- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
- 5Beijing Translational Engineering Center for 3D Printer in Clinical Neuroscience, Beijing, China
Objective: To identify associated risk factors for the angiographic outcomes after direct and combined bypass surgery in moyamoya disease (MMD).
Methods: All direct and combined bypass procedures performed from June 2009 to May 2015 were screened in this prospective cohort study. Patients who acquired presurgical and follow-up catheter angiography were included. Bypass patency and postoperative collateral formation were evaluated. Univariate and multivariate logistic regression analyses were performed to determine the influence factors for bypass patency and postoperative collateral formation.
Results: In total, 188 consecutive bypass procedures were included. After an 18-month median follow-up, the anastomosis patency rate was 88.3%. Postoperative collateral formation was associated with the patency of the anastomosis (Gamma = 0.891, p < 0.001). Multivariate logistic regression analysis showed that presence of hemorrhage (OR, 0.298; 95% CI, 0.125–0.709; p = 0.006) was associated with obstructed anastomosis. Among the 188 bypass surgeries, 125 (63.2%) hemispheres had good postoperative collateral formation and 85 (36.8%) had poor postoperative collateral formation. Multivariate logistic regression analysis showed that younger age at operation (OR, 2.396; 95% CI, 1.231–4.664; p = 0.010) was associated with good postoperative collateral formation, while the poor postoperative collateral formation was related to presence of hemorrhage (OR, 0.329; 95% CI, 0.143–0.758; p = 0.009) and dilated anterior choroidal artery (OR, 0.472; 95% CI, 0.240–0.929; p = 0.030).
Conclusions: This study has demonstrated that presence of hemorrhage predicts lower patency rates. Younger age at operation was associated with good postoperative collateral formation, while the poor postoperative collateral formation was related to presence of hemorrhage and dilated anterior choroidal artery.
Introduction
Moyamoya disease (MMD) is a chronic cerebrovascular disorder, which is characterized by progressive occlusion of the bilateral distal internal carotid arteries, making for neurological/neurocognitive impairment and recurrent stroke (1, 2). Bypass surgery is considered to be the treatment for improving neurological/neurocognitive status and secondary stroke prevention in MMD patients (3, 4).
Over the years, hundreds of studies have examined the efficacy of bypass surgery in MMD patients (3–6). In these studies, angiographic outcomes are the decisive factor for the success of the intervention and quality of life of the patients (7, 8). Although clinical outcome of MMD has been well-documented (9, 10), a paucity study has monitored the angiographic outcomes following bypass using postoperative digital subtraction angiography (DSA) and identified the associated risk factors for the angiographic outcomes in MMD (11).
The identification of associated risk factors for the angiographic outcomes after direct bypass (DB) and combined bypass (CB) would help the surgeons to find out which MMD patient is more suitable for the bypass procedures. Therefore, we performed this prospective study to analyze the angiographic outcomes of bypass surgery for MMD.
Materials and Methods
Patient Data
Our previous trial was a single-center registry, prospective cohort study to evaluate the effects of different surgical modalities on the clinical outcome of MMD (12). Patients with moyamoya syndrome were ruled out. The report of the previous trial showed that CB and DB are more effective at preventing recurrent ischemic strokes than indirect bypass (IB), and there was no difference in preventing recurrent hemorrhage. Meanwhile, we designed a cohort study using the longitudinal data on patients allocated to the DB and CB. Patients who received pre-surgical and follow-up DSA were collected and reviewed. The study was approved by the institutional review board of Beijing Tiantan Hospital, Capital Medical University. Written informed consent for research purposes was obtained from all patients at admission.
Surgical Modalities
To start with, all bypasses were performed by two surgeons (D.Z. and R.W.) with over 10 years of experience in cerebrovascular surgery. DB and CB were the favored surgical procedures for most patients at our stroke center, for they could be used in children, adult, ischemic, or hemorrhagic patients (13). DB was performed as end-to-side anastomosis of branch of the superficial temporal artery (STA) to cortical branches of middle cerebral artery (MCA). As for CB, two types of procedures have been chosen; the first one is combined DB and encephalodurosynangiosis (EDS) and the second one combined DB and encephaloduroarteriosynangiosis (EDAS). To be specific, for EDS, dura was cut in a radial fashion, inverted, and inserted underneath the bone edge of the craniotomy, while EDAS was a combination of EDS and STA branch sutured onto the brain surface. In the end, bypass patency was routinely examined using intraoperative indocyanine green angiography after anastomosis, and patients had routinely received computed tomography to detect postoperative hemorrhage or stroke on the first day after surgery.
Radiological Evaluations
The examination of computed tomography perfusion (CTP) was conducted for patients in no >6 months before surgical modalities (14). CTP parameters include regional cerebral blood volume (rCBV), regional cerebral blood flow (rCBF), mean transit time (MTT), and time to peak (TTP). The stages of preinfarction period were made as the following: Stage I, TTP was delayed, and MTT, rCBF, and rCBV were normal; Stage II, TTP and MTT were delayed, rCBF was normal, and rCBV was normal or slightly increased; Stage III, TTP and MTT were delayed, rCBF was decreased, and rCBV was normal or slightly decreased; Stage IV, TTP and MTT were delayed, and rCBF and rCBV were decreased. Preoperative and postoperative DSA were evaluated by two neurosurgeons and one radiologist, who were not involved in the surgery and blinded to clinical information. Preoperative DSA was performed at 1 month before surgery. Surgical hemispheres were evaluated based on Suzuki stage (15). The anterior choroidal artery (AChA) in surgical hemispheres was assessed (16): Normal, normal AChA; Dilated, dilated AChA with distal branching or abnormal branches. Posterior communicating artery (PComA) was recorded (16): Negative, normal or dilated PComA; Positive, dilated PComA with abnormal branch extensions (Figure 1). Follow-up DSA was performed at 6 to 12 months after surgery. Patency of the anastomosis at follow-up was evaluated (11): Occluded, complete proximal occlusion of STA and disappearance of MCA branches; Stenosed, thin and stenosed STA with a few visible MCA branches; Patent: patent or dilated STA with patent or even dilated MCA branches (Figure 2). Based on the above, Occluded and Stenosed were defined as “Obstructed” angiographic outcome and Patent was defined as “Unobstructed” angiographic outcome. Finally, postoperative collateral formation was evaluated with the Matsushima scale (17): Level A, more than 2/3 of the MCA distribution; Level B, between 2/3 and 1/3 of the MCA distribution; and Level C, slight or none (Figure 3). Based on the above, A and B were defined as “Good” angiographic outcome and C was defined as “Poor” angiographic outcome.
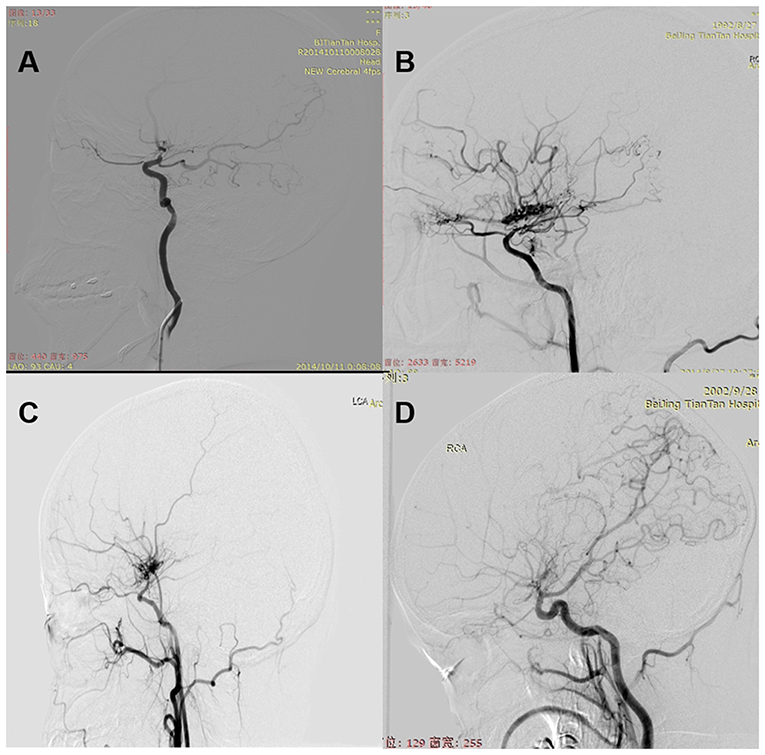
Figure 1. (A) Normal: normal AChA. (B) Dilated: dilated AChA with distal branching or abnormal branches. (C) Negative: normal or dilated PComA. (D) Positive: dilated PComA with abnormal branch extensions.
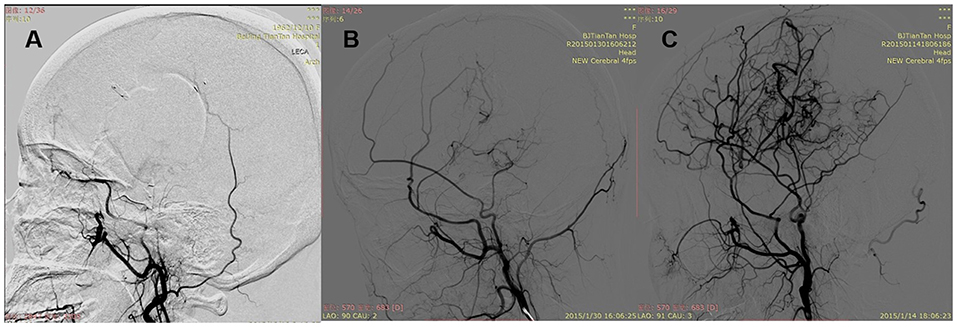
Figure 2. Patency of the anastomosis. (A) Occluded: complete proximal occlusion of STA and disappearance of MCA branches. (B) Stenosed: thin and stenosed STA with a few visible MCA branches. (C) Patent: patent or dilated STA with patent or even dilated MCA branches.
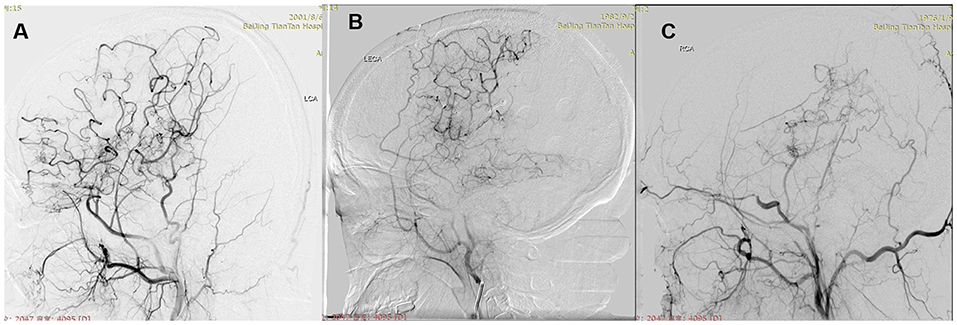
Figure 3. Postoperative collateral formation was evaluated with the Matsushima scale. (A) Level A: more than 2/3 of the MCA distribution. (B) Level B: between 2/3 and 1/3 of the MCA distribution. (C) Level C: slight or none.
Statistical Analysis
All statistical analyses were carried out using SPSS software (Windows version 22.0; IBM). The univariate and multivariate logistic regression model were used to identify which variables were associated with postoperative collateral formation and bypass patency. Odds ratios (OR) with 95% confidence intervals (CIs) are presented. A probability value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
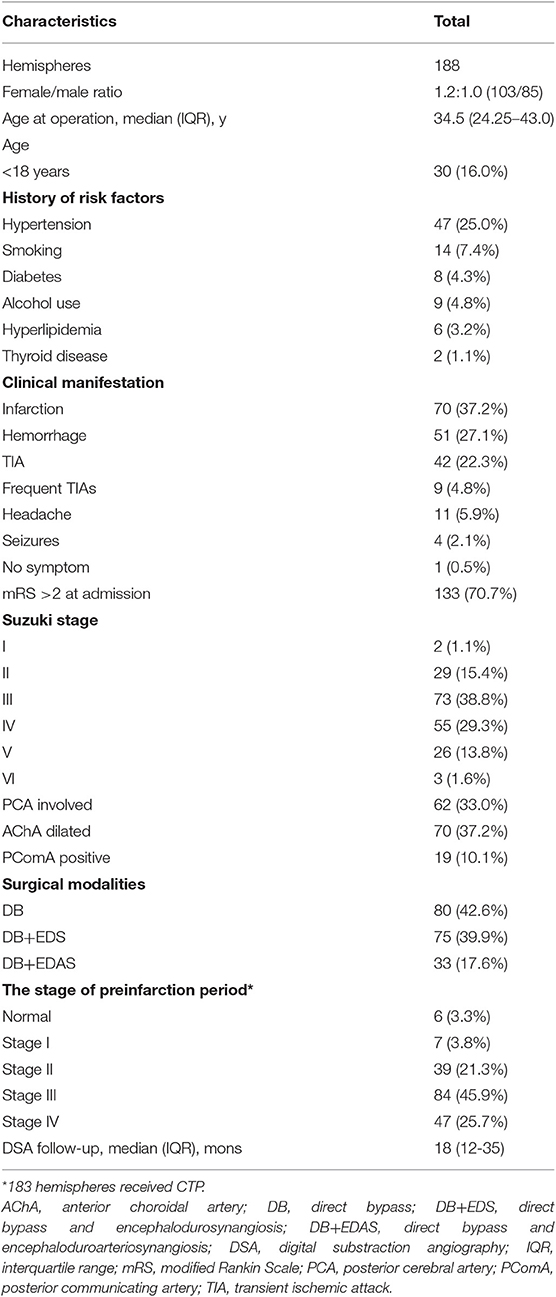
From June 2009 to May 2015, a total of 176 patients (188 hemispheres) at Stroke Center Ward 3 of our institution were included in this study. The median age at the operation was 34.5 years. The female/male ratio was 1.2:1.0. Among the 188 hemispheres, 47 (25.0%) had hypertension, 14 (7.4%) had a history of smoking, 9 (4.8%) had a history of alcohol use, 8 (4.3%) had diabetes, 6 (3.2%) had hyperlipidemia, and 2 (1.1%) had thyroid disease. The most common onset type was infarction (40.6%), followed by hemorrhage (29.0%), transient ischemic attack (TIA, 22.3%), frequent TIAs (≥2 times per month, 4.8%), headache (5.9%), seizures (2.1%), and no symptom (0.5%). Modified Rankin Scale score at admission lower than 2 was observed in 133 hemispheres (Table 1).
Most hemispheres presented with Suzuki Stage 3 (38.8%). Posterior cerebral artery (PCA) involved was observed in 62 (33.0%) hemispheres. Among 188 hemispheres, 70 (37.2%) had dilated AChA, and 19 (10.1%) had positive PComA. In addition, 80 (42.6%) hemispheres were treated with DB, 75 (39.9%) hemispheres received DB+EDS, and 33 (17.6%) hemispheres were treated with DB+EDAS.
Angiographic Outcomes
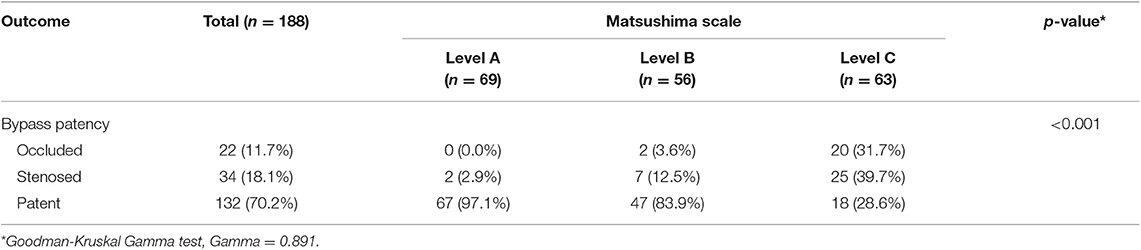
During the 18-month DSA median follow-up, the distribution of patency of the anastomosis was as follows: Occluded, n = 22 (11.7%); Stenosed, n = 34 (18.1%); Patent, n = 132 (70.2%). The anastomosis patency rate was 88.3%. Among a total of 188 hemispheres, 69 (36.7%) achieved Matsushima level A, 56 (29.8%) achieved Matsushima level B, and 63 (33.5%) achieved Matsushima level C. Postoperative collateral formation was associated with the patency of the anastomosis (Gamma = 0.891, p < 0.001). It was notable that although the anastomosis was occluded, two hemispheres treated with DB+EDAS still achieved Matsushima level B (Table 2).
Analysis for Predictive Factors for Patency of the Anastomosis
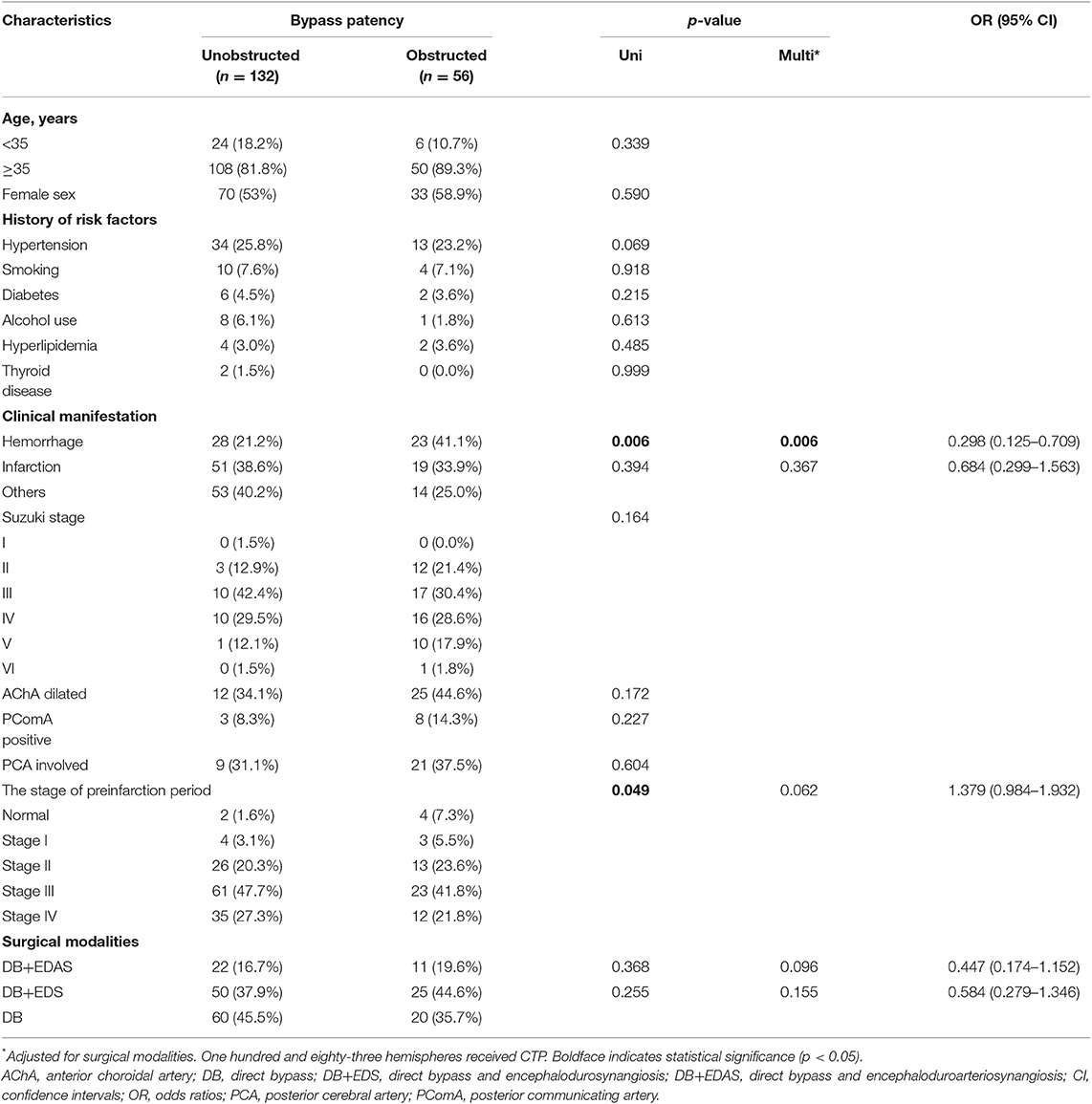
Among the 188 hemispheres that have undergone bypass surgery, 132 (70.2%) hemispheres had unobstructed anastomosis and 56 (29.8%) had obstructed anastomosis. Univariate logistic regression analysis showed that the presence of hemorrhage (OR, 0.322; 95% CI, 0.143–0.721; p = 0.006) and the stage of preinfarction periods (OR, 1.392; 95% CI, 1.002–1.934; p = 0.049) affected patent bypass, and the other factors were found not significantly associated. Multivariate logistic regression analysis showed that presence of hemorrhage (OR, 0.298; 95% CI, 0.125–0.709; p = 0.006) was associated with obstructed anastomosis (Table 3). Furthermore, among 30 pediatric hemispheres, 24 (80.0%) had unobstructed anastomosis, and 6 (20.0%) had obstructed anastomosis. However, univariate logistic regression analysis did not find the factors affecting patent bypass (Supplemental Table 1). In addition, among 158 adult hemispheres, 108 (68.4%) had unobstructed anastomosis, and 50 (31.6%) had obstructed anastomosis. Univariate logistic regression analysis showed that the presence of hemorrhage (OR, 0.329; 95% CI, 0.135–0.801; p = 0.014) was basically the only factor that affected patent bypass, while the other factors were not significantly associated. The other factors were not significantly associated. Multivariate logistic regression analysis showed that presence of hemorrhage (OR, 0.313; 95% CI, 0.126–0.777; p = 0.012) was associated with obstructed anastomosis (Supplemental Table 2).
Analysis for Predictive Factors for Postoperative Collateral Formation
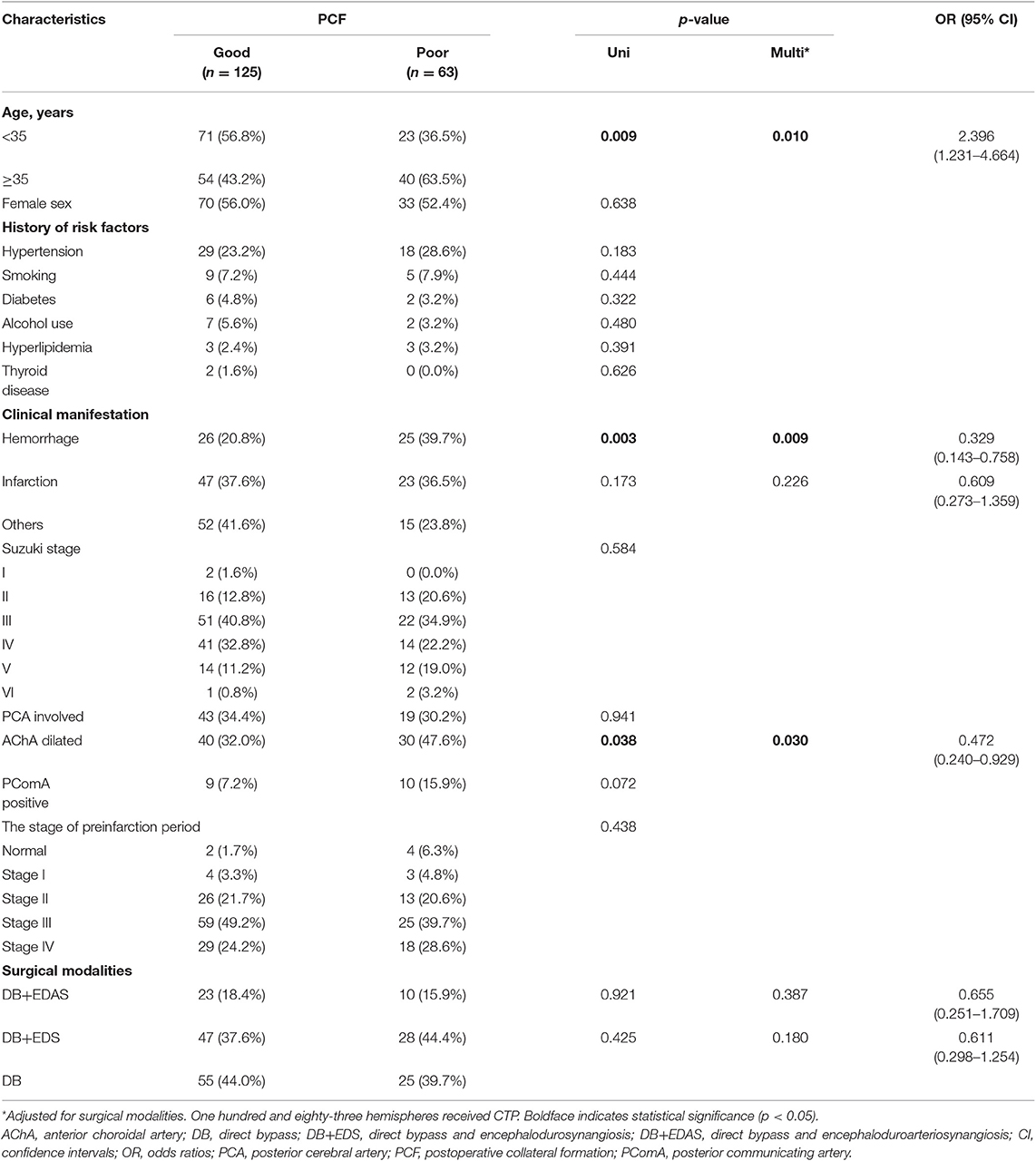
Of the 188 bypass procedures, 125 (63.2%) hemispheres had good postoperative collateral formation and 85 (36.8%) had poor postoperative collateral formation. It could be found from the univariate logistic regression that age is related to the condition of postoperative collateral formation, for patients who had the operation (OR, 2.287; 95% CI, 1.226–4.264; p = 0.009) at a younger age had better postoperative collateral formation. On the other hand, the presence of hemorrhage (OR, 0.300; 95% CI, 0.136–0.664; p = 0.003) and dilated AChA (OR, 0.518; 95% CI, 0.278–0.963; p = 0.038) were identified as predictors of poor postoperative collateral formation (Table 2). Similarly, multivariate logistic regression analysis showed that age is related to the condition of postoperative collateral formation, for patients who had the operation (OR, 2.396; 95% CI, 1.231–4.664; p = 0.010) at a younger age had good (better) postoperative collateral formation, while presence of hemorrhage (OR, 0.329; 95% CI, 0.143–0.758; p = 0.009) and dilated AChA (OR, 0.472; 95% CI, 0.240–0.929; p = 0.030) were associated with poor postoperative collateral formation (Table 4). Besides, among 30 pediatric hemispheres, 25 (83.3%) had good postoperative collateral formation and 5 (16.7%) had poor postoperative collateral formation. Univariate logistic regression analysis did not find the factors that affected postoperative collateral formation (Supplemental Table 3). On the other hand, among 158 adult hemispheres, 100 (63.3%) had good postoperative collateral formation and 58 (36.7%) had poor postoperative collateral formation. Univariate logistic regression analysis showed the presence of hemorrhage (OR, 0.306; 95% CI, 0.128–0.731; p = 0.008) and dilated PComA (OR, 0.257; 95% CI, 0.080–0.832; p = 0.023) could be identified as predictors of poor postoperative collateral formation. Finally, the multivariate logistic regression analysis showed that only presence of hemorrhage (OR, 0.349; 95% CI, 0.141–0.861; p = 0.022) was basically the only factor that associated with poor postoperative collateral formation (Supplemental Table 4).
Discussion
Since the 1970s, STA-MCA anastomosis has been used in MMD patients (18). Successful anastomosis between the donor and recipient arteries can improve blood flow immediately after surgery (4, 19). Bypass surgery contributes in improving neurological/neurocognitive status and secondary stroke prevention in MMD patients. Although the clinical outcomes of bypass surgery in MMD have been well-documented, there is lack of research on angiographic outcomes after bypass surgery for investigating the associated risks of MMD angiographic results using DSA (11). In this study, we performed this prospective study to investigate associated risk factors for the angiographic outcomes after bypass surgery. We found that younger age at operation was associated with good postoperative collateral formation, while the presence of hemorrhage and dilated AChA were associated with poor postoperative collateral formation. Meanwhile, the presence of hemorrhage was the only factor associated with obstructed anastomosis.
Bypass patency is an important determinant factor to evaluate the success of the surgery and the long-term outcome of the patient (8, 11, 20). Although the primary bypass function has been evaluated during surgery, there is a 4–10% chance of early bypass failure (21). Yoon et al. analyzed the long-term patency of 430 bypasses with postoperative imaging and found out that the overall patency rate of bypasses in MMD was 98% (8). The Caucasian Krupp Hospital cohort showed that, with the use of duplex ultrasound, the patency of the STA–MCA bypass at 3 months was 100% (22). In addition, Ha et al. showed that the postoperative patency of single barrel STA-MCA was 88.4% on follow-up imaging (mean, 16.5 months) (23). In our study, the patency of the bypasses was 88.3% on follow-up DSA (median, 18 months), which was similar with the study of Ha et al.
The presence of hemorrhage was the only factor associated with obstructed anastomosis. Yoon et al. conducted 430 consecutive bypasses, and their results revealed that low-flow bypass was associated with higher patency rate. Therefore, they speculated that MMD has high demand to augment blood flow, which encourages bypass patency (8). Interestingly, our recent study showed that hemorrhagic patients suffer less from hypoperfusion (14). Therefore, it is possible that ischemic MMD has higher demand to augment blood flow on the bypasses than the hemorrhagic MMD, which could lead to a conclusion that the hemorrhagic MMD was associated with obstructed anastomosis. However, only univariate logistic regression analysis showed that the stage of preinfarction periods was correlated with patent bypass and multivariate logistic regression analysis showed that preinfarction periods were unrelated to patent bypass. Furthermore, we found that postoperative collateral formation was associated with the patency of the anastomosis (Gamma = 0.891, p < 0.001). Our previous study also showed that bypass patency contributed to good angiographic outcome (11). It was noteworthy that although the anastomosis was occluded, two hemispheres treated with DB+EDAS still achieved Matsushima level B. Likewise, Kim et al. also demonstrated that clinical improvement of non-patent anastomosis can be expected after bypass surgery for adult MMD (20).
Despite the controversy, bypass is one of the main treatments of MMD for preventing recurrent stroke and to improve the prognosis (3, 4, 6, 10). The effect of bypass is based on postoperative collateral formation from the extracranial carotid artery into ischemic brain tissue (14, 24). Besides, multivariate logistic regression analysis showed that the patient who had the operation at a younger age (OR, 2.396; 95% CI, 1.231–4.664; p = 0.010) is more likely to have a better postoperative collateral formation. Previously reported studies had shown that bypass surgery is more effective in younger MMD patients (12). In addition, the presence of hemorrhage (OR, 0.329; 95% CI, 0.143–0.758; p = 0.009) was associated with poor postoperative collateral formation. Compared with the non-surgical group, the results of the Japan Adult Moyamoya Trial revealed that direct bypass undertook certain roles in preventing rebleeding (25). In general, despite aggressive bypass surgery, hemorrhagic MMD patients had higher morbidity and mortality than other types of MMD (26). For instance, in this study, only 28 (54.9%) of 51 had good postoperative collateral formation, which could be an explanation of the worse long-term clinical outcome in hemorrhagic MMD. Moreover, it is interesting for us to know that the dilated AChA (OR, 0.472; 95% CI, 0.240–0.929; p = 0.030) was also associated with poor postoperative collateral formation. It is well-known that the dilatation of AChA was a predictor of hemorrhage in MMD patients (16). A recent study showed that choroidal collaterals are associated with high rebleeding risk in non-surgical cohort and non-hemorrhagic hemispheres (27, 28). However, whether the dilated AChA is associated with poor postoperative collateral formation should be further verified.
DB is thought to provide immediate blood flow by the STA-MCA anastomosis. Meanwhile, indirect surgery takes more time to improve cerebral blood flow, which requires approximately about 3 months for neoangiogenesis from connective tissue (4, 19). DB provided early augmentation of blood flow, whereas the indirect surgery provided a more durable long-term neoangiogenesis, indicating a complementary association between the two procedures (29). Theoretically, CB may have better angiographic outcomes than DB. Many studies confirmed that DB and CB were more effective than indirect surgery in preventing recurrent stroke in adult (10, 30). However, for pediatric patients, indirect surgery can yield similar results with DB and CB (31), but there are few studies on superior surgical modality between DB and CB up until now. In our study, DB and CB had similar angiographic outcomes (postoperative collateral formation and bypass patency). Even so, we hold the opinion that abandoning CB for MMD is unwise, for the neoangiogenesis from indirect surgery might bring additional blood supply and remedy for patients who had STA-MCA anastomosis occlusion.
Limitation
The present study had a few limitations. First, it is a non-randomized controlled study. Selection bias in choosing the bypasses (DB or CB) may exist. Second, this is a single neurosurgery center study, and referral and selection bias may exist. Third, not all patients have done follow-up DSA due to differences in medical conditions, which might lead to biased results. Besides, long-term follow-up DSA was not available. Therefore, we were unable to investigate the long-term angiographic outcomes of the bypasses. Further studies on long-term angiographic outcomes of the bypasses are needed to confirm our conclusions.
Conclusion
This study has demonstrated that the presence of hemorrhage predicts lower patency rates. Besides, the study also found out the factors that affect the postoperative collateral formation. Younger age at operation was associated with good postoperative collateral formation, while presence of hemorrhage and dilated AChA were associated with poor postoperative collateral formation.
Data Availability Statement
The datasets analyzed in this manuscript are not publicly available. Requests to access the datasets should be directed to PG, Z2VwZWljb25nQDE2My5jb20=.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB of Beijing Tiantan Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
PG and QZ: conception, design, analysis, and interpretation of data. PG, XY, XL, JW, and XD: acquisition of data. PG: drafting the article. RW, YZ, and DZ: technical support and surgery. JZ: approved the final version of the manuscript on behalf of all authors. JZ and QZ: study supervision. All authors critically revised the article and reviewed the submitted version of the manuscript.
Funding
This study was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2015BAI12B04), the Beijing Municipal Organization Department talents project (2015000021469G219), the Beijing Municipal ST Commission (D161100003816005), the National Natural Science Foundation of China (81701137), the Beijing Municipal Administration of Hospitals' Mission Plan (SML20150501), and the Program of Beijing Municipal Science and Technology Commission (Z13110200680000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.01267/full#supplementary-material
References
1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. doi: 10.1056/NEJMra0804622
2. Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. (2016) 18:2–11. doi: 10.5853/jos.2015.01627
3. Acker G, Fekonja L, Vajkoczy P. Surgical management of moyamoya disease. Stroke. (2018) 49:476–82. doi: 10.1161/STROKEAHA.117.018563
4. Kim T, Oh CW, Bang JS, Kim JE, Cho WS. Moyamoya disease: treatment and outcomes. J Stroke. (2016) 18:21–30. doi: 10.5853/jos.2015.01739
5. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. (2008) 7:1056–66. doi: 10.1016/S1474-4422(08)70240-0
6. Pandey P, Steinberg GK. Neurosurgical advances in the treatment of moyamoya disease. Stroke. (2011) 42:3304–10. doi: 10.1161/STROKEAHA.110.598565
7. Matano F, Murai Y, Tateyama K, Tamaki T, Mizunari T, Matsukawa H, et al. Long-term patency of superficial temporal artery to middle cerebral artery bypass for cerebral atherosclerotic disease: factors determining the bypass patent. Neurosurg Rev. (2016) 39:655–61. doi: 10.1007/s10143-016-0736-5
8. Yoon S, Burkhardt JK, Lawton MT. Long-term patency in cerebral revascularization surgery: an analysis of a consecutive series of 430 bypasses. J Neurosurg. (2018) 1–8. doi: 10.3171/2018.3.JNS172158. [Epub ahead of print].
9. Duan L, Bao XY, Yang WZ, Shi WC, Li DS, Zhang ZS, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke. (2012) 43:56–60. doi: 10.1161/STROKEAHA.111.621300
10. Jeon JP, Kim JE, Cho WS, Bang JS, Son YJ, Oh CW. Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults. J Neurosurg. (2018) 128:793–9. doi: 10.3171/2016.11.JNS161688
11. Zhao Y, Yu S, Lu J, Yu L, Li J, Zhang Y, et al. Direct bypass surgery vs. combined bypass surgery for hemorrhagic moyamoya disease: a comparison of angiographic outcomes. Front Neurol. (2018) 9:1121. doi: 10.3389/fneur.2018.01121
12. Deng X, Gao F, Zhang D, Zhang Y, Wang R, Wang S, et al. Effects of different surgical modalities on the clinical outcome of patients with moyamoya disease: a prospective cohort study. J Neurosurg. (2018) 128:1327–37. doi: 10.3171/2016.12.JNS162626
13. Deng X, Gao F, Zhang D, Zhang Y, Wang R, Wang S, et al. Direct versus indirect bypasses for adult ischemic-type moyamoya disease: a propensity score-matched analysis. J Neurosurg. (2018) 128:1785–91. doi: 10.3171/2017.2.JNS162405
14. Yin H, Liu X, Zhang D, Zhang Y, Wang R, Zhao M, et al. A novel staging system to evaluate cerebral hypoperfusion in patients with moyamoya disease. Stroke. (2018) 49:2837–43. doi: 10.1161/STROKEAHA.118.022628
15. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. (1969) 20:288–99. doi: 10.1001/archneur.1969.00480090076012
16. Morioka M, Hamada J, Kawano T, Todaka T, Yano S, Kai Y, et al. Angiographic dilatation and branch extension of the anterior choroidal and posterior communicating arteries are predictors of hemorrhage in adult moyamoya patients. Stroke. (2003) 34:90–5. doi: 10.1161/01.STR.0000047120.67507.0D
17. Matsushima T, Inoue T, Suzuki SO, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients–comparison between the results of indirect and direct revascularization procedures. Neurosurgery. (1992) 31:401–5. doi: 10.1097/00006123-199209000-00003
18. Karasawa J, Kikuchi H, Furuse S, Kawamura J, Sakaki T. Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg. (1978) 49:679–88. doi: 10.3171/jns.1978.49.5.0679
19. Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, Son YJ, et al. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke. (2014) 45:3025–31. doi: 10.1161/STROKEAHA.114.005624
20. Kim SH, Lee H, Yoo M, Jin S, Lee S, Choi BS, et al. Angiographic and clinical outcomes of non-patent anastomosis after bypass surgery in adult moyamoya disease. Acta Neurochir. (2019) 161:379–84. doi: 10.1007/s00701-018-3733-3
21. Scharf J, Schmiedek P, Kemmling A, Gerigk L, Groden C, Horn P. Spontaneous recanalization of occluded standard extracranial-intracranial arterial bypass. Cerebrovasc Dis. (2007) 23:175–80. doi: 10.1159/000097056
22. Kraemer M, Karakaya R, Matsushige T, Graf J, Albrecht P, Hartung HP, et al. Efficacy of STA-MCA bypass surgery in moyamoya angiopathy: long-term follow-up of the Caucasian Krupp Hospital cohort with 81 procedures. J Neurol. (2018) 265:2425–33. doi: 10.1007/s00415-018-9031-4
23. Ha M, Choi CH, Lee JI, Cha SH, Lee SW, Ko JK. The efficacy of single barrel superficial temporal artery-middle cerebral artery bypass in treatment of adult patients with ischemic-type moyamoya disease. J Cerebrovasc Endovasc Neurosurg. (2016) 18:239–46. doi: 10.7461/jcen.2016.18.3.239
24. Lee S, Yun TJ, Yoo RE, Yoon BW, Kang KM, Choi SH, et al. Monitoring cerebral perfusion changes after revascularization in patients with moyamoya disease by using arterial spin-labeling MR imaging. Radiology. (2018) 288:565–72. doi: 10.1148/radiol.2018170509
25. Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. (2014) 45:1415–21. doi: 10.1161/STROKEAHA.113.004386
26. Jiang H, Ni W, Xu B, Lei Y, Tian Y, Xu F, et al. Outcome in adult patients with hemorrhagic moyamoya disease after combined extracranial-intracranial bypass. J Neurosurg. (2014) 121:1048–55. doi: 10.3171/2014.7.JNS132434
27. Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M, et al. High rebleeding risk associated with choroidal collateral vessels in hemorrhagic moyamoya disease: analysis of a nonsurgical cohort in the Japan Adult Moyamoya Trial. J Neurosurg. (2018) 1–8. doi: 10.3171/2017.9.JNS17576. [Epub ahead of print].
28. Funaki T, Takahashi JC, Houkin K, Kuroda S, Fujimura M, Tomata Y, et al. Effect of choroidal collateral vessels on de novo hemorrhage in moyamoya disease: analysis of nonhemorrhagic hemispheres in the Japan Adult Moyamoya Trial. J Neurosurg. (2019) 1–7. doi: 10.3171/2018.10.JNS181139. [Epub ahead of print].
29. Zhao J, Liu H, Zou Y, Zhang W, He S. Clinical and angiographic outcomes after combined direct and indirect bypass in adult patients with moyamoya disease: a retrospective study of 76 procedures. Exp Ther Med. (2018) 15:3570–6. doi: 10.3892/etm.2018.5850
30. Qian C, Yu X, Li J, Chen J, Wang L, Chen G. The efficacy of surgical treatment for the secondary prevention of stroke in symptomatic moyamoya disease: a meta-analysis. Medicine. (2015) 94:e2218. doi: 10.1097/MD.0000000000002218
Keywords: moyamoya disease, direct and combined bypass, risk factors, bypass patency, postoperative collateral formation
Citation: Ge P, Ye X, Liu X, Deng X, Wang J, Wang R, Zhang Y, Zhang D, Zhang Q and Zhao J (2019) Angiographic Outcomes of Direct and Combined Bypass Surgery in Moyamoya Disease. Front. Neurol. 10:1267. doi: 10.3389/fneur.2019.01267
Received: 23 June 2019; Accepted: 15 November 2019;
Published: 03 December 2019.
Edited by:
Ayrton R. Massaro, Hospital Sírio-Libanês, BrazilReviewed by:
Yasushi Takagi, Tokushima University, JapanJi Hoon Phi, Seoul National University Children's Hospital, South Korea
Copyright © 2019 Ge, Ye, Liu, Deng, Wang, Wang, Zhang, Zhang, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jizong Zhao, emhhb2p6MjA1QDE2My5jb20=
 Peicong Ge
Peicong Ge Xun Ye1,2,3,4,5
Xun Ye1,2,3,4,5 Xingju Liu
Xingju Liu Rong Wang
Rong Wang Dong Zhang
Dong Zhang Qian Zhang
Qian Zhang Jizong Zhao
Jizong Zhao