- 1Department of Neurology, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Radiology, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
Posterior reversible encephalopathy syndrome (PRES) is a reversible neuroradiological syndrome characterized by reversible vasogenic edema. The pathophysiological mechanism is still unclear, but PRES may be triggered by various etiologies. To date, only a few PRES cases linked to cerebrospinal fluid (CSF) hypovolemia were reported. The association between PRES and CSF hypovolemia needs to be explored. We presented a case of PRES with CSF hypovolemia as a result of an inadvertent dural puncture and reviewed the literature to identify the clinical characterization and pathophysiological mechanism of PRES following CSF hypovolemia. A total of 31 cases of PRES-CSF hypovolemia was included for analysis. The median age was 33 years, with a notable female predominance (87.1%). Fifteen patients (48.4%) didn't have either a history of hypertension nor an episode of hypertension. The most common cause of CSF hypovolemia was epidural or lumbar puncture (n = 21), followed by CSF shunt (n = 6). The median interval between the procedure leading to CSF hypovolemia and PRES was 4 days. Seizure, altered mental state, and headache were the most frequent presenting symptom. The parietooccipital pattern was most frequent (71.0%). Conservative management remains the mainstay of treatment with excellent outcomes. Three patients had a second episode of PRES. CSF hypovolemia is a plausible cause of PRES via a unique pathophysiologic mechanism including arterial hyperperfusion and venous dysfunction. Patients with CSF hypovolemia is more susceptible to PRES, which is potentially life-threatening. Given that CSF hypovolemia is a common complication of anesthetic, neurological, and neurosurgical procedures, PRES should be early considered for prompt diagnosis and appropriate management.
Introduction
Posterior reversible encephalopathy syndrome (PRES), initially described by Hinchey et al. in 1996 (1), refers to a reversible clinical and neuroradiological syndrome characterized by acute headache, seizures, visual disturbances, impaired consciousness, focal neurological deficits, or combinations of them (2). The typical finding in neuroimaging is reversible vasogenic edema in subcortical white matter dominating in the bilateral posterior parieto-occipital region (2, 3). An increasing number of predisposing factors for PRES have been recognized including eclampsia, hypertensive crisis, organ transplantation, sepsis, subarachnoid hemorrhage (SAH), autoimmune disorders, renal insufficiency, and various immunosuppressive drugs (2, 4). The mechanism of PRES remains controversial. Hypertension/hyperperfusion theory and vasoconstriction/hypoperfusion theory have been commonly proposed to explain the pathophysiology of PRES (2, 5).
Cerebrospinal fluid (CSF) hypovolemia, which is used to be referred to as intracranial hypotension (IH) synonymously, is increasingly recognized as a critical but often a misdiagnosed cause of new-onset cephalalgia (6, 7). Usually, it included IH, but it was not an unequivocal definition of IH as a normal or even an increased CSF pressure was not rare in reported cases (8). It is usually triggered by dural puncture, lumbar puncture, spinal surgery, lumboperitoneal shunt, or other spontaneous reasons (6). Atypical clinical presentations including non-orthostatic headaches, visual defects, neurocognitive decline, epilepsy, and focal neurological deficits, which are similar to PRES, have already been reported. Recently, the association between PRES and CSF hypovolemia has started to emerge in the neurology (9–17), neurosurgery (18–24), and anesthesiology literature (10–13, 16, 19, 25–35). However, the association between PRES and CSF hypovolemia has not been fully elucidated.
To our knowledge, there was no systematic review exploring the pathogenesis, clinical and imaging characteristics, and management of PRES in patients with CSF hypovolemia. Herein, a case of PRES who suffered CSF hypovolemia after an inadvertent dural puncture was presented with potential evidence of hyperperfusion. Then, a systematic analysis of published literature was undertaken to reveal the possible association between PRES and CSF hypovolemia.
Methods
The information of the patient from the department of Neurology of our hospital was collected for a preliminary analysis. The additional 30 cases (29 articles) in the PubMed and Web of Science database from inception to July 2019 using a combination with “PRES” and various terms related to CSF hypovolemia or high risks of CSF hypovolemia including “cerebrospinal fluid hypovolemia,” “intracranial hypotension,” “CSF leakage,” “epidural puncture,” “epidural anesthesia,” “spinal puncture,” “spinal anesthesia,” “lumbar puncture,” “cerebrospinal fluid shunt,” “spinal surgery,” and “cranial surgery.” A standardized form was applied to collect clinical information from each eligible article including demographic characteristics, related medical history, the probable cause of CSF hypovolemia, clinical manifestations, magnetic resonance (MR) findings (both PRES and CSF hypovolemia), treatment, and clinical outcome. The flow diagram was shown in the Supplementary Material.
Written informed consent for participation, data collection, and publication was obtained from the patient. Because this is a case report and review of literature, no research legal, and ethical approval is required.
Case Presentation
A 30-year-old woman, gravida 3 para 0, without a previous history of hypertension, presented to the Department of Obstetrics at 40 weeks' gestation. Laboratory investigations at admission remained within the normal range. Epidural analgesia was planned for painless labor. An inadvertent dural puncture occurred in the first procedure. Then, no complication was found in the repeated epidural procedure. Her blood pressure remained consistently normal throughout labor, delivery, and the immediate postpartum period. Two hours after delivery, she complained of mild neck pain that resolved after receiving 2,000 ml Ringer's solution.
On postpartum day 2, she developed a moderate postural occipital headache. In the absence of other focal neurological deficits, postdural puncture headache was diagnosed. The patient was managed with non-steroidal anti-inflammatory agents, hydration, and strictly bed rest. The epidural blood patch (EBP) was recommended as the following therapeutic measure, but the patient refused. On postpartum day 3, the patient complained of progressively worsening postural headache, nausea, and photophobia. The patient had to keep a recumbent posture to relief. The blood pressure was noted elevate to an average level of 140/85 mmHg and a highest-level of 178/96 mmHg. Nifedipine was taken to control hypertension. Then, the blood pressure was under 150/90 mmHg. On the early morning of postpartum day 4, the patient became confused when she waked up and turned to a supine position with a blood pressure of 131/90 mmHg. After a few minutes, she had a generalized tonic-clonic seizure which was controlled by diazepam. After she regained consciousness, she complained of diplopia and severe headache in occipital and left frontal region. Neurological examination revealed left abducens nerve palsy, right hemianesthesia, horizontal nystagmus, right tongue paralysis, and right Babinski sign. Diazepam and magnesium sulfate were taken with a concern that the patient was developing postpartum eclampsia. Six hours later, brain magnetic resonance imaging revealed vasogenic edema in the bilateral parieto-occipital regions, basal ganglia, and brainstem (Figures 1A–E). Convexity SAH was identified in the left frontal lobes (Figure 1C). MR angiography and venography were negative for aneurysms, venous thrombosis, and cerebral vasospasm (Figures 2A,B). The arterial spin labeling perfusion (ASL) imaging showed hyperperfusion areas in the bilateral occipitoparietal lobe (Figure 2F). On susceptibility-weighted imaging (SWI), the commonly marked hypointensity of the cerebral deep venous system was absent, suggestive of blood oxygen level dependent (BOLD) effect probably induced by cerebral hyperperfusion (Figure 2E). In addition, brain MR showed signs of intracranial hypotension including diffuse enhancement of the dura (Figures 2C,D), mild enlargement of pituitary and dural sinuses (Figure 1F), and slightly sagging of brainstem and cerebellum (Figure 1F). Thus, PRES and IH was the diagnosis. Over the following hours, the patient remained normal blood pressure and seizure-free. Magnesium sulfate infusion and diazepam were stopped. The patient was treated with intravascular rehydration which was used to prevent the progression of IH and SAH-induced cerebrovascular spasm. On postpartum day 14, the patient had a full recovery without any headache and neurological deficits. Follow-up MR imaging showed the complete disappearance of vasogenic edema, venous engorgement and convexity SAH (Figures 3A–D), together with the normalization of the signal of the deep venous system in SWI (Figure 3E) and the cerebral blood flow (CBF) in the bilateral occipitoparietal lobe (Figure 3F).
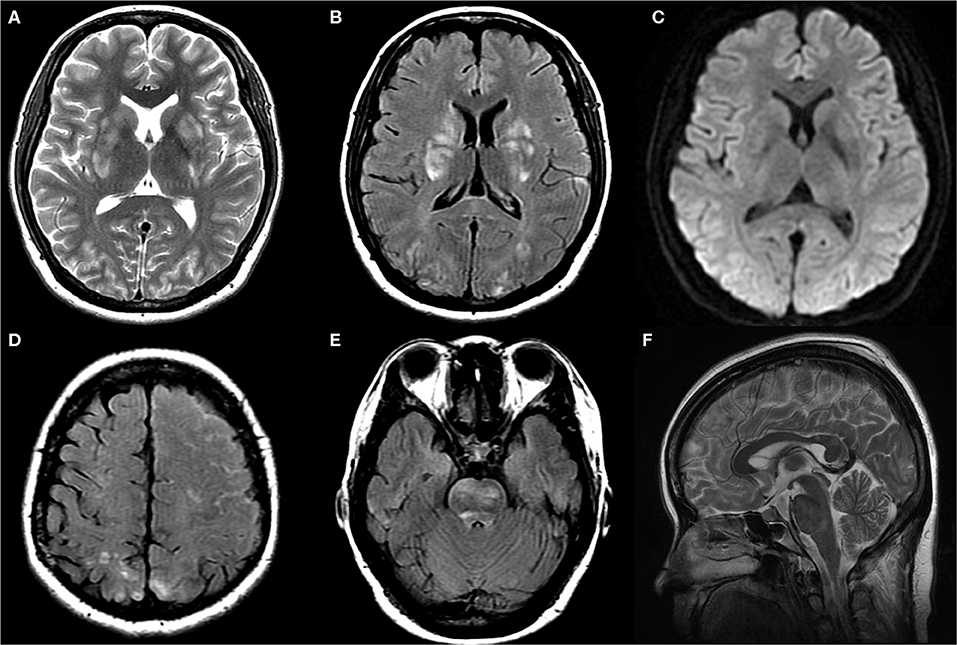
Figure 1. Axial T2WI (A), axial FLAIR (B,D,E), axial DWI (C) and sagittal T2WI (F) images at symptom onset: T2WI, FLAIR, and DWI images demonstrated hyperintensity without diffusion restriction in bilateral parieto-occipital region, basal ganglia, and brainstem. FLAIR images demonstrated left frontoparietal sulcus subarachnoid hemorrhage. Axial T2WI images demonstrated mild enlargement of pituitary and dural sinuses.
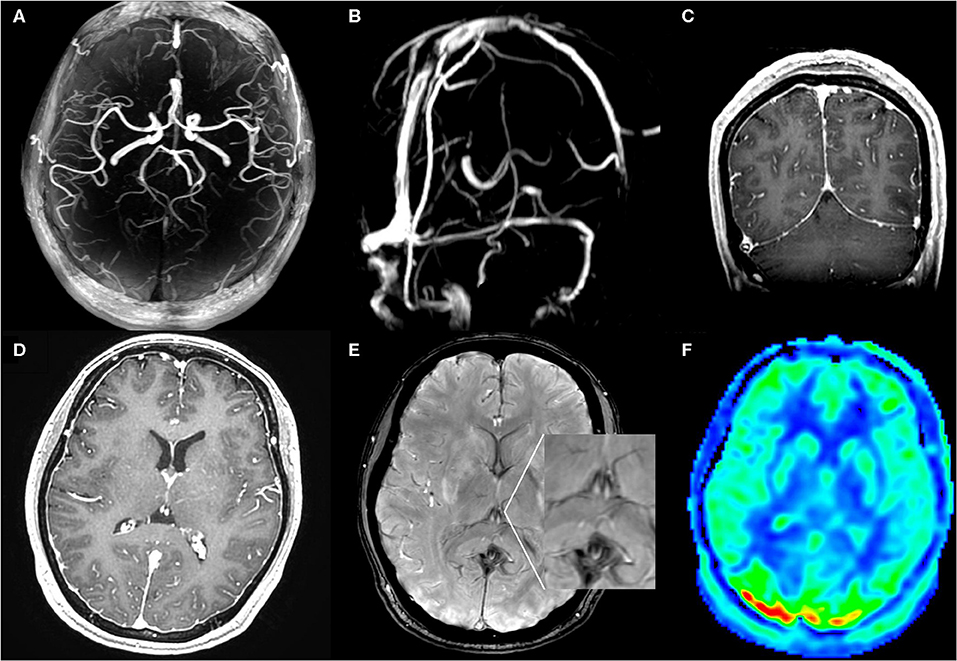
Figure 2. MR angiography (A), MR venography (B), post contrast T1WI (C,D), SWI (E), and ASL (F) MRI images at symptom onset: MR angiography and venography were negative for aneurysms, venous thrombosis, and cerebral vasospasm. SWI images showed lack of the normal hypointensity in deep venous system. Coronal and axial T1WI images with gadolinium-enhancement showed diffuse enhancement of the dura. ASL images showed hyperperfusion areas in bilateral occipitoparietal lobe.
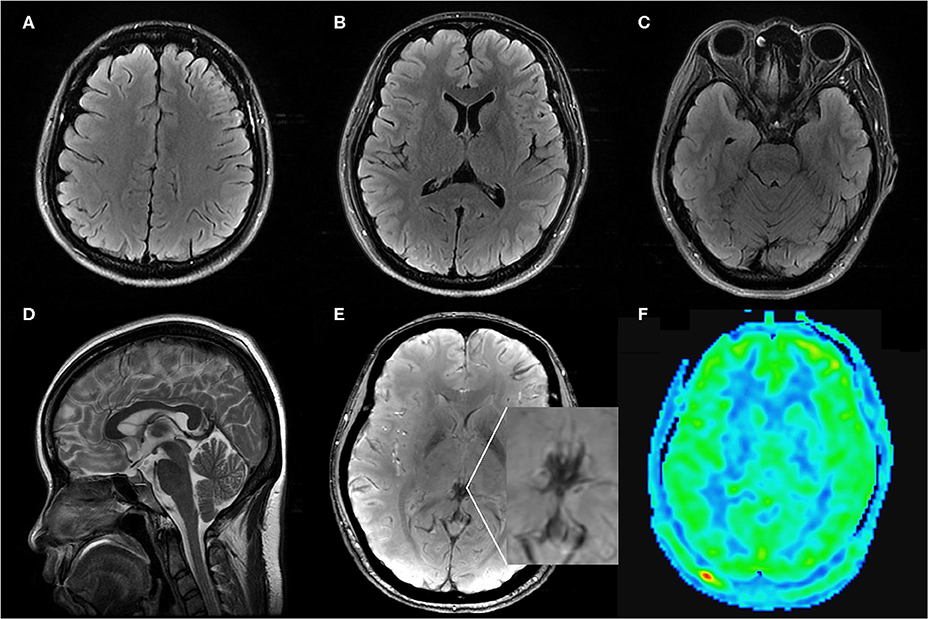
Figure 3. Axial FLAIR (A–C), sagittal T2WI (D), SWI (E), ASL (F) MRI images on follow up: Axial FLAIR imaging demonstrated complete regression of vasogenic edema and convexity SAH. Sagittal T2WI images showed regression of the engorgement of the pituitary and dural sinuses. SWI and ASL images showed the normalization of the signal of the deep venous system and the CBF in bilateral occipitoparietal lobe.
Results
In total, we collected the data on 31 patients (30 patients from literature and our patient) for descriptive analysis. The detailed data of cases were summarized in Table 1.
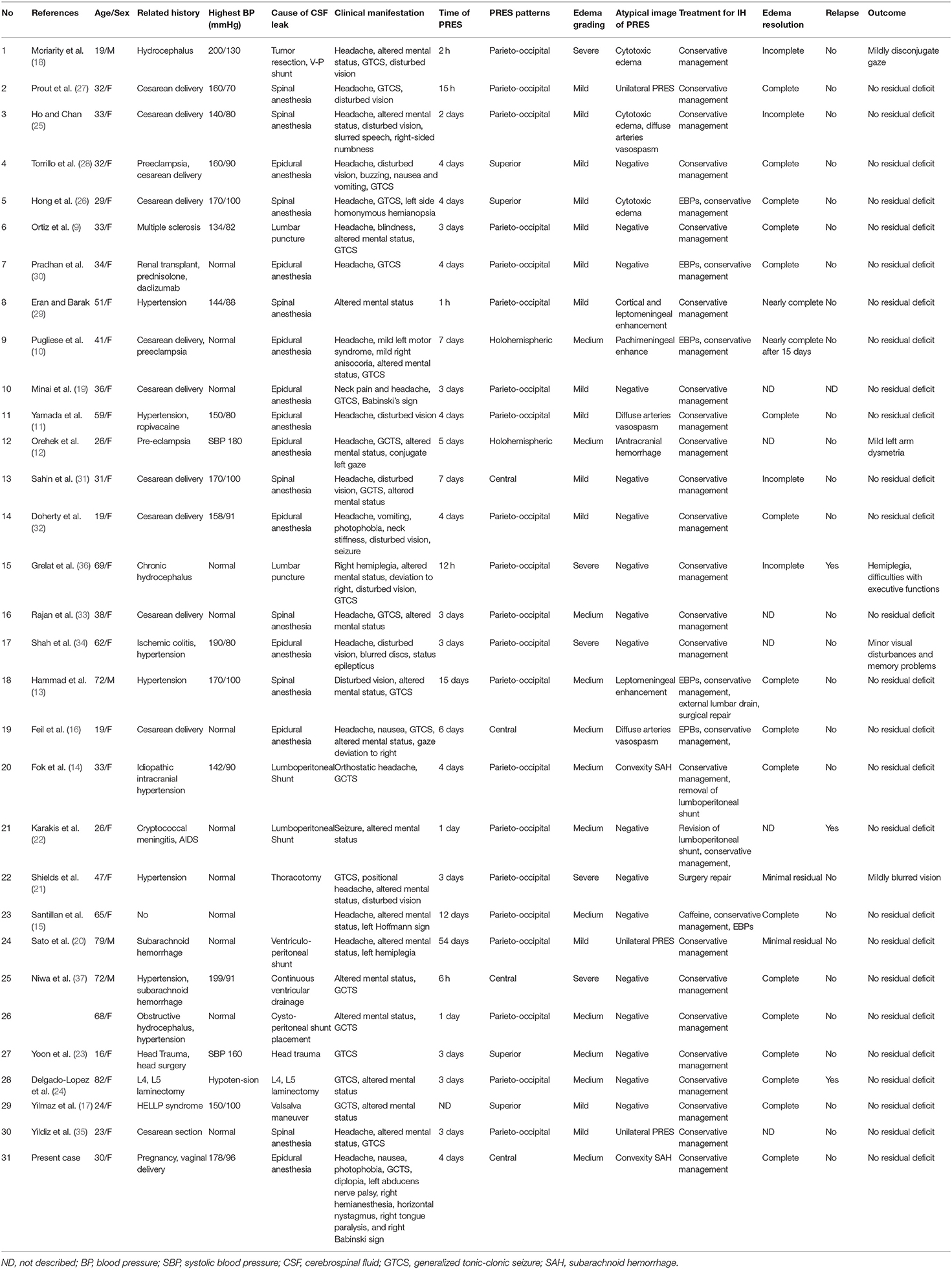
Table 1. Characteristics and clinical manifestations of cases diagnosed with PRES and CSF hypovolemia.
Clinical Characteristics
The clinical characteristics of patients with PRES and CSF hypovolemia were listed in Table 2. The median age was 33 years (range: 16–82 years). There was a female predominance (27 females, 87.1%). Thirty patients were associated with one or more known offending factors, most commonly hypertension (n = 16), pregnancy (n = 14), pre-eclampsia or eclampsia (n = 5), subarachnoid hemorrhage (n = 2). Five patients had a history of hydrocephalus or intracranial hypertension. Fifteen patients (48.4%) didn't have either a history of hypertension nor an episode of hypertension. The reduction of CSF was resulted from epidural or lumbar puncture (n = 21), CSF shunt (n = 6), spinal surgery (n = 2), head trauma (n = 1). Excluding patient 29 who had no exact date of the onset time of PRES (17), the median interval between the procedure leading to CSF reduction and the onset of PRES was 4 days, varying from 2 h to 7 weeks. Headache (71%) was the most common symptom preceding the PRES. Only one patient had a severe elevation of systolic blood pressure more than 200 mmHg. Seizure (83.9%) is the most common neurological symptom in PRES patients with CSF hypovolemia, following by headache (71.0%), altered mental state (64.5%), visual disturbances (41.9%), and hemiparesis (12.9%). Mild edema (51.6%) was most frequent, while the parieto-occipital pattern was most frequent (71.0%). In 80.6% of PRES-CSF hypovolemia patients, follow-up neuroimaging was performed. Of them, complete or nearly complete resolution of edematous lesions was noted in 80.0% of the patients, while 87.1% of the patients had a complete clinical recovery. Three of PRES-CSF hypovolemia patients had a recurrence of PRES after another experience of CSF reduction (22, 24, 36).
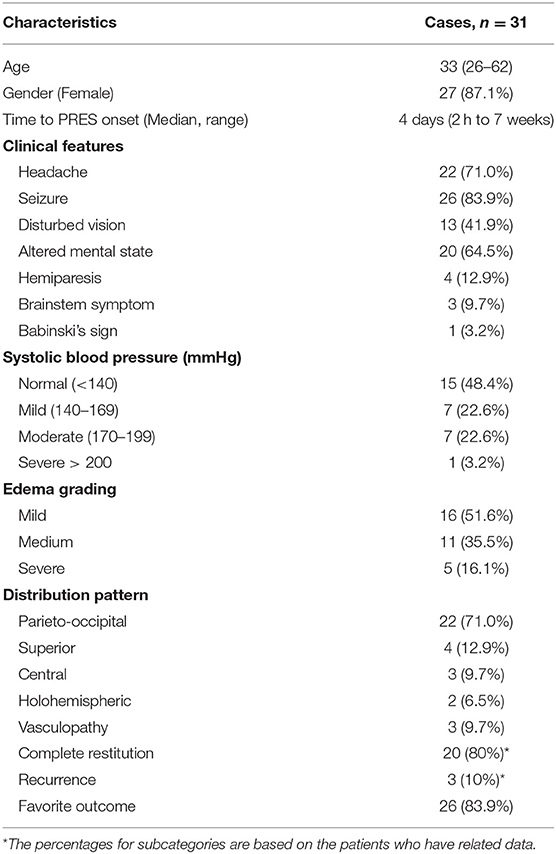
Table 2. Clinical characteristics and neuroimaging manifestations of patients with PRES and CSF hypovolemia.
Discussion
PRES is commonly described as a neuroradiological disease entity characterized by reversible vasogenic edema in the subcortical white matter of bilateral posterior parieto-occipital region with a rapid onset of neurological deficits including seizures, headache, visual disturbances, and altered mental state (2, 4). With the wide application of MR scans, PRES has been much more often recognized in the past decade. The precise pathophysiology underlying PRES is not entirely established. Two contradictory hypotheses are commonly cited (2, 5). The most recognized “Hypertension/hyperperfusion” theory, also called “vasogenic” theory, proposes that severe hypertension, which may overcome the limits of cerebral autoregulation, induces secondary cerebral hyperperfusion leading to an excess of cerebral blood flow, then alterations to the vascular permeability, disruptions to the blood-brain barrier, extravasations of plasma, and subsequent vasogenic edema (2, 5). This concept is primarily supported by the common presence of significant elevation of blood pressure in patients with PRES. Increased perfusion in the vasogenic edema area has been shown in case reports using ASL MRI or CT perfusion (38, 39). Nevertheless, 30–50% of patients with PRES show normal blood pressure or only slightly-to-moderate elevated blood pressure which may not exceed the auto-regulatory limits. The other theory “vasoconstriction/hypoperfusion” theory, or called “endothelial dysfunction” theory, purports that systemic toxicity induces endothelial dysfunction that leads to vascular instability, cerebral vasoconstriction, local hypoperfusion, and subsequent edema (5). This theory is supported by recent vessel imaging and perfusion imaging studies, which have demonstrated diffuse or focal cerebral vasoconstriction, and cerebral hypoperfusion in lesional areas (40). Other proposed theories, such as “cytotoxic” theory, “immunogenic” theory, “neuropeptide” theory, share a similar pathophysiologic mechanism with “vasoconstriction/hypoperfusion” theory (2).
Our case had no stigmata of pre-eclampsia or eclampsia, and the blood pressure maintained normal before and during delivery. She only showed an averaged MAP level of 105 mmHg and a peak mean artery pressure (MAP) level of 123 mmHg after delivery. Did hypertension lead to PRES? Our patient complained postural headache before the changes in blood pressure, and the development of hypertension was following the deterioration of headache. On the other hand, the patient only had a slight elevation of averaged MAP. Even the maximum blood pressure didn't exceed the upper MAP limits of autoregulation. Although puerperium might reduce the threshold of PRES, it is likely that hypertension is not pinpointed as the major cause of PRES. In our review, only 16 patients had hypertension (11–14, 17, 18, 23, 25–29, 31, 32, 34, 37), while only one patient had systolic blood pressure more than 200 mmHg (18). Some patients even experienced hypotension during the development of the disease (11, 24). So, patients with CSF hypovolemia have a different pathophysiological process other than hypertension.
CSF hypovolemia is characterized by orthostatic headaches which almost relive after lying down (6). It was an unequivocal definition of IH characterized by low CSF pressure ( ≤ 60 mmH2O). However, nearly half of the IH patients showed normal CSF pressure (8). Even a few patients showed a CSF pressure of more than 200 mmH2O (8). So, IH is a clinical syndrome resulting from CSF volume depletion. CSF hypovolemia was proposed to replace the definition of IH (7). The neuroradiological features include pachymeningeal enhancement, brain sagging, subdural fluid collections, pituitary hyperemia, and venous distension sign (41). Although the intracranial pressure was not measured in our case, CSF hypovolemia was well-established on clinical and neuroradiological evidence. Grelat et al. (36) reported a case of chronic hydrocephalus who presented PRES after a depletive lumbar puncture. Interestingly, the patient underwent another episode of PRES following emergency ventriculoperitoneal shunt placement. Similarly, Karakis et al. (22) presented a case of PRES in a patient with IH following lumbo-peritoneal shunt, who experienced PRES 1 week later in the setting of CSF hypovolemia resulting from CSF leakage in the lumbo-peritoneal shunt placement site. Both of them had no other trigger factors. So, it is not surprising that CSF hypovolemia plays a key role in the development of PRES via a different pathophysiology independent of hypertension. In our patient, the ASL imaging provided the evidence of cerebral hyperperfusion in basal ganglion and occipital regions. We speculated that CSF hypovolemia combined with a slight elevation of MAP precipitated PRES by inducing cerebral hyperperfusion. Cerebral perfusion pressure (CPP) is dependent on the relationship between MAP and intracranial pressure (ICP). Depends on the cerebral auto-regulation system, CPP varies from 60 to 80 mmHg. Either increased MAP or decreased ICP will lead to an increase in CPP. When the CPP overwhelms the limits of the cerebral auto-regulation system, cerebral hyperperfusion occurs. Therefore, on the base of CSF hypovolemia, either slightly elevated MAP or normal MAP can lead to cerebral hyperperfusion, endothelial dysfunction, and vasogenic edema (13, 15, 22). On the other hand, the cerebral auto-regulation system ensures a steady ICP in the encephalic space as long as possible. In accordance with the Monro–Kellie doctrine, cerebral blood flow and perfusion in cerebral arteries will firstly increase to maintain normal ICP when CSF leak. If the increased cerebral blood flow and perfusion failed to compensate for the loss of CSF completely, dural sinuses, and veins would engorge for increasing the cerebral blood volume which will lead to capillary and venous hypertension. As a result, fluids extravasated into the interstitial space and vasogenic edema occur. In addition, the brain sagging can result in mechanical traction on the vessels, particularly on the veins of Galen and straight sinus (10, 42). Indeed, the velocity of blood flow in the straight sinus was reported to be declined by an average of 47% in supine patients during and shortly after lumbar punctures (43). Therefore, it impairs the deep venous drainage, induces venous hypertension in the deep venous system, and leads to vasogenic edema dominating in the basal ganglia and occipital regions. To summarize, a combination of arterial hyperperfusion and venous dysfunction may be the pathophysiological link between PRES and CSF hypovolemia.
Some authors hypothesized that reversible cerebral vasoconstriction syndrome (RCVS) secondary to the mechanical stimuli of the sagging of the brain and its affiliations would trigger PRES (11, 16, 25). The pathophysiological mechanism and clinical manifestations of PRES and RCVS partially overlap (16). They share similar triggers, including postpartum, drugs, autoimmune disease, and transplantation. The activation of the adrenergic system is presumed to be key of the development of both diseases (16). In the literature, PRES was observed in nearly 9% of the RCVS patients (44). Vasoconstriction was found in up to 30% of patients with PRES (45). However, cerebral vasoconstriction was found only in three of the patients with PRES and CSF hypovolemia. What draws more attention is that the frequency of RCVS in patients with CSF hypovolemia is particularly low. In a MR-angiography study of a series of 56 patients with IH, only one patient was reported to show segmental stenosis of cerebral arteries (46). There was no evidence of RCVS in our case. As a result, we hypothesize that vasoconstriction/hypoperfusion is not the common etiology of PRES in patients with CSF hypovolemia.
In general, PRES is regarded as a benign disease with favorable outcomes (2, 47). Complete resolution of vasogenic edema and full recovery of neurological deficit were observed in 70–90% of patients. In fact, the poor prognosis was reported in nearly 26–36% cases. Meanwhile, the fatal outcome was documented in 8–17% cases (2). Early identification and rational treatments are crucial to reduce morbidity and mortality. The diagnosis of PRES was usually delayed in patients with CSF hypovolemia until the patients presented with epilepsy and encephalopathy. The most common initial clinical presentations of PRES in patients with CSF hypovolemia is headache which usually misleads to a diagnosis of postdural puncture headache, intracranial hypotension, or pain-related headache. In this regard, the symptom of headache was found to be not of value in the diagnosis of PRES in a retrospective study (48). Only the symptoms of visual disturbances, epilepsy, and encephalopathy are the reasonable predictor of PRES. So, in patients with substantial risk factors of CSF hypovolemia including dural puncture, lumbar puncture, lumboperitoneal shunt, ventriculoperitoneal shunt, and spinal surgery, PRES should be early considered when the clinical manifestations (e.g., epilepsy, visual disturbances, impaired consciousness, focal neurological deficits, resistant headache) could not be entirely explained by CSF hypovolemia, hypertension or other medical condition alone. Multi-spectral MRI sequences, including diffusion-weighted imaging (DWI) imaging, ASL imaging, SWI, and MR angiography, should be performed immediately to establish the diagnosis early, and prevent poor prognosis.
Clinical managements of PRES are based on the elimination of underlying trigger factors and immediate control of epilepsy. Due to the differences in pathogenesis, the treatment strategy for patients with CSF hypovolemia may differ from those with other etiology. Compared with other etiologies, PRES patients with CSF hypovolemia were likely to have a shorter median time from CSF loss to PRES onset, which support a direct link between the CSF hypovolemia and PRES. The time between the procedure that incited CSF loss and the ictus of the PRES syndrome may depend on the baseline ICP and the speed of the reduction of CSF volume or ICP. We found that seven patients experienced PRES within 1 day. Of them, five patients had intracranial hypertension before PRES onset; all of them had a rapid loss of CSF or a rapid reduction of ICP. One patient with chronic hydrocephalus developed PRES 2 h after a rapid CSF loss of 50 ml. The other patient developed PRES 6 h after a 2 h inadvertent overdrainage of 200 ml CSF. These two patients experienced PRES recurrence rapidly after another rapid reduction of CSF volume. On the other hand, a marked increase in blood pressure may contribute to the development of PRES. Patients who experienced a systolic blood pressure more than 179 mmHg had a shorter interval of PRES onset. Base on the evidence from the reviewed reports, we propose the following recommendations: First, a precipitous reduction of CSF volume or ICP should be avoided. A graded reduction of ICP is strongly recommended in patients with intracranial hypertension, especially in patients with extremely high CSF pressures. Second, in patients with CSF hypovolemia, the treatment of CSF hypovolemia should be initiated at the early stage of the disease (49). CSF hypovolemia often recovered spontaneously. Conservative medical management could be processed, including strict supine positioning, ample hydration, analgesia, and non-steroidal drugs. Caffeine and steroids should be avoided due to the risks of RCVS which may induce PRES (9, 15). When the conservative measures failed to bring alleviation of the symptoms or in patients who present moderate and severe CSF hypovolemia, epidural blood patching is recommended as the mainstay of first-line treatment (49, 50). Surgical repair should be considered for patients with clearly identified leak sites and no response to non-surgical treatment and EBPs (50). Third, tight blood pressure control is recommended for patients with CSF hypovolemia due to the increased susceptibility to PRES with a slightly elevated MAP or even normal MAP (13, 15).
Conclusion
The present case and reviewed literature highlight the pathophysiological link between PRES and CSF hypovolemia. Both arterial hyperperfusion and venous dysfunction may contribute to the development of PRES in patients with CSF hypovolemia. PRES should be early considered in patients with a high risk of CSF hypovolemia when the clinical manifestations can not be explained by CSF hypovolemia or other conditions alone. Precipitous reduction of CSF should be avoided, while appropriate treatments of CSF hypovolemia should be initiated early. The blood pressure should be strictly controlled in patients with CSF hypovolemia to prevent the development of PRES and improve the clinical outcome.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
FF designed the study. YZ and XW collected clinical data and wrote the manuscript. YC and YL searched the literature and edited the pictures. GZ revised the manuscript. All authors contributed to the manuscript revision and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00591/full#supplementary-material
References
1. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. (1996) 334:494–500. doi: 10.1056/NEJM199602223340803
2. Liman TG, Siebert E, Endres M. Posterior reversible encephalopathy syndrome. Curr Opin Neurol. (2019) 32:25–35. doi: 10.1097/WCO.0000000000000640
3. Brady E, Parikh NS, Navi BB, Gupta A, Schweitzer AD. The imaging spectrum of posterior reversible encephalopathy syndrome: a pictorial review. Clin Imaging. (2018) 47:80–9. doi: 10.1016/j.clinimag.2017.08.008
4. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. (2015) 14:914–25. doi: 10.1016/S1474-4422(15)00111-8
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. (2008) 29:1043–9. doi: 10.3174/ajnr.A0929
6. Chung SJ, Kim JS, Lee MC. Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology. (2000) 55:1321–7. doi: 10.1212/WNL.55.9.1321
7. Mokri B. Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia–evolution of a concept. Mayo Clin Proc. (1999) 74:1113–23. doi: 10.4065/74.11.1113
8. Yao LL, Hu XY. Factors affecting cerebrospinal fluid opening pressure in patients with spontaneous intracranial hypotension. J Zhejiang Univ Sci B. (2017) 18:577–85. doi: 10.1631/jzus.B1600343
9. Ortiz GA, Bianchi NA, Tiede MP, Bhatia RG. Posterior reversible encephalopathy syndrome after intravenous caffeine for post-lumbar puncture headaches. AJNR Am J Neuroradiol. (2009) 30:586–7. doi: 10.3174/ajnr.A1321
10. Pugliese S, Finocchi V, Borgia ML, Nania C, Della Vella B, Pierallini A, et al. Intracranial hypotension and PRES: case report. J Headache Pain. (2010) 11:437–40. doi: 10.1007/s10194-010-0226-z
11. Yamada SM, Kitagawa R, Teramoto A. A case of reversible posterior leukoencephalopathy syndrome with acute hypotension. Neurol Sci. (2011) 32:165–8. doi: 10.1007/s10072-010-0417-z
12. Orehek EK, Burns JD, Koyfman F, Azocar RJ, Holsapple JW, Green DM. Postpartum trifecta: simultaneous eclamptic intracerebral hemorrhage, PRES, and herniation due to intracranial hypotension. Neurocrit Care. (2012) 17:434–8. doi: 10.1007/s12028-012-9742-9
13. Hammad T, DeDent A, Algahtani R, Alastal Y, Elmer L, Medhkour A, et al. Posterior reversible encephalopathy syndrome secondary to CSF leak and intracranial hypotension: a case report and literature review. Case Rep Neurol Med. (2015) 2015:538523. doi: 10.1155/2015/538523
14. Fok A, Chandra RV, Gutman M, Ligtermoet M, Seneviratne U, Kempster P. Posterior reversible encephalopathy syndrome and subarachnoid hemorrhage after lumboperitoneal shunt for fulminant idiopathic intracranial hypertension. J Neuroophthalmol. (2016) 36:164–6. doi: 10.1097/WNO.0000000000000355
15. Santillan A, Aamodt W, Bhavaraju-Sanka R. Pearls & Oy-sters: Spontaneous intracranial hypotension and posterior reversible encephalopathy syndrome. Neurology. (2016) 86:e55–7. doi: 10.1212/WNL.0000000000002349
16. Feil K, Forbrig R, Thaler FS, Conrad J, Heck S, Dorn F, et al. Reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome associated with intracranial hypotension. Neurocrit Care. (2017) 26:103–8. doi: 10.1007/s12028-016-0320-4
17. Yilmaz Z, Voyvoda N, Sirinocak PB, Terzi H. Association of hemolysis, elevated liver enzymes, low platelets syndrome with posterior reversible encephalopathy and intracranial hypotension. Neurol India. (2018) 66:226–7. doi: 10.4103/0028-3886.222856
18. Moriarity JL Jr, Lim M, Storm PB, Beauchamp NJ Jr, Olivi A. Reversible posterior leukoencephalopathy occurring during resection of a posterior fossa tumor: case report and review of the literature. Neurosurgery. (2001) 49:1237–9; discussion 9–40. doi: 10.1227/00006123-200111000-00040
19. Minai FN, Hasan SF, Sheerani M. Post-dural puncture posterior reversible encephalopathy syndrome. J Coll Physicians Surg Pak. (2011) 21:37–9.
20. Sato H, Koizumi T, Sato D, Endo S, Kato S. [Unilateral posterior reversible encephalopathy syndrome after ventriculo-peritoneal shunt for normal pressure hydrocephalus following subarachnoid hemorrhage: a case report]. No Shinkei Geka. (2016) 44:507–15. doi: 10.11477/mf.1436203320
21. Shields LB, Johnson JR, Shields CB. Posterior reversible encephalopathy syndrome following a thoracic discectomy-induced dural leak: case report. J Neurosurg Spine. (2016) 25:586–90. doi: 10.3171/2016.4.SPINE1623
22. Karakis I, Nuccio AH, Amadio JP, Fountain AJ Jr. The Monro-Kellie doctrine in action: posterior reversible leukoencephalopathy syndrome caused by intracranial hypotension from lumboperitoneal shunt placement. World Neurosurg. (2017) 98:868.e11–e5. doi: 10.1016/j.wneu.2016.12.046
23. Yoon JE, Lee CY, Kim HW. Posterior reversible encephalopathy syndrome after head trauma surgery in pediatric patient without any underlying disease. Korean J Neurotrauma. (2017) 13:167–70. doi: 10.13004/kjnt.2017.13.2.167
24. Delgado-Lopez PD, Garces-Perez G, Garcia-Carrasco J, Alonso-Garcia E, Gomez-Menendez AI, Martin-Alonso J. Posterior reversible encephalopathy syndrome with status epilepticus following surgery for lumbar stenosis and spondylolisthesis. World Neurosurg. (2018) 116:309–15. doi: 10.1016/j.wneu.2018.05.174
25. Ho CM, Chan KH. Posterior reversible encephalopathy syndrome with vasospasm in a postpartum woman after postdural puncture headache following spinal anesthesia. Anesth Analg. (2007) 105:770–2. doi: 10.1213/01.ane.0000278128.26896.b2
26. Hong J-Y, Jee YS, Lee IH, Shin JS, Choi HJ. Posterior reversible encephalopathy syndrome after cesarean section under spinal anesthesia -a case report. Korean J Anesthesiol. (2007) 52:S86. doi: 10.4097/kjae.2007.52.6.S86
27. Prout RE, Tuckey JP, Giffen NJ. Reversible posterior leucoencephalopathy syndrome in a peripartum patient. Int J Obstet Anesth. (2007) 16:74–6. doi: 10.1016/j.ijoa.2006.04.012
28. Torrillo TM, Bronster DJ, Beilin Y. Delayed diagnosis of posterior reversible encephalopathy syndrome (PRES) in a parturient with preeclampsia after inadvertent dural puncture. Int J Obstet Anesth. (2007) 16:171–4. doi: 10.1016/j.ijoa.2006.08.015
29. Eran A, Barak M. Posterior reversible encephalopathy syndrome after combined general and spinal anesthesia with intrathecal morphine. Anesth Analg. (2009) 108:609–12. doi: 10.1213/ane.0b013e31818f635e
30. Pradhan A, Jairam A, Kumar RS, Srivastava A, Srivastava A, Sreevastava D, et al. Posterior reversible encephalopathy syndrome posttransplantation: a case report of possible association with cerebrospinal fluid leak after epidural catheterization. Transplant Proc. (2009) 41:1957–60. doi: 10.1016/j.transproceed.2008.12.037
31. Sahin N, Maral J, Celik E, Solak A, Genc B, Kalaycioglu S, et al. Atypical presentation of posterior reversible encephalopathy syndrome after post-dural puncture headache. Turk J Anesthesiol Reanim. (2013) 41:142–5. doi: 10.5152/TJAR.2013.29
32. Doherty H, Hameed S, Ahmed I, Russell IF. Post-dural puncture headache and posterior reversible encephalopathy syndrome: a misdiagnosis or co-presentation? Int J Obstet Anesth. (2014) 23:279–82. doi: 10.1016/j.ijoa.2014.02.003
33. Rajan S, Puthenveettil N, Paul J, Kumar L. Posterior reversible encephalopathy syndrome following caesarean section under spinal anaesthesia. Indian J Anaesth. (2014) 58:762–5. doi: 10.4103/0019-5049.147179
34. Shah R, Kubisz-Pudelko A, Reid J. Posterior reversible encephalopathy syndrome following an inadvertent dural puncture during an emergency laparotomy for ischemic colitis - a case report. Local Reg Anesth. (2014) 7:1–4. doi: 10.2147/LRA.S57660
35. Yildiz E, Öner Ö, Çetiner M, Tokur ME, Koç A, Alan K. Posterior reversible encephalopathy syndrome after cesarean section: a case report. Turk J Intens Care. (2019) 17:226–30. doi: 10.4274/tybd.galenos.2019.19870
36. Grelat M, Debaux JB, Sautreaux JL. Posterior reversible encephalopathy syndrome after depletive lumbar puncture: a case report. J Med Case Rep. (2014) 8:261. doi: 10.1186/1752-1947-8-261
37. Niwa R, Oya S, Nakamura T, Hana T, Matsui T. Rapid intracranial pressure drop as a cause for posterior reversible encephalopathy syndrome: two case reports. Surg Neurol Int. (2017) 8:103. doi: 10.4103/sni.sni_55_17
38. Hedna VS, Stead LG, Bidari S, Patel A, Gottipati A, Favilla CG, et al. Posterior reversible encephalopathy syndrome (PRES) and CT perfusion changes. Int J Emerg Med. (2012) 5:12. doi: 10.1186/1865-1380-5-12
39. Sakashita Y, Hamada T, Machiya T, Yamada M. Acute intermittent porphyria presenting as posterior reversible encephalopathy syndrome with hyperperfusion in bilateral occipital lobes: a case report. J Neurol Sci. (2017) 377:47–9. doi: 10.1016/j.jns.2017.03.052
40. Brubaker LM, Smith JK, Lee YZ, Lin W, Castillo M. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol. (2005) 26:825–30. doi: 10.1080/02841850510021292
41. Urbach H. Intracranial hypotension: clinical presentation, imaging findings, and imaging-guided therapy. Curr Opin Neurol. (2014) 27:414–24. doi: 10.1097/WCO.0000000000000105
42. Savoiardo M, Minati L, Farina L, De Simone T, Aquino D, Mea E, et al. Spontaneous intracranial hypotension with deep brain swelling. Brain J Neurol. (2007) 130:1884–93. doi: 10.1093/brain/awm101
43. Canhao P, Batista P, Falcao F. Lumbar puncture and dural sinus thrombosis–a causal or casual association? Cerebrovasc Dis. (2005) 19:53–6. doi: 10.1159/000081912
44. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain J Neurol. (2007) 130:3091–101. doi: 10.1093/brain/awm256
45. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. (2010) 85:427–32. doi: 10.4065/mcp.2009.0590
46. Schievink WI, Maya MM, Chow W, Louy C. Reversible cerebral vasoconstriction in spontaneous intracranial hypotension. Headache. (2007) 47:284–7. doi: 10.1111/j.1526-4610.2006.00696.x
47. Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry. (2010) 81:773–7. doi: 10.1136/jnnp.2009.189647
48. Faille LD, Fieuws S, Van Paesschen W. Clinical predictors and differential diagnosis of posterior reversible encephalopathy syndrome. Acta Neurol Belg. (2017) 117:469–75. doi: 10.1007/s13760-017-0750-6
49. Lin JP, Zhang SD, He FF, Liu MJ, Ma XX. The status of diagnosis and treatment to intracranial hypotension, including SIH. J Headache Pain. (2017) 18:4. doi: 10.1186/s10194-016-0708-8
Keywords: posterior reversible encephalopathy syndrome, cerebrospinal fluid hypovolemia, intracranial hypotension, dural puncture, epidural analgesia, cerebral hyperperfusion
Citation: Zheng Y, Weng X, Fu F, Cao Y, Li Y, Zheng G and Chen W (2020) Cerebrospinal Fluid Hypovolemia and Posterior Reversible Encephalopathy Syndrome. Front. Neurol. 11:591. doi: 10.3389/fneur.2020.00591
Received: 19 September 2019; Accepted: 22 May 2020;
Published: 23 June 2020.
Edited by:
Bo Gao, Affiliated Hospital of Guizhou Medical University, ChinaReviewed by:
Chenguang Zhou, Fifth Affiliated Hospital of Zhengzhou University, ChinaBrian Jeremy Williams, University of Louisville, United States
Copyright © 2020 Zheng, Weng, Fu, Cao, Li, Zheng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Chen, MTM2NzY3NzUyNjdAMTYzLmNvbQ==
†These authors share first authorship
 Yuan-yuan Zheng1†
Yuan-yuan Zheng1† Xiong-peng Weng
Xiong-peng Weng Guo-qing Zheng
Guo-qing Zheng Wei Chen
Wei Chen