- 1Department of Psychology, Educational Science and Human Movement, University of Palermo, Palermo, Italy
- 2Department of Medical and Surgical Sciences, University “Magna Graecia”, Catanzaro, Italy
- 3Basic Medical Sciences, Neuroscience and Sense Organs Department, University of the Study of Bari “Aldo Moro”, Azienda Ospedaliero-Universitaria Consorziale Policlinico di Bari, Bari, Italy
- 4Clinic of Child and Adolescent Neuropsychiatry, Department of Mental Health, Physical and Preventive Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 5Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 6Department of Legal, Historical, Economic and Social Sciences, University of Catanzaro, Catanzaro, Italy
- 7Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy
- 8Department of Medical, Surgical and Advanced Technologies “G.F. Ingrassia”, University of Catania, Catania, Italy
- 9Institute of Ophthalmology, University of Foggia, Foggia, Italy
- 10Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
- 11Department of Surgical and Biomedical Sciences, University of Perugia, Perugia, Italy
- 12Neurodevelopment Research Lab, Biomedical Research Institute of New Jersey, Cedar Knolls, NJ, United States
- 13Neuroscience Research, MidAtlantic Neonatology Associates, Atlantic Health System, Morristown, NJ, United States
- 14Neuropathology Research, MANA/Biomedical Research Institute of New Jersey, Cedar Knolls, NJ, United States
Children with migraine headaches appear to have a range of sleep disturbances. The aim of the present study was to assess the NREM sleep instability in a population of school-aged individuals affected by migraine without aura (MoA). Thirty-three children with MoA (20 males, 13 females, mean age 10.45 ± 2.06 years) underwent to overnight Polysomnographic (PSG) recordings and Cyclic Alternating Pattern (CAP) analyses accordingly with international criteria. MoA group showed a reduction in sleep duration parameters (TIB, SPT, TST; p ≤ 0.001 for all) and in arousal index during REM sleep and an increase in awakenings per hour (AWK/h) vs. Controls (C) (p = 0.008). In particular, MoA children showed a reduced CAP rate% (p ≤ 0.001), CAP rate% in S1 (p ≤ 0.001) and CAP rate% in SWS (p = 0.004) vs. C. Moreover, A phases distribution were characterized by a reduction in slow wave components (total number CAP A1%, CAP A1 index) (p ≤ 0.001) and an increase of fast components representation (total number of CAP A2% and CAP A3%) (p < 0.001) in MoA vs. C. Moreover, MoA children showed an increased A1 and A2 mean duration (p ≤ 0.001). Our findings show a reduction of arousability in MoA group and lower NREM lower sleep instability associated with MoA in children.
Introduction
Sleep and headache are widely related from a clinical point of view. The biological relationship between sleep and pain processing is not fully understood yet. Presently, a unique hypothesis about the mutual inter-relationship between sleep and primary headaches cannot be presented. In this picture, we can assume that various mechanisms may be responsible for the different clinical features observed in association with headache and sleep (1). However, the connection between sleep disorders and primary headaches is clinically relevant since both conditions tend to establish mutual interrelationships that influence each other (2–4). In this context, the clinical observation raises questions regarding the pathogenesis of these disorders, involving pivotal cerebral structures (i.e., thalamus, hypothalamus, and some brainstem nuclei) and specific neurochemical pathways both in pain perception and sleep regulation.
The hypothalamus is crucial in both headache pathogenesis and sleep-wake cycle regulation because of its connection with the anti-nociceptive system [i.e., medulla oblongata, serotoninergic raphe nucleus, noradrenergic locus coeruleus, and periaqueductal gray matter (PAG)] the stimulation mediated by orexin on ventro-lateral part of PAG, and the inhibition on the anti-nociceptive activity in the caudal trigeminal nucleus (5–7).
In childhood, the most frequent primary headaches could be considered the migraine without aura (MoA) and tension-type headache with a prevalence of 2–17 and 0.9–24%, respectively (8, 9). Children with migraine headaches appear to have more frequent sleep troubles consisting in insufficient sleep, maternal co-sleeping, longer sleep latency, more bedtime resistance, shorter sleep duration, daytime sleepiness, night awakenings, sleep anxiety, parasomnias, and sleep-disordered breathing compared to children from a normative community sample (10).
To date, a limited number of polysomnographic studies carried out on patients with migraine, with no conclusive association about any peculiar characteristics of sleep architecture, although migraine attacks seem to be linked to REM stages and associated with a large amount of deep sleep (11). Moreover, Goder et al. (12) reported that migraine attacks were preceded by a significant decrease in arousals number, REM density, and in beta power band in the slow wave sleep, and by a decrease in alpha power during the first REM period. However, Vendrame et al. evidenced a high prevalence of sleep fragmentation (i.e., sleep disordered breathing, high rate of awakenings) in children with mild or severe migraine with an increasing related to the severity of symptoms (13, 14). In a PSG study Karthik et al. showed significantly lower sleep efficiency, prolonged sleep onset latency, lesser stage 4 and NREM sleep, and a greater number of total awakenings in migraineurs compared to the controls (15). In 2016, Nayak et al. showed a decreased REM arousability as well as a decreased overall CAP rate and CAP cycling in adult patients with migraine as compared to controls (16). In this perspective, we have hypothesized that the sleep parameters (such as macrostructure and microstructure) could be different in children affected by MoA respect of typical developing healthy comparisons (control subjects [C]). Therefore, the aim of the present study was to assess the NREM sleep instability in a population of school- aged individuals affected by MoA vs. C.
Materials and Methods
Study Population
Thirty-three children affected by migraine without aura (MoA) (20 males, 13 females, mean age 10.4 ± 2.0 years) underwent to an overnight PSG recording, after one adaptation night to avoid the first-night effect in the Sleep Laboratory of Child and Adolescent Neuropsychiatry at the Università degli Studi della Campania “Luigi Vanvitelli”, Campania Region, Italy. The diagnosis of migraine was made according to international criteria (17). None of those recruited children had taken prophylactic medication or neither any other regular medication for at least the 2 weeks prior to neither recruitment nor migraine attacks for 48 h at least before the study began.
Following recruitment, to verify the headache characteristics monthly headache frequency and mean headache duration was assessed from daily headache diaries kept by all the children. The headache intensity was assessed on a visual analog (VAS) scale. Exclusion criteria were neurological (i.e., epilepsy, neuromuscular disorders) or psychiatric symptoms (Attention Deficit Hyperactivity Disorder, anxiety, depression, behavior problems), mental retardation (IQ ≤ 70), borderline intellectual functioning (IQ ranging from 71 to 84), and referred signs suggestive for the presence of sleep-related breathing disorders (i.e., habitual snoring, nocturnal apneas), for periodic limb movement disorder (i.e., nocturnal hyperkinesias) and recurrent parasomnias (>3 episodes per week).
In order to compare the data from MoA children with a control group, 52 healthy children (C) (29 males, 23 females, mean age 9.9 ± 2.4 years) were enrolled from the Campania and Sicily regions schools. The subjects of both groups were recruited from the same urban area, were all of Caucasian origin and had middle socioeconomic status.
The investigation was carried out in accordance with the principles of the Declaration of Helsinki (18). All adult subjects provided written informed consent and a parent or guardian of any child participant provided written informed consent on their behalf. All procedures were performed in accordance with International guidelines and were approved by Scientific Committee of University of Palermo (n° 2015-001160-19).
Polysomnographic Evaluation (PSG)
Full overnight PSG recordings were performed according to international criteria (19–21), started at the subject's usual bedtime and continued until spontaneous morning awakening. The PSG scoring was visually analyzed by means of Hypnolab 1.2 sleep software analysis (SWS Soft, Italy) and the following conventional sleep parameters were evaluated:
1) Time in bed (TIB);
2) Sleep period time (SPT);
3) Total sleep time (TST);
4) Sleep latency (SL);
5) First REM latency (FRL);
6) Number of stage shifts/hour (SS/h); Number of awakenings/hour (AWN/h);
7) Sleep efficiency (SE%);
8) Percentage of SPT spent in wakefulness after sleep onset (WASO%);
9) Percentage of SPT spent in sleep stages 1 (N1%), 2 (N2%), slow-wave sleep (N3%), and REM sleep (REM%).
Moreover, the Arousal Index during the REM sleep was calculated.
About respiratory parameters, central, obstructive and hypopnea events were counted according to the standard criteria (22) considering as abnormal an Apnea/Hypopnea index (AHI) >1 (23). Moreover, periodic limb movements (PLMs) events were identified (24) and a PLMI≥5 was considered abnormal.
Cyclic Alternating Pattern (CAP) Analysis
CAP was scored following the standard criteria defined by Terzano et al. (25). CAP A phases have been subdivided into a 3-stage hierarchy of arousal strength: A1 is defined as the A phase with synchronized EEG patterns (intermittent alpha rhythm in stage 1 and sequences of K complexes or delta bursts in the other NREM stages) associated with mild or trivial polygraphic variations; A2 is defined as the A phase with desynchronized EEG patterns preceded by or mixed with slow high-voltage waves (K complexes with alpha and beta activities, K alpha, and arousals with slow-wave synchronization) linked to a moderate increase of muscle tone and/or cardiorespiratory rate; and A3 as the A phase with desynchronized EEG patterns alone (transient activation phases or arousals) or exceeding two thirds of the phase A length and coupled with a remarkable enhancement of muscle tone and/or cardiorespiratory rate (25).
The following CAP parameters were measured:
• CAP time (temporal sum of all CAP sequences) in NREM sleep;
• The CAP rate (percentage of total NREM sleep time occupied by CAP sequences);
• The number and duration of CAP cycles; the number and duration of CAP sequences;
• The number, duration, and percentage of A phases (including the phase A subtypes);
• A1 index (number of A1 phases per hour of NREM sleep);
• A2 index (number of A2 phases per hour of NREM sleep);
• A3 index (number of A3 phases per hour of NREM sleep);
• and the number and duration of B phases.
Statistical Analysis
The comparisons between sleep architecture and CAP parameters, obtained in MoA children and typically developing children (C), were carried out by the Mann–Whitney U test. Bonferroni correction was applied. P-values <0.01 were considered statistically significant. STATISTICA (data analysis software system), version 6, StatSoft, Inc. (2001) was used for all statistical tests.
Results
The two groups (MoA and C) were matched for age, sex, and z-score Body Mass Index (z- BMI) (Table 1). The migraine characteristics such as frequency, intensity and duration of attacks were showed in Table 1. None of the children with migraine in our series were affected by a migraine attack during the sleep study.
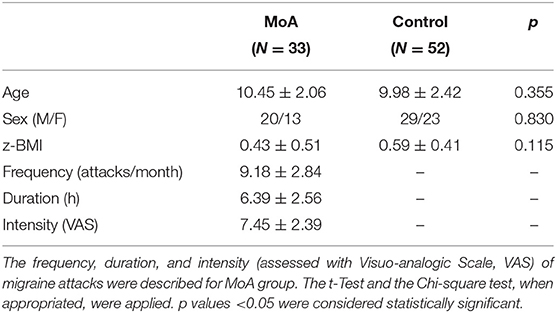
Table 1. The comparison between migraine without aura (MoA) and typically developing children (Control) groups in age, sex distribution, and z-score Body Mass Index (z-BMI).
As for the macrostructural findings, MoA group showed significant reduction in sleep duration parameters (TIB, SPT, TST; p ≤ 0.001 for all) and a significant increase in awakenings per hours (AWK/h) vs. C (p = 0.008; Table 2). Moreover, the Arousal Index during REM sleep was lower in MoA vs. C children (p < 0.001; Table 2).
As for the NREM sleep analysis, MoA children showed a reducing in CAP rate% (p ≤ 0.001), CAP rate% in N1 (p ≤ 0.001) and in CAP rate% in SWS (p = 0.004) vs. C. Moreover, the A phases distribution were characterized by significant reduction in slow wave components (Total number CAP A1%, CAP A1 index) (p ≤ 0.001) and an increasing in fast components representation (Total number of CAP A2% and CAP A3%) in comparison to C. MoA children show also an increased A1 and A2 mean duration (p ≤ 0.001; Table 3) in comparison to the healthy control group (C).
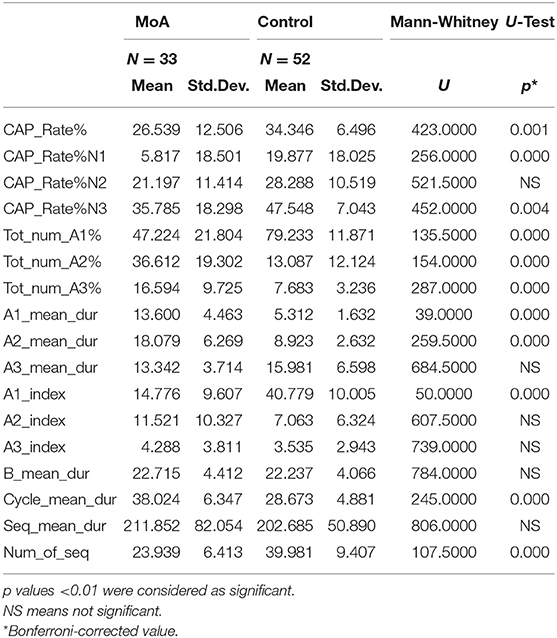
Table 3. The mean differences, evaluated with the Mann-Whitney-U test, among children affected by migraine without aura (MoA) and control group in the following parameters: CAP refers to cyclic alternating pattern; CAP rate (percentage of total NREM sleep time occupied by CAP sequences); percentage and duration of each A phase subtype; A1 index (number of phases A1 per hour of NREM sleep, and of N1, N2, and N3 sleep stage); A2 index (number of phases A2 per hour of NREM sleep, and of N1, N2, and N3 sleep stage); A3 index (number of phases A3 per hour of NREM sleep, and of N1, N2, and N3 sleep stage); duration of B phases; number and duration of CAP sequences.
Discussion
Several reports in the medical literature suggest the existence of a correlation and/or comorbidity between sleep disorders and headache linked to putative common pathophysiological substrates. In general, it has well-known that specific headache disorders, like paroxysmal hemicrania, cluster headache, and hypnic headache may be related to the rapid eye movement sleep (REM) or to obstructive sleep apnea syndrome (OSAS) (4). The details of the relationship mutual relationship between headache and sleep regulation are not still clearly understood, but it is known that sleep may be related to the occurrence of some headache syndromes while headache could cause or sustain various degrees of sleep disturbance (i.e., parasomnias, sleep disordered breathing, sleep-wake transition disorders). To date, in pediatric populations few studies seem to indicate a suggestive association between headaches and sleep disturbances, including primary snoring, obstructive sleep apnea, and NREM parasomnias although these data are mainly derived from questionnaire-based studies (8, 26–30).
On the other hand, clinical, and experimental data indicate that the thalamus may be considered as the key structure for migraine pathophysiology. EEG studies have shown that interictal migraine have low thalamo/thalamocortical transmission associated with low brainstem activation (31). In this picture, we could explain the low arousal index during REM sleep reported in children affected by MoA respect of healthy controls.
Moreover, some reports have showed that children affected by migraine may exhibit disrupted sleep architecture, such as abnormalities in total sleep time (TST) and sleep latency (SOL) compared with healthy control subjects (30). Conversely, the previous PSG study by Vendrame et al. (13) showed an important alteration/disruption in sleep in children affected by migraine and chronic migraine linked to the presence of sleep-disordered breathing, shortened TST, and high SOL, even if no healthy controls were used for comparisons.
About these alterations in TST and SOL, the Authors suggested that because some children may find relief from migraine attacks with daytime naps (or the sleep could be useful to stop the attacks), the attacks occurred during the daytime may impact the normal sleep-wake cycle (32). The severity and frequency of headache attacks may negatively affect sleep architecture provoking sleep disruptions and REM sleep percentages, as confirmed in adult subjects with migraine (33). Moreover, in adults, the reduction in REM sleep and number of arousals during REM was reported during the night preceding the migraine (34), and in this perspective a shorter sleep latency during the night before a migraine attack was observed also in children, suggesting a sort of decreasing in cortical activation the night before the onset of headache (35, 36).
Our findings seem to partially confirm some of the results reported previously such as the reduction in TST and SPT, but not in SOL and stages percentages, which in our sample, were not significantly different from healthy controls. As for the sleep disruption, our results seem to confirm the observation that children with migraine tend to show a higher rate of awakenings per hour respect of controls.
As for the NREM sleep instability analysis, the main finding of our study was the reduction in CAP rate percentage and also in N1 and N3. In our population, the CAP A1 representation was reduced in the total number and index, but with a prolonged duration than controls, and the CAP A2 and CAP A3 higher in the total number, and the CAP A2 with a longer duration in MoA vs. C. Our results are substantially in line with the data found in the study conducted in 2016 by Nayak et al. (16) on a sample of adults with MoA. In their findings the overall CAP rate, the number of CAP cycles and phase B duration was lower among migraineurs while the total phase A and phase 1 duration were increased.
Moreover, our findings confirm the reduction in CAP rate evidenced by Della Marca et al. in adults with frequent MoA (37). From this point of view, the reduction in oscillatory components during sleep in our sample could be reflecting a general hypoactivity of the arousal systems. Each of these systems has ascending projections to the cortex (which stimulate cortical activation and induce fast EEG activity) and descending projections to the spinal cord (which stimulate motor activation and induce high EMG activity) (38) and are located within the brainstem, the thalamus, the hypothalamus, and the basal forebrain (39). These areas could be considered actually as the generators of the migraine attacks (40, 41).
In our sample the CAP reduction involved prevalently the A1 phases subtypes, less so the high-frequency EEG arousals. One main role of CAP A1 fluctuations is to buffer the effect of perturbations occurring during NREM sleep (37). It can therefore be speculated that the reduction of CAP expresses a reduced efficacy of such mechanisms of processing of incoming inputs during sleep in migraine. Finally, we have to consider that to the best our knowledge, this is the first attempt to evaluate NREM instability and CAP parameters in children affected by migraine without aura compared with a control group.
In conclusion, the reduction of arousability and lower NREM sleep instability seem to be associated with MoA in children. These findings may have clinical implications. However, further studies are needed for a better comprehension of the pathophysiological mechanisms underlying the link between migraine and NREM sleep and to investigate possible consequent clinical implications and preventive treatments.
Ethics Statement
The investigation was carried out in accordance with the principles of the Declaration of Helsinki (18). The Departmental Ethics Committee approved the study. Ethics committee protocol and approval was not considered as necessary, because the evaluation done is part of the clinical routine normally performed for children and adolescents in our Unit referred for migraine without aura.
Author Contributions
MR, RM, FO, DS, FP, IB, GM, DI, and MC: substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MR, RM, FO, DS, FP, IB, GM, BG, ME, FS, GD, CL, MS, VR, PM, DI, and MC: drafting the work or revising it critically for important intellectual content and final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Evers S. Sleep and headache: the biological basis. Headache. (2010) 50:1246–51. doi: 10.1111/j.1526-4610.2010.01730.x
2. Yokoyama M, Yokoyama T, Funazu K, Yamashita T, Kondo S, Hosoai H, et al. Associations between headache and stress, alcohol drinking, exercise, sleep, and comorbid health conditions in a Japanese population. J Headache Pain. (2009) 10:177–85. doi: 10.1007/s10194-009-0113-7
3. Seidel S, Hartl T, Weber M, Matterey S, Paul A, Riederer F, et al. PAMINA study group. Quality of sleep, fatigue and daytime sleepiness in migraine–a controlled study. Cephalalgia. (2009) 29:662–9. doi: 10.1111/j.1468-2982.2008.01784.x
4. Mitsikostas DD, Viskos A, Papadopoulos D. Sleep and headache: the clinical relationship. Headache. (2010) 50:1233–45. doi: 10.1111/j.1526-4610.2010.01729.x
5. Dosi C, Riccioni A, Della Corte M, Novelli L, Ferri R, Bruni O. Comorbidities of sleep disorders in childhood and adolescence: focus on migraine. Nat Sci Sleep. (2013) 5:77–85. doi: 10.2147/NSS.S34840
6. Rainero I, Rubino E, Gallone S, Fenoglio P, Picci LR, Giobbe L, et al. Evidence for an association between migraine and the hypocretin receptor 1 gene. J Headache Pain. (2011) 12:193–9. doi: 10.1007/s10194-011-0314-8
7. Messina A, Bitetti I, Precenzano F, Iacono D, Messina G, Roccella M, et al. Non-rapid eye movement sleep parasomnias and migraine: a role of orexinergic projections. Front Neurol. (2018) 9:95. doi: 10.3389/fneur.2018.00095
8. Lateef TM, Merikangas KR, He J, Kalaydjian A, Khoromi S, Knight E, et al. Headache in a national sample of American children: prevalence and comorbidity. J Child Neurol. (2009) 24:536.e43. doi: 10.1177/0883073808327831
9. Miller VA, Palermo TM, Powers SW, Scher MS, Hershey AD. Migraine headaches and sleep disturbances in children. Headache. (2003) 43:362–8. doi: 10.1046/j.1526-4610.2003.03071.x
10. Dexter JD. The relationship between disorders of arousal from sleep and migraine. Headache. (1986) 26:322.
11. Dexter J, Weitzman ED. The relationship of nocturnal headaches to sleep stage patterns. Neurology. (1970) 20:513–8. doi: 10.1212/WNL.20.5.513
12. Goder R, Fritzer G, Kapsokalyvas A, Kropp P, Niederberger U, Strenge H, et al. Polysomnographic findings in nights preceding a migraine attack. Cephalalgia. (2001) 21:31–7. doi: 10.1046/j.1468-2982.2001.00141.x
13. Vendrame M, Kaleyias J, Valencia I, Legido A, Kothare SV. Polysomnographic findings in children with headaches. Pediatr Neurol. (2008) 39:6–11. doi: 10.1016/j.pediatrneurol.2008.03.007
14. Bruni O, Miano S, Galli F, Verrillo E, Guidetti V. Sleep apnea in childhood migraine. J Headache Pain. (2000) 1:169–72. doi: 10.1007/s101940070039
15. Karthik N, Sinha S, Taly AB, Kulkarni GB, Ramachandraiah CT, Rao S. Alteration in polysomnographic profile in ‘migraine without aura’ compared to healthy controls. Sleep Med. (2013) 14:211–41. doi: 10.1016/j.sleep.2012.10.019
16. Nayak C, Sinha S, Nagappa M, Nagaraj K, Kulkarni GB, Thennarasu K, et al. Study of sleep microstructure in patients of migraine without aura. Sleep Breath. (2016) 20:263–9. doi: 10.1007/s11325-015-1207-x
17. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2018) 38:1–2. doi: 10.1177/0333102417738202
18. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 27:2191–4. doi: 10.1001/jama.2013.281053
19. Jasper HH. The 10–20 electrode system of the international federation. Electroencephalogr Clin Neurophysiol. (1958) 10:370–5.
20. Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, 1st Edn. Westchester, IL: American Academy of Sleep Medicine (2007).
21. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med. (2012) 8:597–619. doi: 10.5664/jcsm.2172
22. American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. (1996) 153:866–78. doi: 10.1164/ajrccm.153.2.8564147
23. Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of the literature. Pediatr. Pulmonol. (2005) 40:22–30. doi: 10.1002/ppul.20236
24. Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. (2006) 7:175–83. doi: 10.1016/j.sleep.2006.01.001
25. Terzano MG, Parrino L, Smerieri A, Chervin R, Chokroverty S, Guilleminault C, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. (2002) 2:187–99. doi: 10.1016/S1389-9457(02)00003-5
26. De Giorgis G, Miletto R, Iannuccelli M, Camuffo M, Scerni S. Headache in association with sleep disorders in children: a psycodiagnostic evaluation and controlled clinical study–L-5-HTP versus placebo. Drugs Exp Clin Res. (1987) 13:425–33.
27. Carotenuto M, Guidetti V, Ruju F, Galli F, Tagliente FR, Pascotto A. Headache disorders as risk factors for sleep disturbances in school aged children. J Headache Pain. (2005) 6:268–70. doi: 10.1007/s10194-005-0204-z
28. Dexter JD. The relationship between stage III, IV, REM sleep and arousals with migraine. Headache. (1979) 19:364–9. doi: 10.1111/j.1526-4610.1979.hed1907364.x
29. Isik U, Ersu RH, Ay P, Save D, Arman AR, Karakoc F, et al. Prevalence of headache and its association with sleep disorders in children. Pediatr Neurol. (2007) 36:146–51. doi: 10.1016/j.pediatrneurol.2006.11.006
30. Bruni O, Fabrizi P, Ottaviano S, Cortesi F, Giannotti F, Guidetti V. Prevalence of sleep disorders in childhood and adolescence with headache: a case- control study. Cephalalgia. (1997) 17:492–8. doi: 10.1046/j.1468-2982.1997.1704492.x
31. Porcaro C, Di Lorenzo G, Seri S, Pierelli F, Tecchio F, Coppola G. Impaired brainstem and thalamic high-frequency oscillatory EEG activity in migraine between attacks. Cephalalgia. (2017) 37:915–26. doi: 10.1177/0333102416657146
32. Bursztein C, Steinberg T, Sadeh A. Sleep, sleepiness, and behavior problems in children with headache. J Child Neurol. (2006) 21:1012–9. doi: 10.1177/7010.2006.00239
34. Dodick DW, Eross EJ, Parish JM, Silber M. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache. (2003) 43:282–9. doi: 10.1046/j.1526-4610.2003.03055.x
35. Bruni O, Russo PM, Violani C, Guidetti V. Sleep and migraine: An actigraphic study. Cephalalgia. (2004) 24:134–9. doi: 10.1111/j.1468-2982.2004.00657.x
36. Heng K, Wirrell E. Sleep disturbance in children with migraine. J Child Neurol. (2006) 21:761–6. doi: 10.1177/08830738060210092201
37. Della Marca G, Vollono C, Rubino M, Di Trapani G, Mariotti P, Tonali PA. Dysfunction of arousal systems in sleep-related migraine without aura. Cephalalgia. (2006) 26:857–64. doi: 10.1111/j.1468-2982.2005.01037.x
39. Montagna P. Hypothalamus, sleep and headaches. Neurol Sci. (2006) 27(Suppl 2):S138–43. doi: 10.1007/s10072-006-0589-8
40. Halasz P, Terzano M, Parrino L, Bodizs R. The nature of arousal in sleep. J Sleep Res. (2004) 13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x
41. Coppola G, Di Renzo A, Tinelli E, Lepre C, Di Lorenzo C, Di Lorenzo G, et al. Thalamo-cortical network activity between migraine attacks: Insights from MRI-based microstructural and functional resting-state network correlation analysis. J Headache Pain. (2016) 17:100. doi: 10.1186/s10194-016-0693-y
Keywords: migraine without aura (MoA), NREM sleep instability, cyclic alternating pattern (CAP) analysis, sleep macrostructure, full overnight polysomnography
Citation: Roccella M, Marotta R, Operto FF, Smirni D, Precenzano F, Bitetti I, Messina G, Sessa F, Di Mizio G, Loreto C, Salerno M, Russo V, Murabito P, Gallai B, Esposito M, Iacono D and Carotenuto M (2019) NREM Sleep Instability in Pediatric Migraine Without Aura. Front. Neurol. 10:932. doi: 10.3389/fneur.2019.00932
Received: 31 December 2018; Accepted: 12 August 2019;
Published: 27 August 2019.
Edited by:
Massimiliano Valeriani, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Maurizio Elia, Oasi Maria SS. Association ONLUS (IRCCS), ItalyValerio Brunetti, Catholic University of the Sacred Heart, Rome, Italy
Copyright © 2019 Roccella, Marotta, Operto, Smirni, Precenzano, Bitetti, Messina, Sessa, Di Mizio, Loreto, Salerno, Russo, Murabito, Gallai, Esposito, Iacono and Carotenuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Messina, R2lvdmFubmkubWVzc2luYUB1bmlmZy5pdA==; Diego Iacono, ZGllZ28uaWFjb25vQGF0bGFudGljaGVhbHRoLm9yZw==; aWFjb25vQGJyaW5qLm9yZw==
†These authors have contributed equally to this work
 Michele Roccella
Michele Roccella Rosa Marotta
Rosa Marotta Francesca Felicia Operto
Francesca Felicia Operto Daniela Smirni
Daniela Smirni Francesco Precenzano4
Francesco Precenzano4 Ilaria Bitetti
Ilaria Bitetti Giovanni Messina
Giovanni Messina Francesco Sessa
Francesco Sessa Giulio Di Mizio
Giulio Di Mizio Carla Loreto
Carla Loreto Monica Salerno
Monica Salerno Vincenzo Russo
Vincenzo Russo Maria Esposito
Maria Esposito Diego Iacono
Diego Iacono Marco Carotenuto
Marco Carotenuto