- 1College of Resources and Environmental Sciences, China Agricultural University, Beijing, China
- 2Beijing Key Laboratory of Biodiversity and Organic Farming, China Agricultural University, Beijing, China
- 3State Key Laboratory of Nutrient Use and Management, College of Resources and Environmental Sciences, China Agricultural University, Beijing, China
Introduction: Microbial agents, mainly bacterial strains, are widely used in agriculture, their often limited efficacy underscores the critical need for resilient fungal alternatives. A novel strain of Penicillium kloeckeri with potential agricultural applications was isolated and purified.
Methods and Results: Here, the genome of P. kloeckeri was sequenced and function predicted. The assembled P. kloeckeri genome had a 34.28 Mb genome size with 41.87% GC content, and revealed functional genes associated with Indole-3-Acetic Acid (IAA), daidzein, siderophore, and organic acid secretion, suggesting its role in plant growth promotion and soil improvement. Metabolite analysis further confirmed daidzein and amino acid production. Under salt stress conditions, P. kloeckeri inoculation (YJKS) significantly improved soybean growth compared to the salt-stressed control (YCK). Specifically, inoculation increased stem diameter by 17.79% and dramatically enhanced root length (99.95%), root area (111.96%), root volume (114.80%), and root biomass (14.97%). Furthermore, in saline alkali soil, P. kloeckeri inoculation effectively reduced soil pH, improve soil physical structure, and enhance nutrient availability.
Discussion: P. kloeckeri is a promising new fungal strain with potential agricultural applications. This study provides a foundational genomic resource and initial evidence for its agricultural utility, warranting further investigation into its underlying mechanisms and commercial viability.
1 Introduction
Soil microorganisms represent one of the most diverse biological communities on Earth. A single gram of soil can contain billions of microbial cells and thousands of distinct species (Ma et al., 2023). These communities include bacteria, fungi, actinomycetes, and algae, which interact with one another and with plants to form complex ecological networks (Zhang et al., 2021; Ruiu, 2020; Shen et al., 2024). Over 450 million years of co-evolution have shaped the relationship between plants and microorganisms, giving microbes a central role in ecosystem functioning. (Cordovez et al., 2019; Hassani et al., 2018; Coban et al., 2022). Within agricultural systems, microorganisms support plant growth by supplying nutrients, improving the rhizosphere environment, and enhancing resistance to environmental stresses (Ku et al., 2024; Shi et al., 2024; Zheng et al., 2024). Consequently, they are considered indispensable for soil health, crop productivity, and agricultural sustainability (Gao et al., 2024). In current agricultural practice, most commercial microbial products are based on bacteria. Plant Growth-Promoting Rhizobacteria (PGPR) have been widely tested for their ability to stimulate plant growth and improve soil fertility. For example, nitrogen-fixing bacteria were used to increase nitrogen use efficiency (Chen et al., 2024) while phosphorus-solubilizing bacteria enhanced the availability of insoluble phosphorus sources (Chang et al., 2024). However, fungi, despite their ecological importance and functional versatility, remain underrepresented in microbial inoculants (Stukenbrock and Gurr, 2023). Research shows that fungi act as decomposers, symbionts, and pathogens in natural ecosystems (Yang et al., 2017), and they also contribute to soil structure improvement, water-use efficiency, and plant stress resistance in agricultural soils (Li et al., 2020). Mycorrhizal fungi, for instance, increase nutrient uptake and enhance plant tolerance to drought, salinity, and pests (Wang et al., 2024). Compared with bacteria, fungi may provide additional benefits due to their unique cellular structures and greater tolerance to environmental stresses (Wang and Kuzyakov, 2024). Nevertheless, their practical application is still limited, as fungal strains accounted for only 14.4% of newly registered microbial fertilizers between January and June 2024, whereas Bacillus-based products dominated the market (Sánchez et al., 2024). These observations highlight the substantial but underexploited potential of fungi in agriculture and underscore the need for further research and development.
Fungi are key players in natural ecosystems because of their unique biological traits and extensive biomass in soil (Coleine et al., 2022; Jiao et al., 2022). Existing data indicate that in the top 30 cm of soil, the total length of fungal hyphae per cubic centimeter of soil averages 1,020 meters (Sokol et al., 2022). Their hyphal networks not only establish symbiotic associations with plants but also contribute substantially to nutrient cycling. As decomposers, fungi convert complex organic matter into accessible nutrients, while as symbionts or pathogens they engage in intricate interactions with plants, animals, and other microorganisms, thereby maintaining ecosystem stability (Wu et al., 2024; Genre et al., 2020). Within this group, Penicillium is particularly important due to its species richness, global distribution, and strong secondary metabolic capacity. These fungi secrete organic acids and enzymes that dissolve insoluble phosphorus and potassium, regulate soil pH, and improve soil fertility (Jo et al., 2023; Zhang et al., 2024). In addition, their hyphal growth and exudates promote soil aggregate formation, enhance soil structure, and improve nutrient availability (Wu et al., 2023). Penicillium also exhibits high cellulase activity, supporting carbon turnover and nutrient cycling (Jiang et al., 2020). Beyond soil fertility, fungi contribute to plant resilience under stress conditions. Their metabolites can stimulate lignification in plant cell walls, induce stress-related compounds, and improve tolerance to biological stress, drought, salinity, and temperature extremes (Sánchez et al., 2024). For example, Penicillium citrinum YW322 suppressed ginseng root rot by inhibiting Aspergillus spp. and modulating rhizosphere microbial communities (Wang et al., 2022). Similarly, Paria et al. (2022) utilized the ability of Aspergillus penicillioides (F12) to produce large amounts of exopolysaccharides (EPS). This ability allowed the bioremediation of heavy metals, such as lead, in wastewater. The process achieved a remarkable adsorption rate of 73.14%. These findings demonstrate that fungi hold considerable promise for application in challenging environments such as saline-alkaline or acidic soils. However, most studies remain descriptive, and the underlying molecular mechanisms of fungal-mediated growth promotion and stress tolerance are still poorly understood (Rana et al., 2024). In order to further reveal this mechanism and process, changes and integration in the research methods are necessary.
WGS has been widely applied to improve species identification, predict functional genes, and reconstruct metabolic pathways (Liu et al., 2022; Ramsamy et al., 2020; Kweon et al., 2020). These applications provided valuable insights into microbial functions such as organic matter degradation, nitrogen fixation, and stress resistance, thereby highlighting the essential ecological roles of microorganisms in soil and plants (Chetverikov et al., 2023; Merker et al., 2020; Fan et al., 2024; Zhu et al., 2024). However, WGS alone is insufficient to fully resolve questions related to metabolic adaptability and regulatory mechanisms under environmental stress (Nagy et al., 2023). To complement genomic data, researchers have used metabolomics to capture dynamic changes in small-molecule metabolites. This approach linked gene expression to phenotypic traits and extended the mechanistic understanding gained from genome sequencing (Cui et al., 2024; Ferrell et al., 2024). For instance, the endophytic bacteria were used to reveal the molecular mechanism of alleviating the nutritional limitation of rapeseed growth under low phosphorus environment by multi-omics combined methods (Liu et al., 2024). These findings suggest that integrating WGS with metabolomics and other omics approaches is essential for dissecting the complex regulatory mechanisms of fungi. Such combined strategies not only bridge the gap between genotype and phenotype but also lay a robust foundation for the development of fungal inoculants with improved agricultural applications.
In this study, a strain of P. kloeckeri with potential agricultural applications is investigated through an integrated approach that combines WGS, metabolomic profiling, and phenotypic validation. WGS provides the complete genomic sequence, enabling the identification of functional genes, regulatory elements, and agriculturally relevant pathways. Metabolomic analysis uncovers metabolic responses under salt stress, while phenotypic experiments confirm the effects of this strain on soybean growth promotion and salt tolerance. By linking genomic potential with metabolic activity and observable phenotypes, this study offers new insights into the molecular mechanisms underlying fungal-mediated plant growth promotion and soil improvement.
2 Materials and methods
2.1 Fungal strains and culture conditions
The fungal strain was isolated from the rhizosphere of soybean plants in the soybean-growing region of Heilongjiang Province, China. Isolation and purification of the strain was performed in the Soil Ecology Laboratory of China Agricultural University. The strain was cultured on Potato Dextrose Agar (PDA) plates at 25 °C for 7 days. Conidia from the 7 day old PDA cultures were harvested by washing the plates with sterile distilled water.
2.2 Genome sequencing and assembly
Conidia of the strain were inoculated into sterilized PDA liquid medium and incubated at 25 °C with shaking at 200 r/min for 48 h. The resulting mycelia were collected by filtration, washed three times with sterile water, and subsequently frozen and ground in liquid nitrogen. Genomic DNA was extracted using the Ezup Column Fungal DNA Extraction Kit (Sangon Biotech, Shanghai, China) and further purified with the Genomic DNA Purification Kit (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. Genome sequencing was carried out in two stages using Illumina and PacBio technologies. Illumina sequencing was performed on a HiSeq2000 instrument using two genomic libraries: 180 bp (paired-end) and 2 Kb (mate-pair) insert sizes, with 100 bp read length. Second, PacBio sequencing technology (model RSII and P4C2 chemistry) was used to produce long reads (>1 Kb).
Genome assembly was performed in two steps. Firstly, Illumina reads were assembled with ALLPATHS-LG (version r43019) (Genre et al., 2020) at 200 × coverage to generate high-quality contigs and scaffolds. Secondly, scaffolding was improved using PacBio long reads with SSPACE-LongRead (version 1.1). Assembly quality was assessed using using CEGMA (Paria et al., 2022) by aligning 248 highly conserved eukaryotic proteins to estimate genome completeness. Additionally, standard assembly metrics such as N50, the number of contigs, and BUSCO completeness (Simão et al., 2015) were also calculated to further evaluate the quality of the assembly. The N50 value was 1,559,217 bp, the total number of contigs was 53, and the BUSCO completeness score was 99.1% complete (3513 genes), with only 0.8% missing (31 genes) (using the fungi_odb9 database with BUSCO v3.0.2). The raw sequencing data were deposited in the NCBI Sequence Read Archive under accession number PRJNA1297971.
2.3 Gene prediction and functional annotation
Protein-coding gene prediction was performed using Diamond (v0.9.10.111). The predicted protein sequences were compared against multiple databases, including NR, eggNOG, KEGG, Swiss-Prot, GO, P450, and TCDB. The threshold for sequence alignment was set at 1e-6. GO annotation was performed using InterPro (v66.0). KEGG Orthology (KO) and pathway annotation were carried out using the KEGG KAAS (v2.1) automated annotation system. Annotated KO terms were subsequently mapped to KEGG pathways. KOG annotation was performed using eggNOG-mapper with the eggNOG (v4.5) database. In addition, RNA secondary structure prediction was performed to improve the functional annotation of non-coding RNAs (Wang et al., 2025), following recent methodological advances that integrate deep learning with structural context analysis.
2.4 Untargeted metabolomics
2.4.1 Metabolites extraction
One milliliter of PDB culture supernatant of P. kloeckeri was freeze-dried. The dried sample was reconstituted in 100 μL of 80% aqueous methanol. Samples were incubated on ice for 5 min and then were centrifuged at 15,000 rpm, 4 °C for 5 min. Some of supernatant was diluted to final concentration containing 53% methanol by LC-MS grade water. The samples were subsequently transferred to a fresh Eppendorf tube and then were centrifuged at 15,000 g, 4 °C for 15 min. Finally, the supernatant was injected into the LC-MS/MS system analysis. For QC samples, equal volume of samples were taken from each experimental sample and mix them as QC samples. For blank samples, 53% methanol aqueous solution instead of experimental samples, and the pretreatment process was the same as that of experimental samples.
2.4.2 UHPLC-MS/MS analysis
UHPLC–MS/MS non-targeted metabolomics of P. kloeckeri was performed using a Thermo Vanquish UHPLC system coupled with an Orbitrap Q Exactive HF (Biozeron Co., Shanghai). Samples were separated on a Hypersil Gold C18 column (100 × 2.1 mm, 1.9 μm) at 0.2 mL/min using a 17 min linear gradient (positive mode: 0.1% FA/H2O–MeOH; negative mode: 5 mM ammonium acetate buffer pH 9–MeOH). The mass spectrometer operated in both polarities with a spray voltage of 3.2 kV, capillary temperature of 320 °C, and standard gas settings. Raw data were processed in Compound Discoverer 3.1 for peak alignment, picking, and quantitation (RT tolerance 0.2 min; mass tolerance 5 ppm; S/N ≥3; minimum intensity 100,000), followed by total spectral intensity normalization. Metabolite annotation was achieved by matching MS2 data to mzCloud, mzVault, and MassList databases.
2.5 Apparent experiment
2.5.1 Soybean hydroponic experiment
Hydroponic system. Wash Penicillium koeckeri spores with sterile water to prepare spore suspension. Using sterilized 1/2MS medium and adding NaCl, the final concentration of NaCl in the hydroponic solution was 80 mmol/L, simulating salt stress; Using a 650 mL transparent plastic bucket as the hydroponic device, containing 550 mL of culture medium.
Soybean seed germination. Disinfect the surface of soybean seeds with 10% hydrogen peroxide (soak for 20 min), rinse several times with deionized water, and place the soybean seeds on moist filter paper and sprout in a constant temperature incubator at 25 °C. Wait for the sprouts to reach about 3 cm before transplanting. Soak the spore suspension for 1 h before transplanting in the bacterial treatment group.
Experimental design. Three treatments were set up: CK (normal condition with 1/2 MS medium), YCK (salt stress control with 1/2 MS medium containing 80 mmol/L NaCl), and YJKS (inoculation of P. kloeckeri under salt stress). The NaCl concentration (80 mmol/L) was determined based on preliminary experiments, which showed that this concentration effectively inhibited soybean seed germination without causing complete seedling failure, thereby providing a reliable salt stress condition for the study. Each treatment included 5 biological replicates (5 sets of hydroponic systems and 15 soybean seeds per pot.) Samples were collected after 20 d of hydroponic cultivation. Measure plant biomass and perform root scanning using Epson Scan2.
2.5.2 Experiment on improving saline alkali soil with P. kloeckeri
Materials. Soda saline alkali soil was collected from a typical disposal area in China (115°30′-115°5′E, 36°55′-37°10′N) and air-dried and sieved through a 200-mesh sieve. P. kloeckeri was cultivated according to the previously mentioned.
Experimental design and physicochemical properties. The experimental groups were set up as follows: (i) CK: Soda saline alkali soil only; (ii) JKS: Soil inoculated with P. kloeckeri, with a spore concentration of 107 spores per gram of soil. The spore concentration was selected based on preliminary experiments, which indicated that this concentration effectively promoted soybean seed germination. Each treatment was performed with 5 biological replicates, and the experiments were repeated over a 2 m period. Additionally, the spore suspension of P. kloeckeri was replenished every 15 d to ensure consistent microbial inoculation.
Physicochemical Properties. pH were conducted by a pH meter (REX PHS-3E, China) in deionized water (ratio of water/solid = 5:1); Soil organic matter was measured using potassium dichromate external heating method; The spectrophotometric method is employed for the rapid determination of phosphorus (Storie, 1957); Classification and statistics of soil macroaggregates using wet screening method (Six et al., 2000).
2.6 Data analysis
Metabolites were annotated against the KEGG, HMDB, and LIPID Maps databases. Multivariate analyses, including PCA and PLS-DA, were performed using metaX, and univariate t-tests were applied to assess statistical significance. Differential metabolites were defined as those with VIP >1, P < 0.05, and |fold change| ≥2. Volcano plots and hierarchical clustering heatmaps (Pheatmap, R) were used to visualize differential metabolites. Pearson correlation analysis (corr and corrplot, R) was conducted, with P < 0.05 considered significant. Pathway enrichment analysis of differential metabolites was performed using KEGG, with pathways deemed significantly enriched at P < 0.05. One-way ANOVA was performed using IBM Statistical Product and Service Solutions (SPSS) Statistics for Windows (version 13), followed by post hoc Tukey's HSD test to assess the separation between sample means. P < 0.05 was considered significant. Some data visualizations were analyzed using Origin 2024 and Graphpad Prism 9.5.
2.7 Limitations
Methodological Constraints: Potential limitations were associated with the use of only two sequencing technologies (Illumina and PacBio), which might have introduced biases in the genomic assembly process. In addition, the resolution of untargeted metabolomics could have been influenced by the quality and complexity of the analyzed samples.
Scope of Findings: The applicability of the findings was limited to the specific strain of P. kloeckeri and the experimental conditions employed, and might not have fully represented the diversity of fungal species or the outcomes under different environmental conditions. Moreover, as the study focused on a single stressor (salt stress), interactions with other environmental factors could have provided further insights into the underlying microbial mechanisms.
3 Results
3.1 Genome features of P. kloeckeri
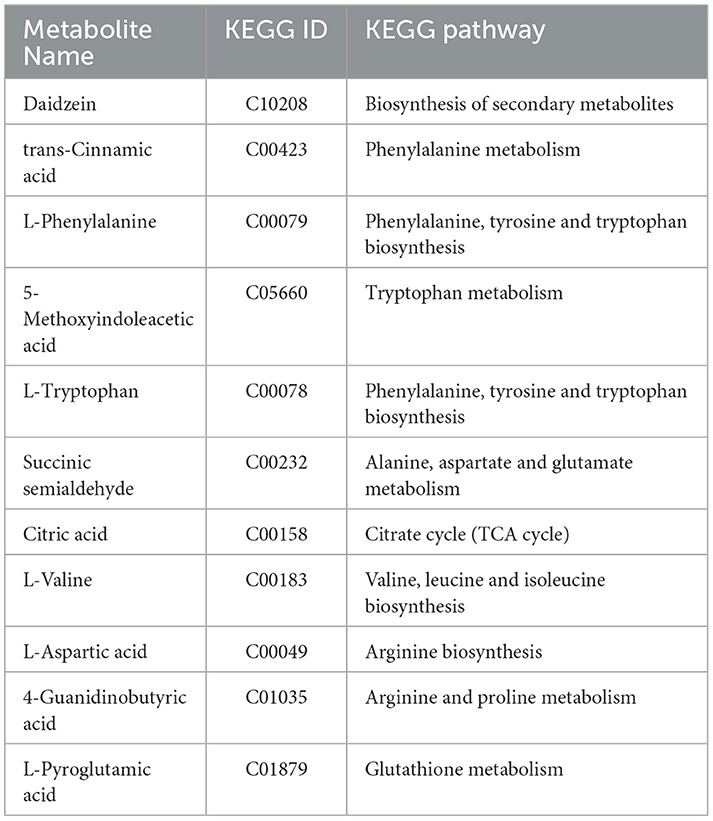
The total number of genomic bases in P. kloeckeri is 4,392,696,002 bp, and the original total number of reads is 29,090,702. The genome size is 34.28 Mb with 41.87% GC content, a minimum sequence length of 55 bp, and a maximum sequence length of 287,510 bp. There are a total of 11,123 protein-coding genes in the genome of P. kloeckeri, with a total gene sequence length of 17,387,264 bp and an average length of 1,563.1 bp per gene. The total number of exons is 31,880, with an average of 2.8 exons per gene and a total exon length of 15,992,151 bp. The proportion of total exon length to genome length is 42.32%, with an average exon length of 501.6 bp and an average intron length of 67.2 bp. The total length of CDS is 15,992,151 bp, and the proportion of CDS to the genome is 42.32%. The average length of CDS is 1,437.7 bp (Figure 1A).
Figure 1. Genomic information, functional annotation, and phylogenetic tree of P. kloeckeri. (A) Whole genome circle diagram: From inside out: First lap: GC sketch (the GC content); Second circle: G + C content; Third circle: Carbohydrate-active enzymes (CAZy); Circle 4: Clusters of Orthologous Groups (COG) to which each coding DNA sequence (CDS) on the negative strand belongs; Circle 5: COG to which each CDS on the positive strand belongs; Circle 6: Scale.; (B) Clusters of KOG (eukaryotic orthologous groups) annotation; (C) Gene count distributions of carbohydrate-active enzyme (CAZy) families; (D) Phylogenetic tree.
The eggNOG classification annotation shows that a total of 10,253 proteins obtain COG functional annotation, accounting for 92.18% of all genes. Excluding unknown functional genes, the highest number of genes involved in carbohydrate transport and metabolism is 898, accounting for 8.1%. There are 533 genes related to posttranslational modification, protein turnover, and chaperones, accounting for 4.79%. Additionally, there are 432 genes involved in amino acid transport and metabolism, accounting for 3.9%. Energy production and conversion includes 428 genes, accounting for 3.8% (Figure 1B).
The CAZy database includes carbohydrate-related enzyme families that catalyze carbohydrate degradation, modification, and biosynthesis. The CAZymes analysis reveals that glycoside hydrolases (GHs) are the major family, with 341 genes, followed by carbohydrate esterases (CEs) with 123 genes, and glycosyl transferases (GTs) with 104 genes (Figure 1C).
Phylogenetic analysis confirms the evolutionary position of the strain within Penicillium by comparing the obtained 18S rDNA sequences with those of related species, resulting in the construction of a phylogenetic tree (Figure 1D). The sequence alignment reveals that the strain with the highest homology is KT385771.1, corresponding to P. kloeckeri, with a sequence identity of 99.21%. The strain P. kloeckeri used in this study has been deposited at the China General Microbiological Culture Collection Center (CGMCC) under accession number CGMCC No. 41049.
3.2 The foundation of P. kloeckeri in agricultural applications
3.2.1 Molecular basis of plant growth promotion and stress resistance in P. kloeckeri
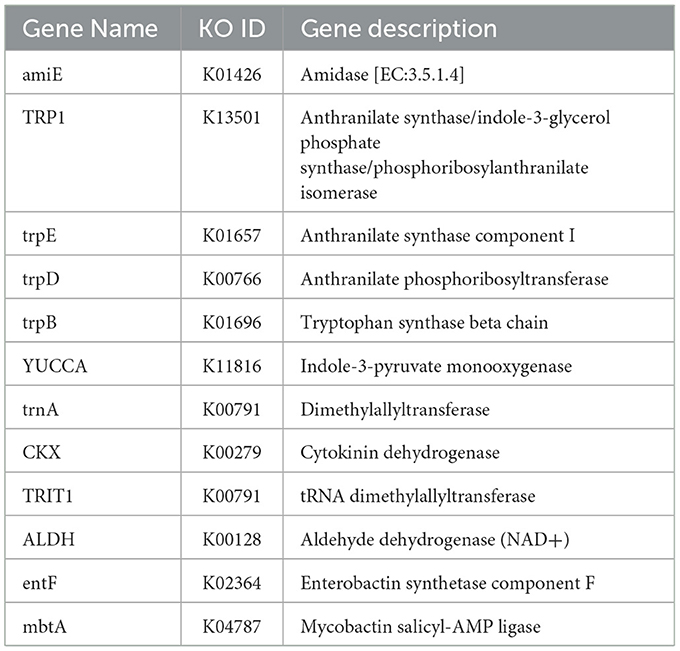
In the genome of P. kloeckeri, six key genes related to IAA synthesis have been identified, including amiE, TRP1, trpBED, and YUCCA. In addition, the key enzyme acetaldehyde dehydrogenase (NAD+), which catalyzes IAA synthesis using tryptophan as a precursor, was also detected. Genes associated with zeaxanthin metabolism, such as trnA, CKX, and TRIT1, were likewise identified (Table 1).
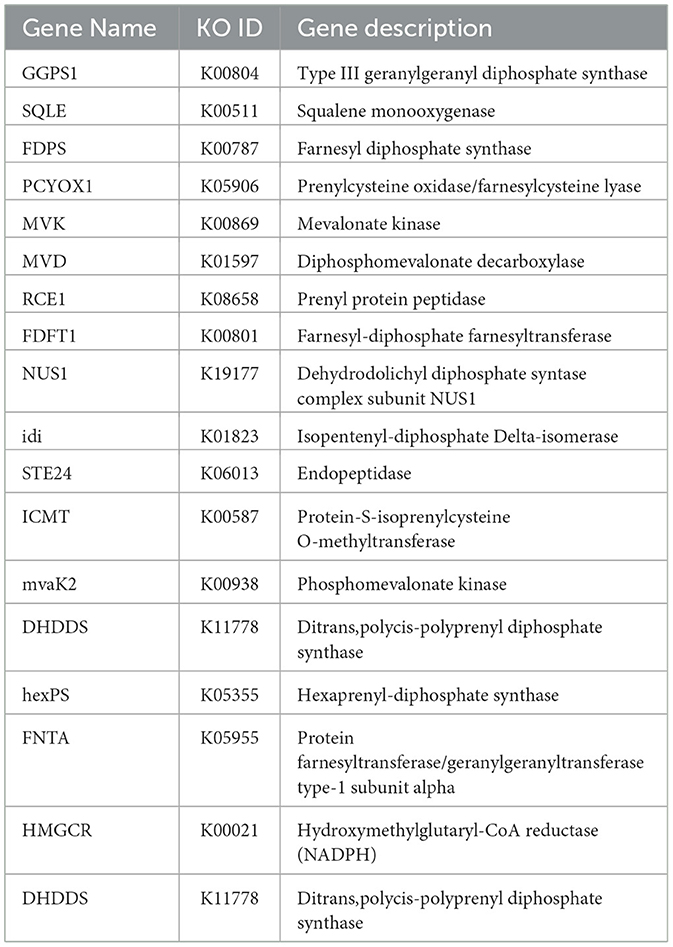
In the genome of P. kloeckeri, 18 genes associated with terpenoid biosynthesis were identified. These genes, including GGPS1, SQLE, FDPS, PCYOX1, MVK, MVD, RCE1, FDFT1, and NUS1, are primarily involved in the regulation of the terpenoid backbone biosynthesis pathway (Table 2).
3.2.2 Molecular basis for improving saline alkali soil with P. kloeckeri
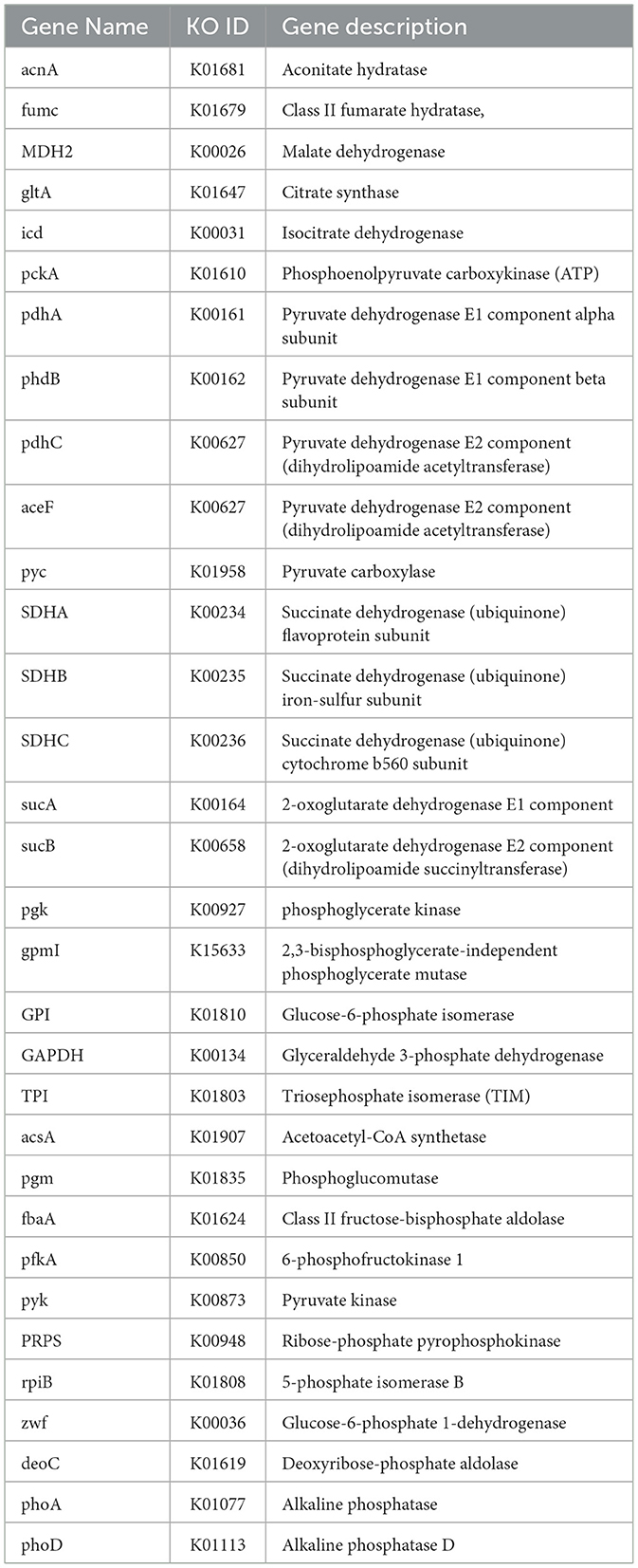
KEGG-based metabolic pathway analysis revealed that the genome of P. kloeckeri contains 32 genes related to organic acid biosynthesis. These genes are involved in glycolysis (EMP), the Tricarboxylic Acid (TCA) cycle, the pentose phosphate pathway, and oxidative phosphorylation. In addition, genes encoding alkaline phosphatases, including phoA and phoD, were identified (Table 3).
3.2.3 Metabolites of P. kloeckeri
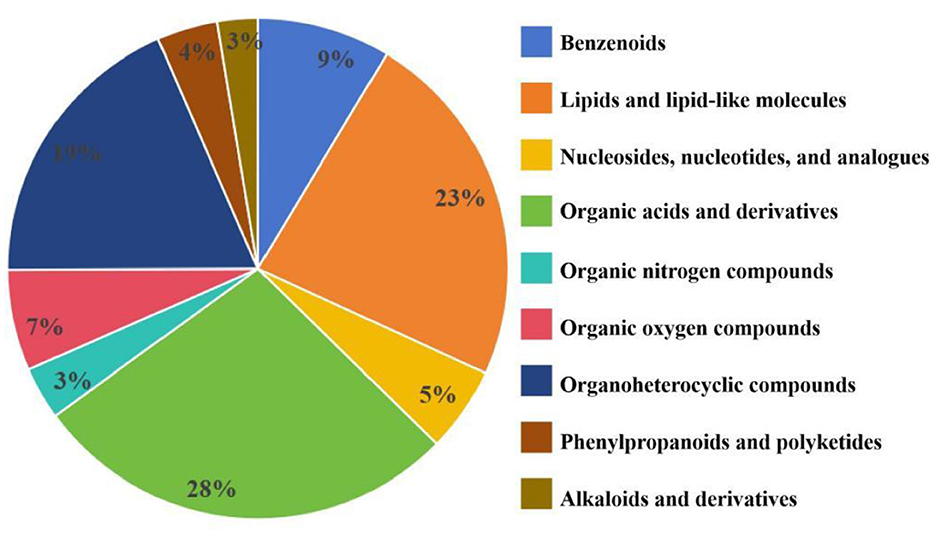
Through identification of the metabolites produced by P. kloeckeri, it is found that the main metabolites include benzene ring compounds, lipids and lipid molecules, nucleosides, nucleotides, and analogs, organic acids and their derivatives, organic nitrogen compounds, organic oxygen compounds, organic heterocyclic compounds, phenylpropanoids, polyketones, and alkaloids and their derivatives. Among these, organic acids and their derivatives comprise the most substances (Figure 2). Metabolite analysis of P. kloeckeri revealed that it produces a variety of compounds that promote plant growth and stress resistance, including daidzein, L-phenylalanine, L-tryptophan, and L-valine (Table 4).
3.3 Verification test
3.3.1 The effect of P. kloeckeri on soybean growth promotion under salt alkali stress
Compared with normal cultivation conditions, salt stress significantly inhibited plant growth. Under salt stress, plant height, stem diameter, shoot and root fresh weight, and chlorophyll content decreased by 13.27%, 0.54%, 21.78%, and 0.61%, respectively. Under salt stress, plants inoculated with P. kloeckeri showed increases in plant height (33.31%), stem diameter (17.79%), shoot fresh weight (38.39%), underground fresh weight (14.97%), and chlorophyll content (14.31%) compared with the non-inoculated control (Figures 3A–E, G). Root growth was also inhibited by salt stress, with root length, root area, and root volume reduced by 16.37%, 21.73%, and 26.68%, respectively. However, inoculation with P. kloeckeri markedly mitigated this inhibition. After inoculation, root length, root area, and root volume increased by 99.95%, 111.96%, and 114.80%, respectively (Figures 3F, H, I).
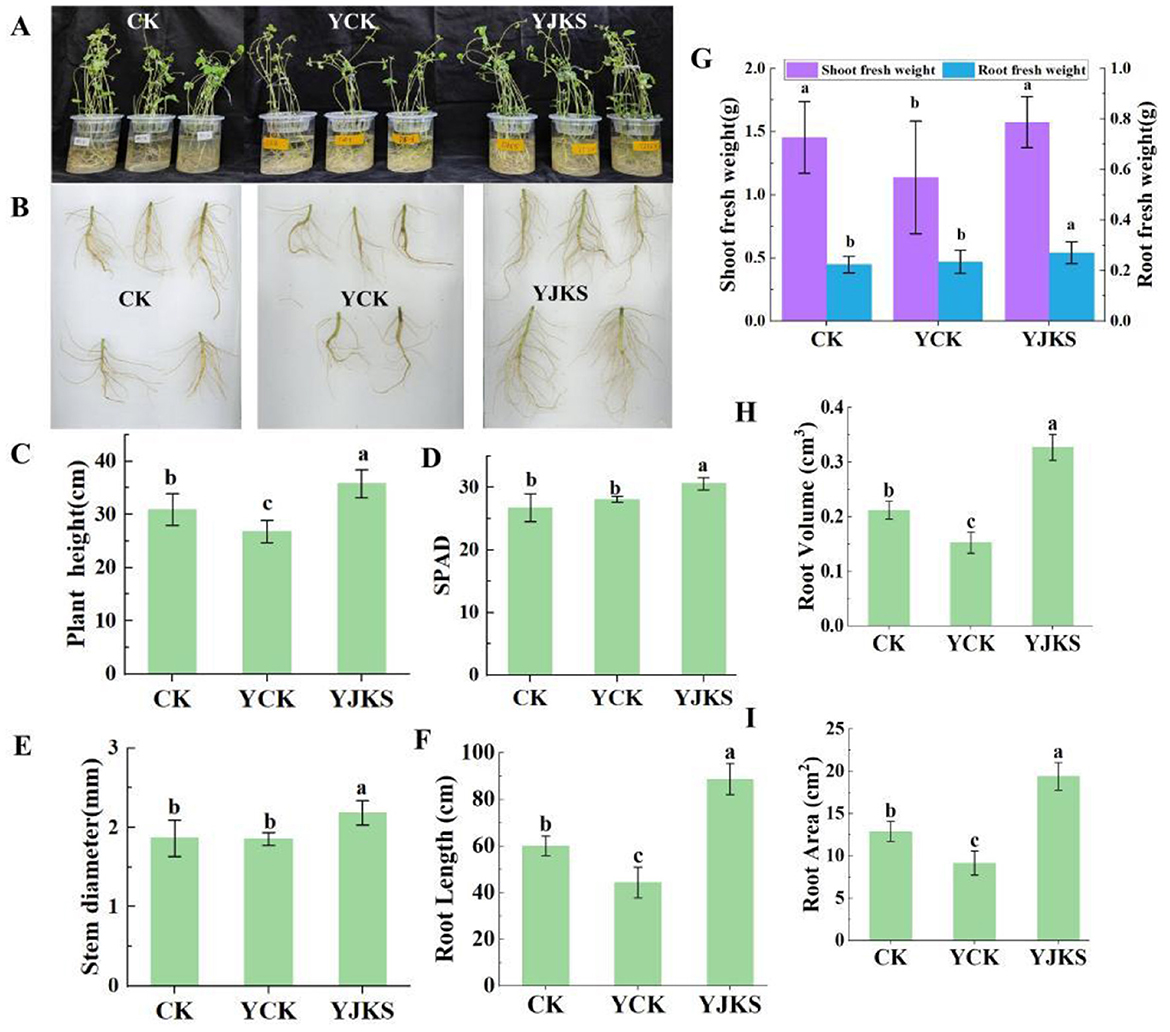
Figure 3. The growth status of soybean plants under different treatments (A); Overall growth situation; (B); Soybean root, Plant height (C); Chlorophyll content of soybean plants (D); Soybean plant stem diameter (E); Root Length (F); Soybean shoot and root biomass (G); Root Volume (H); Root Area (I) One-way ANOVA test was conducted to test the significance of difference in habitat niche breadth.
3.3.2 The effect of P. kloeckeri on saline alkali soil
In the validation experiment on saline alkali soil improvement, inoculation with P. kloeckeri significantly reduced soil pH by 1.83% compared with the control (CK). It also increased the proportion of soil macroaggregates and microaggregates by 11.33% and 52.44%, respectively. In addition, soil organic matter content increased by 7.43%, and available phosphorus content increased by 30.17%. (Table 5).
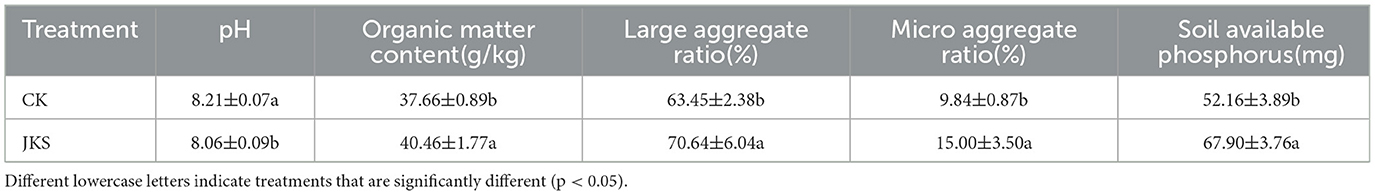
Table 5. The effect of inoculation with P. kloeckeri on the physicochemical properties of soda saline alkali soil.
4 Discussion
Recent studies increasingly emphasize the potential of Plant Rhizosphere Growth-Promoting Microbes (PGPMs), with many efforts focused on identifying and screening effective strains (Gamalero and Glick, 2020; Samet et al., 2022). These microbes have already found broad applications in agriculture. The mechanisms by which PGPMs act can generally be divided into direct and indirect effects. Direct mechanisms involve metabolite secretion and nutrient mobilization (e.g., auxins, siderophores, phosphorus solubilization, potassium mobilization, nitrogen fixation). Indirect mechanisms improve the root microenvironment, such as alleviating saline alkaline stress or suppressing pathogens (Liu et al., 2025). Unlike the majority of microbial agents in China, which are dominated by bacteria such as Bacillus and Pseudomonas, our focus on P. kloeckeri highlights the potential of fungal PGPMs as alternative or complementary bioinoculants. Our genome analysis indicates that P. kloeckeri likely integrates both mechanisms.
4.1 Growth-promoting factors
Auxin plays a central role in root development and overall plant growth. Previous studies show that microbial IAA enhances root elongation and lateral root formation, facilitating nutrient uptake (Bunsupa et al., 2017; Pereira et al., 2021). In the genome of P. kloeckeri, we identified six genes related to Indole-3-Acetic Acid (IAA) synthesis, including amiE, TRP1, trpBED, and YUCCA. These genes encode enzymes that use tryptophan as a precursor, such as aldehyde dehydrogenase and zeaxanthin-related enzymes (trnA, CKX, TRIT1). Then, amino acids further contribute to growth promotion. For example, tryptophan is directly linked to auxin synthesis (Yao et al., 2025), glycine promotes photosynthesis in peach trees by regulating sucrose enzyme metabolic activity (Li et al., 2021). Genomic and metabolite analysis of P. kloeckeri showed that the strain had genes for synthesizing these amino acids, suggesting that it may use a similar mechanism to promote plant growth. Under mild salt stress, Penicillium citrinum was able to promote chlorophyll synthesis and plant growth, which is similar to the results of Galeano et al. (2023) on Penicillium chrysogemun promoting corn under salt stress.
4.2 Stress resistance
The genome of P. kloeckeri (34.28 Mb) is several times larger than those of Bacillus subtilis (4.0 Mb) and Pseudomonas species (6.5 Mb) (Li et al., 2020). A larger genome suggests a broader repertoire of genes that may regulate stress responses and plant-microbe interactions. Abiotic stress tolerance is another critical function of PGPMs. In P. kloeckeri, eighteen genes involved in terpenoid biosynthesis were identified. Terpenoids are widely recognized as defense-related metabolites that modulate stress signaling. In addition, amino acids such as proline and lysine also play protective roles. Proline accumulation stabilizes proteins and membranes under salt or drought stress, while lysine participates in nitrogen assimilation and chlorophyll synthesis. The presence of these metabolic pathways in P. kloeckeri indicates that it may directly enhance plant stress resistance, consistent with findings in other PGPMs like Trichoderma harzianum (Catalá et al., 2014). Functional annotation revealed that genes related to secondary metabolite biosynthesis form the second largest category in P. kloeckeri. This feature contrasts with many bacterial PGPMs and may explain its strong metabolic flexibility. Secondary metabolites such as EPS, VOCs, and compatible solutes (proline, trehalose, glycine betaine) are well documented to modulate genes including SOS1, HKT1, antioxidant proteins, and ethylene biosynthesis genes (Hassani et al., 2018; Kim et al., 2021). Such mechanisms enhance plant tolerance to abiotic stress and are consistent with our findings. In addition to secondary metabolites, P. kloeckeri possesses extensive carbohydrate-active enzyme (CAZyme) genes, including glycoside hydrolases (341 genes), carbohydrate esterases (123 genes), and glycosyltransferases (104 genes). It provides support for the degradation and utilization of complex carbohydrates of P. kloeckeri to obtain more energy and carbon sources (Starke et al., 2021; Zhang et al., 2025) and can also help Penicillium kermitense to occupy ecological advantages in the competitive ecological environment.
4.3 Soil improvement potential
Soil nutrient availability and pH balance are key determinants of crop productivity in saline alkaline conditions. P. kloeckeri harbors thirty two genes related to organic acid synthesis, spanning multiple metabolic pathways such as glycolysis, the TCA cycle, the pentose phosphate pathway, and oxidative phosphorylation. Importantly, we identified phoA and phoD, which encode alkaline phosphatases. These enzymes are known to release inorganic phosphate from organic compounds, thereby improving phosphorus availability in soil (Liu et al., 2025). By secreting organic acids, P. kloeckeri can also reduce soil pH, a mechanism previously described in Penicillium oxalicum (Jiang et al., 2020). Penicillium bairei is recognized for its strong phosphorus-solubilizing capabilities (Brazhnikova et al., 2022). In addition, Penicillium oxalicum increased micro aggregate stability in acidic soils, enhanced phosphorus dissolution, and reduced lead bioavailability (Tong et al., 2024). Thus, the combination of acid production and phosphatase activity positions P. kloeckeri as a promising agent for saline alkaline soil improvement.
5 Conclusion
In this study, whole genome sequencing and comparative genomic analysis of P. kloeckeri were performed. Functional genes associated with auxin (IAA) synthesis, daidzein production, siderophore secretion, and organic acid metabolism were identified. These findings emphasize the potential role of P. kloeckeri in promoting plant growth and improving soil health. Metabolite profiling confirmed the production of daidzein and multiple amino acids, which supports the genomic predictions. Pot experiments further validated the beneficial effects of P. kloeckeri. Under salt stress, P. kloeckeri inoculation significantly enhanced soybean performance, with stem diameter increasing by 17.79%, and root length, area, and volume improving by nearly 100-115% compared to the uninoculated control. Additionally, inoculation improved saline alkaline soil conditions by reducing pH, enhancing soil aggregate stability, increasing organic matter content, and boosting available phosphorus levels. These findings position P. kloeckeri as a promising fungal inoculant with dual benefits for plant growth and soil health, particularly under stress conditions. Future research should focus on elucidating the molecular mechanisms that mediate these effects, conducting field-scale trials to validate its robustness in diverse environments, and developing strategies for its large-scale commercial application in sustainable agriculture.
Data availability statement
The datasets presented in this study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/sra, accession number PRJNA1297971.
Author contributions
WC: Formal analysis, Methodology, Writing – original draft. YW: Data curation, Formal analysis, Visualization, Writing – original draft. BN: Conceptualization, Supervision, Writing – review & editing. JC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2023YFD1901303) and National Natural Science Foundation of China (32301448) and supported by The 2115 Talent Development Program of China Agricultural University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1675497/full#supplementary-material
References
Brazhnikova, Y. V., Shaposhnikov, A. I., Sazanova, A. L., Belimov, A. A., Mukasheva, T. D., and Ignatova, L. V. (2022). Phosphate mobilization by culturable fungi and their capacity to increase soil P availability and promote barley growth. Curr. Microbiol. 79:240. doi: 10.1007/s00284-022-02926-1
Bunsupa, S., Yamazaki, M., and Saito, K. (2017). Lysine-derived alkaloids: overview and update on biosynthesis and medicinal applications with emphasis on quinolizidine alkaloids. Mini Rev. Med. Chem. 17, 1002-1012. doi: 10.2174/1389557516666160506151213
Catalá, R., López-Cobollo, R., Mar Castellano, M., Angosto, T., Alonso, J. M., Ecker, J. R., et al. (2014). The arabidopsis 14-3-3 protein rare cold inducible 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 26, 3326–3342. doi: 10.1105/tpc.114.127605
Chang, D., Song, Y., Liang, H., Liu, R., Cai, C., Lv, S., et al. (2024). Planting Chinese milk vetch with phosphate-solubilizing bacteria inoculation enhances phosphorus turnover by altering the structure of the phoD-harboring bacteria community. Eur. J. Soil Biol. 123:103678. doi: 10.1016/j.ejsobi.2024.103678
Chen, Y., Li, Y., Fu, Y., Jia, L., Li, L., Xu, Z., et al. (2024). The beneficial rhizobacterium bacillus velezensis SQR9 regulates plant nitrogen uptake via an endogenous signaling pathway. J. Exp. Botany 75, 3388–3400. doi: 10.1093/jxb/erae125
Chetverikov, S., Hkudaygulov, G., Sharipov, D., Starikov, S., and Chetverikova, D. (2023). Biodegradation potential of C7-C10 perfluorocarboxylic acids and data from the genome of a new strain of pseudomonas mosselii 5(3). Toxics 11:1001. doi: 10.3390/toxics11121001
Coban, O., De Deyn, G. B., and Van Der Ploeg, M. (2022). Soil microbiota as game-changers in restoration of degraded lands. Science 375:0725. doi: 10.1126/science.abe0725
Coleine, C., Stajich, J. E., and Selbmann, L. (2022). Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 37, 517–528. doi: 10.1016/j.tree.2022.02.002
Cordovez, V., Dini-Andreote, F., Carrión, V. J., and Raaijmakers, J. M. (2019). Ecology and evolution of plant microbiomes. Ann. Rev. Microbiol. 73, 69–88. doi: 10.1146/annurev-micro-090817-062524
Cui, P., Li, X., Huang, C., and Lin, D. (2024). Metabolomics-driven discovery of therapeutic targets for cancer cachexia. J. Cachexia, Sarcopenia Muscle 15, 781–793. doi: 10.1002/jcsm.13465
Fan, L., Liu, S., Dai, H., Yuan, L., Yang, Z., and Jiao, X. (2024). Genotype-phenotype evaluation of the heterogeneity in biofilm formation by diverse bacillus licheniformis strains isolated from dairy products. Int. J. Food Microbiol. 416:110660. doi: 10.1016/j.ijfoodmicro.2024.110660
Ferrell, M., Wang, Z., Anderson, J. T., Li, X. S., Witkowski, M., DiDonato, J. A., et al. (2024). A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat. Med. 30, 424–434. doi: 10.1038/s41591-023-02793-8
Galeano, R. M. S., Silva, S. M., Yonekawa, M. K. A., De Alencar Guimarães, N. C., Giannesi, G. C., Masui, D. C., et al. (2023). Penicillium chrysogenum strain 34-P promotes plant growth and improves initial development of maize under saline conditions. Rhizosphere 26:100710. doi: 10.1016/j.rhisph.2023.100710
Gamalero, E., and Glick, B. R. (2020). The use of plant growth-promoting bacteria to prevent nematode damage to plants. Biology 9:381. doi: 10.3390/biology9110381
Gao, C., Bezemer, T. M., De Vries, F. T., and Van Bodegom, P. M. (2024). Trade-offs in soil microbial functions and soil health in agroecosystems. Trends Ecol. Evol. 39, 895–903. doi: 10.1016/j.tree.2024.05.013
Genre, A., Lanfranco, L., Perotto, S., and Bonfante, P. (2020). Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18, 649–660. doi: 10.1038/s41579-020-0402-3
Hassani, M. A., Durán, P., and Hacquard, S. (2018). Microbial interactions within the plant holobiont. Microbiome 6:58. doi: 10.1186/s40168-018-0445-0
Jiang, Y., Tian, J., and Ge, F. (2020). New insight into carboxylic acid metabolisms and pH regulations during insoluble phosphate solubilisation process by penicillium oxalicum PSF-4. Curr. Microbiol. 77, 4095–4103. doi: 10.1007/s00284-020-02238-2
Jiao, S., Chu, H., Zhang, B., Wei, X., Chen, W., and Wei, G. (2022). Linking soil fungi to bacterial community assembly in arid ecosystems. iMeta 1:e2. doi: 10.1002/imt2.2
Jo, C., Zhang, J., Tam, J. M., Church, G. M., Khalil, A. S., Segrè, D., et al. (2023). Unlocking the magic in mycelium: using synthetic biology to optimize filamentous fungi for biomanufacturing and sustainability. Mater. Today Bio 19:100560. doi: 10.1016/j.mtbio.2023.100560
Kim, D. R., Jeon, C. W., Cho, G., Thomashow, L. S., Weller, D. M., Paik, M. J., et al. (2021). Glutamic acid reshapes the plant microbiota to protect plants against pathogens. Microbiome 9:244. doi: 10.1186/s40168-021-01186-8
Ku, Y., Liao, Y., Chiou, S., Lam, H., and Chan, C. (2024). From trade-off to synergy: microbial insights into enhancing plant growth and immunity. Plant Biotechnol. J. 22, 2461–2471. doi: 10.1111/pbi.14360
Kweon, O. J., Lim, Y. K., Kim, H. R., Kim, T.-H., Ha, S., and Lee, M. K. (2020). Isolation of a novel species in the genus cupriavidus from a patient with sepsis using whole genome sequencing. PLoS ONE 15:e0232850. doi: 10.1371/journal.pone.0232850
Li, F., Zhang, S., Wang, Y., Li, Y., Li, P., Chen, L., et al. (2020). Rare fungus, mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 151:108017. doi: 10.1016/j.soilbio.2020.108017
Li, Y., Lv, Y., Lian, M., Peng, F., and Xiao, Y. (2021). Effects of combined glycine and urea fertilizer application on the photosynthesis, sucrose metabolism, and fruit development of peach. Sci. Horticult. 289:110504. doi: 10.1016/j.scienta.2021.110504
Liu, C., Bai, Z., Luo, Y., Zhang, Y., Wang, Y., Liu, H., et al. (2024). Multiomics dissection of brassica napus L. lateral roots and endophytes interactions under phosphorus starvation. Nat. Commun. 15:9732. doi: 10.1038/s41467-024-54112-5
Liu, D. M., Huang, Y. Y., and Liang, M. H. (2022). Analysis of the probiotic characteristics and adaptability of lactiplantibacillus plantarum DMDL 9010 to gastrointestinal environment by complete genome sequencing and corresponding phenotypes. LWT 158:113129. doi: 10.1016/j.lwt.2022.113129
Liu, S., Zhang, X., Wang, H., Kuzyakov, Y., Pan, J., Chen, F., et al. (2025). Phosphorus-transforming microbes enhance phosphatase catalytic efficiency to alleviate phosphorus limitation under nitrogen and phosphorus additions in subtropical forest soil. Soil Biol. Biochem. 209:109915. doi: 10.1016/j.soilbio.2025.109915
Liu, Y., Jia, B., Ren, Y., Xun, W., Stefanic, P., Yang, T., et al. (2025). Bacterial social interactions in synthetic bacillus consortia enhance plant growth. iMeta 4:e70053. doi: 10.1002/imt2.70053
Ma, B., Lu, C., Wang, Y., Yu, J., Zhao, K., Xue, R., et al. (2023). A genomic catalogue of soil microbiomes boosts mining of biodiversity and genetic resources. Nat. Commun. 14:7318. doi: 10.1038/s41467-023-43000-z
Merker, M., Kohl, T. A., Barilar, I., Andres, S., Fowler, P. W., Chryssanthou, E., et al. (2020). Phylogenetically informative mutations in genes implicated in antibiotic resistance in mycobacterium tuberculosis complex. Genome Med. 12:27. doi: 10.1186/s13073-020-00726-5
Nagy, L. G., Vonk, P. J., Künzler, M., Földi, C., Virágh, M., Ohm, R. A., et al. (2023). Lessons on fruiting body morphogenesis from genomes and transcriptomes of agaricomycetes. Stud. Mycol. 104, 1–85. doi: 10.3114/sim.2022.104.01
Paria, K., Pyne, S., and Chakraborty, S. K. (2022). Optimization of heavy metal (lead) remedial activities of fungi Aspergillus penicillioides (F12) through extra cellular polymeric substances. Chemosphere 286:131874. doi: 10.1016/j.chemosphere.2021.131874
Pereira, E. G., Amaral, M. B., Bucher, C. A., Santos, L. A., Fernandes, M. S., and Vieira Rossetto, C. A. (2021). Proline osmopriming improves the root architecture, nitrogen content and growth of rice seedlings. Biocatal. Agric. Biotechnol. 33:101998. doi: 10.1016/j.bcab.2021.101998
Ramsamy, Y., Mlisana, K. P., Amoako, D. G., Abia, A. L. K., Allam, M., Ismail, A., et al. (2020). Comparative pathogenomics of aeromonas veronii from pigs in south africa: dominance of the novel ST657 clone. Microorganisms 8:2008. doi: 10.3390/microorganisms8122008
Rana, B., Chandola, R., Sanwal, P., and Joshi, G. K. (2024). Unveiling the microbial communities and metabolic pathways of keem, a traditional starter culture, through whole-genome sequencing. Sci. Rep. 14:4031. doi: 10.1038/s41598-024-53350-3
Ruiu, L. (2020). Plant-growth-promoting bacteria (PGPB) against insects and other agricultural pests. Agronomy 10:861. doi: 10.3390/agronomy10060861
Samet, M., Ghazala, I., Karray, F., Abid, C., Chiab, N., Nouri-Ellouz, O., et al. (2022). Isolation of bacterial strains from compost teas and screening of their PGPR properties on potato plants. Environ. Sci. Pollut. Res. 29, 75365–75379. doi: 10.1007/s11356-022-21046-8
Sánchez, A., Oiza, N., Artola, A., Font, X., Barrena, R., Moral-Vico, J., et al. (2024). Solid-state fermentation: a review of its opportunities and challenges in the framework of circular bioeconomy. J. Chem. Eng. Theor. Appl. Chem. 81, 51–57. doi: 10.55815/424209
Shen, J., Wang, M., and Wang, E. (2024). Exploitation of the microbiome for crop breeding. Nat. Plants 10, 533–534. doi: 10.1038/s41477-024-01657-4
Shi, X., Zhao, Y., Xu, M., Ma, L., Adams, J. M., and Shi, Y. (2024). Insights into plant–microbe interactions in the rhizosphere to promote sustainable agriculture in the new crops era. New Crops 1:100004. doi: 10.1016/j.ncrops.2023.11.002
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V., and Zdobnov, E. M. (2015). BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212. doi: 10.1093/bioinformatics/btv351
Six, J., Paustian, K., Elliott, E. T., and Combrink, C. (2000). Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 64, 681–689. doi: 10.2136/sssaj2000.642681x
Sokol, N. W., Slessarev, E., Marschmann, G. L., Nicolas, A., Blazewicz, S. J., Brodie, E. L., et al. (2022). Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430. doi: 10.1038/s41579-022-00695-z
Starke, R., Mondéjar, R. L., Human, Z. R., Navrátilová, D., Štursová, M., Větrovský, T., et al. (2021). Niche differentiation of bacteria and fungi in carbon and nitrogen cycling of different habitats in a temperate coniferous forest: a metaproteomic approach. Soil Biol. Biochem. 155:108170. doi: 10.1016/j.soilbio.2021.108170
Storie, R. E. (1957). Methods of chemical analysis for soil survey samples. Agron. J. 49:1956. doi: 10.2134/agronj1957.00021962004900040024x
Stukenbrock, E., and Gurr, S. (2023). Address the growing urgency of fungal disease in crops. Nature 617, 31–34. doi: 10.1038/d41586-023-01465-4
Tong, J., Wu, H., Jiang, X., Ruan, C., Li, W., Zhang, H., et al. (2024). Dual regulatory role of penicillium oxalicum SL2 in soil: phosphorus solubilization and pb stabilization. Environ. Sci. Technol. 58, 603–616. doi: 10.1021/acs.est.3c08881
Wang, C., and Kuzyakov, Y. (2024). Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 18:wrae073. doi: 10.1093/ismejo/wrae073
Wang, L., Zhang, Y., Wang, Y., Suo, M., Wu, H., Zhao, M., et al. (2022). Inoculation with penicillium citrinum aids ginseng in resisting fusarium oxysporum by regulating the root and rhizosphere microbial communities. Rhizosphere 22:100535. doi: 10.1016/j.rhisph.2022.100535
Wang, Y., Shen, Y., Li, J., Wang, T., Peng, J., and Shang, X. (2025). Enhanced RNA secondary structure prediction through integrative deep learning and structural context analysis. Nucleic Acids Res. 53:533. doi: 10.1093/nar/gkaf533
Wang, Z., Zhang, S., Liang, J., Chen, H., Jiang, Z., Hu, W., et al. (2024). Rhizophagus irregularis regulates RiCPSI and RiCARI expression to influence plant drought tolerance. Plant Physiol. 197:645. doi: 10.1093/plphys/kiae645
Wu, H., Cui, H., Fu, C., Li, R., Qi, F., Liu, Z., et al. (2024). Unveiling the crucial role of soil microorganisms in carbon cycling: a review. Sci. Total Environ. 909:168627. doi: 10.1016/j.scitotenv.2023.168627
Wu, Y., Deng, D., Jiang, J., Li, F., Zeng, J., Guo, X., et al. (2023). Ca-driven stable regulatory of alkalinity within desilication products: experimental, modeling, transformation mechanism and DFT study. Sci. Total Environ. 868:161708. doi: 10.1016/j.scitotenv.2023.161708
Yang, T., Adams, J. M., Shi, Y., He, J., Jing, X., Chen, L., et al. (2017). Soil fungal diversity in natural grasslands of the tibetan plateau: associations with plant diversity and productivity. New Phytol. 215, 756–765. doi: 10.1111/nph.14606
Yao, J., Cui, R., Fang, B., Wang, S., Ye, X., Liu, Z., et al. (2025). Transcription factor BnaA1.WRKY53 is involved in regulation of auxin-induced leaf curling under boron deficiency in brassica napus. Crop J. 13, 1068–1080. doi: 10.1016/j.cj.2025.05.001
Zhang, M., Zhang, L., Li, J., Huang, S., Wang, S., Zhao, Y., et al. (2025). Nitrogen-shaped microbiotas with nutrient competition accelerate early-stage residue decomposition in agricultural soils. Nat. Commun. 16:5793. doi: 10.1038/s41467-025-60948-2
Zhang, N., Nunan, N., Hirsch, P. R., Sun, B., Zhou, J., and Liang, Y. (2021). Theory of microbial coexistence in promoting soil–plant ecosystem health. Biol. Fertil. Soils 57, 897–911. doi: 10.1007/s00374-021-01586-w
Zhang, Y., Gao, H., Zhao, D., Chen, X., Zhu, F., Li, Y., et al. (2024). Microbially-driven alkaline regulation: organic acid secretion behavior of penicillium oxalicum and charge neutralization in bauxite residue. Environ. Res. 240:117489. doi: 10.1016/j.envres.2023.117489
Zheng, Y., Cao, X., Zhou, Y., Ma, S., Wang, Y., Li, Z., et al. (2024). Purines enrich root-associated pseudomonas and improve wild soybean growth under salt stress. Nat. Commun. 15:3520. doi: 10.1038/s41467-024-47773-9
Keywords: Penicillium kloeckeri, whole-genome sequencing, untargeted metabolomics, soybean hydroponic experiment, saline alkali soil
Citation: Cui W, Wu Y, Ni B and Cao J (2025) Whole genome sequence of Penicillium kloeckeri and insight into its growth-promoting, saline alkaline tolerance properties. Front. Microbiol. 16:1675497. doi: 10.3389/fmicb.2025.1675497
Received: 29 July 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Mudasir A. Dar, Jiangsu University, ChinaReviewed by:
Abdul Basit, University of Jhang, PakistanChunli Zeng, Nanjing Agricultural University, China
Copyright © 2025 Cui, Wu, Ni and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Cao, amlhLmNhb0BjYXUuZWR1LmNu
 Wenxiao Cui1,2,3
Wenxiao Cui1,2,3 Yafen Wu
Yafen Wu Bin Ni
Bin Ni Jia Cao
Jia Cao