- Department of Urology Surgery, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, China
Urolithiasis affects 2–20% of the global population and recurs frequently. Emerging evidence positions the gut–kidney axis as a central driver of stone formation. This review synthesizes epidemiological data, comparative metagenomic analyzes, and mechanistic studies to demonstrate that stone formers exhibit reduced α-diversity, depletion of oxalate-degrading taxa (e.g., Oxalobacter, Lactobacillus, Bifidobacterium), and enrichment of pro-inflammatory genera (Escherichia, Bacteroides). Microbial metabolites—oxalate, short-chain fatty acids, p-cresol, and secondary bile acids—modulate intestinal oxalate transport, systemic inflammation, and renal crystal nucleation. Therapeutic modulation via targeted probiotics, prebiotics, engineered Lactobacillus, or fecal microbiota transplantation restores oxalate homeostasis and attenuates nephrolithiasis in rodent models; however, human efficacy remains preliminary. Large-scale multi-omics cohorts and randomized controlled intervention trials are imperative to translate gut-centric strategies into precision urology.
1 Introduction
Urolithiasis is a widespread disease, affecting 2–20% of the global population (Kachkoul et al., 2023). Its prevalence is on the rise, closely following socioeconomic development (Kachkoul et al., 2023). Geographical and racial variations are notable; for instance, in Sub-Saharan Africa, the pooled prevalence among hospital—visiting patients was 9.4% (95% CI = 4.9–14%) (Kassaw et al., 2024). In Asia, the prevalence ranges from 1 to 19.1% across different regions (Liu et al., 2018). Calcium—type stones, especially calcium oxalate, are the most common, accounting for over 80% of cases (Kachkoul et al., 2023). The global burden of urolithiasis, as measured by incidence and disability-adjusted life—years, has shown a decrease in high Socio-Demographic Index countries but an increase in low and low-middle Socio-Demographic Index countries from 1990 to 2019 (Li et al., 2023). Calcium-type stones, particularly calcium oxalate, account for over 80% of cases, underscoring their predominant role in the disease landscape (Nazarian et al., 2025). The recurrence rate of urolithiasis is also a critical concern, with studies indicating that approximately 50% of patients experience at least one recurrence within five to ten years. This recurring nature not only exacerbates the physical suffering of patients but also imposes a substantial economic burden on healthcare systems, necessitating frequent medical interventions, hospitalizations, and long-term monitoring (Tamborino et al., 2024; Karagöz et al., 2022).
The etiology of urolithiasis is multifactorial, with traditional risk factors playing a pivotal role. Dietary habits are among the most well-established contributors (Siener et al., 2023). A diet rich in oxalate, sodium, and animal protein, coupled with inadequate fluid intake, significantly elevates the risk of stone formation. For instance, excessive consumption of oxalate-containing foods such as spinach, nuts, and rhubarb can increase urinary oxalate levels, promoting the precipitation of calcium oxalate crystals (Yang et al., 2024). Similarly, a high-sodium diet can enhance urinary calcium excretion, fostering stone development (Odermatt, 2011). Metabolic abnormalities, including hypercalciuria, hyperoxaluria, and hypocitraturia, are also well-documented risk factors. These imbalances disrupt the delicate equilibrium of urinary components, favoring crystal nucleation and growth (Owino et al., 2023). Genetic predisposition further complicates the risk profile, with monogenic diseases accounting for a higher proportion of stone formers in children and adolescents (Monico and Milliner, 2011). Additionally, obesity, diabetes, and metabolic syndrome have been increasingly linked to urolithiasis. Obesity, for example, may alter urinary acidification and increase urinary calcium excretion, thereby promoting stone formation (Trinchieri et al., 2017). However, despite our understanding of these traditional risk factors, their individual contributions and complex interactions in the pathogenesis of urolithiasis remain areas of active investigation and are not yet fully elucidated.
Recent research has spotlighted the gut-kidney axis as a novel and promising avenue for understanding and managing urolithiasis (Gao et al., 2022). This axis encapsulates the intricate two-way communication between the gut microbiota and the kidneys (Rui-Zhi et al., 2020). In the context of urolithiasis, the gut microbiota emerges as a key player that can influence stone formation through multiple pathways. First and foremost, gut microbes are intricately involved in oxalate metabolism. Certain bacteria, such as Oxalobacter formigenes, possess the unique ability to degrade oxalate in the gut (Stanford et al., 2020). When the population of these beneficial bacteria dwindles, the absorption of dietary oxalate may increase, subsequently raising urinary oxalate levels and the risk of calcium oxalate stone formation (Stanford et al., 2020). Recent reviews and conference data (2024–2025) consolidate the view that food-grade Lactobacillus and Bifidobacterium isolates behave as “generalist oxalotrophs” which constitutively express oxalyl-CoA decarboxylase (oxc) and formyl-CoA transferase (frc), enzymes that cleave dietary oxalate into CO₂ and formate within the intestinal lumen. Al-Kabe and Niamah (2024) surveyed global starter-culture research and noted that over 70% of tested Lactobacillus spp. and all B. longum subsp. infantis strains reduced soluble oxalate by >50% in fermented soymilk within 24 h, an activity that correlated with transcriptional up-regulation of oxc and concomitant lowering of luminal oxalate absorption in a Caco-2 transport model. Expanding these findings, the same group screened 42 Iraqi dairy-derived lactobacilli and reported in 2025 that L. plantarum IQ-6 and L. paracasei IQ-15 combined (10⁹ CFU/day) decreased urinary oxalate excretion by 32% in a rat hyperoxaluric model, while maintaining high gastrointestinal survival (pH 3, 0.3% bile) and full antibiotic susceptibility profiles compatible with probiotic criteria. Collectively, these works corroborate earlier metagenomic observations that enrichment of Lactobacillus/Bifidobacterium communities is inversely associated with 24 h urinary oxalate in human stone formers and provide mechanistic proof that live biotherapeutics targeting oxalate breakdown remain a feasible preventive strategy against calcium oxalate nephrolithiasis (Al-Kabe and Niamah, 2025). Beyond direct oxalate metabolism, the gut microbiota can indirectly impact urolithiasis via metabolic byproducts. For example, the production of short-chain fatty acids (SCFAs) through the fermentation of dietary fibers can influence systemic inflammation and immune responses. These effects, in turn, may create a conducive environment for stone formation within the urinary tract (Hong et al., 2023). Furthermore, the gut microbiota plays a crucial role in immune modulation. Dysbiosis, or imbalance, of the gut microbiota can lead to an aberrant immune response. This may trigger chronic low-grade inflammation in the urinary tract, promoting the aggregation of crystal-forming substances and the development of stones (Levy et al., 2017). Despite these emerging insights, our understanding of the gut-kidney axis in urolithiasis remains incomplete, offering a fertile ground for further exploration.
The gut-kidney axis represents a complex interplay between the gut microbiota and the kidneys. In urolithiasis, this axis has gained significant attention. Gut microbiota can influence urolithiasis through various mechanisms, including direct oxalate metabolism, indirect metabolic pathways, and immune modulation (Tsuji et al., 2024). Dysbiosis of the gut microbiota may lead to an increase in uremic toxins, which can affect renal function and contribute to stone formation. This review aims to synthesize the latest evidence on the gut microbiota’s roles in urolithiasis, exploring both direct and indirect mechanisms and evaluating their therapeutic potential. By bridging the gap between experimental findings and clinical practice, we aspire to facilitate the development of innovative approaches that can ultimately improve patient outcomes and reduce the burden of urolithiasis on healthcare systems globally.
2 Gut microbiota and urolithiasis
The complex relationship between the gut microbiota and urolithiasis has been gradually unveiled in recent research. The gut microbiota, a vital community of microorganisms in the gastrointestinal tract, is now recognized as a significant factor influencing stone formation (Yuan et al., 2023). Understanding this relationship is key to exploring novel therapeutic approaches for urolithiasis.
2.1 Composition and function of gut microbiota
The gut microbiota is a complex ecological community comprised of trillions of microorganisms, including bacteria, fungi, viruses, and other microbes, with bacteria being the most predominant and extensively studied component (Vemuri et al., 2020). These microorganisms primarily reside in the large intestine and are distributed across different phyla, with the most common ones being Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. The gut microbiota composition varies among individuals due to factors such as genetics, diet, age, lifestyle, and antibiotic use, and it can also change over time within the same individual (Vemuri et al., 2020).
The gut microbiota plays a crucial role in maintaining host health through various functions. In terms of nutrition metabolism, it helps in the breakdown of complex carbohydrates, proteins, and lipids that are otherwise indigestible by human enzymes (Valdes et al., 2018). For instance, gut bacteria ferment dietary fiber into SCFAs like acetate, propionate, and butyrate, which serve as energy sources for colonocytes and other cells in the body. These SCFAs also have anti-inflammatory properties and contribute to the maintenance of gut barrier function (Ramos Meyers et al., 2022). Additionally, the gut microbiota is involved in the synthesis of certain vitamins, such as vitamin K and some B vitamins, which are essential for various physiological processes (Ellis et al., 2021).
The gut microbiota plays a vital role in the development and regulation of the host immune system. It interacts with immune cells in the gut-associated lymphoid tissue, helping to shape the maturation of immune cells and modulating immune responses (Yoo et al., 2020). A healthy gut microbiota promotes the production of antibodies and cytokines, which are crucial for defending against pathogens and maintaining immune homeostasis (Gomaa, 2020). Furthermore, the gut microbiota contributes to the maintenance of gut barrier function by stimulating the production of mucus and tight junction proteins, thereby preventing the translocation of harmful substances and pathogens into the systemic circulation (Di Vincenzo et al., 2024).
2.2 Differences in gut microbiota between stone formers and healthy individuals
Numerous studies have compared the gut microbiota composition of individuals with urolithiasis and healthy controls, revealing significant differences (Ticinesi et al., 2018; Zampini et al., 2019; Lee et al., 2025). Overall, stone formers tend to exhibit reduced gut microbiota diversity compared to healthy individuals. A lower diversity of gut microbiota is often associated with dysbiosis, a state of microbial imbalance that can disrupt the normal functioning of the gut ecosystem and contribute to various diseases (Al et al., 2023).
At the phylum level, some studies have reported alterations in the relative abundances of Firmicutes and Bacteroidetes in stone formers (Liu et al., 2023). For example, a meta-analysis by Yuan et al. found that patients with urolithiasis had a higher abundance of Bacteroides and Escherichia, while Prevotella was more abundant in healthy controls. These shifts in phylum-level composition may reflect broader changes in the gut microbial community structure that are linked to stone formation (Yuan et al., 2023). At the genus level, differences are also evident. Stone formers often show a reduced abundance of certain beneficial bacteria, such as Faecalibacterium, Enterobacter, and Dorea. Faecalibacterium prausnitzii, a well-known anti-inflammatory bacterium that produces butyrate, is typically depleted in stone formers. In contrast, potentially pathogenic or pro-inflammatory bacteria, such as Escherichia coli and other Enterobacteriaceae members, may be enriched in the gut microbiota of stone formers (Stern et al., 2016) (Figure 1).
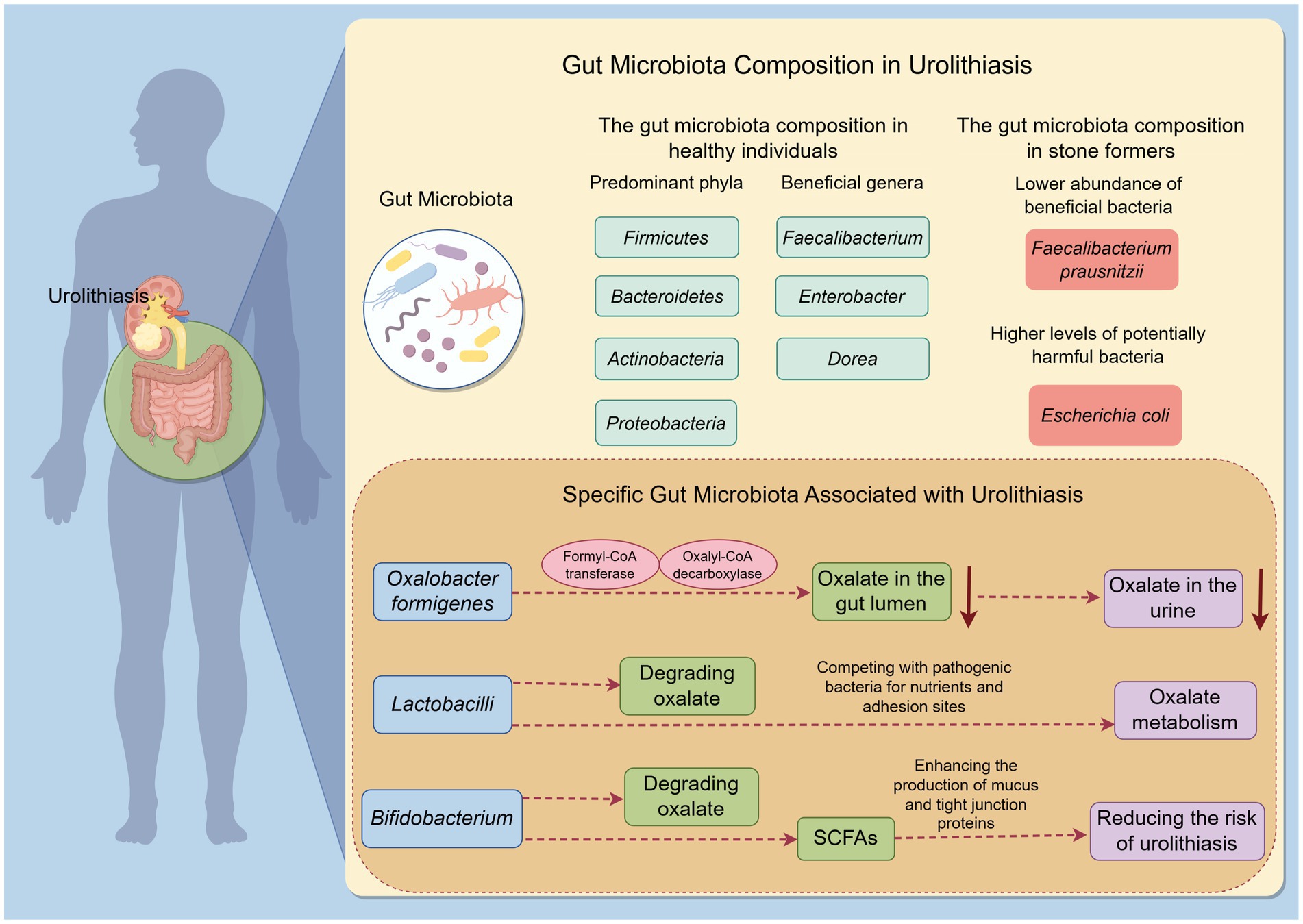
Figure 1. Gut microbiota composition and functional differences in urolithiasis by Figdraw. This figure illustrates the differences in gut microbiota composition and function between stone formers and healthy individuals. It highlights the reduced diversity and specific alterations in beneficial and potentially harmful bacteria in stone formers. The figure also emphasizes the functional differences related to oxalate metabolism.
Moreover, specific functional alterations related to oxalate metabolism have been observed in the gut microbiota of stone formers. Metagenomics studies have revealed that stone formers have a lower representation of genes associated with oxalate degradation compared to healthy individuals (Al et al., 2023). This reduction in oxalate-metabolizing capacity may lead to increased oxalate absorption in the gut and subsequently higher urinary oxalate excretion, which is a known risk factor for calcium oxalate stone formation.
2.3 Specific gut microbiota associated with urolithiasis
Oxalobacter formigenes, a gram-negative, anaerobic bacterium, has a unique capability to degrade oxalate. It utilizes oxalate as its sole carbon and energy source through the action of two key enzymes: formyl-CoA transferase and oxalyl-CoA decarboxylase. By degrading oxalate in the gut lumen, this bacterium reduces the amount of oxalate available for absorption into the bloodstream and subsequent excretion in urine (Miller and Dearing, 2013) (Figure 1). A meta-analysis found that the presence of Oxalobacter formigenes was associated with a lower risk of calcium oxalate stone formation (Kachroo et al., 2021). However, its role in stone formation remains somewhat controversial. Some studies have shown that not all individuals colonized with Oxalobacter formigenes are protected from stone formation, suggesting that other factors and microbes also play important roles in this process (Miller and Dearing, 2013).
Lactobacilli are a genus of gram-positive, facultative anaerobic bacteria commonly found in the human gut. Some species of Lactobacilli have been shown to possess oxalate-degrading activity (Turroni et al., 2007). For example, Lactobacillus acidophilus and Lacticaseibacillus casei have demonstrated the ability to degrade oxalate in vitro. Lactobacilli may also influence oxalate metabolism indirectly by competing with pathogenic bacteria for nutrients and adhesion sites in the gut, thereby maintaining a healthy gut microbiota balance (Di Cerbo et al., 2016). Additionally, Lactobacilli are known for their immunomodulatory effects. They can enhance the production of anti-inflammatory cytokines and strengthen the gut barrier function. By reducing inflammation and oxidative stress in the gut and kidneys, Lactobacilli may contribute to a lower risk of stone formation (Di Cerbo et al., 2016) (Figure 1).
Bifidobacterium is another genus of beneficial gut bacteria that have been implicated in oxalate metabolism. Certain Bifidobacterium species, such as Bifidobacterium bifidum and Bifidobacterium longum, have shown oxalate-degrading capability. Bifidobacterium also plays a significant role in maintaining gut barrier function. They produce SCFAs that nourish colonocytes and enhance the production of mucus and tight junction proteins (O'Callaghan and van Sinderen, 2016). Furthermore, Bifidobacterium has immunomodulatory properties, helping to regulate immune responses and reduce inflammation in the gut. A healthy population of Bifidobacterium in the gut may thus help prevent the translocation of harmful substances and pathogens into the systemic circulation, which could have indirect beneficial effects on kidney health and reduce the risk of urolithiasis (Gavzy et al., 2023) (Figure 1).
3 Metabolites in the gut-kidney axis and urolithiasis
The metabolites produced by the gut microbiota serve as crucial mediators in the gut-kidney axis and play a central role in urolithiasis pathogenesis. These metabolites, such as oxalate and SCFAs, directly impact renal crystal formation and inflammation (Mehta et al., 2016). Investigating their mechanisms of action and therapeutic potential offers promising avenues for managing urolithiasis (Table 1).
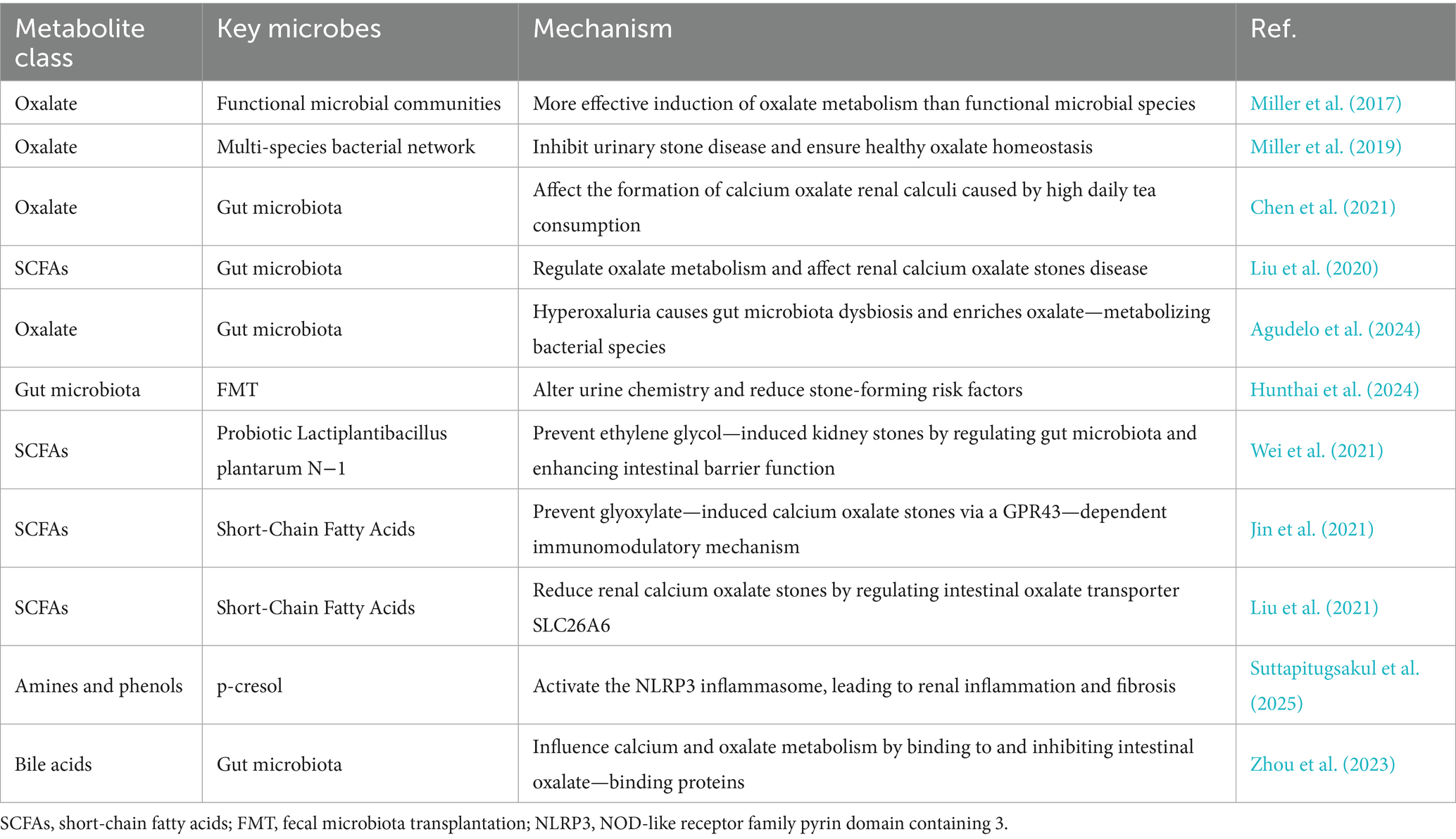
Table 1. Gut microbiota-derived metabolites in urolithiasis: mechanisms and therapeutic implications.
3.1 Oxalate metabolism
Oxalate is a key component in the formation of calcium oxalate stones. The gut plays a crucial role in oxalate metabolism. Dietary oxalate is absorbed in the intestine, and the gut microbiota can degrade oxalate, thereby reducing its absorption and systemic exposure. The gut microbiota’s role in oxalate metabolism extends beyond Oxalobacter formigenes (Falony, 2018). Miller et al. found that functional microbial communities are more effective than individual functional microbial species in inducing oxalate metabolism in vivo, suggesting that a diverse gut microbiota may have a more significant impact on oxalate homeostasis (Miller et al., 2017). Additionally, Miller et al. showed that a multi-species bacterial network can inhibit urinary stone disease and maintain healthy oxalate homeostasis (Miller et al., 2019). Chen et al. also demonstrated that gut microbiota can affect the formation of calcium oxalate renal calculi caused by high daily tea consumption (Chen et al., 2021).
In patients with urolithiasis, gut microbiota dysbiosis may lead to reduced oxalate-degrading capacity, resulting in increased oxalate absorption and elevated urinary oxalate levels, which in turn increase the risk of calcium oxalate stone formation. Liu et al. explored the relationship between gut microbiota and short-chain fatty acids in renal calcium oxalate stone disease and found that gut microbiota dysbiosis is associated with altered oxalate metabolism (Liu et al., 2020). Agudelo et al. discovered that hyperoxaluria can cause gut microbiota dysbiosis and selectively enrich oxalate—metabolizing bacterial species in recurrent kidney stone formers (Agudelo et al., 2024).
Probiotics or prebiotics may modulate the gut microbiota to enhance oxalate degradation. A randomized, controlled trial demonstrated that lactic acid bacteria could reduce idiopathic hyperoxaluria (Liu et al., 2020). Fecal microbiota transplantation (FMT) has also shown potential in modifying urine chemistry risk factors for urinary stone disease. Hunthai et al. (2024) found that FMT could alter urine chemistry and reduce stone-forming risk factors. However, further research is needed to determine the optimal probiotics, prebiotics, and FMT protocols for preventing and treating urolithiasis.
3.2 Short-chain fatty acids
SCFAs, such as acetate, propionate, and butyrate, are produced by the gut microbiota through the fermentation of dietary fiber. These SCFAs have various physiological functions, including regulating gut barrier function, immune responses, and energy metabolism (Mukhopadhya and Louis, 2025). Multiple studies have shown that SCFAs can enhance gut barrier function by promoting the synthesis of mucin and tight junction proteins, thereby preventing the translocation of bacterial products and toxins into the bloodstream (Xiao et al., 2024; Liu et al., 2021). This is significant for urolithiasis, as gut barrier dysfunction may allow bacterial products and toxins to enter the systemic circulation, potentially inducing systemic inflammation and oxidative stress, which contribute to kidney stone formation. Wei et al. found that probiotic Lactiplantibacillus plantarum N-1 can prevent ethylene glycol—induced kidney stones by regulating gut microbiota and enhancing intestinal barrier function (Wei et al., 2021). Other research has also demonstrated the role of SCFAs in maintaining gut integrity and preventing inflammatory conditions that may contribute to stone formation (Tian et al., 2022).
SCFAs play a role in immune regulation by modulating the differentiation and function of immune cells, such as T regulatory cells and macrophages. They can reduce inflammation in the gut and systemically. Inflammation is a key factor in kidney stone formation, as it can promote crystal adhesion and aggregation. Jin et al. showed that short-chain fatty acids can prevent glyoxylate—induced calcium oxalate stones via a GPR43—dependent immunomodulatory mechanism (Jin et al., 2021). Butyrate, as an anti-inflammatory substance, may indirectly affect kidney stone formation by influencing gut inflammation. SCFAs can also regulate the expression of intestinal oxalate transporters, such as SLC26A6, thereby affecting oxalate absorption and excretion. Liu et al. found that short-chain fatty acids reduce renal calcium oxalate stones by regulating the expression of intestinal oxalate transporter SLC26A6 (Liu et al., 2021).
However, the specific mechanisms by which SCFAs regulate gut barrier function, immune responses, and energy metabolism in the context of urolithiasis require further investigation. Additionally, factors such as the types and doses of SCFAs, as well as individual differences in gut microbiota, may influence their effects on urolithiasis.
3.3 Other metabolites
Besides oxalate and SCFAs, the gut microbiota produces various other metabolites, such as amines, phenols, and bile acids, which may also impact kidney function and urolithiasis (Mukhopadhya and Louis, 2025). Amines and phenols, such as p-cresol and indoxyl sulfate, are derived from the gut microbiota’s metabolism of proteins and amino acids. These metabolites can be absorbed into the bloodstream and excreted in urine (McFarlane et al., 2022). At high concentrations, they may induce oxidative stress and inflammation in the kidneys, promoting kidney stone formation. Suttapitugsakul et al. found that p-cresol can activate the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, leading to renal inflammation and fibrosis, which may increase the risk of kidney stones (Suttapitugsakul et al., 2025). Other study has also highlighted the role of these metabolites in inducing oxidative stress and inflammation in renal tubular epithelial cells, contributing to kidney damage and stone formation (Tian et al., 2025).
Bile acids, synthesized by the liver and further metabolized by gut microbiota, play a role in lipid digestion and absorption. They can also act as signaling molecules to regulate glucose and lipid metabolism. Recent a study suggest that bile acids may influence calcium and oxalate metabolism. For example, secondary bile acids can bind to and inhibit the activity of intestinal oxalate—binding proteins, reducing oxalate absorption (Zhou et al., 2023). However, the specific mechanisms by which bile acids affect urolithiasis remain unclear and warrant further exploration. Research in this area is ongoing to better understand the complex interactions between gut microbiota—derived metabolites and their effects on kidney stone formation.
In conclusion, gut microbiota metabolites play a crucial role in the gut-kidney axis and urolithiasis. Oxalate metabolism, SCFAs, and other metabolites collectively influence kidney stone formation through multiple mechanisms. However, current research on the role of gut microbiota metabolites in urolithiasis is still limited, with many mechanisms remaining to be elucidated. Future studies should focus on employing multi-omics technologies to comprehensively analyze the types and functions of gut microbiota metabolites in urolithiasis, revealing their metabolic networks and regulatory mechanisms. Conducting clinical trials to evaluate the efficacy and safety of probiotics, prebiotics, FMT, and other interventions in modulating gut microbiota metabolites for urolithiasis prevention and treatment is also essential. Exploring the potential of gut microbiota metabolites as biomarkers for urolithiasis diagnosis and prognosis, as well as investigating the interactions between gut microbiota metabolites and host genetics, diet, and lifestyle factors to develop personalized prevention and treatment strategies for urolithiasis, are promising research directions. By strengthening research in these areas, we aim to deepen our understanding of the role of gut microbiota metabolites in urolithiasis and promote the translation of research findings into clinical applications, ultimately improving the quality of life for patients with urolithiasis.
4 Mechanisms linking gut microbiota, metabolites, and urolithiasis
The gut microbiota and its metabolites interact through various mechanisms to influence urolithiasis. These include direct effects on oxalate metabolism and indirect effects via inflammation and immunity (Kim et al., 2022). Additionally, the gut microbiota’s impact on gut barrier function plays a significant role in stone formation. Understanding these intricate mechanisms is essential for developing targeted therapeutic strategies.
4.1 Direct effects
The gut microbiota can directly influence urolithiasis through various mechanisms. Certain bacteria possess the ability to degrade oxalate, a key component in calcium oxalate stones, which account for approximately 75% of kidney stones (Hunthai et al., 2024). Oxalobacter formigenes, a gram-negative anaerobic bacterium, specializes in oxalate degradation using formyl-CoA transferase and oxalyl-CoA decarboxylase. Its presence in the gut reduces the amount of oxalate available for absorption and subsequent urinary excretion (Chmiel et al., 2022). However, trials involving oxalate-degrading probiotics, such as those containing Oxalobacter formigenes, have not produced definitive findings. One major factor contributing to this limitation is the high sensitivity of Oxalobacter formigenes to antibiotics, which are commonly used in clinical settings (Lange et al., 2012) (Figure 2). Besides, some studies have shown that not all individuals colonized with Oxalobacter formigenes are protected from stone formation, suggesting that other factors and microbes also play important roles in this process.
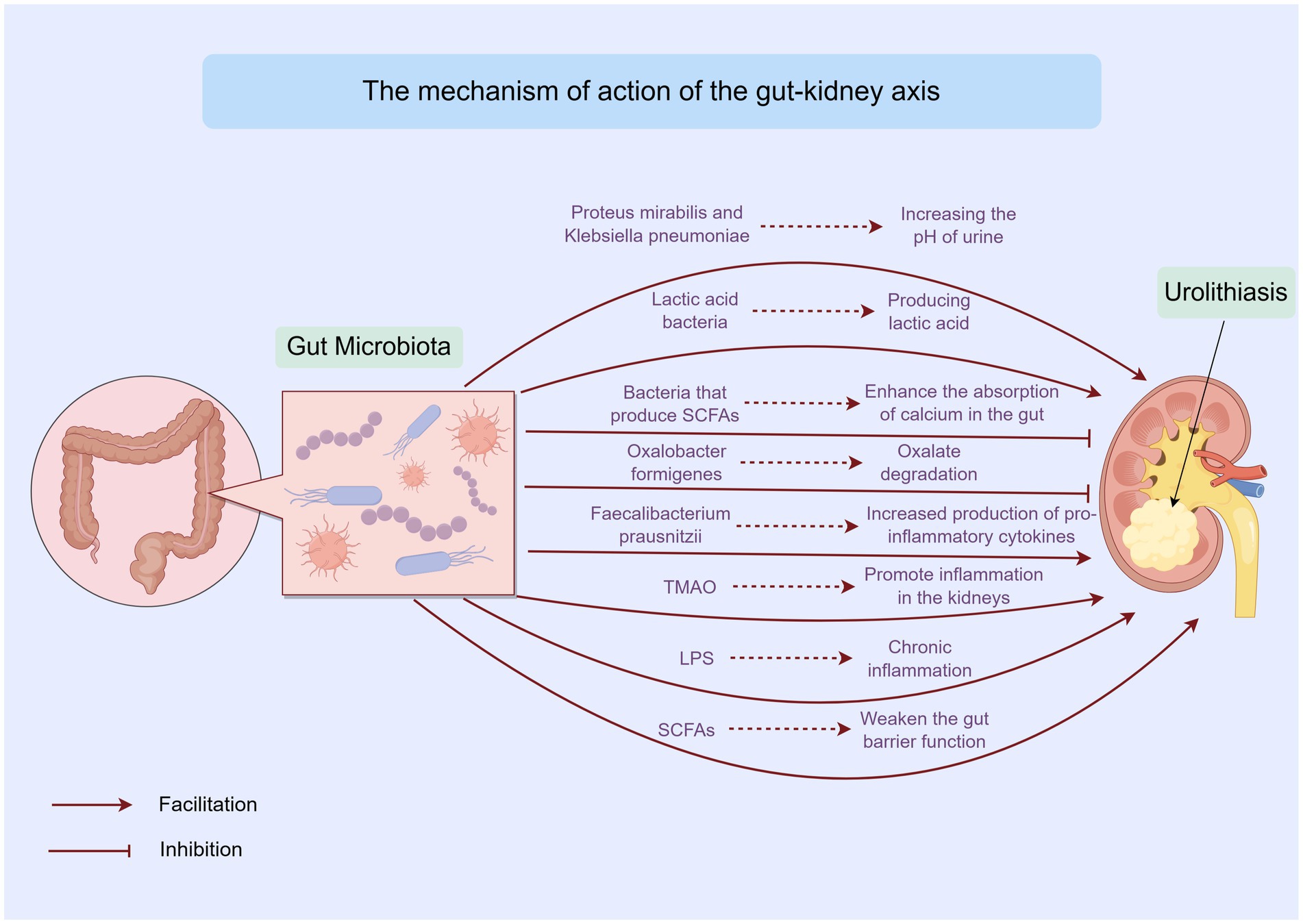
Figure 2. Mechanisms linking gut microbiota, metabolites, and urolithiasis by Figdraw. This figure outlines the direct and indirect mechanisms through which gut microbiota and its metabolites influence urolithiasis. It includes the impact of oxalate metabolism, SCFAs, and other metabolites on kidney stone formation.
In addition to oxalate-degrading bacteria, some gut bacteria can affect the physical and chemical properties of urine, thereby influencing stone formation and growth. For instance, certain bacteria can alter the pH of urine. Urease-producing bacteria, such as Proteus mirabilis and Klebsiella pneumoniae, can hydrolyze urea into ammonia, increasing the pH of urine. This makes the urine more alkaline, which may promote the formation of struvite stones (Jung et al., 2023). Conversely, certain lactic acid bacteria are capable of producing lactic acid, which reduces the pH of the intestinal environment and may potentially influence the absorption and excretion of urinary stone components (Wan et al., 2024).
Moreover, gut bacteria can also influence the concentration of substances in urine. Some bacteria can affect the absorption of minerals and nutrients in the gut, thereby altering the levels of these substances in the blood and urine. For example, bacteria that produce SCFAs can enhance the absorption of calcium in the gut. This may reduce the amount of calcium available to combine with oxalate in the urine, thus lowering the risk of calcium oxalate stone formation (Ticinesi et al., 2018).
4.2 Indirect effects via inflammation and immunity
Growing evidence suggests that the gut microbiota can modulate systemic inflammation and immune responses, which in turn affect kidney health and stone formation. The gut microbiota plays a vital role in maintaining the balance of the immune system. When the gut microbiota is dysbiotic, it can lead to an imbalance in the production of pro-inflammatory and anti-inflammatory cytokines (Wastyk et al., 2021; De Luca et al., 2025) (Figure 2). For example, a decrease in beneficial bacteria such as Faecalibacterium prausnitzii, which has anti-inflammatory properties, can result in increased production of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These cytokines can enter the bloodstream and reach the kidneys, promoting inflammation in the renal tissues and creating a favorable environment for stone formation (Yuan et al., 2023).
The production of certain metabolites by the gut microbiota can also influence inflammation and immune responses. For instance, trimethylamine N-oxide (TMAO), a metabolite derived from dietary choline and L-carnitine, has been associated with an increased risk of cardiovascular disease and chronic kidney disease. Elevated levels of TMAO may promote inflammation in the kidneys and affect the metabolism of urinary stone components, thereby contributing to stone formation (Capolongo et al., 2023). Additionally, lipopolysaccharides (LPS), which are components of the outer membrane of gram-negative bacteria, can trigger strong immune responses when they enter the bloodstream. Dysbiosis of the gut microbiota can increase the permeability of the gut barrier, allowing more LPS to translocate into the systemic circulation. This can lead to chronic inflammation, which may exacerbate kidney damage and increase the risk of urolithiasis (Wei et al., 2021) (Figure 2).
The gut microbiota can also influence the function of immune cells. Regulatory T cells (Tregs), which play a crucial role in maintaining immune tolerance and suppressing excessive immune responses, can be modulated by certain gut bacteria (Bhutta et al., 2024). A decrease in the abundance of Tregs due to gut microbiota dysbiosis may result in unchecked immune responses in the kidneys, leading to tissue damage and the promotion of stone formation. Furthermore, the gut microbiota can affect the maturation and function of dendritic cells, which are important antigen-presenting cells. Dysregulated dendritic cell activity can lead to abnormal activation of T cells and B cells, contributing to autoimmune and inflammatory responses in the kidneys and other organs (Wilson et al., 2022).
Recent evidence demonstrates that Bifidobacterium longum subsp. infantis Iraq-Basrah 3-derived exopolysaccharides (EPS) significantly attenuate LPS-induced IL-6 and TNF-α secretion in THP-1 macrophages, highlighting the immunomodulatory potential of specific commensal strains (Hussein et al., 2025). This finding is particularly relevant to urolithiasis, as elevated IL-6 and TNF-α promote renal tubular epithelial–mesenchymal transition and osteogenic differentiation, thereby facilitating Randall plaque formation and calcium oxalate nucleation (Suttapitugsakul et al., 2025). Furthermore, the EPS from B. longum subsp. infantis enhances regulatory T-cell (Treg) expansion while suppressing Th17 responses via TLR2/MyD88-dependent signaling, a pathway that has been independently shown to reduce renal calcium oxalate crystal deposition in murine models (Jin et al., 2021). Consequently, depletion of Bifidobacterium spp. in stone formers—consistently reported in 16S rRNA meta-analyze—may remove a critical brake on IL-17–driven neutrophil recruitment and subsequent reactive oxygen species (ROS)-mediated crystal adhesion to renal epithelia (Yuan et al., 2023). Collectively, these data position commensal-derived EPS as candidate microbe-based therapeutics capable of restoring the Treg/Th17 balance and mitigating inflammation-facilitated stone formation.
4.3 Interaction with the gut barrier function
The gut barrier function is essential for maintaining the integrity of the gut and preventing the translocation of harmful substances and pathogens into the systemic circulation. The gut microbiota plays a crucial role in maintaining the gut barrier function (Barbara et al., 2021). Beneficial bacteria such as Lactobacilli and Bifidobacterium can enhance the production of mucus and tight junction proteins, thereby strengthening the gut barrier. They also compete with pathogenic bacteria for adhesion sites and nutrients, preventing the overgrowth of harmful microbes that can disrupt the gut barrier (Segui-Perez et al., 2025) (Figure 2).
When the gut microbiota is dysbiotic, the gut barrier function can become compromised. This can lead to increased gut permeability, allowing bacteria, bacterial fragments, and metabolites to enter the bloodstream (Dmytriv et al., 2024). These substances can trigger systemic inflammation and immune responses, which may have detrimental effects on kidney health. For example, the translocation of bacterial endotoxins such as LPS can activate immune cells in the kidneys, leading to the production of inflammatory mediators and oxidative stress (Sun et al., 2022; Zhong et al., 2020). This can result in kidney damage and promote the formation of stones. Furthermore, the increased absorption of oxalate due to impaired gut barrier function can elevate urinary oxalate levels, increasing the risk of calcium oxalate stone formation.
In addition to the direct effects of gut microbiota on the gut barrier, certain metabolites produced by the gut microbiota can also influence gut barrier function. SCFAs, such as butyrate, propionate, and acetate, are important metabolites that help maintain the integrity of the gut barrier. Butyrate, in particular, serves as the primary energy source for colonocytes and promotes the production of mucus and tight junction proteins (Liang et al., 2022; Gasaly et al., 2021). A decrease in the production of SCFAs due to gut microbiota dysbiosis can weaken the gut barrier function, making the gut more susceptible to inflammation and the translocation of harmful substances (Yang et al., 2020; Pohl et al., 2022). This can ultimately affect kidney health and contribute to the development of urolithiasis (Figure 2).
Overall, the gut microbiota and its metabolites are intricately linked to urolithiasis through a variety of direct and indirect mechanisms. Understanding these complex interactions can provide valuable insights into the pathogenesis of urolithiasis and pave the way for the development of novel therapeutic strategies targeting the gut microbiota and its metabolites. However, further research is needed to fully elucidate these mechanisms and to overcome the limitations of current studies, such as small sample sizes and confounding factors, in order to establish more effective and targeted interventions for the prevention and treatment of urolithiasis.
5 Therapeutic implications of gut microbiota modulation
Modulating the gut microbiota presents a novel therapeutic approach for urolithiasis prevention and treatment. Probiotics, prebiotics, FMT, and dietary interventions have shown potential in altering the gut microbiota composition and function (Jung et al., 2023; Zheng and Cao, 2024) (Table 2). Exploring their efficacy and safety in clinical settings is crucial for translating these findings into effective clinical applications.
5.1 Probiotics and prebiotics
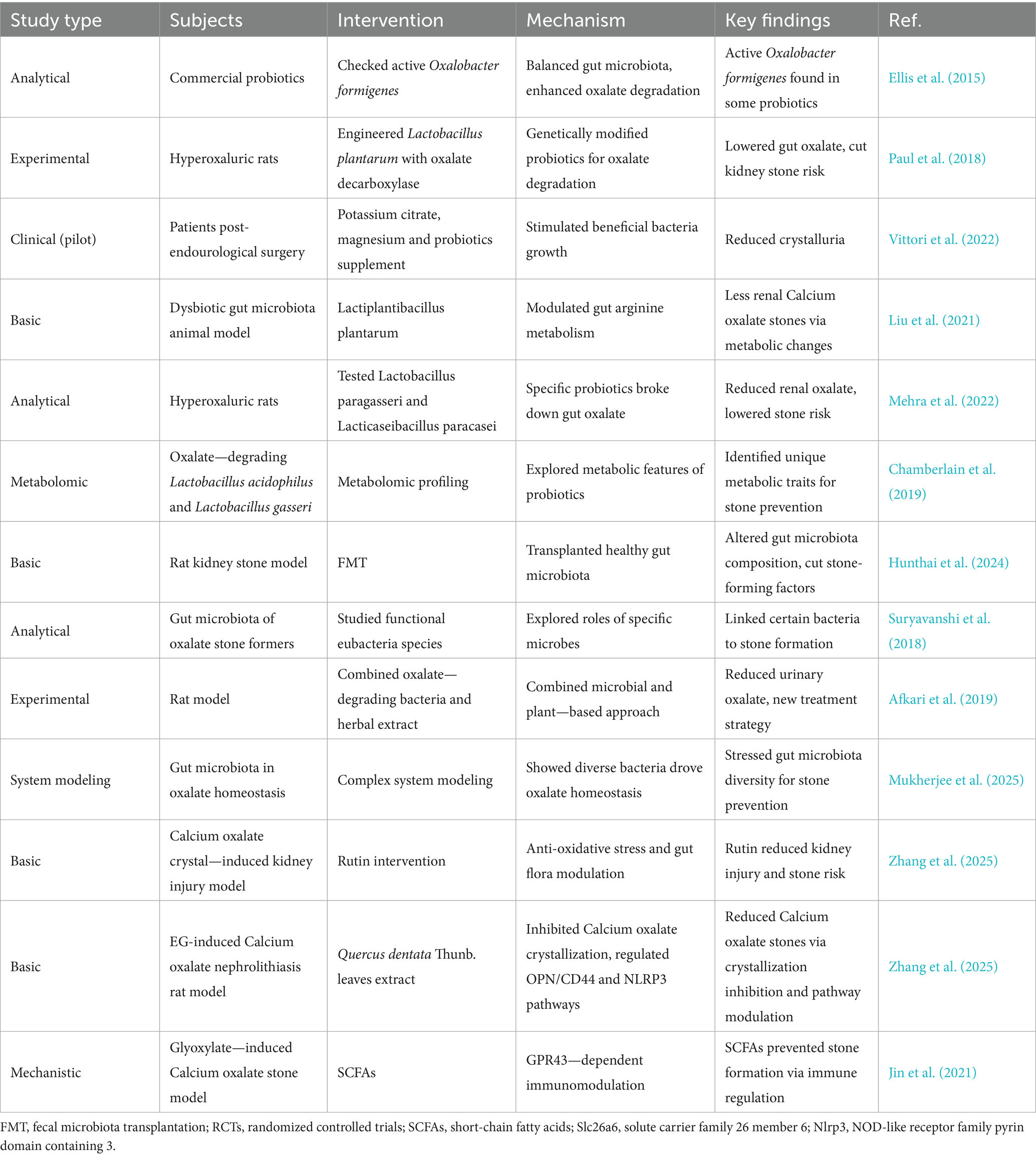
Probiotics and prebiotics have emerged as promising therapeutic options for urolithiasis by modulating the gut microbiota. Probiotics can restore the balance of the gut microbiota and enhance the degradation of oxalate (Taheri et al., 2024; Wigner et al., 2022). For instance, Ellis et al. (2015) found viable Oxalobacter formigenes in some commercial kidney stone probiotic supplements. Paul et al. (2018) developed a designer probiotic Lactobacillus plantarum expressing oxalate decarboxylase. Prebiotics can selectively stimulate the growth of beneficial bacteria. Vittori et al. (2022) reported that food supplementation based on potassium citrate, magnesium, and probiotics reduced crystalluria in patients undergoing endourological surgery for stone disease. However, more research is needed to determine the optimal strains, dosages, and treatment durations for urolithiasis.
Liu et al. (2021) demonstrated that Lactiplantibacillus plantarum could reduce renal calcium oxalate stones by regulating arginine metabolism in the gut microbiota. Wei et al. (2021) showed that probiotic Lactiplantibacillus plantarum N-1 could prevent ethylene glycol-induced kidney stones by regulating gut microbiota and enhancing intestinal barrier function. Mehra et al. (2022) analyzed and characterized Lactobacillus paragasseri and Lacticaseibacillus paracasei. Chamberlain et al. (2019) conducted metabolomic profiling of oxalate-degrading probiotic Lactobacillus acidophilus and Lactobacillus gasseri.
5.2 FMT
FMT involves transferring the gut microbiota from a healthy donor to a patient and could potentially re-establish a healthy gut microbiota composition in urolithiasis patients. Hunthai et al. (2024) explored the role of FMT in a rat model of kidney stone disease and found that it altered the gut microbiota composition, suggesting a potential therapeutic effect. By introducing a diverse and healthy gut microbiota, FMT may help reduce the production of uremic toxins and restore normal metabolic processes related to stone formation. However, challenges such as donor selection, standardization of the procedure, and potential risks of infection need to be addressed before its widespread use in urolithiasis treatment. Suryavanshi et al. (2018) studied functional eubacteria species along with trans-domain gut inhabitants and their role in oxalate stone disease. Afkari et al. (2019) used a combination of oxalate-degrading bacteria and herbal extract to reduce urinary oxalate in a rat model.
5.3 Dietary interventions
Dietary interventions play a significant role in modulating the gut microbiota and preventing urolithiasis. A diet rich in fiber can promote the growth of beneficial gut bacteria and increase the production of SCFAs. For example, increasing the intake of fruits, vegetables, and whole grains can provide prebiotic substrates for the gut microbiota. Mukherjee et al. (2025) revealed that oxalate homeostasis is driven by diverse oxalate-degrading bacteria, highlighting the importance of dietary choices in managing gut microbiota for stone prevention. Additionally, reducing the intake of high-oxalate foods, such as spinach, rhubarb, and nuts, can lower the risk of hyperoxaluria. In patients with autosomal dominant polycystic kidney disease, dietary regimens like caloric restriction, intermittent fasting, and ketogenic diets are being investigated for their potential to slow disease progression, which may also be relevant to urolithiasis.
Zhang et al. (2025) showed that rutin ameliorated calcium oxalate crystal-induced kidney injury through anti-oxidative stress and modulation of intestinal flora. Zhang et al. (2025) highlighted the potential of Quercus dentata Thunb. leaves extract in inhibiting Calcium oxalate crystallization and ameliorating ethylene glycol-induced Calcium oxalate kidney stones via the OPN/CD44 and NLRP3 pathways. Chen et al. (2021) suggested that gut microbiota affect the formation of calcium oxalate renal calculi caused by high daily tea consumption. Jin et al. (2021) found that SCFAs could prevent glyoxylate-induced calcium oxalate stones by GPR43-dependent immunomodulatory mechanisms.
In conclusion, modulation of the gut microbiota through probiotics, prebiotics, FMT, and dietary interventions offers novel therapeutic approaches for urolithiasis. However, further research is needed to optimize these strategies and overcome existing limitations.
6 Research limitations and future directions
Despite the valuable insights gained from current research on the gut-kidney axis in urolithiasis, several limitations exist. These include small sample sizes, heterogeneous study populations, and methodological inconsistencies. Addressing these limitations through large-scale cohort studies, multi-omics integration, and well-designed intervention trials will enhance our understanding and pave the way for more effective therapeutic strategies.
6.1 Limitations in study designs and methodologies
The research on the gut-kidney axis in urolithiasis faces several limitations that may impact the reliability and generalizability of the findings. One major issue is the small sample size in many studies, which can compromise statistical power and make it harder to detect significant differences between groups (Yuan et al., 2023). This also limits the ability to perform subgroup analyzes, which could provide insights into the role of factors such as age, sex, and genetic background.
The heterogeneity of study populations is another challenge. Urolithiasis patients are diverse in terms of stone composition, metabolic abnormalities, and geographical and racial backgrounds (Reed and Hsi, 2024). However, many studies do not adequately account for this diversity, raising questions about the broad applicability of the findings. This heterogeneity can also introduce confounding variables that may obscure the true relationship between gut microbiota and urolithiasis. The majority of studies are observational, which makes it difficult to establish causality. Observational studies can identify associations between gut microbiota and urolithiasis, but they cannot determine whether changes in gut microbiota directly influence stone formation or if these changes are merely secondary effects of the disease. The reliance on self-reported dietary and lifestyle data in these studies also increases the risk of recall bias and inaccuracies.
The dynamic nature of the gut microbiota poses additional methodological challenges. The composition of gut microbiota can vary significantly over time due to factors such as diet, stress, and antibiotic use (Zhang et al., 2022). However, many studies only provide a single snapshot of the gut microbiota, failing to capture its temporal variability. This can lead to an incomplete understanding of how changes in gut microbiota over time contribute to the development and progression of urolithiasis. Moreover, the lack of standardized methodologies for assessing gut microbiota can affect the comparability and replicability of results. Differences in sample collection, processing, and analysis techniques can lead to inconsistencies in findings (Ducarmon et al., 2020). For example, variations in DNA extraction methods, sequencing platforms, and bioinformatics pipelines can significantly impact the identification and quantification of microbial species (Lim et al., 2018).
6.2 Future research directions
To deepen our understanding of the gut-kidney axis in urolithiasis and develop effective therapeutic strategies, several future research directions should be explored. Large-scale cohort studies are necessary to address the limitations of small sample sizes and heterogeneity (Yuan et al., 2023; Kachroo et al., 2021). These studies should include diverse urolithiasis patient populations, covering different stone compositions, metabolic profiles, and demographic backgrounds (Reed and Hsi, 2024; Liu et al., 2023). Longitudinal follow-up of these cohorts can enhance our understanding of the temporal relationships between gut microbiota changes and stone formation. Establishing standardized protocols for data collection and analysis will ensure consistency across studies.
The integration of multi-omics technologies offers a powerful approach to comprehensively analyze the gut microbiota and its metabolites in urolithiasis. Metagenomics can reveal the genetic potential of microbial communities, while metatranscriptomics and metaproteomics can shed light on their functional activity (Liu et al., 2023; Zhang et al., 2023). Metabolomics can identify and quantify the small molecules produced by gut microbiota, which may serve as intermediates in the gut-kidney axis. This integrated analysis can help identify key microbial functions and metabolic pathways involved in stone formation and may uncover new therapeutic targets (Gao et al., 2022).
Well-designed intervention studies are crucial to establish causality and evaluate the efficacy of gut microbiota modulation in preventing and treating urolithiasis. Randomized controlled trials (RCTs) of probiotics, prebiotics, and FMT should be conducted with clearly defined endpoints, such as changes in urinary stone risk factors, stone recurrence rates, and patient-reported outcomes. Mechanistic studies are also needed to clarify how gut microbiota and its metabolites influence urolithiasis. Animal models can be useful for studying these mechanisms in a controlled setting, but it is important to ensure that findings are translatable to humans.
Personalized treatment approaches based on individual gut microbiota profiles represent a promising avenue for future research. By understanding how variations in gut microbiota composition and function influence an individual’s risk of urolithiasis and response to treatment, clinicians can tailor interventions to meet each patient’s specific needs. This may involve the use of precision medicine strategies, such as microbiota-based biomarkers for disease stratification and treatment selection. Furthermore, further exploration of the interactions between gut microbiota, host genetics, diet, and lifestyle factors are essential to fully understand the complex etiology of urolithiasis. These interactions likely play a significant role in modulating the gut-kidney axis and may provide opportunities for developing more effective prevention and treatment strategies that consider the whole-person context.
In summary, while existing research on the gut-kidney axis in urolithiasis has provided valuable insights, there are limitations that need to be addressed. By conducting large-scale cohort studies, utilizing multi-omics technologies, performing well-designed intervention trials, pursuing personalized treatment approaches, and investigating the interplay of multiple factors, we can enhance our understanding of this complex relationship and ultimately improve the clinical management of urolithiasis.
7 Conclusion
The gut-kidney axis represents a pivotal mechanism in the pathogenesis of urolithiasis, with gut microbiota and their metabolites playing central roles in modulating stone formation. Dysbiosis contributes to elevated urinary oxalate, systemic inflammation, and impaired gut barrier function, thereby promoting calcium oxalate crystallization. Microbiota-derived metabolites, including short-chain fatty acids and oxalate-degrading agents, are key mediators in these processes. Emerging therapeutic strategies, such as probiotics, prebiotics, and fecal microbiota transplantation, offer promising avenues for prevention and treatment. However, further large-scale clinical trials and mechanistic studies are essential to validate efficacy and optimize protocols. Integration of multi-omics approaches and personalized interventions based on individual microbiota profiles may enhance therapeutic outcomes. Continued research is warranted to translate these findings into clinical practice and reduce the global burden of urolithiasis.
Author contributions
DL: Writing – original draft, Writing – review & editing. ZL: Writing – original draft, Writing – review & editing. WL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Research Project of the First People’s Hospital of Baiyin City (Title: Study on the Correlation between the Degree of Renal Colic Pain and Clinical Factors) Project Number: 2021YK-09.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afkari, R., Feizabadi, M. M., Ansari-Moghadam, A., Safari, T., and Bokaeian, M. (2019). Simultaneous use of oxalate-degrading bacteria and herbal extract to reduce the urinary oxalate in a rat model: a new strategy. Int. Braz J Urol 45, 1249–1259. doi: 10.1590/s1677-5538.Ibju.2019.0167
Agudelo, J., Mukherjee, S., Suryavanshi, M., Ljubetic, B., Lindenbaum, M. M., and Miller, A. W. (2024). Mechanism of nephrolithiasis: does the microbiome play a role? Eur. Urol. Focus 10, 902–905. doi: 10.1016/j.euf.2024.11.010
Al, K. F., Joris, B. R., Daisley, B. A., Chmiel, J. A., Bjazevic, J., Reid, G., et al. (2023). Multi-site microbiota alteration is a hallmark of kidney stone formation. Microbiome 11:263. doi: 10.1186/s40168-023-01703-x
Al-Kabe, S. H., and Niamah, A. K. (2024). Current trends and technological advancements in the use of oxalate-degrading bacteria as starters in fermented foods-a review. Life 14:1338. doi: 10.3390/life14101338
Al-Kabe, S. H., and Niamah, A. K. (2025). Oxalate degrading screening and probiotic properties of lactic acid bacteria isolates. IOP Conf. Ser. Earth Environ. Sci. 1487:012121. doi: 10.1088/1755-1315/1487/1/012121
Barbara, G., Barbaro, M. R., Fuschi, D., Palombo, M., Falangone, F., Cremon, C., et al. (2021). Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 8:718356. doi: 10.3389/fnut.2021.718356
Bhutta, N. K., Xu, X., Jian, C., Wang, Y., Liu, Y., Sun, J., et al. (2024). Gut microbiota mediated T cells regulation and autoimmune diseases. Front. Microbiol. 15:1477187. doi: 10.3389/fmicb.2024.1477187
Capolongo, G., Ferraro, P. M., and Unwin, R. (2023). Inflammation and kidney stones: cause and effect? Curr. Opin. Urol. 33, 129–135. doi: 10.1097/mou.0000000000001066
Chamberlain, C. A., Hatch, M., and Garrett, T. J. (2019). Metabolomic profiling of oxalate-degrading probiotic Lactobacillus acidophilus and Lactobacillus gasseri. PLoS One 14:e0222393. doi: 10.1371/journal.pone.0222393
Chen, F., Bao, X., Liu, S., Ye, K., Xiang, S., Yu, L., et al. (2021). Gut microbiota affect the formation of calcium oxalate renal calculi caused by high daily tea consumption. Appl. Microbiol. Biotechnol. 105, 789–802. doi: 10.1007/s00253-020-11086-w
Chmiel, J. A., Carr, C., Stuivenberg, G. A., Venema, R., Chanyi, R. M., Al, K. F., et al. (2022). New perspectives on an old grouping: the genomic and phenotypic variability of Oxalobacter formigenes and the implications for calcium oxalate stone prevention. Front. Microbiol. 13:1011102. doi: 10.3389/fmicb.2022.1011102
De Luca, R., Arrè, V., Nardone, S., Incerpi, S., Giannelli, G., Trivedi, P., et al. (2025). Gastrointestinal microbiota and inflammasomes interplay in health and disease: a gut feeling. Gut. doi: 10.1136/gutjnl-2025-334938
Di Cerbo, A., Palmieri, B., Aponte, M., Morales-Medina, J. C., and Iannitti, T. (2016). Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 69, 187–203. doi: 10.1136/jclinpath-2015-202976
Di Vincenzo, F., Del Gaudio, A., Petito, V., Lopetuso, L. R., and Scaldaferri, F. (2024). Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern. Emerg. Med. 19, 275–293. doi: 10.1007/s11739-023-03374-w
Dmytriv, T. R., Storey, K. B., and Lushchak, V. I. (2024). Intestinal barrier permeability: the influence of gut microbiota, nutrition, and exercise. Front. Physiol. 15:1380713. doi: 10.3389/fphys.2024.1380713
Ducarmon, Q. R., Hornung, B. V. H., Geelen, A. R., Kuijper, E. J., and Zwittink, R. D. (2020). Toward standards in clinical microbiota studies: comparison of three DNA extraction methods and two bioinformatic pipelines. mSystems 5:e00547-19. doi: 10.1128/mSystems.00547-19
Ellis, J. L., Karl, J. P., Oliverio, A. M., Fu, X., Soares, J. W., Wolfe, B. E., et al. (2021). Dietary vitamin K is remodeled by gut microbiota and influences community composition. Gut Microbes 13, 1–16. doi: 10.1080/19490976.2021.1887721
Ellis, M. L., Shaw, K. J., Jackson, S. B., Daniel, S. L., and Knight, J. (2015). Analysis of commercial kidney stone probiotic supplements. Urology 85, 517–521. doi: 10.1016/j.urology.2014.11.013
Falony, G. (2018). Beyond Oxalobacter: the gut microbiota and kidney stone formation. Gut 67, 2078–2079. doi: 10.1136/gutjnl-2018-316639
Gao, H., Lin, J., Xiong, F., Yu, Z., Pan, S., and Huang, Y. (2022). Urinary microbial and Metabolomic profiles in kidney stone disease. Front. Cell. Infect. Microbiol. 12:953392. doi: 10.3389/fcimb.2022.953392
Gasaly, N., de Vos, P., and Hermoso, M. A. (2021). Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 12:658354. doi: 10.3389/fimmu.2021.658354
Gavzy, S. J., Kensiski, A., Lee, Z. L., Mongodin, E. F., Ma, B., and Bromberg, J. S. (2023). Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 15:2291164. doi: 10.1080/19490976.2023.2291164
Gomaa, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113, 2019–2040. doi: 10.1007/s10482-020-01474-7
Hong, S. Y., Xia, Q. D., Yang, Y. Y., Li, C., Zhang, J. Q., Xu, J. Z., et al. (2023). The role of microbiome: a novel insight into urolithiasis. Crit. Rev. Microbiol. 49, 177–196. doi: 10.1080/1040841x.2022.2045899
Hunthai, S., Usawachintachit, M., Taweevisit, M., Srisa-Art, M., Anegkamol, W., Tosukhowong, P., et al. (2024). Unraveling the role of gut microbiota by fecal microbiota transplantation in rat model of kidney stone disease. Sci. Rep. 14:21924. doi: 10.1038/s41598-024-72694-4
Hussein, K. A., Niamah, A. K., and Majeed, K. R. (2025). Immunomodulation capability and cytotoxicity activities of novel exopolysaccharides produced by a new local strain, Bifidobacterium longum subsp. infantis strain Iraq-Basrah 3. J. App. Biol. Biotech. 13, 104–111. doi: 10.7324/JABB.2024.183750
Jin, X., Jian, Z., Chen, X., Ma, Y., Ma, H., Liu, Y., et al. (2021). Short chain fatty acids prevent Glyoxylate-induced calcium oxalate stones by GPR43-dependent immunomodulatory mechanism. Front. Immunol. 12:729382. doi: 10.3389/fimmu.2021.729382
Jung, H. D., Cho, S., and Lee, J. Y. (2023). Update on the effect of the urinary microbiome on Urolithiasis. Diagnostics (Basel) 13:951. doi: 10.3390/diagnostics13050951
Kachkoul, R., Touimi, G. B., El Mouhri, G., El Habbani, R., Mohim, M., and Lahrichi, A. (2023). Urolithiasis: history, epidemiology, aetiologic factors and management. Malays. J. Pathol. 45, 333–352.
Kachroo, N., Lange, D., Penniston, K. L., Stern, J., Tasian, G., Bajic, P., et al. (2021). Meta-analysis of clinical microbiome studies in urolithiasis reveal age, stone composition, and study location as the predominant factors in urolithiasis-associated microbiome composition. MBio 12:e0200721. doi: 10.1128/mBio.02007-21
Karagöz, M. A., Güven, S., Tefik, T., Gökçe, M., Kiremit, M. C., Atar, F. A., et al. (2022). Attitudes of urologists on metabolic evaluation for urolithiasis: outcomes of a global survey from 57 countries. Urolithiasis 50, 711–720. doi: 10.1007/s00240-022-01362-x
Kassaw, A. B., Belete, M., Assefa, E. M., and Tareke, A. A. (2024). Prevalence and clinical patterns of urolithiasis in sub-saharan Africa: a systematic review and meta-analysis of observational studies. BMC Nephrol. 25:334. doi: 10.1186/s12882-024-03780-y
Kim, H. N., Kim, J. H., Chang, Y., Yang, D., Joo, K. J., Cho, Y. S., et al. (2022). Gut microbiota and the prevalence and incidence of renal stones. Sci. Rep. 12:3732. doi: 10.1038/s41598-022-07796-y
Lange, J. N., Wood, K. D., Wong, H., Otto, R., Mufarrij, P. W., Knight, J., et al. (2012). Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology 79, 1286–1289. doi: 10.1016/j.urology.2011.11.017
Lee, H. Y., Lin, C. Y., Juan, Y. S., Wu, W. J., Cho, S. Y., and Wu, D. C. (2025). The influence and relationship of dysbiosis in the urinary microbiota on patients with urolithiasis. Urolithiasis 53:53. doi: 10.1007/s00240-025-01724-1
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A., and Elinav, E. (2017). Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232. doi: 10.1038/nri.2017.7
Li, J., Zhao, Y., Xiong, Z., and Yang, G. (2023). Global, regional, and National Incidence and disability-adjusted life-years for Urolithiasis in 195 countries and territories, 1990-2019: results from the global burden of disease study 2019. J. Clin. Med. 12:1048. doi: 10.3390/jcm12031048
Liang, L., Liu, L., Zhou, W., Yang, C., Mai, G., Li, H., et al. (2022). Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. (Lond.) 136, 291–307. doi: 10.1042/cs20210778
Lim, M. Y., Song, E. J., Kim, S. H., Lee, J., and Nam, Y. D. (2018). Comparison of DNA extraction methods for human gut microbial community profiling. Syst. Appl. Microbiol. 41, 151–157. doi: 10.1016/j.syapm.2017.11.008
Liu, Y., Chen, Y., Liao, B., Luo, D., Wang, K., Li, H., et al. (2018). Epidemiology of urolithiasis in Asia. Asian J. Urol. 5, 205–214. doi: 10.1016/j.ajur.2018.08.007
Liu, H., Hu, Q., Yan, Q., Hao, Z., and Liang, C. (2023). Alterations in urinary microbiota composition in urolithiasis patients: insights from 16S rRNA gene sequencing. Front. Cell. Infect. Microbiol. 13:1266446. doi: 10.3389/fcimb.2023.1266446
Liu, Y., Jin, X., Hong, H. G., Xiang, L., Jiang, Q., Ma, Y., et al. (2020). The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 34, 11200–11214. doi: 10.1096/fj.202000786R
Liu, Y., Jin, X., Ma, Y., Jian, Z., Wei, Z., Xiang, L., et al. (2021). Short-chain fatty acids reduced renal calcium oxalate stones by regulating the expression of intestinal oxalate transporter SLC26A6. mSystems 6:e01045-21. doi: 10.1128/mSystems.01045-21
Liu, Y., Jin, X., Tian, L., Jian, Z., Ma, Y., Cheng, L., et al. (2021). Lactiplantibacillus plantarum reduced renal calcium oxalate stones by regulating arginine metabolism in gut microbiota. Front. Microbiol. 12:743097. doi: 10.3389/fmicb.2021.743097
Liu, P., Wang, Y., Yang, G., Zhang, Q., Meng, L., Xin, Y., et al. (2021). The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 165:105420. doi: 10.1016/j.phrs.2021.105420
Liu, M., Zhang, Y., Wu, J., Gao, M., Zhu, Z., and Chen, H. (2023). Causal relationship between kidney stones and gut microbiota contributes to the gut-kidney axis: a two-sample Mendelian randomization study. Front. Microbiol. 14:1204311. doi: 10.3389/fmicb.2023.1204311
McFarlane, C., Krishnasamy, R., Stanton, T., Savill, E., Snelson, M., Mihala, G., et al. (2022). Diet quality and protein-bound Uraemic toxins: investigation of novel risk factors and the role of microbiome in chronic kidney disease. J. Ren. Nutr. 32, 542–551. doi: 10.1053/j.jrn.2021.10.003
Mehra, Y., Rajesh, N. G., and Viswanathan, P. (2022). Analysis and characterization of Lactobacillus paragasseri and Lacticaseibacillus paracasei: two probiotic Bacteria that can degrade intestinal oxalate in Hyperoxaluric rats. Probiotics Antimicrob. Proteins 14, 854–872. doi: 10.1007/s12602-022-09958-w
Mehta, M., Goldfarb, D. S., and Nazzal, L. (2016). The role of the microbiome in kidney stone formation. Int. J. Surg. 36, 607–612. doi: 10.1016/j.ijsu.2016.11.024
Miller, A. W., Choy, D., Penniston, K. L., and Lange, D. (2019). Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 96, 180–188. doi: 10.1016/j.kint.2019.02.012
Miller, A. W., Dale, C., and Dearing, M. D. (2017). The induction of oxalate metabolism in vivo is more effective with functional microbial communities than with functional microbial species. mSystems 2:e00088-17. doi: 10.1128/mSystems.00088-17
Miller, A. W., and Dearing, D. (2013). The metabolic and ecological interactions of oxalate-degrading bacteria in the mammalian gut. Pathogens 2, 636–652. doi: 10.3390/pathogens2040636
Monico, C. G., and Milliner, D. S. (2011). Genetic determinants of urolithiasis. Nat. Rev. Nephrol. 8, 151–162. doi: 10.1038/nrneph.2011.211
Mukherjee, S. D., Batagello, C. A., Adler, A., Agudelo, J., Zampini, A., Suryavanshi, M., et al. (2025). Complex system modelling reveals oxalate homeostasis is driven by diverse oxalate-degrading bacteria. bioRxiv. doi: 10.1101/2024.10.28.620613
Mukhopadhya, I., and Louis, P. (2025). Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat. Rev. Microbiol. 23, 635–651. doi: 10.1038/s41579-025-01183-w
Nazarian, R., Lin, N., Thaker, S., Yang, R., Wong, G. C. L., and Scotland, K. B. (2025). What causes calcium oxalate kidney stones to form? An update on recent advances. Uro 5:6. doi: 10.3390/uro5010006
O'Callaghan, A., and van Sinderen, D. (2016). Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 7:925. doi: 10.3389/fmicb.2016.00925
Odermatt, A. (2011). The western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Renal Physiol. 301, F919–F931. doi: 10.1152/ajprenal.00068.2011
Owino, C., Mutugi, A., and Tang, J. (2023). Hyperoxaluria – a major metabolic risk for kidney stone disease. R. I. Med. J. (2013) 106, 14–19.
Paul, E., Albert, A., Ponnusamy, S., Mishra, S. R., Vignesh, A. G., Sivakumar, S. M., et al. (2018). Designer probiotic Lactobacillus plantarum expressing oxalate decarboxylase developed using group II intron degrades intestinal oxalate in hyperoxaluric rats. Microbiol. Res. 215, 65–75. doi: 10.1016/j.micres.2018.06.009
Pohl, K., Moodley, P., and Dhanda, A. (2022). The effect of increasing intestinal short-chain fatty acid concentration on gut permeability and liver injury in the context of liver disease: a systematic review. J. Gastroenterol. Hepatol. 37, 1498–1506. doi: 10.1111/jgh.15899
Ramos Meyers, G., Samouda, H., and Bohn, T. (2022). Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients 14:5361. doi: 10.3390/nu14245361
Reed, A. M., and Hsi, R. S. (2024). Diversity, equity, and inclusion in Urolithiasis clinical trials: representative enrollment by race, ethnicity, and sex. Urol. Pract. 11, 685–691. doi: 10.1097/upj.0000000000000595
Rui-Zhi, T., Hui, D., Jian-Chun, L., Xia, Z., Xiao-Jia, W., Dan, W., et al. (2020). Astragalus mongholicus Bunge and Panax Notoginseng formula (a&P) combined with Bifidobacterium contribute a Renoprotective effect in chronic kidney disease through inhibiting macrophage inflammatory response in kidney and intestine. Front. Physiol. 11:583668. doi: 10.3389/fphys.2020.583668
Segui-Perez, C., Huang, L. Z. X., Paganelli, F. L., Lievens, E., and Strijbis, K. (2025). Probiotic Bifidobacterium bifidum strains desialylate MUC13 and increase intestinal epithelial barrier function. Sci. Rep. 15:8778. doi: 10.1038/s41598-025-92125-2
Siener, R., Löhr, P., and Hesse, A. (2023). Urinary risk profile, impact of diet, and risk of calcium oxalate Urolithiasis in idiopathic uric acid stone disease. Nutrients 15:572. doi: 10.3390/nu15030572
Stanford, J., Charlton, K., Stefoska-Needham, A., Ibrahim, R., and Lambert, K. (2020). The gut microbiota profile of adults with kidney disease and kidney stones: a systematic review of the literature. BMC Nephrol. 21:215. doi: 10.1186/s12882-020-01805-w
Stern, J. M., Moazami, S., Qiu, Y., Kurland, I., Chen, Z., Agalliu, I., et al. (2016). Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407. doi: 10.1007/s00240-016-0882-9
Sun, Y., Chen, X., Xie, Y., Wang, Y., Zhang, Q., Lu, Y., et al. (2022). TRPM7 promotes lipopolysaccharide-induced inflammatory dysfunction in renal tubular epithelial cells. Immun. Inflamm. Dis. 10:e641. doi: 10.1002/iid3.641
Suryavanshi, M. V., Bhute, S. S., Gune, R. P., and Shouche, Y. S. (2018). Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep. 8:16598. doi: 10.1038/s41598-018-33773-5
Suttapitugsakul, S., Sassanarakkit, S., Peerapen, P., and Thongboonkerd, V. (2025). Integrated proteomics reveals enrichment of oxidative stress and inflammatory proteins in the urine and stone matrix of calcium oxalate stone formers. Urolithiasis 53:25. doi: 10.1007/s00240-025-01697-1
Taheri, H., Feizabadi, M. M., Keikha, R., and Afkari, R. (2024). Therapeutic effects of probiotics and herbal medications on oxalate nephrolithiasis: a mini systematic review. Iran J. Microbiol. 16, 4–18. doi: 10.18502/ijm.v16i1.14866
Tamborino, F., Cicchetti, R., Mascitti, M., Litterio, G., Orsini, A., Ferretti, S., et al. (2024). Pathophysiology and Main molecular mechanisms of urinary stone formation and recurrence. Int. J. Mol. Sci. 25:3075. doi: 10.3390/ijms25053075
Tian, L., Liu, Y., Xu, X., Jiao, P., Hu, G., Cui, Y., et al. (2022). Lactiplantibacillus plantarum J-15 reduced calcium oxalate kidney stones by regulating intestinal microbiota, metabolism, and inflammation in rats. FASEB J. 36:e22340. doi: 10.1096/fj.202101972RR
Tian, Y., Ye, Z., Wang, X., Guan, H., Liu, W., Duan, X., et al. (2025). MOF-818 Nanozyme suppresses calcium oxalate kidney stones by alleviating oxidative stress and inflammatory injury. Adv. Healthc. Mater. 14:e2401574. doi: 10.1002/adhm.202401574
Ticinesi, A., Milani, C., Guerra, A., Allegri, F., Lauretani, F., Nouvenne, A., et al. (2018). Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106. doi: 10.1136/gutjnl-2017-315734
Trinchieri, A., Croppi, E., and Montanari, E. (2017). Obesity and urolithiasis: evidence of regional influences. Urolithiasis 45, 271–278. doi: 10.1007/s00240-016-0908-3
Tsuji, K., Uchida, N., Nakanoh, H., Fukushima, K., Haraguchi, S., Kitamura, S., et al. (2024). The gut-kidney Axis in chronic kidney diseases. Diagnostics (Basel) 15:21. doi: 10.3390/diagnostics15010021
Turroni, S., Vitali, B., Bendazzoli, C., Candela, M., Gotti, R., Federici, F., et al. (2007). Oxalate consumption by lactobacilli: evaluation of oxalyl-CoA decarboxylase and formyl-CoA transferase activity in Lactobacillus acidophilus. J. Appl. Microbiol. 103, 1600–1609. doi: 10.1111/j.1365-2672.2007.03388.x
Valdes, A. M., Walter, J., Segal, E., and Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ 361:k2179. doi: 10.1136/bmj.k2179
Vemuri, R., Shankar, E. M., Chieppa, M., Eri, R., and Kavanagh, K. (2020). Beyond just bacteria: functional biomes in the gut ecosystem including virome, mycobiome, archaeome and helminths. Microorganisms 8:483. doi: 10.3390/microorganisms8040483
Vittori, M., Signoretti, M., Cipriani, C., Antonucci, M., Ferraro, P. M., and Bove, P. (2022). Food supplementation based on potassium citrate, magnesium and probiotics reduces crystalluria in patients undergoing endourological surgery for stone disease: report from a pilot study. Urolithiasis 50, 651–652. doi: 10.1007/s00240-022-01348-9
Wan, W., Wu, W., Amier, Y., Li, X., Yang, J., Huang, Y., et al. (2024). Engineered microorganisms: a new direction in kidney stone prevention and treatment. Synth. Syst. Biotechnol. 9, 294–303. doi: 10.1016/j.synbio.2024.02.005
Wastyk, H. C., Fragiadakis, G. K., Perelman, D., Dahan, D., Merrill, B. D., Yu, F. B., et al. (2021). Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14. doi: 10.1016/j.cell.2021.06.019
Wei, Z., Cui, Y., Tian, L., Liu, Y., Yu, Y., Jin, X., et al. (2021). Probiotic Lactiplantibacillus plantarum N-1 could prevent ethylene glycol-induced kidney stones by regulating gut microbiota and enhancing intestinal barrier function. FASEB J. 35:e21937. doi: 10.1096/fj.202100887RR
Wigner, P., Bijak, M., and Saluk-Bijak, J. (2022). Probiotics in the prevention of the calcium oxalate Urolithiasis. Cells 11:284. doi: 10.3390/cells11020284
Wilson, K. R., Gressier, E., McConville, M. J., and Bedoui, S. (2022). Microbial metabolites in the maturation and activation of dendritic cells and their relevance for respiratory immunity. Front. Immunol. 13:897462. doi: 10.3389/fimmu.2022.897462
Xiao, J., Guo, X., and Wang, Z. (2024). Crosstalk between hypoxia-inducible factor-1α and short-chain fatty acids in inflammatory bowel disease: key clues toward unraveling the mystery. Front. Immunol. 15:1385907. doi: 10.3389/fimmu.2024.1385907
Yang, Q., Ouyang, J., Sun, F., and Yang, J. (2020). Short-chain fatty acids: a soldier fighting against inflammation and protecting from tumorigenesis in people with diabetes. Front. Immunol. 11:590685. doi: 10.3389/fimmu.2020.590685
Yang, L., Wang, L., Liu, Y., Bao, E., Wang, J., Xia, L., et al. (2024). Causal associations between 45 dietary intake habits and urolithiasis: insights from genetic studies. Transl. Androl. Urol. 13, 1074–1084. doi: 10.21037/tau-24-79
Yoo, J. Y., Groer, M., Dutra, S. V. O., Sarkar, A., and McSkimming, D. I. (2020). Gut microbiota and immune system interactions. Microorganisms 8:1587. doi: 10.3390/microorganisms8101587
Yuan, T., Xia, Y., Li, B., Yu, W., Rao, T., Ye, Z., et al. (2023). Gut microbiota in patients with kidney stones: a systematic review and meta-analysis. BMC Microbiol. 23:143. doi: 10.1186/s12866-023-02891-0
Zampini, A., Nguyen, A. H., Rose, E., Monga, M., and Miller, A. W. (2019). Defining Dysbiosis in patients with Urolithiasis. Sci. Rep. 9:5425. doi: 10.1038/s41598-019-41977-6
Zhang, W., Han, N., Zhang, T., Qiang, Y., Peng, X., Li, X., et al. (2022). The spatial features and temporal changes in the gut microbiota of a healthy Chinese population. Microbiol. Spectr. 10:e0131022. doi: 10.1128/spectrum.01310-22
Zhang, Q. Z., Xu, H. Y., Feng, C. Y., Zhang, C. H., Ren, K., Pan, Y. N., et al. (2025). Quercus dentata Thunb. Leaves extract inhibits CaOx crystallization and ameliorates ethylene glycol-induced CaOx kidney stones via the OPN/CD44 and NLRP3 pathways. J. Ethnopharmacol. 337:118839. doi: 10.1016/j.jep.2024.118839
Zhang, H., Yang, H., Du, S., Ren, J., Qiao, G., and Ren, J. (2025). Rutin ameliorates calcium oxalate crystal-induced kidney injury through anti-oxidative stress and modulation of intestinal flora. Urolithiasis 53:50. doi: 10.1007/s00240-025-01726-z
Zhang, R., Zhao, W., Zhao, R., Zhao, Y., Zhang, Y., and Liang, X. (2023). Causal relationship in gut microbiota and upper urinary urolithiasis using Mendelian randomization. Front. Microbiol. 14:1170793. doi: 10.3389/fmicb.2023.1170793
Zheng, Z., and Cao, X. (2024). Association of dietary live microbe intake with kidney stone disease in US adults: a real-world cross-sectional study. Front. Nutr. 11:1463352. doi: 10.3389/fnut.2024.1463352
Zhong, Y., Wu, S., Yang, Y., Li, G. Q., Meng, L., Zheng, Q. Y., et al. (2020). LIGHT aggravates sepsis-associated acute kidney injury via TLR4-MyD88-NF-κB pathway. J. Cell. Mol. Med. 24, 11936–11948. doi: 10.1111/jcmm.15815
Keywords: gut-kidney axis, urolithiasis, gut microbiota, oxalate metabolism, short-chain fatty acids, probiotics, prebiotics, fecal microbiota transplantation
Citation: Li D, Li Z and Liu W (2025) The gut-kidney axis in urolithiasis: roles of gut microbiota, metabolites, and therapeutic implications. Front. Microbiol. 16:1655808. doi: 10.3389/fmicb.2025.1655808
Edited by:
Mohamed Ezzat Abdin, Agricultural Research Center, EgyptReviewed by:
Matteo Vittori, Policlinico Tor Vergata, ItalyAlaa Kareem Niamah, University of Basrah, Iraq
Copyright © 2025 Li, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Liu, NTI3Nzk0NjczQHFxLmNvbQ==
 Dong Li
Dong Li Zehong Li
Zehong Li