Abstract
Background: Intracranial hemorrhage (ICH) is a common complication in adults treated with extracorporeal membrane oxygenation (ECMO).
Objectives: The aim of this study was to conduct a systematic review of the literature on the incidence, outcome and predictors of ECMO-associated ICH in adult patients, supplemented by a narrative review of its pathophysiology, management and future perspectives.
Methods: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews and www.clinicaltrials.gov were systematically searched. Studies that reported incidence, outcome or predictors of ECMO-associated ICH in adults (≥18 years) were eligible for inclusion.
Results: Twenty five articles were included in the systematic review. The incidence of ECMO-associated ICH varied between 1.8 and 21 %. Mortality rates in ICH-cohorts varied between 32 and 100 %, with a relative risk of mortality of 1.27–4.43 compared to non-ICH cohorts. An increased risk of ICH was associated with ECMO-duration, antithrombotic therapy, altered intrinsic coagulation, renal failure, need of blood products, rapid hypercapnia at ECMO initiation, and even pre-ECMO morbidity.
Conclusions: ICH is a common complication in adults treated with ECMO and associated with increased mortality. Treating an ICH during ECMO represents a balance between pro- and anticoagulatory demands. Neurosurgical treatment is associated with severe morbidity, but has been successful in selected cases. Future studies should aim at investigating the validity and feasibility of non-invasive monitoring in early detection of ECMO-associated ICH.
Background
Rationale
Extracorporeal membrane oxygenation (ECMO) is being used more frequently in adults (1–5), and is now recognized as an important part in the treatment of severe reversible refractory respiratory and/or circulatory failure (1–3). However, the treatment itself is associated with significant morbidity and mortality (6), and intracranial hemorrhage (ICH) is one of the most frequent serious adverse events occurring during ECMO support (7–9). In fact, during the H1N1 pandemic in Australia and New Zealand, ICH was the most common cause of death among ECMO treated patients (10). Despite this, there are no established guidelines on its detection, prevention or management (11).
Objectives
The aim of this study was to review the literature on ICH in ECMO-treated adult patients. This was performed by conducting a systematic review of the literature on the incidence, outcome, and predictors of ECMO-associated ICH in adults, supplemented by a narrative review of its pathophysiology, management, and future perspectives.
Methods
Search strategy and selection criteria
This review was performed by searching the following databases from their dates of inception until January 2017: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, and the clinical trial registry www.clinicaltrials.gov. A search strategy for MEDLINE and EMBASE was decided on (Supplementary File 1), with a similar search strategy utilized for the other databases. There was no specific restriction on study methodological quality. The titles and abstracts were independently screened to determine if they met the inclusion criteria. Full texts of the chosen articles were assessed to confirm this. Reference lists of relevant articles were screened for additional studies.
Inclusion and exclusion criteria
All studies that reported incidence, outcome or predictors of ECMO-associated ICH in adults (≥18 years) were included. ICH was defined as an intraparenchymal hemorrhage (IPH), subdural hemorrhage (SDH), and/or subarachnoid hemorrhage (SAH). Studies were excluded if they were non-English or if it was impossible to deduce the data specifically related to ICH in ECMO-treated adults—for example if they failed to specify patient age, or had grouped ICH and other neurological complications (e.g., ischemic stroke) together and analyzed these as one entity.
Data abstraction
Using a customized form, data were extracted from the included articles and stored in an electronic database. Where applicable, the following data were abstracted: study design, study length, amount of patients included, percentage of patients treated with V-A ECMO, amount of ICH cases, ICH characteristics, outcome studied, mortality rate, and risk factors for ICH development.
Data analysis
A systematic analysis was performed comparing mortality rates in ICH vs. non-ICH adult ECMO cohorts, with data reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12) (Supplementary File 2). We presented the binary data as risk ratios with 95% confidence intervals and p-values. Additional systematic analyses, including meta-analysis, were not performed due to the heterogeneity of data and study design of the included articles. The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Results
Study selection and characteristics
The initial literature search yielded 2,985 articles. 2,942 articles were excluded following removal of duplicates and title and abstract review. Following full-text review of the remaining 43 articles, 25 were included in the systematic analysis (Figure 1).
Figure 1
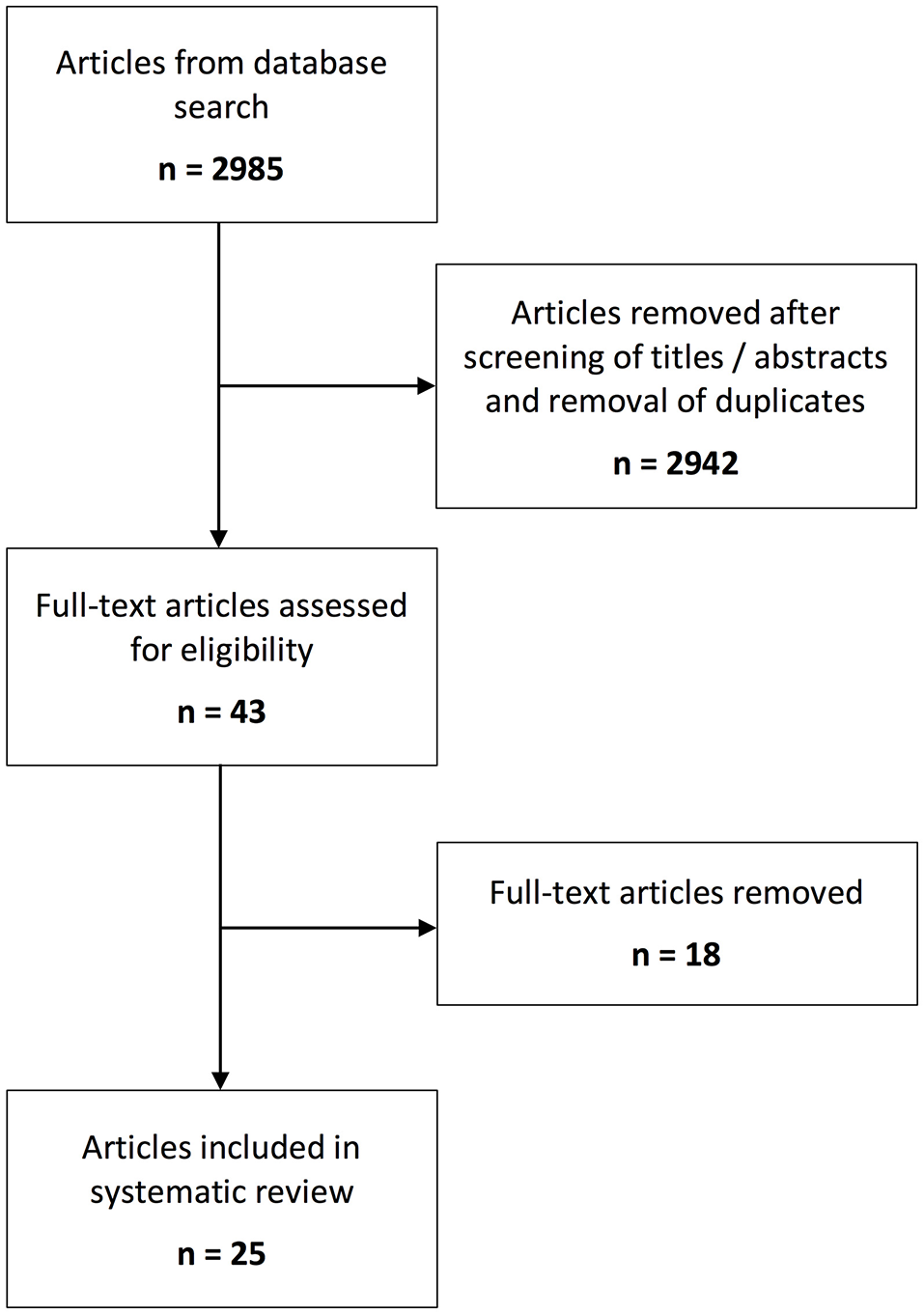
Schematic overview of the number of identified records for the systematic steps of the review process.
Synthesized findings
The reported incidence of ICH in adults during ECMO varied between 1.8 and 21% (4, 5, 13–22) (Table 1). The vast majority of studies were retrospective, and only one prospective cohort study was included.
Table 1
| Study | Study design | Study length (years) | Patients (n) | % V-A ECMO | ICH (n) | ICH rate (%) | ICH characteristics |
|---|---|---|---|---|---|---|---|
| Fletcher-Sandersjöö et al. 23 | Single-center, retrospective | 10.8 | 253 | 36 | 54 | 21.3 | 76% intraparenchymal hemorrhage (IPH), 2% subdural hemorrhage (SDH), 22% subarachnoid hemorrhage (SAH) |
| Kasirajan et al. 14 | Single-center, retrospective | 5.0 | 74 | 100 | 14 | 18.9 | ICH was defined as IPH and/or intraventricular hemorrhage |
| Klinzing et al. 15 | Single-center, retrospective | 6.0 | 74 | 27 | 8 | 10.8 | 25% >1 type, 88% IPH, 38% SAH |
| Lockie et al. 16 | Single-center, retrospective | 4.2 | 250 | 0 | 41 | 16.4 | 37% > 1 type, 20% large IPH, 39% petechial IPH, 56% SAH, 2% SDH |
| Lorusso et al. 18 | Multicenter, ELSO data registry | 22.0 | 4,522 | 100 | 80 | 1.8 | NR |
| Lorusso et al. 17 | Multicenter, ELSO data registry | 24.0 | 4,988 | 0 | 181 | 3.6 | NR |
| Luyt et al. 19 | Single-center, retrospective | 7.0 | 135 | 0 | 10 | 7.5 | NR |
| Nasr and Rabinstein 4 | Multicenter, Nationwide inpatient sample | 11.0 | 8,397 | NR | 239 | 2.9 | NR |
| Omar et al. 20 | Single-center, retrospective | 7.0 | 154 | 81 | 12 | 7.8 | NR |
| Paden et al. 21 | Multicenter, ELSO data registry | NR | NR | NR | NR | 3.9 | NR |
| Rastan et al. 24 | Single-center, prospective | 12.0 | 517 | 100 | 19 | 3.7 | NR |
| Smedira et al. 22 | Single-center, retrospective | 7.5 | 202 | 100 | 13 | 6.4 | NR |
Studies reporting ICH rates in adult ECMO cohorts.
ICH, intracranial hemorrhage; IPH, intraparenchymal hemorrhage; SDH, subdural hemorrhage; SAH, subarachnoid hemorrhage; ECMO, extracorporeal membrane oxygenation; ELSO, Extracorporeal Life Support Organization; V-A, venoarterial; NR, not reported
Survival rates in adult ECMO patients who developed ICH was low, with a reported mortality rate of 32–100% (7, 10, 14–17, 19, 20, 23, 25). A systematic analysis comparing outcome between ICH and non-ICH cohorts is presented in Table 2. Of these, the two largest studies (n > 200) reported a 2.91 and 1.48 relative risk of mortality comparing their ICH and non-ICH cohort (13, 16), while the two studies including the smallest cohorts reported the highest relative risk [4.43 and 3.28, respectively] (10, 25) (Table 2).
Table 2
| Study | Study design | Patients (n) | % V-A ECMO | Outcome studied | ICH | No ICH | RR | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-survivors (n) | Survivors (n) | Non-survivors (n) | Survivors (n) | ||||||||
| Aubron et al. 7 | Multicenter, retrospective | 149 | 74 | Hospital mortality | 5 | 0 | 48 | 96 | 3.00 | 2.38–3.78 | <0.0001 |
| Davies et al. 10 | Multicenter, retrospective | 68 | 7 | ICU mortality | 6 | 0 | 14 | 48 | 4.43 | 2.79–7.02 | <0.0001 |
| Fletcher-Sandersjöö et al. 23 | Single-center, retrospective | 253 | 36 | 30 day mortality | 44 | 10 | 52 | 134 | 2.91 | 2.24–3.79 | <0.0001 |
| Kasirajan et al. 14 | Single-center, retrospective | 74 | 100 | ICU mortality | 13 | 1 | 36 | 24 | 1.55 | 1.20–1.99 | 0.001 |
| Klinzing et al. 15 | Single-center, retrospective | 74 | 27 | 6 month mortality | 7 | 1 | 35 | 31 | 1.65 | 1.17–2.33 | 0.005 |
| Lockie et al. 16 | Single-center, retrospective | 250 | 100 | ICU mortality | 13 | 28 | 44 | 162 | 1.48 | 0.88–2.50 | 0.137 |
| Luyt et al. 19 | Single-center, retrospective | 135 | 0 | ICU mortality | 7 | 3 | 46 | 81 | 1.93 | 1.21–3.08 | 0.006 |
| Omar et al. 20 | Single-center, retrospective | 154 | 81 | Hospital mortality | 10 | 2 | 93 | 49 | 1.27 | 0.96–1.68 | 0.092 |
| Patroniti et al. 25 | Multicenter, retrospective | 60 | 2 | ICU mortality | 1 | 0 | 18 | 41 | 3.28 | 2.23–4.82 | <0.0001 |
Systematic analysis of mortality rates between ICH and non-ICH adult ECMO cohorts.
ECMO, extracorporeal membrane oxygenation; ICH, intracranial hemorrhage; V-A, venoarterial; RR, risk ratio; CI, confidence interval
While many studies on ICH predictors combined ischemic and hemorrhagic stroke in outcome models (15, 17, 18, 20), four studies analyzed predictors of solely ICH in adult ECMO patients (13, 14, 19, 20). They found that an increased risk of ICH was associated with ECMO-duration (20), pre- and per-ECMO antithrombotic therapy (defined as antithrombotic therapy administered prior to, or during, ECMO treatment) (13, 14), altered intrinsic coagulation (13, 14, 20), renal failure (19), and even pre-ECMO morbidity (13), to name a few. The results are summarized in Table 3. A meta-analysis of ICH predictors was not performed due to the heterogeneity of data.
Table 3
| Risk factor | Study |
|---|---|
| ECMO duration | Omar et al. 20 |
| Female gender | Kasirajan et al. 14 |
| Pre-admission antithrombotic therapy | Fletcher-Sandersjöö et al. 23 |
| Pre-cannulation SOFA coagulation score | Fletcher-Sandersjöö et al. 23 |
| Thrombocytopenia | Kasirajan et al. 14, Fletcher-Sandersjöö et al. 23 |
| Extracranial bleeding | Omar et al. 20, Fletcher-Sandersjöö et al. 23 |
| Platelet transfusion volume | Omar et al. 20, Fletcher-Sandersjöö et al. 23 |
| RBC transfusion volume | Fletcher-Sandersjöö et al. 23 |
| ACT-levels | Omar et al. 20 |
| Use of heparin | Kasirajan et al. 14 |
| Dialysis | Kasirajan et al. 14 |
| Renal failure at admission | Luyt et al. 19 |
| Hypercreatinemia | Kasirajan et al. 14 |
| PaCO2 decrease at ECMO initiation | Luyt et al. 19 |
| PaO2 increase at ECMO initiation | Luyt et al. 19 |
Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation.
ECMO, extracorporeal membrane oxygenation; SOFA, sepsis organ failure assessment (also known as Severity Organ Failure Assessment); RBC, red blood cell; ACT, activated clotting time; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen
Risk of bias
Using the “RTI” item bank (26), bias was assessed in each study that was included in the systematic analysis. Each risk of bias item was graded as either Low-risk or High-risk (Supplementary File 3).
Discussion
Summary of main findings
We conducted a systematic review of the incidence, outcome and predictors of ECMO associated ICH in adults. Twenty-five articles were included. We found an ICH-incidence between 1.8 and 21%. Developing an ICH was associated with a mortality of 32–100%, with a relative risk of mortality of 1.27–4.43 in patients that developed ICH as compared to those that did not. To the best of our knowledge, this is the first review of ICH in ECMO-treated adult patients and contributes findings that are important for patient management and future study design.
Incidence
The reported incidence of ICH in adults during ECMO varied between 1.8 and 21 % (4, 5, 13–22) (Table 1). It is important to note that sedatives and muscle relaxants used during ECMO can mask symptoms of brain injury, resulting in the fact that several ECMO-associated ICH diagnoses are made in the absence of neurological symptoms (15, 23, 27). Moreover, while a CT scan is the gold standard to detect an ICH, in some cases ICH was only detected on magnetic resonance imaging (MRI) (28, 29) or post-mortem autopsies (24, 30). If these tools had been used more frequently, the incidence would presumably have been higher in many studies. Solely relying on neurological assessment before performing a CT scan may, therefore, not be sensitive enough (27, 31). To combat this, some centers perform routine screening cerebral CT scans (13, 16, 31), even though it involves exposure to radiation and potential risks associated with transportation of ECMO patients (32, 33). Centers performing regular screening CT scans had among the highest rate of ICH of the studies included in the analysis (13, 16) (Table 1). Thus, we presume the variation of reported ICH incidence is influenced by centers' routines for performing brain imaging, as well as differences in risk factors between the analyzed cohorts and variability in enrollment criteria.
Outcome
Survival from ECMO is generally poor, with studies from the Extracorporeal Life Support Organization (ELSO) registry reporting a mortality rate of 38% in V-V ECMO, and 57% in V-A ECMO, patients without neurological complications (17, 18). The systematic review showed a relative risk of mortality of 1.27–4.43 in patients that developed ICH, as compared to those that did not. Only two of the studies showed a non-significant difference (Table 2). Interestingly, one of them was from a center that performed cerebral CT scans at ECMO initiation and adjusted the anti-thrombotic regimen accordingly in those with an ICH present (16). The reasons behind the variance in mortality rates between studies are presumably due to differences in ICH risk factors, variability in enrollment criteria, and variation in clinical thresholds to use CT imaging. For example, a center performing screening CT scans would be more likely to diagnose an ICH with a minor effect on outcome. Unfortunately, the type of ICH and whether it was symptomatic was not described in most of the included studies and therefore not analyzed. The presence of an ICH during ECMO may also result in withdrawal of further therapy (23), thereby introducing a selection bias as it may be considered futile to escalate management in ECMO patients with ICH. Thus, our results indicate that ICH in ECMO patients is generally associated with increased mortality, but that the mortality rates varied between studies.
Pathophysiology
Alteration in hemostasis is likely a significant mechanism behind ECMO-associated ICH development. ECMO support in and of itself results in thrombocytopenia, factor XIII deficiency, acquired von Willebrand syndrome, fibrinogen deficiency, and pump-induced platelet dysfunction (14, 17, 34–42). Additionally, activation of factor X, and the ensuing production of thrombin, may contribute to further imbalance through consumption of clotting factors (41). All of the above is probably exacerbated by the systemic anticoagulation used to facilitate ECMO (43, 44), and the lack of change in ECMO anticoagulation regimens might be one of the reasons behind the stagnation in rates of ICH occurrence and mortality (17).
Another contributing factor is the systemic inflammatory response that can develop due to activation of circulating blood cells in the ECMO circuit (45), causing simultaneous thrombocytopenia and consumptive coagulopathy (34), as well as the possibility of embolus formation from the cannula that can lead to ischemic stroke in venoarterial (V-A) ECMO and ensuing ICH (27). In a porcine venovenous (V-V) ECMO model, ECMO treatment resulted in increased pro-inflammatory response in cerebral tissue vs. non-ECMO treated animals, highlighting the fact that brain tissue specific inflammation may play a role in the pathophysiology of ICH development (46).
Pre-ECMO factors may also play a role in ICH development. In V-A ECMO, factors related to cardiogenic shock (for example low cerebral blood flow, hypoxia, acidosis, and hemostatic disorders due to liver failure) and reperfusion injury at ECMO initiation can precipitate brain injury (30, 44). In V-V ECMO, abrupt CO2, or O2 changes at ECMO cannulation can disrupt cerebral perfusion (47, 48), which is further decreased by the use of potent sedatives (49), and has been linked to cerebral desaturation during ECMO initiation (50, 51), as well as impairing cerebral autoregulation which in turn can precede ischemic stroke leading to ICH (19, 52, 53).
Finally, it's noteworthy that given the arbitrary timing of CT-imaging in adult ECMO patients, ICH may result from lesion development before ECMO treatment has even commenced. Supporting this, in one study where cerebral CT scans were performed in all adult ECMO patients at the start of treatment, as many as 16% had an ICH present (16).
Thus, it is likely that ICH etiology is multifactorial, including pre-ECMO morbidity, hemostasis and inflammation.
Does V-A ECMO increase the risk of ICH?
It is believed that patients on V-A ECMO are more prone to bleeding complications compared to V-V patients, both due to difference in the underlying clinical condition and comorbidities (7) as well as the ECMO treatment itself. In our systematic review, we could not see any indication that V-A ECMO neither caused an increased risk of ICH, nor that these ICH patients had an increased mortality (Tables 1, 2). However, even though we could not find any association, this is often a matter for debate in the literature. On one hand, it is believed that patients on V-A ECMO are at increased risk of systemic thromboembolism from thrombus formation within the ECMO unit (19), since the blood is returned directly into the arteries without the lungs functioning as a filter. This has been confirmed by studies from the ELSO Registry (17, 18). Moreover, during V-A ECMO cerebral perfusion is mainly non-pulsatile (unless combined with an intra-aortic balloon pump) (54), although it is unclear whether this affects the risk of ICH. In addition to differences in ECMO treatment itself, there is a difference in the underlying clinical condition and comorbidities (7). Despite this, one study from the ELSO Registry found that ICH was twice as likely to occur in V-V ECMO patients compared to V-A (17, 18), and single-center studies of V-A and V-V ECMO adult patients have not identified ECMO-mode as a predictor of ICH development (13, 20). Thus, additional research is needed to determine if V-A ECMO really does increase the risk of ICH in adult ECMO patients.
Management
Treating an ICH during ECMO represents a difficult balance between pro- and anticoagulatory demands. Hematoma components, patient characteristics, and other predictors of outcome need to be assessed to determine the best course of action (23). Only one single-center retrospective cohort study has described the management of ICH in adults during ECMO support (23). In this study, a decision was made to withdraw life-sustaining ECMO therapy in 42% of the ICH patients, no intervention was undertaken in 18% because the ICH was deemed to be of minimal clinical importance, and 40% of patients had medical and/or surgical treatment. Interestingly, in patients where no intervention was done there were no deaths attributed to the ICH, presumably due to smaller hematoma volumes and/or non-critical ICH location (23).
While surgical management may be indicated when an ICH occurs during ECMO, the associated anticoagulation presents a considerable risk. Moreover, time restraints allow limited opportunity for pre-operative optimization of coagulation, other than immediate heparin reversal, to decrease intra- and postoperative blood loss and hematoma progression. We have identified nine cases of surgical intervention in adult patients with ECMO-associated ICH (23, 55–58), with two survivors (23, 57). Thus, neurosurgical intervention in patients with ongoing anticoagulation is extremely hazardous, but the two successful case reports suggest that it might be an option in well-selected patients where no other management strategies are available.
Future perspectives
Given the increasing utilization of ECMO and the poor outcomes associated with ICH, more research is needed to determine the best way to prevent ICH from occurring and/or progressing. A better understanding of the pathophysiology and predictors of ECMO-associated ICH will facilitate identification of patients who are more prone to developing the complication, and where more rigorous neurological checks, earlier weaning from ECMO, or alternatives for anticoagulation could be considered.
Hemostasis
There have been several successful case reports of V-V and V-A ECMO treatment without systemic anticoagulation in hemorrhagic patients (59–61), as well as on patients requiring ECMO after traumatic brain injury (62–65). Moreover, a study comparing anticoagulation guided with activated clotting time (ACT) levels of 180–220 s (s) vs. 140–160 s showed no difference in oxygenator failure caused by clotting (66). In another study of adult V-V ECMO patients, prophylactic correction of coagulation factor deficiencies led to a reduction in the rate of ICH (34). In rabbits, recombinant fully human antibody 3F7, which interferes with activated factor XII (FXIIa) mediated coagulation, provided thrombo-protection as efficiently as heparin but did not impair the hemostatic capacity or increase bleeding from wounds, suggesting that FXIIa targeting could work as a anticoagulation strategy not associated with excess bleeding (67). Another V-V ECMO study evaluated a protocol where 61 patients were assigned subcutaneous enoxaparin exclusively, and no cases of ICH or oxygenator change due to clotting were observed (68). Lastly, the pilot study for the HELP-ECMO study, evaluating normal vs. low-dose heparin in adult patients, resulted in significant decreases in daily activated partial thromboplastin time and anti-Xa levels but no difference in thromboembolic or bleeding events, thus supporting the feasibility of a phase III study evaluating the effectiveness of low-dose anticoagulation in adult patients during ECMO (69).
Considering the fact that ECMO causes platelet dysfunction (42), which can lead to the development of ICH even in the absence of thrombocytopenia (70), one should also consider the value of performing regular platelet function tests (such as platelet aggregometry, Multiplate®) during treatment, which has revealed different temporal trajectories of platelet receptor activity following traumatic brain injury (71). Tentative evidence from smaller studies indicate that multiplate values during ECMO may facilitate in the detection of patients at risk of bleeding events (72, 73).
Non-invasive neurological monitoring
There is a difficulty associated with neurological assessment of ECMO patients, and the fact that invasive monitoring procedures are associated with a high risk of uncontrolled bleeding and death (23), non-invasive neurological monitoring could provide a suitable option for these patients in order to detect ICH development and initiate eventual treatment efforts at an earlier stage. This includes, but is not limited to, protein biomarkers of brain injury (74), cerebral near infrared spectroscopy (NIRS) (75), and transcranial doppler (TCD) to assess dynamics of intracerebral vessels and to assess the intracranial pressure (ICP) (76).
S100B, a biomarker used to monitor treatment effect and detect secondary brain damage (77–79), or rule out traumatic ICH after moderate-to-mild TBI (80), has been assessed during ECMO in a few smaller studies. A case-control study showed increased S100B levels in ECMO-treated infants with ICH up to 72 h before they were detectable on a cranial ultrasound (81). Another case-control study of 15 ECMO-treated patients found that S100B was significantly higher in the group with cerebral complications (82). Bembea et al. found that the biomarkers S100B, neuron specific enolase (NSE), glial fibrillary acidic protein (GFAP), and monocyte chemoattractant protein 1 (MCP1) levels were higher in non-survivors and in patients with unfavorable outcome in neonatal and pediatric ECMO cohorts (83). Another study showed that GFAP was elevated 1–2 days before neurological injury was diagnosed using neuroimaging in pediatric ECMO patients (84). High NSE levels after extracorporeal cardiopulmonary resuscitation have also been shown to correspond to poor neurological outcome and mortality (85). Thus, there is tentative evidence of biomarkers' utility to detect ICH during ECMO treatment, but larger trials investigating their clinical usefulness as part of a decision algorithm to perform a head CT or other imaging to detect ECMO-associated ICH in adults are warranted.
NIRS uses near-infrared light to measure trends in cerebral oxygenation (86). In neonatal ECMO patients, case series have reported decreased brain tissue oxygenation in patients that later demonstrated cerebral injury on neuroimaging (87). Only one study has studied cerebral NIRS in adults, finding that cerebral oxygenation significantly responded to hemodynamic intervention and that non-responders were more likely to have ischemic cerebral complications (88).
We have not found any studies that have evaluated the use of TCD for detection of ICH in adult ECMO patients. However, in a pediatric ECMO study TCD detected abnormally high blood flow velocities several days prior to detection of an ICH (89), thus warranting further studies into the clinical utility of TCD to predict ICH in ECMO patients.
Limitations
There are some limitations to this study that should be mentioned. First and foremost, ECMO cohorts carry an inherent heterogeneity due to factors such as variations in centers' patient acceptance criteria and disease panorama. Moreover, due to a lack of data we were not able to analyze several established predictors of ICH mortality, such as hematoma volume, hematoma location, and secondary complications (90–92). We also included several forms of ICH (i.e., IPH, SDH, and SAH) in common analysis. Thus, we were not able to perform more extensive systematic analysis, such as meta-analysis of mortality rates or risk factors for ICH development.
Conclusion
ICH is a common complication in adults treated with ECMO and associated with increased mortality. Evidence shows that it is to some extent caused by both pre-ECMO morbidity and ECMO-induced disruption of hemostasis. Treating an ICH during ECMO represents a complicated balance between pro- and anticoagulatory demands. Neurosurgical treatment is associated with severe morbidity, but has been successful in selected cases. A better understanding of the pathophysiology and predictors of ECMO-associated ICH may help reduce its incidence. Moreover, prospective trials are warranted to investigate the validity and feasibility of non-invasive neuromonitoring in early detection of the complication.
Statements
Author contributions
AF-S, ET, and JB study design. AF-S data collection. AF-S and ET statistical analysis. All authors data interpretation, revision and approval of manuscript. AF-S draft of manuscript. BB study supervision.
Funding
ET is supported by post-doctoral scholarships from the Swedish Society for Medical Research. The funder had no role in the design of the study or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers SP and NO and handling Editor declared their shared affiliation.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00548/full#supplementary-material
- ACT
Activated clotting time
- CT
Computed tomography
- ECMO
Extracorporeal membrane oxygenation
- ELSO
Extracorporeal Life Support Organization
- FXIIa
Activated factor XII
- GFAP
Glial fibrillary acidic protein
- ICP
Intracranial pressure
- IPH
Intraparenchymal pressure
- MCP1
Monocyte chemoattractant protein 1
- MRI
Magnetic resonance imaging
- NIRS
Near infrared spectroscopy
- NSE
Neuron specific enolase
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-
- SAH
Subarachnoid hemorrhage
- SDH
Subdural hemorrhage
- TCD
Transcranial doppler
- V-A
Venoarterial
- V-V
Venovenous.
Abbreviations
References
1.
GerkeAKTangFCavanaughJEDoerschugKCPolgreenPM. Increased trend in extracorporeal membrane oxygenation use by adults in the United States since 2007. BMC Res Notes (2015) 8: 686. 10.1186/s13104-015-1678-7
2.
McCarthyFHMcDermottKMKiniVGutscheJTWaldJWXieD. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002-2012. Semin Thorac Cardiovasc Surg. (2015) 27:81–8. 10.1053/j.semtcvs.2015.07.005
3.
SauerCMYuhDDBondeP. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J (2015) 61:31–6. 10.1097/MAT.0000000000000160
4.
NasrDMRabinsteinAA. Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol. (2015) 11:383–9. 10.3988/jcn.2015.11.4.383
5.
RastanAJDegeAMohrMDollNFalkVWaltherTet al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. (2010) 139:302–11.e1. 10.1016/j.jtcvs.2009.10.043
6.
AubronCChengACPilcherDLeongTMagrinGCooperDJet al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care (2013) 17:R73. 10.1186/cc12681
7.
AubronCDePuydtJBelonFBaileyMSchmidtMSheldrakeJet al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care (2016) 6: 97. 10.1186/s13613-016-0196-7
8.
GattinoniLCarlessoELangerT. Clinical review: extracorporeal membrane oxygenation. Crit Care (2011) 15:243. 10.1186/cc10490
9.
CombesABacchettaMBrodieDMüllerTPellegrinoV. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care (2012) 18:99–104. 10.1097/MCC.0b013e32834ef412
10.
DaviesAJonesDBaileyMBecaJBellomoRBlackwellNet al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA (2009) 302:1888. 10.1001/jama.2009.1535
11.
XieAMdBLoPYanTDForrestP. Neurologic complications of extracorporeal membrane oxygenation: a review. J Cardiothorac Vasc Anesth. (2017) 31:1836–46. 10.1053/j.jvca.2017.03.001
12.
PanicNLeonciniEde BelvisGRicciardiWBocciaS. Evaluation of the Endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE (2013) 8:e83138. 10.1371/journal.pone.0083138
13.
FletcherSandersjöö ABartekJThelinEPErikssonAElmi-TeranderABromanMet al. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care (2017) 5:27. 10.1186/s40560-017-0223-2
14.
KasirajanVSmediraNGMccarthyJFCasselmanFBoparaiNMcCarthyPM. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardio-thoracic Surg. (1999) 15:508–14. 10.1016/S1010-7940(99)00061-5
15.
KlinzingSWengerUStrettiFSteigerPRushingEJSchwarzUet al. Neurologic injury with severe adult respiratory distress syndrome in patients undergoing extracorporeal membrane oxygenation. Anesth Analg. (2017) 125:1544–8. 10.1213/ANE.0000000000002431
16.
LockieCJAGillonSABarrettNATaylorDMazumderAParameshKet al. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med. (2017) 45:1642–9. 10.1097/CCM.0000000000002579
17.
LorussoRGelsominoSPariseODi MauroMBariliFGeskesGet al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. (2017) 45:1389–97. 10.1097/CCM.0000000000002502
18.
LorussoRBariliFDi MauroMGelsominoSPariseORycusPTet al. In-Hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. (2016) 44:e964–72. 10.1097/CCM.0000000000001865
19.
LuytC-EBréchotNDemondionPJovanovicTHékimianGLebretonGet al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. (2016) 42:897–907. 10.1007/s00134-016-4318-3
20.
OmarHRMirsaeidiMMangarDCamporesiEM. Duration of ECMO is an independent predictor of intracranial hemorrhage occurring during ECMO support. ASAIO J. (2016) 62:634–6. 10.1097/MAT.0000000000000368
21.
PadenMLConradSARycusPTThiagarajanRR. Extracorporeal life support organization registry report 2012. ASAIO J (2013) 59:202–10. 10.1097/MAT.0b013e3182904a52
22.
SmediraNGMoazamiNGoldingCMMcCarthyPMApperson-HansenCBlackstoneEHet al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. (2001) 122:92–102. 10.1067/mtc.2001.114351
23.
Fletcher-SandersjööAThelinEPBartekJElmi-TeranderABromanMBellanderB-M. Management of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation (ECMO): an observational cohort study. PLoS ONE (2017) 12:e0190365. 10.1371/journal.pone.0190365
24.
RastanAJLachmannNWaltherTDollNGradistanacTGommertJFet al. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO). Int J Artif Organs (2006) 29:1121–31. 10.1177/039139880602901205
25.
PatronitiNZangrilloAPappalardoFPerisACianchiGBraschiAet al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. (2011) 37:1447–57. 10.1007/s00134-011-2301-6
26.
ViswanathanMBerkmanND. Item Bank for Assessment of Risk of Bias and Precision for Observational Studies of Interventions or Exposures. Agency for Healthcare Research and Quality (US) (2011).
27.
RisnesIWagnerKNomeTSundetKJensenJHynåsIAet al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. (2006) 81:1401–7. 10.1016/j.athoracsur.2005.10.008
28.
LiebeskindDSSanossianNSapoMLSaverJL. Cerebral microbleeds after use of extracorporeal membrane oxygenation in children. J Neuroimaging (2013) 23:75–8. 10.1111/j.1552-6569.2012.00723.x
29.
Le GuennecLBertrandALaurentCRozeHChastreJCombesAet al. Diffuse cerebral microbleeds after extracorporeal membrane oxygenation support. Am J Respir Crit Care Med. (2015) 191:594–6. 10.1164/rccm.201411-2118LE
30.
MateenFJMuralidharanRShinoharaRParisiJSchearsGWijdicksE. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. (2011) 68:1543–9. 10.1001/archneurol.2011.209
31.
LidegranMKMosskinMRingertzHGFrencknerBPLindénVB. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: clinical benefits in diagnosis and treatment. Acad Radiol. (2007) 14:62–71. 10.1016/j.acra.2006.10.004
32.
MALovellMYMudaliarPKlineberg. Intrahospital transport of critically ill patients: complications and difficulties. Anaesth Intensive Care (2001) 29:400–5.
33.
AndrewsPJDPiperIRDeardenNMMillerJD. Secondary insults during intrahospital transport of head-injured patients. Lancet (1990) 335:327–30. 10.1016/0140-6736(90)90614-B
34.
KalbhennJWittauNSchmutzAZiegerBSchmidtRKalbhennJ. Identification of acquired coagulation disorders and effects of target-controlled coagulation factor substitution on the incidence and severity of spontaneous intracranial bleeding during veno-venous ECMO therapy. Perfusion (2015) 30:675–82. 10.1177/0267659115579714
35.
KalbhennJSchmidtRNakamuraLSchellingJRosenfelderSZiegerB. Early diagnosis of acquired von Willebrand Syndrome (AVWS) is elementary for clinical practice in patients treated with ECMO therapy. J Atheroscler Thromb. (2015) 22:265–71. 10.5551/jat.27268
36.
HeilmannCGeisenUBeyersdorfFNakamuraLBenkCTrummerGet al. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Intensive Care Med. (2012) 38:62–8. 10.1007/s00134-011-2370-6
37.
McmanusMLKevyS VBowerLKHickeyPR. Coagulation factor deficiencies during initiation of extracorporeal membrane oxygenation. J Pediatr (1994) 126:900–4. 10.1016/S0022-3476(95)70205-9
38.
HirthlerMAABlackwellEAbbeDDoe-ChapmanRLeClair SmithCGoldthornJet al. Coagulation parameter instability as an early predictor of intracranial hemorrhage during extracorporeal membrane oxygenation. J Pediatr Surg. (1992) 27:40–3. 10.1016/0022-3468(92)90101-C
39.
EsperSALevyJHWatersJHWelsbyIJ. Extracorporeal membrane oxygenation in the adult. Anesth Analg. (2014) 118:731–43. 10.1213/ANE.0000000000000115
40.
HamptonCRVerrierED. Systemic consequences of ventricular assist devices: alterations of coagulation, immune function, inflammation, and the neuroendocrine system. Artif Organs (2002) 26:902–8. 10.1046/j.1525-1594.2002.07122.x
41.
RaitenJMWongZZSpeldeALittlejohnJEAugoustidesJGTGutscheJT. Anticoagulation and transfusion therapy in patients requiring extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. (2017) 31:1051–9. 10.1053/j.jvca.2016.08.011
42.
HalaweishIColeACooleyELynchWRHaftJW. Roller and centrifugal pumps: a retrospective comparison of bleeding complications in extracorporeal membrane oxygenation. ASAIO J (2015) 61:496–501. 10.1097/MAT.0000000000000243
43.
Extracorporeal Life Support Organization. ELSO Anticoagulation Guideline. Available online at: https://www.elso.org/Resources/Guidelines.aspx [Accessed January 29, 2018]
44.
ChowFCEdlowBLFroschMPCopenWAGreerDM. Outcome in patients with H1N1 influenza and cerebrovascular injury treated with extracorporeal membrane oxygenation. Neurocrit Care (2011) 15:156–60. 10.1007/s12028-011-9534-7
45.
GraberLCQuillinanNMarrotteEJMcDonaghDLBartelsK. Neurocognitive outcomes after extracorporeal membrane oxygenation. Best Pract Res Clin Anaesthesiol. (2015) 29:125–35. 10.1016/j.bpa.2015.03.004
46.
ChenQYuWShiJShenJHuYGaoTet al. The effect of venovenous extra-corporeal membrane oxygenation (ECMO) therapy on immune inflammatory response of cerebral tissues in porcine model. J Cardiothorac Surg. (2013) 8:186. 10.1186/1749-8090-8-186
47.
LassenNAChristensenMS. Physiology of cerebral blood flow. Br J Anaesth. (1976) 48:719–34. 10.1093/bja/48.8.719
48.
MengLGelbAW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology (2015) 122:196–205. 10.1097/ALN.0000000000000506
49.
OddoMCrippaIAMehtaSMenonDPayenJ-FTacconeFSet al. Optimizing sedation in patients with acute brain injury. Crit Care (2016) 20:128. 10.1186/s13054-016-1294-5
50.
MuellenbachRMKilgensteinCKrankePKüstermannJKredelMRoewerNet al. Effects of venovenous extracorporeal membrane oxygenation on cerebral oxygenation in hypercapnic ARDS. Perfusion (2014) 29:139–41. 10.1177/0267659113497073
51.
KredelMLubnowMWestermaierTMüllerTPhilippALotzCet al. Cerebral tissue oxygenation during the initiation of venovenous ECMO. ASAIO J (2014) 60:694–700. 10.1097/MAT.0000000000000128
52.
ShortBLou. The effect of extracorporeal life support on the brain: a focus on ECMO. Semin Perinatol. (2005) 29:45–50. 10.1053/j.semperi.2005.02.007
53.
GrazianiLJGringlasMBaumgartS. Cerebrovascular complications and neurodevelopmental sequelae of neonatal ECMO. Clin Perinatol. (1997) 24:655–75. 10.1016/S0095-5108(18)30163-5
54.
HolleyDGShortBLKarrSSMartinGR. Mechanisms of change in cardiac performance in infants undergoing extracorporeal membrane oxygenation. Crit Care Med. (1994) 22:1865–70. 10.1097/00003246-199411000-00024
55.
KrausMWeisRAlwardtCLanzaLBirchBRamakrisnaH. Veno-venous extracorporeal membrane oxygenation: anesthetic management for massive intracranial hemorrhage in H1N1 infection. Open J Anesthesiol. (2015) 5:251–6. 10.4236/ojanes.2015.512045
56.
FactoraFNFBustamanteSSpiottaAAvitsianR. Intracranial hemorrhage surgery on patients on mechanical circulatory support: a case series. J Neurosurg Anesthesiol. (2011) 23:30–4. 10.1097/ANA.0b013e3181eee55e
57.
FrieseneckerBEPeerRRiederJLirkPKnotzerHHasibederWRet al. Craniotomy during ECMO in a severely traumatized patient. Acta Neurochir (2005) 147:993–6. 10.1007/s00701-005-0568-5
58.
KrenzlinHRosenthalCWolfSViereckeJKowskiAHetzerRet al. Surgical treatment of intraparenchymal hemorrhage during mechanical circulatory support for heart-failure - a single-centre experience. Acta Neurochir (2014) 156:1729–34. 10.1007/s00701-014-2141-6
59.
HerbertDGBuscherHNairP. Prolonged venovenous extracorporeal membrane oxygenation without anticoagulation: a case of Goodpasture syndrome-related pulmonary haemorrhage. Crit Care Resusc. (2014) 16:69–72.
60.
WenP-HChanWChenY-CChenY-LChanC-PLinP-Y. Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: a case report and literature review. World J Emerg Surg. (2015) 10:15. 10.1186/s13017-015-0006-9
61.
CroninBMausTPretoriusVNguyenLJohnsonDOvandoJet al. Case 13 - 2014: management of pulmonary hemorrhage after pulmonary endarterectomy with venovenous extracorporeal membrane oxygenation without systemic anticoagulation. J Cardiothorac Vasc Anesth. (2014) 28:1667–76. 10.1053/j.jvca.2014.07.018
62.
MuellenbachRMKredelMKunzeEKrankePKuestermannJBrackAet al. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. (2012) 72:1444–7. 10.1097/TA.0b013e31824d68e3
63.
YenT-SLiauC-CChenY-SChaoA. Extracorporeal membrane oxygenation resuscitation for traumatic brain injury after decompressive craniotomy. Clin Neurol Neurosurg. (2008) 110:295–7. 10.1016/j.clineuro.2007.10.017
64.
BruzekAKVegaRAMathernBE. Extracorporeal membrane oxygenation support as a life-saving measure for acute respiratory distress syndrome after craniectomy. J Neurosurg Anesthesiol. (2014) 26:259–60. 10.1097/ANA.0b013e3182a5d0fd
65.
ZhouRLiuBLinKWangRQinZLiaoRet al. ECMO support for right main bronchial disruption in multiple trauma patient with brain injury — a case report and literature review. Perfusion (2015) 30:403–6. 10.1177/0267659114554326
66.
YeoHJKimDHJeonDKimYSChoWH. Low-dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med. (2015) 41:2020–1. 10.1007/s00134-015-4015-7
67.
LarssonMRayzmanVNolteMWNickelKFBjörkqvistJJämsäAet al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. (2014) 6:222ra17. 10.1126/scitranslmed.3006804
68.
KruegerKSchmutzAZiegerBKalbhennJ. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs (2017) 41:186–92. 10.1111/aor.12737
69.
McQuiltenZAubronCBaileyMBoardJBuhrHDennisMet al. Low-dose heparin in critically ill patients undergoing extracorporeal membrane oxygenation - the help-ECMO pilot randomised controlled trial. Blood (2016) 128:3822.
70.
NekludovMBellanderB-MMBlombäckMWallenHNBlombackMWallenHN. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma (2007) 24:1699–706. 10.1089/neu.2007.0322
71.
LindbladCThelinEPNekludovMFrostellANelsonDWSvenssonMet al. Assessment of platelet function in traumatic brain injury—a retrospective observational study in the neuro-critical care setting. Front Neurol (2018) 9:15. 10.3389/fneur.2018.00015
72.
NairPHoechterDJBuscherHVenkateshKWhittamSJosephJet al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth. (2015) 29:288–96. 10.1053/j.jvca.2014.06.006
73.
Velik-SalchnerCMaierSInnerhoferPKolbitschCStreifWMittermayrMet al. An assessment of cardiopulmonary bypass-induced changes in platelet function using whole blood and classical light transmission aggregometry: the results of a pilot study. Anesth Analg. (2009) 108:1747–54. 10.1213/ane.0b013e3181a198ac
74.
ThelinEPZeilerFAErcoleAMondelloSBükiABellanderB-Met al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front Neurol (2017) 8:300. 10.3389/fneur.2017.00300
75.
SaloniaRBellMJKochanekPMBergerRP. The utility of near infrared spectroscopy in detecting intracranial hemorrhage in children. J Neurotrauma (2012) 29:1047–53. 10.1089/neu.2011.1890
76.
RobbaCCardimDTajsicTPietersenJBulmanMDonnellyJet al. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: a prospective observational study. PLoS Med. (2017) 14:e1002356. 10.1371/journal.pmed.1002356
77.
ThelinEPNelsonDWBellanderB-M. Secondary peaks of S100B in serum relate to subsequent radiological pathology in traumatic brain injury. Neurocrit Care (2014) 20:217–29. 10.1007/s12028-013-9916-0
78.
ErcoleAThelinEPHolstABellanderBMNelsonDW. Kinetic modelling of serum S100b after traumatic brain injury. BMC Neurol. (2016) 16:93. 10.1186/s12883-016-0614-3
79.
ThelinEPNelsonDWBellanderB-M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir (2017) 159:209–25. 10.1007/s00701-016-3046-3
80.
UndénLCalcagnileOUndénJReinstrupPBazarianJ. Validation of the Scandinavian guidelines for initial management of minimal, mild and moderate traumatic brain injury in adults. BMC Med. (2015) 13:292. 10.1186/s12916-015-0533-y
81.
GazzoloDMasettiPMeliMGrutzfeldDMichettiF. Elevated S100B protein as an early indicator of intracranial haemorrhage in infants subjected to extracorporeal membrane oxygenation. Acta Paediatr. (2002) 91:218–21. 10.1111/j.1651-2227.2002.tb01698.x
82.
NguyenDNHuyghensLWellensFSchiettecatteJSmitzJVincentJ-L. Serum S100B protein could help to detect cerebral complications associated with extracorporeal membrane oxygenation (ECMO). Neurocrit Care (2014) 20:367–74. 10.1007/s12028-013-9874-6
83.
BembeaMMRizkallaNFreedyJBaraschNVaidyaDPronovostPJet al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med. (2015) 43:2202–11. 10.1097/CCM.0000000000001145
84.
BembeaMMSavageWStrouseJJSchwartzJMGrahamEThompsonCBet al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. (2011) 12:572–9. 10.1097/PCC.0b013e3181fe3ec7
85.
FloerchingerBPhilippAFoltanMKeyserACamboniDLubnowMet al. Neuron-specific enolase serum levels predict severe neuronal injury after extracorporeal life support in resuscitation. Eur J Cardiothorac Surg. (2014) 45:496–501. 10.1093/ejcts/ezt370
86.
LinNFlibotteJLichtDJ. Neuromonitoring in the neonatal ECMO patient. Semin Perinatol. (2018) 42:111–21. 10.1053/j.semperi.2017.12.007
87.
ClairM-PRambaudJFlahaultAGuedjRGuilbertJGuellecIDurandyADemoulinMJeanSMitanchezDet al. Prognostic value of cerebral tissue oxygen saturation during neonatal extracorporeal membrane oxygenation. PLoS ONE (2017) 12:e0172991. 10.1371/journal.pone.0172991
88.
WongJKSmithTNPitcherHTHiroseHCavarocchiNC. Cerebral and lower limb near-infrared spectroscopy in adults on extracorporeal membrane oxygenation. Artif Organs (2012) 36:659–67. 10.1111/j.1525-1594.2012.01496.x
89.
O'BrienNFHallMW. Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Pediatr Crit Care Med. (2013) 14:e126–34. 10.1097/PCC.0b013e3182712d62
90.
LantiguaHOrtega-GutierrezSSchmidtJMLeeKBadjatiaNAgarwalSet al. Subarachnoid hemorrhage: who dies, and why?Crit Care (2015) 19:309. 10.1186/s13054-015-1036-0
91.
HemphillJCBonovichDCBesmertisLManleyGTJohnstonSC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke (2001) 32:891–7. 10.1161/01.STR.32.4.891
92.
SafatliDAGüntherASchlattmannPSchwarzFKalffREwaldC. Predictors of 30-day mortality in patients with spontaneous primary intracerebral hemorrhage. Surg Neurol Int. (2016) 7:510–7. 10.4103/2152-7806.187493
Summary
Keywords
intracranial hemorrhage, intracerebral hemorrhage, brain injury, neurological injury, extracorporeal membrane oxygenation, extracorporeal life support, adults
Citation
Fletcher-Sandersjöö A, Thelin EP, Bartek J Jr., Broman M, Sallisalmi M, Elmi-Terander A and Bellander B-M (2018) Incidence, Outcome, and Predictors of Intracranial Hemorrhage in Adult Patients on Extracorporeal Membrane Oxygenation: A Systematic and Narrative Review. Front. Neurol. 9:548. doi: 10.3389/fneur.2018.00548
Received
16 April 2018
Accepted
19 June 2018
Published
06 July 2018
Volume
9 - 2018
Edited by
Rajeev Kumar Garg, Rush University, United States
Reviewed by
Ryan Matthew Martin, University of California, Davis, United States; Sebastian Pollandt, Rush University, United States; Nick Osteraas, Rush Medical College, United States
Updates
Copyright
© 2018 Fletcher-Sandersjöö, Thelin, Bartek, Broman, Sallisalmi, Elmi-Terander, Bellander.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Fletcher-Sandersjöö alexander.sandersjoo@gmail.com
This article was submitted to Neurocritical and Neurohospitalist Care, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.