- 1Pellegrino Center for Clinical Bioethics, Georgetown University Medical Center, Washington, DC, United States
- 2Department of Oncology, Georgetown University Medical Center, Washington, DC, United States
- 3Departments of Neurology and Biochemistry, Georgetown University Medical Center, Washington, DC, United States
Central neurotrauma, such as spinal cord injury or traumatic brain injury, can damage critical axonal pathways and neurons and lead to partial to complete loss of neural function that is difficult to address in the mature central nervous system. Improvement and innovation in the development, manufacture, and delivery of stem-cell based therapies, as well as the continued exploration of newer forms of stem cells, have allowed the professional and public spheres to resolve technical and ethical questions that previously hindered stem cell research for central nervous system injury. Recent in vitro and in vivo models have demonstrated the potential that reprogrammed autologous stem cells, in particular, have to restore functionality and induce regeneration—while potentially mitigating technical issues of immunogenicity, rejection, and ethical issues of embryonic derivation. These newer stem-cell based approaches are not, however, without concerns and problems of safety, efficacy, use and distribution. This review is an assessment of the current state of the science, the potential solutions that have been and are currently being explored, and the problems and questions that arise from what appears to be a promising way forward (i.e., autologous stem cell-based therapies)—for the purpose of advancing the research for much-needed therapeutic interventions for central neurotrauma.
Neurotrauma: An Overview
Neurotrauma is defined as neurological insult that results in the disturbance of neural circuitry through disruption of axonal pathways and/or neural cell damage or loss (1). Injuries to the central nervous system (CNS) include, but are not limited to, spinal cord injury (SCI), traumatic brain injury (TBI), and stroke (1–4).
The epidemiological impact of neurotrauma is significant. Annually, there are approximately 12,000 cases of SCI in the United States alone (5), with injury being incurred by compression, contusion, laceration and/or partial or complete severing of the cord (6). There are 1.5–2 million cases of traumatic brain injury (TBI) per year in the US, with 75,000–100,000 of these cases being classified as severe (7, 8). Approximately 795,000 strokes occur annually in the US, with 87% of these being ischemic and 10% due to intracranial hemorrhage (9). Neurotrauma can incur both short and long-term, partial or complete loss of neurological function, depending on the type and severity of injuries (7, 8, 10). Pathophysiological features can include sensory and/or autonomic impairment, muscle weakness, and/or decreased control of movement, decreased endurance, muscle spasms, and hypertonicity, and are typically reflective of the site(s) and extent of neurological damage (6–8, 11). Onset of signs and symptoms resulting from neurotrauma can begin immediately following injury, or in some cases (of mild to moderate insult, with iterative neurological involvement) can be latent, and can persist for months, years and/or durably (i.e., - permanently).
The short- and long-term effects of neurotrauma can extend beyond the biophysiological to detrimentally affect quality of life (i.e., employment opportunities, relationships, ability to partake in recreational activities, etc.) (12–14). Post-trauma care and management of symptoms can also be economically burdensome for individuals and families affected by SCI, TBI or stroke. For example, in the US, the first year of care following an SCI injury, can incur individual costs ranging from US$123,000 to US$423,000 (15). Lifetime costs for an individual injured at 25 years of age have been estimated to reach US$2.7 million, depending on the severity of the injury incurred (16).
While we recognize that SCI, TBI, and stroke each and all have differing therapeutic targets, and methods, and have been, and may be therapeutically approached in distinct ways, herein we take a heterogeneous approach to include all of these conditions in this review, as stem cells are and may be used in a variety of therapeutic applications. As well, we place specific emphasis on SCI, recognizing the particular potential for application of stem cell-based therapies in this context, and acknowledge and address that TBI and stroke have been shown to share certain pathophysiological processes and clinical targets, which have been supportive of stem-cell based interventions (3, 17, 18).
The Therapeutic/Recuperative Problem
Patterns of recovery of function following neurotrauma generally reflect neurons' incapacity for replication (i.e., post-mitotic stability). It is noteworthy that some areas of the brain have shown regenerative capacity (i.e., neurogenesis) (19–21) through initiation and migration of neural progenitor cells. Regions that have exhibited progenitor cell activation and resulting neurogenesis include the hippocampal subgranular zone (22–24) and dentate gyrus [(25); however, for contrasting report, see (26)], the periventricular area, the olfactory bulb (27–30), the subventricular zone (31–33), and the central canal of the spinal cord (34–37). However, progenitor cells appear to be isolated to these regions, and are not ubiquitous in the CNS. The majority of mature neurons of the CNS are terminally differentiated (TD), can no longer undergo mitosis, and are considered to be outside of the cell cycle (38). While there is some debate whether neurogenesis may persist into and throughout adulthood in certain brain areas [e.g., the dentate gyrus; (25, 26, 39)]; such mitotic capacity, even if limited to key periods in development, is certainly not neuroanatomically ubiquitous. In mammals (including humans) cortical neurons become mature, post-mitotic cells early on during development (40), and maintain the stable post-mitotic state for decades (41). Further evidence suggests that the human neocortex does not acquire any additional neurons after birth (41), and that brain tumors are not derived from mature, TD neurons, but rather, from aberrant neural stem cells found in the areas of the brain with regenerative ability (38, 42). Neuronal cell death also typically occurs when TD neurons are induced to re-enter the cell cycle, either experimentally or due to cell stress (38, 43).
Following neural insult and injury, the terminal zones of severed neurons swell into dystrophic growth cones and have been shown to have some regenerative capabilities when in the appropriate environment(s) (44–47). Neurons of the dorsal root ganglia, for instance, have axons in both the peripheral neural system (PNS) and the CNS; however, only those ending in the PNS have capacity for regeneration (48). The inability of CNS neurons to regenerate neural fibers is related to the environment of the adult CNS following injury (49). When a neuronal fiber is severed, axonal regrowth is initially inhibited by the presence of myelin-associated inhibitors in the glial environment [see (50, 51) for overview]. These myelin-associated inhibitors are released by both intact oligodendrocytes and myelin structures that may have been damaged by injury (47, 52–55). Identified inhibitors include: Nogo, myelin-associated glycoprotein (Mag), oligodendrocyte myelin glycoprotein (Omgp), ephrin B3 and transmembrane semaphoring 4D (Sema4D) (54, 56).
Following CNS injury, microglia, oligodendrocyte precursors, meningeal cells, and astrocytes are also recruited to the site. Activation of local inflammatory processes and induction of lipid-collagen matrices lead to gliosis and formation of a glial scar, which provides a physical barrier and further inhibits regrowth of the axon and outgrowth of neurites beyond the lesion site (1, 47, 54). Moreover, the recruited astrocytes enter a reactive state, in which they up-regulate and release chondroitin sulfate proteoglycans (CSPGs), creating a chemical gradient at the site of injury that is inhibitory to regrowth (47, 57, 58). Additional evidence suggests that the glial scar may also provide stability to the site of injury in the CNS, preventing further cellular degeneration, isolating the inflammatory response, and repairing the blood-brain barrier at the injury site (59–61).
Thus, the adult nervous system is largely unable to regenerate following irreparable neuronal damage and/or loss (1, 62–64). This presents challenges (viz. a “therapeutic/recuperative problem”) to developing effective interventions for neural insult, and is an increasing clinical (and socio-economic) concern as the incidence of neurological injury and global prevalence of neurodegenerative disease rise (1, 6, 38, 47, 54, 65).
Cell-Based Approaches to Central Neural Repair and Regeneration
A variety of strategies have been developed to treat CNS injury and disease. In general, methods for the repair and possible regeneration of the CNS entail pharmacological, structural, and cell-based approaches, either singularly or in combination. Although pharmacological approaches have demonstrated therapeutic benefit for management of symptoms and functional rehabilitation associated with SCI (66), TBI (67), and stroke (68, 69), not all have been proven safe or effective in reparative or regenerative capacities in clinical application. Structural approaches, while perhaps limited in their reparative and regenerative capacities when employed in isolation, have demonstrated some success when employed in combination with cell-based approaches (70–74). We focus herein, however, on cell-based approaches to repair and possible regeneration of the injured CNS that have shown potential in preclinical and early clinical studies.
Cell-based therapies can have a variety (i.e., direct, indirect, or both types) of effects. Such therapies can be used to facilitate regeneration of neurons via implantation of specifically active cells in the adult CNS. Direct cell-based therapies generally involve use of implanted cells to replace or repair damaged neuronal and/or glial cells and tissue. Indirect effects involve use of implanted cells to contribute biomolecular and biochemical factors that modify the neural micro- and/or macroenvironment, providing trophic support that facilitates neural repair and regeneration (6, 75–77). Some cell-based therapies have both indirect and direct effects. Bone-marrow derived mesenchymal stem cells (BM-MSCs), for instance, exhibit immunosuppressive qualities and the ability to promote the proliferation of endogenous cells following stroke, in animal models (78–81). These cells are also capable of trans-differentiation into various neural cell types, such as astrocytes, oligodendrocytes, and neurons, both in vitro and in vivo, and in in vivo animal models have been shown to demonstrate migratory capacity and actions in the CNS (82–92).
Stem Cells
Stem cell-based therapies for neural regeneration and repair garnered attention after the identification of specific regions of the adult human brain capable of maintaining the capacity for neuroregeneration throughout the human adult lifespan (6, 77, 93–95).
Stem cell-based techniques have been increasingly innovative, with relatively rapid advances enabling the potential to combine stem-cell therapies with previously explored pharmacological, structural, and even other cell-based methods (96–99). For example, stem cells could be modified to deliver biomolecules or to replace damaged neurons, astrocytes, oligodendrocytes, etc. and thereby act directly and/or indirectly, as noted above (100).
As illustrated in Table 1, embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), neural stem/progenitor cells (NSCs), and induced pluripotent stem cells (iPSCs) have all been explored for use in cell therapies for neuroregeneration in a variety of models and applications.
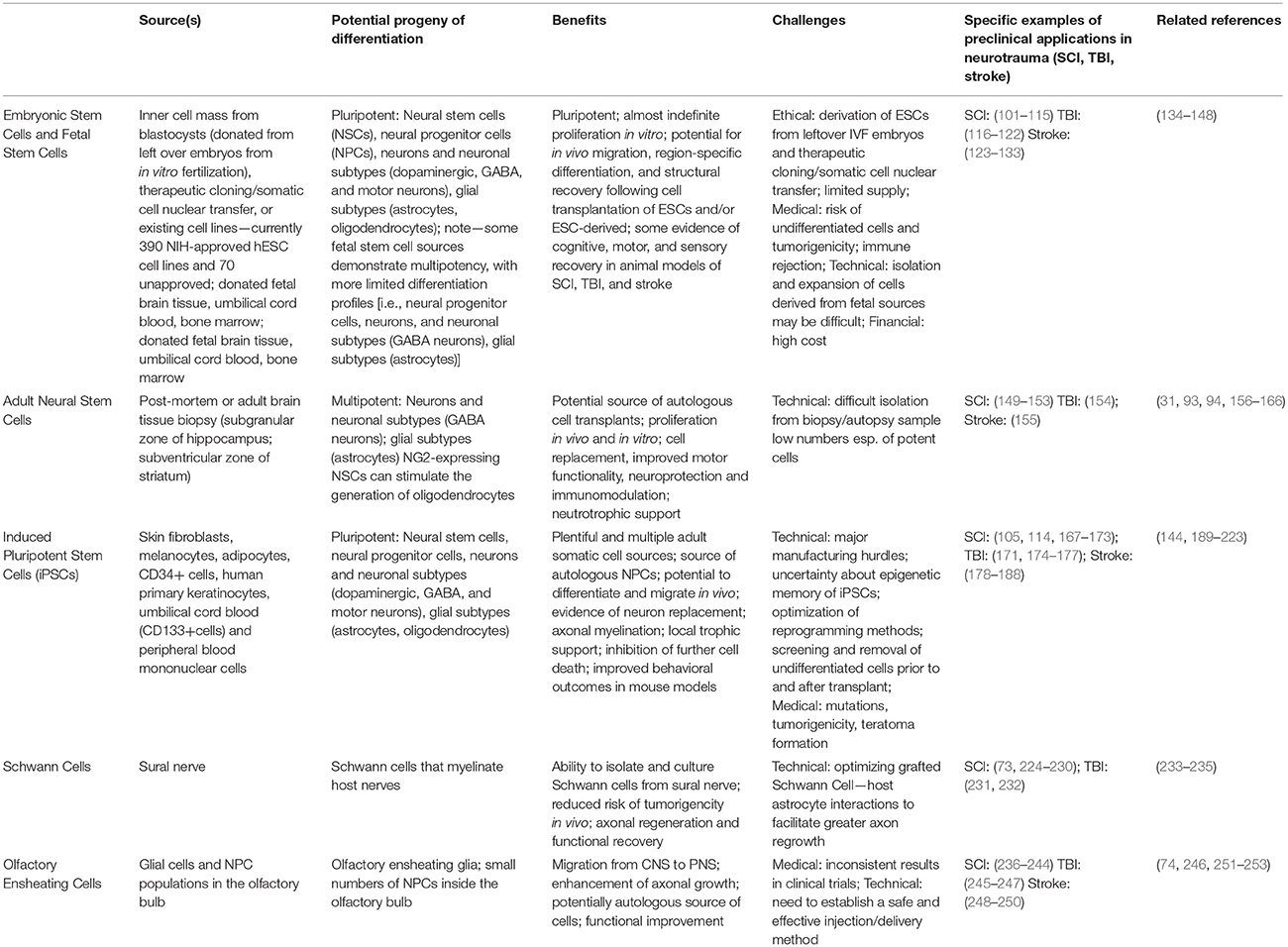
Table 1. Stem cell types (in addition to Schwann Cells and olfactory ensheating cells) being explored as treatment strategies for neuroregeneration and repair in neurotrauma (SCI, TBI, and stroke).
Embryonic Stem Cells (ESCs), Fetal Stem Cells and Derivatives
ESCs were first cultured and isolated from mice (134), but can now be derived from donated human blastocysts following in-vitro fertilization (IVF) procedures (135, 136), somatic cell nuclear transfer (137), human or mice fetal brains (120, 122), or existing hESC lines (there are currently 390 NIH-approved hESC and 70 unapproved cell lines1.
ESCs are pluripotent and can proliferate almost indefinitely in vitro (135, 138, 254). Furthermore, ESCs have potential to differentiate into any cell type, including neurotransmitter or growth factor-secreting cells, neural stem cells (NSCs) and neural progenitor cells that can be further differentiated into neuronal subtypes, and/or glia (e.g., oligodendrocytes, astrocytes) capable of effecting roles in facilitating neural repair and/or regeneration (117, 120, 121, 139, 254, 255).
Early preclinical studies employing in vivo mouse models demonstrated the ability of hESC-derived neural progenitor cells to integrate into host parenchyma, migrate along established pathways in the brain, and differentiate according to region-specific cues (254). Various cell transplantation applications of hESC-derived, as well as mouse or human fetal-derived NSCs, in animal models of TBI suggest the potential of these cells to migrate to injured regions of the brain, differentiate into neurons and neuronal subtypes, and improve cognitive and motor functional recovery in the injured brain (121, 122, 139). Transplanted ESC-derived cells in ischemic animal models (e.g., rats subject to middle cerebral artery occlusion (MCAO)) have also demonstrated the ability to differentiate in vivo and to improve structural, functional, behavioral, and motor and sensory repair (123–125). NSCs and NPCs derived from ESCs have also been applied in preclinical animal models of stroke (126–131) with marked improvements in the size of the infarct area, the level of differentiation into neurons and neuronal cell types post-transplantation, and improved behavioral deficits (256).
Transplanted ESC-derived NSCs have demonstrated functional and structural improvement in animal models of SCI, as well (101, 102, 104–107, 257, 258). An ongoing phase I/II study (NCT02302157) is investigating the application of human ESC-derived oligodendrocyte progenitor cells (OPCs) in subjects with subacute cervical SCI; this clinical trial is supported, in part, by preclinical evidence of the safety and ability of these cells to promote neurite outgrowth in vitro and to facilitate myelination in vivo in rodent models of thoracic SCI (103).
Risks of inappropriate differentiation (i.e., teratoma and tumor formation), as well as technical issues arising from the possibility of immunological and graft rejection of the implanted tissues are major challenges that are still to be overcome (140–142). As well, ethical controversies surrounding the source of ESCs has led to alternative sources of pluripotent stem cells being explored (143, 144).2
Fetally-derived NSCs can be isolated from fetal brain tissue, with minute quantities also reported in bone marrow and umbilical cord blood (259). While these cells readily give rise to neurons, astrocytes, and oligodendrocytes, they are less versatile and proliferative than ESCs because they maintain some of the characteristics of the region of the brain from which they were derived3 (100, 145). Transplantation of human fetal stem cells and their derivatives have shown promise in: increasing neuronal survival and decreasing lesion size in animal models of TBI through increased angiogenesis and reduced astrogliosis (122, 147); and, neuronal regrowth, functional and structural improvement following transplant in animal models of SCI (108–110). Clinical trials involving fetal stem cells or their derivatives for the treatment of SCI and stroke are completed and ongoing. A Phase I study is underway that is investigating the safety and efficacy of human fetal spinal cord-derived NSCs (NSI-566) for the treatment of chronic SCI (NCT01772810), and a Phase II study investigating the effect of human central nervous system stem cells (HuCNS-SCs) on patients with thoracic spinal cord injury has been completed (NCT01321333). Yet another Phase I/II clinical trial is ongoing that is evaluating the effect of NSI-566 cells on motor deficits in stroke patients (NCT03296618).
Despite such progress in preclinical and clinical fields, ESC- and fetally-derived stem cells have given rise to ethical concerns regarding the source and process of their procurement, technical considerations focal to possible risks incurred during transplantation, and unregulated tissue growth in situ [for overview, see (260)].
A Focus on Neural Stem Cells and Neural Progenitor Cells: Possibilities and Problems
NSCs are found in the developing and adult CNS in a number of brain regions [i.e., cortex, hippocampus, olfactory bulb, striatum, ventricles, midbrain, cerebellum, spinal cord, and retina, (31, 93, 94, 157–161). NSCs can be derived from ESCs, fetal tissues (as discussed in prior section), iPSCs, and adult brain tissue1 (160, 261). NSC differentiation (e.g., into neurons, astrocytes, and/or oligodendrocytes) is dependent, in part upon exposure to particular growth and environmental factors.
The use of adult NSCs/NPCs (derived from biopsy tissue from brain or spinal cord) in cell therapies could, if validated in preclinical proof-of-concept studies, allow for autologous cell transplantation and repair by endogenous NSCs, thereby potentially minimizing risks of incompatibility. Early studies in animal models of SCI and TBI have suggested the potential viability of this approach (isolation, in vitro expansion, and transplantation), either alone or in combination with other methods (e.g., bone marrow-derived MSCs) for provision of neuroprotection, immunomodulation and facilitation of re-myelination and functional recovery of the injured spinal cord (149–151, 153, 154, 156).
In transplantation-based therapy, NSCs or NPCs are implanted within defined areas of the CNS1. Transplanted NSCs and NPCs facilitate neuroregeneration and therapeutic plasticity in the injured CNS via processes of neuroprotection [i.e., the “bystander” “mechanism” in SCI, see (262) for overview; (132, 155) for ischemic stroke], neurotrophic support (262); immunomodulation (164), and cell replacement and integration (111). Broadly speaking, neuroprotection is the activation of pathways that prevent further neuronal cell death, while neurogenesis involves proliferation and differentiation of endogenous neural stem and progenitor cells (259, 262), or implanted NSCs capable of cell replacement. Lu et al. (113) found improved motor functionality following SCI in rodents, when rat- and human-derived NPCs, human ESC-derived NSCs, and human iPSC-derived NSCs were implanted at injury sites (112, 114, 115, 263). Specifically, it was observed that these cells were able to extend multiple axons over long distances, and form synapses with host neurons (112, 264). Research suggests that endogenous NSCs and the immune system share secreted mediators (chemokines, cytokines) and receptors relevant both to inflammation and the maintenance, structure, and function of endogenous neural stem cell niches in the brain (165, 166, 265). Transplanted exogenous NSCs may modulate local immune responses and secrete growth factors that are conducive to neuronal growth (132, 133). Indeed, both in vitro and in vivo proof-of-concept studies have demonstrated the potential of NSCs as a cell-based therapy for SCI (266–268), stroke (269), and TBI (266, 268, 269), based, at least in part, upon the capability for defined differentiation and target specific integration and functional activity of these cells when introduced at or proximate to sites of neural injury (259).
However, NSCs and NPCs from adult tissues cannot be easily engaged to generate certain types of neurons [e.g., dopamine or motor neurons; (162)]2, nor are they easily isolated or expanded from the regions of the human brain that contain NSCs [i.e., the subgranular zone of the hippocampus, and the subventricular zone of the striatum; (94)], in part, because they are present in rather low numbers (100). Moreover, despite the viability and potential value of transplanted NSCs as a therapeutic approach, gaps exist in knowledge about the activity and effect(s) of NSCs in vivo with regard to migration mechanisms and the optimum number of NSCs needed to facilitate regeneration in different types of lesions (166, 259). The method and timing of delivery, and potential interactions between the transplanted NSCs and the host immune system may also impact the induction and extent of neuroregenerative effects produced by these cells (160, 166).
The CNS environment at the site of neural injury in SCI, TBI, or stroke is not conducive to neuroregeneration (270). This is due to the activation of inhibitory pathways, glial scar formation, and the lack of guiding astrocytes essential for axonal regrowth (6). As a result, many applications of NSCs have resulted in poor cell survival, failed integration into host tissue, and poor, if not uncontrolled, differentiation of cells (6, 153, 271). Additionally, while NSC transplants derived from ESCs have shown increased risk for formation of tumor growth compared to NSCs that have been derived from either fetal or adult brain tissues, that is not to say that these cells do not also have associated risks of tumor formation1 (100, 260). Furthermore, given that a source of NSCs is from ESCs and fetal tissues, research and potential therapies using these stem cells generate concerns about derivation, cost, supply, and accessibility of these cells (11, 272, 273). In sum, these challenges, as well as mixed evidence of safety and efficacy in animal studies (152, 153, 274), have hindered translation of adult brain-derived NSCs/NPCs into clinical therapies (100, 259, 260).
Translation of Stem Cell Therapies
Ongoing Trials
There are currently no stem cell therapies for neural injuries (namely, SCI, TBI, or stroke) that have received US FDA market approval for clinical application in humans, although there are 19 ongoing trials investigating stem cell therapies for SCI (as of May 2018; see Table 2 for details)3. Four of these are Phase 1, seven are Phase 1/2, six are Phase 2, one is phase 2/3, and one is unspecified. There are also 6 trials investigating stem cell therapies for TBI, and 22 trials investigating stem cell therapies for application to stroke4.
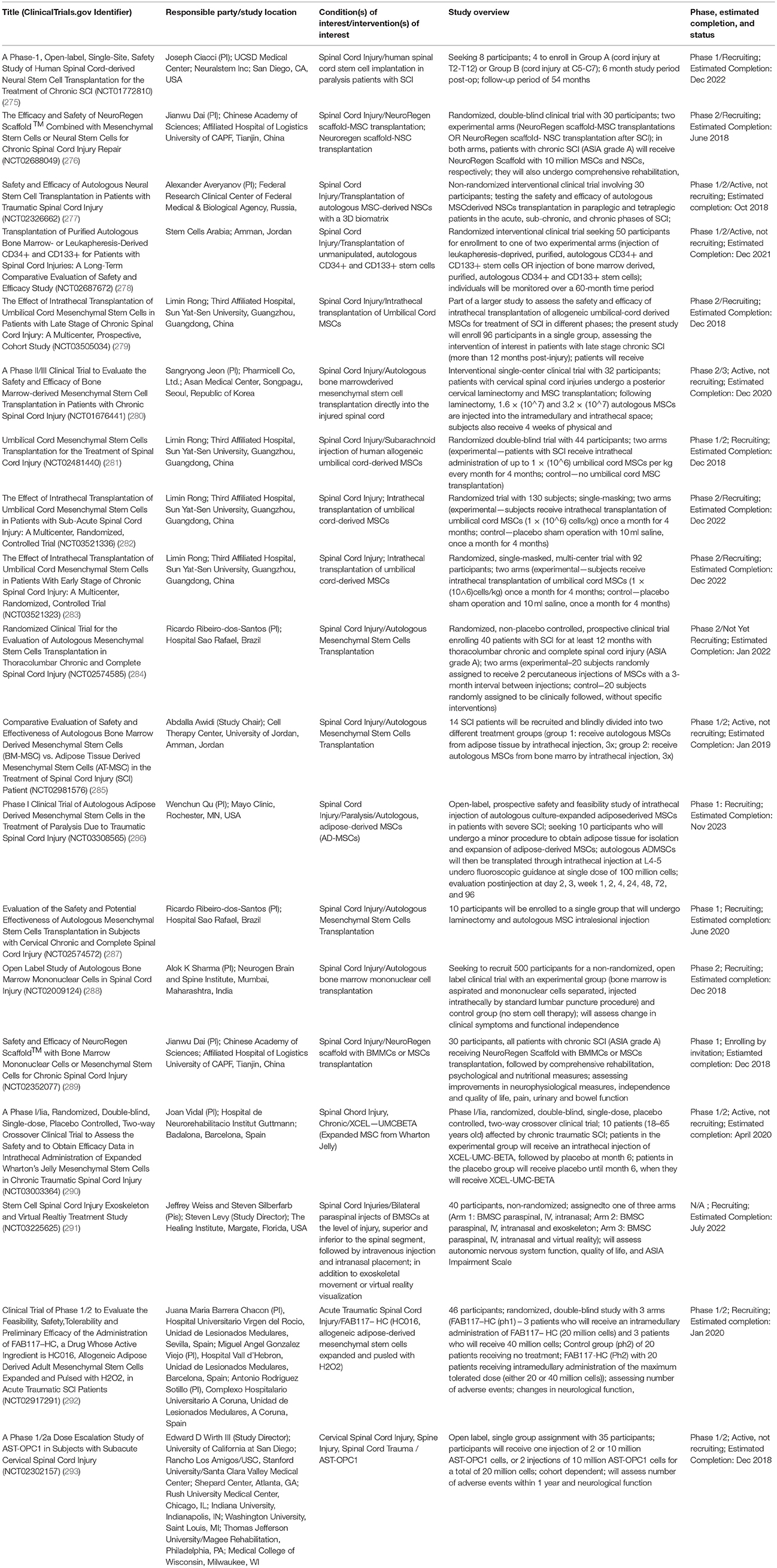
Table 2. Ongoing clinical trials as of May 2018 investigating stem cell therapies for SCI (Source:Clinicaltrials.gov).
The majority (12) of the studies are investigating the safety and efficacy of MSCs for treatment of SCI, with the remainder utilizing NSCs [NCT01772810 (275) and NCT02326662 (277)], NSCs and MSCs (NCT02688049) (276), CD34+ and CD133+ stem cells (NCT02687672) (278), bone marrow mononuclear cells (BMMCs) (NCT02009124) (288), BMMCs and MSCs (NCT02352077) (289), and AST-OPC1 [human ESC-derived oligodendrocyte progenitor cells (OPCs)] (NCT02302157) (293). Seven (7) of these trials use allogeneic stem cells products [NCT03505034 (279), NCT02481440 (281), NCT02917291 (292), NCT03521336 (282), NCT03521323 (283), NCT03003364 (290), NCT02302157 (293)], 9 use autologous stem cells (NCT02326662 (277), NCT01676441 (280), NCT02687672 (278), NCT02574585 (284), NCT02981576 (285), NCT03308565 (286), NCT02574572 (287), NCT03225625 (291), NCT02009124) (288), and 3 are unspecified [NCT01772810 (275), NCT02688049 (276), NCT02352077 (289)]4.
Here we highlight several of these ongoing trials pertaining to stem cell therapies for SCI to demonstrate the types of studies being conducted. NCT02326662 (277) is a non-randomized interventional study assessing the safety and efficacy of MSC-derived autologous NSC transplantation for individuals with traumatic SCI (NCT02326662). The aim is to recruit 30 participants (paraplegic and tetraplegic patients in the acute, sub-chronic, and chronic phases of SCI), who will receive transplants of autologous MSC-derived NSCs by intraspinal and intrathecal injection, along with RMx Biomatrix scaffolding as needed. NCT02688049 is a Phase 1/2 randomized, double-blind clinical trial assessing the efficacy and safety of NeuroRegen Scaffold™ when combined with MSCs or NSCs for chronic SCI repair. It is a two-arm study, with patients in one arm receiving the NeuroRegen Scaffold™ combined with MSC transplantation therapy following SCI, and those in the other arm receiving NeuroRegen Scaffold™ combined with NSC transplantation following SCI (NCT02688049). NCT01676441 is a Phase 2/3 clinical trial being conducted in the Republic of Korea, that aims to evaluate the safety and efficacy of bone marrow-derived MSCs when transplanted into patients with chronic SCI. All participants (32) will receive MSC transplants directly to the injured spinal cord lesion site via laminectomy, and following recovery from the procedure, will undergo 4 weeks of physical and occupational therapy (NCT01676441). Participants will undergo magnetic resonance and diffusion tensor imaging and electromyography and nerve conduction testing at 6 months post-operatively, and be assessed for motor and sensory function as well as adverse events (using the American Spinal Injury Association (ASIA) scale) 12 months post-operatively (NCT01676441)4.
Reported Results
At this writing, 13 trails involving stem cell therapies for SCI on ClinicalTrials.gov have been completed, although the results from all of these studies are not yet available. Park et al. (294) reported long-term outcomes (i.e., changes in motor power grade of extremities, MRI, and electrophysiological recordings) of 3 SCI patients who received intramedullary transplantations of autologous bone marrow-derived MSCs (sourced from the iliac bone of patients). The patients were identified from an initial cohort of 10 patients as those who showed evidence of improvement in their activities of daily living (ADL) at 6 months following MSC- transplant (295). Patients had received direct injections of MSCs (8 × 106) to the spinal cord, in addition to introduction of MSCs (4 × 107) to the intradural space. At 4 and 8 weeks post-operatively, patients received additional injections of MSCs (5 × 107) via lumbar puncture. In this long-term follow-up, none of the 3 patients exhibited tumorigenesis or complications associated with the intramedullary injection of MSCs. Additionally, 2 of the 3 patients showed evidence of gradual improvement in motor power of the upper extremities, as well as evidence of the disappearance of cavity margins, which may suggest the ability of BM-derived MSCs to diminish glial scarring (294). The results of this study do not contradict those of previous studies [e.g., (296, 297); see (6) for an overview of completed trials involving stem cells for SCI], which similarly found slight motor improvements without significant complications associated with MSC transplantation in humans with SCI (294). The results of such studies suggest that stem cell therapies can be especially beneficial for functional recovery in the acute and subacute stages of SCI, but that improvements incurred during the later and chronic stages of SCI are characteristically less significant (6).
Clinical trials involving stem cell therapies for TBI and stroke have also been completed. Results for MSC transplants in TBI patients have been promising, with both (298) and (299) reporting enhancement of neurological function and recovery among adults and pediatric patients following injury. Kalladka et al. (300) recently reported on the use of human fetal brain-derived NSCs (CTX0E03) in patients with chronic ischemic stroke in a phase 1 study. This single-site, dose-escalation study found improved neurological function and no immunological or cell transplant-related adverse events in a sample of 13 men over the age of 60 who received intracerebral implants of (2, 5, 10, or 20 million) NSCs, up to 2 years post-transplant (300).
More Recent Approaches: iPSCs and Reprogrammed Autologous Cells
Alternatives to ESC and fetally-derived cell-based transplantation therapies received renewed attention upon the discovery that adult somatic cells could be induced to become pluripotent stem cells (190). Adult somatic cells include MSC derived from bone marrow, adipose, or epidermal tissues (301, 302). NSCs may also be induced from amniotic fluid stem cells (193). Induced pluripotent stem cells (iPSCs) have shown some promise in their ability to generate patient-specific, autologous pluripotent cells and tissues, although their ability to retain all autologous characteristics has recently come into question (194, 195). While iPSCs have been predominantly been used in animal and in vitro models, these studies have both significantly contributed to an understanding of neurodegenerative diseases, and are being viewed as significant resource tools for discovering novel therapeutic strategies against neurodegenerative disease and particular types of neurological injury (144, 303).
iPSCs
Researchers successfully demonstrated that induced pluripotent stem cells (iPSCs) could be generated from mouse embryonic fibroblasts (190) and from adult fibroblasts (189, 191, 192, 199) that are cultured in the presence of some combination of four transcription factors (Oct4, Sox2, Klf2, and c-Myc; or Oct4, Sox2, Nanog, and Lin28)—rather than from embryonically- or fetally-derived cells (11, 144, 190, 191). Since then, iPSCs have become an attractive source of pluripotent stem cells for use in neural replacement and regeneration therapies, given their potential to generate a “virtually limitless supply” of autologous iPSCs—thus resolving ethical concerns regarding the source of stem cells and risks associated with immunological rejection in transplant recipients (11, 196). In neural therapies, iPSCs and their progeny can facilitate regeneration of neurons, induce axonal remyelination, provide trophic support and immunomodulation, and modify the extracellular microenvironment (105, 167, 177, 187).
Reprogramming and Differentiation of iPSCs for Neural Insult
The first step in generating iPSCs is to select an appropriate type of donor cell (201). Currently, the majority (i.e., 80%) of published studies of cellular reprogramming employ fibroblasts in that they are easy to obtain, purify, maintain, and have significant potential for autologous transplantation (11, 191, 273). Other somatic donor cells include: melanocytes, adipocytes, CD34+ cells, mesenchymal stem cells, human primary keratinocytes, umbilical cord blood CD133+ cells, and peripheral blood mononuclear cells (199, 208, 273, 304–307).
Each of these cell types has a characteristic differentiation profile related to its epigenetic memory (201, 308, 309). Such “memory” (e.g., particular patterns of DNA methylation, histone acetylation and phosphorylation) can influence patterns of expression and differentiation profiles of the resultant pluripotent stem cells (201, 309). Cells of different origins can also differ in their reprogramming efficiencies (11, 310). When designing cell therapies for neurotrauma, it is important to consider the donor cell source in light of the ease of isolation/expansion, the differentiation profile, the pathology of the CNS injury of interest, and the intended destination of the cell therapy in the injured patient.
Skin fibroblasts, for instance, are easily obtained but can require more time, resources, and manipulation to generate iPSCs due to their low programming efficiency (311, 312). Given the increased time required for reprogramming, they also have potential for increased mutations in vitro. Keratinocytes and melanocytes show therapeutic potential as sources of iPSCs: both are relatively easy to obtain from skin biopsies or plucked hairs, have relatively short reprogramming periods and high reprogramming efficiencies (203). Some research suggests the potential for these cells to be reprogrammed to neural stem cells, though further research is needed to improve the reprogramming process and validate its potential for differentiation and neuroregenerative applications (11, 313). Use of CD133+ cells from umbilical cord blood has been explored due to these cells ‘relatively high reprogramming efficiency and pluripotency (205); however, their use in therapies for CNS insult remains questionable, as there remains a need to characterize their NSC differentiation potential and optimal culture conditions (11, 314). CD34+ cells, derived from bone marrow and umbilical cord blood, are not recommended for clinical use due to their low availability and low reprogramming efficiency (206, 207). Stem cells derived from adipose tissue show notable promise as a reliable source of iPSCs: they are easily obtained during liposuction procedures, require fewer genomic changes to generate iPSCs, and the reprogramming period is both relatively short and efficient (208, 315).
Since the publication of studies by (189), a number of protocols for the development of iPSCs have become available that use these various adult-derived donor cells (some autologous) and employ different combinations of transcription factors (TFs) and delivery methods—with the goal of finding a protocol that optimally balances efficiency, safety and related risks (201, 311–321). Some TF delivery methods involve the use of viruses, such as lentiviruses, to integrate viral DNA into replicating regions of the host genome (322). The use of viral vectors to deliver reprogramming factors can, however, lead to chromosomal disruption, expression of transgenes, and/or mutations (322). More recent “scarless” technologies have been introduced that use episomal vectors, synthetic mRNAs and Sendai viruses that do not integrate to the genome during reprogramming of adult somatic cells into iPSCs (198, 323–325). Kim et al. (199) developed a vector-free method to generate human iPSCs directly from human fibroblasts through delivery of reprogramming proteins (Oct4, Sox2, Klf4, and c-Myc) fused to cell penetrating peptides capable of crossing the cell membrane (199). These “scarless” and vector-free systems reduce potential risks of chromosomal disruption associated with DNA transfection and genome manipulation.
Studies in animal models of SCI that involve transplantation of human NSCs derived from iPSCs also suggest that difficulties remain in ensuring in vivo differentiation of cells into appropriate neural cell lineages and with tumorigenicity—both of which may hinder successful cell engraftment and functional recovery (326, 327). Evidence suggests that the local CNS microenvironment and pro-inflammatory factors (and associated neuroinflammation) can influence transplanted cell engraftment, differentiation and survival (326). In light of this, optimization of protocols for human cell reprogramming and the generation of iPSCs for use in clinical trials remains a work in progress in an attempt to achieve balance in safety and efficiency when applied in humans for certain types of neurotrauma (11, 144, 328).
Direct Reprogramming and Transdifferentiation of Autologous Somatic Cells
Cell reprogramming studies published in the late twentieth and early twenty-first centuries that demonstrated transdifferentation challenged the widespread notion of predetermined cell fates (329–332). Indeed, NPCs/NSCs can be generated from iPSCs, but can also be directly generated from somatic cells (e.g., fibroblasts) via a process known as direct reprogramming (333, 334). Lujan et al. (335) showed the direct conversion of MEFs to NPCs using reprogramming factors (Sox2, FoxG1, and Brn2). Somatic cells can also be transdifferentiated to become mature neuronal cells [e.g., neurons, glia; (336, 337)]. Vierbuchen et al. (336), for instance, demonstrated that mouse embryonic fibroblasts (MEFs) could be directly converted to neurons. The same and other iterative combinations of factors (e.g., Musashi-1 (Msi-1)5) and techniques (e.g., silencing of donor cell-specific transcriptional programs, chromatin remodeling) to optimize cell transdifferentiation, generation and stability have been further identified (339–343).
Direct reprogramming via chromatin remodeling involves opening the chromatin in somatic cells, and facilitating sustained exposure to a set of transcription factors that effectively converts the somatic cells to NSC/NPCs; chromatin remodeling enables NSCs/NPCs to maintain their new identities and bypass the intermediate pluripotent state (337, 344, 345). For direct reprogramming approaches, bypassing the intermediate pluripotent state reduces the associated reprogramming risks of genetic and epigenetic abnormalities (346, 347). Continuing research is further exploring those combinations of TFs that could be most effective in direct reprogramming somatic cells into neuronal subtypes (e.g., dopaminergic, GABA-ergic, glutamatergic, and motor neurons), as well as distinct types of glial cells [i.e., oligodendrocytes, astrocytes, and Schwann cells; (325, 348)].
Possible Applications of Autologous iPSC-Derived Cell Types and drNPCs
NPCs have potential to participate in and facilitate central neural regeneration and repair by functioning to replace lost or damaged neurons, prompt axonal remyelination, and provide local trophic support (111, 132, 155, 262). Autologous iPSC-NPCs could be generated using a patient's somatic cells, and reprogrammed via dual SMAD inhibition (209, 349), embryoid body formation and differentiation into neural rosettes (210), or via the piggybac transposon system paired with induction of the NOTCH pathway (211, 324). Dimos et al. (350) generated autologous iPSCs from patient-specific fibroblasts that were then differentiated into motor neurons. As previously mentioned, drNPCs may also be generated via direct reprogramming of autologous somatic cells. In mouse models of SCI, transplanted iPSC-NPCs induced axonal remyelination and regeneration, inhibition of (further) cell death, and improvements in a number of behavioral outcomes (169, 170, 172). These cells have also shown the ability to differentiate in vivo into neurons and glial cells, migrate (169), and effectively integrate to host tissue within the CNS (114).
Issues, Questions, Problems, and Possible Solutions
Optimization of iPSC Production and Differentiation
Several important questions remain regarding reprogramming of human cells that must be considered pursuant to their use in clinical applications. For example, it will be important to discern to what extent iPSCs retain epigenetic memory of their donor cell types, and whether the induced pluripotency is equivalent to that seen in ESCs (144, 309, 351). It is also necessary to determine the extent to which different reprogramming methods (choice of donor cell and iPSC line, cell selection during propagation, and culture conditions) influences genetic variability and functionality of the differentiated cells (144, 310). While genetic variability between iPSC lines could theoretically be resolved via gene editing, human pluripotent stem cells have demonstrated resilience to conventional gene targeting methods, and even those methods that have worked (352–354) have been shown to be both inefficient and time consuming (144). Advancements made in the use of novel gene-editing systems, such as CRISPR/Cas-9 and its variants, may offer a possible solution to this demonstrated resilience in iPSCs to targeted gene-editing (144, 355).
Mutations, Tumorigenicity, and Teratoma Formation
Concerns regarding mutations and tumorigencitiy are focal to any consideration of the development and use of reprogrammed, differentiated iPSCs. iPSCs can acquire mutations during both the reprogramming process and in vitro expansion; furthermore, some protocols for generation of iPSCs call for the use of c-Myc—a proto-oncogene associated with various types of cancers (325, 356, 357). Risk of tumorigenicity and accumulation of genetic alterations might be reduced by selecting appropriate cell source(s) for the generation of iPSCs (i.e. adipose cells, keratinocytes, or melanocytes) (11, 310). Direct reprogramming may also offer a way to reduce these risks, as reprogrammed cells do not require an intermediate pluripotent stage (329–332).
Yet, even with sufficient controls for mutation during these phases of development, there remains a definitive need for an efficient technique for evaluating differentiation and screening for mutagenesis and potential tumor- and/or teratoma formation both prior to and following transplantation. Antibodies that target high-risk cell subpopulations and selective in vivo ablation of transplanted cells are potential solutions being explored to reduce this risk (358), and further studies are needed to more fully define the effectiveness of this, and other methods of insuring stability of these cells.
Immunological Interactions and Possible Rejection
A growing body of literature describes the numerous pathways of communication that exist between the immune system and the CNS—namely, the role that such communication has in the CNS' ability to maintain homeostasis and to react to neurological injury and trauma [e.g., neuroinflammation, (359–362)]. Communication between these two vital systems is mediated primarily through immune regulatory molecules that are released by cells residing in the CNS, such as microglia, mast cells, astrocytes, and oligodendrocytes (361–365).
Following neurotrauma, the CNS undergoes pathophysiological changes that both affect and are affected by the innate and adaptive arms of the immune system. After acute stroke, for instance, brain ischaemia, damage to tissue and subsequent release of pro-inflammatory factors (e.g., matrix metalloproteinase-3 (MMP-3), ATP, alpha-synuclein, and neuromelanin, (366, 367)) can activate innate immune (via leukocytes, toll-like receptors, and the lectin pathway) and neuroinflammatory responses, such as the release of cytokines and activation of resident microglial cells (359, 365, 368). T and B cells mediate the adaptive immune responses to neurological damage, providing both potentially protective and potentially damaging autoimmune/reactive effects on neural tissue, if inflammation is prolonged and unregulated [(362, 365, 369–371); for a thorough overview of the innate and adaptive immune responses to neurotrauma, see (359, 371)].
For cell therapies intended to treat the effects of neurotrauma, neuroinflammation and the potential for an immune response must be considered. In some cases, the administration of antibiotics, immunosuppresants, or other small-molecule anti-inflammatory agents [e.g., N-palmitoylethanolamine (PEA)] may be required, particularly following or alongside transplant or injection of tissue/cells into injured patients (362, 372, 373). However, one must also consider the effect that administration of such immunosuppressants may have on the stem cell therapies, cell differentiation and cell engraftment, as well as the optimum time for delivery of these substances (374).
In theory, the use of autologous adult somatic cells to generate iPSCs and progeny would avoid or at least significantly reduce immunogenicity in stem cell transplant therapies. However, Zhao et al. (375) suggested that transplantable stem cells could be subject to aberrant DNA methylation during the reprogramming process, and that such changes to the DNA might trigger an immune response after transplantation in the host. Other potential sources of immunogenicity may include culture reagents, protein expression, maturity of transplanted cells, or factors of the micro- or macro-environment at the site of transplantation (376). Others have explored the potential immunogenicity of autologous iPSCs using mouse (377, 378) and primate models (379), and these studies appear to contradict the results reported by Zhao et al. (375). Such ambiguity establishes the need to more stringently assess potential sources of immunogenicity in iPSC-derived cell therapies prior to their clinical use.
Safety and Use: Addressing Ethical, Legal and Social Issues
Interest in stem cell research is sustained by the potential contribution that these cells and their progeny can make to transplant-based therapies for neurological repair and regeneration (260, 380, 381). Indeed, research suggests that stem cell transplantation affords potential as a therapeutic strategy for central neural injury and disease (260, 382, 383). Initially focused on use of ESCs as sources of pluripotent stem cells, the field was soon rife with ethical debate related to controversies surrounding the sources of ESCs, implications relevant to the status of early-stage human embryos (380), limited supply, high cost, and associated risks of tumorigenicity and immune rejection after transplantation.
In light of this, more recent basic and preclinical studies of regenerative therapies for CNS insult have shifted toward use of iPSCs and their derivatives that are generated from adult somatic cells. As noted above, iPSC-derived and directly reprogrammed neuronal and glial cell types can be generated from cell sources that are not (or at least less) controversial and more readily available. Such cell sources can be autologous—derived from tissue samples directly from patients—and thus, can eliminate or significantly reduce risks of immunological rejection after transplantation, and may appeal to current incentives for personalized and precision medicine. As well, developments have been made in reducing time spent in vitro during the reprogramming processes, improving selection of cell type, and increasing efficiency of methods to remove harmful cells pre- and post-transplantation, which, when taken together, can reduce risks of tumorigencity in iPSC transplant-based therapies.
To be sure, autologous reprogrammed cells have a number of attributes that make them attractive and potentially viable alternatives (to ESCs and fetal stem cells) in cell-based therapeutic approaches for particular types of CNS injury and disease. Yet, while this approach has potential to fulfill therapeutic goals of enabling partial to complete recovery of defined functions, ethico-legal and social issues persist. As described in detail elsewhere (260, 384, 385) such issues involve the unknowns of intermediate and long-term use of new techniques and technologies in practice. For example, (1) how such unknowns impact the validity and contingencies of informed consent; (2) the need for continuity of research and clinical care (to address emerging issues consequential to sustained use-in-practice); (3) legal liabilities and process of claims if/when adverse events occur; and (4) provision/distribution of resources and services (to furnish cells, as well clinical interventions, and ongoing support) in and across various populations in need.
We have proposed a risk assessment approach to more specifically define and address the issues, questions and problems generated by possible uses of emerging neuroscientific and neurotechnological developments (386, 387); but address does not assure resolution. While some concerns relate to safety and effectiveness (e.g., possibilities of unanticipated consequences, runaway effects) with regard for patients' welfare (i.e., via integrity of informed consent and availability of sustainable clinical care), others reflect recognition of problematic distribution of medical goods [for overview, see (388)]. Both safety and distribution can be met, to some extent, by rigorous quality control and expediency in the manufacture and delivery of cells. Still, it remains to be determined if the type and extent of broad-based medical care (i.e., trained personnel and institutional resources) can and/or will be made available to perform the procedures and insure sustained post-implantation evaluations and care.
We have opined, and re-iterate here, that any consideration of the ethics of biomedicine must regard economic factors, and in many cases, economics of biomedical research and care are determined by policy and law (389, 390). Here discussion of the viability and value of NSCs—or any biomedical technique and/or technology—centers upon the interactive roles of regulatory policy in establishing both standards of care and fiscal (i.e., insurance) subsidy of these approaches in patient care. Suffice it to note that the gravitas—and international relevance and importance—of this situation is such that a complete discussion of economic and policy issues focal to NSCs or translational neuroscience would be significant, and is therefore beyond the scope of this paper.
Conclusions and Future Vistas
Research and possible use of stem cell therapies toward mitigating the effects of neurotrauma, and inducing neurological repair and regeneration are gaining momentum and attention—in both the professional and public spheres. Emerging techniques and technologies of cell development, manufacture and delivery are progressing at a considerable pace. A number of previously contentious technical and ethical issues and questions have been met and resolved, at least in part, by the advent of ever newer forms of stem cells. Of these, autologous stem cells appear to offer a number of advantages (e.g., immunological compatibility and lack of rejection, personalized therapy, non-fetal, or embryonic derivation) that would suggest their value in and for clinical use in effecting neurological repair and regeneration (following neurotrauma and in treatment of certain neurological diseases). Yet, it is likely that there will not be a single stem cell therapy for CNS injury, but instead a more customizable (pathology- and patient-specific) approach.
Regardless of iteration or approach, it will be important and necessary to develop and insure high quality, secure and rapid throughput manufacturing processes that enable time- and cost-efficient delivery of these cells. But manufacture is but one cog in a multi-spoked wheel of biomedical research and care. As noted, if developments in the brain sciences are to be translated into safe, affordable and accessible clinical care that maintain high quality, then medicine, as well as economics, regulatory policy and law must be aligned in order to support and sustain meaningful benefit to patients in need. Simply put, the prevalence and multi-dimensional burden of neurological injuries and neurodegenerative diseases demand the further exploration and pursuit of NSCs—and other therapeutic strategies—that are both promising and feasible.
Author Contributions
JG and KF developed the outline and structure of the work. SW conducted the literature review and drafted the initial manuscript. JG and KF oversaw subsequent revisions. JG, SW, and KF contributed and approved final edits and revisions to the manuscript for publication.
Funding
This work was supported in part by federal funds UL1TR001409 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of the Department of Health and Human Services, part of the Roadmap Initiative, Re-Engineering the Clinical Research Enterprise (JG); by the European Union's Horizon 2020 Research and Innovation Programme (JG); the AEHS Foundation and Project Neuro-HOPE (JG); and by an unrestricted service agreement from Fortuna Fix Inc. (JG and KF).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^National Institutes of Health, “NIH Human Embryonic Stem Cell Registry,” 4 May 2018, accessed May 2018, https://grants.nih.gov/stem_cells/registry/current.htm.).
2. ^Mattis V, Svendsen S, Sareen D, and Svendsen C, “Neural stem cells,” Nature Neuroscience, 2010, https://www.stemcell.com/media/files/wallchart/WA10008-Neural_Stem_Cells.pdf.
3. ^FDA, “FDA Warns About Stem Cell Claims,” 6 Jan 2012, accessed 8 Aug 2017, https://www.fda.gov/forconsumers/consumerupdates/ucm286155.htm#Regulation.
4. ^ClinicalTrials.gov, accessed May 2018, www.clinicaltrials.gov.
5. ^Musashi-1(Ms-1) is found to upregulate in injured ependymal cells in certain adult amphibians and lizards, and thought to play a role in regeneration of the injured spinal cord (143, 144, 338, 339).
References
1. Struzyna LA, Adewole DO, Gordian-Velez WJ, Govola MR, Burrell JC, Katiyar KS, et al. Anatomically inspired three-dimensional micro-tissue engineered neural networks for nervous system reconstruction, modulation, and modeling. J Vis Exp. (2017). 123:e55609. doi: 10.3791/55609
2. Shoichet MS, Tate CC, Baumann MD, LaPlaca MC. Strategies for regeneration and repair in the injured central nervous system. In: Reichert WM, editor. Indwelling Neural Implants: Strategies for Contending With the In Vivo Environment. Boca Raton, FL: CRC Press/Taylor & Francis (2008).
3. Giordano J, Waters P. What might renewed focus in brain research mean for the prevention and care of brain injury and its effects. In: Giordano J, Waters P, editor. Brain Injury: Spectrum Effects and Implications. Arlington, VA: Potomac Institute Press (2013). p. xiii–xxiii.
4. Giordano J, Walter J, Wurzman R. Acquired brain injury and its manifestation as spectrum disorder: implications for the diagnosis and treatment of chronic pain and ohter neuropsychiatric features. In: Giordano J, Waters P, editor. Brain Injury Spectrum Effects and Implications. Arlington VA: Potomac Institue Press (2013). p. 272–85.
5. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts Figures at a Glance. (2013). Available online at: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202013.pdf
6. Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. (2015) 10:726–42. doi: 10.4103/1673-5374.156966
7. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. (2013) 246:35–43. doi: 10.1016/j.expneurol.2012.01.013
8. Bose P, Hou J, Thompson FJ. Chapter 14: Traumatic Brain Injury (TBI)- Induced Spasticity. In Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects (Boca Raton, FL: CRC Press/Taylor & Francis).
9. Lloyd-Jones D, Adams R, Brown T, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics 2010 update: a report from the American Heart Association. Circulation (2010) 121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667
10. Faravelli I, Corti S. MicroRNA-Directed Neuronal Reprogramming as a Therapeutic Strategy for Neurological Diseases. Mol Neurobiol. (2017) 55:4428–36. doi: 10.1007/s12035-017-0671-7
11. Khazaei M, Ahuja CS, Fehlings MG. Induced pluripotent stem cells for traumatic spinal cord injury. Front Cell Dev Biol. (2017) 4:152. doi: 10.3389/fcell.2016.00152
12. Burckhardt CS, Anderson KL. The Quality of Life Scale (QOLS): Reliability, Validity, and Utilization. Health Qual Life Outcomes (2003) 1:60. doi: 10.1186/1477-7525-1-60
13. Di Battista A, Soo C, Catroppa C, Anderson V. Quality of life in children and adolescents post-TBI: a systematic review and meta-analysis. J Neurotrauma. (2012) 29:1717–27. doi: 10.1089/neu.2011.2157
14. Grauwmeijer E, Heijenbrok-Kal MH, Peppel LD, Hartjes CJ, Haitsma IK, de Koning I, et al. Cognition, health-related quality of life, and depression ten years after moderate to severe traumatic brain injury: a prospective cohort study. J Neurotrauma. (2018) 35:1543–1551. doi: 10.1089/neu.2017.5404
15. DeVivo MJ, Chen Y, Mennemeyer ST, Deutsch A. Costs of care following spinal cord injury. Top Spinal Cord Inj Rehabil. (2011) 16:1–9. doi: 10.1310/sci1604-1
16. Cao Y, Chen Y, DeVivo MJ. Lifetime direct costs after spinal cord injury. Top Spinal Cord Inj Rehab. (2011) 16:10–6. doi: 10.1310/sci1604-10
17. Hayakata T, Shiozaki T, Hosotubo H, Kieko F, Yamashita T, Tanaka H, et al. Changes in CSF and S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. (2004) 22:102–7. doi: 10.1097/01.shk.0000131193.80038.f1
18. Nortje J, Menon DK. (2004). Traumatic brain injury: physiology, mechanisms, and outcome. Curr Opin Neurol. (2008) 17:711–8. doi: 10.1097/00019052-200412000-00011
20. Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA (1983) 80:2390–4.
21. Paton JA, O'Laughlin BE, Nottebohm F. Cells born in adult canary forebrain are local interneurons. J Neurosci. (1985) 5:3088–93.
23. Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. (1988) 72:399–406.
24. Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience (1993) 56:337–44.
25. Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. (2018) 22:589–599.e5 doi: 10.1016/j.stem.2018.03.015
26. Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. (2018) 555:377–81. doi: 10.1038/nature25975
27. Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. (2003) 6:507–18. doi: 10.1038/nn1048
28. Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron (2004) 41:683–6. doi: 10.1016/S0896-6273(04)00111-4
29. Kosaka T, Kosaka K. Tyrosine hydroxylase-positive GABAergic juxtaglomerular neurons are the main source of the interglomerular connections in the mouse main olfactory bulb. Neurosci Res. (2008) 60:349–54. doi: 10.1016/j.neures.2007.11.012
30. Adam Y, Mizrahi A. Circuit formation and maintenance - perspectives from the mammalian olfactory bulb. Curr Opin Neurobiol. (2010) 20:134–40. doi: 10.1016/j.conb.2009.11.001
31. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. (1996) 16:7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996
32. Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. (1999) 9:135–41.
33. Gage FH. Mammalian neural stem cells. Science (2000) 287:1433–8. doi: 10.1126/science.287.5457.1433
34. Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. (2000) 20:2218–28. doi: 10.1523/JNEUROSCI.20-06-02218.2000
35. Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma (2002) 19:223–38. doi: 10.1089/08977150252806974
36. Lie DC, Song H, Colamarino SA, Ming G, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. (2004) 44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631
37. Gao Y, Yang Z, Li X. Regeneration strategies after the adult mammalian central nervous system injury-biomaterials. Regen Biomater. (2016) 3:115–22. doi: 10.1093/rb/rbw004
38. Aranda-Anzaldo A. The post-mitotic state in neurons correlates with a stable nuclear higher-order structure. Commun Integr Biol. (2012) 5:134–9. doi: 10.4161/cib.18761
39. Andreae LC. Adult neurogenesis in humans: Dogma overturned again and again? Sci Transl Med. (2018) 10:eaat3893. doi: 10.1126/scitranslmed.aat3893
40. Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science (1974) 183:425–7. doi: 10.1126/science.183.4123.425
41. Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. (2006) 103:12564–8. doi: 10.1073/pnas.0605177103
42. Rakic P. Neuroscience. No more cortical neurons for you. Science (2006) 313:928–9. doi: 10.1126/science.1131713
43. Park DS, Obeidat A, Giovanni A, Greene LA. Cell cycle regulators in neuronal death evoked by excitotoxic stress: implications for neurodegeneration and its treatment. Neurobiol Aging (2000) 21:771–81. doi: 10.1016/S0197-4580(00)00220-7
44. Ramon Y, Cajal S. Degeneration and Regeneration of the Nervous System. Oxford, UK: Clarendon Press (1928).
45. Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. (2004) 24:6531–9. doi: 10.1523/JNEUROSCI.0994-04.2004
46. David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science (1981) 214:931–3.
47. Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. (2006) 7:617–27. doi: 10.1038/nrn1956
48. Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. (1985) 5:2415–23. doi: 10.1523/JNEUROSCI.05-09-02415.1985
49. Renfranz PJ, Cunningham MG, McKay RD. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell (1991) 66:713–29. doi: 10.1016/0092-8674(91)90116-G
50. Calabrese EJ, Calabrese V, Giordano J. Role of hormesis in functional performance and protection of neural systems. Brain Circ. (2017) 3:1–13. doi: 10.4103/2394-8108.203257
51. Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature (1990) 343:269–72. doi: 10.1038/343269a0
52. Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. (1989) 9:1126–33. doi: 10.1523/JNEUROSCI.09-04-01126.1989
53. Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. (1990) 10:3837–48. doi: 10.1523/JNEUROSCI.10-12-03837.1990
54. Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature Rev Neurosci. (2003) 4:703–13. doi: 10.1038/nrn1195
55. Yiu G, He Z. Signaling mechanisms of the myelin inhibitors of axon regeneration. Curr Opin Neurobiol. (2003) 13:545–51. doi: 10.1016/j.conb.2003.09.006
56. He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu Rev Neurosci. (2004) 27:341–68. doi: 10.1146/annurev.neuro.27.070203.144340
57. Menet V, Prieto M, Privat A, Gimenez Y, Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA. (2003) 100:8999–9004. doi: 10.1073/pnas.1533187100
58. Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev Neurosci. (2004) 5:146–56. doi: 10.1038/nrn1326
59. Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron (1999) 23:297–308. doi: 10.1016/S0896-6273(00)80781-3
60. Faulkner JR, Hermann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. (2004) 24:2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004
61. Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. (2006). Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129(Pt 10):2761–2772. doi: 10.1093/brain/awl165
62. Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. (2004) 101:17528–32. doi: 10.1073/pnas.0407893101
63. Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. (2004) 27:145–67. doi: 10.1146/annurev.neuro.27.070203.144308
64. Mu L, Berti L, Masserdotti G, Covic M, Michaelidis TM, Doberauer K, et al. SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J Neurosci. (2012) 32:3067–80. doi: 10.1523/JNEUROSCI.4679-11.2012
65. Huebner E, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. (2009) 48:339–51. doi: 10.1007/400_2009_19
66. Kabu S, Gao Y, Kwon BK, Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Control Rel. (2016) 219:141–54. doi: 10.1016/j.jconrel.2015.08.060
67. Diaz-Arrastia R, Kochanek PM, Bergold P, Kenney K, Marx CE, Grimes JB, et al. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the department of defense neurotrauma pharmacology workgroup. J Neurotrauma (2014) 31:135–58. doi: 10.1089/neu.2013.3019
68. Bansal S, Sangha KS, Khatri P. Drug treatment of acute ischemic stroke. Am J Cardiovasc Drugs (2014) 13:57–69. doi: 10.1007/s40256-013-0007-6
69. Cramer SC. Drugs to enhance motor recovery after stroke. Stroke (2015) 46:2998–3005. doi: 10.1161/STROKEAHA.115.007433
70. Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering – a review. Carbohydr Polym. (2013) 92:1262–79. doi: 10.1016/j.carbpol.2012.10.028
71. Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials (2013) 34:5937–46. doi: 10.1016/j.biomaterials.2013.04.047
72. Liu T, Xu J, Chan BP, Chew SY. Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair. J Biomed Mater Res A. (2012) 100:236–42. doi: 10.1002/jbm.a.33271
73. Sun F, Shi T, Zhou T, Dong D, Xie J, Wang R, et al. 3D poly(lactic-co-glycolic acid) scaffolds for treating spinal cord injury. J Biomed Nanotechnol. (2017) 13:290–302. doi: 10.1166/jbn.2017.2348
74. Tang ZP, Liu N, Li ZW, Xie XW, Chen Y, Shi YH, et al. in vitro evaluation of the compatibility of a novel collagen-heparin sulfate biological scaffold with olfactory ensheathing cells. Chin Med J (Engl). (2010) 123:1299–304. doi: 10.3760/cma.j.issn.0366-6999.2010.10.014
75. Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell (2009) 4:507–10. doi: 10.1016/j.stem.2009.05.008
76. Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. (2010) 37:275–83. doi: 10.1016/j.nbd.2009.10.003
77. Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. (2012) 122:3824–34. doi: 10.1172/JCI64124
78. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. (2003) 73:778–86. doi: 10.1002/jnr.10691
79. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology (2002) 59:514–23. doi: 10.1212/WNL.59.4.514
80. Galindo LT, Filippo TR, Semedo P, Ariza CB, Moreira CM, Camara NO, et al. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. (2011) 2011:564089. doi: 10.1155/2011/564089
81. Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, et al. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. (2010) 10:1496–500. doi: 10.1016/j.intimp.2010.06.019
82. Anbari F, Khalili MA, Bahrami AR, Khoradmehr A, Sadeghian F, Fesahat F, et al. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen Res. (2014) 9:919–923. doi: 10.4103/1673-5374.133133
83. Ritfeld GJ, Nandoe Tewarie RD, Vajn K, Rahiem ST, Hurtado A, Wendell DF, et al. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. (2012) 21:1561–75. doi: 10.3727/096368912X640484
84. Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells (2007) 25:1737–1745. doi: 10.1634/stemcells.2007-0054
85. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. (1999) 96:10711–6. doi: 10.1073/pnas.96.19.10711
86. Schmidt A, Ladage D, Steingen C, Brixius K, Schinköthe T, Klinz FJ, et al. Mesenchymal stem cells transmigrate over the endothelial barrier. Eur J Cell Biol. (2006) 85:1179–88. doi: 10.1016/j.ejcb.2006.05.015
87. Matsushita T, Kibayashi T, Katayama T, Yamashita Y, Suzuki S, Kawamata J, et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. (2011) 502:41–5. doi: 10.1016/j.neulet.2011.07.021
88. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie C, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. (2002) 174:11–20. doi: 10.1006/exnr.2001.7853
89. Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. (2003) 101:2999–3001. doi: 10.1182/blood-2002-06-1830
90. Yang ZJ, Ma DC, Wang W, Xu SL, Zhang YQ, Chen B, et al. Experimental study of bone marrow-derived mesenchymal stem cells combined with hepatocyte growth factor transplantation via noninfarct-relative artery in acute myocardial infarction. Gene Ther. (2006) 13:1564–8. doi: 10.1038/sj.gt.3302820
91. Yang Z, Duan H, Mo L, Qiao H, Li X. The effect of the dosage of NT-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials (2010) 31:4846–54. doi: 10.1016/j.biomaterials.2010.02.015
92. Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. (2012) 4:161ra150. doi: 10.1126/scitranslmed.3004660
93. Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. (1998) 36:249–66.
94. Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. (1993) 90:2074–7. doi: 10.1073/pnas.90.5.2074
95. English D, Sharma NK, Sharma K, Anand A. Neural stem cells-trends and advances. J Cell Biochem. (2013) 114:764–72. doi: 10.1002/jcb.24436
96. Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. (2005) 25:1169–78. doi: 10.1523/JNEUROSCI.3562-04.2005
97. Oraee-Yazdani S, Hafizi M, Atashi A, Ashrafi F, Seddighi AS, Hashemi SM, et al. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal Cord (2016) 54:102–9. doi: 10.1038/sc.2015.142
98. Bento AR, Quelhas P, Oliveira MJ, Pego AP, Amaral IF. Three-dimensional culture of single embryonic stem-derived neural/stem progenitor cells in fibrin hydrogels: neuronal network formation and matrix remodeling. J Tiss Eng Regener Med. (2017) 11, 3494–3507. doi: 10.1002/term.2262
99. Mukhamedshina YO, Akhmetzyanova ER, Kostennikov AA, Zakirova EY, Galieva LR, Garanina EE, et al. Adipose-derived mesenchymal stem cell application combined with fibrin matrix promotes structural and functional recovery following spinal cord injury in rats. Front Pharmacol. (2018) 9:343. doi: 10.3389/fphar.2018.00343
100. Ormerod BK, Palmer TD, Caldwell MA. Neurodegeneration and cell replacement. Phil Trans R Soc B. (2008) 363:153–70. doi: 10.1098/rstb.2006.2018
101. McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. (1999) 5:1410–2. doi: 10.1038/70986
102. Bottai D, Cigognini D, Madaschi L, Adami R, Nicora E, Menarini M, et al. Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Exp Neurol. (2010) 223:452–63. doi: 10.1016/j.expneurol.2010.01.010
103. Priest CA, Manley NC, Denham J, Wirth ED III, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. (2015) 10:939–58. doi: 10.2217/rme.15.57
104. Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. (2005) 25:4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005
105. Salewski RP, Mitchell RA, Shen C, Fehlings MG. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. (2015) 24:36–50. doi: 10.1089/scd.2014.0096
106. Kumagi G, Okada Y, Yamane J, Nagoshi N, Kitamura K, Mukaino M, et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS ONE (2009) 4:e7706. doi: 10.1371/journal.pone.0007706
107. Marques SA, Almedia FM, Fernandes AM, dos Santos Souza C, Cadilhe DV, Rehen SK, et al. Predifferentiated embryonic stem cells promote functional recovery after spinal cord compressive injury. Brain Res. (2010) 1349:115–28. doi: 10.1016/j.brainres.2010.06.028
108. Reier PJ, Bregman BS, Wujek JR. Intrapsinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. (1986) 247:275–96. doi: 10.1002/cne.902470302
109. Houlé JD, Reier PJ. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol. (1988) 269:535–47. doi: 10.1002/cne.902690406
110. Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophics. J Neurosci. (2001) 21:9334–44. doi: 10.1523/JNEUROSCI.21-23-09334.2001
111. Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore connectivity across the injured spinal cord. J Neurosci. (2011) 31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011
112. Lu P, Wang Y, Graham L, McHale K, Wu D, Brock J, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell (2012) 150:1264–73. doi: 10.1016/j.cell.2012.08.020
113. Lu P, Ceto S, Wang Y, Graham L, Wu D, Kumamaru H, et al. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J Clin Invest. (2017) 127:3287–99. doi: 10.1172/JCI92955
114. Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. (2016) 22:479–87. doi: 10.1038/nm.4066
115. Iwai H, Shimada H, Nishimura S, Kobayashi Y, Itakura G, Hori K, et al. Allogeneic neural stem/progenitor cells derived from embryonic stem cells promote functional recovery after transplantation into injured spinal cord of nonhuman primates. Stem Cells Transl Med. (2015) 4:708–19. doi: 10.5966/sctm.2014-0215
116. Hoane MR, Becerra GD, Shank JE, Tatko L, Pak ES, Smith M, et al. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J Neurotrauma (2004) 21:163–74. doi: 10.1089/089771504322778622
117. Shear DA, Tate MC, Archer DR, Hoffman SW, Hulce VD, Laplaca MC, et al. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res. (2004) 1026:11–22. doi: 10.1016/j.brainres.2004.07.087
118. Riess P, Molcanyi M, Bentz K, Maegele M, Simanski C, Carlitscheck C, et al. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause, tumors. J Neurotrauma (2007) 24:216–25. doi: 10.1089/neu.2006.0141
119. Dobrowolski S, Lepski G. Stem cells in traumatic brain injury. Am J Neurosci. (2013) 4:13–24. doi: 10.3844/ajnsp.2013.13.24
120. Wennersten A, Meier X, Holmin S, Wahlberg L, Mathiesen T. Proliferation, migration, and differentiation of human neural stem/progenitor cells after transplantation into a rat model of traumatic brain injury. J Neurosurg. (2004) 100:88–96. doi: 10.3171/jns.2004.100.1.0088
121. Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol. (2006) 201:281–92. doi: 10.1016/j.expneurol.2006.04.039
122. Skardelly M, Gaber K, Burdack S, Scheidt F, Hilbig H, Boltze J, et al. Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J Neurotrauma. (2011) 28:401–14. doi: 10.1089/neu.2010.1526
123. Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. (2005) 19:183–93. doi: 10.1016/j.nbd.2004.12.016
124. Yanagisawa D, Qi M, Kim DH, Kitamura Y, Inden M, Tsuchiya D, et al. Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci Lett. (2006) 407:74–9. doi: 10.1016/j.neulet.2006.08.007
125. Tae-Hoon L, Yoon-Seok L. Transplantation of mouse embryonic stem cell after middle cerebral artery occlusion. Acta Cir Bras. (2012) 27:333–9. doi: 10.1590/S0102-86502012000400009
126. Bühnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain (2006) 129(Pt 12):3238–48. doi: 10.1093/brain/awl261
127. Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. (2006) 26:906–14. doi: 10.1038/sj.jcbfm.9600247
128. Kim DY, Park SH, Lee SU, Choi DH, Park HW, Paek SH, et al. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. (2007) 58:164–75. doi: 10.1016/j.neures.2007.02.016
129. Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isaacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells (2007) 25:411–8. doi: 10.1634/stemcells.2006-0380
130. Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS ONE (2008) 3:e1644. doi: 10.1371/journal.pone.0001644
131. Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. (2009) 29:562–74. doi: 10.1111/j.1460-9568.2008.06599.x
132. Jin K, Xie L, Mao X, Greenberg MB, Moore A, Peng B, et al. Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res. (2011) 1374:56–62. doi: 10.1016/j.brainres.2010.12.037
133. Jablonska A, Drela K, Wojcik-Stanaszek L, Janowski M, Zalewski T, Lukomska B. Short-lived human umbilical cord-blood-derived neural stem cells influence the endogenous secretome and increase the number of endogenous neural progenitors in a rat model of lacunar stroke. Mol Neurobiol. (2016) 53:6413–25. doi: 10.1007/s12035-015-9530-6
134. Evans MJ. The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morphol. (1972) 28:163–76.
135. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–47. doi: 10.1126/science.282.5391.1145
136. Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. (2000) 18:399–404. doi: 10.1038/74447
137. Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, et al. Somatic cell nuclear transfer. Nature (2002) 419:583–6. doi: 10.1038/nature01079
138. 1Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol Med. (2000) 6:88–95.
139. Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, et al. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. (2002) 51:1043–52.
140. Webowetski-Ogilvie TE, Bosse M, Stewart M, Schnerch A, Ramos-Mejia V, Rouleau A, et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol. (2009) 27:91–7. doi: 10.1038/nbt.1516
141. Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. (2009) 27:743–5. doi: 10.1038/nbt.1554
142. Sadowski D, Kiel ME, Apicella M, Arriola AG, Chen CP, McKinnon RD. Teratogenic potential in cultures optimized for oligodendrocyte development from mouse embryonic stem cells. Stem Cells Dev. (2010) 19:1343–53. doi: 10.1089/scd.2009.0520
143. Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. (2013) 23:70–80. doi: 10.1038/cr.2012.171
144. Hockemeyer D, Jaenisch R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell. (2016) 18:573–86. doi: 10.1016/j.stem.2016.04.013
145. Dalamagkas K, Tsintou M, Seifalian AM. Stem cells for spinal cord injuries bearing translational potential. Neural Regen Res. (2018) 13:35–42. doi: 10.4103/1673-5374.224360
146. Fisher LJ, Gage FH. Grafting in the mammalian central nervous system. Physiol Rev. (1993) 73:583–616.
147. Skardelly M, Gaber K, Burdack S, Scheidt F, Schuhmann MU, Hilbig H, et al. Transient but not permanent benefit of neuronal progenitor cell therapy after traumatic brain injury: potential causes and translational consequences. Front Cell Neurosci. (2014) 8:318. doi: 10.3389/fncel.2014.00318
148. Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, et al. in vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. (2005) 92:1265–76. doi: 10.1111/j.1471-4159.2004.03006.x
149. Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. (2001) 167:48–58. doi: 10.1006/exnr.2000.7536
150. Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. (2006) 26:3377–89. doi: 10.1523/JNEUROSCI.4184-05.2006
151. Pfeifer K, Vroemen M, Caloni M, Aigner L, Bogdahn U, Weidner N. Autologous adult rodent neural progenitor cell transplantation represents a feasible strategy to promote structural repair in the chronically injured spinal cord. Regen Med. (2006) 1:255–66. doi: 10.2217/17460751.1.2.255
152. Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. (2007) 24:835–45. doi: 10.1089/neu.2006.3771
153. Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, et al. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. (2008) 155:760–70. doi: 10.1016/j.neuroscience.2008.05.042
154. Sullivan GM, Armstrong RC. Transplanted adult neural stem cells express sonic hedgehog in vivo and suppress white matter neuroinflammation after experimental traumatic brain injury. Stem Cells Int. (2017) 2017:9342534. doi: 10.1155/2017/9342534
155. Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. (2009) 133:3483. doi: 10.1093/brain/awp174
156. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. (1992) 255:1707–10. doi: 10.1126/science.1553558
157. Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. (1965) 207:953–6. doi: 10.1038/207953a0
158. Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. (1999) 19:8487–97. doi: 10.1523/JNEUROSCI.19-19-08487.1999
159. Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. (2002) 22:6639–49. doi: 10.1523/JNEUROSCI.22-15-06639.2002
160. Yin X, Li L, Zhang X, Yang Y, Chai Y, Han X, et al. Development of neural stem cells at different sites of fetus brain of different gestational age. J Clin Exp Pathol. (2013) 6:2757–64.
161. Ruggieri M, Riboldi G, Brajkovic S, Bucchia M, Bresolin N, Comi GP, et al. Induced neural stem cells: methods of reprogramming and potential therapeutic applications. Prog Neurobiol. (2014) 114:15–24. doi: 10.1016/j.pneurobio.2013.11.001
162. Martino GV, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev. (2011) 91:1281–304. doi: 10.1152/physrev.00032.2010
163. Liu Y, Yi XC, Guo G, Long QF, Wang XA, Zhong J, et al. Basic fibroblast growth factor increases the transplantation-mediated therapeutic effect of bone mesenchymal stem cells following traumatic brain injury. Mol Med Rep. (2014) 9:333–9. doi: 10.3892/mmr.2013.1803
164. Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. (2005) 436:266–71. doi: 10.1038/nature03889
165. Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, et al. (2008). Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain 131(Pt 10), 2564–78. doi: 10.1093/brain/awn198
166. Ottoboni L, Merlini A, Martino G. Neural stem cell plasticity: advantages in therapy for the injured central nervous system. Front Cell Dev Biol. (2017) 12:52. doi: 10.3389/fcell.2017.00052
167. Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA. (2010) 107:12704. doi: 10.1073/pnas.0910106107
168. Hayashi K, Hashimoto M, Koda M, Naito AT, Murata A, Okawa A, et al. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell-derived astrocytes in a rat spinal cord injury model. J Neurosurg Spine. (2011) 15:582–93. doi: 10.3171/2011.7.SPINE10775
169. Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells (2012) 30:1163–73. doi: 10.1002/stem.1083
170. Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE (2012) 7:e52787. doi: 10.1371/journal.pone.0052787
171. Tang H, Sha H, Sun H, Wu X, Xie L, Wang P, et al. Tracking induced pluripotent stem cells-derived neural stem cells in the central nervous system of rats and monkeys. Cell Reprogram. (2013) 15:435–42. doi: 10.1089/cell.2012.0081
172. Nutt SE, Chang E-A, Suhr ST, Schlosser LO, Mondello SE, Moritz CT, et al. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol. (2013) 248:491–503. doi: 10.1016/j.expneurol.2013.07.010
173. Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, et al. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell. (2016) 19:544–57. doi: 10.1016/j.stem.2016.08.020
174. Dunkerson J, Moritz KE, Young J, Pionk T, Fink K, Rossignol J, et al. Combining enriched environment and induced pluripotent stem cell therapy results in improved cognitive and motor function following traumatic brain injury. Nestor Neurol Neurosci. (2014) 32:675–87.
175. Gao X, Wang X, Xiong W, Chen J. in vivo reprogramming reactive glia into iPSCs to produce new neurons in the cortex following traumatic brain injury. Sci Rep. (2016) 6:22490. doi: 10.1038/srep22490
176. Wei ZZ, Lee JH, Zhang Y, Zhu YB, Deveau TC, Gu X, et al. Intracranial transplantation of hypoxia-preconditioned iPSC-derived neural progenitor cells alleviates neuropsychiatric defects after traumatic brain injury in juvenile rats. Cell Transplant. (2016) 25:797–809. doi: 10.3727/096368916X690403
177. Lyu Q, Zhang ZB, Fu SJ, Xiong LL, Liu J, Wang TH. Microarray expression profile of IncRNAs and mRNAs in rats with traumatic brain injury after A2B5+ cell transplantation. Cell Transplant. (2017) 26:1622–35. doi: 10.1177/0963689717723014
178. Chen SJ, Chang CM, Tsai SK, Chang YL, Chou SJ, Huang SS, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. (2010) 19:1757–67. doi: 10.1089/scd.2009.0452
179. Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. iPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells. (2014) 32:3075–87. doi: 10.1002/stem.1802
180. Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. (2010) 30:1487–93. doi: 10.1038/jcbfm.2010.32
181. Chang DJ, Lee N, Park IH, Choi C, Jeon I, Kwon J, et al. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant. (2013) 22:1427–40. doi: 10.3727/096368912X657314
182. Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Transl Med. (2015) 4:841–51. doi: 10.5966/sctm.2014-0184
183. Jensen MB, Yan H, Krishnaney-Davison R, Al Sawaf A, Zhang SC. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J Stroke Cerebrovasc Dis. (2013) 22:304–8. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.008
184. Jiang M, Lv L, Ji H, Yang X, Zhu W, Cai L, et al. (2011). Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 354: 67–75. doi: 10.1007/s11010-011-0806-5
185. Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. (2014) 24:7053–62. doi: 10.1002/adfm.201401483
186. Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, et al. (2013). Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 136(Pt 12):3561–3577. doi: 10.1093/brain/awt278
187. Tornero D, Tsupykov O, Granmo M, Rodriguez C, Groening-Hansen M, Thelin J, et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. (2017) 140:692–706. doi: 10.1093/brain/aww347
188. Chan TM, Harn HJ, Lin HP, Chiu SC, Lin PC, Wang HI, et al. The use of ADSCs as a treatment for chronic stroke. Cell Transplant. (2014) 23:541–7. doi: 10.3727/096368914X678409
189. Yamanaka Y, Ralston A, Stephenson RO, Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. (2006) 235:2301–14. doi: 10.1002/dvdy.20844
190. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. (2006) 126:663–76. doi: 10.1016/j.cell.2006.07.024
191. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72. doi: 10.1016/j.cell.2007.11.019
192. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. (2007) 318:1917–20. doi: 10.1126/science.1151526
193. Chang YJ, Su HL, Hsu LF, Huang PJ, Wang TH, Cheng FC, et al. Isolation of human neural stem cells from the amniotic fluid with diagnosed neural tube defects. Stem Cells Dev. (2015) 24:1740–50. doi: 10.1089/scd.2014.0516
194. Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, Lin T, et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell (2015) 17:353–9. doi: 10.1016/j.stem.2015.07.021
195. Deng J, Zhang Y, Xie Y, Zhang L, Tang P. Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cells Int. (2018) 2018:5653787. doi: 10.1155/2018/5653787
196. Ahuja CS, Fehlings M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. (2016) 5:914–24. doi: 10.5966/sctm.2015-0381
197. Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. (2011) 29:443–8. doi: 10.1038/nbt.1862
198. Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science (2009) 324:797–801. doi: 10.1126/science.1172482
199. Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. (2009) 4:472–6. doi: 10.1016/j.stem.2009.05.005
200. Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. (2010) 7:618–30. doi: 10.1016/j.stem.2010.08.012
201. Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. (2011) 29:1117–9. doi: 10.1038/nbt.2052
202. Chen J, Lin M, Foxe JJ, Pedrosa E, Hrabovsky A, Carroll R, et al. Transcriptome comparison of human neurons generated using induced pluripotent stem cells derived from dental pulp and skin fibroblasts. PLoS ONE. (2013) 8:e75682. doi: 10.1371/journal.pone.0075682
203. Aasen T, Izpisúa Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. (2010) 5:371–82. doi: 10.1038/nprot.2009.241
204. Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. (2009) 122:3502–10. doi: 10.1242/jcs.054783
205. Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodríguez-Pizà I, Vassena R, et al. (2009). Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 5, 353–357. doi: 10.1016/j.stem.2009.09.008
206. Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, et al. Generation of induced pluripotent stem cells from human blood. Blood. (2009) 113:5476–9. doi: 10.1182/blood-2009-02-204800
207. Ramos-Mejía V, Montes R, Bueno C, Ayllón V, Real PJ, Rodríguez R, et al. Residual expression of the reprogramming factors prevents differentiation of iPSC generated from human fibroblasts and cord blood CD34+ progenitors. PLoS ONE (2012) 7:e35824. doi: 10.1371/journal.pone.0035824
208. Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA. (2009) 106:15720–5. doi: 10.1073/pnas.0908450106
209. Chambers SM, Tchieu J, Studer L. Build-a-Brain. Cell Stem Cell (2013) 13:377–8. doi: 10.1016/j.stem.2013.09.010
210. Muratore CR, Srikanth P, Callahan DG, Young-Pearse TL. Comparison and optimization of hiPSC forebrain cortical differentiation protocols. PLoS ONE (2014) 9:e105807. doi: 10.1371/journal.pone.0105807
211. Salewski RP, Buttigieg J, Mitchell RA, van der Kooy D, Nagy A, Fehlings MG. The generation of definitive neural stem cells from PiggyBac transposon-induced pluripotent stem cells can be enhanced by induction of the NOTCH signaling pathway. Stem Cells Dev. (2013) 22:383–96. doi: 10.1089/scd.2012.0218
212. Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. (2013). Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 78, 785–798. doi: 10.1016/j.neuron.2013.05.029
213. Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. (2012) 7:1836–46. doi: 10.1038/nprot.2012.116
214. Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. (2013) 12:573–86. doi: 10.1016/j.stem.2013.04.005
215. Jha BS, Rao M, Malik N. Motor neuron differentiation from pluripotent stem cells and other intermediate proliferative precursors that can be discriminated by lineage specific reporters. Stem Cell Rev. (2015) 11:194–204. doi: 10.1007/s12015-014-9541-0
216. Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. (2009) 27:806–11. doi: 10.1002/stem.31
217. Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, et al. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy. (2012) 14:584–97. doi: 10.3109/14653249.2012.658913
218. Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of Multilineage-differentiating Stress-enduring (Muse) cells. Nat Protoc. (2013) 8:1391–415. doi: 10.1038/nprot.2013.076
219. Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, et al. Multilineage-differentiating Stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA. (2011) 108:9875–80. doi: 10.1073/pnas.1100816108
220. Yamauchi T, Kuroda Y, Morita T, Shichinohe H, Houkin K, Dezawa M, et al. Therapeutic effects of human Multilineage-differentiating stress enduring (MUSE) cell transplantation into infarct brain of mice. PLoS ONE (2015) 10:e0116009. doi: 10.1371/journal.pone.0116009
221. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells (2004) 22:649–58. doi: 10.1634/stemcells.22-5-649
222. Yuan Y, Pan S, Sun Z, Dan Q, Liu J. Brain-derived neurotrophic factor-modified umbilical cord mesenchymal stem cell transplantation improves neurological deficits in rats with traumatic brain injury. Int J Neurosci. (2014) 124:524–31. doi: 10.3109/00207454.2013.859144
223. Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. (2005) 102:18171–6. doi: 10.1073/pnas.0508945102
224. Ghosh M, Tuesta LM, Puentes R, Patel S, Melendez K, El Maarouf A, et al. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia. (2012) 60:979–92. doi: 10.1002/glia.22330
225. Kanno H, Pressman Y, Moody A, Berg R, Muir EM, Rogers JH, et al. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosc. (2014) 34:1838–55. doi: 10.1523/JNEUROSCI.2661-13.2014
226. Anderson KD, Guest JD, Dietrich WD, Bunge MB, Curiel R, Dididze M, et al. Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. J. Neurotrauma. (2017) 34:2950–63. doi: 10.1089/neu.2016.4895
227. Lee YS, Wu S, Arinzeh TL, Bunge MB. Transplantation of Schwann Cells inside PVDF-TrFE conduits to bridge transected rat spinal cord stumps to promote axon regeneration across the gap. J Vis Exp. (2017) e56077. doi: 10.3791/56077
228. Lee YS, Funk LH, Lee JK, Bunge MB. Macrophage depletion and Schwann cell transplantation reduce cyst size after rat contusive spinal cord injury. Neural Regen Res. (2018) 13:684–91. doi: 10.4103/1673-5374.230295
229. Namjoo Z, Morandi F, Aryanpour R, Piryaei A, Joghataei MT, Abbasi Y, et al. Combined effects of rat Schwann cells and 17(Beta)-estradiol in a spinal cord injury model. Metal Brain Dis (2018). 33:1229–42. doi: 10.1007/s11011-018-0220-8
230. Dai Y, Hill CE. Transplantation of adult rat Schwann cells into the injured spinal cord. Methods Mol Biol. (2018) 1739:409–38. doi: 10.1007/978-1-4939-7649-2_28
231. Chen L, Fan X, Jin G, Wan X, Qiu R, Yi G, et al. Treatment of rat with traumatic brain injury and MR tracing in vivo via combined transplantation of bone marrow stroll cells labeled with super paramagnetic iron oxide and Schwann cells. J Biomed Nanotechnol. (2014) 10:205–15. doi: 10.1166/jbn.2014.1765
232. Xu HS, Ma C, Cao L, Wang JJ, Fan XX. Study of co-transplantation of SPIO labeled bone marrow stromal stem cells and Schwann cells for treating traumatic brain injury in rats and in vivo tracing of magnetically labeled cells by MRI. Eur Rev Med Pharmacol Sci. (2014) 18:520–5.
233. Brooks AE, Athauda G, Bunge MB, Khan A. Culture and expansion of rodent and porcine Schwann Cells for preclinical animal studies. Methods Mol Biol. (2018) 1739:111–26. doi: 10.1007/978-1-4939-7649-2_7
234. Santamaria AJ, Solano JP, Benavides FD, Guest JD. Intraspinal delivery of Schwann Cells for spinal cord injury. Methods Mol Biol. (2018) 1739:467–84. doi: 10.1007/978-1-4939-7649-2_31
235. de la Garza-Castro O, Martinez-Rodriguez HG, Sanchez-Gonzalez SG, Vidal-Torres O, Arreola-Romero A, de la Garza-Pineda O, et al. Schwann cell precursor transplant in a rat spinal cord injury model. Rev Invest Clin. (2018) 70:88–95. doi: 10.24875/RIC.18002466
236. Ramon-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exper Neurol. (1994) 127:232–244. doi: 10.1006/exnr.1994.1099
237. Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science (1997) 277:2000–2. doi: 10.1126/science.277.5334.2000
238. Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. (1994) 18:3803–15. doi: 10.1523/JNEUROSCI.18-10-03803.1998
239. Lopez-Vales R, Fores J, Verdu E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis. (2006) 21:57–68. doi: 10.1016/j.nbd.2005.06.011
240. Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain (2008) 131(Pt 9):2376–86. doi: 10.1093/brain/awn173
241. Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. (2001) 889: 344–57. doi: 10.1016/S0006-8993(00)03235-2
242. Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron (2000) 25:425–35. doi: 10.1016/S0896-6273(00)80905-8
243. Tabakow P, Jarmundowicz W, Czapiga B, Fortuna W, Miedzybrodzki R, Czyz M, et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. (2013) 22:1591–612. doi: 10.3727/096368912X663532
244. Zadroga A, Jezierska-Wozniak K, Czarzasta J, Monika B, Wojtkiewicz J, Wojciech M. Therapeutic potential of olfactory ensheathing cells and mesenchymal stem cells in spinal cord injuries. Stem Cells Int. (2017) 2017:3978595. doi: 10.1155/2017/9438310
245. Wang YC, Xia QJ, Ba YC, Wang TY, Li N, Zou Y, et al. Transplantation of olfactory ensheathing cells promotes the recovery of neurological functions in rats with traumatic brain injury associated with down regulation of Bad. Cytotherapy (2014) 16:1000–10. doi: 10.1016/j.jcyt.2013.12.009
246. Liu SJ, Zou Y, Belegu V, Lv LY, Lin N, Wang TY, et al. Co-grafting of neural stem cells with olfactory ensheathing cells promotes neuronal restoration in traumatic brain injury with an anti-inflammatory mechanism. J Neuroinflamm. (2014) 11:66. doi: 10.1186/1742-2094-11-66
247. Fu XM, Liu SJ, Dan QQ, Wang YP, Lin N, Lv LY, et al. Combined bone mesenchymal stem cell and olfactory ensheathing transplantation promotes neural repair associated with CNTF expression in traumatic brain-injured rats. Cell Transplant. (2015) 24:1533–44. doi: 10.3727/096368914X679345
248. Shyu WC, Liu DD, Lin SZ, Li WW, Su CY, Chang YC, et al. Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke. J Clin Invest. (2008) 118:2482–95. doi: 10.1172/JCI34363
249. Shi X, Kang Y, Hu Q, Chen C, Yang L, Wang K, et al. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. (2010) 1317:257–67. doi: 10.1016/j.brainres.2009.12.061
250. Augestad IL, Nyman AKG, Costa AI, Barnett SC, Sandvig A, Haberg AK, et al. Effects of neural stem cell and olfactory ensheathing cell co-transplants on tissue remodeling after transient focal cerebral ischemia in the adult rat. Neurochem Res. (2017) 42:1599–609. doi: 10.1007/s11064-016-2098-3
251. Ramon-Cueto A, Nieto-Sampedro M. Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience (1992) 47:213–220. doi: 10.1016/0306-4522(92)90134-N
252. Roet KC, Franssen EH, de Bree FM, Essing AH, Zijstra SJ, Fagoe ND, et al. A multilevel screening strategy defines a molecular fingerprint of pro regenerative olfactory ensheathing cells and identifies SCARB2, a protein that improves regenerative sprouting of injured sensory spinal axons. J Neurosci. (2013) 33:11116–35. doi: 10.1523/JNEUROSCI.1002-13.2013
253. Su Z, He C. Olfactory ensheathing cells: biology in neural development and regeneration. Prog Neurobiol. (2010) 92:517–32. doi: 10.1016/j.pneurobio.2010.08.008
254. Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. (2001) 19:1134–40. doi: 10.1038/nbt1201-1134
255. Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. (2008) 132:661–80. doi: 10.1016/j.cell.2008.02.008
256. Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. (2014) 2014:468748. doi: 10.1155/2014/468748
257. Chiba S, Iwasaki Y, Sekino H, Suzuki N. Transplantation of motorneuron-enriched neural cells derived from mouse embryonic stem cells improves motor function of hemiplegic mice. Cell Transplant. (2003) 12:457–68. doi: 10.3727/000000003108747019
258. Kerr DA, Llado J, Shamblott MJ, Maragakis NJ, Irani DN, Crawford TO, et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci. (2003) 23:5131–40. doi: 10.1523/JNEUROSCI.23-12-05131.2003
259. Gincberg G, Arien-Zakay H, Lazarovici P, Lelkes PI. Neural stem cells: therapeutic potential for neurodegenerative diseases. British Medical Bulletin. (2012) 104:7–19. doi: 10.1093/bmb/lds024
260. Giordano J. Neuroethical issues in neurogenetics and neurotransplantation technology – the need for pragmatism and preparedness in practice and policy. Studies Ethics Law Technol. (2011) 4. doi: 10.2202/1941-6008.1152
261. Azari H, Osborne GW, Yasuda T, Golmohammadi MG, Rahman M, Deleyrolle LP, et al. Purification of immature neuronal cells from neural stem cell progeny. PLoS ONE (2011) 6:e20941. doi: 10.1371/journal.pone.0020941
262. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. (2006) 7:395–406. doi: 10.1038/nrn1908
263. Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, et al. Grafted human-induced pluripotent stem cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. (2011) 108:16825–30. doi: 10.1073/pnas.1108077108
264. Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. (2014) 83:789–96. doi: 10.1016/j.neuron.2014.07.014
265. Pourabdolhossein F, Gil-Perotin S, Garcia-Belda P, Dauphin A, Mozafari S, Tepavcevic V, et al. Inflammatory demyelination induces ependymal modifications concomitant to activation of adult (SVZ) stem cell proliferation. Glia. (2017) 65:756–72. doi: 10.1002/glia.23124
266. Madhavan L, Collier TJ. A synergistic approach for neural repair: cell transplantation and induction of endogenous precursor cell activity. Neuropharmacology. (2010) 58:835–44. doi: 10.1016/j.neuropharm.2009.10.005
267. Carletti B, Piemonte F, Rossi F. Neuroprotection: the emerging concept of restorative neural stem cell biology for the treatment of neurodegenerative diseases. Curr Neuropharmacol. (2011) 9:313–7. doi: 10.2174/157015911795596603
268. Yoneyama M, Shiba T, Hasebe S, Ogita K. Adult neurogenesis is regulated by endogenous factors produced during neurodegeneration. J Pharmacol Sci. (2011) 115:425–32. doi: 10.1254/jphs.11R02CP
269. Arien-Zakay H, Lecht S, Nagler A, Lazarovici P. Human umbilical cord blood stem cells: rational use as a neuroprotectant in ischemic brain disease. Int J Mol Sci. (2010) 11:3513–28. doi: 10.3390/ijms11093513
270. Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, Salani G, et al. (2012). Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 135 (Pt 2), 447–460. doi: 10.1093/brain/awr339
271. Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials (2013) 34:3775–83. doi: 10.1016/j.biomaterials.2013.02.002
272. Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. (2009) 30:204–13. doi: 10.1210/er.2008-0031
273. Bahmad H, Hadadeh O, Chamaa F, Cheaito K, Darwish B, Makkawi AK, et al. Modeling human neurological and neurodegenerative diseases: from induced pluripotent stem cells to neuronal differentiation and its applications in neurotrauma. Front Mol Neurosci. (2017) 10:50. doi: 10.3389/fnmol.2017.00050
274. Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. (2011) 28:1611–82. doi: 10.1089/neu.2009.1177
275. A Phase 1, Open-label Single-site, Safety Study of Human Spinal Cord-derived Neural Stem Cell Transplantation for the Treatment of Chronic SCI. Available online at: https://clinicaltrials.gov/ct2/show/NCT01772810?term=NCT01772810andrank=1 (Identification No. NCT01772810)
276. The Efficacy and Safety of NeuroRegen Scaffold (TM) Combined with Mesenchymal Stem Cells or Neural Stem Cells for Chronic Spinal Cord Injury Repair. Available online at: https://clinicaltrials.gov/ct2/show/NCT02688049?term=NCT02688049&rank=1 (Identification No. NCT02688049)
277. Safety, and Efficacy of Autologous Neural Stem Cell Transplantation in Patients With Traumatic Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT02326662?term=NCT02326662andrank=1 (Identification No. NCT02326662)
278. Transplantation, of Purified Autologous Bone Marrow- or Leukapheresis-Derived CD34+ and CD133+ for Patients With Spinal Cord Injuries: A Long-Term Comparative Evaluation of Safety and Efficacy Study. Available online at: https://clinicaltrials.gov/ct2/show/NCT02687672?term=NCT02687672andrank=1 (identification No. NCT02687672)
279. The Effect of Intrathecal Transplantation of Umbilical Cord Mesenchymal Stem Cells in Patients With Late Stage of Chronic Spinal Cord Injury: A Multicenter, Prospective, Cohort Study. Available online at: https://clinicaltrials.gov/ct2/show/NCT03505034?term=NCT03505034andrank=1. (Identification No. NCT03505034)
280. A Phase II/III Clinical Trial to Evaluate the Safety and Efficacy of Bone Marrow-derived Mesenchymal Stem Cell Transplantation in Patients With Chronic Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT01676441?term=NCT01676441andrank=1 (Identification No. NCT01676441)
281. Umbilical Cord Mesenchymal Stem Cells Transplantation for the Treatment of Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT02481440?term=NCT02481440andrank=1 (Identification No. NCT02481440)
282. The Effect of Intrathecal Transplantation of Umbilical Cord Mesenchymal Stem Cells in Patients With Sub-Acute Spinal Cord Injury: A Multicenter, Randomized, Controlled Trial. Available online at: https://clinicaltrials.gov/ct2/show/NCT03521336?term=NCT03521336andrank=1 (Identification No. NCT03521336)
283. The Effect of Intrathecal Transplantation of Umbilical Cord Mesenchymal Stem Cells in Patients With Early Stage of Chronic Spinal Cord Injury: A Multicenter, Randomized, Controlled Trial. Available online at: https://clinicaltrials.gov/ct2/show/NCT03521323?term=NCT03521323andrank=1 (Identification No. NCT03521323)
284. Randomized Clinical Trial for the Evaluation of Autologous Mesenchymal Stem Cells Transplantation in Thoracolumbar Chronic and Complete Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT02574585?term=NCT02574585andrank=1 (identification No. NCT02574585)
285. Comparative Evaluation of Safety and Effectiveness of Autologous Bone Marrow Derived Mesenchymal Stem Cells (BM-MSC) vs Adipose Tissue Derived Mesenchymal Stem Cells (AT-MSC) in the Treatment of Spinal Cord Injury (SCI) Patient. Available online at: https://clinicaltrials.gov/ct2/show/NCT02981576?term=NCT02981576&rank=1. (Identification No. NCT02981576)
286. Phase I Clinical Trial of Autologous Adipose Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT03308565?term=NCT03308565andrank=1 (Identification No. NCT03308565)
287. Evaluation of the Safety and Potential Effectiveness of Autologous Mesenchymal Stem Cells Transplantation in Subjects With Cervical Chronic and Complete Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT02574572?term=NCT02574572andrank=1 (Identification No. NCT02574572)
288. Open Label Study of Autologous Bone Marrow Mononuclear Cells in Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT02009124?term=NCT02009124andrank=1 (Identification No. NCT02009124)
289. Safety and Efficacy of NeuroRegen Scaffold™ With Bone Marrow Mononuclear Cells or Mesenchymal Stem Cells for Chronic Spinal Cord Injury Repair. Available online at: https://clinicaltrials.gov/ct2/show/NCT02352077?term=NCT02352077andrank=1 (Identification No. NCT02352077)
290. A, Phase I/IIa, Randomized Double-blind Single-dose Placebo, Controlled, Two-Way, Crossover Clinical Trial to Assess the Safety and to Obtain Efficacy Data in Intrathecal Administration of Expanded Wharton's Jelly Mesenchymal Stem Cells in Chronic Traumatic Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT03003364?term=NCT03003364andrank=1 (Identification No. NCT03003364)
291. Stem Cell Spinal Cord Injury Exoskeleton and Virtual Reality Treatment Study. Available online at: https://clinicaltrials.gov/ct2/show/NCT03225625?term=NCT03225625andrank=1 (Identification No. NCT03225625)
292. Clinical, Trial of Phase 1/2 to Evaluate the Feasibility, Safety Tolerability, and Preliminary Efficacy of the Administration of FAB117-HC, a, Drug Whose Active Ingredient is HC016, Allogeneic, Adipose Derived Adult Mesenchymal Stem Cells Expanded and Pulsed With H2O2, in, Acute Traumatic SCI Patients. Available online at: https://clinicaltrials.gov/ct2/show/NCT02917291?term=NCT02917291andrank=1 (Identification No. NCT02917291)
293. A, Phase 1/2a Dose Escalation Study of AST-OPC1 in Subjects With Subacute Cervical Spinal Cord Injury. Available online at: https://clinicaltrials.gov/ct2/show/NCT02302157?term=NCT02302157andrank=1 (Identification No. NCT02302157)
294. Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. (2012) 70:1238–47. doi: 10.1227/NEU.0b013e31824387f9
295. Jeon SR, Park JH, Lee JH, Kim DY, Kim HS, Sung IY, et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng Regen Med. (2010) 7:316–22.
296. Saito F, Nakatani T, Iwase M, Maeda Y, Hirakawa A, Murao Y, et al. Spinal cord injury treatment with intrathecal autologous bone marrow stromal cell transplantation: the first clinical trial case report. J Trauma. (2008) 64:53–9. doi: 10.1097/TA.0b013e31815b847d
297. Pal R, Venkataramana NK, Balaraju S, Jan M, Chandra R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. (2009) 11:897–911. doi: 10.3109/14653240903253857
298. Zhang ZX, Guan LX, Zhang K, Zhang Q, Dai LJ. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. (2008) 10:134–9. doi: 10.1080/14653240701883061
299. Cox CS, Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery (2011) 68:588–600. doi: 10.1227/NEU.0b013e318207734c
300. Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet (2016) 388:787–96. doi: 10.1016/S0140-6736(16)30513-X
301. Ma K, Fox L, Shi G, Shen J, Liu Q, Pappas JD, et al. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol Res. (2011) 33:1083–93. doi: 10.1179/1743132811Y.0000000053
302. Lee OK, Kuo TK, Chen W-M, Lee K-D, Hsieh S-L, Chen T-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. (2004) 103:1669–75. doi: 10.1182/blood-2003-05-1670
303. Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature (2012) 481:295–305. doi: 10.1038/nature10761
304. Haase A, Ulmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. (2009) 5:434–41. doi: 10.1016/j.stem.2009.08.021
305. Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. (2008) 26:1276–84. doi: 10.1038/nbt.1503
306. Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. (2010) 7:15–9. doi: 10.1016/j.stem.2010.06.004
307. Takenaka C, Nishishita N, Takada N, Jakt LM, Kawabata S. Effective generation of iPS cells from CD34+ cord blood cells by inhibition of p53. Exp Hematol. (2010) 38:154–62. doi: 10.1016/j.exphem.2009.11.003
308. Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. (2011) 471:68–73. doi: 10.1038/nature09798
309. Boland MJ, Nazor KL, Loring JF. Epigenetic regulation of pluripotency and differentiation. Circ Res. (2014) 115:311–24. doi: 10.1161/CIRCRESAHA.115.301517
310. Omole AE, Fakoya AOJ. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ. (2018) 6:e4370. doi: 10.7717/peerj.4370
311. Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. (2008) 3:1180–6. doi: 10.1038/nprot.2008.92
312. Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature (2009) 460:49–52. doi: 10.1038/nature08180
313. Zabierowski SE, Baubet V, Himes B, Li L, Fukunaga-Kalabis M, Patel S, et al. Direct reprogramming of melanocytes to neural crest stem-like cells by one defined factor. Stem Cells. (2011) 29:1752–62. doi: 10.1002/stem.740
314. New SEP, Alvarez-Gonzalez C, Vagaska B, Gomez SG, Bulstrode NW, Madrigal A, et al. A matter of identity – Phenotype and differentiation potential of human somatic stem cells. Stem Cell Res. (2015) 15:1–13. doi: 10.1016/j.scr.2015.04.003
315. Qu X, Liu T, Song K, Li X, Ge D. Induced pluripotent stem cells generated from human adipose-derived stem cells using a non-viral polycistronic plasmid in feeder-free conditions. PLoS ONE (2012) 7:e48161. doi: 10.1371/journal.pone.0048161
316. Nakagawa M, Koyangi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. (2008) 26:101–6. doi: 10.1038/nbt1374
317. Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. (2008) 3:475–9. doi: 10.1016/j.stem.2008.10.002
318. Meng X, Neises A, Su R-J, Payne KJ, Ritter L, Gridley DS, et al. Efficient reprogramming of human cord blood CD34+ cells into induced pluripotent stem cells with OCT4 and SOX2 alone. Mol Ther. (2012) 20:408–16. doi: 10.1038/mt.2011.258
319. Buganim Y, Faddah DA, Cheng AW, Itskovich S, Markoulaki S, Ganz K, et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell (2012) 150:1209–22. doi: 10.1016/j.cell.2012.08.023
320. Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. (2013) 502:462–71. doi: 10.1038/nature12749
321. Takahashi K, Yamanaka S. A developmental framework for induced pluripotency. Development (2015) 142:3274–85. doi: 10.1242/dev.114249
322. Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. (2008) 3:340–345. doi: 10.1016/j.stem.2008.08.003
323. Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature (2009) 458:771–5. doi: 10.1038/nature07864
324. Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, et al. piggBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature (2009) 458:766–70. doi: 10.1038/nature07863
325. Broccoli V. Chapter 2: Reprogramming of somatic cells: iPS and iN cells. Prog Brain Res. (2017) 230:53–68. doi: 10.1016/bs.pbr.2016.12.009
326. Lopez-Serrano C, Torres-Espin A, Hernandez J, Alvarez-Palomo AB, Requena J, Gasull X, et al. Effects of the post-spinal cord injury microenvironment on the differentiation capacity of human neural stem cells derived from induced pluripotent stem cells. Cell Transplant. (2016) 25:1833–52. doi: 10.3727/096368916X691312
327. Gervois P, Wolfs E, Ratajczak J, Dillen Y, Vangansewinkel T, Hilkens P, et al. Stem cell-based therapies for ischemic stroke: preclinical results and the potential of imaging-assisted evaluation of donor cell fate and mechanisms of brain regeneration. Med Res Rev. (2016) 36:1080–126. doi: 10.1002/med.21400
328. Brouwer M, Zhou H, Kasri NN. Choices for induction of pluripotency: recent developments in human induced pluripotent stem cell reprogramming strategies. Stem Cell Rev. (2016) 12:54–72. doi: 10.1007/s12015-015-9622-8
329. Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science (1999) 283:534–7. doi: 10.1126/science.283.5401.534
330. Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, et al. Generalized potential of adult neural stem cells. Science (2000) 288:1660–3. doi: 10.1126/science.288.5471.1660
331. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science (2000) 290:1775–9. doi: 10.1126/science.290.5497.1775
332. Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell (2001) 105:829–41. doi: 10.1016/S0092-8674(01)00409-3
333. Shahbazi E, Moradi S, Nemati S, Satarian L, Basiri M, Gourabi H, et al. Conversion of human fibroblasts to stably self-renewing neural stem cells with a single zinc-finger transcription factor. Stem Cell Reports (2016) 6:539–51. doi: 10.1016/j.stemcr.2016.02.013
334. Dametti S, Faravelli I, Ruggieri M, Ramirez A, Nizzardo M, Corti S. Experimental advances towards neural regeneration from induced stem cells to direct in vivo reprogramming. Mol Neurobiol. (2016) 53:2124–31. doi: 10.1007/s12035-015-9181-7
335. Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. (2012) 109:2527–32. doi: 10.1073/pnas.1121003109
336. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature (2010) 463:1035–41. doi: 10.1038/nature08797
337. Wapinski OL, Lee QY, Chen AC, Li R, Corces MR, Ang CE, et al. Rapid chromatin switch in the direct reprogramming of fibroblasts to neurons. Cell Rep. (2017) 20:3236–47. doi: 10.1016/j.celrep.2017.09.011
338. Chernoff EAG, Sato K, Salfity HVN, Sarria DA, Belecky-Adams T. Musashi and plasticity of Xenopus and Axolotl Spinal Cord Ependymal Cells. Front Cell Neurosci. (2018) 12:45. doi: 10.3389/fncel.2018.00045
339. Gilbert EAB, Vickaryous MK. Neural stem/progenitor cells are activated during tail regeneration in the leopard gecko (Eublepharis macularius). J Comp Neurol. (2017) 526:285–309. doi: 10.1002/cne.24335
340. Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell (2012) 10:465–72. doi: 10.1016/j.stem.2012.02.021
341. Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell (2012) 11:100–9. doi: 10.1016/j.stem.2012.05.018
342. Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, et al. Hierarchical mechanisms for transcription factor-mediated reprogramming of fibroblasts to neurons. Cell (2013) 155, 621–635. doi: 10.1016/j.cell.2013.09.028
343. Zou Q, Yan Q, Zhong J, Wang K, Sun H, Yi X, et al. Direct conversion of human fibroblasts into neuronal restricted progenitors. J Biol Chem. (2014) 289:5250–60. doi: 10.1074/jbc.M113.516112
344. Ma T, Xie M, Laurent T, Ding S. Progress in the Reprogramming of Somatic Cells. Circ Res. (2013) 112:562–74. doi: 10.1161/CIRCRESAHA.111.249235
345. Kelaini S, Cochrane A, Margariti A. Direct reprogramming of adult cells: avoiding the pluripotent state. Stem Cells Cloning. (2014) 7:19–29. doi: 10.2147/SCCAA.S38006
346. Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature (2011) 471:63–7. doi: 10.1038/nature09805
347. Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, et al. Copy number variation and selection during reprogramming to pluripotency. Nature (2011) 471:58–62. doi: 10.1038/nature09871
348. An N, Xu H, Gao WQ, Yang H. Direct conversion of somatic cells into induced neurons. Mol Neurobiol. (2018) 55:642–51. doi: 10.1007/s12035-016-0350-0
349. Chambers SM, Mica Y, Lee G, Studer L, Tomishima MJ. Dual-SMAD inhibition/WNT activation-based methods to induce neural crest and derivatives from human pluripotent stem cells. In: Turksen K, editor. Human Embryonic Stem Cell Protocols. New York, NY: Springer (2017). p. 329–43.
350. Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Inducted pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science (2008) 321:1218–1221. doi: 10.1126/science.1158799
351. Brix J, Zhou Y, Luo Y. The epigenetic reprogramming roadmap in generation of iPSCs from somatic cells. J Genet Genomics (2015) 42:661–70. doi: 10.1016/j.jgg.2015.10.001
352. Costa M, Dottori M, Sourris K, Jamshidi P, Hatzistavrou T, Davis R, et al. A method for genetic modification of human embryonic stem cells using electroporation. Nat Protoc. (2007) 2:792–6. doi: 10.1038/nprot.2007.105
353. Davis RP, Costa M, Grandela C, Holland AM, Hatzistavrou T, Micallef SJ, et al. A protocol for removal of antibiotic resistance cassettes from human embryonic stem cells genetically modified by homologous recombination or transgenesis. Nat Protoc. (2008) 3:1550–1558. doi: 10.1038/nprot.2008.146
354. Ruby KM, Zheng B. Gene targeting in a HUES line of human embryonic stem cells via electroporation. Stem Cells (2009) 27:1496–506. doi: 10.1002/stem.73
355. Bressan RB, Dewari PS, Kalantzaki M, Gangoso E, Matjusaitis M, et al. Efficient CRISPR/Cas9-assisted gene targeting enables rapid and precise genetic manipulation of mammalian neural stem cells. Development. (2017) 144:635–48. doi: 10.1242/dev.140855
356. Ji J, Ng SH, Sharma V, Neculai D, Hussein S, Sam M, et al. Elevated coding mutation rate during the reprogramming of human somatic cells into induced pluripotent stem cells. Stem Cells (2012) 30:435–40. doi: 10.1002/stem.1011
357. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature (2007) 448:313–7. doi: 10.1038/nature05934
358. Wu C, Hong SG, Winkler T, Spencer DM, Jares A, Ichwan B, et al. Development of an inducible caspase-9 safety switch for pluripotent stem cell-based therapies. Mol Ther Methods Clin Dev. (2014) 1:14053. doi: 10.1038/mtm.2014.53
359. Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. (2012) 12:623–35. doi: 10.1038/nri3265
360. Le Thuc O, Blondeau N, Nahon JL, Rovere C. The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Ann NY Acad Sci. (2015) 1351:127–40. doi: 10.1111/nyas.12855
361. Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. (2013) 4:18. doi: 10.3389/fneur.2013.00018
362. Skaper SD, Facci L, Zusso M, Giusti P. An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci. (2018) 12:72. doi: 10.3389/fncel.2018.00072
363. Posfai B, Cserep C, Orsolitis B, Denes A. New insights into microglia-neuron interactions: a neuron's perspective. Neuroscience (2018) S0306-4522:30320–30328. doi: 10.1016/j.neuroscience.2018.04.046
364. Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S. Suppression of brain mast cells degranulation inhibits microglial activation and central nervous system inflammation. Mol Neurobiol. (2017) 54:997–1007. doi: 10.1007/s12035-016-9720-x
365. Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. (2012) 8:401–10. doi: 10.1038/nrneurol.2012.98
366. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. (2007) 8:57–69. doi: 10.1038/nrn2038
367. Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, et al. ADP and AMP induce interleukin-1β release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. (2002) 22:3061–9. doi: 10.1523/JNEUROSCI.22-08-03061.2002
368. Miro-Mur F, Urra X, Gallizioli M, Chamorro A, Planas AM. Antigen presentation after stroke. Neurotherapeutics. (2016) 13:719–28. doi: 10.1007/s13311-016-0469-8
369. Pais TF, Figueiredo C, Peixoto R, Braz MH, Chatterjee S. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflamm. (2008) 5:43. doi: 10.1186/1742-2094-5-43
370. Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation (2006) 113:2105–12. doi: 10.1161/CIRCULATIONAHA.105.593046
371. Amor S, Woodroofe MN. Innate and adaptive immune responses in neurodegeneration and repair. Immunology (2014) 141:287–91. doi: 10.1111/imm.12134
372. Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. (2017) 174:1349–65. doi: 10.1111/bph.13580
373. Paladini A, Fusco M, Cenacchi T, Schievano C, Prioli A, Varrassi G. Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: a pooled data meta-analysis. Pain Physician. (2016) 19:11–24.
374. Sontag CJ, Nguyen HX, Kamei N, Uchida N, Anderson AJ, Cummings BJ. Immunosuppressants affect human neural stem cells in vitro but no in an in vivo model of spinal cord injury. Stem Cells Transl Med. (2013) 2:731–44. doi: 10.5966/sctm.2012-0175
375. Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature (2011) 474:212–5. doi: 10.1038/nature10135
376. Scheiner ZS, Talib S, Feigal EG. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J Biol Chem. (2014) 289:4571–7. doi: 10.1074/jbc.R113.509588
377. Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature (2013) 494:100–4. doi: 10.1038/nature11807
378. Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell (2013) 12:407–12. doi: 10.1016/j.stem.2013.01.006
379. Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, et al. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural stem cells in the brain of a non-human primate. Stem Cell Reports (2013) 1:283–92. doi: 10.1016/j.stemcr.2013.08.007
381. Swaab DF. Developments in neuroscience. In: Giordano J, Gordijn B, editors. Scientific and Philosophical Perspectives in Neuroethics Cambridge: Cambridge University Press (2010). p. 1–36.
382. Boer GJ. Transplantation and xenotransplantation. In: Giordano J, Gordijn B, editors. Scientific and Philosophical Perspectives in Neuroethics, Cambridge: Cambridge University Press (2010). p. 190–216.
383. Meloni R, Mallet J, Faucon-Biguet N. Brain gene transfer and brain implants. Stud Ethics Law Technol. (2011) 4. doi: 10.2202/1941-6008.1141
384. Giordano J. Neurotechnology. In: Ten Have H, editor. Global Encyclopedia of Bioethics. New York, NY: Springer (2016). doi: 10.1007/978-3-319-09483-0
385. Giordano J. Neurotechnological progress: the need for neuroethics. In: Gonzalez F, editor. The Next Step: Exponential Life. Barcelona: BBVA Press (2016). p. 298–312.
386. Giordano J. A preparatory neuroethical approach to assessing developments in neurotechnology. AMA J Ethics (2015) 17:56–61. doi: 10.1001/virtualmentor.2015.17.1.msoc1-1501
387. Giordano J. Toward an operational neuroethical risk analysis and mitigation paradigm for emerging neuroscience and technology (neuroS/T). Exp Neurol. (2017) 287:492–5. doi: 10.1016/j.expneurol.2016.07.016
388. Giordano J. Conditions for consent to the use of neurotechnology: a preparatory neuroethical approach to risk assessment and reduction. AJOB Neurosci. (2015) 6:12–4. doi: 10.1080/21507740.2015.1094557
389. Giordano J, Shook JR. Minding brain science in medicine: on the need for neuroethical engagement for guidance of neuroscience in clinical contexts. Ethics Biol Engineer Med. (2015) 6:37–42. doi: 10.1615/EthicsBiologyEngMed.2015015333
Keywords: nervous system trauma, neural stem cells, autologous transplantation, regeneration, cell and tissue based therapy, spinal cord injury, traumatic brain injury
Citation: Wu S, FitzGerald KT and Giordano J (2018) On the Viability and Potential Value of Stem Cells for Repair and Treatment of Central Neurotrauma: Overview and Speculations. Front. Neurol. 9:602. doi: 10.3389/fneur.2018.00602
Received: 25 January 2018; Accepted: 06 July 2018;
Published: 13 August 2018.
Edited by:
Vassilis E. Koliatsos, Johns Hopkins University, United StatesReviewed by:
Martin L. Doughty, Uniformed Services University, United StatesHassan Azari, Shiraz University of Medical Sciences, Iran
Dong Sun, Virginia Commonwealth University, United States
Copyright © 2018 Wu, FitzGerald and Giordano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Giordano, SmFtZXMuZ2lvcmRhbm9AZ2VvcmdldG93bi5lZHU=
 Samantha Wu
Samantha Wu Kevin T. FitzGerald
Kevin T. FitzGerald James Giordano
James Giordano