- 1Department of Physiotherapy, Bayero University, Kano, Nigeria
- 2Department of Physiotherapy and Rehabilitation Sciences, University of Antwerp, Antwerp, Belgium
- 3Department of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Ordu University, Ordu, Turkey
- 4Department of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Mehmet Akif University, Burdur, Turkey
- 5Department of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Ege University, Izmir, Turkey
- 6Department of Physiotherapy, University of Ibadan, Ibadan, Nigeria
Background: There is emerging evidence that Guillain–Barré syndrome (GBS) may be associated with coronavirus disease 2019 (COVID-19) infection. The aim of this review was to investigate the strength of the evidence.
Method: The review was registered in PROSPERO (CDR42020184822). Three electronic databases, MEDLINE, PubMed, and Web of Science, and three preprint servers, MedRvix, ChemRvix, and BioRvix, were searched from December 2019 to 24th September 2020. Studies were included if they were on COVID-19 and of any design. Articles that are reviews or opinion were excluded. The selection process was carried out using EndNote and Rayyan software. The main outcomes in the study were study design, sample size, sex, age, overall GBS symptoms, other COVID-19 symptoms, comorbidity, timing between infection and the onset of neurological symptoms, CT, MRI, and EMG results. Methodological quality of the studies was assessed using the McMaster Critical Review Form. The collected data was analyzed using qualitative synthesis.
Findings: Fifty-one high-quality studies (mostly) consisting of 83 patients were included in the study. All of the patients (except in a very few) in the included studies had confirmed diagnosis of COVID-19. Similarly, the diagnosis of GBS was based on standard clinical, electrophysiological, and cerebrospinal fluid (CSF) criteria.
Conclusion: GBS may be associated with COVID-19, and therefore, testing for COVID-19 is recommended in patients presenting with GBS during this pandemic.
Introduction
The novel coronavirus was first reported in Wuhan, China, in December 2019, and the world has since grappled under the effect of the coronavirus disease 2019 (COVID-19) pandemic with more than three million confirmed cases (1). The infection primarily affects the respiratory epithelium (2). However, evidence of its capability of affecting other cells, tissues, organs, and systems are currently emerging (3, 4). One of these systems is the nervous system (3). Although the mechanisms through which COVID-19 affects the nervous system are to date poorly understood, it is believed that direct infection injury, immune-mediated injury, systemic hypoxia as a result of severe pneumonia, and expression of angiotensin-converting enzyme 2 (ACE 2), the receptor for COVID-19 in the nervous system, may play a role (3, 5–9). This is because all the aforementioned mechanisms can cause damage to the nervous system and ultimately impair its functions (10). In addition, it is believed that intranasal inoculation of COVID-19 could cause damage to the olfactory epithelium and bulb (11). Furthermore, the virus can access the olfactory bulb via the peripheral neurons (12). This is because the olfactory neurons are directly exposed to the external environment at the sites of the dendritic nerve terminals (13). Thus, there could be spread of the virus through axonal transport as well as from the olfactory and trigeminal nerve endings in the nasal epithelium (14).
In addition, it was previously postulated that an outbreak of an infectious disease may trigger Guillain–Barré syndrome (GBS) (15). Consequently, one of the potential neurological complications during COVID-19 could be GBS. To buttress the above postulation, infections by viruses such as Zika, influenza, cytomegalo, and Epstein–Barr have been implicated in the pathogenesis of GBS (16, 17). Similarly, there is currently emerging evidence that COVID-19 is associated with GBS (18, 19). In general, GBS is clinically characterized by the absence of reflexes and increased concentration of cerebrospinal fluid protein that progress very rapidly (20). Neurophysiologically, what are seen are small action potentials, prolonged distal motor latency, delayed F waves, and conduction block (21). The aim of this systematic review is to summarize the evidence on the association between GBS and COVID-19 infection.
Methods
Strategy and Selection Criteria
The study design was a systematic review whose protocol was registered in PROSPERO, a registry for systematic reviews owned and managed by the University of York in the United Kingdom. The registration number is CDR42020184822. In addition, the study was carried out accordance with the criteria set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (22).
Studies of any design were included if they were on COVID-19 and had reported GBS as a neurological complication of the disease. In addition, the included studies were only those published in English from December 2019 to 24th September 2020. Reviews and opinion articles were excluded from the study.
Three electronic databases, MEDLINE, PubMed, and Web of Science, and three electronic preprint servers, MedRvix, ChemRvix, and BioRvix, were searched from December 2019 to 24th September 2020. Similarly, the lists of references of the included studies were screened and Google search was also carried out for any relevant papers. Coronavirus, signs and symptoms, and Guillain–Barré syndrome were used as some of the key search terms. However, during the search, these key search terms were modified according to the requirements of each database and appropriate Boolean operators were used to combine the terms. The strategy was adopted from our previous systematic review on neurological and musculoskeletal features of COVID-19 (23). The search was carried out by one of the reviewers (MST). See Appendix 1 for the details of the search strategy used in MEDLINE.
Duplicates were removed using EndNote and Rayyan software. Similarly, the Rayyan software was used for the selection of the eligible studies (24). Two reviewers (SAC and NE), researchers who have experience in doing systematic review, performed the study selection. In case of any disagreement in the selection process, consensus discussion and/or a third reviewer (MST) was used to resolve it. The selection was done according to the study inclusion and exclusion criteria.
Data Analysis
A data extraction form was used to extract the data from the included studies. The data extracted include the study title; study design; authors; year of publication; country; sample size; sex; age; overall GBS symptoms the patients present with; other COVID-19 symptoms; comorbidity; timing between infection and the onset of neurological symptoms; treatment received; diagnostic criteria for GBS and COVID-19; other examinations such as CT, MRI, and EMG carried out to confirm the presence of GBS; and mortality status. Data on electrophysiological subtypes were also extracted. The data extraction was carried out by SAC, NE, and AA independently and consensus was achieved through discussion.
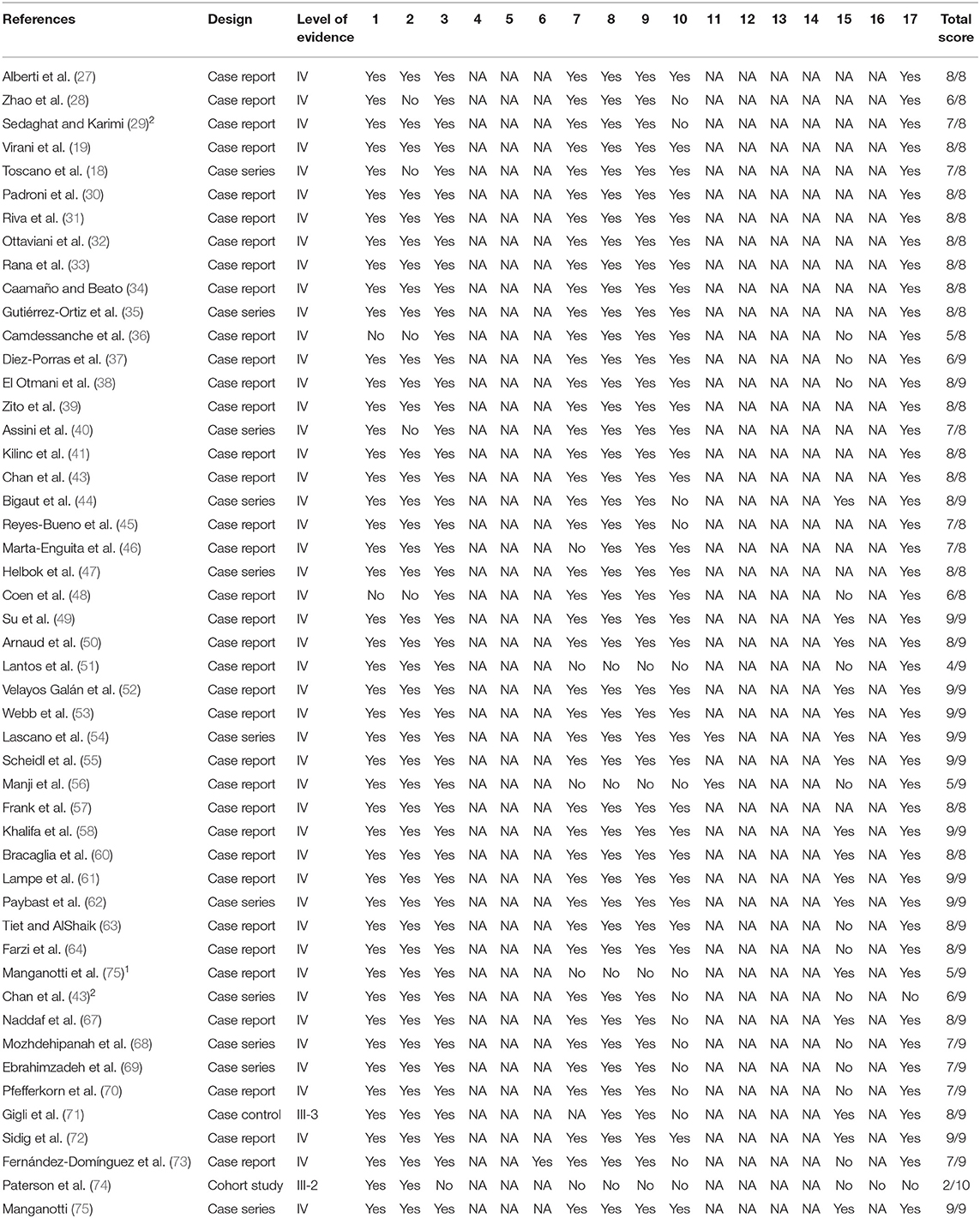
The methodological quality of the included studies was appraised independently by AA and OD and confirmed by WS and ST using the Modified McMaster Critical Review Form. The form consists of 17 items that assesses study purpose, literature review, study design, study sample, reliability, and validity of the study outcomes, interventions given, results, and conclusions (25). Each of the items is scored on a four-point scale, represented by yes, no, not addressed, and not applicable. When the answer to a particular item is no or not addressed, a score of zero is allocated, and when it is yes, a score of one is allocated. However, no score is allocated when the item is not applicable to a particular study design such as case reports or observational studies. The total scores from the appraisal can then be classified as poor, fair, good, or excellent quality when they are 1/4 or less, ≤ 2/4, ≥2/4 but ≤ 3/4 and >3/4–4/4 of the total score, respectively. When there were any disputes between the assessors, it was resolved through consensus discussion and/or through contacting a third reviewer. Similarly, the National Health and Medical Research Council's (NHMRC) evidence hierarchy was used to determine the level of evidence (26).
Qualitative synthesis which involves narrative synthesis of the characteristics and findings of the included studies was used for the data analysis. Some of the results of the synthesis were reported in the form of sum, frequency and percentage, mean and standard deviation, study flow chart, and summary tables.
Role of the Funding Source
There was no funding source for this study. However, any information about access to data and responsibility for submission can be directed to the corresponding author (AA).
Result
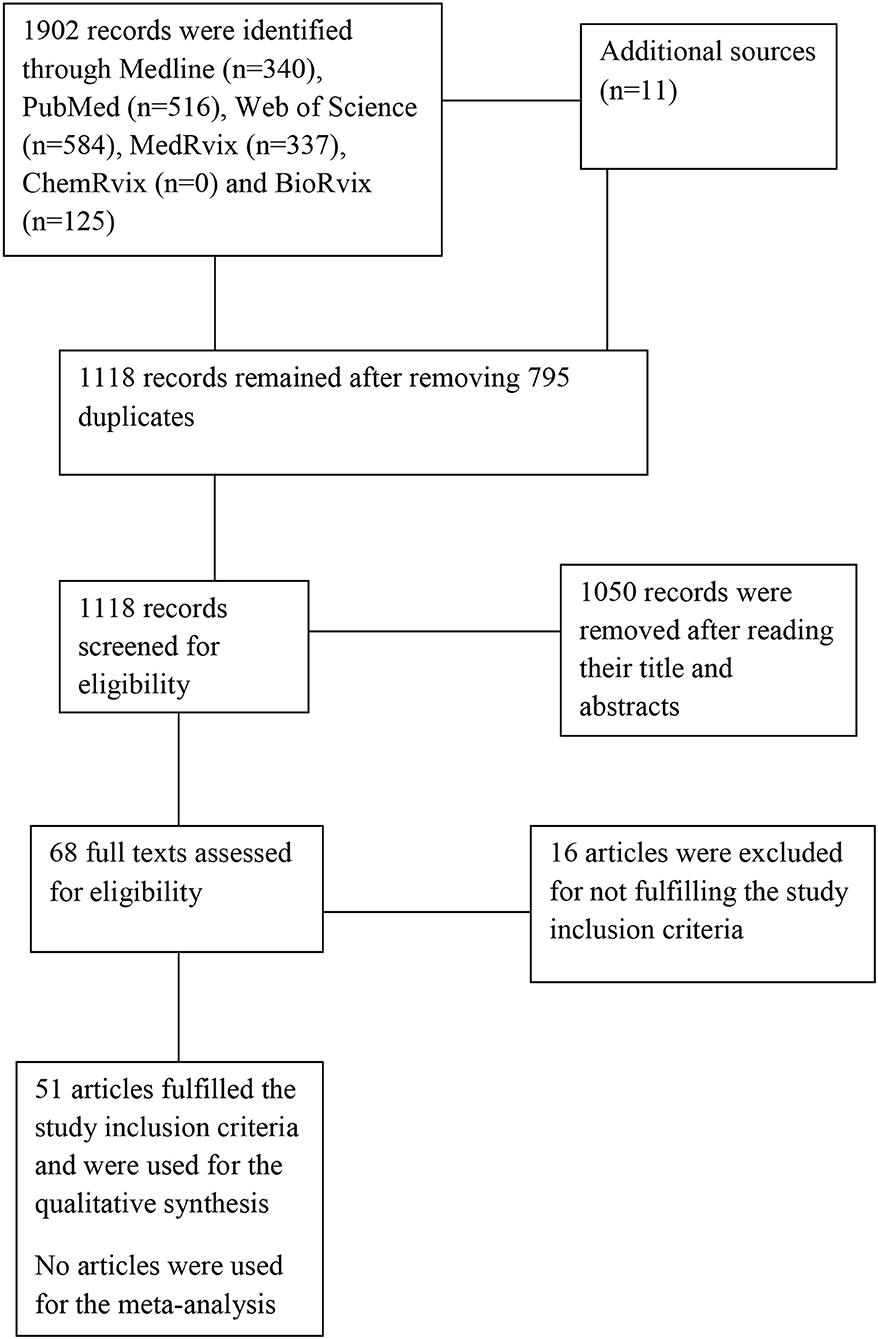
The search yielded a total of 1,913 hits in which only 51 articles were eligible for inclusion in the review (18, 19, 27–75). See Figure 1 for the PRISMA flowchart.
The total number of participants in the included studies was 83 in which 60 were male and 23 were female. However, five out of the total number of participants were children (56–58, 62, 68). Consequently, the age range of the patients was between 11 and 94 years.
The countries where the studies were carried out were Italy (18, 27, 30–32, 39, 40, 60, 65, 71, 75), China (21), Iran (22, 62, 64, 68, 69), Morocco (38), Netherlands (41), Turkey (42), Canada (43), Austria (47), Switzerland (48, 54), Spain (34, 35, 37, 45, 46, 52, 73), United States (19, 33, 49, 59, 66), France (36, 44, 50), UK (53, 74), Germany (55, 61, 70), Tanzania (56), Brazil (57), Saudi Arabia (58), and Sudan (72). However, in a few of the studies and the patients, GBS symptoms preceded the symptoms of COVID-19 infection with a range of 7–10 days (27, 28, 44). In addition, in some of the studies and the patients, it could not be determined whether COVID-19 symptoms preceded GBS or vice versa (42, 43, 47, 57, 60, 62, 71).
All the studies used reverse transcription polymerase chain reaction (RT-PCR) for the diagnosis of COVID-19. In some of the studies, nasal swab RT-PCR tested negative (31, 39, 45, 47, 48). However, in some of the studies, IgG was positive using the ELISA technique (48, 57, 67, 75).
Some of the studies tested for antiganglioside antibodies (18, 35–37, 39–41, 44, 45, 48–50, 61, 65–67, 71, 73, 75). The test was positive for the antibodies GD1b-IgG, IgM for GM2 and GD3, and a weak IgG band for GT1b and GD1a in some patients in only five studies (35, 37, 66, 67, 71). However, the GM1 antibody was in the equivocal range in one study (51). In addition, in all the studies in which real-time polymerase chain reaction assay of the cerebrospinal fluid (CSF) was performed, COVID-19 was only detected in the CSF in one study (47).
COVID-19 symptoms the patients presented with were dyspnea (19, 27, 29, 46, 50, 54, 63, 64, 68, 69, 75), pharygitis (18, 69, 72), fever (18, 19, 27, 28, 30–37, 39, 40, 42–44, 46, 47, 50, 51, 56, 58, 59, 61, 64–67, 69–72, 75), anosmia or hyposmia (18, 31, 40, 44, 47, 65, 68, 75), cough (18, 19, 25, 28–30, 32, 34, 35, 37–41, 44–48, 50, 53, 55, 56, 58, 59, 61, 63–65, 68–71, 75), ageusia or dysgeusia (18, 31, 39, 40, 44, 47, 55, 59, 65, 67, 75), chest pain (28, 54), diarrhea (19, 28, 35, 44, 45, 49, 50, 52, 54, 59, 71), headache (31, 35, 47, 53, 72), myalgia (31, 37, 44, 47, 48, 53, 54, 58, 62, 63, 67, 68), fatigue (32, 47, 48, 59, 72), rhinorrhea (33), odynophagia (33, 45, 54), chills (33, 49, 51, 54), night sweats (33), malaise (35, 53), anorexia (49), asthenia (44, 67), unspecified upper respiratory symptoms (52, 58, 66), low back pain (35), nausea (54, 59), vomiting (54), vasovagal syncope (54), arthralgia (54), sinonasal congestion (59), dizziness (62), and gastrointestinal symptoms (75).
Magnetic resonance imaging (MRI) of the brain showed normal findings in some of the studies (28, 30, 34, 40, 41, 57, 65). However, in some of the studies, it revealed enhancement of some cranial nerves such as the facial nerve (CN VII) bilaterally, CN III, CN V, and CN VI (18, 42, 43, 51, 59). For the spine, MRI showed normal findings in some of the studies (18, 19, 31, 33, 51, 57), mild herniation of two intervertebral discs, and enhancement or thickening and hyperintensity of brachial, caudal, and lumbosacral nerves and roots in some of the patients (18, 29, 44, 54, 58, 70). However, brain CT showed normal findings in some of the patients (27, 34, 43, 49, 50).
Lung computed tomography (CT) revealed ground-glass opacities in one or both lungs (27, 29–33, 36, 38, 42, 43, 47, 50, 52, 68, 69, 71, 72), bilateral pleural effusion and bilateral basilar opacities (29), diffused consolidation (27, 29, 42, 50, 72), patchy subsegmental faint opacifications with an atelectasis in the lingula (58), peri-bronchovascular thickening (71), bilateral interstitial infiltrates (70, 71), mild lung involvement (46), and normal findings (60).
Chest X-ray revealed diffuse heterogeneous infiltration in both lungs (43), mild bibasilar atelectasis and patchy consolidations (49), bilateral basilar opacifications (56), bilateral paracardiac and basal veiling opacities (58), patchy bilateral air space opacities without lobar consolidation (59), and no conspicuous findings (61).
Chest auscultation revealed bilateral diffuse crackles consistent with pneumonia (43, 50, 69) and bilateral crepitations to the mid-zones on lung auscultation (53).
The laboratory investigations showed elevated level of interleukin-6 and interleukin-8 (31, 60, 75), raised creatinine level (29), elevated level of creatine kinase (64), decreased creatinine level (49), raised creatinine phosphokinaselevel (60, 61), increased level of D-dimer (43, 46, 58), thrombocytopenia (46, 54), high level of fibrinogen (46), elevated brinogen level (47), elevated lactate level (63), increased lactate dehydrogenase level (47, 60), lymphocytosis (43, 68), raised C-reactive protein level (39, 42, 44, 47, 53, 60, 63, 64, 68), eosinopenia (38), leucopenia (54, 56, 61), lymphopenia (42, 53, 59, 64, 68), lymphocytopenia (38, 40, 54, 60), thrombocythemia (53), increased erythrocyte sedimentation rate (47, 64, 68), elevated glucose level (64), leukocytosis (40, 49), deceased potassium level (49), raised alanine transaminase level (49), and raised glutamic oxaloacetic transaminase and glutamic pyruvic transaminase (60).
Similarly, neurophysiological tests indicated abnormality in some of the studies.
Comorbidities reported in the studies were diabetes mellitus (22, 37, 50, 54, 64), Clostridium difficile colitis (23), hypertension (27, 31, 32, 37, 49, 53, 59, 62, 73), abdominal aortic aneurysm (27), lung cancer (27), hyperlipidemia (32), restless leg syndrome (32), chronic back pain (32), coronary artery disease (49), alcohol abuse (49), rheumatoid arthritis (38), left eye strabismus (51), prediabetes (59), class 1 obesity (59), and follicular lymphoma (73). See Supplementary Table 1 for the characteristic of the included studies.
Most of the studies are level VI evidence studies; only two studies are level IV evidence studies (71, 74). However, most of the studies had excellent methodological qualities. See Table 1 for the level of evidence and the methodological quality of the included studies.
Discussion
Eleven articles with good and excellent methodological quality, consisting of a total number of 16 patients, were included in the review. In the reviewed studies, COVID-19 infection was confirmed using RT-PCR. GBS was confirmed using clinical examinations including muscle strength testing, neurophysiological and laboratory examinations, and CSF analysis, all of which showed abnormality consistent with the diagnostic criteria for GBS (76). The coexistence of GBS in these patients is not surprising since infections by Zika, influenza, cytomegalo, and Epstein–Barr viruses have been implicated in the etiology of GBS (16, 17). Consequently, it was hypothesized that GBS may be triggered especially during an outbreak of an infectious illness (15).
Although the mechanisms through which COVID-19 infection affects the nervous system are still poorly understood, there are some theoretical mechanisms through which infection is believed to injure the nervous system. One of the theories is that infectious agents such as viruses can get into the nervous system through blood circulation and/or retrograde neuronal pathways and infect the peripheral nerves (6). These have, however, not yet been reported in patients with COVID-19 as scientists are still working to understand its pathogenesis (77). Secondly, severe pneumonia, one of the classic symptoms of COVID-19, can result in systemic hypoxia that can deprive the nerves of oxygen and vital nutrients which may eventually result in the accumulation of toxic substances that are capable of damaging neurons (7, 10). Thirdly, the special affinity COVID-19 has for ACE 2 which is also noted to be present in the nervous system, the skeletal muscles, other tissues, and organs may as well serve as the reason for the neuronal pathology such as GBS seen in people with the disease (9, 78).
In addition, the neuronal damage may be caused by immune response-related injury. This is because, in response to an infection, levels of inflammatory cytokines such as the interleukin- 6 are raised. Similarly, activities of T lymphocytes, macrophages, and endothelial cells also increase. These can result in vascular leakage, activation of complement and coagulation cascade, and eventually end organ damage (5).
Similarly, in the reviewed studies, there were reports of impaired arterial blood gases indicating severe hypoxia, raised white cell counts, impaired erythrocyte sedimentation rate, and increased CSF protein levels, which are markers of pathology that can result in neuronal injury. Therefore, it is possible that GBS in patients with COVID-19 is caused by a number of different mechanisms including immune-mediated injury. However, in two of the studies, GBS symptoms preceded the COVID-19 symptoms (27, 28). This should be noted for the early diagnosis of COVID-19, and any patients presenting with GBS should be evaluated for the disease especially during the pandemic. This is consistent with the reports of many studies as patients with COVID-19 may not present with the classical symptoms of the disease such as fever and cough at the early stage, but may present with other symptoms such as anosmia and impaired taste sensation (3, 79).
Although in one of the reviewed studies, the participant had ataxia, it is difficult to say this was caused by the direct affectation of the cerebellum. This is because the cerebellum has not been reported to have ACE 2 receptors (80). This review has some strengths. Firstly, the comorbidities reported in the studies are not known to cause GBS. Secondly, the reviewed studies had confirmatory diagnosis of COVID-19 in the patients. Thirdly, outcomes such as from neurophysiological and clinical examinations and laboratory investigations were used in the diagnosis of GBS. These strengthened our claims for the association or relationship between COVID-19 infection and GBS. However, one of the limitations of the review is that, in most of the studies, the recovery outcomes of the patients were not clearly reported. This can undermine the quality of the reports. In addition, the reviewed studies were only those published in English. Therefore, it is possible that we missed very relevant information from studies published in other languages.
Conclusion
COVID-19 infection affects the nervous system and can trigger GBS since there is consistency in the findings of the reviewed studies. Thus, patients presenting with GBS should be evaluated for COVID-19 as soon as possible especially during this pandemic.
Panel: Research in Context
Evidence Before This Study
There has been speculation on COVID-19 potential to affect the nervous system. In particular, there were few case reports on patients with COVID-19 presenting with Guillain–Barré syndrome. Three electronic databases, MEDLINE, PubMed, and Web of Science, and three preprint servers, MedRvix, ChemRvix, and BioRvix, were searched from December 2019 to 24th September 2020. Studies were included if they were on COVID-19 and of any design. Reviews and opinion articles were excluded. The key search terms used were coronavirus, signs and symptoms, and Guillain–Barré syndrome. Methodological quality of the studies was assessed using the McMaster Critical Review Form.
Added Value of This Study
The study found converging evidence from different studies reporting on Guillain–Barré syndrome in patients with COVID-19.
Implications of All the Available Evidence
Patients with COVID-19 may present with Guillain–Barré syndrome. Therefore, any patient presenting with this syndrome should be evaluated for COVID-19. This will help with the early diagnosis, treatment plan, and rehabilitation to help curb the spread of the disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author Contributions
MS searched the literature. SC and NE selected the studies with input from MS and AA extracted the study data with input from SC and NE. AA and OD assessed the methodological quality of the studies with input from SC, WS, and ST. AA did the data analysis and interpretation and drafted the manuscript with input from SC, WS, and ST. SC, MS, NE, OD, WS, and ST critically reviewed the drafted manuscript. All the authors approved the manuscript for submission and contributed in designing the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the authors and journals that published the reviewed articles for their efforts in the fight against the COVID-19 pandemic.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.566308/full#supplementary-material
References
1. WHO. (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed April 11, 2020).
2. Mason RJ. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J. (2020) 2000607. doi: 10.1183/13993003.00607-2020
3. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
4. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. (2020) 92:548–51.doi: 10.1002/jmv.25722
5. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
6. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dube M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2019) 12:14. doi: 10.3390/v12010014
7. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. (2020) 77:8–12. doi: 10.1016/j.jocn.2020.05.017
8. Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L, et al. Acute cerebrovascular disease following covid-19: a single center, retrospective, observational study. SSRN. (2020). doi: 10.2139/ssrn.3550025
9. Chen R, Wang K, Yu J, Chen Z, Wen C, Xu Z, et al. The spatial and cell-type distribution of SARSCoV-2 receptor ACE2 in human and mouse brain. bioRxiv. (2020). doi: 10.1101/2020.04.07.030650
10. Tu H, Tu S, Gao S, Shao A, Sheng J. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. (2020) 81:19. doi: 10.1016/j.jinf.2020.04.011
11. Imam SA, Lao WP, Reddy P, Nguyen SA, Schlosser RJ. Is SARS-CoV-2 (COVID-19) postviral olfactory dysfunction (PVOD) different from other PVOD? World J Otorhinolaryngol Head Neck Surg. (2020). doi: 10.1016/j.wjorl.2020.05.004
12. Briguglio M, Bona A, Porta M, Dell'Osso B, Pregliasco FE, Banfi G. Disentangling the hypothesis of host dysosmia and SARS-CoV-2: the bait symptom that hides neglected neurophysiological routes. Front Physiol. (2020) 11:671. doi: 10.3389/fphys.2020.00671
13. Mori I, Nishiyama Y, Yokochi T, Kimura Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. (2005) 11:129–37. doi: 10.1080/13550280590922793
14. Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. (2020) 27:1764–73. doi: 10.1111/ene.14277
15. Jacobs BC, Rothbarth PH, van der Meché FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurol. (1998) 51:1110–5. doi: 10.1212/WNL.51.4.1110
16. Kim JE, Heo JH, Kim HO, Song S-H, Park S-S, Park T-H, et al. Neurological complications during treatment of Middle East Respiratory syndrome. J Clin Neurol. (2017) 13:227–33. doi: 10.3988/jcn.2017.13.3.227
17. Sharma K, Tengsupakul S, Sanchez O, Phaltas R, Maertens P. Guillain-Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: a case report. SAGE Open Med Case Rep. (2019) 7:2050313X19838750. doi: 10.1177/2050313X19838750
18. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–barré syndrome associated with SARS-CoV-2. N Engl J Med. (2020) NEJMc2009191. doi: 10.1056/NEJMc2009191
19. Virani A, Rabold E, Hanson T, Haag A, Elrufay R, Cheema T, et al. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. (2020) 2020:e00771. doi: 10.1016/j.idcr.2020.e00771
20. Guillain G, Barré J, Strohl A. Sur un syndrome de radiculo-nevrite avec hyperalbuminose du liquide cephalorachidien sans reaction cellulaire. Remarques sur les characteres clinique et graphique des reflexes tendinaux. Bull Memor Soc Med Hop Paris. (1916) 40:1462–70.
21. Cornblath DR. Electrophysiology in Guillain-Barré syndrome. Ann. Neurol. (1990) 27:S17–20. doi: 10.1002/ana.410270706
22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
23. Abdullahi A, Candan SA, Abba MA, Hassan AB, Alshehri MA, Afemefuna EV, et al. Neurological and musculoskeletal features of Covid-19: a systematic review and meta-analysis. Front Neurol. (2020) 11:687. doi: 10.3389/fneur.2020.00687
24. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
25. Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, et al. Chemokine upregulation in SARS coronavirus infected human monocyte derived dendritic cells. Blood. (2005) 106:2366–76. doi: 10.1182/blood-2004-10-4166
26. Council-NHaMR. NHMRC Levels of Evidence and Grades for Recommendations for Guideline Developers. Canberra, ACT: National Health and Medical Research Council (2009).
27. Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. (2020). 7:e741. doi: 10.1212/NXI.0000000000000741
28. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
29. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. (2020) 76:233–5. doi: 10.1016/j.jocn.2020.04.062
30. Padroni M, Mastrangelo V, Asioli GM, Pavolucci L, Abu-Rumeileh S, Maria GraPiscaglia MG, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. (2020) 1–3. doi: 10.1007/s00415-020-09849-6
31. Riva N, Russo T, Falzone YM, Strollo M, Amadio S, Del Carro U, et al. Post-infectious Guillain–Barré syndrome related to SARS-CoV-2 infection: a case report. J Neurol. (2020). doi: 10.1007/s00415-020-09907-z. [Epub ahead of print].
32. Ottaviani D, Boso F, Tranquillini E, Gapeni I, Pedrotti G, Cozzio S, et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. (2020) 41:1351–4. doi: 10.21203/rs.3.rs-24886/v1
33. Rana S, Lima AA, Chandra R, Valeriano J, Desai T, Freiberg W, et al. Novel coronavirus (covid-19)-associated Guillain–barre' syndrome: case report. J Clin Neuromuscular Dis. (2020) 21:240–2. doi: 10.1097/CND.0000000000000309
34. Caamaño DSJ, Beato RA. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. (2020) 77:230–2. doi: 10.1016/j.jocn.2020.05.016
35. Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurol. (2020) 95:e601–5. doi: 10.1212/WNL.0000000000009619
36. Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce guillain-barré syndrome. Rev Neurol. (2020) 176:516–8. doi: 10.1016/j.neurol.2020.04.003
37. Diez-Porras L, Vergés E, Gil F, Vidal MJ, Massons J, Arboix A. Guillain-Barré-Strohl syndrome and COVID-19: case report and literature review. Neuromuscular Disord. (2020) 30:859–61. doi: 10.1016/j.nmd.2020.08.354
38. El Otmani H, El Moutawakil B, Rafai M-A, El Benna N, El Kettani C, Soussi M, et al. Covid-19 and guillain-barré syndrome: more than a coincidence! Rev Neurol. (2020) 176:518–9. doi: 10.1016/j.neurol.2020.04.007
39. Zito A, Alfonsi E, Franciotta D, Todisco M, Gastaldi M, Cotta Ramusino M, et al. COVID-19 and Guillain–Barré syndrome: a case report and review of literature. Front Neurol. (2020) 11:909. doi: 10.3389/fneur.2020.00909
40. Assini A, Benedetti L, Di Maio S, Schirinzi E, Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. (2020) 41:1657–8. doi: 10.1007/s10072-020-04484-5
41. Kilinc D, van de Pasch S, Doets AY, Jacobs BC, van Vliet J, Garssen MPJ. Guillain-barré syndrome after SARS-CoV-2 infection. Eur J Neurol. (2020) 47:852–4. doi: 10.1111/ene.14398
42. Oguz-Akarsu E, Ozpar R, Mirzayev H, Acet-Ozturk NA, Hakyemez B, Ediger D, et al. Guillain–barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. (2020) 62:E54–7. doi: 10.1002/mus.26992
43. Chan JL, Ebadi H, Sarna JR. Guillain-barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can J Neurol Sci. (2020) 1–3. doi: 10.1017/cjn.2020.106
44. Bigaut K, Mallaret M, Baloglu S, Nemoz B, Morand P, Baicry F, et al. Guillain- Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflammation. (2020) 7:e785. doi: 10.1212/NXI.0000000000000785
45. Reyes-Bueno JA, García-Trujillo L, Urbaneja P, Ciano-Petersen NL, Postigo-Pozo MJ, Martínez-Tomás C, et al. Miller-fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. (2020) 27:1759-61. doi: 10.1111/ene.14383
46. Marta-Enguita J, Rubio-Baines I, Gastón-Zubimendi I. Fatal guillain-barre syndrome after infection with SARS-CoV-2. Neurologia. (2020) 35:265–7. doi: 10.1016/j.nrleng.2020.04.004
47. Helbok R, Beer R, Löscher W, Boesch S, Reindl M, Hornung R, et al. Guillainbarré syndrome in a patient with antibodies against SARS-COV 2. Eur J Neurol. (2020) 27:1754–6. doi: 10.1111/ene.14388
48. Coen M, Jeanson G, Culebras Almeida LA, Hübers A, Stierlin F, Najjar I, et al. Guillain-barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. (2020) 87:111–2. doi: 10.1016/j.bbi.2020.04.074
49. Su XW, Palka S V, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2 associated guillain-barre syndrome with dysautonomia. Muscle Nerve. (2020) 62:E48–9. doi: 10.1002/mus.26988
50. Arnaud S, Budowski C, Ng Wing Tin S, Degos B. Post SARSCoV-2 Guillain-Barré syndrome. Clin Neurophysiol. (2020) 131:1652–4. doi: 10.1016/j.clinph.2020.05.003
51. Lantos JE, Strauss SB, Lin E. COVID-19–associated miller fisher syndrome: MRI findings. Am J Neuroradiol. (2020) 41:1184–6. doi: 10.3174/ajnr.A6609
52. Velayos Galán A, del Saz Saucedo P, Peinado Postigo F, Botia Paniagua E. Guillain-barré syndrome associated with SARS-CoV-2 infection. Neurologia. (2020) 35:e00771 doi: 10.1016/j.nrleng.2020.04.006
53. Webb S, Wallace VCJ, Martin-Lopez D, Yogarajah M. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. (2020) 13:e236182. doi: 10.1136/bcr-2020-236182
54. Lascano AM, Epiney J-B, Coen M, Serratrice J, Bernard-Valnet R, Lalive PH, et al. SARS-CoV-2 and guillain-barré syndrome: AIDP variant with favorable outcome. Eur J Neurol. (2020) 27:1751–3. doi: 10.1111/ene.14368
55. Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. (2020) 25:204–7. doi: 10.1111/jns.12382
56. Manji HK, George MU, Mkopi NP, Manji KP. Guillain-Barré syndrome associated with COVID-19 infection. Pan Afr Med J. (2020) 35:118. doi: 10.11604/pamj.supp.2020.35.2.25003
57. Frank CHM, Almeida TVR, Marques EA, Monteiro OD-S, Feitoza PVS, Borba MGS, et al. Guillain- Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. (2020) 20:ee00771. doi: 10.1093/tropej/fmaa044
58. Khalifa M, Zakaria F, Ragab Y, Saad A, Bamaga A, Emad Y, et al. Guillain-Barre syndrome associated with SARS-CoV-2 detection and a COVID-19 infection in a child. J Pediatric Infect Dis Soc. (2020) 9:510–3. doi: 10.1093/jpids/piaa086
59. Hutchins KL, Jansen JH, Comer AD, Scheer RV, Zahn GS, Capps AE, et al. COVID- 19-associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. Am J Neuroradiol. (2020) 41:1701–11. doi: 10.3174/ajnr.A6654
60. Bracaglia M, Naldi I, Govoni A, Ventura DB, de Massis P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J Neurol. (2020) 267:3166–8. doi: 10.1007/s00415-020-10014-2
61. Lampe A, Winschel A, Lang C, Steiner T. Guillain-Barré syndrome and SARS-CoV-2. Neurol Res Pract. (2020) 2:19. doi: 10.1186/s42466-020-00066-0
62. Paybast S, Gorji R, Mavandadi S. Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist. (2020) 25:101–3. doi: 10.1097/NRL.0000000000000291
63. Tiet MY, AlShaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. (2020) 13:e236536. doi: 10.1136/bcr-2020-236536
64. Farzi MA, Ayromlou H, Jahanbakhsh N, Bavil HP, Janzadeh A, Shayan FK. Guillain- Barré syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. (2020) 346:577294. doi: 10.1016/j.jneuroim.2020.577294
65. Manganotti P, Pesavento V, Buoite SA, Bonzi L, Campagnolo E, Bellavita G, et al. Miller fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neurovirol. (2020) 1–2. doi: 10.1007/s13365-020-00858-9
66. Chan M, Han SC, Kelly S, Tamimi M, Giglio B, Lewis A, et al. A case series of Guillain- Barré syndrome following Covid-19 infection in New York. Neurol Clin Pract. (2020). doi: 10.1212/CPJ.0000000000000880
67. Naddaf E, Laughlin RS, Klein C J, Toledano M, Theel ES, Binnicker MJ, et al. Guillain-Barre syndrome in a patient with evidence of recent SARS-CoV-2 infection. Mayo Clin Proc. (2020) 95:1799–801. doi: 10.1016/j.mayocp.2020.05.029
68. Mozhdehipanah H, Paybast S, Gorji R. Guillain-Barré syndrome as a neurological complication of COVID-19 infection: a case series and review of the literature. Int Clin Neurosci J. (2020) 7:156–61. doi: 10.34172/icnj.2020.18
69. Ebrahimzadeh SA, Ghoreishi A, Rahimian N. Guillain- Barré syndrome associated with the coronavirus disease 2019 (COVID-19). Neurol Clin Pract. (2020)76:233–5. doi: 10.1212/CPJ.0000000000000879
70. Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, Aufenanger J, Nowak-Machen M, Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. (2020) 1–2. doi: 10.21203/rs.3.rs-26238/v1
71. Gigli GL, Bax F, Marini A, Pellitteri G, Scalise A, Surcinelli A, et al. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J Neurol. (2020) 1–3. doi: 10.1007/s00415-020-09911-3
72. Sidig A, Abbasher K, Abbasher H, Digna MF, El Sayed M, Abbasher H, et al. COVID-19 and Guillain-Barre syndrome case report. J Neurol Neurobiol. (2020) 6. doi: 10.21203/rs.3.rs-48327/v1
73. Fernández-Domínguez J, Ameijide-Sanluis E, García-Cabo C, García-Rodríguez R, Mateos V, et al. Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19). J Neurol. (2020) 1–2. doi: 10.1007/s00415-020-09912-2
74. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. (2020) 143:3104–20. doi: 10.1093/brain/awaa240
75. Manganotti P, Bellavita G, D'Acunto L, Tommasini V, Fabris M, Sartori A, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. (2020). doi: 10.1002/jmv.26289. [Epub ahead of print].
76. Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically Ill patients. Chest. (2002)121:1262–8. doi: 10.1378/chest.121.4.1262
77. Uncini A, Kuwabara S. The electrodiagnosis of Guillain- Barré syndrome subtypes: where do we stand? Clin Neurophysiol. (2018) 129:2586–93. doi: 10.1016/j.clinph.2018.09.025
78. Zhiwen H, Zhongliang Y, Qi L, Yongfeng H. Infodemiological study on COVID-19 epidemic and COVID-19 infodemic. Preprints. (2020). doi: 10.4103/jfmpc.jfmpc_1797_20
79. Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev. (2015) 35:437–63. doi: 10.1002/med.21343
80. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. (2020) 58:299–301. doi: 10.4193/Rhin20.114
Appendix 1
Keywords: COVID-19, Guillain Barre syndrome (GBS), electromyography, olfactory bulb, cytokines storms, reactive protein, physiotherapy, intravenous immunoglobulin
Citation: Abdullahi A, Candan SA, Soysal Tomruk M, Elibol N, Dada O, Truijen S and Saeys W (2021) Is Guillain–Barré Syndrome Associated With COVID-19 Infection? A Systemic Review of the Evidence. Front. Neurol. 11:566308. doi: 10.3389/fneur.2020.566308
Received: 27 May 2020; Accepted: 18 November 2020;
Published: 13 January 2021.
Edited by:
U. K. Misra, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaReviewed by:
Mritunjai Singh, All India Institute of Medical Sciences Raipur, IndiaRajesh Kumar Singh, All India Institute of Medical Sciences, India
Copyright © 2021 Abdullahi, Candan, Soysal Tomruk, Elibol, Dada, Truijen and Saeys. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Auwal Abdullahi, YWFiZHVsbGFoaS5wdGhAYnVrLmVkdS5uZw==
 Auwal Abdullahi
Auwal Abdullahi Sevim Acaroz Candan
Sevim Acaroz Candan Melda Soysal Tomruk
Melda Soysal Tomruk Nuray Elibol5
Nuray Elibol5 Olumide Dada
Olumide Dada