- 1Department of Neurology, National Taiwan University Hospital, Taipei, Taiwan
- 2Department of Neurology, National Taiwan University Hospital Yunlin Branch, Yunlin, Taiwan
- 3Graduate Institute of Anatomy and Cell Biology, National Taiwan University Hospital College of Medicine, Taipei, Taiwan
Objective: Miller Fisher syndrome (MFS) is predominantly a clinical diagnosis, with classic triad of ophthalmoplegia, ataxia, and generalized reduced reflexes. Previous studies in chronic and acute immune-mediated neuropathies indicated that ultrasound, may help to detect changes that could correspond with disease activity. We studied the feasibility of serial nerve ultrasound in MFS, using a healthy controls.
Methods: All MFS patients (n = 5) and healthy controls (n = 18), underwent a standardized sonographic protocol that evaluated nerve sizes of facial, large arm and leg nerves, and spinal nerve roots. All MFS patients underwent routine ancillary investigations, including electrodiagnostic testing and for presence of anti-GQ1b antibodies. In addition, four MFS patients had 2nd, and 3rd clinical and sonographic evaluation at 14 and 90 days from onset.
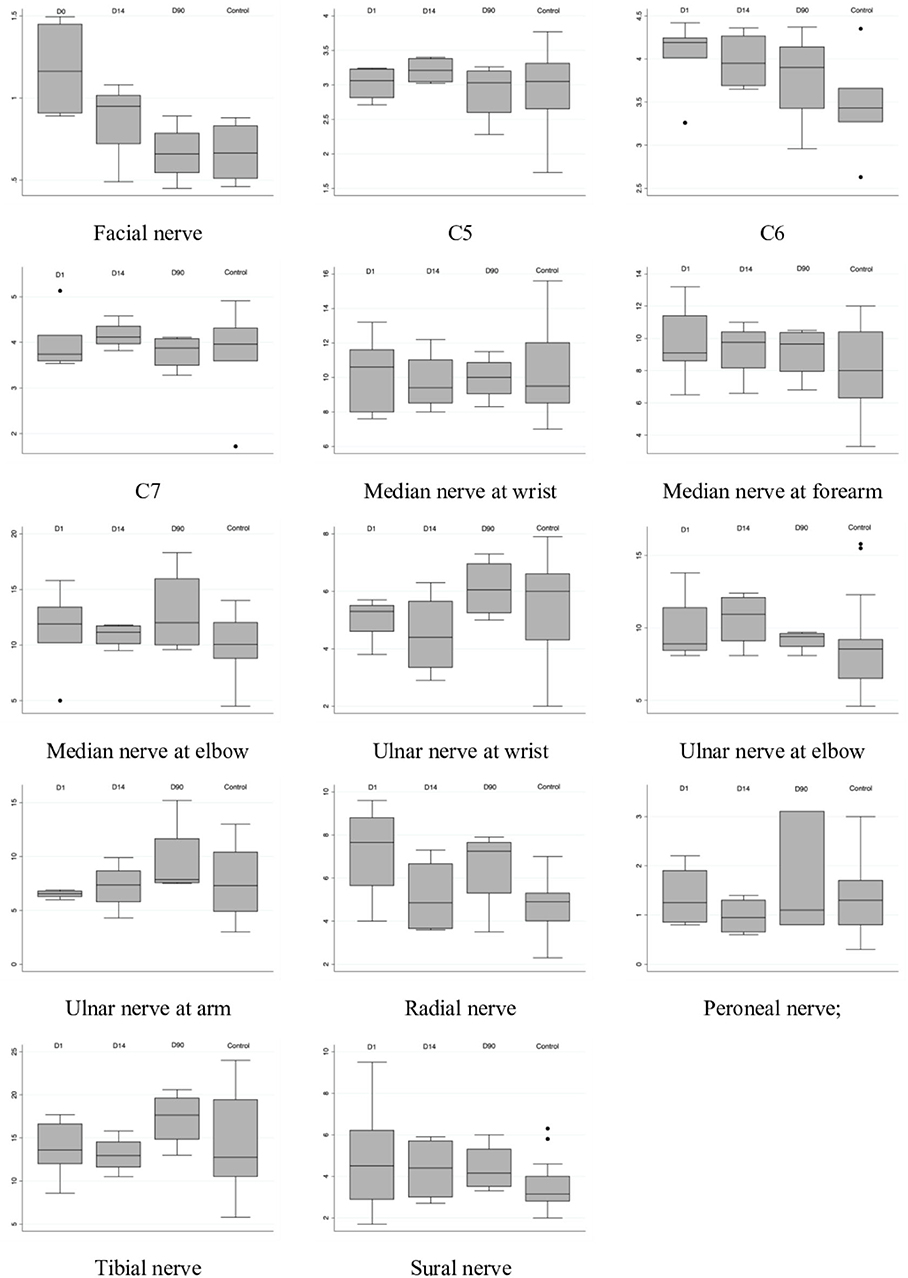
Results: The width of the facial nerve was significantly larger in the MFS group than in the control group (MFS: 1.19 ± 0.31 mm vs. normal: 0.67 ± 0.13 mm, P = 0.01). The size of the cervical roots and the nerves in the limbs were similar between the two groups. Two patients' facial nerve size subsided with time, but the decrease in other nerves' sizes were not obvious.
Conclusion: Our study showed that serial nerve ultrasound studies are feasible in MFS, and can capture changes in facial nerve size that could complement routine diagnostic tests. Further studies are warranted to determine and compare its test characteristics in MFS.
Introduction
Miller Fisher syndrome (MFS) is a variant of Guillain-Barre syndrome (GBS)-spectrum disorder, which is a post-infectious monophasic neuropathy (1). The incidence of GBS is between 1.1 and 1.8/100,000/year (2). There are several GBS spectrum disorder variants (acute inflammatory demyelinating polyradiculoneuropathies, acute motor axonal neuropathy, acute motor and sensory axonal neuropathy, and MFS), with distinct geographic distribution of prevalence. The proportion of MFS in the GBS spectrum disorder is higher in Asia (18–26%) than in Western countries (3–5%) (2, 3). The cardinal features of MFS include ophthalmoplegia, ataxia, and hyporeflexia. However, limb paresthesia and facial palsy are not uncommon in pure MFS (1, 4), and MFS could occasionally overlap with another variant of GBS, such as the pharyngeal-cervical-brachial variant (1, 5).
Miller Fisher syndrome and other GBS-spectrum disorders are essentially a clinical diagnosis, while the nerve conduction studies (NCS) and cerebrospinal fluid (CSF) studies often reported non-specific findings in the early phase of GBS-spectrum disorders (6, 7). In routine practice, the laboratory tests are predominantly used to exclude other causes. An objective examination is needed to increase the confidence of a neurologist to diagnose MFS. The contribution of ganglioside antibodies in routine diagnostic setting of GBS and its' variants is limited, as testing for their presence is time consuming, and do not influence treatment early decisions. In addition, availability and performance of different assays tot test for ganglioside antibodies is highly variable. The anti-GQ1b antibody has been reported to be positive in 70–90% of MFS patients (8, 9). In other words, MFS is usually diagnosed by symptoms and neurological examinations.
Magnetic resonance imaging (MRI) had been investigated in GBS, and contrast enhancement had been reported in some studies (10, 11). However, the resolution of MRI was not enough to evaluate the size change in GBS. Recently, morphological studies using sonography have become a popular tool in addition to the classic electrophysiological studies in clinical practice. Nerve enlargement could be clearly seen in entrapment neuropathy with sonography by an experienced operator, but there are few sonography studies on GBS (12–14); those on MFS are even rarer (15). The facial nerve has been investigated by sonography in patients with Bell's palsy (16–19), however, the use of facial nerve sonography has not been explored in MFS before. Therefore, we performed and systematic study to explore sonographic involvement of facial nerves in MFS, and compare this with spinal nerve roots (C5–C7), large arm and leg nerves.
Materials and Methods
Subject Recruitment
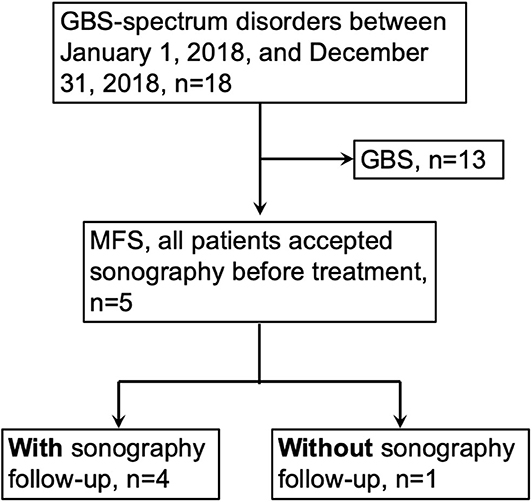
This study (201705058RIND) was approved by the Ethics Committee of the National Taiwan University Hospital (NTUH), Taipei, Taiwan, and informed consent was obtained before all procedures were carried out. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We used the GBS consensus criteria for diagnosis of MFS: (1) existence of ophthalmoplegia and ataxia with or without areflexia/hyporeflexia; (2) monophasic disease course with an interval between onset and nadir ranging from 12 h to 28 days, followed by a clinical plateau; and (3) alternative diagnosis is excluded. (1) We recruited patients with MFS between January 1, 2018, and December 31, 2018, in our hospital (Figure 1). The inclusion and exclusion criteria of this study were (1) fulfilled the MFS diagnostic criteria mentioned above, (2) accepted the nerve ultrasound study, and (3) no diabetes mellitus, chronic kidney disease, prior Bell's palsy, family history of hereditary diseases, chronic infection, and exposure of toxin. The diagnosis of MFS was made by the primary neurologists, and Dr. HWH was the primary neurologist of case 1, 4, and 5. We recruited the control groups from the colleagues in our department and from the volunteers in our hospital. The neurological examinations were performed by a board neurologist. The inclusion and exclusion criteria of the control group included the following: (1) aged at least 20 years old and <65 years old, (2) no neuropathic symptoms or signs, (3) no abnormality on nerve conduction studies, and (4) no diabetes mellitus, chronic kidney disease, prior Bell's palsy, family history of hereditary diseases, chronic infection, and exposure of toxin.
Nerve Conduction Studies
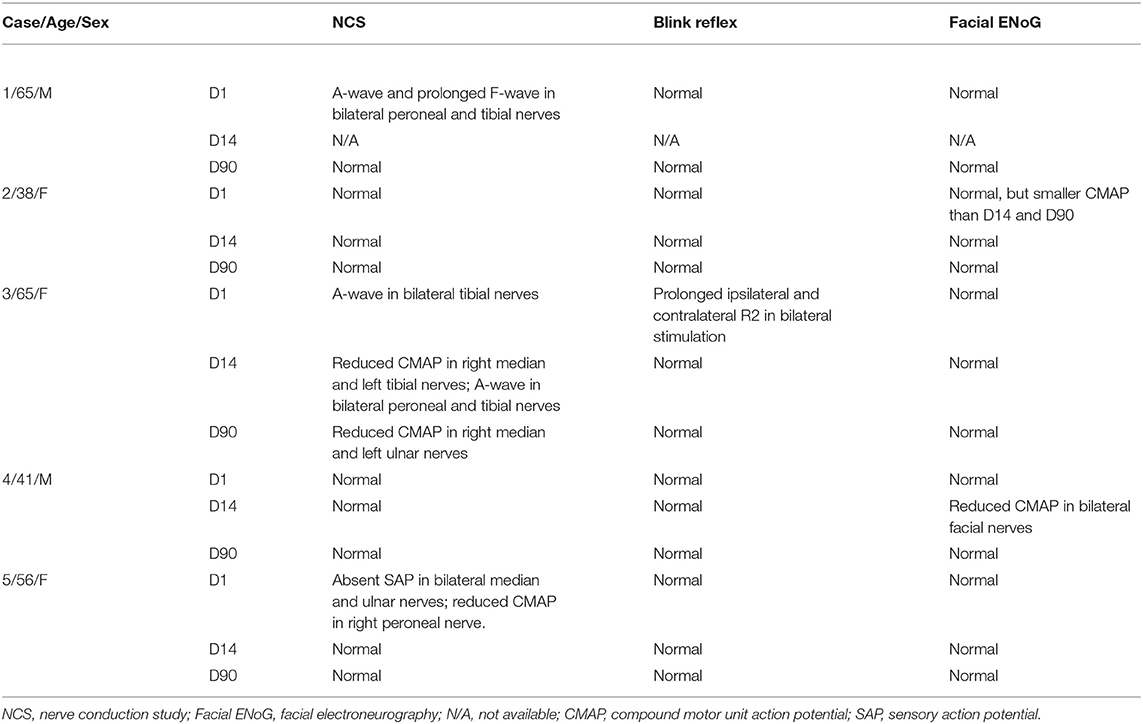
Nerve conduction studies were performed with a Nicolet Viking IV Electromyographer (Madison, WI) as per standardized methods in our laboratory (Appendix). The studied nerves included the facial, sural, peroneal, tibial, median, and ulnar (motor and sensory) nerves. Abnormal results in the NCS were defined as reduced amplitudes of compound motor action potentials (CMAPs) or sensory nerve action potentials (SNAPs), conduction block, prolonged distal latencies, slowing of the nerve conduction velocity (NCV), or prolonged minimal F-wave latency (20). The blink reflex study was also performed with recording of R1 and R2 latency. Ipsilateral R1 latency over 13 microseconds, ipsilateral R2 latency over 36 microseconds, or contralateral R2 latency over 38 microseconds was defined abnormal according our own laboratory reference value. The NCS and blink reflex were performed on admission (before treatment), 14 days after first evaluation, and 90 days after first evaluation for the MFS patients.
Nerve Ultrasound
A nerve ultrasound was performed using 3–12 MHz linear probe with an Affiniti 70G [Philips Medical Instruments, Bothell, WA] by the neurologist (Dr. HWH) in both patient and control groups. The specific MSK mode was not available when the study was performed, so the modes we used (Nerve/Dr. HWH/C Roots mode) were adjusted from the official “Vasc carotid mode” to view the targeted structure most clearly. The examination was not blinded because of the obvious neurological deficit in the MFS group. The cross-sectional area (CSA) was measured with “tracing” methods at the inner border of the hyperechoic rim of the nerve. The CSA was sampled at the nerve of the limbs according to the standardized protocol in our department: median nerve at the wrist, forearm, and elbow; ulnar nerve at the wrist, elbow, and arm; radial nerve at the outlet of the spiral groove; tibial nerve at the medial malleolus; and sural nerve at the lateral malleolus (Appendix). The cervical roots and facial nerves were measured according to previous studies (18, 21). A longitudinal view of the nerve root was observed with the probe placed at the lateral side of the neck. The maximum nerve root width was measured 2 cm distal to the transverse process at the C5, C6, and C7 levels. The nerve of the limbs and spinal nerve was sampled at the dominant hand side. The probe was then placed transversely just beneath the ear lobule to measure the facial nerve inside the parotid gland. The maximal width (inclusion of the hyperechoic border) of the facial nerve was measured according to the previous study (18). The mean width of the facial nerves of both sides was calculated. The duplex signal was checked frequently to prevent mistaking vessels as nerves in all measurements. The ultrasound was also performed on admission (before treatment), 14 days after first evaluation, and 90 days after first evaluation for the MFS patients. All measurements were performed without zoom magnification. In order to evaluate the reliability, we repeated the measurements in all sampled nerves in 10 subjects. The test-retest intra-rater reliability was evaluated by intraclass correlation coefficient, two-way random-effect model, and showed substantial reliability (Appendix).
Statistical Analysis
Numerical variables were expressed as the mean ± SD and were compared with t-tests if the data followed a Gaussian distribution. If the sample size was small, the numerical variables were compared using a non-parametric test (Wilcoxon rank sum test). Fisher's exact test was used to compare categorical data. All analyses were performed using Stata software (StataCorp LP, College Station, TX). The results were considered significant at p < 0.05.
Results
Clinical, Serological, and Electrophysiological Data
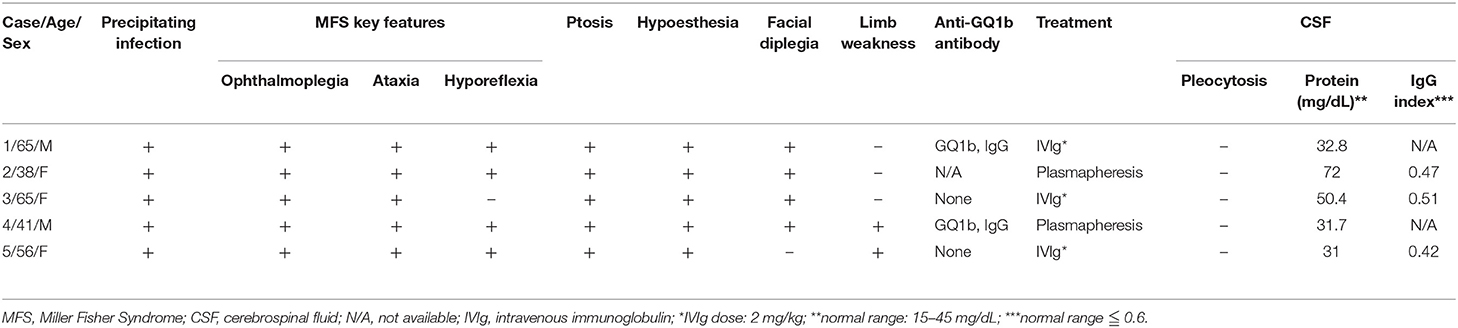
In out hospital, there were 19 patients with GBS-spectrum disorders between January 1, 2018, and December 31, 2018, and there were 5 patients fulfilling the criteria for MFS. All patients presented to our hospital and accepted the thorough examinations within 1 week after symptoms onset. The details of the clinical, serological, and electrophysiological data are summarized in Tables 1, 2. The average age was 53.6 ± 12.9 years, and 3 patients were female. All patients had ophthalmoplegia, ptosis, and ataxia, while facial diplegia and hyporeflexia were noted in 4 patients. Although one patient had hyperreflexia, we still categorized her as MFS rather than overlap with Bickerstaff brainstem encephalitis because of a lack of hypersomnolence (1). In the cerebrospinal fluid study, no patients had pleocytosis, and two patients had elevated total protein. In the electrophysiological study 2 weeks after the disease onset, no abnormality was noted in CMAP, SNAP, and NCV in the nerves of the limbs. There were abnormalities in some parameters, but the abnormality rate was all smaller than 50%: A-wave presence of the lower limbs (n = 2), prolonged minimal F-wave latency of the lower limbs (n = 1), prolonged R2 latency in the blink study (n = 1), and reduced compound motor action potentials in the facial nerve (n = 1). Three patients accepted intravenous immunoglobulin, and two patients accepted plasmapheresis. Anti-ganglioside antibodies were checked in four patients, and only two patients had anti-GQ1b IgG antibody. Taken together, our patients presented with core symptoms of MFS, but nearly all had facial weakness and sensory deficits which is infrequent to typical MFS. This particular set of patients likely reflect more severe phenotype of MFS.
Comparison Between the MFS Group and Control Group Before Treatment
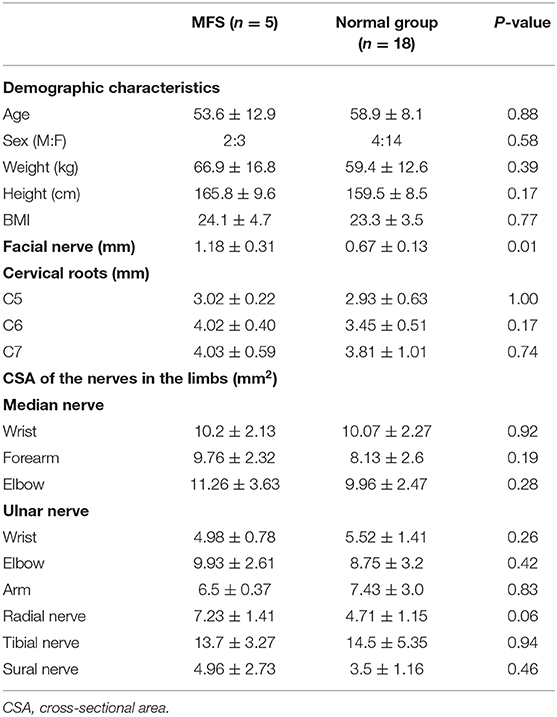
All patients underwent sonography before the treatment. However, the facial nerve of one patient (Patient 4) was not sampled because the measurement of facial nerve was not included in the protocol at that time. The spinal nerve at the C5 level was not observed in another patient (Patient 3) because of technical issue. The details of the study and the comparison with the control group are summarized in Table 3. The demographic characteristics were the same between the two groups. The width of the facial nerve in the MFS group was 1.19 ± 0.31 mm, which was significantly larger than in the control group (0.67 ± 0.13 mm, P = 0.01) (Figure 2). In the cervical roots, although the width of the cervical roots was larger in the MFS group, no comparisons showed significant differences between the two groups. The CSAs of the nerves in the limbs were similar between the two groups.
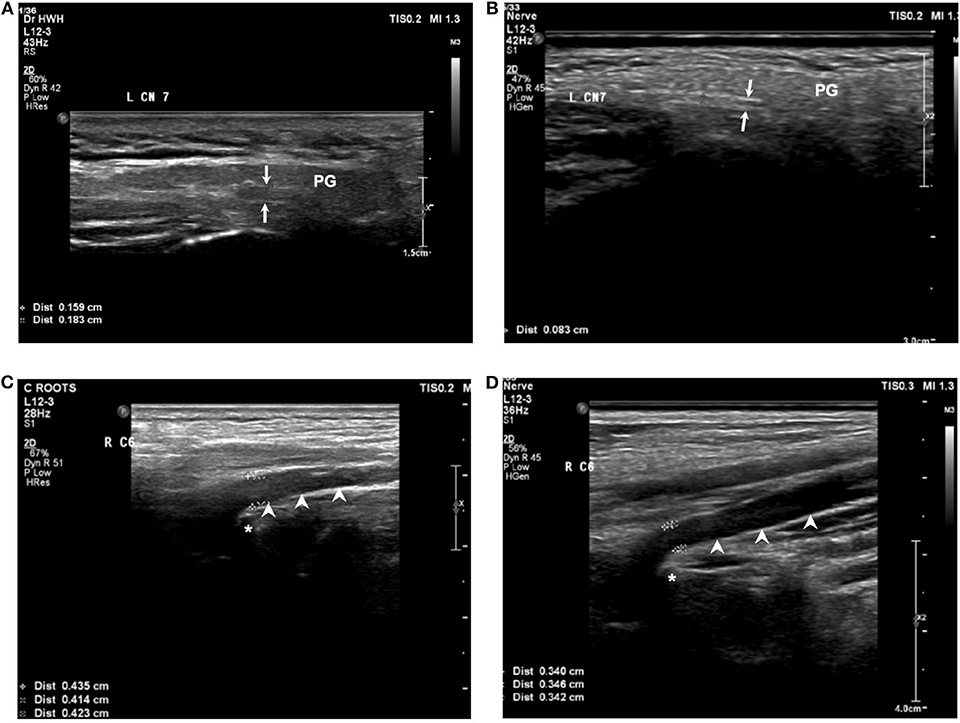
Figure 2. Sonography of facial nerve and cervical root at C6 level for the MFS and normal group at baseline. The width of the facial nerve (arrows) in a patient (A) with MFS was larger than in a control (B). The width of the cervical root (arrowheads) at C6 level in a patient with MFS (C) and a control (D). PG, parotid gland; asterisk: transverse process of the vertebrae.
Serial Follow-Up
The symptoms improved gradually in all patients, and the above abnormal findings in the electrophysiological study also improved in all patients by the follow-up examination 3 months later. Four patients underwent serial follow-up with sonography. The serial sonography data are summarized in Figures 3, 4. The facial nerve size decreased with time in 2 patients, but one patient was similar in the follow-up, though that patient's nerve size was within normal limits at the disease onset.
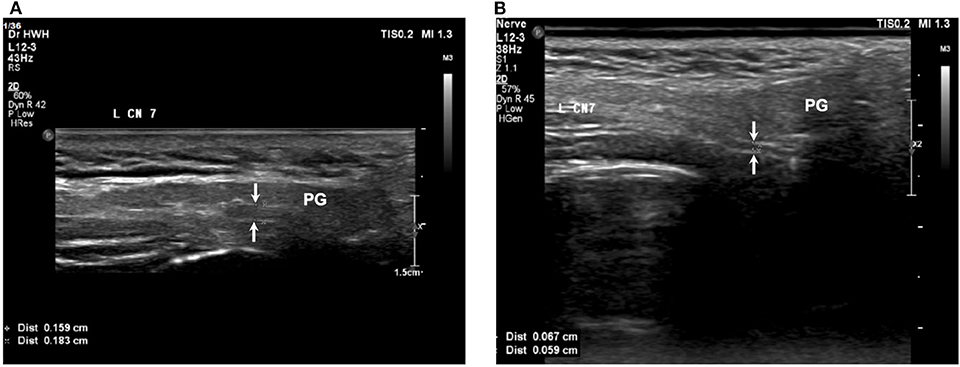
Figure 4. The facial nerve size subsided with time in a patient with MFS. (A) Before treatment, (B) 90 days after treatment. Arrows: facial nerve; PG, parotid gland.
Discussion
This study demonstrated the presence of an enlarged facial nerve in patients of MFS. The facial nerve width decreased in parallel with the clinical symptoms in two of our patients.
There is only one study exploring ultrasound in MFS patients. In 2015, Decard et al. reported two cases of MFS with enlargement of the vagus nerve, spinal nerves, and/or peripheral nerves (15). The enlargement improved 2 weeks later, and the clinical symptoms also improved. The enlargement of the cervical root in patients with MFS indicated involvement of nerves other than brain and cranial nerves, which may explain the generalized hyporeflexia in MFS. We proposed the nerve in four limbs may be involved in MFS even the symptom is not obvious. Because hyporeflexia is in the diagnostic triad of MFS and distal limb numbness is often noted in patient with MFS, we thought the peripheral nerves or nerve root should also be involved. A thorough nerve ultrasound study with specific ultrasound scores (such as UPSS-ultrasound pattern sum score) could help diagnose different diseases (22, 23). However, the facial nerve was not evaluated in the previous studies, so we included the facial nerve and spinal nerve into our protocol. Notably, the spinal nerve size was not significantly different between the two groups in our study. To explain the different results, we compared the patient characteristics and methodology between our study and Decard's report. In view of patient characteristic, both their patients had limb weakness, and was classified as MFS-plus variant of GBS, and only two patients in our group had limb weakness. The age is also older in our group (Our patient group: 53.6 ± 12.9 vs. Decard's reports: 46 and 17 year-old). In methodology, both of us followed the same protocol (21, 24). Actually, the diameter of spinal nerve of C5 and C6 was not obviously different between patients of Decard's group and ours. Instead, the value from our control group was markedly larger than the reference value of spinal nerve of Decard's group. The reference value of spinal nerve of Decard's group was derived from Japanese adults, and Decard's group is from Europe (The race was not mentioned in their report) (15, 24). If we compared our control group with the Japanese adults' normal value, the age is markedly order than Japanese reference values (Our control group: 53.6 ± 12.9 vs. Japanese adults: 35.4 ± 9.7), while there was no obvious difference in height, weight, and BMI (24). As a result, age, clinical symptoms, and races may serve as an important factor for the different results.
MRI occasionally shows enhancement of the cranial nerve in patients with MFS, but the resolution of MRI makes the evaluation of nerve enlargement challenging. The resolution of ultrasound makes the quantitative evaluation of nerve enlargement possible. Several studies have evaluated the facial nerve in patients with Bell's palsy by ultrasound (16, 17, 19). Tawfik et al. reported an enlarged facial nerve in patients with Bell's palsy compared with controls and a significant side-to-side difference as well (17). Facial nerve ultrasound had not been studied in patients with MFS previously. Our study is the first to demonstrate the use of facial nerve ultrasound in GBS-spectrum disorders.
In contrast to MFS, GBS and CIDP have been studied with sonography. CIDP is a chronic inflammatory polyneuropathy with an “onion bulb” formation on pathology (25). Repetitive demyelination and remyelination make the nerve enlarged and can be observed by MRI or ultrasound. The focal enlargement, especially on the non-entrapment site and proximal portion of the nerves and spinal nerves, could be clearly observed on ultrasound and is very different from the presentation of usual entrapment neuropathy (26, 27). Besides, the cranial nerve hypertrophy had been reported in patients with CIDP, but only being studied by MRI. Our study provided an easy, inexpensive and non-invasive way to evaluate the facial nerve in CIDP at both relapse and remitting state. In GBS, enlargement was observed in different parts of the nerve, and the enlargement subsided in parallel with the clinical symptoms (13, 28). In other words, the nerve size decreased as the symptoms decreased. Interestingly, several studies reported the enlargement of the cervical spinal nerve at the early phase of GBS, and it may reflect the radiculopathy pattern in NCSs in the early phase of GBS (13). However, the nerve enlargement in GBS was smaller than in CIDP (29), suggesting the different pathogenesis underlying the nerve enlargement in CIDP and GBS. Besides, facial nerve involvement was frequently noted in clinical presentation and by MRI in patients with GBS, but facial nerve hypertrophy was not reported before. This may be related to that MRI resolution is not enough to show the facial nerve enlargement in GBS.
The pathogenesis underlying the facial nerve enlargement may be related to nerve edema or “onion-bulb” formation. Because remyelination takes times, early nerve enlargement and improvement within months in GBS-spectrum disorders should be related to edematous changes rather than an “onion-bulb” formation. The prognosis is good in most MFS patients, so biopsy or autopsy studies are rare. Instead, pathology of GBS had been investigated widely by sural nerve biopsy or autopsy. In addition to patchy demyelination and perivascular mononuclear inflammatory infiltration throughout the peripheral nerve system, endoneurial and subperineural edema were also noted in acute inflammatory demyelinating polyneuropathy (AIDP). In acute motor axonal neuropathy/acute motor and sensory axonal neuropathy (AMAN/AMSAN), primary axonal degeneration with a paucity of inflammatory lymphocytic infiltration is the main pathology finding, and edema is less frequently reported (25, 30). One sonographic study showed that the nerve root width was significantly larger in an AIDP group than in patients with AMAN and unclassified conditions (14), which also supported the nerve edema should be related to the nerve enlargement. Because sonographic study of GBS is still limited and the patient number is small in each study, further studies integrating clinical, electrophysiological, imaging, and pathology evaluations are needed.
There were some limitations to our study. First, facial palsy is not the cardinal feature of MFS, and there was only one patient without facial palsy in our cohort. However, the spinal nerve enlargement and multiple cranial nerve enhancements on MRI in patients without clinical symptoms (31, 32) suggest that the nerve could be involved without symptoms. Further study of MFS patients without facial palsy could clarify the usefulness of facial nerve ultrasound. Second, the sonography was performed by a single operator (Dr. HWH) who was not blinded to the diagnosis of the patients. The single operator prevents the inter-rater variation, but the open-label study makes the measurement having some bias. An operator blinded to the diagnosis could ameliorate the bias, but the examination is still hard to be totally blinded for an experienced neurologist because of the obvious symptoms of MFS. In addition, the reliability of nerve sonography is not evaluated, and these should be evaluated in the future study. A disease control group (such as Bell's palsy, CIDP, and Charcot-Marie-Tooth disease) could also help optimize the nerve sonography. Third, the nerve ultrasound study was performed with a relatively low frequency range (3–12 MHz) probe. The low frequency range probe still could see the superficial structure but with poorer resolution, but it could depict the spinal nerves more clearly than pure high frequency probe (>15 MHz). The better way is buying a wide rage frequency probe (such as L5–18 or L4–18 MHz probes in Philips Affinity 70) or two probes with different frequency ranges, but it is not available in our department. Instead, we chose the lower frequency probe for covering all structures we want to study. Our ultrasound mode was not an official musculoskeletal mode, but a mode derived from the official “Vasc carotid mode,” whose setting was adjusted by the Philip official technician. Fourth, the study didn't include the disease control group to see whether facial nerve enlargement is specific to MFS. We considered the facial nerve enlargement is not specific to MFS, because previous studies on Bell's palsy also reported enlarged facial nerve (16, 17, 19). Last, the number of patients is small and the symptoms are not homogeneous, and some patients had overlap with GBS. The GBS is a rare disease, and the MFS is even more rare. It is hard to recruit enough patients to achieve the statistic power in a single hospital. We used a non-parametric test, which is more strict than parametric test to perform the statistics. The small number may also explain the discrepancy in the spinal nerve width between our study and the previous report (15). Besides, an extensive sonography study including facial nerve, spinal nerves and nerve of the limbs would clarify the nerve involvement in MFS, whereas the generalized hyporeflexia is the cardinal feature. Multicenter and international collaboration is needed for investigating sonography use in MFS/GBS, but also for the prediction of clinical course and treatment response.
Conclusion
Sonography revealed an enlarged facial nerve at the onset of MFS, which decreased 3 months after the disease onset. Further validation is needed to confirm nerve ultrasound as an objective tool to evaluate MFS.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the National Taiwan University Hospital, Taipei, Taiwan. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-WH, C-CC, and S-TH conceived and planned the studies and wrote the manuscripts with input from all authors. All authors contributed to data acquisition and analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was initiated by the principal investigator only without other external funding. This manuscript was edited by American Journal Experts.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00865/full#supplementary-material
References
1. Wakerley BR, Uncini A, Yuki N. Guillain-Barre and Miller Fisher syndromes–new diagnostic classification. Nat Rev Neurol. (2014) 10:537–44. doi: 10.1038/nrneurol.2014.138
2. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barre syndrome worldwide. A systematic literature review. Neuroepidemiology. (2009) 32:150–63. doi: 10.1159/000184748
3. Lyu RK, Tang LM, Cheng SY, Hsu WC, Chen ST. Guillain-Barre syndrome in Taiwan: a clinical study of 167 patients. J Neurol Neurosurg Psychiatr. (1997) 63:494–500. doi: 10.1136/jnnp.63.4.494
4. Berlit P, Rakicky J. The Miller Fisher syndrome. Review of the literature. J Clin Neuroophthalmol. (1992) 12:57–63.
5. Wakerley BR, Yuki N. Pharyngeal-cervical-brachial variant of Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry. (2014) 85:339. doi: 10.1136/jnnp-2013-305397
6. Arányi Z, Kovács T, Sipos I, Bereczki D. Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings. Eur J Neurol. (2012) 19:15–e13. doi: 10.1111/j.1468-1331.2011.03445.x
7. Kuwabara S, Sekiguchi Y, Misawa S. Electrophysiology in Fisher syndrome. Clin Neurophysiol. (2017) 128:215–9. doi: 10.1016/j.clinph.2016.11.009
8. Suzuki T, Chiba A, Kusunoki S, Chikuda M, Fujita T, Misu K. Anti-GQ1b ganglioside antibody and ophthalmoplegia of undetermined cause. Br J Ophthalmol. (1998) 82:916–8. doi: 10.1136/bjo.82.8.916
9. Ito M, Kuwabara S, Odaka M, Misawa S, Koga M, Hirata K, et al. Bickerstaff's brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. (2008) 255:674–82. doi: 10.1007/s00415-008-0775-0
10. Fulbright RK, Erdum E, Sze G, Byrne T. Cranial nerve enhancement in the Guillain-Barré syndrome. Am J Neuroradiol. (1995) 16:923–5.
11. Byun WM, Park WK, Park BH, Ahn SH, Hwang MS, Chang JC. Guillain-Barré syndrome: MR imaging findings of the spine in eight patients. Radiology. (1998) 208:137–41. doi: 10.1148/radiology.208.1.9646804
12. Mori A, Nodera H, Takamatsu N, Maruyama-Saladini K, Osaki Y, Shimatani Y, et al. Sonographic evaluation of peripheral nerves in subtypes of Guillain-Barrand#xe9; syndrome. J Neurol Sci. (2016) 364:154–9. doi: 10.1016/j.jns.2016.03.042
13. Berciano J. Spinal nerve involvement in early Guillain-Barre syndrome: the Haymaker and Kernohan's legacy. J Neurol Sci. (2017) 382:1–9. doi: 10.1016/j.jns.2017.09.017
14. Iizuka N, Nomoto S, Kurokawa S, Kasai H, Itaya K, Ichikawa H. Ultrasonographic appearance of nerve roots in patients with guillain-barre syndrome (GBS): comparison between acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN). J Neurol Sci. (2017) 381:491. doi: 10.1016/j.jns.2017.08.3592
15. Decard BF, Fladt J, Axer H, Fischer D, Grimm A. Nerve ultrasound in Miller Fisher variant of Guillain-Barre syndrome. Muscle Nerve. (2015) 52:1106–10. doi: 10.1002/mus.24753
16. Lo YL, Fook-Chong S, Leoh TH, Dan YF, Lee MP, Gan HY, et al. High-resolution ultrasound in the evaluation and prognosis of Bell's palsy. Eur J Neurol. (2010) 17:885–9. doi: 10.1111/j.1468-1331.2010.02950.x
17. Tawfik EA, Walker FO, Cartwright MS. A pilot study of diagnostic neuromuscular ultrasound in Bell's Palsy. J Neuroimaging. (2015) 25:564–70. doi: 10.1111/jon.12269
18. Tawfik EA, Walker FO, Cartwright MS. Neuromuscular ultrasound of cranial nerves. J Clin Neurol. (2015) 11:109–21. doi: 10.3988/jcn.2015.11.2.109
19. Li S, Guo RJ, Liang XN, Wu Y, Cao W, Zhang ZP, et al. High-frequency ultrasound as an adjunct to neural electrophysiology: evaluation and prognosis of Bell's palsy. Exp Ther Med. (2016) 11:77–82. doi: 10.3892/etm.2015.2878
20. Chao CC, Tsai LK, Chiou YH, Tseng MT, Hsieh ST, Chang SC, et al. Peripheral nerve disease in SARS:: report of a case. Neurology. (2003) 61:1820–1. doi: 10.1212/01.WNL.0000099171.26943.D0
21. Matsuoka N, Kohriyama T, Ochi K, Nishitani M, Sueda Y, Mimori Y, et al. Detection of cervical nerve root hypertrophy by ultrasonography in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci. (2004) 219:15–21. doi: 10.1016/j.jns.2003.11.011
22. Grimm A, Décard BF, Axer H, Fuhr P. The Ultrasound pattern sum score - UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin Neurophysiol. (2015) 126:2216–25. doi: 10.1016/j.clinph.2015.01.011
23. Grimm A, Rattay TW, Winter N, Axer H. Peripheral nerve ultrasound scoring systems: benchmarking and comparative analysis. J Neurol. (2017) 264:243–53. doi: 10.1007/s00415-016-8305-y
24. Sugimoto T, Ochi K, Hosomi N, Mukai T, Ueno H, Takahashi T, et al. Ultrasonographic reference sizes of the median and ulnar nerves and the cervical nerve roots in healthy Japanese adults. Ultrasound Med Biol. (2013) 39:1560–70. doi: 10.1016/j.ultrasmedbio.2013.03.031
25. Ubogu EE. Inflammatory neuropathies: pathology, molecular markers and targets for specific therapeutic intervention. Acta Neuropathol. (2015) 130:445–68. doi: 10.1007/s00401-015-1466-4
26. Jang JH, Cho CS, Yang KS, Seok HY, Kim BJ. Pattern analysis of nerve enlargement using ultrasonography in chronic inflammatory demyelinating polyneuropathy. Clin Neurophysiol. (2014) 125:1893–9. doi: 10.1016/j.clinph.2013.12.115
27. Padua L, Granata G, Sabatelli M, Inghilleri M, Lucchetta M, Luigetti M, et al. Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin Neurophysiol. (2014) 125:160–5. doi: 10.1016/j.clinph.2013.07.023
28. Razali SNO, Arumugam T, Yuki N, Rozalli FI, Goh KJ, Shahrizaila N. Serial peripheral nerve ultrasound in Guillain-Barre syndrome. Clin Neurophysiol. (2016) 127:1652–6. doi: 10.1016/j.clinph.2015.06.030
29. Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. (2009) 40:960–6. doi: 10.1002/mus.21431
30. Nakano Y, Kanda T. Pathology of Guillain–Barré syndrome. Clin Exp Neuroimmunol. (2016) 7:312–9. doi: 10.1111/cen3.12342
31. Hattori M, Takada K, Yamada K, Kamimoto K, Mitake S. [A case of Miller Fisher syndrome with gadolinium-enhancing lesions in the cranial nerves and the cauda equina on magnetic resonance imaging]. Rinsho Shinkeigaku. (1999) 39:1054–8.
Keywords: Miller Fisher syndrome, nerve sonography, facial nerve, Guillain-Barre syndrome, follow-up
Citation: Hsueh H-W, Chang K-C, Chao C-C and Hsieh S-T (2020) A Pilot Study on Serial Nerve Ultrasound in Miller Fisher Syndrome. Front. Neurol. 11:865. doi: 10.3389/fneur.2020.00865
Received: 13 November 2019; Accepted: 07 July 2020;
Published: 14 August 2020.
Edited by:
Chiara Briani, University of Padua, ItalyReviewed by:
Stephan Goedee, UMC Utrecht, NetherlandsDaniele Coraci, Catholic University of the Sacred Heart, Italy
Copyright © 2020 Hsueh, Chang, Chao and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung-Tsang Hsieh, c2hzaWVoQG50dS5lZHUudHc=
 Hsueh-Wen Hsueh
Hsueh-Wen Hsueh Kai-Chieh Chang2
Kai-Chieh Chang2 Sung-Tsang Hsieh
Sung-Tsang Hsieh