- 1Key Laboratory of Animal Epidemiology and Zoonosis, Ministry of Agriculture, National Animal Transmissible Spongiform Encephalopathy Laboratory, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2Department of Pathology, Faculty of Veterinary Sciences, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
- 3State Key Laboratory for Infectious Disease Prevention and Control, Chinese Center for Disease Control and Prevention, National Institute for Viral Disease Control and Prevention, Beijing, China
Background: The current diagnosis method for Creutzfeldt-Jakob disease (CJD) is post-mortem examination, so early detection of CJD has been historically problematic. Auxiliary detection of CJD based on changes in levels of components of the cerebrospinal fluid (CSF) has become a focus of research. In other neurodegenerative diseases such as Alzheimer's disease (AD), cell-free mitochondrial DNA (mtDNA) in the CSF of patients may serve as a biomarker that could facilitate early diagnosis and studies of the mechanisms underlying the disease.
Methods: In this study, the cell-free mitochondrial DNA in the CSF of patients with sCJD and control patients was compared by digital droplet PCR.
Results: The cell-free mitochondrial DNA copy number in the CSF of sCJD patients was significantly increased in comparison with that of the control group, and this difference was pathologically related to CJD.
Conclusion: Therefore, we speculate that changes in cerebrospinal fluid mitochondrial DNA copy number play an important role in the study of CJD mechanism and diagnosis.
Introduction
Creutzfeldt-Jakob disease (CJD) is a neurodegenerative disease associated with an abnormal accumulation of proteinaceous infectious particles (prions) in neurons (1). CJD is the most frequent human transmissible spongiform encephalopathies (2–4). CJD can be confirmed by subjecting brain tissue from CJD patients to western blotting after digestion with proteases that is a demonstration of the protease resistance of the prion protein accumulated, or to use immunohistochemistry, abnormal PrP can be identified by its abundance, location and morphology (5). A diagnosis of pre-mortem CJD can be made based on autosomal dominant pathogenic mutations in the human prion protein gene (PRNP) (6) and using real-time quaking induced conversion (RT-QuIC) which detected the accumulation of misfolded PrP in the brain, CSF, or nasal brushings (7), combined with clinical manifestations, medical history, epidemiological reports, EEG, and MRI (8, 9). In recent years, non-invasive and less invasive detection methods have been used to diagnose CJD, and this idea can also be applied to the study of the mechanisms underlying CJD. CSF is in direct contact with the brain parenchyma and can indirectly reflect certain pathological indications of the central nervous system (10). Changes in the abundance of many proteins (14-3-3 protein, tau et al.) in CSF can be used in the diagnosis of CJD (11–14). RT-QuIC has proved to be a very valuable diagnostic CSF test for sCJD (7). The cell-free mitochondrial DNA content in CSF was found to be changed in a specific manner in patients with AD and PD, and such changes were distinct to particular neurodegenerative diseases (15–19). These findings suggest that particular changes in mitochondrial DNA content may be related to the mechanisms underlying specific diseases. In comparison with other neurodegenerative diseases, there have been few studies of changes in mitochondrial DNA in the CSF of patients with CJD (16).
In this study, we examined the mitochondrial DNA concentration in the CSF of sCJD patients, with the goal of determining the mechanisms underlying the development and progression of CJD and identifying if changes in cerebrospinal fluid mitochondrial DNA copy number could be suitable for diagnosing the disease.
Materials and Methods
Subjects
CSF samples from 20 age-and sex ratio-matched sCJD and 13 age-and sex ratio-matched non-CJD controls were presented to the Chinese center for disease control and prevention. Both the control group and the CJD patient in the sample were Chinese. The average age of the sCJD and control patients was ~55 years, and the ratio of males to females was nearly 1:1. The clinical symptoms of the subjects were: rapid progressive dementia, myoclonus, akinetic mutism, disturbance of consciousness, mental symptoms, etc. Detailed samples background information was shown in Table 1. The diagnosis of sCJD refers to the CJD diagnostic criteria standardized by the Chinese Center for Disease Control and Prevention. The CJD diagnostic criteria unified by the centers for disease control are as follows: ① Suspected cases are based on the exclusion of other diseases, and meet the following clinical manifestations 2 or more: rapid progressive dementia, passive silence, myoclonus, visual/cerebellar dysfunction, cone system/extrapyramidal dysfunction. ② The clinical cases are basically consistent with the suspected diagnosis cases, and meet any of the following items: EEG shows periodic three-phase waves during the course of the disease; laboratory tests for positive detection of 14-3-3 protein in cerebrospinal fluid (CSF); Early nuclear magnetic resonance imaging revealed abnormally high signals in the putamen/tail caudate nucleus, and diffusion-weighted images (DWI) showed a “ribbon” sign of symmetry/asymmetry cortex (or cortex). ③ The confirmed cases should meet one of the following items: spongiform lesions found in brain histopathology; immunohistochemical detection of brain tissue, presence of protease-resistant PrPsc deposition; protein immunoblotting to detect brain tissue, protease-resistant PrPsc.
Detection of CSF Mitochondrial DNA Copy Number in sCJD Patients
Digital Droplet Polymerase Chain Reaction (ddPCR)
The CSF mtDNA copy number of sCJD patients and non-CJD samples was directly determined by ddPCR. In this experiment, an amplification product containing 85 base pairs (mtDNA-85) was the only product generated. Three replicates were performed in 96-well plates. The sequences of the amplified mtDNA primer and probe were shown in Supplementary Table 1-1 and Date Supplementary Table 1-2. The amplification reaction system and amplification reaction procedure were shown in Date Supplementary Tables 1-3, 1-4. After the amplification reaction was complete, the QX200 Droplet Reader was used to read the signal, after which the data were analyzed.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism 7 software to compare the mitochondrial DNA copy number in CSF samples from the control group and sCJD patients. At the same time, the factors of gender, age, and symptoms were analyzed to assess their relationship with the CSF mitochondrial DNA copy number. The results are expressed as the mean mtDNA concentration in CSF (mtDNA copy/20 μL ± SEM). The statistical significance was set at p < 0.05.
Results
Mitochondrial DNA Copy Number of the CSF of sCJD Patients (Calculated per 20 μL Copy Number)
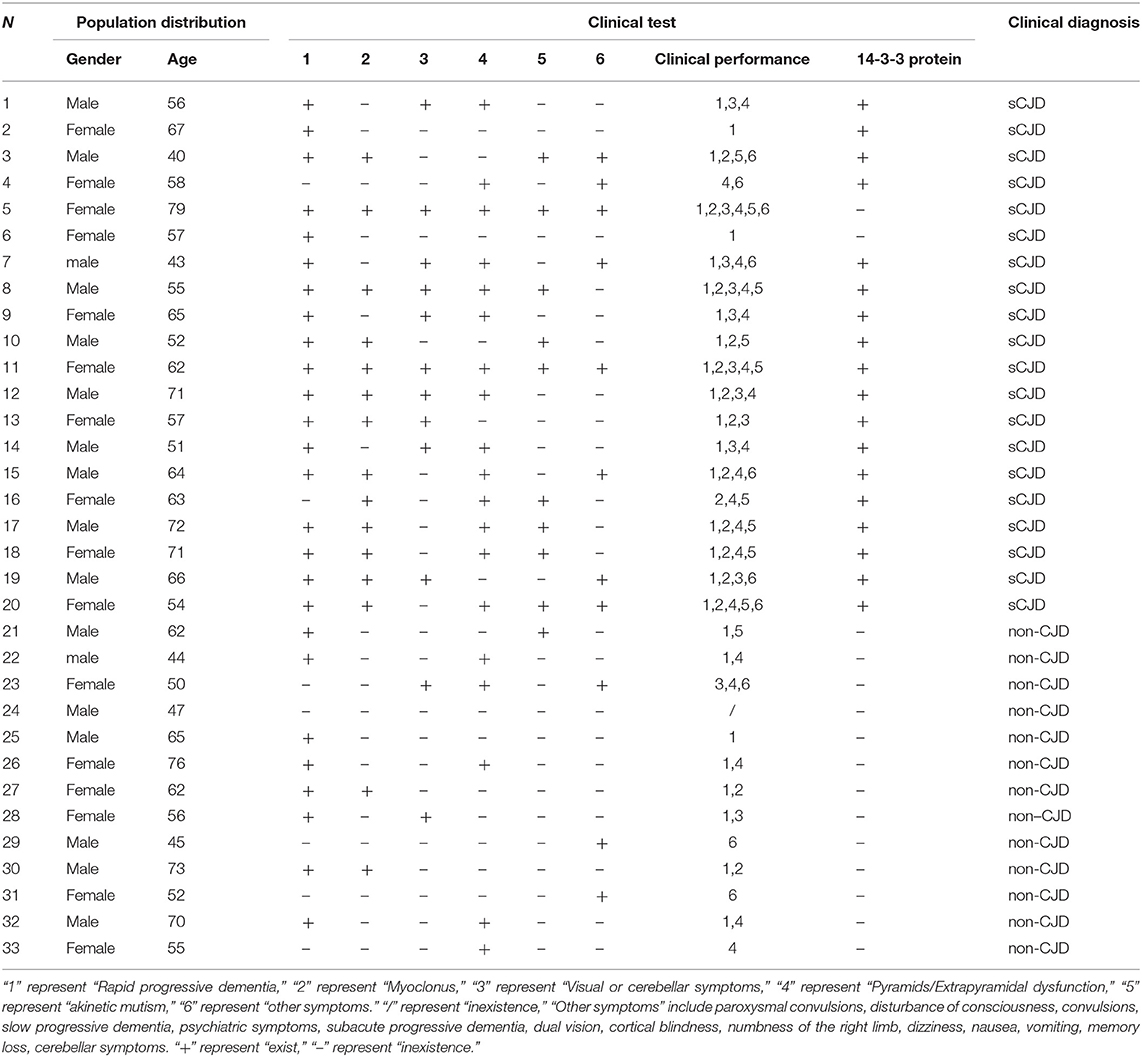
The amplified mitochondrial DNA copy number of CSF in the experimental group and the control group results are shown in Supplementary Table 2. The mitochondrial DNA copy number of the CSF of sCJD patients (404.9 ± 142.2 copies/20 μL) was significantly increased in comparison with that of the control group (264.3 ± 78.62 copies/20 μL) (Figure 1A).
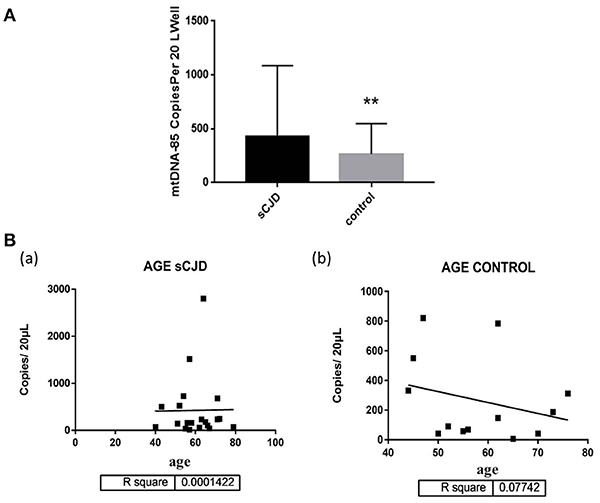
Figure 1. (A) The mitochondrial DNA copy number of the CSF of the control group (n = 13) and patients with sCJD (n = 21). Subjects were divided into the following groups: possible sCJD patients (black bar) and non-CJD subjects (gray bar). The results are expressed as mean ± SEM. The mtDNA copy number of the CSF is expressed as mtDNA copies/20 μL. *P < 0.05; **P < 0.01, represents the sCJD group are significantly different from the control group, by unpaired t-test. (B) mitochondrial DNA copy numbers of the CSF of sCJD patients/non-patients of different ages. The correlation between age and the cell-free mitochondrial DNA copy number of the CSF was assessed for the experimental and control groups (a,b). There was no correlation between age and disease, By liner regression graph and pearson correlation analysis.
Correlation Between Changes in Mitochondrial DNA Copy Number and age in Cerebrospinal Fluid in Patients With Creutzfeldt-Jakob
We further evaluated the association between changes in mitochondrial DNA copy number and age in cerebrospinal fluid in CJD patients. The mitochondrial DNA copy number of the CSF was not associated with age in the sCJD or control group (Figure 1B).
Correlation Between Changes in Mitochondrial DNA Copy Number and Gender in Cerebrospinal Fluid in Patients With Creutzfeldt-Jakob
We also evaluated the association between changes in cerebrospinal fluid mitochondrial DNA copy number and gender in Creutzfeldt-Jakob patients. The mitochondrial DNA copy number of male sCJD patients (480.2 ± 263.1 copies/20 μL) was significantly higher than that of the control group (208.1 ± 103.9 copies/20 μL), and this difference was significant. The mitochondrial DNA copy number of female sCJD patients (367.3 ± 151.1 copies/20 μL) was higher than that of the control group (330 ± 124.2 copies/20 μL), but this difference was not significant. In the control group, the cell-free mitochondrial DNA copy number of the CSF of female patients (330 ± 124.2 copies/20 μL) was higher than that of male patients (208.1 ± 103.9 copies/20 μL), but this difference was not significant. Among the sCJD patients, the mitochondrial DNA copy number of the male patients (480.2 ± 263.1 copies/20 μL) was higher than that of the female patients (367.3 ± 151.1 copies/20 μL), but this difference was not significant (Figure 2).
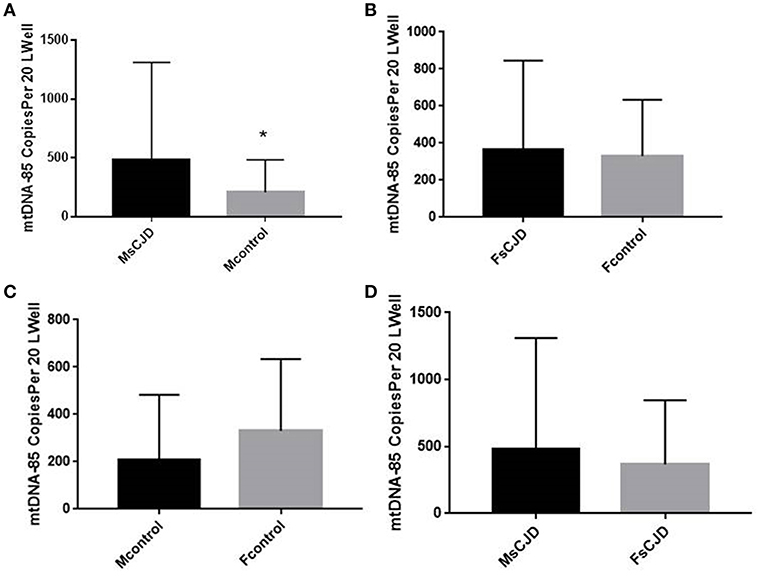
Figure 2. Copies of mtDNA in CSF and correlation with gender. (A) The mitochondrial DNA copy number of the male sCJD patients was significantly higher than that of the control group. (B) The mitochondrial DNA copy number of the female sCJD patients (n = 11) was higher than that of the control group (n = 10), but this difference was not significant. (C) In the control group, the cell-free mitochondrial DNA copy number of female patients (n = 6) was higher than that of male patients (n = 7), but this difference was not significant. (D) Among sCJD patients, the mitochondrial DNA copy number of male patients was higher than that of female patients, but this difference was not significant. The results are expressed as mean ± SEM. The mtDNA copy number of the CSF is expressed as mtDNA copies/20 μL. *P < 0.05; **P < 0.01, represents the MsCJD group are significantly different from the control group, by unpaired t-test.
Correlation Between Changes in Mitochondrial DNA Copy Number and Clinical Symptoms in Cerebrospinal Fluid in Patients With Creutzfeldt-Jakob
We next evaluated the association between changes in cerebrospinal fluid mitochondrial DNA copy number and clinical symptoms in Creutzfeldt-Jakob patients. Clinical symptoms include rapid progressive dementia, myoclonus, akinetic mutism, visual/cerebellar symptoms, pyramidal/extrapyramidal dysfunction, mental symptoms. The mitochondrial DNA copy numbers of the CSF of the experimental and control groups were not significantly associated with differences in clinical symptoms (Figure 3).
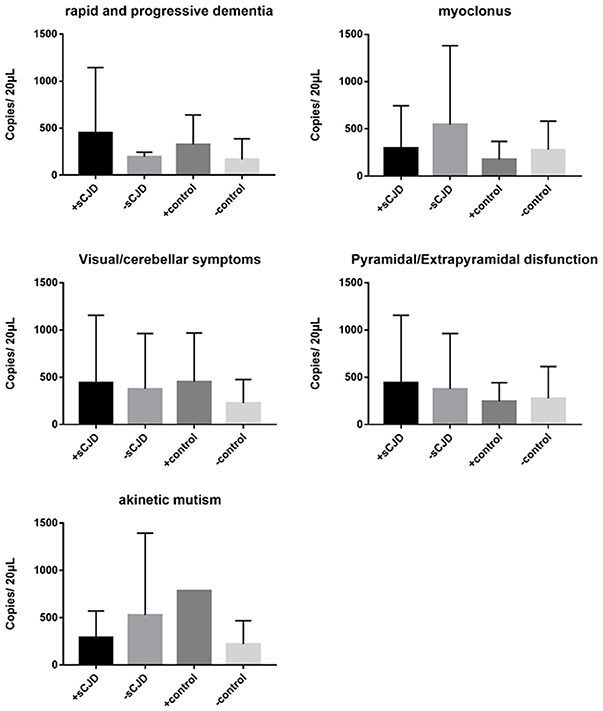
Figure 3. The correlation between clinical symptoms and the cell-free mitochondrial DNA copy number of the CSF was analyzed for the experimental and control groups. The CSF mitochondrial DNA copy number was not found to be related to CJD symptoms. The results are expressed as mean ± SEM. The mtDNA copy number of the CSF is expressed as mtDNA copies/20 μL. *P < 0.05; **P < 0.01, represents the sCJD group are significantly different from the control group, by unpaired t-test.
Correlation Between Changes in Mitochondrial DNA Copy Number and 14-3-3 Protein in Cerebrospinal Fluid in Patients With Creutzfeldt-Jakob
Finally, we examined the association between the copy number of mitochondrial DNA and 14-3-3 protein in CSF. The mitochondrial DNA copy number of the 14-3-3 protein positive group (466.3 ± 161.9 copies/20 μL) was significantly higher than that of the 14-3-3 protein negative group (234.5 ± 70.81 copies/20 μL). Statistical analysis results were shown in Supplementary Figure 1.
Discussion
Here, we investigated changes in the concentration of mtDNA in CSF from sCJD patients. In comparison with the control group with general neurological symptoms, the increase in the mitochondrial DNA copy number of the CSF of patients with sCJD was significant. Therefore, we speculate that changes in cerebrospinal fluid mitochondrial DNA copy number play an important role in the study of CJD mechanism and diagnosis. Our data support previous work where, other than in AD, all the other non-AD type dementia, including sCJD have an increased of relative mtDNA copy numbers (16). They also found that the 14-3-3 protein was not associated with significant differences in mitochondrial DNA copy number in CSF. However, our experimental results showed that the mitochondrial DNA copy number was significantly increased in the 14-3-3 protein-positive group compared to the 14-3-3 protein-negative group, and in 20 cases of CJD cerebrospinal fluid samples, 18 samples of 14-3-3 protein were positive. In general, this result indicated that the change in mitochondrial DNA copy number has a certain correlation with 14-3-3 protein. We hypothesized that changes in mitochondrial DNA copy number are important for the diagnosis of CJD.
This study did not find a relationship between cell-free mitochondrial DNA content in CSF and the age/clinical neurological symptoms of CJD or non-CJD patients, which is similar to PD patients (18). However, Wei et al.'s research indicated that changes in mitochondrial DNA copy number in brain samples from Creutzfeldt-Jakob patients were strongly positively correlated with age (20). We speculate that because our sample size is small and the age span is small, our sample age is concentrated around 55 years old. The mitochondrial DNA copy number changes in this period of time are relatively small. Although they were different, they were not significant between each other, and the results obtained were one-sided.
The cell-free mitochondrial DNA copy number of the CSF of male patients was significantly increased compared with that of the control group, and it was significantly correlated with sCJD. However, there was no correlation between mitochondrial DNA copy number and sCJD in female patients. Therefore, changes in cerebrospinal fluid mitochondrial DNA copy number in male CJD patients play a better role than in female patients in studying CJD mechanisms and diagnosis. This phenomenon has also been observed in PD, AD, and Huntington's disease patients, in which mitochondrial DNA in men is more closely associated with the disease (18, 21–23).
We speculate that the extent of the increase in mitochondrial DNA in CSF directly reflects neuronal damage. When mitochondria are dysfunctional, an insufficient energy supply leads to neuronal degeneration, and an increased mitochondrial DNA copy number in CSF may be a response to neuronal mitochondrial dysfunction to maintain neuronal energy requirements. This hypothesis is consistent with the conclusion of Wei et al. (20) they observed a strongly positive correlation between age and mtDNA copy number in CJD, this could reflect compensatory mitochondrial biogenesis in older subjects. There are no effective prevention or treatment methods for most neurodegenerative diseases. Changes in the number of mitochondrial DNA copies are associated with several neurodegenerative diseases, including sCJD, PD, and AD, so we hypothesize that drugs that regulate the mtDNA copy number in neurons may have therapeutic effects in patients with certain neurodegenerative diseases.
Conclusions
The mtDNA-85 copy number in cerebrospinal fluid of patients with sCJD was significantly different from that of the control group, we speculate that changes in cerebrospinal fluid mitochondrial DNA copy number play an important role in the study of CJD mechanism and diagnosis. Due to the small number of samples we collected, so our results are indeed limited, we need a larger sample to further verify this result.
Data Availability
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Ethics Statement
The study was ethically approved by the Institutional Ethics committee of the Chinese Center for Disease Control and Prevention. Written informed consent was obtained from each participant.
Author Contributions
JL, YD, SS, and LY conceived the study. WW, XZ, ML, and QS collected the CSF samples and clinical data. JL, YD, ZG, HG, HZ, XW, and DY performed the experiments and data analyses. LY and DZ provided intellectual inputs. JL and YD wrote the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by National Key Research and Development Program (Project No. 2017YFC1200500, No.2017YFD0501600), 948 projects (2014-S9).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Xiaoping Dong, QS, and Wei Zhou from CDC for their assistance with the sample preparation and many helps from Capital Medical University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.00645/full#supplementary-material
Abbreviations
AD, Alzheimer's disease; CJD, Creutzfeldt-Jakob disease; CSF, cerebrospinal fluid; ddPCR, digital droplet polymerase chain reaction; mtDNA, mitochondrial DNA; PD, Parkinson's Disease; sCJD, Sporadic Creutzfeldt-Jakob disease; TSEs, Transmissible spongiform encephalopathies.
References
1. Chen C, Xiao D, Zhou W, Shi Q, Zhang HF, Zhang J, et al. Global protein differential expression profiling of cerebrospinal fluid samples pooled from Chinese sporadic CJD and non-CJD patients. Mol Neurobiol. (2014) 49:290–302. doi: 10.1007/s12035-013-8519-2
2. Liberski PP. Historical overview of prion diseases: a view from afar. Folia Neuropathol. (2012) 50:1–12.
3. Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. (2012) 11:618–28. doi: 10.1016/S1474-4422(12)70063-7
4. Zerr I, Parchi P. Sporadic Creutzfeldt-Jakob disease. Handb Clin Neurol. (2018) 153:155–74. doi: 10.1016/B978-0-444-63945-5.00009-X
5. Thompson AGB, Mead SH. Review: fluid biomarkers in the human prion diseases. Mol Cell Neurosci. (2018) 97:81–92. doi: 10.1016/j.mcn.2018.12.003
6. Wadsworth JDF, Adamson G, Joiner S, Brock L, Powell C, Linehan JM, et al. Methods for molecular diagnosis of human Prion disease. Methods Mol Biol. (2017) 1658:311–46. doi: 10.1007/978-1-4939-7244-9_22
7. Orru CD, Groveman BR, Hughson AG, Manca M, Raymond LD, Raymond GJ, et al. RT-QuIC assays for prion disease detection and diagnostics. Methods Mol Biol. (2017) 1658:185–203. doi: 10.1007/978-1-4939-7244-9_14
8. Aguzzi A. Prion diseases of humans and farm animals: epidemiology, genetics, and pathogenesis. J Neurochem. (2006) 97:1726–39. doi: 10.1111/j.1471-4159.2006.03909.x
9. Summers DM, Collie DA, Zeidler M, Will RG. The pulvinar sign in variant Creutzfeldt-Jakob disease. Arch Neurol. (2004) 61:446–7. doi: 10.1001/archneur.61.3.446
10. Dislich B, Wohlrab F, Bachhuber T, Muller SA, Kuhn PH, Hogl S, et al. Label-free quantitative proteomics of mouse cerebrospinal fluid detects beta-Site APP cleaving enzyme (BACE1) protease substrates in vivo. Mol Cell Proteomics. (2015) 14:2550–63. doi: 10.1074/mcp.M114.041533
11. Ermann N, Lewczuk P, Schmitz M, Lange P, Knipper T, Goebel S, et al. CSF nonphosphorylated Tau as a biomarker for the discrimination of AD from CJD. Ann Clin Transl Neurol. (2018) 5:883–7. doi: 10.1002/acn3.584
12. Mahmoudi R, Manckoundia P, Morrone I, Lang PO, Drame M, Novella JL. Atypical case of Alzheimer's disease mimicking Creutzfeldt-Jakob disease: interest of cerebrospinal fluid biomarkers in the differential diagnosis. J Am Geriatr Soc. (2010) 58:1821–3. doi: 10.1111/j.1532-5415.2010.03051.x
13. Schmitz M, Villar-Pique A, Llorens F, Gmitterova K, Hermann P, Varges D, et al. Cerebrospinal fluid total and phosphorylated alpha-synuclein in patients with Creutzfeldt-Jakob disease and synucleinopathy. Mol Neurobiol. (2018) 56:3476–83. doi: 10.1007/s12035-018-1313-4
14. Toman E, Harrisson S, Belli T. Biomarkers in traumatic brain injury: a review. J R Army Med Corps. (2016) 162:103–8. doi: 10.1136/jramc-2015-000517
15. Podlesniy P, Figueiro-Silva J, Llado A, Antonell A, Sanchez-Valle R, Alcolea D, et al. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol. (2013) 74:655–68. doi: 10.1002/ana.23955
16. Podlesniy P, Llorens F, Golanska E, Sikorska B, Liberski P, Zerr I, et al. Mitochondrial DNA differentiates Alzheimer's disease from Creutzfeldt-Jakob disease. Alzheimers Dement. (2016) 12:546–55. doi: 10.1016/j.jalz.2015.12.011
17. Podlesniy P, Vilas D, Taylor P, Shaw LM, Tolosa E, Trullas R. Mitochondrial DNA in CSF distinguishes LRRK2 from idiopathic Parkinson's disease. Neurobiol Dis. (2016) 94:10–7. doi: 10.1016/j.nbd.2016.05.019
18. Pyle A, Anugrha H, Kurzawa-Akanbi M, Yarnall A, Burn D, Hudson G. Reduced mitochondrial DNA copy number is a biomarker of Parkinson's disease. Neurobiol Aging. (2016) 38:216 e7–10. doi: 10.1016/j.neurobiolaging.2015.10.033
19. Pyle A, Brennan R, Kurzawa-Akanbi M, Yarnall A, Thouin A, Mollenhauer B, et al. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson's disease. Ann Neurol. (2015) 78:1000–4. doi: 10.1002/ana.24515
20. Wei W, Keogh MJ, Wilson I, Coxhead J, Ryan S, Rollinson S, et al. Mitochondrial DNA point mutations and relative copy number in 1363 disease and control human brains. Acta Neuropathol Commun. (2017) 5:13. doi: 10.1186/s40478-017-0419-7
21. Petersen MH, Budtz-Jorgensen E, Sorensen SA, Nielsen JE, Hjermind LE, Vinther-Jensen T, et al. Reduction in mitochondrial DNA copy number in peripheral leukocytes after onset of Huntington's disease. Mitochondrion. (2014) 17:14–21. doi: 10.1016/j.mito.2014.05.001
22. Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JP Jr. Mitochondrial DNA copy numbers in pyramidal neurons are decreased and mitochondrial biogenesis transcriptome signaling is disrupted in Alzheimer's disease hippocampi. J Alzheimers Dis. (2014) 40:319–30. doi: 10.3233/JAD-131715
Keywords: mtDNA, Creutzfeldt-Jakob disease, cerebrospinal fluid, diagnosis, prion disease
Citation: Li J, Duan Y, Zhao D, Shah SZA, Wu W, Zhang X, Lai M, Guan Z, Yang D, Wu X, Gao H, Zhao H, Shi Q and Yang L (2019) Detection of Cell-Free Mitochondrial DNA in Cerebrospinal Fluid of Creutzfeldt-Jakob Patients. Front. Neurol. 10:645. doi: 10.3389/fneur.2019.00645
Received: 25 March 2019; Accepted: 31 May 2019;
Published: 21 June 2019.
Edited by:
Thomas Skripuletz, Hannover Medical School, GermanyReviewed by:
Todd Hulgan, Vanderbilt University, United StatesSabina Capellari, University of Bologna, Italy
Wenquan Zou, Case Western Reserve University, United States
Copyright © 2019 Li, Duan, Zhao, Shah, Wu, Zhang, Lai, Guan, Yang, Wu, Gao, Zhao, Shi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Shi, c2hpcWk3NkAxMjYuY29t; Lifeng Yang, eWFuZ2xmQGNhdS5lZHUuY24=
†These authors have contributed equally to this work
 Jie Li1†
Jie Li1† Lifeng Yang
Lifeng Yang