- 1Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Neurology, China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 3Central Laboratory, Department of Neurology and Institute of Neurology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 4Key Laboratory of the Ministry of Education for Medicinal Resources and Natural Pharmaceutical Chemistry, National Engineering Laboratory for Resource Development of Endangered Crude Drugs in Northwest of China, College of Life Sciences, Shaanxi Normal University, Xi'an, China
To explore Dawson's fingers in cerebral small vessel disease (CSVD) and factors related to the development of Dawson's finger, we collected and analyzed clinical data of 65 patients with CSVD. We found a venous abnormality feature called Dawson's fingers around the ventricles in magnetic resonance images (MRIs) of 20 out of 65 patients with CSVD (30. 8%). A significant association between Dawson's fingers and diabetes mellitus (DM) was also detected (30 vs. 8.9%, P < 0.05). CSVD patients with Dawson's fingers had significantly increased cerebral microbleeds (CMB) (44.2 vs. 75.0%, p < 0.05), lacunae (66.7 vs. 95.0%, p < 0.05), and white matter hyperintensity (WMH) (p < 0.05) damage, and these patients exhibited significant cognitive domain impairment as assessed via Montreal Cognitive Assessment (MoCA) (18.9 ± 1.8 vs. 24.0 ± 0.8, p < 0.05) and Mini-Mental State Examination (MMSE) (24.5 ± 1.1 vs. 26.6 ± 0.6, p < 0.05). Our results show a distinctly high incidence of Dawson's fingers in CSVD patients and identify a significant association with DM, thus yielding insights about the appropriate use of Dawson's fingers, a venous imaging marker, to explore the basic pathophysiology of CSVD.
Introduction
Cerebral small vessel disease (CSVD) refers to a broad class of cerebrovascular diseases that affect the small arterioles, venules, and capillaries with a variety of different causes (1, 2). Age-related and hypertension-related CSVD are the most common forms (3, 4). Despite its diverse pathogenesis modes, the consequences of CSVD on brain parenchyma elicit similar neuroimaging markers, including lacunes, white matter hyperintensity (WMH), enlarged perivascular space (EPVS), and cerebral microbleeds (CMB) (2). In general, small vessels cannot be visualized in computed tomography angiography (CTA) or magnetic resonance angiography (MRA), but the corresponding parenchymal lesions of small vessels can be captured on magnetic resonance imaging (MRI). The STandards for Reporting Vascular changes on nEuroimaging (STRIVE) is an international neuroimaging standard that classifies and defines CSVD markers (5). For the diagnosis of CSVD, MRI is more specific than clinical criteria. The characteristics of neuroimaging markers are indicative of etiological analysis (5). Notably, arteriolosclerosis is associated with the pathogenesis of sporadic CSVD, yet relatively little attention has been focused on potential roles of venules in CSVD. Thus, the role of venules in the pathogenesis of CSVD, as well as the potential for use of venule abnormalities as imaging markers for diagnosing CSVD, remain unclear.
Central vein sign (CVS), a vein positioned centrally in brain white matter lesions (WMLs), has been considered to be an imaging marker for multiple sclerosis (MS) (6–8), a chronic demyelination and neurodegenerative disease caused by central nervous system (CNS) inflammation. Some studies show that CVS can distinguish MS from mimic diseases (9, 10) including CSVD (11–16). Dawson's fingers are elongated, flame-shaped lesions perpendicular to the lateral ventricle wall on fluid attenuated inversion recovery (FLAIR)/T2 weighted images. T2-weighted/FLAIR MRI allows visibility of venule within WMLs, thus representing inflammatory activity surrounding venule (17). It makes sense that Dawson's fingers are widely used as important imaging markers for the diagnosis and differential diagnosis of MS (18, 19). Complicating matters, CSVD shares some imaging features with MS, including white matter demyelination and brain atrophy. Demyelination around the ventricle is a useful imaging marker for distinguishing MS from other diseases (18). Dawson's fingers, indicating perivascular inflammation around veins and venules, has close pathological association with CVS. Previous studies showed Dawson's fingers can distinguish MS from neuromyelitis optica spectrum disorder (18, 20) and MOG antibody disease (20), but the condition of Dawson's fingers in CSVD is still unknown.
This study aimed to (1) determine the proportion of Dawson's fingers in CSVD; (2) explore the factors contributing to Dawson's fingers in patients with CSVD; (3) determine the imaging and clinical characteristic of CSVD patients with Dawson's fingers; and (4) explore if Dawson's fingers can be used as a specific sign to distinguish MS from CSVD.
Methods
Patients and Population
This was a prospective observational study. Patients were consecutively recruited from Tianjin Medical University General Hospital and Beijing Tiantan Hospital. Only patients with clinically manifested CSVD and at least one of the imaging features (lacuna, WMH, EPVS, and CMBs) were eligible to participate in this study. MS was diagnosed using 2017 McDonald criteria. Main exclusion criteria were patients with (1) unidentified vascular risk factors for CSVD such as a history of hypertension, hypercholesterolemia, diabetes mellitus (DM), smoking, and alcohol consumption; (2) evidence of other diseases that could potentially also cause WMLs; and (3) contraindications to MRI scanning.
Standard Protocol Approvals, Registrations, and Patient Consents
The Ethics Committees of Tianjin Medical University and Beijing Tiantan Hospital approved this study. We obtained informed consent from all the participants.
Image Acquisition Protocol
All brain MRI was performed on a 3.0-T scanner (Magnetom Trio Tim; Siemens). The routing sequences included T2, FLAIR, T1, diffusion-weighted imaging (DWI), and susceptibility-weighted imaging (SWI) (Supplemental Table 1). All MRIs were assessed for the presence, location, and size of lesions (WMH, enlarged perivascular space [EPVS], lacunes, microbleeds) according to the STandards for Reporting Vascular Changes on nEuroimaging (STRIVE) recommendations. The total SVD score (range 0–4) was calculated according to the individual imaging features by awarding points as follows: 1 for any lacuna, 1 for any microbleed, 1 for moderate-to-severe EPVS in the basal ganglia (EPVS >10), and 1 for WMHs (deep tissue: Fazekas score 2 or 3 and/or periventricular: Fazekas score 3). Individual WM lesion masks were manually delineated based on their T2 images using MRIcro software (http://www.cabiatl.com/mricro/mricro/). WMH lesion volume was calculated within the masks.
Dawson's finger, an elongated, flame-shaped WMH lesion perpendicular to the lateral ventricle wall, was here defined based on T2/FLAIR images. All assessments were executed independently by two trained neurologists who were blinded to the clinical details. The interclass correlation coefficient was 0.86. If there was a discrepancy between the two raters, assessment from a third rater was used.
Clinical Assessment
Each patient underwent clinical assessment by two neurologists. The demographic factors, clinical factors, and vascular risk factors evaluated included the following: age, sex, hypertension [systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure (DBP) >90 mmHg], DM (plasma glucose 2 h after a meal ≥11.1 mmol/L or fasting plasma glucose ≥ 7.0 mmol/L), hyperlipidemia (triglycerides >1.7 mmol/L or serum total cholesterol level >5.72 mmol/L), Modified Rankin Scale (mRS), and disease duration, current smoking (21). Laboratory examination results also included atrial fibrillation, as well as assessment of cognition [Montreal Cognitive Assessment (MoCA) and Mini-mental State Examination (MMSE)] of the CSVD patients. All clinical and laboratory factors were fully assessed in all participants.
Statistical Analysis
Continuous variables with normal distributions, such as age and lesion volumes, are presented as the mean ± standard error of mean. All continuous variables were compared using the Student's t-tests for independent samples. Those with non-normal distributions are presented as medians, and interquartile range (IQR) for non-parametric data are quoted. In the univariable analyses, we used χ2-tests for lacunes, CMBs, WMH, and EPVS (Fisher's exact test when the expected value was <5) between patients with or without Dawson's fingers. For variables that were non-normally distributed, Mann-Whitney or the Kruskal-Wallis tests were used, as appropriate, or the data were log transformed for parametric testing. ROC curves were used to evaluate the diagnostic value of Dawson's fingers. Statistical Product and Service Solutions (SPSS) for Windows version 17.0 software (Chicago, IL, USA) was used for the analyses. Values of p < 0.05 were considered statistically significant.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon request.
Result
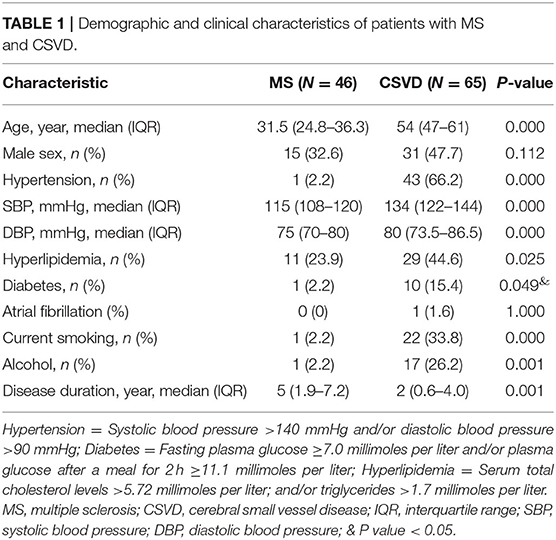
Dawson's Fingers Appear in Patients With CSVD
Most often, CSVD is considered to be an arterial-related disease based only on radiological phenotypes; signs of CSVD on conventional MRI markers include CMB, lacunes, WMH, and moderate to severe EPVS (5). However, minimal attention has been paid to venous abnormalities in patients with CSVD. To verify the condition of venous vessels in patients with CSVD, we assessed the appearance of Dawson's fingers among 65 patients with CSVD. The baseline characteristics of the CSVD and MS groups are displayed in Table 1. We found a venous abnormality feature identified as Dawson's fingers around the ventricles in the MRI of CSVD patients (Figure 1). Dawson's fingers were present in 20 of 65 patients with CSVD (30.8%). There were, however, more patients with MS (32/46) than CSVD (20/65) that had Dawson's fingers (69.6 vs. 30.8%, P < 0.001); thus, Dawson's fingers can still be used as a neuroimaging marker to distinguish between CSVD and MS. The area under the ROC curve for Dawson's fingers was 0.694 (P = 0.001) (sensitivity 71% and specificity 69%).
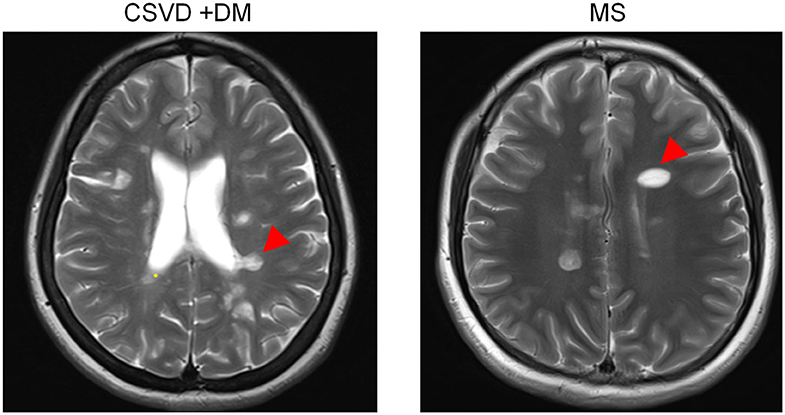
Figure 1. Dawson's fingers of cerebral small vessel disease (CSVD) patients with diabetes mellitus (DM). The venous abnormality feature Dawson's fingers around the ventricles in the fluid attenuated inversion recovery (FLAIR) of both multiple sclerosis (MS) and CSVD patients (arrowheads). Dawson's fingers can be used as a neuroimaging marker of MS and can distinguish between CSVD and MS, but the utility of this marker decreased in patients with DM.
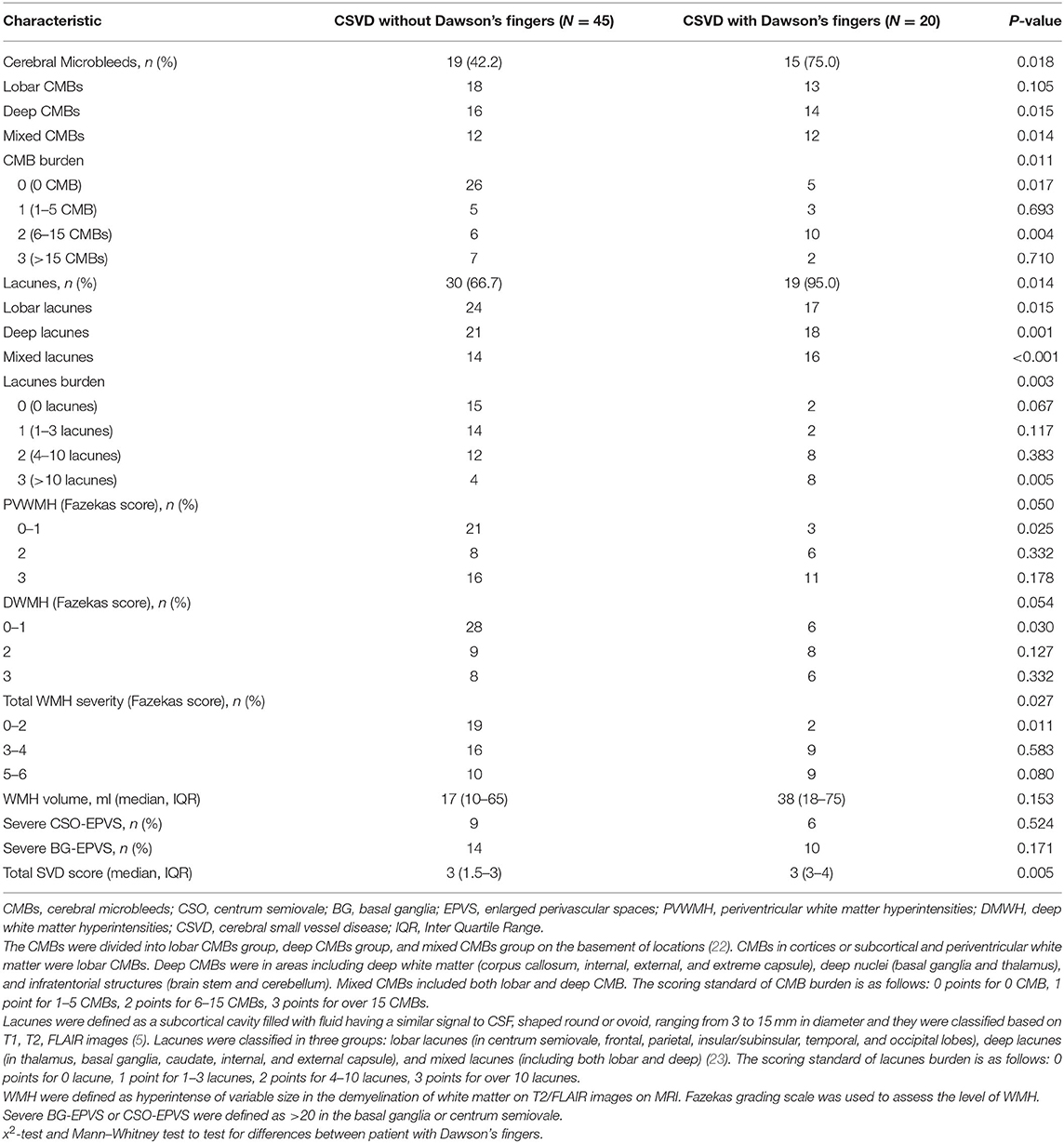
Demographics and Vascular Risk Factors in Patients With and Without Dawson's Fingers
To explore the factors contributing to Dawson's fingers in CSVD, we performed demographic and vascular risk factor analysis of CSVD patients with and without Dawson's fingers. With the exception of patients with DM, these analyses did not identify differences between CSVD patients with Dawson's fingers compared to patients without Dawson's fingers in demographic factors, number of attacks, or disease duration, or with other vascular risk factors (e.g., hypertension, hyperlipidemia). There was a statistically significant difference between DM status and CSVD patients with (6/20) or without (4/45) Dawson's fingers (30 vs. 8.9%, P < 0.05, Table 2). The utility of Dawson's fingers to distinguish between CSVD from MS (32/46) decreased in CSVD patients with DM (6/10) (AUC = 0.454; P > 0.05).
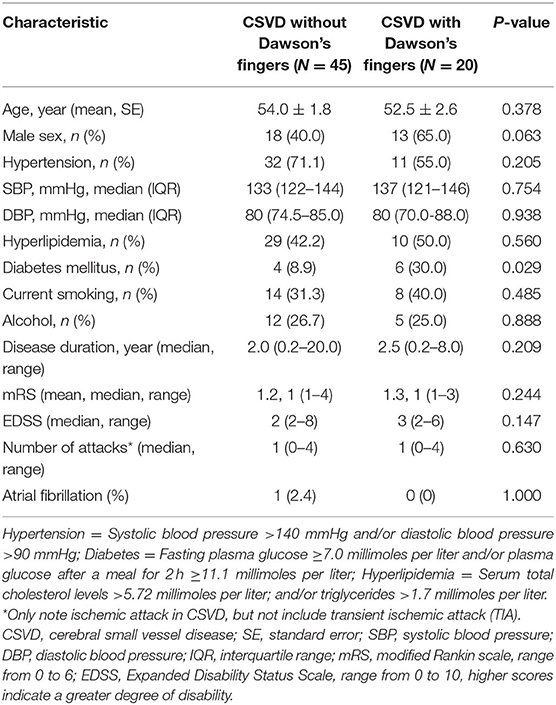
Table 2. Demographic and clinical characteristics of CSVD patients with or without Dawson's fingers.
Dawson's Fingers Affect Cognition and Imaging in Patients With CSVD
Dawson's fingers appear in patients with CSVD and are related to DM. To observe the impact of Dawson's fingers on clinical function and imaging of CSVD patients, we carefully analyzed imaging for all patients. WMH, lacunes, and CMB have a statistically significant difference between CSVD with and without Dawson's fingers, especially mixed lacunes (p < 0.001, Table 3). Then, we analyzed the function of CSVD with and without Dawson's fingers. No differences in mRS (1.3 ± 0.1 vs. 1.2 ± 0.1, p = 0.244) were observed, however, MoCA (18.9 ± 1.8 vs. 24.0 ± 0.8, p < 0.05) and MMSE (24.5 ± 1.1 vs. 26.6 ± 0.6, p < 0.05) were significantly different between CSVD with and without Dawson's fingers (Figure 2). Therefore, the cognitive level of CSVD patients with Dawson's fingers is significantly decreased compared to CSVD patients where Dawson's fingers were not detected.
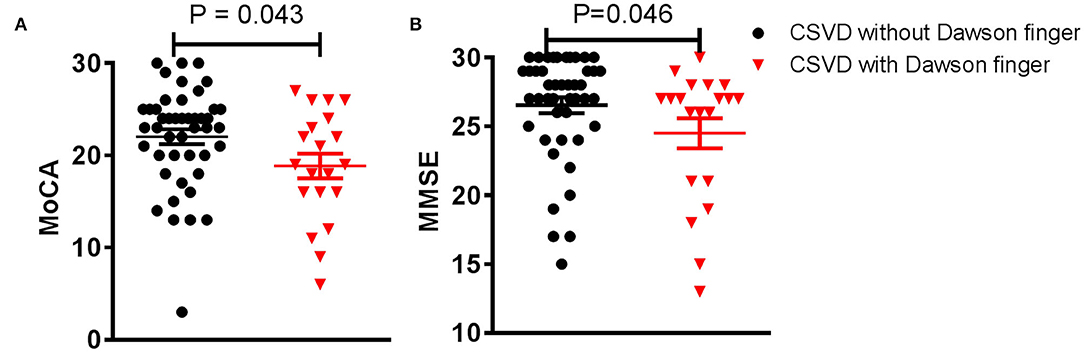
Figure 2. Dawson's fingers are associated with cognitive impairment in small vessel disease (CSVD) patients. (A,B) Montreal Cognitive Assessment (MoCA) and Mini-mental State Examination (MMSE) scores of CSVD patients with Dawson's fingers were lower compared to those without Dawson's fingers (p < 0.05). The data are presented as the mean ± standard error of mean (SEM).
Discussion
CSVD mainly refers to a group of diseases in which arterioles are damaged; whether there is venous injury has been controversial. Dawson's fingers are an important imaging marker in the diagnosis of MS. To determine whether there is a venous abnormality in CSVD, we collected and analyzed the clinical data of small arteriosclerotic CSVD and found a venous abnormality feature (Dawson's fingers) around the ventricles in the MRI of 30.8% of patients. Statistical analysis revealed an association between Dawson's fingers and DM in the CSVD patients of our cohort. Moreover, we found that Dawson's fingers are associated with decreased cognitive ability.
CSVD is typically considered to be a small artery system-related disease (24). The most prevalent etiological type for sporadic CSVD is arteriolosclerosis caused by aging or vascular risk factors (1, 25). The pathological feature of arteriosclerosis is narrowing of the lumen and thickening of the vessel wall, and atherosclerosis has a strong relationship with the clinical symptoms of CSVD such as subsequent cognitive decline and neuroimaging features, e.g., CMB (26). Further, previous work has established that high blood pressure is as an independent risk factor for CSVD (27). It is notable that relatively few studies of CSVD have focused on veins. CVS, as a venous sign, is a common imaging feature identified with MS, which is only present in 6–7% proportion of CSVD patients. However, Dawson's fingers, another venous sign, were present in more than 30% of patients with CSVD in this study. These results clearly indicate that venous problems can occur amongst CSVD patients, thus supporting an important role for venous problems in the pathogenesis of CSVD.
The pathogenesis of Dawson's fingers corresponds to the scope of perivenous inflammation, which is perpendicular to lateral ventricles along subependymal veins (28). As noted, Dawson's fingers can be used as an imaging marker in differential diagnosis of MS from its mimics. Additionally, it has been reported that Dawson's fingers can also help differentiate other autoimmune demyelinating diseases from MS (18, 29, 30). However, a common challenge in the clinic is difficulties in distinguishing MS from CSVD on the basis of MRI appearance. For example, in middle-aged or elderly patients, CSVD has a high prevalence of leukoencephalopathy (31), so a variety of imaging markers are used to distinguish MS from CSVD. In our study, 20 of 65 CSVD patients (30.8%) had Dawson's fingers, whereas 32 of 46 MS patients (69.6%) had Dawson's fingers, and the difference in proportions between MS and CSVD groups was significant. Thus, despite its apparently abnormally high prevalence in CSVD patients in our cohort, Dawson's fingers can still be used to distinguish between MS and CSVD. However, our data also shows that the utility of this marker decreased in patients with DM. Therefore, careful consideration of this and other imaging markers for the differential diagnosis of MS and CSVD, and especially in patients with DM, merits attention.
DM can contribute to the development of CSVD by initiating and accelerating the development of arteriolosclerosis (26). It is known that CSVD patients with DM exhibit CMB, WMH, and lacunes more commonly than do CSVD patients without DM (32). It is also long been known that DM can cause venous damage (33). Our study showed significantly higher prevalence of Dawson's fingers in CSVD patients with DM. This result suggested that DM may be associated with the occurrence of venous problems in CSVD, but this needs to be confirmed in a large longitudinal study.
Our study has some limitations, including its nature as an explorative study with a relatively small patient cohort. Therefore, we encourage researchers to use caution in directly extrapolating our results to other research; future studies should include additional participants. In addition, this study did not conduct any follow-up scientific explorations of the potential pathogenic mechanism of Dawson's fingers in CSVD patients. We did rigorously evaluate the clinical data of all CSVD patients. Our work also therefore suggests that future examinations of the pathophysiology of CSVD should pay attention to venous abnormalities and should certainly consider potential impacts from diabetes.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Tianjin Medical University and Beijing Tiantan Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
YF conceptualized the study, acquired funding for this study, and designed the study. YF, ZZ, and LY collected the data. LY, YY, WZ, and AL analyzed the data. YF, WZ, and AL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81771279) the Professor Academic Development Fund of Fujian Medical University (No. JS15012) and the Shaanxi Province Natural Science Foundation Research Project (2018JM7016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our patients for participating in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00669/full#supplementary-material
References
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
2. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. (2013) 12:483–97. doi: 10.1016/S1474-4422(13)70060-7
3. Rostrup E, Gouw AA, Vrenken H, van Straaten EC, Ropele S, Pantoni L, et al. The spatial distribution of age-related white matter changes as a function of vascular risk factors–results from the LADIS study. NeuroImage. (2012) 60:1597–607. doi: 10.1016/j.neuroimage.2012.01.106
4. Munoz Maniega S, Chappell FM, Valdes Hernandez MC, Armitage PA, Makin SD, Heye AK, et al. Integrity of normal-appearing white matter: influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. (2017) 37:644–56. doi: 10.1177/0271678X16635657
5. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
6. Maggi P, Absinta M, Sati P, Perrotta G, Massacesi L, Dachy B, et al. The “central vein sign” in patients with diagnostic “red flags” for multiple sclerosis: a prospective multicenter 3T study. Mult Scler. (2020) 26:421–32. doi: 10.1177/1352458519876031
7. Clarke MA, Samaraweera AP, Falah Y, Pitiot A, Allen CM, Dineen RA, et al. Single test to ARrive at multiple sclerosis (STAR-MS) diagnosis: a prospective pilot study assessing the accuracy of the central vein sign in predicting multiple sclerosis in cases of diagnostic uncertainty. Mult Scler. (2020) 26:433–41. doi: 10.1177/1352458519882282
8. Sinnecker T, Clarke MA, Meier D, Enzinger C, Calabrese M, De Stefano N, et al. Evaluation of the central vein sign as a diagnostic imaging biomarker in multiple sclerosis. JAMA Neurol. (2019) 76:1446–56. doi: 10.1001/jamaneurol.2019.2478
9. Cortese R, Magnollay L, Tur C, Abdel-Aziz K, Jacob A, De Angelis F, et al. Value of the central vein sign at 3T to differentiate MS from seropositive NMOSD. Neurology. (2018) 90:e1183–e90. doi: 10.1212/WNL.0000000000005256
10. Maggi P, Absinta M, Grammatico M, Vuolo L, Emmi G, Carlucci G, et al. Central vein sign differentiates multiple sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol. (2018) 83:283–94. doi: 10.1002/ana.25146
11. Samaraweera APR, Falah Y, Pitiot A, Dineen RA, Morgan PS, Evangelou N. The MRI central vein marker; differentiating PPMS from RRMS and ischemic SVD. Neurol Neuroimmunol Neuroinflamm. (2018) 5:e496. doi: 10.1212/NXI.0000000000000496
12. Samaraweera AP, Clarke MA, Whitehead A, Falah Y, Driver ID, Dineen RA, et al. The central vein sign in multiple sclerosis lesions is present irrespective of the T2* sequence at 3 T. J Neuroimaging. (2017) 27:114–21. doi: 10.1111/jon.12367
13. Tallantyre EC, Dixon JE, Donaldson I, Owens T, Morgan PS, Morris PG, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. (2011) 76:534–9. doi: 10.1212/WNL.0b013e31820b7630
14. Mistry N, Abdel-Fahim R, Samaraweera A, Mougin O, Tallantyre E, Tench C, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler. (2016) 22:1289–96. doi: 10.1177/1352458515616700
15. Kilsdonk ID, Wattjes MP, Lopez-Soriano A, Kuijer JP, de Jong MC, de Graaf WL, et al. Improved differentiation between MS and vascular brain lesions using FLAIR* at 7 tesla. Eur Radiol. (2014) 24:841–9. doi: 10.1007/s00330-013-3080-y
16. Lane JI, Bolster B, Campeau NG, Welker KM, Gilbertson JR. Characterization of multiple sclerosis plaques using susceptibility-weighted imaging at 1.5 T: can perivenular localization improve specificity of imaging criteria? J Comp Assist Tomogr. (2015) 39:317–20. doi: 10.1097/RCT.0000000000000233
17. Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. (2000) 47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q
18. Raz E, Loh JP, Saba L, Omari M, Herbert J, Lui Y, et al. Periventricular lesions help differentiate neuromyelitis optica spectrum disorders from multiple sclerosis. Mult Scler Int. (2014) 2014:986923. doi: 10.1155/2014/986923
19. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. (2011) 69:292–302. doi: 10.1002/ana.22366
20. Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. (2017) 140:617–27. doi: 10.1093/brain/aww350
21. Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. (2015) 132:1104–12. doi: 10.1161/CIRCULATIONAHA.115.016371
22. Wang Y, Jiang Y, Suo C, Yuan Z, Xu K, Yang Q, et al. Deep/mixed cerebral microbleeds are associated with cognitive dysfunction through thalamocortical connectivity disruption: the Taizhou Imaging Study. NeuroImage Clin. (2019) 22:101749. doi: 10.1016/j.nicl.2019.101749
23. Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. (2017) 88:2162–8. doi: 10.1212/WNL.0000000000004007
24. Chen X, Wang J, Shan Y, Cai W, Liu S, Hu M, et al. Cerebral small vessel disease: neuroimaging markers and clinical implication. J Neurol. (2019) 266:2347–62. doi: 10.1007/s00415-018-9077-3
25. Basile AM, Pantoni L, Pracucci G, Asplund K, Chabriat H, Erkinjuntti T, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (leukoaraiosis and disability in the elderly) study. Cerebrovasc Dis. (2006) 21:315–22. doi: 10.1159/000091536
26. Arboix A, Font A, Garro C, Garcia-Eroles L, Comes E, Massons J. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosurg Psychiatry. (2007) 78:1392–4. doi: 10.1136/jnnp.2007.119776
27. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. (2014) 83:1228–34. doi: 10.1212/WNL.0000000000000837
28. Hashemi RH, Bradley WG Jr., Chen DY, Jordan JE, Queralt JA, et al. Suspected multiple sclerosis: MR imaging with a thin-section fast FLAIR pulse sequence. Radiology. (1995) 196:505–10. doi: 10.1148/radiology.196.2.7617868
29. Horowitz AL, Kaplan RD, Grewe G, White RT, Salberg LM. The ovoid lesion: a new MR observation in patients with multiple sclerosis. AJNR Am J Neuroradiol. (1989) 10:303–5.
30. Pantano P, Bernardi S, Tinelli E, Pontecorvo S, Lenzi D, Raz E, et al. Impaired cortical deactivation during hand movement in the relapsing phase of multiple sclerosis: a cross-sectional and longitudinal fMRI study. Mult Scler. (2011) 17:1177–84. doi: 10.1177/1352458511411757
31. Aliaga ES, Barkhof F. MRI mimics of multiple sclerosis. Handb Clin Neurol. (2014) 122:291–316. doi: 10.1016/B978-0-444-52001-2.00012-1
32. Thorn LM, Shams S, Gordin D, Liebkind R, Forsblom C, Summanen P, et al. Clinical and MRI features of cerebral small-vessel disease in type 1 diabetes. Diabetes Care. (2019) 42:327–30. doi: 10.2337/dc18-1302
Keywords: Dawson's fingers, cerebral small vessel disease, multiple sclerosis, diabetes mellitus, magnetic resonance imaging
Citation: Lv A, Zhang Z, Fu Y, Yan Y, Yang L and Zhu W (2020) Dawson's Fingers in Cerebral Small Vessel Disease. Front. Neurol. 11:669. doi: 10.3389/fneur.2020.00669
Received: 08 December 2019; Accepted: 03 June 2020;
Published: 24 July 2020.
Edited by:
Yulin Ge, New York University, United StatesReviewed by:
Robert Zivadinov, University at Buffalo, United StatesMaria Marcella Lagana, Fondazione Don Carlo Gnocchi Onlus (IRCCS), Italy
Copyright © 2020 Lv, Zhang, Fu, Yan, Yang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yang, eWFuZ2xpMjAwMUB0bXUuZWR1LmNu; Wenli Zhu, d2VubGl6aHUxOEAxMjYuY29t
 Aowei Lv
Aowei Lv Zaiqiang Zhang2
Zaiqiang Zhang2 Ying Fu
Ying Fu Yaping Yan
Yaping Yan Wenli Zhu
Wenli Zhu