- Key Laboratory of Cognition and Personality (MOE), Faculty of Psychology, Southwest University, Chongqing, China
Sleep deprivation (SD) has been reported to severely affect executive function, and interindividual differences in these effects may contribute to the SD-associated cognition impairment. However, it is unclear how individual differences in chronotypes (morning-type, MT; evening-type, ET) influence neurobehavioral functions after SD. To address this question, we used functional magnetic resonance imaging (fMRI) to evaluate whether 24 h of SD differentially affect response inhibition, a core component of executive function, in MT and ET individuals. Accordingly, MT and ET participants were instructed to follow their preferred 7–9-h sleep schedule for 2 weeks at home both prior to and throughout the course of the study, and then performed a go/no-go task during fMRI scanning at 08:00 a.m. both at rested wakefulness (RW) and following SD. We also examined whether the neurobehavioral inhibition differences in the chronotypes in each session can be predicted by subjective ratings (sleepiness, mood, and task) or objective attention. Behaviorally, SD led to an increased response time of go trials (hit RT), more attentional lapses, higher subjective sleepiness, and worse mood indices, but it did not impair the accuracy of go trials (hit rate) and no-go trials (stop rate). Regardless of the presence of SD, ET individuals exhibited a lower stop rate, higher subjective ratings of sleepiness, exhausted mood, and task difficulty in comparison with MT individuals. On the neural level, SD resulted in decreased inhibition-related activation of the right lateral inferior frontal gyrus (rIFG) in MT individuals and increased rIFG activation in ET individuals. Moreover, the rIFG activation in ET individuals after SD was positively correlated to the subjective ratings of sleepiness and effort put into the task, which was considered as a compensatory response to the adverse effects of SD. These findings suggest that individual differences in inhibition-related cerebral activation after SD are influenced by chronotypes. In addition, ET individuals may be vulnerable to response inhibition. Thus, it is essential to take into consideration the chronotype in SD research and sleep medicine.
Introduction
Sleep deprivation (SD) is commonplace in modern society, and there is increasing neuroimaging evidence suggesting that the prefrontal cortex may be particularly susceptible to the impacts of sleep loss due to its extensive use during normal waking (1). Accordingly, SD should particularly impair complex executive functions that rely on the prefrontal regions (2). However, studies on this assumption have yielded inconsistent results, with some groups reporting impairments in executive function tasks during SD (3–5) and others failing to find such effects (6, 7). These inconsistencies may be attributable to interindividual differences. For instance, earlier studies reported that individuals who are better able to maintain inhibitory efficiency exhibit a larger activation in the prefrontal cortex as a compensatory response to SD relative to those whose inhibitory efficiency declines (8).
The concept of chronotype relies on the subjective preference for activities in the morning or evening (morning- [MT] or evening- [ET] type). MT individuals are most alert in the early morning and prefer to go to sleep and wake up early. By contrast, ET individuals are most alert toward later in the evening and prefer to go to sleep and wake up late (9, 10). Individuals with different chronotypes differ in their homeostatic sleep regulation; the build-up (11) and dissipation (12) rate of sleep pressure are faster in MT individuals than in ET individuals. Even under conditions of sleep fragmentation (5-min awakenings every 30 min), MT individuals exhibit increased homeostatic response (13). In a normal waking day, ET individuals are more capable of maintaining alertness (14) and executive function (15) by recruiting arousal-promoting brain structures with increasing homeostatic sleep pressure. However, few studies have directly examined the interindividual chronotype differences (MT vs. ET) in the neurobehavioral responses to an elevation in sleep pressure triggered by total SD (16). Therefore, the present study employed functional magnetic resonance imaging (fMRI) to evaluate whether 24-h total SD differentially affects the neurobehavioral differences in response inhibition, a core component of executive function, between MT and ET individuals. To investigate this question, MT and ET participants underwent scanning while performing a go/no-go task in both rested wakefulness (RW) and SD conditions. Furthermore, the study aimed to examine whether the subjective ratings (sleepiness, mood, and task) and objective attention (psychomotor vigilance) of chronotypes in each session reflect the neurobehavioral differences in response inhibition.
On the basis of previous findings, we expected that SD impairs inhibition-related neurobehavioral responses, such as poor inhibition performance (8) and impaired frontoparietal network activities (17), which are especially located in the right lateral inferior frontal gyrus (rIFG) and critical for successful response inhibition (18, 19). Furthermore, we hypothesized that SD would differentially impact the neurobehavioral changes of chronotypes and that the subjective ratings and objective attention of participants in each session would predict to some extent the neurobehavioral responses to inhibition.
Methods
Subjects
Participants were recruited from students who completed the self-reported Morningness–Eveningness Questionnaire (20) at Southwest University. The inclusion criteria were as follows: (1) age, 18–30 years; (2) normal or corrected-to-normal vision; (3) right-handedness; and (4) a regular sleep-wake schedule that includes 7–9 h of total sleep time. Exclusion criteria were as follows: (1) self-reported history of psychiatric, neurologic, or sleep disorders; (2) drug or alcohol abuse, excessive caffeine (>5 cups of coffee per day) or nicotine (>5 cigarettes per day) intake; (3) travel across more than two time zones within 3 months before the study; and (4) presence of contraindications for fMRI.
The chronotype was determined by the Chinese version of the Morningness–Eveningness Questionnaire (21, 22) which has good psychometric properties. After the answers had been checked and scored by the experimenters (score >62, MT participant; score < 50, ET participant), 26 MT individuals (mean score = 64.2 ± 3.4) and 22 ET individuals (mean score = 40.0 ± 4.2) were recruited for this study. Three participants (MT, 2; ET, 1) were excluded from data analysis because of excessive head movement and the presence of behavioral outliers. This study received approval from the Institutional Review Board at the Southwest University, Chongqing and followed the principles of the Declaration of Helsinki. All participants gave written informed consent before the experiment and were compensated for their participation.
Experimental Procedure
The participants visited the laboratory three times. At their first visit, the participants underwent the screening process, were informed of the study requirements, and practiced the task. The participants filled out the Pittsburgh Sleep Quality Index [PSQI; (23)], Epworth Sleepiness Scale (24), the positive and negative affective schedule (25), the self-rating depression scale (26), the self-rating anxiety scale (27), the NEO Five-Factor Inventory (28), the Barrett Impulsiveness Scale-11 [BIS; (29)], and the Dysexecutive Questionnaire [DEX; (30)]. Only participants with regular sleep habits (self-reported sleep for 7–9 h per night) were invited to take part in the following experiments. The participants were then instructed to follow their preferred 7–9-h sleep-wake schedule at home for at least 2 weeks both prior to and throughout the course of the study. Compliance was verified by using sleep diaries. In addition, alcohol, nicotine, and caffeine intake, napping, and intense physical activity were forbidden for at least 24 h before scanning.
Participants were scanned twice with a week between the scans. The order of the two scanning sessions was randomized and counterbalanced (31, 32). The two sessions were conducted 1 week apart to minimize the possible residual effects of SD. In the RW session, participants underwent scanning at 08:00 a.m. after a night of normal sleep at home. Before the experiment, the participants were instructed to sleep about 7–9 h the night, get up at least 1 h prior to the beginning of scanning, and arrived at the laboratory at 07:30 a.m. to prepare for the following scanning. In the SD session, participants were monitored by the two experimenters in the laboratory from 10:00 p.m. until scanning began at 08:00 a.m. For both sessions, lighting conditions were carefully controlled at a steady low level, and exposure to sunlight was avoided. During the SD session, at every hour from 10:00 p.m., the participants performed the 10-min version of the Psychomotor Vigilance Task [PVT; (33, 34)], responded to the Karolinska Sleepiness Scale [KSS; (35)] as well as a mood-related Likert-type rating scale (range, 0–10) which was defined by the items motivated–unmotivated, fresh–exhausted, elated–depressed, congenial–irritable, relaxed–stressed, and calm–anxious (8). For the rest of the time, participants were kept awake with non-strenuous activities like reading, watching movies, and conversing with the experimenters. In addition, regular snacks were available. Prior to the scanning, subjects carried out the PVT, KSS, the mood rating, and had a task practice. Then, participants performed the task immediately in the fMRI scanner. After task completion but still in the scanner, participants were asked to complete the KSS, the mood rating, and the 10-point Likert scales (36) to assess the following task-related factors: task difficulty, ability to concentrate, effort put into the task, and motivation to perform the task well. The number of lapses (RTs >500 ms) and mean RT in PVT were treated as indexes of psychomotor vigilance (33).
Task
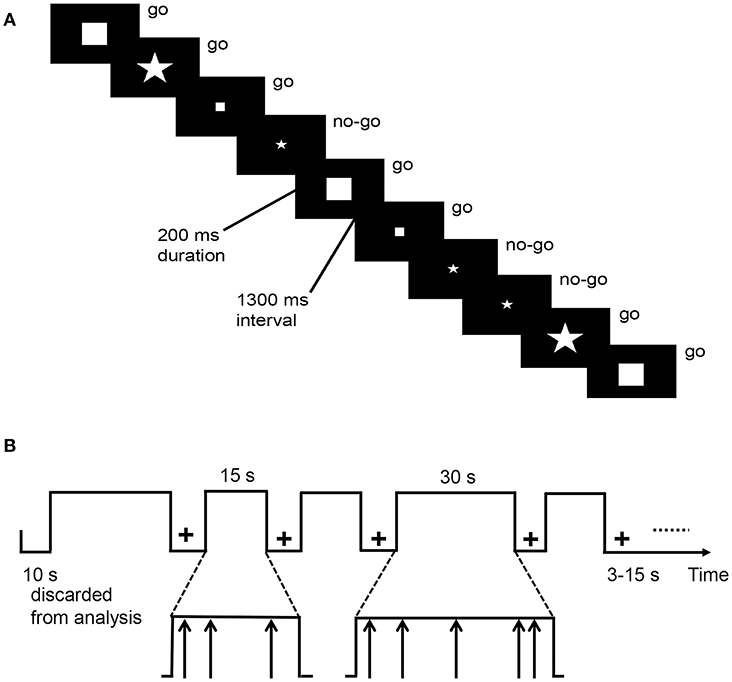
The go/no-go task requires from the participant continual responses to stimuli while bearing in mind to refrain from responding to a specific but less frequently presented stimulus. This task [Figure 1; (36, 37)] alternated between task blocks and resting blocks. During resting blocks, a fixation cross appeared in the center of the screen. During task blocks, stimuli were exhibited by an event-related design and four shapes (go stimuli: large square, small square, large pentagon; the no-go stimulus: small pentagon) were presented one at a time in the center of the screen. Once the subjects observed a go stimulus, they had to press a button using the right finger as soon as possible. However, they were required to refrain from responding when they observed the no-go stimulus. Task blocks were in total 270 s long, in which each of five task blocks lasted 30 s and another eight blocks lasted 15 s. Resting blocks were in total 114 s and varied between 3 and 15 s (mean = 8.8 s). Stimuli appeared for 200 ms every 1,500 ms. There were 180 stimuli in total, of which 75% were go stimuli. The task lasted 6 min 24 s. The response time of go trials (hit RT), as well as the accuracy of go (hit rate) and no-go (stop rate) trials, were assessed.
fMRI Data Acquisition
Images were collected on a 3-Tesla MR scanner (Siemens Magnetom Trio TIM; Erlangen, Germany). A magnetization-prepared gradient echo sequence was employed to acquire T1-weighted anatomical images: TR = 1,900 ms, TE = 2.52 ms, flip angle = 9°, FOV = 250 × 250 mm2, in-plane resolution = 0.98 × 0.98 mm, slices = 176, thickness = 1 mm. A single-shot gradient echo-planar imaging (EPI) sequence was employed to acquire task-based functional T2*-weighted images: TR = 2,000 ms, TE = 30 ms, bandwidth = 2,232 Hz/pixel, flip angle = 90°, FOV = 220 × 220 mm2, matrix size = 64 × 64, slices = 32, thickness = 3 mm, inter-slice gap = 1 mm.
fMRI Data Analysis
We used SPM12 and DPABI2.1 to analyze the functional data (38, 39). For each participant, the first five images were discarded due to non-steady magnetization, the rest of the functional images were corrected for slice timing and spatially realigned using six parameters of head motion. The structural images were co-registered to the EPI mean image and segmented into white matter, gray matter, and cerebrospinal fluid. The functional data were normalized to a Montreal Neurological Institute (MNI) space with a voxel size of 3 × 3 × 3 mm3 and spatially smoothed using a Gaussian kernel with 8 mm full width at half maximum.
In the first-level analysis, a statistical analytical design was estimated using the general linear model (GLM). Five regressors were created (rest; go success, GS; go error, GE; no-go success, NGS; and no-go error, NGE) after convolution with the canonical hemodynamic response function [HRF; (40)]. Six realignment parameters were included in the model to attribute to the residual variance, and a high-pass filter of 128 s was used to remove possible effects of low-frequency changes. Sex and age differences were controlled as covariates. Subsequently, we probed the inhibition-related cerebral activations using the contrast of NGS and GS at the group level [two-tailed Gaussian random field correction, voxel level: p < 0.001, cluster level: p < 0.05; (41)]. The group analysis targeted the interaction effects between chronotype and session on response inhibition. In addition, using functional MRI results, the regions-of-interest (ROIs) were defined by a sphere of 6 mm radius around the centers of the peak coordinates of inhibition-related areas. For the ROI analysis using MarsBar (42), individual β values were extracted. To assess the interaction effect on response inhibition, repeated-measures analysis of variance (ANOVA) based on the ROIs were carried out using SPSS Statistics 20.0, followed by Tukey's post hoc tests.
Results
Participants
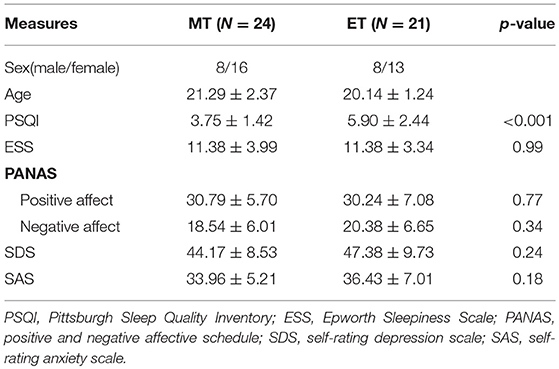
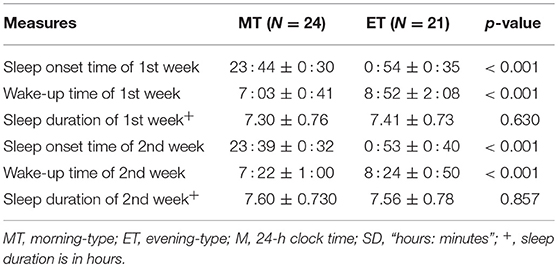
The features of the participants are specified in Table 1. Global and several component (sleep quality, latency, and disturbance; Table S1) scores on the PSQI were significantly higher for ET individuals, with higher scores indicating more severe complaints. In addition, ET individuals showed marginally significantly higher values for the non-planning factor [t(43) = −1.81, p = 0.08] on the BIS and the inhibition factor [t(43) = −1.78, p = 0.08] on the DEX (Table S1). According to the sleep diaries (Table 2), chronotypes showed significant differences in sleeping and waking time but not in sleep duration.
Behavioral Findings-Subjective and Objective Measures
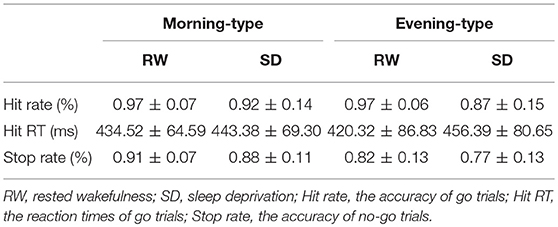
For the go/no-go task, repeated-measures ANOVAs for the accuracy and RT data were carried out (Table 3). A main effect of session was noted on the hit RT [F(1, 43) = 4.34, p < 0.05] after controlling for the covariates of sex and age, i.e., the hit RT was significantly higher following SD than at RW. However, the hit rate [F(1, 43) = 2.43, p = 0.13] and stop rate [F(1, 43) = 0.27, p = 0.61] showed no significant main effects of session. A main effect of chronotype was found on the stop rate [F(1, 43) = 8.65, p < 0.01] after controlling for the covariates of sex and age. In other words, ET individuals showed a significantly lower stop rate than MT individuals. However, the hit rate [F(1, 43) = 0.18, p = 0.68] and hit RT [F(1, 43) = 0.01, p = 0.93] showed no significant main effects of chronotype. The interaction effect of chronotype and session on hit rate [F(1, 43) = 0.34, p = 0.57], hit RT [F(1, 43) = 0.52, p = 0.48], and stop rate [F(1, 43) = 0.25, p = 0.62] were not significant.
With respect to the PVT, we focused on the number of lapses [transformed lapses: ; (33)] as the primary outcome to assess vigilance. Repeated-measures ANOVAs (Table S2) showed a significant main effect of session [F(1, 43) = 67.66, p < 0.001] and a marginally significant main effect of chronotype [F(1, 43) = 3.88, p = 0.06] on the transformed lapses. As expected, the number of lapses after SD were more than those after RW. In addition, more lapses (trend level) occurred in ET individuals than in MT individuals. Next, to explore the differences in lapses during SD between MT and ET individuals, an independent-samples t-test (MT vs. ET) was performed on transformed lapses (values between 11:00 p.m. and 07:00 a.m.) during the SD session (Figure 2). Five subjects were eliminated from the analysis (1 from the MT and 4 from the ET) since they failed to complete the PVT hourly on the SD night because of a technical error. The findings revealed more lapses among ET individuals at 02:00 a.m. [t(1, 38) = −2.10, p < 0.05], 03:00 a.m. [t(1, 38) = −2.17, p < 0.05], and 06:00 a.m. [t(1, 38) = −1.78, p = 0.08, marginally significant] compared to MT individuals (Figure 2). Thus, ET individuals may be at a disadvantage while completing the PVT in the SD condition.
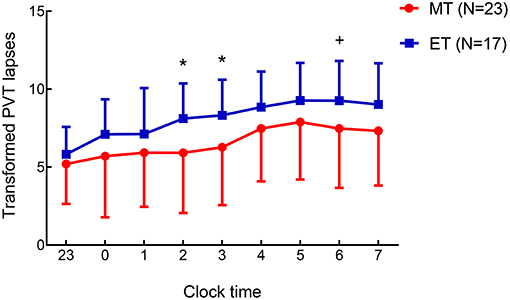
Figure 2. Mean ± standard deviation change in the transformed Psychomotor Vigilance Task (PVT) lapses () determined hourly during the period from 11:00 p.m. to 07:00 a.m. on the 24-h sleep deprivation (SD) night in morning-type (MT) and evening-type (ET) participants. The differences (MT vs. ET) on transformed PVT lapses were investigated using t-tests for independent samples. Five participants were eliminated from the analysis (1 from the MT, 4 from the ET) owing to failure to complete the PVT hourly at the SD night. Condition effect *p < 0.05; +: the effect was marginally significant.
A repeated-measures ANOVA was also performed on the subjective measures (ratings of sleepiness, mood, and task). The ratings for sleepiness and each item on mood just before each scanning session differed significantly between the two sessions (Table S2). In comparison with RW, subjects presented increased sleepiness and decreased mood parameters after SD. We also observed a main effect of session on ratings of sleepiness, mood, and task [effect on task difficulty was marginally significant, F(1, 43) = 3.38, p = 0.07] during the scanning (Table S3). Thus, SD significantly changed almost all subjective ratings of sleepiness, mood, and task. Importantly, the main effect of chronotype on the ratings of sleepiness [F(1, 43) = 10.38, p < 0.01], fresh–exhausted mood [F(1, 43) = 4.91, p < 0.05], and task difficulty [F(1, 43) = 9.52, p < 0.01] were statistically significant, with ET individuals showing significantly higher values than MT individuals.
fMRI Results
A repeated-measures ANOVA for chronotype (MT vs. ET) and session (RW vs. SD) revealed a significant interaction effect between the two factors (two-tailed Gaussian random field correction, voxel level: p < 0.001, cluster level: p < 0.05) in the right lateral inferior frontal gyrus region [rIFG: Brodmann area 46, peak coordinate (45, 54, 12); peak intensity: 18.26; number of voxels: 47; Figure 3A], which was closely associated with the execution of response inhibition.
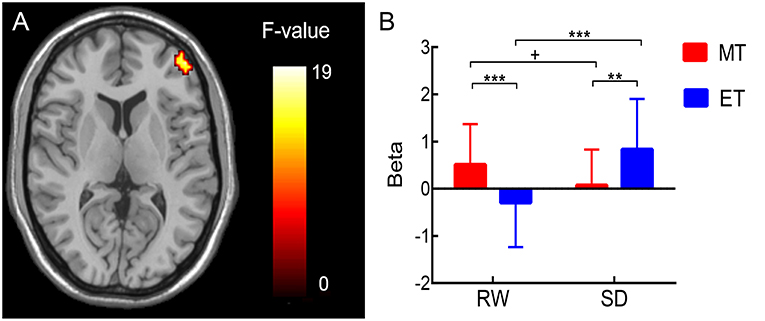
Figure 3. (A) Brain regions showing an interaction effect between chronotype and session during response inhibition (NGS vs. GS) in the right lateral inferior frontal gyrus [rIFG; Brodmann area 46, peak coordinate (45, 54, 12); peak intensity: 18.26; number of voxels: 47; two-tailed Gaussian random field correction, voxel level: p < 0.001, cluster level: p < 0.05]. (B) For ROI analysis, the results showed that inhibition-related (NGS vs. GS) response in rIFG decreased from the rested wakefulness (RW) session to the sleep deprivation (SD) session in morning-type (MT) participants, whereas rIFG activity significantly increased from the RW session to the SD session in evening-type (ET) participants. Condition effect **p < 0.01, ***p < 0.005; +: the effect was marginally significant.
For the ROI analysis, there was a significant chronotype × session interaction effect on the rIFG region [F(1, 43) = 17.86, p < 0.001; Figure 3B]. Then, a simple effect analysis were performed. The results (Figure 3B) were as follows: in MT participants, the cerebral responses induced by successful inhibition (NGS > GS) in the rIFG decreased [t(1, 23) = 2.01, p = 0.06] in SD compared to RW sessions; in ET participants, the rIFG activity significantly increased [t(1, 20) = −3.66, p < 0.005] in SD compared to RW sessions. In addition, rIFG activity in MT participants was significantly higher compared with ET participants at RW [t(1, 43) = 3.02, p < 0.005], whereas rIFG activity in ET participants was significantly higher compared with MT participants after SD [t(1, 43) = −2.78, p < 0.01].
Brain-Behavior Correlation Results
To examine whether the subjective ratings (sleepiness, mood, and task-related factors) and objective task performance (PVT and go/no-go task) are predictive of the inhibition-related rIFG activation in each session, we performed a correlation analysis between behavioral indices (subjective ratings, objective task performance) and rIFG activation at RW and following SD in the two chronotypes. In MT individuals, rIFG activity was positively correlated with subjective ratings for task difficulty (r = 0.45, p < 0.05) and negatively correlated with hit RT (r = −0.43, p < 0.05) during the RW session. However, rIFG activity and behavior indices showed no correlation during the SD session. Among ET individuals, rIFG activity was negatively correlated to the mean RT of the PVT during the RW session (r = −0.45, p < 0.05) and positively correlated to subjective ratings of sleepiness just before the scanning (r = 0.44, p = 0.06) and the effort put into the task (r = 0.55, p < 0.05; the correlation analysis was computed with 19 data pairs due to two outliers of neural activity in the rIFG) during the SD session (Figure 4). Obviously, a relationship between rIFG activity and attention (hit RT of the go/no-go task or mean RT of the PVT) was only detectable during RW but not after SD. This suggests that SD altered the association between inhibition-related activation and attention.
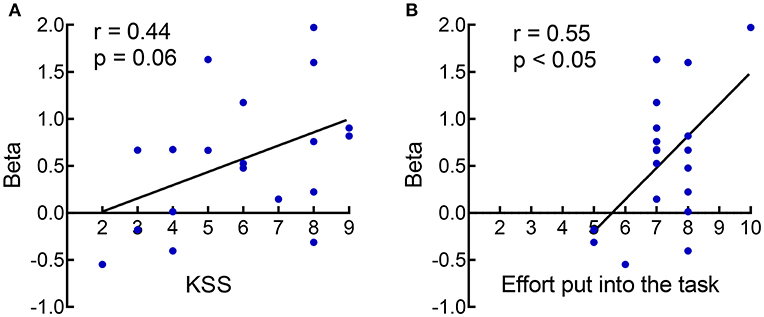
Figure 4. The correlation analysis showed that the rIFG activity in ET participants was positively related to subjective ratings of sleepiness [(A); Karolinska Sleepiness Scale, KSS] and the effort put into the task (B) after SD.
Discussion
We investigated the interindividual differences in the neurobehavioral functions associated with response inhibition between MT and ET individuals after SD. Behaviorally, SD led to an increase in hit RT, more attentional lapses, increased sleepiness, and worse mood indices. However, SD did neither impair the hit rate nor the stop rate. Regardless of the presence of SD, ET individuals demonstrated a lower stop rate, as well as higher subjective ratings of sleepiness, exhausted mood, and task difficulty compared to MT individuals. On the neural level, SD resulted in decreased inhibition-related rIFG activation in MT individuals and in increased rIFG activation in ET individuals. Moreover, the rIFG activation in ET individuals after SD was positively correlated to the subjective ratings of sleepiness and effort put into the task. These findings suggest that ET individuals demonstrate an increased rIFG activation after SD, which is consistent with previous studies (8, 37) and can be interpreted as a compensatory response to SD. Together, the present findings thus provide a new chronotype-related perspective to explore the differential SD-induced effects on cognition.
Consistent with the results of previous studies (37, 43), the subjects in this study experienced a significant increase in hit RT after SD. Studies have shown that SD leads to a general slowing of response times of attention (44, 45), and a meta-analysis reported that after SD, reaction time is more vulnerable than accuracy (46). As expected, the increase in hit RT was accompanied by increased lapses in vigilance, greater sleepiness, and worse mood, which is consistent with previous research (43, 47). SD did not impair the inhibition performance in the study, which could be attributable to the fact that scanning was not performed in the early morning hours which are regarded as a sensitive time-window for SD (48). Studies have suggested that SD should particularly impair executive functions (2, 3), such as response inhibition (5, 8, 37, 43). By contrast, other studies have shown that the inhibition performance does not differ between RW and SD sessions (49, 50). In the future, more studies are necessary to identify which components of executive functions are reliably impaired by sleep loss.
Interestingly, in comparison to MT individuals, ET individuals showed a significantly lower stop rate during RW and following SD. This finding suggested that ET individuals were probably particularly vulnerable to response inhibition. Furthermore, ET individuals scored higher in non-planning factors on the BIS and the inhibition factor on the DEX. Previous studies have shown that ET individuals are correlated with increased impulsivity, enhanced disinhibition, and impaired response inhibition (trend level) in comparison to MT individuals (51–53). However, another study could not confirm an effect of chronotype on the inhibition performance assessed by the stop-signal task in a synchronous effect paradigm (54). We hypothesized that differences in task paradigm, experimental design, and study population may have contributed to the dissimilarity in findings. Additionally, the heterogeneous factors defining impulsivity, which represents a broad concept, predict psychological outcomes (55), and inhibitory control is not a unitary construct (56). Considering the complex interactions between behavioral inhibitory control and the self-reported trait impulsivity (56), future studies should further examine the detailed relationships among chronotype, inhibitory control, and impulsivity (57).
In our study, ET individuals also scored higher than MT individuals in the subjective ratings for sleepiness, exhausted mood, and task difficulty, regardless of whether the participants underwent SD. Additionally, ET individuals exhibited in comparison to MT individuals a worse subjective sleep quality as assessed by the PSQI, which is consistent with previous findings (58–60). We hypothesized that the increases in sleepiness and exhausted mood in ET individuals, which may be attributable to poorer sleep quality, could have contributed to the higher ratings for task difficulty and the impaired inhibition performance. Due to common social standards in everyday life, ET individuals have to work in the morning which conflicts with their preferred time of activities. This social jetlag, i.e., the asynchrony between social and biological rhythms, occurs chronically throughout an individual's learning and working life (61), which directly leads to less sleep in ET individuals on weekdays (62–64) and probably influences the sleep quality and pattern. Therefore, environmental factors such as early work or school starting times may result for ET individuals in sleep and circadian disturbances like social jetlag or disturbed sleep that act on neuropsychological mechanisms such as response inhibition or impulsivity (65). Moreover, researchers highlight the importance of utilizing longitudinal studies to specifically determine the direction of effects among chronotypes, social jetlag, and psychological outcomes in the future (66).
On the neural level, the whole-brain activation results indicated significant interaction effects in the rIFG region which is consistent with prior studies (36, 37). Previous findings suggest that the role of the rIFG is critical for inhibiting response trends (18) and is related to both response and attentional control (67, 68). The rIFG has also been characterized as a “brake,” and it can be initiated in both total (to outright suppress a response) and partial (to pause the response) conditions (18).
Crucially, the current study revealed significant interaction effects of chronotype and SD on the cerebral activation patterns of response inhibition, i.e., a decreased rIFG activation pattern in MT participants but an increased rIFG activation in ET participants for SD in comparison to RW. The prevailing hypothesis proposes that functioning of the frontal lobe is particularly affected by sleep loss (1). In the present study, a decreased prefrontal activity during SD was only apparent in MT participants. By contrast, ET participants demonstrated increased rIFG activation following SD, which was positively related to subjective ratings of sleepiness (trend level) and effort put into the task. Researchers have insisted that effort is closely linked to the concept of motivation, and the degree of effort is probably particularly elevated when individuals are motivated or perceive the trends of poor task performance (69). In addition, the PVT findings indicated more attentional lapses among ET individuals during the SD night, especially at 02:00 a.m., 03:00 a.m., and 06:00 a.m. (trend level), compared to the corresponding values for MT individuals. Thus, the effects of SD appear to differ across cognitive domains in the two chronotypes. ET individuals may show vulnerabilities on sustained attention, but could also show increased inhibition-related cerebral activation as a compensatory response after SD. However, the results were inconsistent with the previous study (16), in which participants were instructed to follow a fixed sleep-wake schedule (different from personal preferred sleep schedules). Then, both chronotypes performed a simple reaction time test hourly during 36 h of extended wakefulness under constant routine. Consequently, ET individuals maintained optimal alertness (fastest 10% reaction time) throughout the night, but MT individuals did not. We surmised that differences in experimental design, study population, and dependent variable may have contributed to the dissimilarity in findings. Importantly, the individual differences of chronotypes in sustained attention after SD should be explored in a larger sample size in the future. Finally, we hypothesized that the ET individuals in our study probably experienced a negative impact on attention after SD. In addition, these individuals demonstrated increased sleepiness, more exhausted mood, higher task difficulty, and poorer inhibition performance, thus needing to put more effort into the task which leads to increased rIFG activation acting as a compensatory response. In SD session, the time of testing started at 08:00 a.m. which differed from the preferential time of ET individuals. This adverse circadian time may have exaggerated the compensatory trend. However, this response in ET individuals was not sufficient to reverse the adverse effects on inhibition performance in SD. Alternatively, the short task duration caused differences in cerebral activation after SD without detectable behavioral changes (48). By contrast, MT individuals exhibited relatively decreased sleepiness, fresh mood, lower task difficulty, and better inhibition performance. We hypothesized that MT individuals may have experienced only a subtle negative SD impact and detected that the task was not difficult, hence it was unnecessary to increase the brain activation to maintain performance.
Recent studies have persistently indicated significant individual differences in homeostatic sleep responses and cognitive performances after SD (70, 71), and the PERIOD3 (PER3) polymorphism has been related to individual differences after total SD (72, 73). Compared to individuals with the shorter allele (PER34/4), those expressing the longer allele (PER35/5) showed a greater cognitive decline (72–74) and a more widespread reduction in task-related (working memory task, 3-back) cortical activations following SD (75). It has been suggested that PER35/5 may mediate the differential susceptibility via its impact on sleep homeostasis (72, 76). Furthermore, the PER3 polymorphism is correlated with the chronotype, PER35/5 is associated with MT and PER34/4 with ET (77–79), although the correlation of chronotypes and PER3 genotypes is not consistently verified (80, 81). Meanwhile, it is essential to note an assumption in which the interactions among genotype, phenotype, and social constraints should be taken into consideration (48). In the present study, ET individuals perhaps exhibited vulnerabilities on sustained attention after SD, which was probably influenced by social constraints such as social jetlag. Compared with the abundance of studies focusing on individual genotype-related differences after SD, studies addressing phenotype-related differences are relatively rare. To better reflect real-life situations and to acquire more information about social jetlag, it is essential to pay more attention in the future to individual chronotype-linked differences in SD-related neurobehavioral studies, which could be assessed using the Munich Chronotype Questionnaire (82).
The present results should be understood in the context of several limitations. First, the sample size in our study was relatively small. Therefore, the results need to be verified in a larger sample. Second, the task were scheduled according to external clock time, not according to the personalized preferential waketime of the participants. Consequently, the masking effects on circadian and homeostatic processes for the two chronotypes were not fully controlled. Moreover, the physiological circadian and homeostatic indicators of participants were not assessed in the study. Therefore, an exact correlation of the SD-induced inhibition impairment with the chronotype-associated circadian and homeostatic changes could not be established. Future studies should schedule the testing periods according to individual time plans and consider utilizing a combination of techniques from the fields of physiology, psychology, and cognitive neuroscience, especially when focusing on a chronotype-related difference in the SD paradigm. Third, the total SD is probably not the most appropriate paradigm for chronotypes, and future studies should verify the differences in chronotypes by means of a chronic sleep restriction paradigm. It will also be interesting to explore the chronotype-related differences in recovery from sleep loss. Many studies have targeted the differences between subjects in susceptibility to the neurobehavioral changes after SD within a cognitive domain, and future studies should pay more attention to within-subjects and between-domain differences in susceptibility (83).
In summary, these findings indicate that ET individuals were vulnerable to inhibition, and the poorer inhibition performance was accompanied by higher subjective ratings of sleepiness, exhausted mood, and task difficulty. In addition, ET individuals exhibited a worse attention performance on the SD night. Importantly, the individual differences in inhibition-related cerebral activation after SD were influenced by chronotypes, with decreased rIFG activation in MT individuals, but increased rIFG activation in ET individuals, which was considered as a compensatory mechanism to cope with the SD-induced adverse effects such as more attentional lapses, although changes in regional responses preceded the behavioral modifications in the present study. Accordingly, it is essential to take into consideration the chronotype in SD-related neurobehavioral research and sleep medicine in the future.
Ethics Statement
This study was approved by the Institutional Review Board at the Southwest University, Chongqing and conformed to the tenets of the Declaration of Helsinki. All subjects provided written informed consent prior to the experiment and were compensated for their time.
Author Contributions
JS, PF, XW, and YZ conceived and designed the study. JS, XW, BL, YS, and YL carried out the study. JS and PF performed the data analysis. JS wrote the main manuscript text whereas YZ, PF, and BL revised it critically prior to submission for publication.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities (SWU1709106), China.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.00514/full#supplementary-material
References
1. Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults—a model for healthy aging? Sleep. (2000) 23:1067–73. doi: 10.1016/S0304-3959(00)00354-7
2. Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psycho Appl. (2000) 6:236–49. doi: 10.1111/j.0030-1299.2005.13339.x
3. Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. (2001) 5:463–75. doi: 10.1053/smrv.2001.0203
4. Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. (2002) 6:475–81. doi: 10.1016/S1364-6613(02)01992-7
5. Sagaspe P, Taillard J, Amieva H, Beck A, Rascol O, Dartigues J, et al. Influence of age, circadian and homeostatic processes on inhibitory motor control: a go/nogo task study. PLoS ONE. (2012) 7:e39410. doi: 10.1371/journal.pone.0039410
6. Pace-Schott EF, Hutcherson CA, Bemporad B, Morgan A, Kumar A, Hobson JA, et al. Failure to find executive function deficits following one night's total sleep deprivation in university students under naturalistic conditions. Behav Sleep Med. (2009) 7:136–63. doi: 10.1080/15402000902976671
7. Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. (2010) 33:47–57. doi: 10.1093/sleep/33.1.47
8. Chuah YL, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. (2006) 26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006
9. Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. (2012) 29:1153–75. doi: 10.3109/07420528.2012.719971
10. Song J, Zheng Y. Circadian typology and mental health. Adv Psychol Sci. (2014) 22:1446–55. doi: 10.13031/trans.56.10273
11. Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res. (2003) 12:275–82. doi: 10.1046/j.0962-1105.2003.00369.x
12. Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res. (2006) 15:162–6. doi: 10.1111/j.1365-2869.2006.00532.x
13. Mongrain V, Dumont M. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. Sleep. (2007) 30:773–80. doi: 10.1016/j.seizure.2007.02.001
14. Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, et al. Homeostatic sleep pressure and responses to sustained attentionin the suprachiasmatic area. Science. (2009) 324:516–9. doi: 10.1126/science.1167337
15. Schmidt C, Peigneux P, Leclercq Y, Sterpenich V, Vandewalle G, Phillips C, et al. Circadian preference modulates the neural substrate of conflict processing across the day. PLoS ONE. (2012) 7:e29658. doi: 10.1371/journal.pone.0029658
16. Taillard J, Philip P, Claustrat B, Capelli A, Coste O, Chaumet G, et al. Time course of neurobehavioral alertness during extended wakefulness in morning- and evening-type healthy sleepers. Chronobiol Int. (2011) 28:520–7. doi: 10.3109/07420528.2011.590
17. Zhang S, Li CR. Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp. (2012) 33:89–104. doi: 10.1002/hbm.21197
18. Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. (2014) 18:177–85. doi: 10.1016/j.tics.2013.12.003
19. Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. (1999) 96:8301–6. doi: 10.1073/pnas.96.14.8301
20. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. (1976) 4:97–110.
21. Zhang B, Hao YL, Wing YK. The reliability and validity of Chinese version Morningness/Eveningness Questionnaire. Chinese J Behav Med Sci. (2006) 15:856–8. doi: 10.3760/cma.j.issn.1674-6554.2006.09.044
22. Li SX, Li QQ, Wang XF, Liu LJ, Liu Y, Zhang LX, et al. Preliminary test for the chinese version of the morningness-eveningness questionnaire. Sleep Biol Rhythm. (2011) 9:19–23. doi: 10.1111/j.1479-8425.2010.00480.x
23. Buysse DJ, Reynolds IIICF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiat Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
24. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
25. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. (1988) 54:1063e1070. doi: 10.1037/0022-3514.54.6.1063
26. Zung WW. A self-rating depression scale. Arch Gen Psychiat. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
27. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
28. McCrae RR, Costa PT. Validation of the five-factor model of personality across instruments and observers. J Pers Soc Psychol. (1987) 52:81–90. doi: 10.1037/0022-3514.52.1.81
29. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. (1995) 51:768–74. doi: 10.1002/1097-4679(199511)51:63.0.CO;2-1
30. Chan RC. Dysexecutive symptoms among a non-clinical sample: a study with the use of the Dysexecutive questionnaire. Br J Psychol. (2001) 92:551–65. doi: 10.1348/000712601162338
31. Van Dongen HP. Brain activation patterns and individual differences in working memory impairment during sleep deprivation. Sleep. (2005) 28:386–8. doi: 10.1016/j.seizure.2005.01.010
32. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. (2003) 26:117–26. doi: 10.1093/sleep/26.2.117
33. Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. (1997) 20:267–77. doi: 10.1093/sleep/20.4.267
34. Gui D, Xu S, Zhu S, Fang Z, Spaeth AM, Xin Y, et al. Resting spontaneous activity in the default mode network predicts performance decline during prolonged attention workload. NeuroImage. (2015) 120:323–30. doi: 10.1016/j.neuroimage.2015.07.030
35. Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. In J Neurosci. (1990) 52:29–37. doi: 10.3109/00207459008994241
36. Ayalon L, Ancoliisrael S, Drummond SP. Altered brain activation during response inhibition in obstructive sleep apnea. J Sleep Res. (2009) 18:204–8. doi: 10.1111/j.1365-2869.2008.00707.x
37. Almklov EL, Drummond SP, Orff H, Alhassoon OM. The effects of sleep deprivation on brain functioning in older adults. Behav Sleep Med. (2015) 13:324–45. doi: 10.1080/15402002.2014.905474
38. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. (1994) 2:189–210. doi: 10.1002/hbm.460020402
39. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing and analysis for (Resting-State). Brain Imag Neuroinform. (2016) 14:339–51. doi: 10.1007/s12021-016-9299-4
40. Lindquist MA. The statistical analysis of fMRI data. Stat Sci. (2008) 23:439–64. doi: 10.1214/09-STS282
41. Chen X, Lu B, Yan CG. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. (2018) 3:300–18. doi: 10.1002/hbm.23843
42. Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. (2002) 16:S497. doi: 10.1016/S1053-8119(02)90013-3
43. Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. (2006) 15:261–5. doi: 10.1111/j.1365-2869.2006.00535.x
44. Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. (2010) 136:375–89. doi: 10.1037/a0018883
45. Waters F, Bucks RS. Neuropsychological effects of sleep loss: implication for neuropsychologists. J Int Neuropsychol Soc. (2011) 17:571–86. doi: 10.1017/s1355617711000610
46. Koslowsky M, Babkoff H. Meta-analysis of the relationship between total sleep deprivation and performance. Chronobiol Int. (1992) 9:132–6. doi: 10.3109/07420529209064524
47. Daniela T, Alessandro C, Giuseppe C, Fabio M, Cristina M. Lack of sleep affects the evaluation of emotional stimuli. Brain Res Bull. (2010) 82:104–8. doi: 10.1016/j.brainresbull.2010.01.014
48. Archer SN, Schmidt C, Vandewalle G, Dijk DJ. Phenotyping of per3 variants reveals widespread effects on circadian preference, sleep regulation, and health. Sleep Med Rev. (2017) 40:109–26. doi: 10.1016/j.smrv.2017.10.008
49. Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. (2003) 14:473–86. doi: 10.1111/1467-9280.02456
50. Zhao R, Zhang X, Fei N, Zhu Y, Sun J, Liu P, et al. Decreased cortical and subcortical response to inhibition control after sleep deprivation. Brain Imaging Behav. (2018). doi: 10.1007/s11682-018-9868-2. [Epub ahead of print].
51. Russo PM, Leone L, Penolazzi B, Natale V. Circadian preference and the big five: The role of impulsivity and sensation seeking. Chronobiol Int. (2012) 29:1121–6. doi: 10.3109/07420528.2012.706768
52. Hwang JY, Kang SG, Gwak AR, Park J, Lee YJ. The associations of morningness-eveningness with anger and impulsivity in the general population. Chronobiol Int. (2016) 33:200–9. doi: 10.3109/07420528.2015.1128947
53. Kang JI, Park CI, Sohn SY, Kim HW, Namkoong K, Kim SJ. Circadian preference and trait impulsivity, sensation-seeking and response inhibition in healthy young adults. Chronobiol Int. (2015) 32:235–41. doi: 10.3109/07420528.2014.965313
54. Song J, Feng P, Zhao X, Xu W, Xiao L, Zhou J, et al. Chronotype regulates the neural basis of response inhibition during the daytime. Chronobiol Int. (2018) 35:208–18. doi: 10.1080/07420528.2017.1392550
55. Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assess. (2015) 27:1129–46. doi: 10.1037/pas0000111.supp
56. Roberts W, Fillmore MT, Milich R. Linking impulsivity and inhibitory control using manual and oculomotor response inhibition tasks. Acta Psychol. (2011) 138:419–28. doi: 10.1016/j.actpsy.2011.09.002
57. Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. (2013) 214:357–64. doi: 10.1016/j.pscychresns.2013.08.005
58. Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. (2002) 11:191–9. doi: 10.1046/j.1365-2869.2002.00302.x
59. Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: same genes? A study of young adult twins Chronobiol Int. (2010) 27:278–96. doi: 10.3109/07420521003663801
60. Önder I, Beşoluk S, Iskender M, Masal E, Demirhan E. Circadian preferences, sleep quality and sleep patterns, personality, academic motivation and academic achievement of university students. Learn Individ Differ. (2014) 32:184–92. doi: 10.1016/j.lindif.2014.02.003
61. Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes. (2015) 39:842–8. doi: 10.1038/ijo.2014.201
62. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. (2006) 23:497–509. doi: 10.1080/07420520500545979
63. Lang CJ, Reynolds AC, Appleton SL, Taylor AW, Gill TK, Mcevoy RD, et al. Sociodemographic and behavioural correlates of social jetlag in Australian adults: results from the 2016 National Sleep Health Foundation Study. Sleep Med. (2018) 51:133–9. doi: 10.1016/j.sleep.2018.06.014
64. Zerbini G, Kantermann T, Merrow M. Strategies to decrease social jetlag: Reducing evening blue light advances sleep and melatonin. Eur J Neurosci. (2018). doi: 10.1111/ejn.14293. [Epub ahead of print].
65. Taylor BJ, Hasler BP. Chronotype and mental health: recent advances. Curr Psychiatry Rep. (2018) 20:59–69. doi: 10.1007/s11920-018-0925-8
66. Tavernier R, Munroe M, Willoughby T. Perceived morningness–eveningness predicts academic adjustment and substance use across university, but social jetlag is not to blame. Chronobiol Int. (2015) 32:1233–45. doi: 10.3109/07420528.2015.1085062
67. Dodds CM, Morein-Zamir S, Robbins TW. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb Cortex. (2011) 21:1155–65. doi: 10.1093/cercor/bhq187
68. Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. (2010) 50:1313–9. doi: 10.1016/j.neuroimage.2009.12.109
69. Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. (2006) 51:145–60. doi: 10.1016/j.brainresrev.2005.11.002
70. Van Dongen PA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. (2004) 27:423–33. doi: 10.1093/sleep/27.3.423
71. Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS ONE. (2009) 4:e5874. doi: 10.1371/journal.pone.0005874
72. Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. (2007) 17:613–8. doi: 10.1016/j.cub.2007.01.073
73. Maire M, Reichert CF, Gabel V, Viola AU, Strobel W, Krebs J, et al. Sleep ability mediates individual differences in the vulnerability to sleep loss: evidence from a PER3 polymorphism. Cortex. (2014) 52:47–59. doi: 10.1016/j.cortex.2013.11.008
74. Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. (2008) 31:1159–67. doi: 10.5665/sleep/31.8.1159
75. Vandewalle G, Archer SN, Wuillaume C, Balteau E, Degueldre C, Luxen A, et al. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J Neurosci. (2009) 29:7948–56. doi: 10.1523/JNEUROSCI.0229-09.2009
76. Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. (2010) 14:151–60. doi: 10.1016/j.smrv.2009.07.002
77. Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. (2003) 26:413–5. doi: 10.1093/sleep/26.4.413
78. Jones KHS, Ellis J, Schantz MV, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the per3 gene and preferred timing of sleep and waking activities. J Sleep Res. (2007) 16:12–6. doi: 10.1111/j.1365-2869.2007.00561.x
79. Kunorozva L, Stephenson KJ, Rae DE, Roden LC. Chronotype and period3 variable number tandem repeat polymorphism in individual sports athletes. Chronobiol Int. (2012) 29:1004–10. doi: 10.3109/07420528.2012.719966
80. Barclay NL, Eley TC, Mill J, Wong CC, Zavos HM, Archer SN, et al. Sleep quality and diurnal preference in a sample of young adults: associations with 5httlpr, per3, and clock 3111. Am J Med Genet. (2011) 156:681–90. doi: 10.1002/ajmg.b.31210
81. Henst RH, Jaspers RT, Roden LC, Rae DE. A chronotype comparison of South African and Dutch marathon runners: the role of scheduled race start times and effects on performance. Chronobiol Int. (2015) 32:858–68. doi: 10.3109/07420528.2015.1048870
82. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. (2003) 18:80–90. doi: 10.1177/0748730402239679
Keywords: chronotype, sleep deprivation, response inhibition, interindividual difference, go/no-go task, inferior frontal gyrus, functional magnetic resonance imaging
Citation: Song J, Feng P, Wu X, Li B, Su Y, Liu Y and Zheng Y (2019) Individual Differences in the Neural Basis of Response Inhibition After Sleep Deprivation Are Mediated by Chronotype. Front. Neurol. 10:514. doi: 10.3389/fneur.2019.00514
Received: 17 December 2018; Accepted: 30 April 2019;
Published: 15 May 2019.
Edited by:
Birendra N. Mallick, Jawaharlal Nehru University, IndiaReviewed by:
Timo Partonen, National Institute for Health and Welfare, FinlandJacques Taillard, USR3413 Sommeil, Addiction et Neuropsychiatrie (SANPSY), France
Copyright © 2019 Song, Feng, Wu, Li, Su, Liu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zheng, emhlbmd5QHN3dS5lZHUuY24=
 Jingjing Song
Jingjing Song Pan Feng
Pan Feng Xin Wu
Xin Wu Yanchen Su
Yanchen Su Yingjiang Liu
Yingjiang Liu Yong Zheng
Yong Zheng