- 1Department of Neurology, J. Philip Kistler Stroke Research Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 2Department of Radiology, Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States
White matter hyperintensities of presumed vascular origin (WMH) are a prevalent form of cerebral small-vessel disease and an important risk factor for post-stroke cognitive dysfunction. Despite this prevalence, it is not well understood how WMH contributes to post-stroke cognitive dysfunction. Preliminary findings suggest that increasing WMH volume is associated with total hippocampal volume in chronic stroke patients. The hippocampus, however, is a complex structure with distinct subfields that have varying roles in the function of the hippocampal circuitry and unique anatomical projections to different brain regions. For these reasons, an investigation into the relationship between WMH and hippocampal subfield volume may further delineate how WMH predispose to post-stroke cognitive dysfunction. In a prospective study of acute ischemic stroke patients with moderate/severe WMH burden, we assessed the relationship between quantitative WMH burden and hippocampal subfield volumes. Patients underwent a 3T MRI brain within 2–5 days of stroke onset. Total WMH volume was calculated in a semi-automated manner. Mean cortical thickness and hippocampal volumes were measured in the contralesional hemisphere. Total and subfield hippocampal volumes were measured using an automated, high-resolution, ex vivo computational atlas. Linear regression analyses were performed for predictors of total and subfield hippocampal volumes. Forty patients with acute ischemic stroke and moderate/severe white matter hyperintensity burden were included in this analysis. Median WMH volume was 9.0 cm3. Adjusting for intracranial volume and stroke laterality, age (β = −3.7, P < 0.001), hypertension (β = −44.7, P = 0.04), WMH volume (β = −0.89, P = 0.049), and mean cortical thickness (β = 286.2, P = 0.006) were associated with total hippocampal volume. In multivariable analysis, age (β = −3.3, P < 0.001) and cortical thickness (β = 205.2, P = 0.028) remained independently associated with total hippocampal volume. In linear regression for predictors of hippocampal subfield volume, increasing WMH volume was associated with decreased hippocampal-amygdala transition area volume (β = −0.04, P = 0.001). These finding suggest that in ischemic stroke patients, increased WMH burden is associated with selective hippocampal subfield degeneration in the hippocampal-amygdala transition area.
Introduction
Post-stroke cognitive impairment/dysfunction (PSCID) is highly prevalent and associated with poor global outcomes (1–3). Recognition of the factors that predispose to PSCID is therefore clinically important, as early identification of vulnerable patients could guide individualized therapies focused on PSCID prevention and rehabilitation. Along these lines, identifying clinically relevant biomarkers that identify patients at risk for poor stroke outcomes is of major clinical interest (4). White matter hyperintensities of presumed vascular origin (WMH), as a marker of cerebral small-vessel disease, are a recognized risk factor for poor outcomes after ischemic stroke. Specifically, increased burden of WMH has been associated with worse functional outcomes after ischemic stroke (5), poor ischemic tissue outcomes (6), and PSCID (7–10). Regarding PSCID, total WMH burden is an independent determinant of poor cognitive outcomes after ischemic stroke (7, 11, 12). Extending beyond total WMH burden, additional studies have shown that the location of WMH is relevant with periventricular and deep WMH being associated with cognitive performance after stroke (13–16). Moreover, a lesion-symptom mapping-based approach identified the locations of WMH with additional impact on post-stroke cognitive beyond those effects from the acute infarct lesion (10). Finally, in a comparison of patients with minor stroke, the addition of other markers of small-vessel disease to WMH volume did not improve prediction accuracy of PSCID (9). While these observations exemplify the clinical relevance of WMH as a biomarker to identify patients at high risk for poor functional and cognitive outcomes after stroke, it remains unclear how increasing WMH burden predisposes to PSCID.
One potential hypothesis is that there is an interrelationship between cerebral small-vessel disease and neurodegenerative pathology that contributes to the development of PSCID (2, 5, 17). For example, in patients with ischemic stroke or transient ischemic attack, medial temporal lobe atrophy has been shown to be associated with qualitative measures of WMH and PSCID (18). In chronic stroke patients, WMH burden has also been associated with hippocampal atrophy and the late development of PSCID (17, 19). These observations of an association between WMH, as a marker of small-vessel disease, and total hippocampal volume, a radiographic marker associated with neurodegenerative diseases, suggest an interaction between small-vessel disease and neurodegenerative pathology that predisposes to PSCID. These studies, however, were largely in the chronic stages of stroke and focused on total hippocampal volume. In addition, the cytoarchitecture of the hippocampus is segregated whereby there are unique subfields that comprise different parts of the hippocampal circuitry and, based on their projections to different brain regions, have distinct roles for hippocampal function and memory (20, 21). For these reasons, an investigation into the relationship between WMH and the hippocampal subfields in acute ischemic stroke patients holds potential to further characterize the interaction between WMH and hippocampal atrophy and any differential effect on select hippocampal subfields. Secondarily, this investigation in the acute stage may elucidate another mechanism of how WMH predisposes to the development of PSCID.
The aim of this study was to gain further insight into the relationship between cerebral small vessel disease and hippocampal degeneration in an acute ischemic stroke population. We therefore performed an in vivo investigation of hippocampal subfield volumes and WMH volume. Our hypothesis was that because the hippocampus is a heterogeneous structure with distinct subfields with differential roles in learning and memory (22), WMH may be associated with degeneration of select hippocampal subfields.
Materials and Methods
All aspects of this study and the use of human participants were approved by the local Institutional Review Board in October 2013. Written informed consent was obtained from all participating subjects or their surrogates.
Subjects, Inclusion/Exclusion Criteria
Consecutive subjects presenting to our institution and able to undergo a brain MRI within 24 h of stroke onset were screened for eligibility. All subjects with confirmed AIS on MRI that were able to receive gadolinium-based contrast and had radiographic evidence of moderate to severe WMH burden (Fazekas ≥ 2, Figure 1) were eligible for enrollment (23).
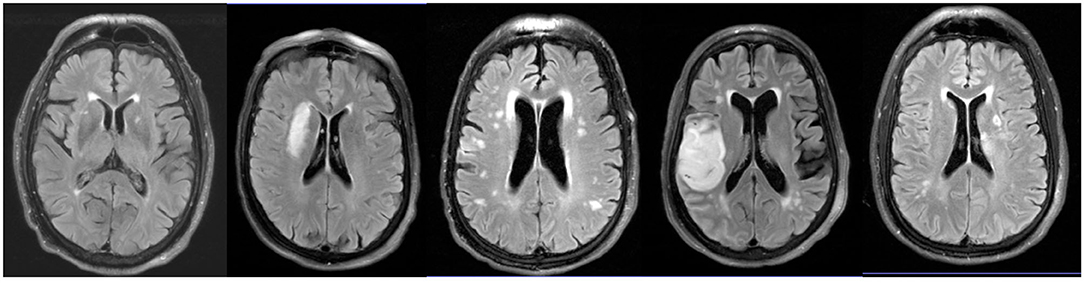
Figure 1. WMH location in AIS patients with moderate to severe WMH. Representative axial FLAIR images of WMH location in AIS patients enrolled in this study.
Clinical Variables
Age, sex, race, prestroke functional status, and medical comorbidities were ascertained from the patient or their surrogate or otherwise from the medical record. Admission NIH stroke scale (NIHSS) was performed by a trained neurologist on clinical service at the time of patient presentation. Stroke subtype was determined according to the Trial of Org 10172 in Acute Stroke Treatment classification by a trained neurologist (24). The modified Rankin scale (mRS) was used to determine functional outcomes at 90 days post-stroke (25).
Imaging Acquisition and Analysis
A research 3-T MRI was obtained 2–5 days post-stroke using a Siemens Skyra scanner. MP-RAGE sequences were acquired with the following parameters: TR/TE/TI = 2,530/1.69/1,100–1,300 ms, flip angle = 7°, 1 mm isotropic resolution. FLAIR sequences were acquired with the following parameters: TR/TE/TI = 9,000/119/2,200–2,500 ms, 5 mm slice thickness with 1 mm gap, and in-plane resolution of 0.86 × 0.86 mm2.
WMH lesions were outlined in both hemispheres in a semi-automated manner on the FLAIR sequences using MRIcro software as described previously (26). Briefly, masks for ipsi- and contralesional WMH were constructed in MRIcro (www.mricro.com) using the axial FLAIR sequence and applying a signal intensity threshold followed by manual editing.
Cortical reconstruction and volumetric segmentation were performed using FreeSurfer 6.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu) as described previously (27). Briefly, this processing includes motion correction and averaging of multiple volumetric T1 weighted images, removal of non-brain tissue, automated Talairach transformation, segmentation of subcortical white matter and deep gray matter volumetric structures, tessellation of the gray matter white matter boundary, automated topology correction, and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders (28, 29). To minimize the influence of the acute stroke on the automated hippocampal segmentation, the hippocampal volumetric estimates and mean cortical thickness were acquired in the hemisphere contralateral to the acute infarct. The algorithm uses a generative framework and Bayesian inference in order to fit a high-resolution probabilistic atlas of the hippocampal subregions—derived from highly detailed manual delineations made on ultra-high resolution ex vivo MRI—to a target MRI scan (30). An advantage of the method is its adaptiveness to variations in MRI contrast and image intensity profiles, which is desirable when processing images with pathology, as in this study. For each subject, the T1-weighted structural image, surface models, and hippocampal segmentation were visually inspected for accuracy and manual corrections were performed for tissue misclassification when necessary. Estimated Total Intracranial Volume (eTIV) was calculated using FreeSurfer version 6.0.
Statistical Analysis
No prestroke disability was defined as a mRS score of 0. Tobacco use was defined as active or any history of smoking. Excellent outcome was defined as mRS < 2 at 90 days post-stroke. The ipsilesional and contralesional WMH volumes were summed to obtain the total WMH volume (WMHv). The point-biserial correlation coefficient was determined for the correlation between WMHv and stroke laterality. Linear regression was performed to determine clinical and radiographic factors associated with hippocampal volume and cortical thickness adjusting for eTIV and stroke laterality (RStudio 1.0.153). For each dependent variable, all variables demonstrating a nominal P-value <0.05 in univariable analysis were incorporated into the multivariable backward stepwise linear regression model. A backward stepwise approach was pursued to identify a reduced model that best explains the data for each variable. For linear regression of hippocampal subfield volumes, eTIV and stroke laterality were again included as covariates to adjust for any effects of head size or laterality on the regression results. The Bonferroni correction was applied for multiple comparisons.
Results
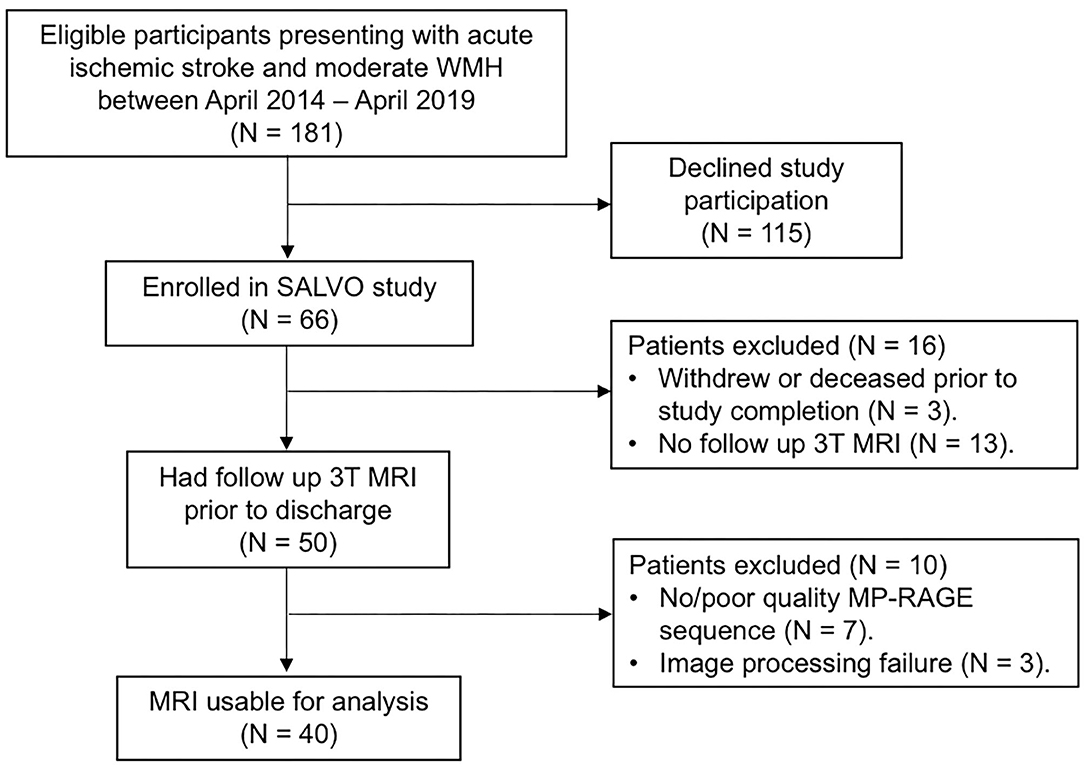
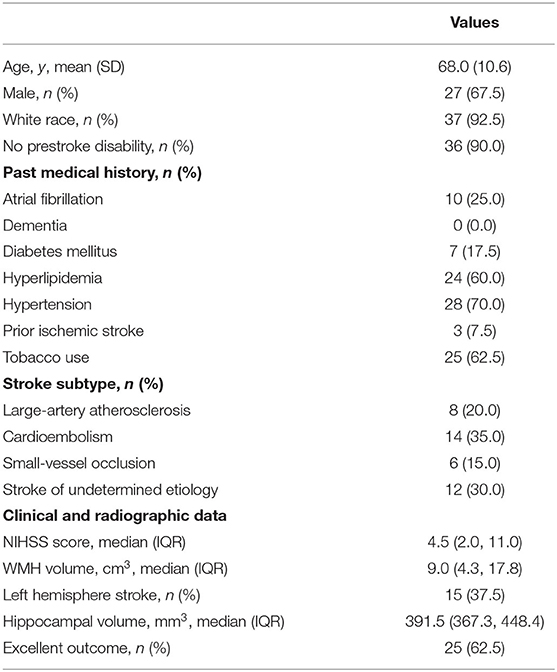
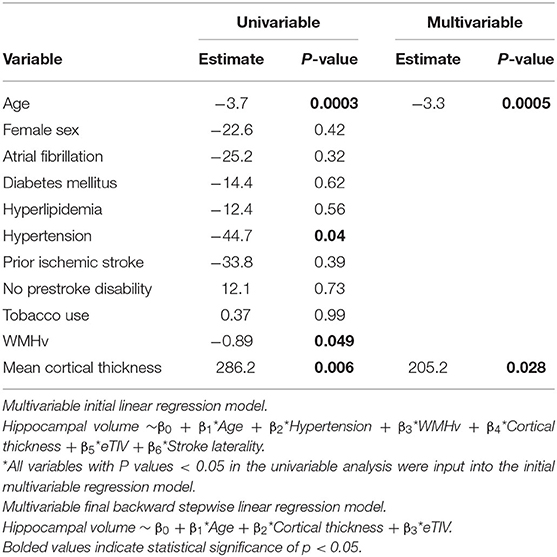
Forty subjects met inclusion criteria for this analysis (Figure 2; see Table 1 for details of baseline demographics). Median NIHSS was 4.5. Median WMH volume was 9.0 cm3 (Supplementary Figure 1). The median Fazekas scores were 2 (interquartile range; IQR 1, 3) for periventricular WMH and 1 (IQR 1, 2) for deep WMH. Strokes attributed to small-vessel occlusion represented 15% of the population. There was no significant correlation between WMHv and stroke laterality (r = −0.06, P = 0.70). In univariable linear regression for predictors of total hippocampal volume, increasing age, hypertension, and WMHv were associated with decreased hippocampal volume, while cortical thickness showed a positive association (Table 2). In multivariable, backward, stepwise linear regression, increasing age and cortical thickness were independent predictors of total hippocampal volume (Table 2).
Age (β = −0.003, P = 0.053) and hypertension (β = −0.08, P = 0.018) were associated with decreased mean cortical thickness in univariable analysis, while adjusting for eTIV and stroke laterality. In the multivariable model, however, only hypertension was a significant independent predictor of mean cortical thickness (β = −0.07, P = 0.043). WMHv was not associated with mean cortical thickness when adjusted for eTIV and stroke laterality (β = −0.0001, P = 0.90).
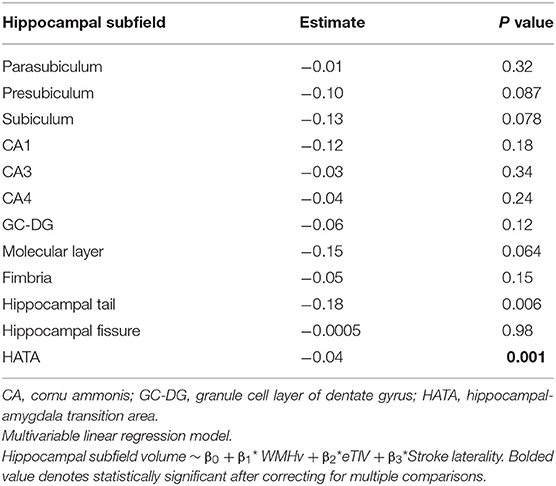
Hippocampal subfield volumes were subsequently segmented (Figure 3). Linear regression for predictors of hippocampal subfield volume demonstrated that WMHv was an independent predictor of Hippocampal Amygdala Transition Area (HATA) volume (Table 3). When a clinical diagnosis of hypertension was included in the regression model, WMHv remained an independent predictor of HATA volume (β = −0.03, P = 0.003).
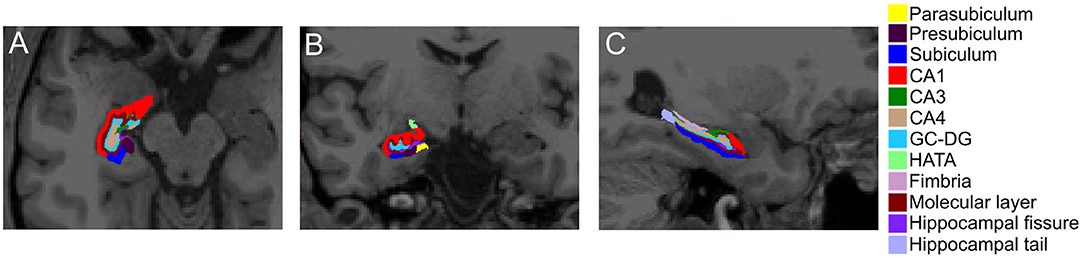
Figure 3. Hippocampal subfield segmentation in the hemisphere contralateral to the acute stroke. Representative image of hippocampal subfield automated segmentations in the (A) axial, (B) coronal, and (C) sagittal orientations. CA, cornu ammonis; GC-DG, granule cell layer of dentate gyrus; HATA, hippocampal-amygdala transition area.
Lastly, we pursued subgroup analysis of the association between WMHv and HATA volume based on stroke laterality. In right hemisphere stroke patients (N = 25), increased WMHv was significantly associated with reduced left HATA volume (β = −0.03, P = 0.008) when adjusting for eTIV. In patients with left hemisphere stroke (N = 15), increased WMHv was associated with decreased right HATA volume (β = −0.03, P = 0.19), but did not achieve statistical significance, when adjusting for eTIV.
Discussion
In a cohort of AIS patients with moderate to severe WMH burden and no prestroke dementia, we show that WMHv is a negative predictor of total hippocampal volume and selectively associated with volume of the HATA subfield. We also show that a clinical diagnosis of hypertension and increasing age are associated with decreased hippocampal volume and mean cortical thickness. There are several important implications of these findings with regards to how WMH and small-vessel disease risk factors (e.g., hypertension) may predispose to PSCID. In addition, these findings provide potential insight into an in vivo relationship between markers of cerebral small vessel and neurodegenerative diseases.
WMH burden has previously been shown to be associated with PSCID (9) and hippocampal atrophy in healthy, community-dwelling adults (31), patients with mild cognitive impairment/Alzheimer's dementia (32, 33), or stroke (18, 34). Analysis of 511 nondemented elderly subjects in the Rotterdam study, a prospective study of community-dwelling adults, showed that qualitative measures of increased WMH were associated with atrophy of the hippocampus and amygdala (31). In patients with stroke, one analysis of individual patient level data from the Virtual International Stroke Trials Archive found that, in a population of patients with predominantly small-vessel occlusive mediated ischemic stroke, markers of small-vessel disease were associated with medial temporal lobe atrophy and PSCID (18). Another retrospective cohort study of patients admitted to a hospital stroke service demonstrated that medial temporal lobe atrophy was associated with verbal memory skills. In another study of chronic stroke patients, medial temporal lobe atrophy correlated with WMH burden and PSCID (19). Based on the role of the hippocampus in cognition, these findings suggest that increasing WMH burden predisposes to PSCID, at least in part, through its association with hippocampal atrophy. Because the hippocampus receives input from the prefrontal and parietal-temporal association areas, one hypothesis is that white matter injury to these projection tracts reduces hippocampal input causing secondary hippocampal atrophy and resultant poor cognitive outcomes.
In support of this hypothesis, longitudinal analysis of 503 nondemented subjects with small-vessel disease, as part of the RUN DMC study, demonstrated that the interaction term of WMH and hippocampal atrophy significantly improved model accuracy for both working and episodic memory (35). Moreover, increased WMH burden was associated with decline in episodic memory and this association was not causally mediated by hippocampal atrophy in mediation analysis (35). These findings suggest that the interaction of small-vessel disease and neurodegenerative pathology may drive PSCID.
We also show the novel finding that increasing WMH burden is selectively associated with decreased volume of the HATA subfield of the hippocampus. Subgroup analysis for stroke laterality, showed that increased volume of the left HATA (e.g., patients with right hemisphere stroke) but not right HATA was associated with decreased WMH burden. Of note, both hemispheres showed an association between increasing HATA volume and decreased WMH burden, however, the lack of statistical significance with the right HATA is likely due to the smaller sample size of left hemisphere stroke patients in this study (N = 15). The association between HATA volume and WMH burden is of clinical interest given the potential to provide insight into the mechanism of how increased chronic white matter structural injury is associated with PSCID and poor functional outcomes (5, 14, 36). Based on the connectivity of the HATA, with projections to the medial amygdala (37), and secondarily the many projections to the amygdala from other brain regions, such as the prefrontal cortex and thalamus, it seems plausible that WMH could manifest clinically by the disruption of critical white matter tracts to hippocampal subfields. Along these lines, abnormalities in hippocampal subfield volumes have been reported in other diseases including bipolar disorder (38), depression (39), and neurodegenerative disorders (40, 41). Specific to the HATA, studies in healthy adults and patients with neurologic disorders have suggested a relationship with cognitive function. One study of 275 healthy adults demonstrated that both immediate and delayed recall scores were associated with HATA volume (42). Furthermore, in patients with Parkinson's disease, HATA volume has been shown to be predictive of the conversion to mild cognitive impairment (43). As the HATA is a key component of the limbic system that plays a critical role in emotional learning and memory and social cognition (44), these findings offer some insight into the mechanisms of how increased WMH burden contributes to PSCID.
We also demonstrate that the application of an ultra-high-resolution computational atlas for quantitative segmentation of hippocampal volumes is feasible in ischemic stroke patients contemporaneous with the index stroke. Hippocampal subfield segmentation performed using an ex vivo ultra-high-resolution atlas, such as we employed here, has been shown to provide a more accurate estimate of hippocampal volume than in vivo atlases or whole hippocampal segmentations (30). As such, incorporation of this approach into post-stroke clinical assessments may have utility in identifying stroke patients particularly vulnerable PSCID development.
There are several limitations of our study that are important to consider when interpreting the overall generalizability including. First, this was a study of a relatively small sample of AIS patients with moderate to severe WMH burden. We would maintain, however, that the inclusion criteria of this study, enriches the cohort for individuals with advanced small-vessel disease that are most at risk for development of PSCID. A second limitation is the absence of specific assessments of cognitive outcomes, which would afford an analysis of hippocampal subfield volumes and post-stroke cognitive function and further inform on the clinical relevance of these findings. Future studies therefore should assess the relationship between white matter structural injury, hippocampal subfield volume, and long-term cognitive outcomes post-stroke. Regarding the neuroimaging approach, while we showed feasibility of performing research grade MRIs in the late acute to subacute phase of ischemic stroke, there is potential bias from the spatial resolution of 3T MRI for hippocampal volume analysis and errors in automated hippocampal volume extraction. We would assert, however, that: (i) the HATA is one of the subregions that the ex vivo atlas extracts most accurately (30), since it shows strong contrast with neighboring CSF (this is, e.g., in contrast with internal subregions such as CA4); (ii) that the similar relationship between WMH burden and hippocampal size in stroke-free (32, 33) and chronic stroke individuals (19) corroborates our findings; and (iii) that the automated hippocampal volume extraction, with visual inspection of segmentation for obvious errors, minimizes inter-rater variability and is therefore a strength of this approach. Clearly, additional studies are needed investigating hippocampal volume at the time of index stroke in relation to longitudinal cognitive assessments.
Conclusions
Increasing WMH burden is selectively associated with decreased HATA volume in AIS patients. The selective association of WMHv, as a marker of cerebral small vessel disease, and the hippocampal subfield volume of the HATA may inform on the in vivo mechanisms of PSCID.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available upon reasonable request to the authors and pending approval of the local Institutional Review Board.
Ethics Statement
The studies involving human participants were reviewed and approved by Massachusetts General Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ME designed and conceptualized study, analyzed the data, data acquisition and interpretation, and drafted the manuscript for intellectual content. PF and A-KG performed data acquisition and revised manuscript for intellectual content. JI interpreted the data and revised the manuscript for intellectual content. OW and NR designed and conceptualized the study, analyzed the data, and revised manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
ME was supported in part by AHA−17CPOST33680102. JI was supported by the European Research Council (ERC) Starting Grant agreement No 677697 (BUNGEE-TOOLS). OW was supported in part by NIH-NINDS P50NS051343, R01NS059775, R01NS063925, R01NS082285, and R01NS086905. NR was supported in part by NIH-NINDS R01NS082285, and R01NS086905.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.588883/full#supplementary-material
References
1. Paolucci S, Antonucci G, Gialloreti LE, Traballesi M, Lubich S, Pratesi L, et al. Predicting stroke inpatient rehabilitation outcome: the prominent role of neuropsychological disorders. Eur Neurol. (1996) 36:385–90. doi: 10.1159/000117298
2. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. (2009) 8:1006–18. doi: 10.1016/S1474-4422(09)70236-4
3. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. (2014) 2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05
4. Tu WJ, Dong X, Zhao SJ, Yang DG, Chen H. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol. (2013) 25:771–8. doi: 10.1111/jne.12052
5. Georgakis MK, Duering M, Wardlaw JM, Dichgans M. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology. (2019) 92:e1298–308. doi: 10.1212/WNL.0000000000007142
6. Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. (2008) 39:1409–13. doi: 10.1161/STROKEAHA.107.501932
7. Wen HM, Mok VC, Fan YH, Lam WW, Tang WK, Wong A, et al. Effect of white matter changes on cognitive impairment in patients with lacunar infarcts. Stroke. (2004) 35:1826–30. doi: 10.1161/01.STR.0000133686.29320.58
8. van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. (2008) 39:2712–9. doi: 10.1161/STROKEAHA.107.513176
9. Molad J, Kliper E, Korczyn AD, Ben Assayag E, Ben Bashat D, Shenhar-Tsarfaty S, et al. Only white matter hyperintensities predicts post-stroke cognitive performances among cerebral small vessel disease markers: results from the TABASCO study. J Alzheimers Dis. (2017) 56:1293–9. doi: 10.3233/JAD-160939
10. Zhao L, Wong A, Luo Y, Liu W, Chu WWC, Abrigo JM, et al. The additional contribution of white matter hyperintensity location to post-stroke cognitive impairment: insights from a multiple-lesion symptom mapping study. Front Neurosci. (2018) 12:290. doi: 10.3389/fnins.2018.00290
11. Kliper E, Ben Assayag E, Tarrasch R, Artzi M, Korczyn AD, Shenhar-Tsarfaty S, et al. Cognitive state following stroke: the predominant role of preexisting white matter lesions. PLoS ONE. (2014) 9:e105461. doi: 10.1371/journal.pone.0105461
12. Zamboni G, Griffanti L, Jenkinson M, Mazzucco S, Li L, Kuker W, et al. White matter imaging correlates of early cognitive impairment detected by the montreal cognitive assessment after transient ischemic attack and minor stroke. Stroke. (2017) 48:1539–47. doi: 10.1161/STROKEAHA.116.016044
13. Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. (2004) 61:1531–4. doi: 10.1001/archneur.61.10.1531
14. Jokinen H, Kalska H, Mantyla R, Ylikoski R, Hietanen M, Pohjasvaara T, et al. White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. J Neurol Neurosurg Psychiatry. (2005) 76:1229–33. doi: 10.1136/jnnp.2004.055657
15. Kang HJ, Stewart R, Park MS, Bae KY, Kim SW, Kim JM, et al. White matter hyperintensities and functional outcomes at 2 weeks and 1 year after stroke. Cerebrovasc Dis. (2013) 35:138–45. doi: 10.1159/000346604
16. Kandiah N, Chander RJ, Lin X, Ng A, Poh YY, Cheong CY, et al. Cognitive impairment after mild stroke: development and validation of the SIGNAL2 risk score. J Alzheimers Dis. (2016) 49:1169–77. doi: 10.3233/JAD-150736
17. Firbank MJ, Burton EJ, Barber R, Stephens S, Kenny RA, Ballard C, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging. (2007) 28:1664–9. doi: 10.1016/j.neurobiolaging.2006.07.009
18. Arba F, Quinn T, Hankey GJ, Ali M, Lees KR, Inzitari D, et al. Cerebral small vessel disease, medial temporal lobe atrophy and cognitive status in patients with ischaemic stroke and transient ischaemic attack. Eur J Neurol. (2017) 24:276–82. doi: 10.1111/ene.13191
19. Akinyemi RO, Firbank M, Ogbole GI, Allan LM, Owolabi MO, Akinyemi JO, et al. Medial temporal lobe atrophy, white matter hyperintensities and cognitive impairment among Nigerian African stroke survivors. BMC Res Notes. (2015) 8:625. doi: 10.1186/s13104-015-1552-7
20. van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. (2009) 10:272–82. doi: 10.1038/nrn2614
21. Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. (2013) 17:230–40. doi: 10.1016/j.tics.2013.03.005
22. Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. (2011) 12:585–601. doi: 10.1038/nrn3085
23. Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. (2002) 13 (Suppl. 2):31–6. doi: 10.1159/000049147
24. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
25. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. (2007) 38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6
26. Etherton MR, Wu O, Cougo P, Giese AK, Cloonan L, Fitzpatrick KM, et al. Structural integrity of normal appearing white matter and sex-specific outcomes after acute ischemic stroke. Stroke. (2017) 48:3387–9. doi: 10.1161/STROKEAHA.117.019258
27. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. (2000) 97:11050–5. doi: 10.1073/pnas.200033797
28. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I Segmentation and surface reconstruction. Neuroimage. (1999) 9:179–94. doi: 10.1006/nimg.1998.0395
29. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. (1999) 9:195–207. doi: 10.1006/nimg.1998.0396
30. Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. (2015) 115:117–37. doi: 10.1016/j.neuroimage.2015.04.042
31. den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. (2005) 64:263–7. doi: 10.1212/01.WNL.0000149641.55751.2E
32. de Leeuw FE, Barkhof F, Scheltens P. White matter lesions and hippocampal atrophy in Alzheimer's disease. Neurology. (2004) 62:310–2. doi: 10.1212/01.WNL.0000103289.03648.AD
33. Fiford CM, Manning EN, Bartlett JW, Cash DM, Malone IB, Ridgway GR, et al. White matter hyperintensities are associated with disproportionate progressive hippocampal atrophy. Hippocampus. (2017) 27:249–62. doi: 10.1002/hipo.22690
34. Kebets V, Gregoire SM, Charidimou A, Barnes J, Rantell K, Brown MM, et al. Prevalence and cognitive impact of medial temporal atrophy in a hospital stroke service: retrospective cohort study. Int J Stroke. (2015) 10:861–7. doi: 10.1111/ijs.12544
35. van Leijsen EMC, Tay J, van Uden IWM, Kooijmans ECM, Bergkamp MI, van der Holst HM, et al. Memory decline in elderly with cerebral small vessel disease explained by temporal interactions between white matter hyperintensities and hippocampal atrophy. Hippocampus. (2019) 29:500–10. doi: 10.1002/hipo.23039
36. Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. (2009) 72:1403–10. doi: 10.1212/WNL.0b013e3181a18823
37. Fudge JL, deCampo DM, Becoats KT. Revisiting the hippocampal-amygdala pathway in primates: association with immature-appearing neurons. Neuroscience. (2012) 212:104–19. doi: 10.1016/j.neuroscience.2012.03.040
38. Cao B, Passos IC, Mwangi B, Amaral-Silva H, Tannous J, Wu MJ, et al. Hippocampal subfield volumes in mood disorders. Mol Psychiatry. (2017) 22:1352–8. doi: 10.1038/mp.2016.262
39. Han KM, Kim A, Kang W, Kang Y, Kang J, Won E, et al. Hippocampal subfield volumes in major depressive disorder and bipolar disorder. Eur Psychiatry. (2019) 57:70–7. doi: 10.1016/j.eurpsy.2019.01.016
40. Delli Pizzi S, Franciotti R, Bubbico G, Thomas A, Onofrj M, Bonanni L. Atrophy of hippocampal subfields and adjacent extrahippocampal structures in dementia with Lewy bodies and Alzheimer's disease. Neurobiol Aging. (2016) 40:103–9. doi: 10.1016/j.neurobiolaging.2016.01.010
41. Mak E, Su L, Williams GB, Watson R, Firbank M, Blamire A, et al. Differential atrophy of hippocampal subfields: a comparative study of dementia with Lewy bodies and Alzheimer disease. Am J Geriatr Psychiatry. (2016) 24:136–43. doi: 10.1016/j.jagp.2015.06.006
42. Zheng F, Cui D, Zhang L, Zhang S, Zhao Y, Liu X, et al. The volume of hippocampal subfields in relation to decline of memory recall across the adult lifespan. Front Aging Neurosci. (2018) 10:320. doi: 10.3389/fnagi.2018.00320
43. Foo H, Mak E, Chander RJ, Ng A, Au WL, Sitoh YY, et al. Associations of hippocampal subfields in the progression of cognitive decline related to Parkinson's disease. Neuroimage Clin. (2017) 14:37–42. doi: 10.1016/j.nicl.2016.12.008
Keywords: acute ischemic stroke, white matter disease, vascular dementia, MRI, hippocampus
Citation: Etherton MR, Fotiadis P, Giese A-K, Iglesias JE, Wu O and Rost NS (2020) White Matter Hyperintensity Burden Is Associated With Hippocampal Subfield Volume in Stroke. Front. Neurol. 11:588883. doi: 10.3389/fneur.2020.588883
Received: 29 July 2020; Accepted: 05 October 2020;
Published: 26 October 2020.
Edited by:
Simone Beretta, San Gerardo Hospital, ItalyReviewed by:
Jialing Liu, University of California, San Francisco, United StatesWen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2020 Etherton, Fotiadis, Giese, Iglesias, Wu and Rost. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark R. Etherton, bWV0aGVydG9uQHBhcnRuZXJzLm9yZw==
 Mark R. Etherton
Mark R. Etherton Panagiotis Fotiadis1
Panagiotis Fotiadis1 Juan E. Iglesias
Juan E. Iglesias Ona Wu
Ona Wu Natalia S. Rost
Natalia S. Rost