- Department of Clinical Laboratories Sciences, College of Applied Medical Sciences, Taif University, Taif, Saudi Arabia
Introduction: Rotavirus infection has been a major health burden among children under 5 years in Saudi Arabia. There is a lack of meta-analysis on epidemiology and genetic diversity of rotavirus in Saudi Arabia.
Methods: We conducted this study to provide a comparative overview of rotavirus infection in Saudi Arabia. We selected published literature between 1985 and 2025.
Results: Epidemiological data were retrieved from 18 articles. In Saudi Arabia, the pooled prevalence of RVA among children under 5 years was 34.3% (95% CI: 2% to 81%, I2 = 98.97%). Overall, G1P[8] (52%, 95% CI: 29% to 69%, I2 = 0%) was reported from the highest number of cases, followed by G2P[4] (18%, 95% CI: 9% to 31%, I2 = 36.86%), G9P[8] (14%, 95% CI: 8% to 27%, I2 = 45.37%), G12P[8] (4%, 95% CI: 2% to 11%, I2 = 0%), and G3P[8] (3%, 95% CI: 1% to 11%, I2 = 99%), respectively. Genotype G2P[4] (41%) became the most prevalent, while the frequency of G1P[8] reduced to 38% and G9P[8] to 6% during 2013–2024. The odds of rotavirus infection increased in the winter season (aOD 2.4, 95% CI: 1.52 to 3.57, p-value 0.005). The odds of rotavirus prevalence were significantly lower after vaccination (aOD 0.56, 95% CI: 0.25 to 0.73, p-value 0.001).
Discussion: This is one of the first meta-analyses to compare the genotypic data of rotaviruses before and after vaccination in Saudi Arabia. This study will provide an overall insight into prevalence, genetic diversity, and seasonality during pre-vaccination and post-vaccination periods and contribute to policy making.
Introduction
Rotavirus is the main (family Reoviridae) pathogen of gastroenteritis among children under 5 years worldwide (Aliabadi et al., 2019; Kotloff et al., 2017; Milivojevic and Milosavljevic, 2020). Every year, approximately 300,000 deaths associated with rotavirus infection are documented globally (Aliabadi et al., 2019). Rotavirus-associated deaths of under-five children have been partially reduced due to vaccination (Coşkun and Kasap, 2019; Hartman et al., 2019; Kotloff, 2017). The emergence of new genotypes and partial coverage of vaccines are still contributing to the high morbidity of rotavirus-associated gastroenteritis (Dennehy, 2015; Kheyami et al., 2006). Globally, rotavirus is one of the major causes of childhood morbidity and mortality. The health burden of rotavirus among children is also reflected in the WHO Eastern Mediterranean Region, with higher cases and mortality than other diarrheal pathogens. The estimated incidence of rotavirus is 7000 cases per 100,000 children under 5 years in Saudi Arabia. According to the WHO, severe and prolonged health conditions are reported from about 25%–30% of rotavirus cases (Kheyami et al., 2006; Troeger et al., 2018).
Rotavirus is a double-stranded (dsRNA, segmented), non-enveloped, RNA virus with an 18.55 kilobase pair genome (Estes and Cohen, 1989). The genome encodes six structural (VP1 to VP6) and six non-structural proteins (NSP1 to NSP6) (Estes and Cohen, 1989; Lefkowitz et al., 2018). Among ten species, rotavirus A is the major human pathogen. Rotaviruses are classified into different genotypes, including the G genotype based on glycoprotein (VP7) and the P genotype based on protease (VP4) (Estes and Cohen, 1989; Lefkowitz et al., 2018). Approximately 36 G-genotypes and 51 P-genotypes are circulating globally (Lefkowitz et al., 2018; Patton, 2012). Rotavirus genotypes G2P[4], G1P[8], G3P[8], G9P[8] and G4P[8] are the most widely documented worldwide. Among the rotavirus P-genotypes, P[4], P[6], and P[8] are the most prevalent globally (Al-Aidaroos et al., 2017; Ashwan et al., 2012; Kawai et al., 2012; Miles et al., 2012; Patton, 2012; Sharif et al., 2023). Rotavirus genotypes G1 to G4 were the most prevalent before 2000, and genotypes G9 and G12 became widespread after 2000 in Asia and the Middle East (Lefkowitz et al., 2018; Patton, 2012; Shaheen, 2019). The genotypic diversity in Saudi Arabia is also high for rotavirus infection, and it has changed over the recent years (Al-Aidaroos et al., 2017; Ashwan et al., 2012; Kawai et al., 2012; Miles et al., 2012; Page et al., 2021; Shaheen, 2019; Sharif et al., 2023).
Saudi Arabia is a temperate country with higher air temperatures and aridity. It occupies around 80% of the Arabian Peninsula and has a population of 34 million (Al-Aidaroos et al., 2017; Ashwan et al., 2012). Rotavirus-associated diarrhea is still a notable health issue in Saudi Arabia. Previous studies have reported the prevalence of rotavirus among diarrheal children between 8% to 65% in Saudi Arabia (Kawai et al., 2012; Patton, 2012; Shaheen, 2019; Sharif et al., 2023). Though the incidence has reduced in some regions, the overall prevalence of rotavirus is still high and underestimated.
The WHO prequalified and internationally accepted rotavirus A vaccines, including RotaTeq (bovine-human reassorted vaccine), and Rotarix (human rotavirus), are available in Saudi Arabia. Since 1 January 2013, rotavirus vaccines have been included in the national immunization program in Saudi Arabia (Kheyami et al., 2006; Troeger et al., 2018). Though rotavirus vaccination has been included in the national immunization program in Saudi Arabia for the last 13 years, meta-data analysis on the genotypic diversity of rotavirus is lacking. Knowing the changing epidemiology and genetic diversity of circulating rotavirus can contribute to the evaluation of the effectiveness of available vaccines. The main aim of this study is to determine the prevalence and genotypic diversity of rotavirus in Saudi Arabia.
Methods
Definitions
This study included data from the previously published epidemiological, virological, and clinical research on rotavirus in Saudi Arabia. The positive case was confirmed by the ELISA-based method before the invention of the RT-PCR laboratory-confirmed test. The prevalence of rotavirus was defined as the proportion of the study population with a rotavirus-positive test at a specific study. We defined the epidemiology of rotavirus as the distribution and determinants of the cases in study populations. The transmission was defined as the spread of the virus from the sources to the susceptible individuals. We followed the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement and Cochrane Collaboration to perform this study (Page et al., 2021).
Case definition
For defining a confirmed rotavirus case, we followed the case definition by the WHO and the final classification guideline. We included the data from the previously published paper confirming the presence of rotavirus by reverse transcriptase polymerase chain reaction (PCR)-based method or an enzyme immunoassay (EIA) method. The specific class of RT-PCR was also determined following the local guidelines and availability of the methods in a particular study population.
Study design
The study was conducted by adopting different strategies. At first, the search strategies and specific objectives were determined, followed by finding appropriate research articles, setting inclusion and exclusion criteria of manuscripts, data collection, quality assessment, screening, data analysis, inclusion of the findings, summarization, and exclusion of irrelevant data. The inclusion criteria consist of several factors, including original research articles, prevalence data, genotypic data, epidemiological data, case studies, outbreak investigation, surveillance work, hospital-based surveillance, case-control studies, and online databases. All of the previous studies on the selected topic missed the parameters for the quality assessment. Hence, the quality reports by the authors were the major source of data for this study.
Search strategy and selection criteria
The relevant data were collected from articles published in peer-reviewed journals. Data were retrieved from Web of Science, Scopus, MEDLINE (through PubMed), EMBASE, ASM journals, The New England Journal of Medicine (NEJM), Cell Press journals, Journals from Saudi Arabian Publishers, and The Lancet with no restriction on language. This study included data from only Saudi Arabia. All published peer-reviewed manuscripts till January 01, 2025, were included in this study.
The search terms included Saudi Arabia, Rotavirus, Human Rotavirus, Infection, Epidemiology, Coinfection, RVA, RVB, RVC, Symptoms, Seasonality, Genotypes, Vaccines, Children, Gastroenteritis, Rapid kit, RT-PCR, Diarrhea, 2000 to 2025, Clinical features, Signs and Symptoms, Cases, Clinical characteristics, Transmission, Prevention, Food-born illness, Water-borne illness, Molecular epidemiology, Genetic diversity, Infants, ELISA, WHO, CDC, Hospitals, Clinics, Morbidity, Mortality, Prevalence, Incidence, Age, Gender, Sex, Population, Acute gastroenteritis and several combination of these terms. Combined and separate searches were performed with these terms on every website, journal page, and database.
Additionally, the Google Scholar database and gray literature were also searched to get all the possible study links. The relevant data were searched in WHO (the World Health Organization), CDC (Centers for Disease Control and Prevention, USA), ProMed, ECDC (European Centers for Disease Control and Prevention), Epicenter, and local health surveillance websites in Saudi Arabia. This study also analyzed and screened data from the yearly update of health data. Preprint databases, namely bioRxiv, SSRN, medRxiv, and AAS Open Research, were searched for any relevant data. In addition, the first thirty pages on the Google Scholar search engine were searched for the search terms. This study integrated the epidemiologic data of rotavirus outbreaks by cumulating studies on incidence, case reports, prevalence over the years, genetic diversity, clinical history, seasonality, geographic distribution, and vaccination.
The author conducted the quality evaluation of selected studies. The repeated and partial data were removed after searching the data sources, journals, published articles, and websites. The modeling of outbreaks and prediction studies, review articles, editorials, correspondences, mini-reviews, book chapters, environmental factors studies, and non-relevant studies to the objectives were excluded. Further, the author performed a critical quality evaluation of the selected manuscripts, identification of duplicated articles, and removal of correspondence or comment on duplicated data multiple times. Articles containing the relevant data were included for all geographic locations in Saudi Arabia, all ethnicities, children under 18 years, all sexes, and clinical features. A full-text analysis of the selected articles was conducted.
The risk of bias in the selected manuscripts was measured using the JBI tool. This JBI tool used nine parameters to assess the bias of the selected studies (Munn et al., 2015). Each parameter was evaluated using the findings as not applicable, unclear, yes, or no. Further, the author also used the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) assessment tool where applicable (Zeng et al., 2015). The SYRCLE tool consists of 10 parameters for assessing various biases. The parameters included selection bias, reporting bias, detection bias, attrition bias, performance bias, and other biases. The bias for the selected parameter was measured by following the previously published scales using the possible results as yes, no, and unclear, for low, high, and unclear bias, respectively (Zeng et al., 2015).
The data on meteorological factors were collected from different servers and websites, including the official website of the Saudi Arabia Meteorological Department1, Weather Forecast Saudi Arabia2, AccuWeather3 meteoblue4, and WeatherOnline5. The sociodemographic factors were taken from the included studies.
Statistical analysis
Descriptive statistical analysis, including frequencies, percentages, mean, and standard deviation (SD), was calculated for selected data of rotavirus-positive cases. The pooled data were reported as proportions with 95% confidence intervals (CI). The heterogeneity of the data was assessed by the I2-test. Values <25% were recorded as mild, 25%–75% as intermediate, and >75% as significant heterogeneity. The overall pooled prevalence was determined using the DerSimonian-Laird method (Zhao, 2013). Forest plots were used to represent genotype-specific pooled prevalence. The pooled prevalence and genotypic variation were determined by using SAS version 9.4. Linear logistic regression analysis was conducted to find out the impact of environmental factors and sociodemographic factors on the odds of rotavirus infection.
Results
Included studies
This study found 7394 previous articles and 252 documents on the search topics in Saudi Arabia. After the first screening, only 156 articles were considered for analyzing the abstracts. From these 156 articles, 55 were excluded based on the exclusion criteria, and a full-text analysis was conducted on 101 articles. After that, 83 articles were excluded from these 101 articles, and 18 were selected for the systematic review (Afifi and Nabiha, 2013; Al-Ayed et al., 2017; Almalki et al., 2022; Alqurayn et al., 2024; Aly et al., 2015; Eifan et al., 2023; El Assouli et al., 1996; Ghazi et al., 2005; Hegazi et al., 2017; Khalil et al., 2015; Kheyami et al., 2008; Meqdam and Thwiny, 2007; Obeid, 2011; Paul et al., 2021; Qadri et al., 1990; Tayeb et al., 2008, 2011; Zaki et al., 2017; Zhao, 2013). Among the 18 selected articles, epidemiological data were taken from 18 articles, prevalence data from 18 articles, clinical symptoms from 10 articles, and genotyping from 7 studies (Figure 1). Finally, 15 studies were included in the meta-analysis, and the other 3 studies were excluded due to the lack of data for meta-analysis.
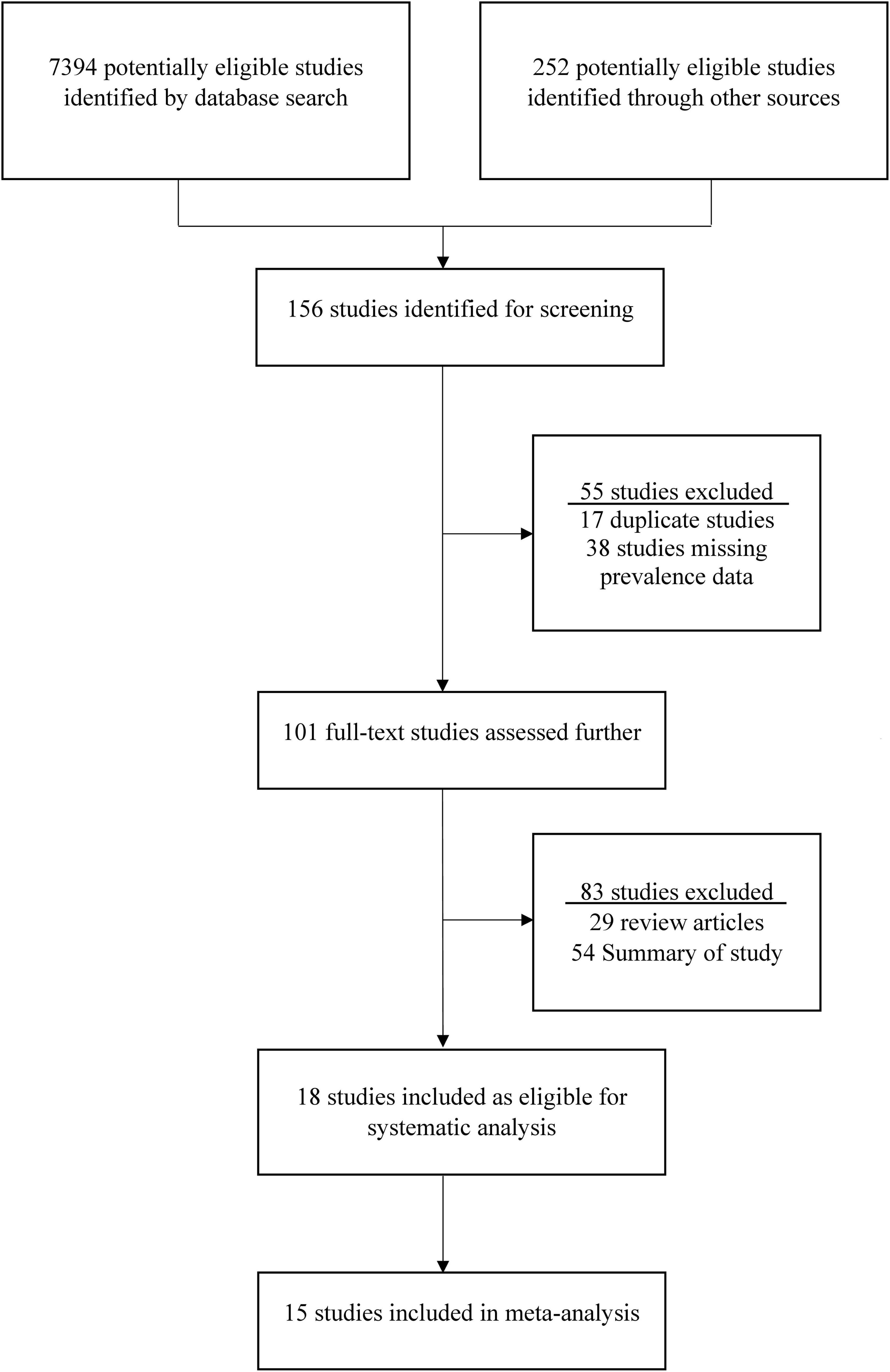
Figure 1. The procedure of selection and screening of studies on rotavirus in Saudi Arabia. The standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement was followed to identify and select the eligible studies.
Prevalence of rotavirus infection in Saudi Arabia
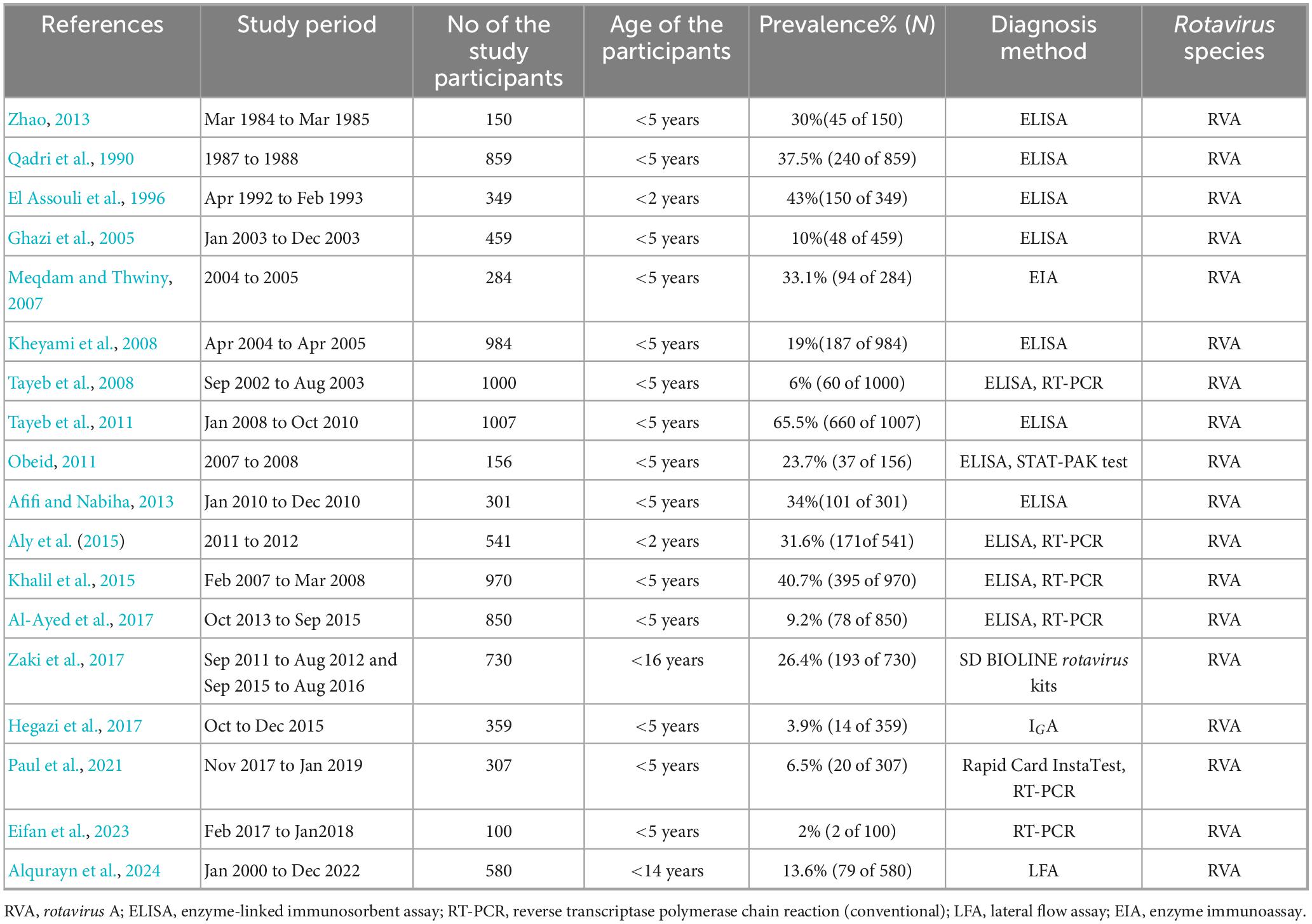
The prevalence of rotavirus A (RVA) in Saudi Arabia has been underreported during the last 40 years. This study found 18 original manuscripts reporting the prevalence of rotavirus from 1985 to 2025. In Saudi Arabia, the pooled prevalence of RVA among children under 5 years was 34.3% (95% CI: 2% to 81%, I2 = 98.97%). Among these 18 studies from 1985 to 2025, RVA was reported in 18 (100%) studies. These studies confirmed the presence of RVA by ELISA (100%) or RT-PCR methods. This study found 100% of the rotavirus cases from hospital surveillance in Saudi Arabia. The higher prevalence of RVA (40% of the gastroenteritis) was documented before 2013. After the start of vaccination in 2013, the prevalence of RVA dropped significantly (from 40 to 8%) among the children in the study regions. The majority of the cases were reported from children under 2 years (81%, 95% CI: 58% to 93% I2 = 65.98%). All of the cases (100%) were from rotavirus A (Table 1).
Distribution of rotavirus cases in Saudi Arabia
Rotavirus infection has been reported from the majority of areas in Saudi Arabia. The first report of confirmed cases was from Dammam during 1984–85, with a higher prevalence of 30% positive children. A higher prevalence of RVA (26%, 95% CI: 24% to 48%, I2 = 2.34%) was reported from Dammam (1987–88), Taif (1992–93), Makkah, Jeddah, and Jizan, as well as from Dammam (2002–10). The prevalence of RVA ranged from 15 to 24% from 2007 to 2013 in Riyadh and 15% in Qassim and Taif of Saudi Arabia. In Dammam and Makkah, the prevalence of RVA was 14% (95% CI: 12% to 24%, I2 = 0%) after the initiation of vaccination in 2013. In Riyadh, Jeddah, and Najran, the prevalence of RVA was reduced below 10% (95% CI: 1% to 9%, I2 = 0%) from 2014 to 2024 (Figure 2).
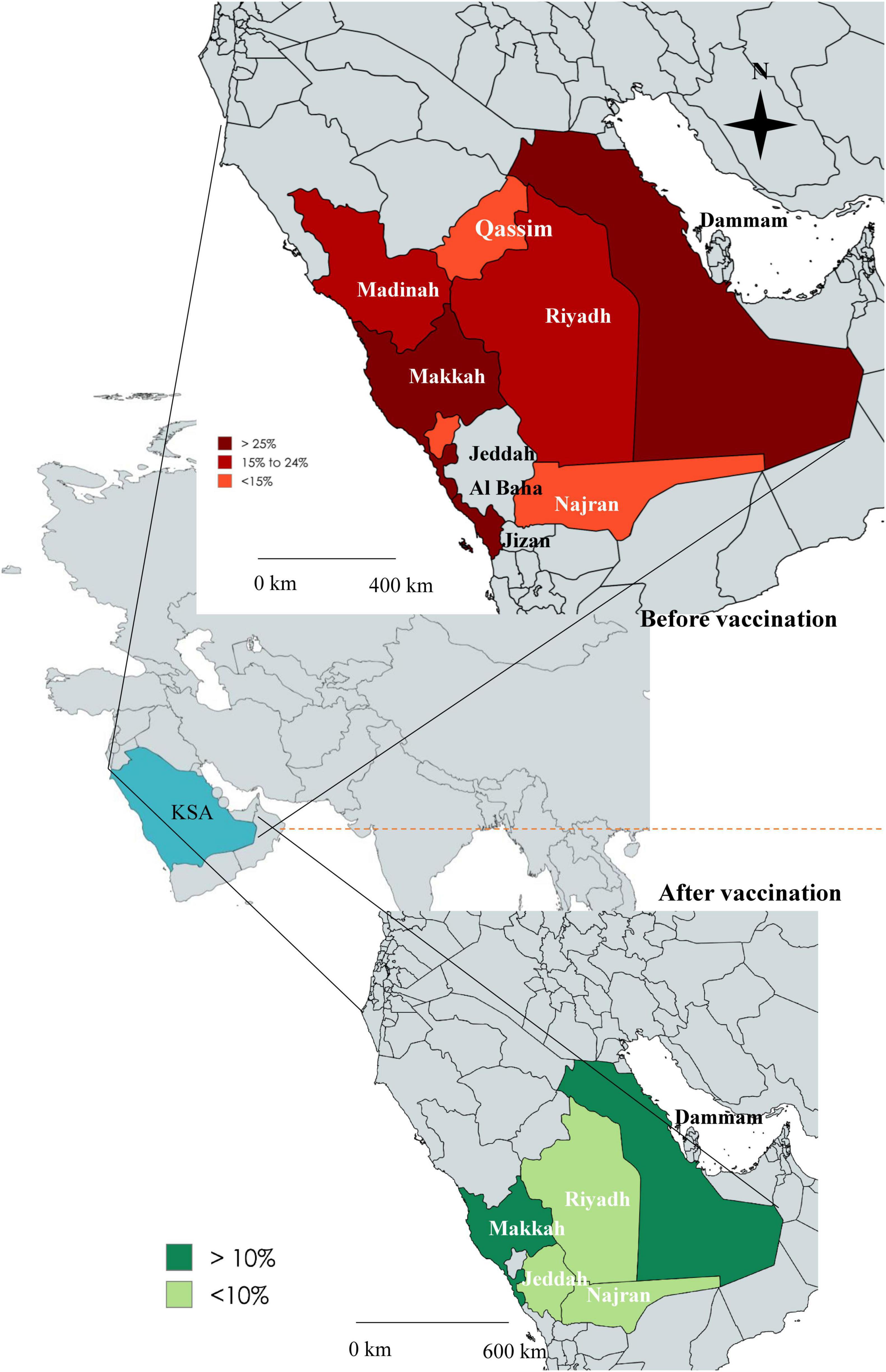
Figure 2. Map of Saudi Arabia showing the comparative prevalence of rotavirus in different regions before vaccination (upper panel) and after vaccination (lower panel).
Genetic diversity of rotavirus A in Saudi Arabia
The studies on the genetic diversity of RVA were less frequent in Saudi Arabia. We found 39% (7 of 18) studies reported the genotypic information. Rotavirus G-typing and P-typing were conducted for 583 isolates. Genotype G1P[8] (48%, 95% CI: 26% to 62%, I2 = 1.39%) was the most prevalent, followed by G2P[4] (21%, 95% CI: 13% to 39%, I2 = 0%), G9P[8] (9%, 95% CI: 2% to 14%, I2 = 76.87%), G3P[8] (3%), and G12P[8] (3%), respectively, during 1995–2004. Genotype G1P[8] (67%, 95% CI: 36% to 79%, I2 = 0%) remained the most prevalent and G9P[8] (24%, 95% CI: 15% to 31%, I2 = 8.93%) replaced G2P[4] (2%) during 2005–2012. Genotype G2P[4] (41%, 95% CI: 26% to 48%, I2 = 2.34%) became the most prevalent from 2013 to 2024, while the frequency of G1P[8] reduced to 38% (95% CI: 12% to 51%, I2 = 97.45%) and G9P[8] to 6% (Figure 3).
![Four-panel data visualization showing rotavirus genotype distribution over time. Panel A: Stacked bar and line chart display genotype changes from 1985-2024, with a legend indicating various genotypes. Panel B: Stacked bar chart for 1995-2024, showing percentages of different genotypes. Panel C: Pie chart details genotype proportions, with G1P[8] as the predominant type at 52%. Panel D: Stacked bar chart for years 1995-2024, showing percentages of P genotypes. Legends include specific genotype identifiers for clarity.](https://www.frontiersin.org/files/Articles/1671607/fmicb-16-1671607-HTML/image_m/fmicb-16-1671607-g003.jpg)
Figure 3. (A) Year-wise genotypic distribution of rotavirus in Saudi Arabia, (B) proportionate frequency of different genotypes of rotavirus in Saudi Arabia, (C) Year-wise prevalence of G-type, rotavirus and (D) Year-wise prevalence of P-type rotavirus. Before vaccination is represented by data from 1995 to 2012, and after vaccination is represented by data from 2013 to 2024.
Overall, G1P[8] (52%, 95% CI: 29% to 69%, I2 = 0%) was reported from the highest number of cases, followed by G2P[4] (17%, 95% CI: 7% to 27%, I2 = 36.86%), G9P[8] (13%, 95% CI: 8% to 25%, I2 = 45.37%), G12P[8] (3%, 95% CI: 2% to 6%, I2 = 0%), and G3P[8] (3%, 95% CI: 1% to 5%, I2 = 99%), respectively (Figure 4). About 4% of the isolates were non-typable, and 3% were mixed. Among the other reported genotypes, G1P[4], G1P[6], G2P[6], G2P[8], G4P[6], G4P[8], G9P[6], and G12P[6] were found in <2% frequency in Saudi Arabia (Figure 3).
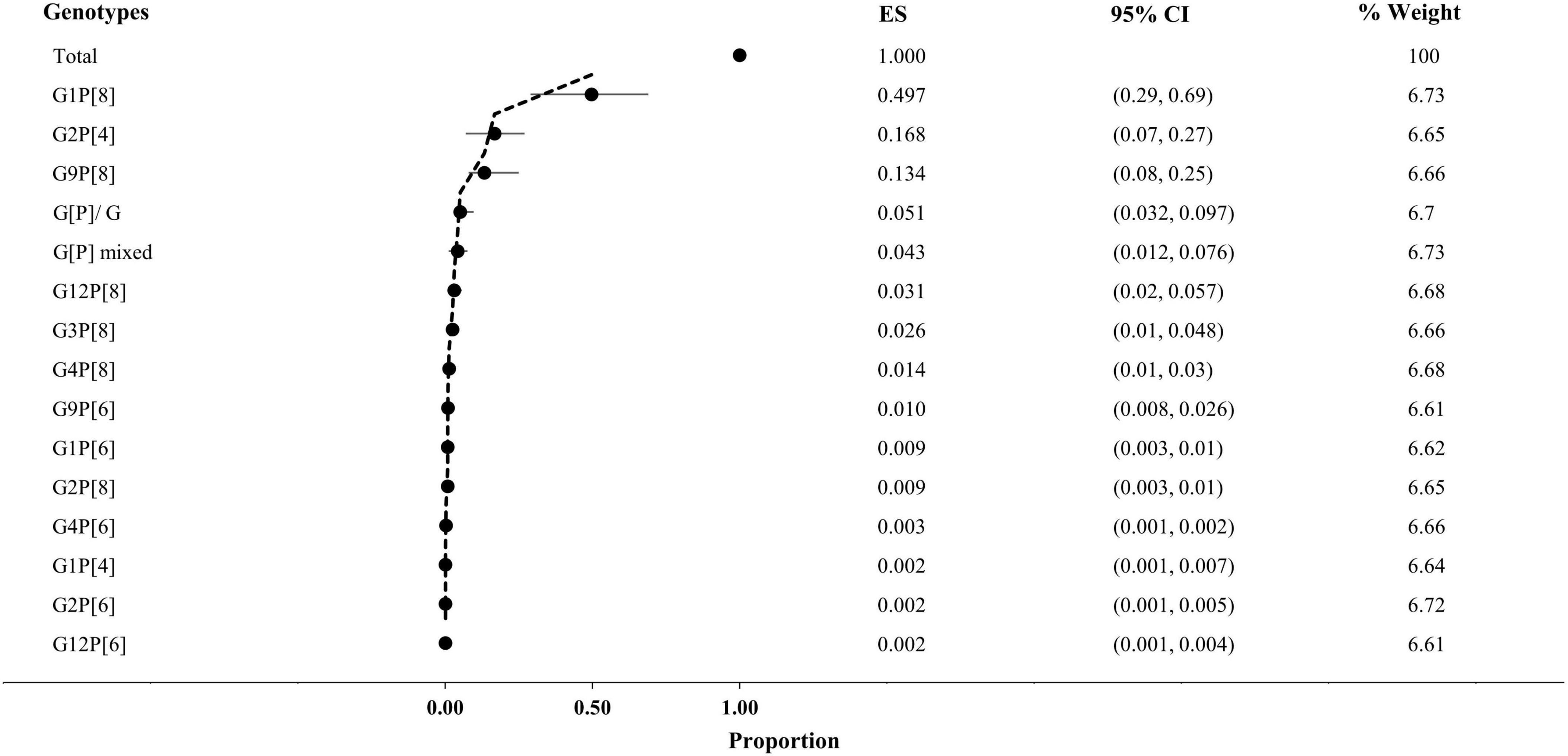
Figure 4. Prevalence of genotypes of rotavirus in Saudi Arabia. CI, confidence interval; ES, effect size (proportion of genotypes); Weight, inverse variance.
Among the 583 G-typed isolates, G1 (52%, 95% CI: 29% to 77%, I2 = 0%) was the most frequently reported, followed by G2 (19%, 95% CI: 8% to 32%, I2 = 32.67%) and G9 (11%, 95% CI: 4% to 25%, I2 = 98%), respectively, from Saudi Arabia. During 1995–2012, G1 (55%) was the most prevalent, followed by G9 (17%), and the prevalence of G2 reduced from 18% to 3% from 1995–2004 to 2005–12. After the start of vaccination in 2013, G2 (42%) became prevalent, followed by G1 (37%) and G9 (11%), respectively (Figure 3).
Among the 583 P-typed isolates, P[8] (78%, 95% CI: 36% to 89%, I2 = 0%) was the most frequently reported, followed by P[4] (17%, 95% CI: 11% to 25%, I2 = 26.21%) and P[6] (3%, 95% CI: 2% to 8%, I2 = 0%), respectively, from Saudi Arabia. During 1995–2012, P[8] (87%) was the most prevalent, followed by P[4] (10%). After the start of vaccination in 2013, the prevalence of P[4] (42%) increased significantly (p-value 0.005), and the prevalence of P[8] reduced from 87 to 55% (Figures 3, 4).
Different genotypes of rotavirus were widely distributed in several regions in Saudi Arabia. Genotype G1P[8] was reported from almost every region included in this study, with a higher prevalence than other genotypes. Genotype G2P[4] was reported in the children with diarrhea from Riyadh, Aseer, Jeddah, Dammam, Jizan, and Makkah.
Seasonality of rotavirus A in Saudi Arabia
The highest prevalence of rotavirus was reported during 2005–2012 (39%), followed by 1985–1994 (37%), 1995–2004 (17%), in the pre-vaccination era. However, after the vaccination program, the prevalence reduced significantly to 14% during 2013–2024 (Figure 5). The data from 2013 to 2024 indicated post-vaccination periods, and from 1985 to 2012 represented pre-vaccination periods. The data on rotavirus seasonality were extracted from 11 studies during 1985–2024 in Saudi Arabia (Figure 5). Rotavirus infection was reported all year round in Saudi Arabia. The odds of rotavirus infection increased in the winter season (aOD 2.4, 95% CI: 1.52 to 3.57, p-value 0.005) with an average temperature of 16 °C. We also analyzed the odds of the prevalence of rotavirus infection after vaccination. The odds of rotavirus prevalence were significantly lower after vaccination (aOD 0.56 95% CI: 0.25 to 0. 73, p-value 0.001). The highest peak of rotavirus infection (70%, 95% CI: 50%–78%, p-value 0.001) was reported from November to March in Saudi Arabia (Figure 5). The pattern of the seasonality of rotavirus was consistent in the majority (89%) of the studies.
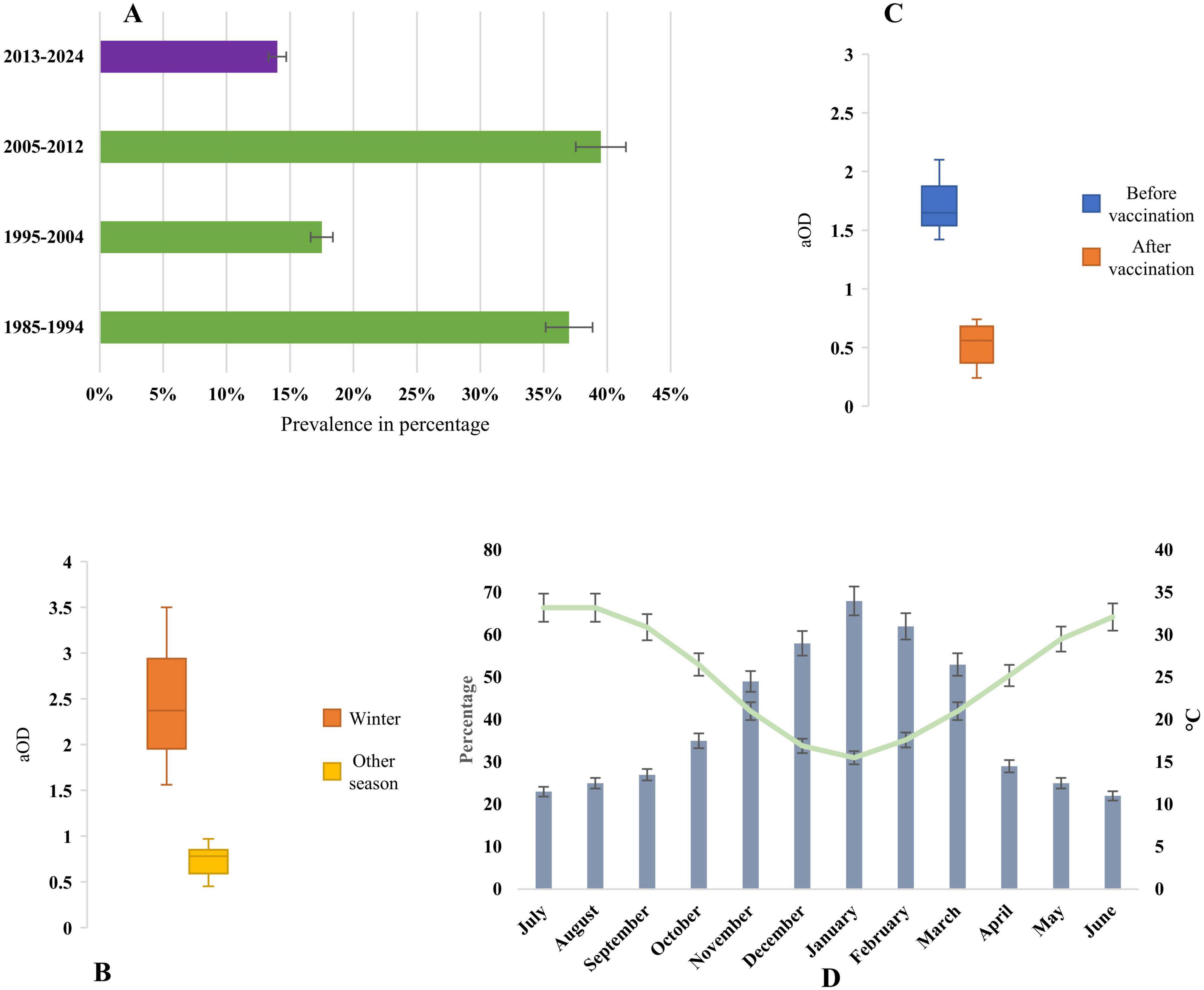
Figure 5. (A) Year-wise prevalence of rotavirus infection in Saudi Arabia, before vaccination is represented by data from 1995 to 2012, and after vaccination is represented by data from 2013 to 2024. (B) Adjusted odds ratio of seasonality of rotavirus, (C) Adjusted odds ratio of prevalence of rotavirus after and before vaccination, and (D) Seasonal prevalence of rotavirus and changes of average day temperature in Saudi Arabia.
Clinical characteristics of rotavirus infection
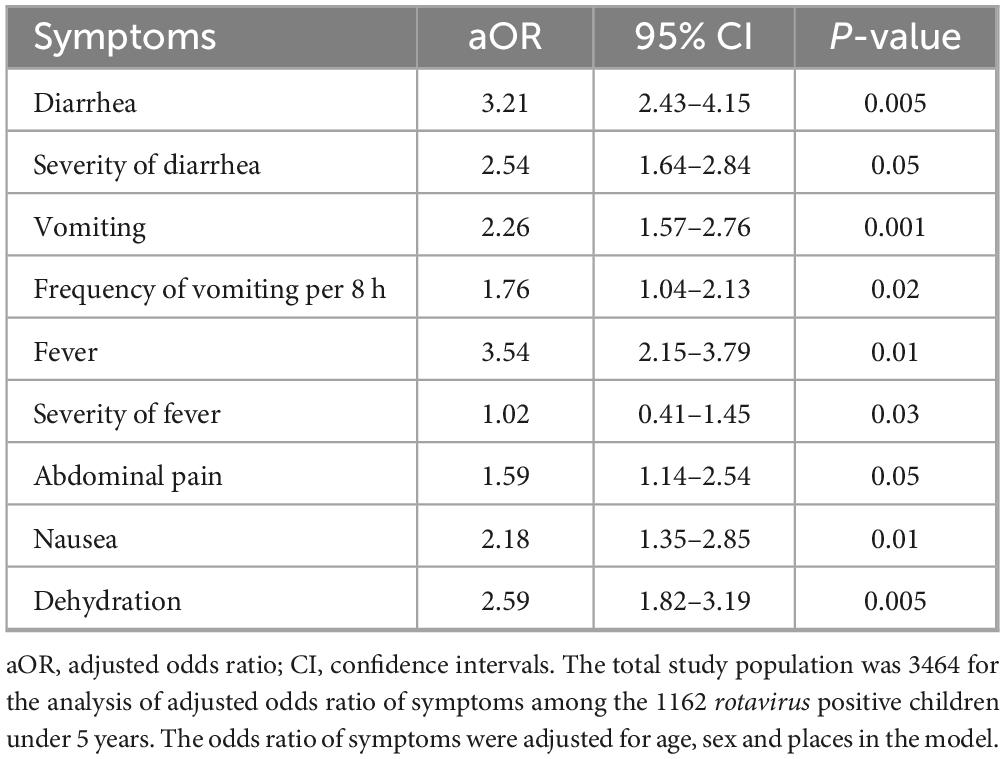
Data on the clinical presentations of rotavirus-infected children were retrieved from 10 studies. About 1162 rotavirus-positive children under 5 years showed different symptoms in Saudi Arabia. Diarrhea (86%, 95% CI: 65% to 96%, p-value 0.01) was the most common symptom followed by dehydration (79%, 95% CI: 45% to 86%, p-value 0.05), vomiting (74%, 95% CI 52% to 78%, p-value 0.03), fever (68%, 95% CI: 39% to 74%, p-value 0.01) and abdominal pain (37%, 95% CI: 33% to 64%, p-value 0.05), respectively. About 100% (10 of 10) of the studies reported diarrhea followed by dehydration (90%, 9 of 10), vomiting (90%, 9 of 10), and fever (80%, 8 of 10), respectively. Rotavirus-positive children had a higher risk of developing severe diarrhea (aOR: 2.54, 95% CI: 1.64 to 2.84, p-value 0.05), dehydration (aOR: 2.59, 95% CI: 1.82 to 3.19, p- value 0.005), and severe vomiting (aOR: 1.76, 95% CI: 1.04 to 2.13, p-value 0.02) (Table 2). We found that children aged below 2 years (aOR: 3.36, 95% CI: 2.59 to 3.76, p-value 0.05) had significantly higher odds of rotavirus infection, and the sex of the children was not associated with the incidence of rotavirus in Saudi Arabia.
Discussion
Rotavirus infection was one of the major causes of child morbidity in Saudi Arabia (Aleid et al., 2024; Almalki et al., 2022; Alqurayn et al., 2024; Eifan et al., 2023; Paul et al., 2021; Troeger et al., 2018). Rotavirus vaccination has been included in the national immunization program since 2013, and Saudi Arabia has one of the highest vaccine coverages for rotavirus (97%) globally (Troeger et al., 2018). However, studies on the genetic diversity, epidemiology, seasonality, and clinical characteristics of rotavirus infection are lacking in Saudi Arabia. We conducted this study to provide a comparative insight into rotavirus infection before and after vaccination in Saudi Arabia. First, this is one of the early studies of meta-analysis of rotavirus before and after vaccination in Saudi Arabia. The pooled prevalence of rotavirus infection was 34.3% (95% CI: 2% to 81%, p-value 0.001). However, we found high heterogeneity in the prevalence data across different studies. There might be several reasons, including some of the studies reporting pre-vaccination data, while others are during and after vaccination data, and regional and seasonal factors affecting the prevalence of rotavirus in Saudi Arabia. We found a reduction of rotavirus prevalence in Saudi Arabia before and after vaccination. This reduction might not only solely represent the impact of vaccination, but also other factors like improved sanitation, early diagnosis, proper water treatment, increased awareness and study reporting strategies might have contributed. The finding is similar to previous original studies in Saudi Arabia and other nearby countries in the Middle Eastern regions reporting a prevalence near 40% and in European children about 43% before vaccination (Arakaki et al., 2021; Damtie et al., 2020; Dey et al., 2020; Giri et al., 2018; Grimprel et al., 2008; Gupta et al., 2019; Güzel et al., 2020; Jagai et al., 2012; Jeong and Kim, 2014; Jesudason et al., 2024; Leshem et al., 2014; Monavari et al., 2017; Ouermi et al., 2017; Sadiq et al., 2019; Sharif et al., 2020a; Tcheremenskaia et al., 2007; Yu et al., 2019; Zhou et al., 2016). However, compared to other meta-analyses and surveillance studies conducted in LMICs like Bangladesh, Turkey, and China, we found a slightly higher prevalence of rotavirus among children under 5 years in Saudi Arabia (Arakaki et al., 2021; Damtie et al., 2020; Dey et al., 2020; Giri et al., 2018; Gupta et al., 2019; Güzel et al., 2020; Jeong and Kim, 2014; Jesudason et al., 2024; Leshem et al., 2014; Monavari et al., 2017; Sharif et al., 2020a; Yu et al., 2019; Zhou et al., 2016). We also found that the prevalence of rotavirus was higher than 10% in diarrheal children in Dammam and Makkah regions after vaccination. These data call for continuous monitoring of the sources of transmission of rotavirus in Saudi Arabia. Though the rotavirus vaccination program in Saudi Arabia is considered successful in reducing the burden of rotavirus diarrhea, reports of lower efficacy of rotavirus vaccines in developing countries and the emergence of non-included genotypes due to vaccine pressure can be future challenges. Second, we found the studies were concentrated on some fixed hospitals in selected regions of Dammam, Riyadh, Makkah, Jeddah, Jizan, Madinah, and Qassim. However, the lack of real-world community-based surveillance data is absent in Saudi Arabia. Among these, hospital-based surveillance, Dammam and Makkah were found to be heavily burdened with rotavirus compared to other regions in Saudi Arabia. We found lower heterogeneity of prevalence of rotavirus in Dammam and Makkah, Riyadh, Jeddah, and Najran after vaccination compared to the overall pooled prevalence. This might be due to the reduction of prevalence, reporting bias and lack of community-based studies. The probable disproportionate prevalence of rotavirus needs further investigation of the drinking water and food, and hygiene habits of the residents of these areas. In previous studies in India, Bangladesh, and China, it was found that contaminated water and foods serve as the main sources of rotavirus transmission and a higher burden of the disease (Dey et al., 2020; Giri et al., 2018; Jesudason et al., 2024; Ouermi et al., 2017; Sadiq et al., 2019; Tcheremenskaia et al., 2007). Third, we found a higher prevalence of co-circulating pathogens of diarrhea among children in Saudi Arabia. About 68% of the studies reported the presence of adenovirus, norovirus, pathogenic E. coli, Salmonella spp, and Shigella spp. Additionally, circulation of less-reported genotypes along with co-infection might pose a higher risk of diarrheal disease burden in the future. Compared to previous studies in developing and developed countries, we also found a similar prevalence of non-rotavirus diarrheal pathogens among the children (Dey et al., 2020, 2021; Giri et al., 2018; Jesudason et al., 2024; Ouermi et al., 2017; Sadiq et al., 2019; Sharif et al., 2020a,b; Tcheremenskaia et al., 2007). However, surveillance and epidemiologic studies are limited in Saudi Arabia compared to other developed and developing countries, which should be increased to reflect the pathogenic diversity after rotavirus vaccination.
Fourth, we found that all of the rotavirus cases in Saudi Arabia were associated with rotavirus A (100%). We also found a great diversity of genotypes among the circulating isolates. The vaccination event had significant effects in changing the frequency of circulating genotypes in Saudi Arabia. Overall, G1P[8], G2P[4], and G9P[8] showed higher prevalence compared to other genotypes. However, after the start of the vaccination program in 2013, the frequency of G1P[8] reduced, and G2P[4] increased in Saudi Arabia. Compared to previous studies, we found that G1P[8] was prevalent in Jordan and the United Arab Emirates, G2P[4] in Oman and Yemen, G2P[6] in Iraq, and G4P[8] in Lebanon before 2010. After the start of vaccination, G1P[8] and G9P[8] were the two major genotypes in the nearby countries, including Yemen, Lebanon, and Egypt (Jesudason et al., 2024). Based on the findings of the nearby countries, our findings also indicated that vaccine-driven selective pressure was one of the major contributors to genotypic shift in Saudi Arabia (Jesudason et al., 2024). Though the findings are similar to previous reports in the Middle East and other countries in South Asia, the prevalence of genotype G9P[8] was found to be significantly lower than in other countries (Arakaki et al., 2021; Giri et al., 2018; Gupta et al., 2019; Güzel et al., 2020; Monavari et al., 2017; Ouermi et al., 2017; Tcheremenskaia et al., 2007; Yu et al., 2019; Zhou et al., 2016). Among the other genotypes, G4P[8], G3P[8], and G12P[8] were found in consistent frequency before and after the vaccination program. The vaccine coverage in the majority of the regions is higher in Saudi Arabia. However, the genotypes G2P[4], G3P[8], G4P[8], and G12P[8] are circulating in relatively higher frequency than other G1 genotypes. This study found higher heterogeneity in the relative frequency of some of the genotypes across different studies. The probable underlying reasons might be the lack of continuous genotyping studies from similar regions, the lack of community-surveillance data, and the lack of a strong national surveillance system. The surveillance study in the northern regions is also lacking. The impact of vaccination and coverage frequency on the circulating genotypes is not well-studied in Saudi Arabia. This study suggests that due to the vaccination by Rotarix in the reported regions, the shifts in genotypes have occurred. These findings strongly resonate with previous findings in developed and developing countries (Arakaki et al., 2021; Damtie et al., 2020; Giri et al., 2018; Grimprel et al., 2008; Gupta et al., 2019; Güzel et al., 2020; Jagai et al., 2012; Jesudason et al., 2024; Leshem et al., 2014; Monavari et al., 2017; Ouermi et al., 2017; Sadiq et al., 2019; Tcheremenskaia et al., 2007; Yu et al., 2019; Zhou et al., 2016). Additionally, we found a higher prevalence of G1, G2, and G9 genotypes and among the P-types, P[8] and P[4] genotypes in Saudi Arabia. The incidence of G2 and G9 increased after vaccination, while the incidence of G1 reduced, which needs further investigation to elucidate the impact of vaccination on genotype distribution.
Fifth, the seasonality of rotavirus infection is well-studied in different regions of the world. However, the seasonality is not well explored in hot and arid regions. We found that rotavirus cases spiked during the winter season, with lower average daily temperatures in Saudi Arabia. This finding is strongly similar to the previous findings globally (Jagai et al., 2012; Sharif et al., 2023). Additionally, we found rotavirus infection had higher odds during the winter in Saudi Arabia. However, some of the included works mentioned higher incidences during June to September with higher day temperatures. These data call for further investigation of the seasonal effects of rotavirus transmission in Saudi Arabia.
We found that rotavirus-positive children suffered from different clinical presentations (Afifi and Nabiha, 2013; Al-Ayed et al., 2017; Almalki et al., 2022; Alqurayn et al., 2024; Aly et al., 2015; Eifan et al., 2023; El Assouli et al., 1996; Ghazi et al., 2005; Hegazi et al., 2017; Khalil et al., 2015; Kheyami et al., 2008; Meqdam and Thwiny, 2007; Obeid, 2011; Paul et al., 2021; Qadri et al., 1990; Tayeb et al., 2008; Tayeb et al., 2011; Zaki et al., 2017). Rotavirus-positive children had significantly higher odds of severe diarrhea, vomiting, and fever in Saudi Arabia. These findings are similar to previous studies in Saudi Arabia and other developing countries (Arakaki et al., 2021; Grimprel et al., 2008; Gupta et al., 2019; Güzel et al., 2020; Jagai et al., 2012; Leshem et al., 2014; Monavari et al., 2017; Ouermi et al., 2017; Sharif et al., 2023; Tcheremenskaia et al., 2007; Yu et al., 2019; Zhou et al., 2016). Among other factors, children aged <2 years had significantly higher odds of rotavirus infection with severe health outcomes. There might be several reasons, including children aged >2 years already having antibodies against rotavirus due to previous infection and stronger immune systems compared to children aged <2 years. Further, the odds of prevalence of rotavirus infection reduced significantly after implementation of vaccination, which strongly resonates with previous studies in developed countries.
There are a number of limitations in this study that can be addressed in the future. Firstly, we could not add any rotavirus vaccination data due to the unavailable of studies. The findings are reported from hospital-based surveillance that may not truly represent the overall community prevalence. To get a more accurate insight into the epidemiology and genetic diversity of rotaviruses, community-based surveillance and hospital surveillance should be combined in the future. Further, we found genotypic data only from seven studies that might underscore the actual diversity in Saudi Arabia. More inclusive studies involving a higher number of samples from community-based surveillance should be conducted to reflect the circulating genotypes in the future. Moreover, phylogenetic and mutational analysis of the isolated genotypes could not be conducted in this study. In addition, the diagnostic methods reported in different papers were either ELISA or RT-PCR. However, we found minimal heterogeneity in regarding the used diagnostic methods. It should be considered with more strict approach in the future studies. In the future, more data from all over Saudi Arabia should be included to create a complete scenario of rotavirus-associated health burden.
Conclusion
This is one of the first meta-analyses including the majority of the studies from 1985 to 2025 in Saudi Arabia. This study will provide an integrated insight into rotavirus epidemiology in Saudi Arabia. We found a higher prevalence of rotavirus before vaccination, and after the start of vaccination, it decreased significantly from 35% to about 2% in some of the regions in Saudi Arabia. However, in some of the regions, the prevalence is still higher than 10%. We also found genotypes G2P[4], and G9P[8] became prevalent over G1P[8] after vaccination. This study calls for more epidemiologic and genotypic analysis of rotavirus, expanding to all of regions in Saudi Arabia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KA: Project administration, Data curation, Conceptualization, Validation, Supervision, Methodology, Writing – review & editing, Resources, Investigation, Writing – original draft, Software, Formal analysis, Visualization, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We extend their appreciation to Khalaf F. Alsharif and Abdulrahman Theyab for their contributions to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Afifi, R., and Nabiha, M. (2013). The burden of Rotavirus gastroenteritis among hospitalized pediatric patients in a tertiary referral hospital in Jeddah. Ann. Saudi Med. 33, 241–246. doi: 10.5144/0256-4947.2013.241
Al-Aidaroos, A., Standaert, B., Meszaros, K., and Shibl, A. (2017). Economic assessment of rotavirus vaccination in Saudi Arabia. J. Infect. Public Health 10, 564–571. doi: 10.1016/j.jiph.2016.11.006
Al-Ayed, M., Asaad, A., Qureshi, M., and Hawan, A. (2017). Epidemiology of group A rotavirus infection after the introduction of monovalent vaccine in the National immunization program of Saudi Arabia. J. Med. Virol. 89, 429–434. doi: 10.1002/jmv.24664
Aleid, M., Aljardan, S., Alqahtani, S., Omar, E., Alqahtani, M., Alzahrani, A., et al. (2024). Epidemiological patterns and clinical consequences of rotavirus gastroenteritis amongst children in Saudi Arabia: A cross-sectional study. J. Adv. Trends Med. Res. 1, 322–328. doi: 10.4103/ATMR.ATMR_69_24
Aliabadi, N., Antoni, S., Mwenda, J., Weldegebriel, G., Biey, J., Cheikh, D., et al. (2019). Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: Findings from the Global rotavirus surveillance network. Lancet Glob. Health 7, e893–e903. doi: 10.1016/S2214-109X(19)30207-4
Almalki, S., Ahmed, W., Elhaj, Y., Akhter, N., Alharbi, R., Alyahyawi, H., et al. (2022). Demographical and clinical features of suspected cases of rotavirus gastroenteritis at Al-Baha region, Saudi Arabia, Saudi. J. Health Sci. 11, 43–47. doi: 10.4103/sjhs.sjhs_38_22
Alqurayn, A., Obeid, O., and Alkharsah, K. (2024). Rotavirus and adenovirus in children evaluated for viral gastroenteritis at a single healthcare center in the Eastern Province of Saudi Arabia: A perspective of two decades. J. Family Community Med. 31, 133–139. doi: 10.4103/jfcm.jfcm_273_23
Aly, M., Al Khairy, A., Al Johani, S., and Balkhy, H. (2015). Unusual rotavirus genotypes among children with acute diarrhea in Saudi Arabia. BMC Infect. Dis. 15:192. doi: 10.1186/s12879-015-0923-y
Arakaki, L., Tollefson, D., Kharono, B., and Drain, P. (2021). Prevalence of rotavirus among older children and adults with diarrhea: A systematic review and meta-analysis. Vaccine 39, 4577–4590. doi: 10.1016/j.vaccine.2021.06.073
Ashwan, M., Salam, A., and Mouselhy, M. (2012). Population growth, structure and distribution in Saudi Arabia. Humanit. Soc. Sci. Rev. 1, 33–46.
Coşkun, U. S. Ş, and Kasap, T. (2019). Frequency of rotavirus and adenovirus in pediatric patients with acute gastroenteritis. J. Contemp. Med. 9, 85–88. doi: 10.16899/gopctd.459823
Damtie, D., Melku, M., Tessema, B., and Vlasova, A. (2020). Prevalence and genetic diversity of rotaviruses among under-five children in Ethiopia: A systematic review and meta-analysis. Viruses 12:62. doi: 10.3390/v12010062
Dennehy, P. (2015). Rotavirus infection: A disease of the past? Infect. Dis. Clin. North Am. 29, 617–635. doi: 10.1016/j.idc.2015.07.002
Dey, S., Sharif, N., Billah, B., Siddique, T., Islam, T., Parvez, A., et al. (2021). Molecular epidemiology and genetic diversity of norovirus infection in children with acute gastroenteritis in Bangladesh, 2014-2019. J. Med. Virol. 93, 3564–3571. doi: 10.1002/jmv.26772
Dey, S., Sharif, N., Sarkar, O., Sarkar, M., Talukder, A., Phan, T., et al. (2020). Molecular epidemiology and surveillance of circulating rotavirus among children with gastroenteritis in Bangladesh during 2014-2019. PLoS One 15:e0242813. doi: 10.1371/journal.pone.0242813
Eifan, S., Nour, I., Hanif, A., Alhetheel, A., and Al-Ashkar, I. (2023). Molecular epidemiology and surveillance of human adenovirus and rotavirus A associated gastroenteritis in Riyadh, Saudi Arabia. Trop. Med. Infect. Dis. 8:279. doi: 10.3390/tropicalmed8050279
El Assouli, S., Banjar, Z., Mohammed, K., Milaat, W., and El Assouli, M. Z. (1996). Genetic and antigenic analysis of human rotavirus prevalent in Al-Taif, Saudi Arabia. J. Trop. Pediatr. 42, 211–219. doi: 10.1093/tropej/42.4.211
Estes, M., and Cohen, J. (1989). Rotavirus gene structure and function. Microbiol. Rev. 53, 410–449. doi: 10.1128/mr.53.4.410-449.1989
Ghazi, H., Khan, M., Telmesani, A., Idress, B., and Mahomed, M. (2005). Rotavirus infection in infants and young children in Makkah, Saudi Arabia. J. Pak. Med. Assoc. 55, 231–234.
Giri, S., Priya Hemavathy, R., Arumugam, R., Sherchand, J., Thu, H., Galagoda, G., et al. (2018). Molecular epidemiology of rotaviruses in the south-east Asian region from 2009 to 2015. Vaccine 36, 7851–7855. doi: 10.1016/j.vaccine.2018.02.092
Grimprel, E., Rodrigo, C., and Desselberger, U. (2008). Rotavirus disease: Impact of coinfections. Pediatr. Infect. Dis. J. 27, S3–S10. doi: 10.1097/INF.0b013e31815eedfa
Gupta, S., Chaudhary, S., Bubber, P., and Ray, P. (2019). Epidemiology and genetic diversity of group A rotavirus in acute diarrhea patients in pre-vaccination era in Himachal Pradesh, India. Vaccine 37, 5350–5356. doi: 10.1016/j.vaccine.2019.07.037
Güzel, M., Akpınar, O., and Kılıç, M. (2020). Prevalence of rotavirus-associated acute gastroenteritis cases in early childhood in Turkey: Meta-analysis. Children 7:159. doi: 10.3390/children7100159
Hartman, S., Brown, E., Loomis, E., and Russell, H. (2019). Gastroenteritis in Children. Am. Fam. Physician 99, 159–165. Available online at: https://www.aafp.org/afp/2019/0201/p159-s1.html
Hegazi, M., Sayed, M., Sindi, H., Bekhit, O., El-Deek, B., Alshoudri, F., et al. (2017). Is rotavirus still a major cause for diarrheal illness in hospitalized pediatric patients after rotavirus vaccine introduction in the Saudi national immunization program? Medicine 96:e6574. doi: 10.1097/MD.0000000000006574
Jagai, J., Sarkar, R., Castronovo, D., Kattula, D., McEntee, J., Ward, H., et al. (2012). Seasonality of rotavirus in South Asia: A meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One 7:e38168. doi: 10.1371/journal.pone.0038168
Jeong, S., and Kim, W. (2014). A systematic review of genetic diversity of human rotavirus circulating in South Korea. Infect. Genet. Evol. 28, 462–469. doi: 10.1016/j.meegid.2014.08.020
Jesudason, T., Sharomi, O., Fleetwood, K., Cheuk, A., Bermudez, M., Schirrmacher, H., et al. (2024). Systematic literature review and meta-analysis on the prevalence of rotavirus genotypes in Europe and the Middle East in the post-licensure period. Hum. Vaccin. Immunother. 20:2389606. doi: 10.1080/21645515.2024.2389606
Kawai, K., O’Brien, M., Goveia, M., Mast, T., and El Khoury, A. (2012). Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: A systematic review. Vaccine 30, 1244–1254. doi: 10.1016/j.vaccine.2011.12.092
Khalil, M., Azhar, E., Kao, M., Al-Kaiedi, N., Alhani, H., Al Olayan, I., et al. (2015). Gastroenteritis attributable to rotavirus in hospitalized Saudi Arabian children in the period 2007-2008. Clin. Epidemiol. 7, 129–137. doi: 10.2147/CLEP.S69502
Kheyami, A., Cunliffe, N., and Hart, C. (2006). Rotavirus infection in Saudi Arabia. Ann. Saudi Med. 26, 184–191. doi: 10.5144/0256-4947.2006.184
Kheyami, A., Nakagomi, T., Nakagomi, O., Dove, W., Hart, C., and Cunliffe, N. (2008). Molecular epidemiology of rotavirus diarrhea among children in Saudi Arabia: First detection of G9 and G12 strains. J. Clin. Microbiol. 46, 1185–1191. doi: 10.1128/JCM.02244-07
Kotloff, K. (2017). The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. North Am. 64, 799–814. doi: 10.1016/j.pcl.2017.03.006
Kotloff, K., Platts-Mills, J., Nasrin, D., Roose, A., Blackwelder, W., and Levine, M. (2017). Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 35, 6783–6789. doi: 10.1016/j.vaccine.2017.07.036
Lefkowitz, E., Dempsey, D., Hendrickson, R., Orton, R., Siddell, S., and Smith, D. (2018). Virus taxonomy: The database of the international committee on taxonomy of viruses (ICTV). Nucleic Acids Res. 46, D708–D717. doi: 10.1093/nar/gkx932
Leshem, E., Lopman, B., Glass, R., Gentsch, J., Bányai, K., Parashar, U., et al. (2014). Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: A systematic review and meta-analysis. Lancet Infect. Dis. 14, 847–856. doi: 10.1016/S1473-3099(14)70832-1
Meqdam, M., and Thwiny, I. (2007). Prevalence of group a rotavirus, enteric adenovirus, norovirus and astrovirus infections among children with acute gastroenteritis in Al-Qassim, Saudi Arabia. Pak. J. Med. Sci. 23:551.
Miles, M., Lewis, K., Kang, G., Parashar, U., and Steele, A. D. (2012). A systematic review of rotavirus strain diversity in India, Bangladesh, and Pakistan. Vaccine 30, A131–A139. doi: 10.1016/j.vaccine.2011.10.002
Milivojevic, V., and Milosavljevic, T. (2020). Burden of gastroduodenal diseases from the global perspective. Curr Treat Options Gastroenterol. 18, 148–157. doi: 10.1007/s11938-020-00277-z
Monavari, S., Hadifar, S., Mostafaei, S., Miri, A., Keshavarz, M., Babaei, F., et al. (2017). Epidemiology of rotavirus in the Iranian children: A systematic review and meta-analysis. J. Glob. Infect. Dis. 9, 66–72. doi: 10.4103/0974-777X.205173
Munn, Z., Moola, S., Lisy, K., Riitano, D., and Tufanaru, C. (2015). Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 13, 147–153. doi: 10.1097/XEB.0000000000000054
Obeid, O. (2011). Characterization of human rotavirus subgroups and serotypes in children under five with acute gastroenteritis in a Saudi Hospital. J. Family Community Med. 18, 22–25. doi: 10.4103/1319-1683.78634
Ouermi, D., Soubeiga, D., Nadembega, W., Sawadogo, P., Zohoncon, T., Obiri-Yeboah, D., et al. (2017). Molecular epidemiology of rotavirus in children under Five in Africa (2006-2016): A systematic review. Pak. J. Biol. Sci. 20, 59–69. doi: 10.3923/pjbs.2017.59.69
Page, M., McKenzie, J., Bossuyt, P., Boutron, I., Hoffmann, T., Mulrow, C., et al. (2021). Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 134, 103–112. doi: 10.1016/j.jclinepi.2021.02.003
Patton, J. (2012). Rotavirus diversity and evolution in the post-vaccine world. Discov. Med. 13, 85–97.
Paul, E., Alzaydani, I., Al-Hakami, A., Hawan, A., Shati, A., Abdelrahim, I., et al. (2021). Identification and genotypic characterization of Rota-virus from children post vaccination in Aseer region of Southwestern Saudi Arabia “An ephemeral experience. Revi. Argentina Clín. Psicol. 30:368. doi: 10.24205/03276716.2020.2034
Qadri, M., Al-Ghamdi, M., and Imadulhaq, M. (1990). Acute diarrheal disease in children under five years of age in the Eastern province of Saudi Arabia. Ann. Saudi Med. 10, 280–284. doi: 10.5144/0256-4947.1990.280
Sadiq, A., Bostan, N., Bokhari, H., Matthijnssens, J., Yinda, K., Raza, S., et al. (2019). Molecular characterization of human group A rotavirus genotypes circulating in Rawalpindi, Islamabad, Pakistan during 2015-2016. PLoS One 14:e0220387. doi: 10.1371/journal.pone.0220387
Shaheen, M. (2019). Rotavirus gastroenteritis among hospitalized children under 5 years of age in the Eastern mediterranean region: A review. East Mediterr Health J. 25, 422–430. doi: 10.26719/emhj.18.054
Sharif, N., Nobel, N., Sakib, N., Liza, S., Khan, S., Billah, B., et al. (2020a). Molecular and epidemiologic analysis of diarrheal pathogens in children with acute gastroenteritis in Bangladesh during 2014-2019. Pediatr. Infect. Dis. J. 39, 580–585. doi: 10.1097/INF.0000000000002637
Sharif, N., Parvez, A., Haque, A., Talukder, A., Ushijima, H., and Dey, S. (2020b). Molecular and epidemiological trends of human bocavirus and adenovirus in children with acute gastroenteritis in Bangladesh during 2015 to 2019. J. Med. Virol. 92, 3194–3201. doi: 10.1002/jmv.25812
Sharif, N., Sharif, N., Khan, A., Azpíroz, I., Diaz, R., Díez, I., et al. (2023). Prevalence and genetic diversity of rotavirus in Bangladesh during pre-vaccination period, 1973-2023: A meta-analysis. Front. Immunol. 14:1289032. doi: 10.3389/fimmu.2023.1289032
Tayeb, H., Balkhy, H., Aljuhani, S., Elbanyan, E., Alalola, S., and Alshaalan, M. (2011). Increased prevalence of rotavirus among children associated gastroenteritis in Riyadh Saudi Arabia. Virol. J. 8, 1–5. doi: 10.1186/1743-422X-8-548
Tayeb, H., Dela Cruz, D., Al-Qahtani, A., Al-Ahdal, M., and Carter, M. (2008). Enteric viruses in pediatric diarrhea in Saudi Arabia. J. Med. Virol. 80, 1919–1929. doi: 10.1002/jmv.21291
Tcheremenskaia, O., Marucci, G., De Petris, S., Ruggeri, F., Dovecar, D., Sternak, S., et al. (2007). Molecular epidemiology of rotavirus in Central and Southeastern Europe. J. Clin. Microbiol. 45, 2197–2204. doi: 10.1128/JCM.00484-07
Troeger, C., Khalil, I., Rao, P., Cao, S., Blacker, B., Ahmed, T., et al. (2018). Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 172, 958–965. doi: 10.1001/jamapediatrics.2018.1960
Yu, J., Lai, S., Geng, Q., Ye, C., Zhang, Z., Zheng, Y., et al. (2019). Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009-2015. J. Infect. 78, 66–74. doi: 10.1016/j.jinf.2018.07.004
Zaki, A., Abousekkien, M., Alkholy, U., and Eid, A. (2017). Effectiveness and impact of rotavirus vaccines in Saudi Arabia: A single hospital-based study. Arab. J. Gastroenterol. 18, 140–143. doi: 10.1016/j.ajg.2017.09.008
Zeng, X., Zhang, Y., Kwong, J., Zhang, C., Li, S., Sun, F., et al. (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 8, 2–10. doi: 10.1111/jebm.12141
Zhao, J. (2013). Identifying and measuring heterogeneity across the studies in meta-analysis. J. Hand Surg. Am. 38, 1449–1450. doi: 10.1016/j.jhsa.2013.05.020
Keywords: rotavirus, genotype, epidemiology, vaccination, Saudi Arabia
Citation: Alzahrani KJ (2025) Epidemiology, genetic diversity and symptom characterization of rotavirus infection in Saudi Arabia, 1985–2024: a meta-analysis. Front. Microbiol. 16:1671607. doi: 10.3389/fmicb.2025.1671607
Received: 23 July 2025; Accepted: 07 October 2025;
Published: 21 October 2025.
Edited by:
Svetlana Khaiboullina, University of Nevada, Reno, United StatesReviewed by:
Asma Sadiq, University of Jhang, PakistanShilu Mathew, Hamad Medical Corporation, Qatar
Copyright © 2025 Alzahrani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khalid J. Alzahrani, QWsuamFtYWFuQHR1LmVkdS5zYQ==
 Khalid J. Alzahrani
Khalid J. Alzahrani