rat model. The former extracted and purified GLP from G. lucidum mycelium, which reversed the relative abundance of harmful bacteria such as Ruminococcus and Proteus, increased the number of beneficial bacteria such as Bacteroides, and finally made mice blood sugar returned to normal level. The latter made a grain fiber fraction which can not be digested in small intestine but can be fermented by microorganisms in colon. Resistant starch (RS) was used as coating material to prepare encapsulated G. lucidum spore polysaccharide (GLS), and the final product capsule was called EGLS. The intervention of EGLS significantly reduced the Proteus community and enhanced the parameters of glucose metabolism and fat metabolism in rats, which was related to enhancing insulin secretion, glycogen synthesis and reducing fat production. However, a kind of compound polysaccharide F31, which is rich in lactic acid bacteria, Bacteroides and Ruminococeae, isolated from the fruiting body of G. lucidum by Shao et al. (2022), can increase the specific pathogen-free (SPF) ratio of Bacteroidetes/Firmicutes (B/F) to 0.6969 (p < 0.01), even close to the normal control (p = 0.9579). Ding (2020) found that PSG could not be completely digested in rat’s stomach and small intestine, but was decomposed into SCFAs under the action of gut flora. PSG could significantly promote the growth of beneficial bacteria such as Bifidobacterium and fecal coccus, inhibit the growth of harmful bacteria such as Enterococcus and Leuconostoc, and change the index content related to T2DM, thus improving T2DM. Yang (2019) also found that GLP can improve low-grade chronic inflammation and reverse systemic insulin resistance induced by HFD in mice by reducing plasma insulin concentration and regulating inflammatory factors; Improve the gut flora structure of mice and enhance the sensitivity of mice to insulin resistance. To sum up, the hypoglycemic effect of GLP is mainly related to the gut flora that causes the increase of blood sugar. It can reduce blood sugar by promoting the increase of beneficial bacteria and the decrease of harmful bacteria, which has a positive effect on reducing inflammation. Therefore, GLP is an excellent potential candidate drug for preventing and treating T2DM.
Specific immunity is the third line of defense in the human body. The gut flora can affect the development and function of immune cells by regulating the functions of macrophages and T cells. In addition, the gut flora may participate in GLP-mediated immune regulation, but whether it plays a role in specific immune regulation needs further verification (Andrew et al., 2023). Lu et al. (2021) compared the changes in the activity of mice spleen lymphocytes between G. lucidum broken spore crude polysaccharide (CPGS) and G. lucidum broken spore polysaccharide (RPGS). CPGS and RPGS enriched Adlercreutzia, Prevotella and the unclassified Desulfovibrionaceae, which are positively related to immune regulation. The beta diversity of mice spleen lymphocytes in the RPGS group changed more obviously than that in the CPGS group. In addition, Liu et al. (2021) established a cardiac dysfunction model by intraperitoneal injection of trimethylamine-N-oxide (TMAO). G. lucidum spore extract (GS) can reduce the blood lipid content of TMAO rats and improve the index level of high density lipoprotein cholesterol (HDL-C) for preventing cardiovascular diseases. The lipophilic components rich in GS regulate the expression of related protein and polysaccharide components by targeting gut flora, so as to control the biotransformation of trimethylamine, thus maintaining the metabolic balance and function of the heart, which may be the potential mechanism of heart protection. Besides, G. lucidum polysaccharide (Liz-H) prepared by Wu et al. (2023) from malt extract agar (MEA) plate can treat cachexia induced by intraperitoneal injection of cisplatin combined with docetaxel (cisplatin+docetaxel), and Liz-H can restore disordered gut flora, reduce body weight and neutrophils in mice, and also alleviate muscle atrophy. It shows that Liz-H is a good chemical protective reagent. GLP plays a good role in promoting immune regulation. Although it provides data proof for G. lucidum’s natural functional food resources, it still needs more pharmacodynamics and clinical verification to establish the safety and feasibility of G. lucidum’s pharmacological action.
Up to now, according to the chemical structure of G. lucidum 1,3-or 1,6-b-d-glucan, GLP can not be digested by host-derived enzymes in human intestine, and there is no direct evidence that GLP can be absorbed by intestine. In view of the fact that there is no suitable polysaccharide digestive enzyme in human intestine, GLP can not be directly absorbed by human intestine. According to the research, we boldly speculate that GLP is likely to be decomposed by gut flora, and then regulate the changes of flora, and then play a therapeutic or auxiliary role in disease treatment.
3.2 Ganoderma lucidum oligosaccharides (GLOs)
Frontiers requires figures to be submitted individually, GLO is a chain-like homogeneous oligosaccharide extracted from G. lucidum polysaccharides, and its structure consists of a disaccharide repeating unit [−4-β-1-Galf(1–6)-O-(β-Glcp)-1-]n = 3,4] (Isaac et al., 2013). GLO, a new functional sugar source, can be absorbed and utilized by beneficial gut flora. Because it cannot be degraded by gastric acid, GLO can play a role in regulating the gut flora (Wang et al., 2012).
Up to now, three groups of studies have confirmed that GLO can regulate different gut flora through in vitro fermentation model. Yang et al. (2020) found that oligosaccharides (UB-GLS and B-GLS) in broken and unbroken G. lucidum spore powder can increase the number of beneficial bacteria such as Bifidobacterium and Lactobacillus, increase the content of SCFAs, and regulate the function of gut flora. Kai et al. (2020) two kinds of oligosaccharides (UGLS-O and BGLS-O) prepared from (crushed and unbroken) G. lucidum spore powder by water extraction and alcohol precipitation can increase the relative abundance of beneficial bacteria such as Prevotella, Faecalibacterium, Bifidobacterium and Lactobacillus, but decrease the relative abundance of Escherichia-Shigella. In addition, Xia et al. (2022) extracted GLO from G. lucidum by ultrasonic-assisted enzymolysis and dextran gel electrophoresis, which can not only increase the beneficial bacteria detected by the first two groups of researchers, but also reduce the relative abundance of harmful bacteria such as Escherichia-Shigella and Dorea. Because GLO structure can not be digested and degraded in oral cavity, but can be used by gut flora in gastrointestinal tract, so GLO can be used as a component of functional food or nutritional products to improve intestinal tract and enhance immunity.
3.3 Ganoderma lucidum triterpenoids (GPs)
Ganoderma triterpene (GLT), also known as “ganoderic acid,” is one of the main active components isolated from G. lucidum (Baby et al., 2015). GLT compounds have high medicinal value and are widely used in cancer treatment, liver protection, prevention and treatment of cardiovascular diseases, epilepsy relief, asthma relief, reducing blood sugar and blood lipid levels, inhibiting platelet aggregation, radioprotection, and other areas; Compared to other active substances, GLP and GLT possess unique pharmacological advantages of more diverse and modifiable structures (Wei et al., 2019).
Diseases of the digestive system affect eating and nourishment. Gut flora affects the energy balance and weight of individuals by affecting the digestion and absorption of food, which has an important influence on the digestion process. Three groups of researchers found that different types of GLT can treat gut flora disordered by ALD, including main triterpenoid ganoderic acid (GA) (Cao et al., 2022), ganoderic acid A (GAA) (Lv et al., 2022) and G. lucidum ethanol extract (GLE) rich in ganoderic acid (Guo et al., 2022) significantly inhibited the abnormal elevated levels of serum TC, TG, LDL-C, AST and ALT, increased the level of serum HDL-C, and promoted the digestion and decomposition of alcohol by regulating different gut flora, thus protecting the liver from excessive accumulation of liver lipids and pathological changes caused by alcohol. The data show that alcohol-induced liver oxidative stress can be significantly improved by diet. Another common digestive system disease IBD is characterized by intestinal mucosal inflammation (Rodríguez-Lago et al., 2018). Yang et al. (2017) using 5% water extract of G. lucidum (WGL) and self-digested water extract of G. lucidum (AWGL) to treat intestinal mucosal epithelial malignant colon cancer achieved good results, which helped these extracts to restore normal colon mucosa by regulating gut flora and greatly reduced the number of cancer cells. However, AWGL significantly increased the level of propionate, which was more beneficial than WGL.
The management of metabolic diseases focuses on a light diet, and G. lucidum, with low fat and high cellulose content, is the first choice for many people. GLT can effectively treat three diseases (hypertension, hyperglycemia and hyperlipidemia) caused by metabolic disorder by regulating specific gut flora, lipid and cholesterol metabolites. Rongkang et al. (2018) found that supplementing 55% ethanol extract of G. lucidum (GL55) can increase the relative abundance of beneficial bacteria such as Ruminococcus beneficial to the metabolic process of gut flora in rats, and reduce the relative abundance of harmful bacteria such as Fusobacterium. Guo et al. (2018) using 95% ethanol to extract ganoderic acid (GL95) to supplement GL95 can improve the symptoms of hyperlipidemia, indicating that triterpenoids from G. lucidum can be used as a new functional food for potential treatment or improvement of hyperlipidemia. GL55, GL95 and GP (Tong et al., 2023) can all reduce serum TG, TC and LDL-C, and GL55 can additionally reduce ALT, FFA and fasting blood glucose levels to inhibit hepatic steatosis. However, GA extracted by Guo et al. (2020) regulates the changes of gut flora, which is different from the first three groups. Barnesiella intestinihominis, Bacteroides plebeins, Akkermansia muciniphila, Lactobacillus murinus, Parabacteroides goldesteinii, Anaerotruncus colihominis, Barnesiella viscericola, and Barnesiella intestinihominis were negatively correlated with the caecal levels of SCFAs (acetic acid, butyric acid and propionic acid), and increase the level of SCFAs in the intestine. To sum up, GLT will affect the gut flora, promote the increase of beneficial bacteria, reduce the number of harmful bacteria, regulate the serum level related to lipid metabolism and liver biochemical indicators, and maintain the intestinal environmental balance. We can realize the role of key microbial system types and important metabolic biomarkers in the occurrence and development of hyperlipidemia, and provide useful information for mining GLT drugs to prevent or treat hyperlipidemia. According to the existing research, we boldly speculate that gut flora may be a potential target for the treatment of dyslipidemia and nonalcoholic fatty liver disease (NAFLD), and GLT can regulate this target to achieve the purpose of treating diseases. We are looking forward to the follow-up research to confirm this speculation.
Peace of mind is important in the management of cardiovascular diseases, and G. lucidum nourishes the heart and calms the nerves. Qizheng et al. (2017) used G. lucidum mycelium fermentation broth (GLFL) to relieve cardiovascular diseases of volunteers to varying degrees and provide protection for human health; However, GLFL decreased the LDL-c of volunteers, increased the number of Firmicutes/Bacteroides and conditional pathogenic bacteria, and decreased the number of Lactococcus belonging to probiotics, which was unfavorable to human body. Therefore, GLFL as a drug needs more rigorous experiments to confirm the dose and quality. We call for re-examination of the dose and results of G. lucidum and its active ingredients in volunteer experiments, which may be different from rats. In addition, the sedative effect of G. lucidum has also been proved by Yao et al. (2021) to regulate gut flora and promote sleep. Taoist bacteria and metabolites rich in ethanol acidic fraction extract (GLAA) from G. lucidum mycelium, including Bifdobacterium, Bifdobacterium animalis, indole-3-carboxylic acid and acetylphosphate were negatively correlated with sleep latency, but positively correlated with sleep time and the hypothalamus 5-hydroxytryptamine concentration. GLAA administration decreased the levels of serum lipopolysaccharide and peptidoglycan in mice. We predict that GLAA can promote sleep in mice by relying on gut flora and serotonin-related pathways, and there is still much room for discussion on the relationship between gut flora and sleep.
Immune system is the fundamental way to cure diseases, and gut flora also has an important influence on the development and function of immune system. G. lucidum ethanol extract (GLE) (Li et al., 2023) is beneficial to gut flora and immune system. Ordinary ciprofloxacin can destroy the intestinal barrier and increase the risk of pathogenic bacterial infection in mice. GLE+ ciprofloxacin combined administration can avoid the intestinal barrier by increasing the abundance of probiotics Lachnospiraceae NK4A136, Ruminococcaceae UGG-014, Lactobacillus, and Parabacteroides. It shows that GLE has great potential in repairing intestinal injury caused by antibiotics. Wu et al. (2020) used supercritical carbon dioxide fluid extraction technology to extract oily lipids from broken G. lucidum spores. G. lucidum spore oil (GLSO) rich in triterpenes and ergosterol can regulate gut flora, enhance phagocytosis of macrophages and cytotoxicity of NK cells, and improve immunity. The above research shows that gut flora has an important influence on the function of immune system. GLT can alleviate the side effects caused by other drug treatments and enhance immunity by regulating gut flora, which is beneficial to physical and mental health.
3.4 Combined application of active components of Ganoderma lucidum and clinical drugs
Su et al. (2018) from the perspective of the role of gut flora in tumor, a mice model of breast cancer was established. Through 16S rRNA sequencing, it was found that G. lucidum spore polysaccharide (SGP) could improve the gut flora imbalance caused by paclitaxel (PTX). It was found that the bacterial abundance of five genera, such as Bacteroides and Ruminococcus, increased significantly, while the abundance of the genera of Acinetobacter and Vibrio desulphulariae, which increased the risk of tumor, decreased.
Obesity is the result of nutritional metabolic disorder, which leads to slow cholesterol metabolism and increased TC concentration in the blood of obese patients, thus leading to high cholesterol levels. María et al. (2016) firstly treated hypercholesterolemia with ethanol-water extract (Gl) from G. lucidum mycelium from Mexico, and compared the advantages and disadvantages with simvastatin. The effect of Gl extract on lipid metabolism was better than simvastatin. The levels of serum TG, TC, LDL-C, liver cholesterol and liver triglyceride in Gl group are closer to the normal level than simvastatin. The decrease of TC level is mediated by α-glucan and β-glucan in Gl, which promotes the absorption of cholesterol in intestinal tract and increases the levels of Lactobacilla ceae family and Lactobacillus. Secondly, Xiong et al. (2022) can also treat hyperlipidemia with G. lucidum protease hydrolysate (GLPH), and reduce the content of Alistair and Clostridium in rat intestine, and improve the disorder of lipid metabolism. G. lucidum polysaccharide extract (GAP), G. lucidum 75% ethanol extract (GAE) (2021) and G. lucidum ethanol extract triterpenoid (GLE) (2022) of Li et al. all promoted the recovery of colitis in a manner dependent on gut flora. GLE also increased the abundance of Helicobacter and Bifidobacterium, enhanced immunity and protected intestinal barrier. It can be seen that the above experiments reveal that G. lucidum has a significant positive regulatory effect on gut flora in metabolic diseases, indicating that G. lucidum has the potential as a lipid-lowering functional food beneficial to metabolism.
Ganoderma lucidum not only promotes body metabolism, but also has a positive effect on body function recovery. Two groups of researchers conducted in vitro fermentation experiments from the perspective of immune function of gut flora. Li et al. (2021) adding Lactobacillus acidophilus and Brevibacterium to the fermentation broth of water extract of G. lucidum fruiting body restored the T lymphocyte activity of dexamethasone (DEX)-induced immunosuppression mice to normal level, and the gut flora imbalance was obviously improved. In contrast, Dai et al. (2022) used G. lucidum tablets (FGL) fermented by Lactobacillus rhamnosus to treat chromium-induced physical dysfunction in SD rats, increased the content of probiotics and stopped diarrhea in rats. These positive changes once again show that G. lucidum can enhance immunity by regulating gut flora and play an immunomodulatory role. The targeted regulation of G. lucidum or G. lucidum combination drugs on gut flora and its metabolites may be the key to enhance the immune function of the body, which provides a reliable basis for the application of probiotic fermented G. lucidum in immune regulation.
4 Conclusion and prospects
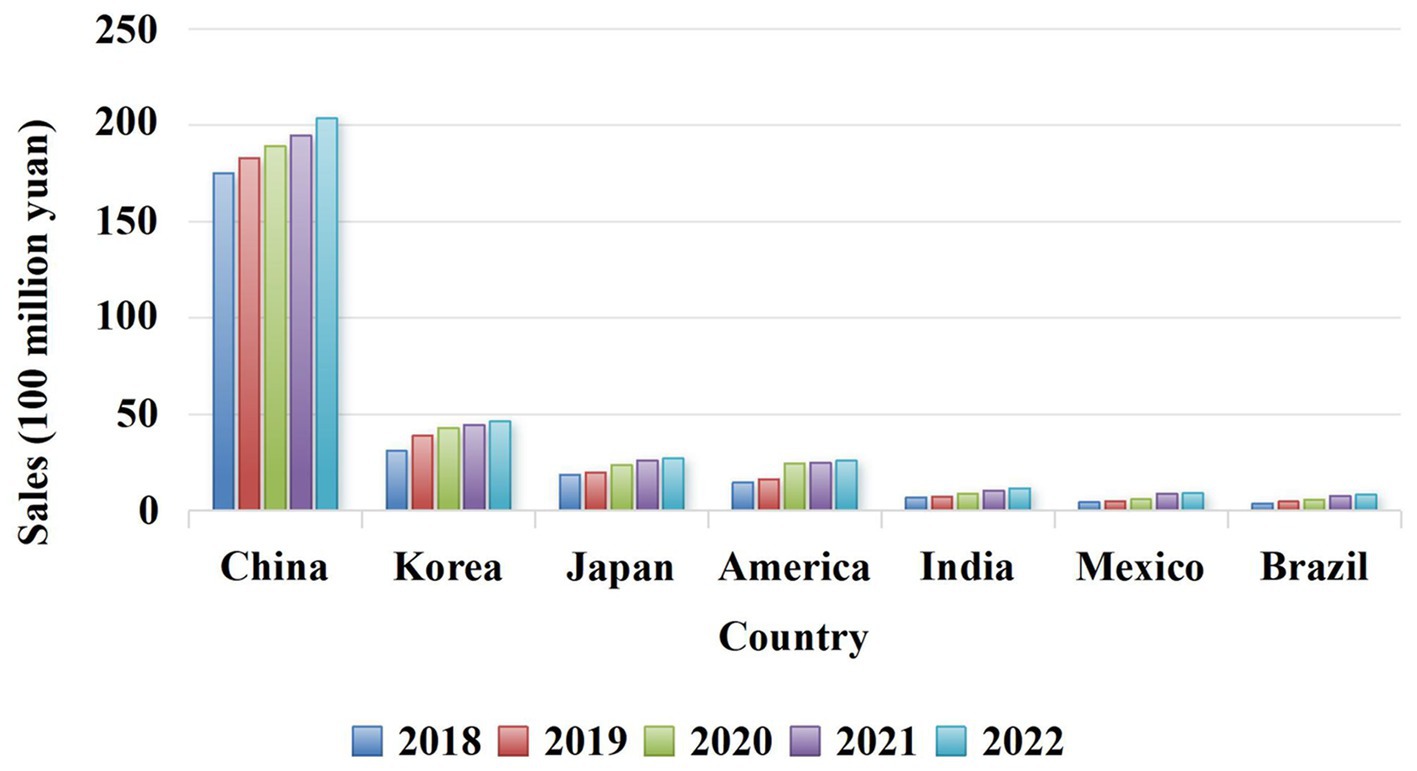
Ganoderma lucidum possesses a variety of biological activities and has no obvious toxicity or side effects as a pharmaceutical ingredient. Because its consumption worldwide is estimated at several thousand tons, and the market is growing rapidly (Liu, 2021; Figure 3). This huge consumption potential and planting ability make G. lucidum can be used to develop high-quality functional drugs and foods in terms of composition, pharmacological action and clinical application (Lai et al., 2004; Fidelis et al., 2023). Therefore, pharmacists, medical scientists, and chemists have attached great importance to this issue. This paper reveals the relationship among the active components of G. lucidum-gut flora-diseases, and summarizes the effects of G. lucidum and its active components on improving related diseases by regulating gut flora. Animal and in vitro experiments show that when the body is infected by pathogenic bacteria or suffers from a disease, the intestinal microecological balance is destroyed, and active components such as G. lucidum polysaccharides and triterpenoids can participate in the body metabolism process from the perspective of regulating gut flora. Most of its manifestations involve increasing the relative abundance of probiotics (such as lactic acid bacteria and Bifidobacteria) and reducing the relative abundance of pathogenic bacteria (such as Escherichia-Shigella and Cladosporium) to maintain the dynamic balance of gut flora in terms of microbial species and abundance (Shuai, 2021). To provide scientific basis for clinical application of G. lucidum.
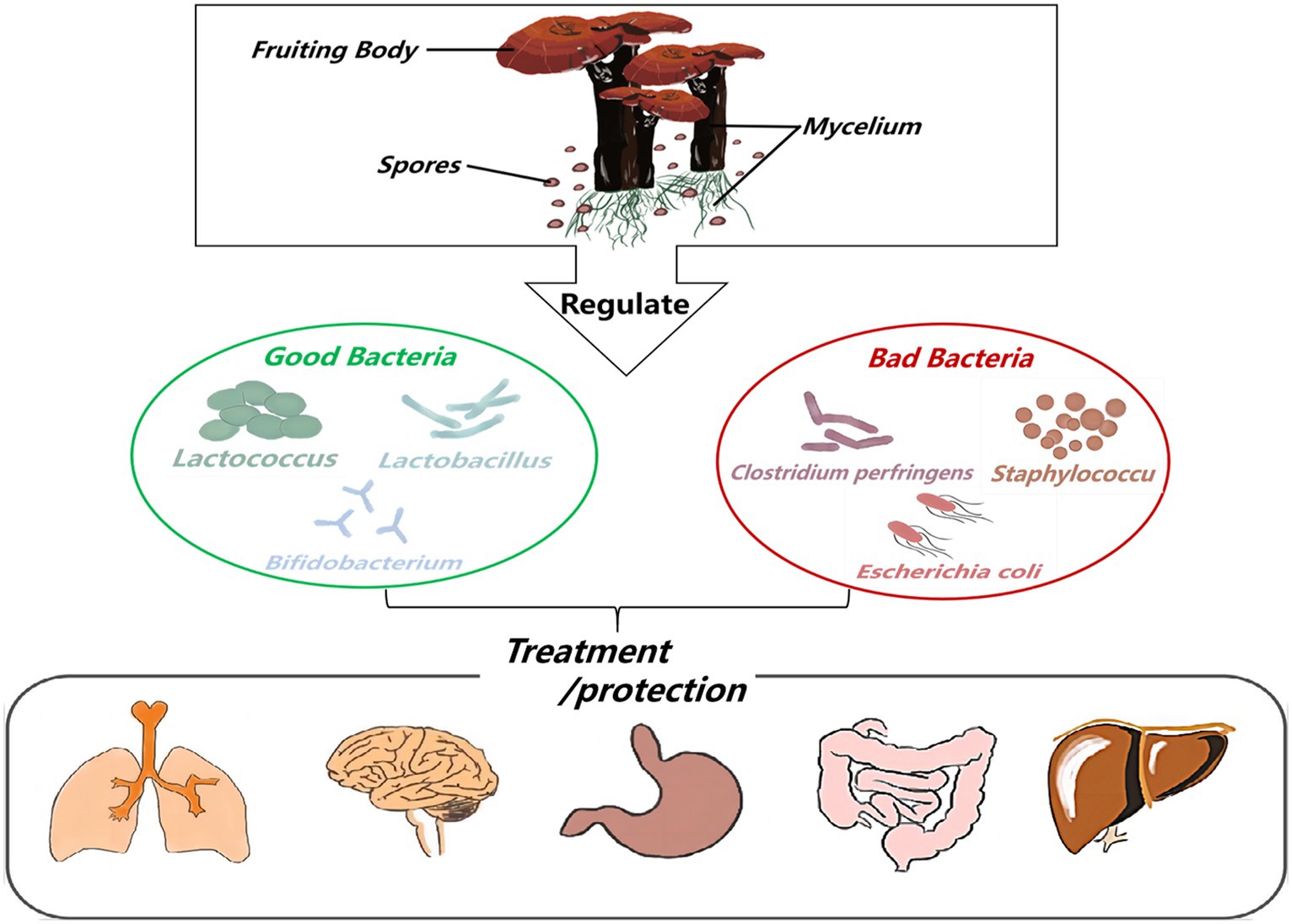
Figure 3. The flow chart of Ganoderma lucidum and its active ingredients for treating diseases or protecting the body by regulating gut flora.
Ganoderma lucidum and its active components exert protective effects by regulating intestinal imbalance, preventing diabetes and nephropathy, and alleviating kidney damage caused by chemotherapy drugs. Basic and applied research on the active components of G. lucidum (He et al., 2023) will certainly promote its use as a medicine or health food in the prevention and treatment of diseases in terms of reasonable diet (Gentile and Weir, 2018), scientific medicine, and health care. Foreign researchers have found that G. lucidum can inhibit intestinal inflammation and relieve symptoms such as intestinal sensitivity. The research on G. lucidum and gut flora in China is also deepening. G. lucidum has antibacterial effect on some pathogens in the intestine, which is helpful to prevent and treat intestinal infections. In a word, the regulating and improving effect of G. lucidum on gut flora has been widely concerned and studied. In the future, with the deepening of research and technological progress, we are expected to have a deeper understanding of the interaction mechanism between G. lucidum and gut flora and provide more effective solutions for intestinal health.
Although G. lucidum has been actively developed in the intestinal field, we should also pay attention to the objective problems that cannot be ignored in its development (El Sheikha, 2022). First, food safety problems in the environment are serious, and G. lucidum is affected by environmental pollution. When extracting G. lucidum components, heavy metal ions are inevitably present; their cytotoxicity must be avoided, and food safety must be guaranteed. Second, there is the problem of purity. There are many active ingredients in G. lucidum, but not all of them are beneficial; therefore, innovations in separation and purification technology are needed. Finally, there is the issue of norms. Extensive processing of G. lucidum has high economic benefits, but there are bound to be problems arising from variations in techniques and counterfeiting. However, from the perspective of food safety and standardization, G. lucidum can be developed to produce cost-effective products for regulating gut flora, improving health care, and enhancing recovery from illness.
Author contributions
XQ: Conceptualization, Writing – original draft, Writing – review & editing. ZF: Data curation, Investigation, Writing – original draft. JZ: Data curation, Writing – review & editing. WZ: Visualization, Writing – review & editing. NZ: Formal analysis, Methodology, Writing – original draft. XW: Conceptualization, Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We greatly appreciate the financial support from the Department of Science and Technology of Jilin Province (No. 20210203131SF).
Acknowledgments
We would like to thank the Editage team for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrew, J. M., Pachnis, V., and Prinz, M. (2023). Boundaries and integration between microbiota, the nervous system, and immunity. Immunity 56, 1712–1726. doi: 10.1016/j.immuni.2023.07.011
Baby, S., Johnson, A. J., and Govindan, B. (2015). Secondary metabolites from Ganoderma. Phytochemistry 114, 66–101. doi: 10.1016/j.phytochem.2015.03.010
Batra, P., Sharma, A. K., and Khajuria, R. (2013). Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes): a bitter mushroom with amazing health benefits. Int. J. Med. Mushrooms 15, 127–143. doi: 10.1615/intjmedmushr.v15.i2.20
Blundell, R., Camilleri, E., Baral, B., Karpiński, T. M., Neza, E., and Atrooz, O. M. (2023). The phytochemistry of ganoderma species and their medicinal potentials. Am. J. Chin. Med. 51, 859–882. doi: 10.1142/S0192415X23500404
Cao, Y. J., Huang, Z. R., You, S. Z., Guo, W. L., Zhang, F., Liu, B., et al. (2022). The protective effects of ganoderic acids from Ganoderma lucidum fruiting body on alcoholic liver injury and intestinal microflora disturbance in mice with excessive alcohol intake. Foods 11:949. doi: 10.3390/foods11070949
Chang, C. J., Lin, C. S., Lu, C. C., Martel, J., Ko, Y. F., Ojcius, D. M., et al. (2015). Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 6:7489. doi: 10.1038/ncomms8489
Chen, M., Xiao, D., Wen, L.-R., Song, Y., Zou, B., Li, L., et al. (2020). Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 155, 890–902. doi: 10.1016/j.ijbiomac.2019.11.047
Chen, W., Fei, M., Lin, Z., Pei, G., Xiangyu, L., Meiping, Z., et al. (2016). Research progress on extraction of effective components and pharmacological activities of Ganoderma lucidum. Anhui Agric. Sci. 8:147-149+202. doi: 10.13989/j.cnki.0517
Dai, Z., Liu, J., Yao, X., Wang, A., Liu, Y., Strappe, P., et al. (2022). Association of gut microbiota characteristics and metabolites reveals the regulation mechanisms under cadmium consumption circumstance. J. Sci. Food Agric. 102, 6737–6748. doi: 10.1002/jsfa.12041
Daniel, B. G., and Ramnik, J. X. (2023). Conditioning of the immune system by the microbiome. Trends Immunol. 44, 499–511. doi: 10.1016/j.it.2023.05.002
Ding, Q. (2020). Discussion on the mechanism of black ganoderma lucidum polysaccharide on type 2 diabetic rats based on intestinal flora. Doctoral thesis, (Nanchang University). doi: 10.27232/d.cnki.gnchu.2020.003506
Dokht, A., Seyed-Mohammadi, S., Teimoori, A., and Asarehzadegan Dezfuli, A. (2022). The role of microbiota in colorectal cancer. Folia Microbiol. 67, 683–691. doi: 10.1007/s12223-022-00978-1
Ehtasham, A., Lim, S., Lamptey, R., Webb, D. R., and Davies, M. J. (2022). Type 2 diabetes. Lancet 400, 1803–1820. doi: 10.1016/s0140-6736(22)01655-5
El Sheikha, A. F. (2022). Nutritional profile and health benefits of Ganoderma lucidum "Lingzhi, Reishi, or Mannentake" as functional foods: current scenario and future perspectives. Foods 11:1030. doi: 10.3390/foods11071030
Faruque, A., Ahmad, F. A., Zeyaullah, M., Alsayegh, A. A., Mahmood, S. E., AlShahrani, A. M., et al. (2023). Ganoderma lucidum: novel insight into Hepatoprotective potential with mechanisms of action. Nutrients 15:1874. doi: 10.3390/nu15081874
Fidelis, A., Wang, Z., Chen, W., Dong, L., and Peng, X. (2023). Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 42, 197–211. doi: 10.1016/j.tibtech.2023.07.008
Gentile, C. L., and Weir, T. L. (2018). The gut microbiota at the intersection of diet and human health. Science 362, 776–780. doi: 10.1126/science.aau5812
Guo, C., Guo, D., Liu, F., Sang, T., Jianjun, W., Guo, C., et al. (2021). Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 267:118231. doi: 10.1016/j.carbpol.2021.118231
Guo, L., Zhang, J., Kan, Q., Song, M., Hou, T., An, S., et al. (2022). Extraction, structural characterization, and immunomodulatory activity of a high molecular weight polysaccharide from Ganoderma lucidum. Front. Nutr. 9:846080. doi: 10.3389/fnut.2022.846080
Guo, W. L., Cao, Y. J., You, S. Z., Wu, Q., Zhang, F., Han, J. Z., et al. (2022). Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 5, 515–530. doi: 10.1016/j.crfs.2022.02.013
Guo, W. L., Guo, J. B., Liu, B., Lu, J. Q., Chen, M., Liu, B., et al. (2020). Ganoderic acid a from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 11, 6818–6833. doi: 10.1039/d0fo00436g
Guo, W. L., Pan, Y. Y., Li, L., Li, T. T., Liu, B., and Lv, X. C. (2018). Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 9, 3419–3431. doi: 10.1039/c8fo00836a
Healey, G., Murphy, R., Butts, C., Brough, L., Whelan, K., and Coad, J. (2018). Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 119, 176–189. doi: 10.1017/S0007114517003440
He, X., Chen, Y., Li, Z., Fang, L., Chen, H., Liang, Z., et al. (2023). Germplasm resources and secondary metabolism regulation in Reishi mushroom (Ganoderma lucidum). Chinese Herb. Med. 15, 376–382. doi: 10.1016/j.chmed.2023.01.005
Hongtao, W. (2022). Research progress on the relationship between intestinal microorganisms and human health and its influencing factors. J. Food Saf. Qual. Inspect. 1, 175–181. doi: 10.19812/J.CNKI.JFSQ11-5956/TS.2022.01.016
Imran, K., Guoxin, H., Xiaoang, L., Waikit, L., Wenrui, X., and Wendy, H. (2018). Mushroom polysaccharides from Ganoderma lucidum and Poria cocos reveal prebiotic functions. J. Funct. Foods 41, 191–201. doi: 10.1016/j.jff.2017.12.046
Isaac, T., Montiel, V. C.-P. E., Rodriguez, V., Aguirre-Moreno, A., León-Rivera, I., del Río-Portilla, F., et al. (2013). Anticonvulsant and neuroprotective effects of oligosaccharides from Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes). Int. J. Med. Mushrooms 15, 555–568. doi: 10.1615/intjmedmushr.v15.i6.40
Jiang, G., Lei, A., Chen, Y., Yu, Q., Xie, J., Yang, Y., et al. (2021a). The protective effects of the Ganoderma atrum polysaccharide against acrylamide-induced inflammation and oxidative damage in rats. Food \u0026amp; function, 12, 397–407.
Jiang, Y., Zhang, N., Zhou, Y., Zhou, Z., Bai, Y., Strappe, P., et al. (2021b). Manipulations of glucose/lipid metabolism and gut microbiota of resistant starch encapsulated Ganoderma lucidum spores in T2DM rats. Food Sci. Biotechnol. 30, 755–764. doi: 10.1007/s10068-021-00908-w
Jie, P., Laihao, L., and Jianwei, M. (2021). Research progress of intestinal flora and human health. J. Shandong Nor. Univ. 4, 337–365. doi: 10.3969/j.issn.1001-4748.2021.04.002
Jie, Z., Xia, H., Zhong, S., Feng, Q., Li, S., Liang, S., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8:845. doi: 10.1038/s41467-017-00900-1
Jin, M., Zhu, Y., Shao, D., Zhao, K., Chunlan, X., Li, Q., et al. (2017). Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int. J. Biol. Macromol. 94, 1–9. doi: 10.1016/j.ijbiomac.2016.09.099
Jin, X., Ruiz Beguerie, J., Sze, D. M., and Chan, G. C. (2016). Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 4:CD007731. doi: 10.1002/14651858.CD007731.pub3
Kai, Y., Yajie, Z., Zhang, S., Ming, C., Xionge, P., Junrong, H., et al. (2020). Preparation of Ganoderma lucidum spore powder oligosaccharide and its function in regulating intestinal flora. Food Ferment. Indus. 9, 37–42. doi: 10.13995/j.cnki.11-
Kovatcheva-Datchary, P., Nilsson, A., Akrami, R., Lee, Y. S., De Vadder, F., Arora, T., et al. (2015). Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 22, 971–982. doi: 10.1016/j.cmet.2015.10.001
La Fata, G., Rastall, R. A., Lacroix, C., Harmsen, H. J. M., Mohajeri, M. H., Weber, P., et al. (2017). Recent development of prebiotic research-statement from an expert workshop. Nutrients 9:1376. doi: 10.3390/nu9121376
Lai, T. Y. Y., Gao, Y., and Zhou, S.-F. (2004). Global Marketing of Medicinal Ling Zhi Mushroom Ganoderma lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromycetideae) products and safety concerns. Int. J. Med. Mushrooms 6, 189–194. doi: 10.1615/intjmedmushr.v6.i2.100
Leng, Y. X., Wang, F., Chen, C., Wan, X., Li, X., Wang, H., et al. (2023). Protective effect of Ganoderma lucidum spore powder on acute liver injury in mice and its regulation of gut microbiota. Front. Biosci. 28:23. doi: 10.31083/j.fbl2802023
Lepakshi, B, Kv, Ratnam, Prabhaker Yadav, C, Saheb, Meera, Pandita, Anu, and Pandita, Deepu (2023). Reishi Mushroom (Ganoderma lucidum). Boca Raton: CRC Press.
Li, D., Wang, P., Wang, P., Xiaosong, H., and Chen, F. (2017). Targeting the gut microbiota by dietary nutrients: a new avenue for human health. Crit. Rev. Food Sci. Nutr. 59, 181–195. doi: 10.1080/10408398.2017.1363708
Li, K., Zhuo, C., Teng, C., Yu, S. L., Wang, X., Yang, H., et al. (2016). Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 93, 904–912. doi: 10.1016/j.ijbiomac.2016.09.029
Li, M., Yu, L., Zhai, Q., Chu, C., Wang, S., Zhao, J., et al. (2023). Combined Ganoderma lucidum polysaccharide and ciprofloxacin therapy alleviates Salmonella enterica infection, protects the intestinal barrier, and regulates gut microbiota. Food Funct. 14, 6896–6913. doi: 10.1039/d3fo00625e
Li, M., Yu, L., Zhai, Q., Liu, B., Zhao, J., Chen, W., et al. (2022). Ganoderma lucidum ethanol extraction promotes dextran sulphate sodium induced colitis recovery and modulation in microbiota. Foods 11:4023. doi: 10.3390/foods11244023
Li, M., Yu, L., Zhai, Q., Liu, B., Zhao, J., Zhang, H., et al. (2021). Ganoderma applanatum polysaccharides and ethanol extracts promote the recovery of colitis through intestinal barrier protection and gut microbiota modulations. Food Funct. 13, 688–701. doi: 10.1039/d1fo03677g
Liu, J., Tan, Y., Cheng, H., Zhang, D., Feng, W., and Peng, C. (2022). Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 13, 1106–1126. doi: 10.14336/ad.2022.0104
Liu, X., Yang, L., Li, G., Jiang, Y., Zhang, G., and Ling, J. (2023). A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 229, 168–180. doi: 10.1016/j.ijbiomac.2022.12.276
Liu, Y., Lai, G., Guo, Y., Tang, X., Shuai, O., Xie, Y., et al. (2021). Protective effect of Ganoderma lucidum spore extract in trimethylamine-N-oxide-induced cardiac dysfunction in rats. J. Food Sci. 86, 546–562. doi: 10.1111/1750-3841.15575
Li, Y., Li, H., Qi, H.-W., Tang, W., Zhang, C., Liu, Z., et al. (2021). Probiotic fermentation of Ganoderma lucidum fruiting body extracts promoted its immunostimulatory activity in mice with dexamethasone-induced immunosuppression. Biomed. Pharmacother. 141:111909. doi: 10.1016/j.biopha.2021.111909
Llewellyn, S. R., Britton, G. J., Contijoch, E. J., Vennaro, O. H., Mortha, A., Colombel, J. F., et al. (2018). Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 154, 1037–1046.e2. doi: 10.1053/j.gastro.2017.11.030
Longgang, G., Hongyu, J., Zhang Yiyao, X., Zhengdi, X. H., and Peishan, X. (2020). Application of Ganoderma lucidum and Ganoderma lucidum control extracts in fingerprint analysis of Ganoderma lucidum samples. China J. Pharm. 5, 349–356. doi: 10.11669/cpj.2020.05.004
Luo, J., Zhang, C., Liu, R., Gao, L., Shiyi, O., Liu, L., et al. (2018). Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J. Funct. Foods 47, 127–135. doi: 10.1016/j.jff.2018.05.041
Lu, S., Li, D., Juanjuan, S., Zhang, E., Chen, S., Chaoqun, Z., et al. (2021). Polysaccharides of Sporoderm-broken spore of Ganoderma lucidum modulate adaptive immune function via gut microbiota regulation. Evid. Based Complement. Alternat. Med. 2021, 1–15. doi: 10.1155/2021/8842062
Lv, X.-C., Guo, W., Li, L., Yu, X., and Liu, B. (2019). Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods 57, 48–58. doi: 10.1016/j.jff.2019.03.043
Lv, X. C., Wu, Q., Cao, Y. J., Lin, Y. C., Guo, W. L., Rao, P. F., et al. (2022). Ganoderic acid a from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition. Food Funct. 13, 5820–5837. doi: 10.1039/d1fo03219d
María, E. M., Martínez-Carrera, D., Torres, N., Sánchez-Tapia, M., Aguilar-Lopez, M., Morales, P., et al. (2016). Hypocholesterolemic properties and prebiotic effects of Mexican Ganoderma lucidum in C57BL/6 mice. PLoS One 11, –e0159631. doi: 10.1371/journal.pone.0159631
Nakagawa, T., Zhu, Q., Tamrakar, S., Amen, Y., Mori, Y., Suhara, H., et al. (2018). Changes in content of triterpenoids and polysaccharides in Ganoderma lingzhi at different growth stages. J. Nat. Med. 72, 734–744. doi: 10.1007/s11418-018-1213-y
O’Keefe, S. J. (2016). Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 13, 691–706. doi: 10.1038/nrgastro.2016.165
Qizheng, W., Zhang, H.-C., Wang, P. G., and Chen, M. (2017). Evaluation of the efficacy and safety of Ganoderma lucidum mycelium-fermented liquid on gut microbiota and its impact on cardiovascular risk factors in human. RSC Adv. 7, 45093–45100. doi: 10.1039/c7ra08087e
Rodríguez-Lago, I., Merino, O., Azagra, I., Maiz, A., Zapata, E., Higuera, R., et al. (2018). Characteristics and progression of preclinical inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 16, 1459–1466. doi: 10.1016/j.cgh.2017.11.006
Rongkang, H., Guo, W., Huang, Z., Li, L., Liu, B., and Lv, X.-C. (2018). Extracts of Ganoderma lucidum attenuate lipid metabolism and modulate gut microbiota in high-fat diet fed rats. J. Funct. Foods 46, 403–412. doi: 10.1016/j.jff.2018.05.020
Sachdev, A., and Pimentel, M. (2013). Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther. Adv. Chronic Dis. 4, 223–231. doi: 10.1177/2040622313496126
Sang, T., Guo, C., Guo, D., Wu, J., Wang, Y., Wang, Y., et al. (2021). Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 256:117594. doi: 10.1016/j.carbpol.2020.117594
Sears, C. L., and Garrett, W. S. (2014). Microbes, microbiota, and colon cancer. Cell Host Microbe 15, 317–328. doi: 10.1016/j.chom.2014.02.007
Shao, W., Xiao, C., Yong, T., Zhang, Y., Huiping, H., Xie, T., et al. (2022). A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 197, 23–38. doi: 10.1016/j.ijbiomac.2021.12.034
Shuai, G. (2021). Master’s degree thesis on preparation of broken rice recombinant rice and its influence on intestinal flora. Harbin Univ. Commerce. 5–6, 33–52 doi: 10.27787/d.cnki.ghrbs.2021.000064
Su, J., Li, D., Chen, Q., Li, M., Su, L., Luo, T., et al. (2018). Anti-breast cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: suppression on tumor metabolism with gut microbiota reshaping. Front. Microbiol. 9:3099. doi: 10.3389/fmicb.2018.03099
Thuy, N. H. L., Vo Linh, T., Le, T. M., Giang, T. T., Huyen, D. T. K., Loc, D. H., et al. (2023). Pharmacological activities and safety of Ganoderma lucidum spores: a systematic review. Cureus 15:e44574. doi: 10.7759/cureus.44574
Tian, B., Liu, R., Xu, T., Cai, M., Mao, R., Huang, L., et al. (2023). Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J. Sci. Food Agric. 103, 3050–3064. doi: 10.1002/jsfa.12404
Tian, B., Zhao, Q., Xing, H., Xu, J., Li, Z., Zhu, H., et al. (2022). Gastroprotective effects of Ganoderma lucidum polysaccharides with different molecular weights on ethanol-induced acute gastric injury in rats. Nutrients 14:1476. doi: 10.3390/nu14071476
Tong, A. J., Hu, R. K., Wu, L. X., Lv, X. C., Li, X., Zhao, L. N., et al. (2020). Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food Biochem. 44:e13109. doi: 10.1111/jfbc.13109
Tong, A., Wu, W., Chen, Z., Wen, J., Jia, R., Liu, B., et al. (2023). Modulation of gut microbiota and lipid metabolism in rats fed high-fat diets by Ganoderma lucidum triterpenoids. Curr. Res. Food Sci. 6:100427. doi: 10.1016/j.crfs.2022.100427
Ventura, M., Turroni, F., Motherway, M. O.' C., MacSharry, J., and van Sinderen, D. (2012). Host–microbe interactions that facilitate gut colonization by commensal bifidobacteria. Trends Microbiol. 20, 467–476. doi: 10.1016/j.tim.2012.07.002
Vigsnæs, L. K., Van den Abbeele, P., Sulek, K., Frandsen, H. L., Steenholdt, C., Brynskov, J., et al. (2013). Microbiotas from UC patients display altered metabolism and reduced ability of LAB to colonize mucus. Sci. Rep. 3:1110. doi: 10.1038/srep01110
Wang, J., Cao, B., Zhao, H., and Feng, J. (2017). Emerging roles of Ganoderma Lucidum in anti-aging. Aging Dis. 8, 691–707. doi: 10.14336/ad.2017.0410
Wang, P., Cai, M., Yang, K., Sun, P., Xu, J., Li, Z., et al. (2023). Phenolics from Dendrobium officinale leaf ameliorate dextran sulfate sodium-induced chronic colitis by regulating gut microbiota and intestinal barrier. J. Agric. Food Chem. 71, 16630–16646. doi: 10.1021/acs.jafc.3c05339
Wang, X., Shuangshuang, J., Yaxin, S., Wang, S., Hongtao, T., Qinghai, S., et al. (2012). Preparation of oligosaccharides from Zizyphus jujuba Mill. and its effect on growth of Bifidobacterium in vitro. J. China Food 9, 28–33. doi: 10.6429/J.1009-7848
Wei, L., Yuzhen, H., Julie,, Jianhong, G., Ying, L., Ningping, T., et al. (2019). Research and application progress of Ganoderma lucidum triterpenoids. Food Sci. 5, 309–315. doi: 10.7506/spkx1002-6630-20180201-021
Wong, S. H., and Yu, J. (2019). Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 16, 690–704. doi: 10.1038/s41575-019-0209-8
Wu, S.-Y., Chu-Chyn, O., Lee, M.-L., Ma, H. I., Kang, Y., Jan, M.-S., et al. (2023). Polysaccharide of Ganoderma lucidum ameliorates cachectic myopathy induced by the combination cisplatin plus docetaxel in mice. Microbiol. Spectrum 11:e0313022. doi: 10.1128/spectrum.03130-22
Wu, X., Cao, J., Li, M., Yao, P., Li, H., Xu, W., et al. (2020). An integrated microbiome and metabolomic analysis identifies immunoenhancing features of Ganoderma lucidum spores oil in mice. Pharmacol. Res. 158:104937. doi: 10.1016/j.phrs.2020.104937
Xia, Q., Zhao, Q., Zhu, H., Cao, Y., Yang, K., Sun, P., et al. (2022). Physicochemical characteristics of Ganoderma lucidum oligosaccharide and its regulatory effect on intestinal flora in vitro fermentation. Food Chem 15:100421. doi: 10.1016/j.fochx.2022.100421
Xie, J., Liu, Y., Chen, B., Zhang, G., Shiyi, O., Luo, J., et al. (2019). Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 63:2–10 doi: 10.29219/fnr.v63.1559
Xinyi, W. (2022). Master’s degree thesis on the development of special diet for the elderly based on the regulation of intestinal flora, Jiangnan University. Available at: http://link-CNKI-net-s.vpn.jlenu.edu.cn/doi/10.27169/d.cnki.gwqgu.2022.100000000026
Xiong, Y., Zhang, F., Li, J., Peng, P.-Z., Liu, B., and Zhao, L. (2022). Ganoderma lucidum protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet fed rats. Food Biosci. 47:101460. doi: 10.1016/j.fbio.2021.101460
Xu, R. (2010). Introduction to Ganoderma lucidum in 2010. p. 34–41, Nianxi Culture and Education Foundation: Taipei.
Yang, J., Martínez, I., Walter, J., Keshavarzian, A., and Rose, D. J. (2013). In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23, 74–81. doi: 10.1016/j.anaerobe.2013.06.012
Yang, K., Zhang, Y., Cai, M., Guan, R., Neng, J., Pi, X., et al. (2020). In vitro prebiotic activities of oligosaccharides from the by-products in Ganoderma lucidum spore polysaccharide extraction. RSC Adv. 10, 14794–14802. doi: 10.1039/c9ra10798c
Yang, X., (2019). Effect of Ganoderma lucidum polysaccharide and its hydrolysate on intestinal mucosal immune function in immunosuppressed mice. Master’s degree thesis, (Shaanxi Normal University). doi: 10.27292/d.cnki.gsxfu.2019.000721
Yang, Y., Nirmagustina, D. E., Kumrungsee, T., Okazaki, Y., Tomotake, H., and Kato, N. (2017). Feeding of the water extract from Ganoderma lingzhi to rats modulates secondary bile acids, intestinal microflora, mucins, and propionate important to colon cancer. Biosci. Biotechnol. Biochem. 81, 1796–1804. doi: 10.1080/09168451.2017.1343117
Yao, C., Wang, Z., Jiang, H., Yan, R., Huang, Q., Wang, Y., et al. (2021). Ganoderma lucidum promotes sleep through a gut microbiota-dependent and serotonin-involved pathway in mice. Sci. Rep. 11:13660. doi: 10.1038/s41598-021-92913-6
Keywords: Ganoderma lucidum, active ingredients, gut flora, medicinal value, regulation
Citation: Qin X, Fang Z, Zhang J, Zhao W, Zheng N and Wang X (2024) Regulatory effect of Ganoderma lucidum and its active components on gut flora in diseases. Front. Microbiol. 15:1362479. doi: 10.3389/fmicb.2024.1362479
Edited by:
Bo Yang, Jiangnan University, ChinaReviewed by:
Baoming Tian, Zhejiang University of Technology, ChinaXinyuan Shi, Beijing University of Chinese Medicine, China
Qingsong Qu, Beijing University of Chinese Medicine, China, in collaboration with reviewer XS
Copyright © 2024 Qin, Fang, Zhang, Zhao, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoe Wang, d2FuZ3hlQGpsZW51LmVkdS5jbg==
 Xinjie Qin
Xinjie Qin Zinan Fang1,2
Zinan Fang1,2