- 1Department of Neurology, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Ophthalmology, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Objective: The objective of the study is to investigate the gender and socioeconomic disparities in the global burden of epilepsy by prevalence and disability-adjusted life-years (DALYs).
Methods: The global, regional, and national gender-specific prevalence and DALYs caused by epilepsy by year and age were extracted from the Global Burden of Disease (GBD) Study 2017. The Gini coefficient and concentration index (CI) were calculated to demonstrate the trends in between-country inequality in the epilepsy burden from 1990 to 2017. Paired Wilcoxon signed rank test, Pearson correlation, and linear regression analyses were performed to analyze the association of gender disparity in epilepsy and socio-demographic index (SDI).
Results: The DALYs number of epilepsies increased from 1990 to 2017 by 13.8%, whereas age-standardized DALY rates showed a substantial reduction (16.1%). Men had a higher epilepsy burden than women of the same period. The epilepsy burden appeared to be higher in countries with lower socioeconomic development (CI < 0). The Gini coefficient decreased from 0.273 in 1995 to 0.259 in 2017, representing a decline in the between-country gap. Age-standardized prevalence and DALY rates of men were higher than those of women in each SDI-based country group (p < 0.0001). Male-minus-female difference (r = −0.5100, p < 0.0001) and male-to-female ratio (r = −0.3087, p < 0.0001) of age-standardized DALY rates were negatively correlated with SDI.
Conclusion: Although global health care of epilepsy is in progress, the epilepsy burden was concentrated in males and developing countries. Our findings highlight the importance of formulating gender-sensitive health policies and providing more services in developing countries.
Introduction
Epilepsy is one of the most common and serious neurological diseases, which remains an important cause of disability and mortality, affecting 50 million people worldwide (1). It is defined as a brain disorder characterized by an enduring predisposition to generate epileptic seizure (2). Epilepsy affects people of all ages, particularly prevalent among infants and older age groups (1). Health care (long-term treatment, hospitalization, and surgery) and social services (social support and health education) lead to high health costs (3). Therefore, in many parts of the world, individuals living with epilepsy and their families suffer from a high economic burden for health systems (4). Besides, the lives of patients with epilepsy are impacted with social stigma and discrimination (5).
Epilepsy burden has been measured by calculating disability-adjusted life-years (DALYs; a summary measure of health loss defined by the sum of years of life lost due to premature mortality and years lived with disability) in the Global Burden of Disease (GBD) Study (6–8). Several studies have analyzed the gender difference and socioeconomic disparity in epilepsy. In the GBD 2015 study (9), epilepsy contributed to 5.0% of total DALYs due to neurological disorders and 1.3% of all deaths, with higher DALY and prevalence rates in males. Worldwide, men had a higher incidence of epilepsy compared with women (10). Men were likely to be vulnerable to common risk factors such as brain damage (11). In addition, women tended to have a lower clinical consultation rate than men, especially in counties with lower socioeconomic status (10, 12). Socioeconomic status is related to inequality in the quality of medical care. Leonardi et al. (13) first attempted to quantify the global disparity in the burden of epilepsy based on the GBD 2000 study. More than 80% of people with epilepsy lived in developing countries where epilepsy was not well-treated (14). The previous studies have demonstrated that the lower socioeconomic status group were more likely to develop epilepsy (15, 16). The incidence of epilepsy in low-/middle-income countries (LMICs) was 139.0 (95% confidence interval 69.4–278.2), while it was 48.9 (95% confidence interval 39.0–61.1) for high-income countries (HICs) (17, 18). This association might be a result of less expenditure on health care, lower education level, as well as a higher incidence of risk factors such as infections and traumatic brain injury (19, 20).
Gender and socioeconomic disparities in epilepsy burden are worth more attention in reducing the progression of epilepsy. Existing studies have focused on the relationship between gender or socioeconomic development and the prevalence and incidence of epilepsy. However, there are few studies quantifying gender and socioeconomic disparities in global epilepsy burden in multiple dimensions. Epilepsy affects people of all ages, sexes, races, income groups, and geographical locations. About half of the people with epilepsy have physical or mental illnesses. Physical and mental comorbidities in people with epilepsy are related to poorer health, increased health care demand, decreased quality of life, and greater social exclusion (9). Our study analyzed the global burden of epilepsy by year, age, sex, geography, and socioeconomic status using prevalence and DALYs. In this study, we aimed to assess the gender differences and compare the epilepsy burden across countries with different socioeconomic status, using the most recent data from the GBD 2017 study.
Methods
Data Source
The GBD category of epilepsy is defined by the International Classification of Diseases (ICD)-10 code G40 and G41. The GBD 2017 study collected data from typical surveys from 195 countries and territories based on 354 diseases and injuries from 1990 to 2017. The GBD collaboration quantified health loss by age, sex, and geography over time, using estimated prevalence and DALYs. Methods to calculate DALYs estimates for the GBD 2017 study have been reported previously (21–24). We extracted data from the Global Health Data Exchange (http://ghdx.healthdata.org/gbd-data-tool) based on the GBD 2017 study, including (1) global total and gender-specific burden due to epilepsy, containing prevalence and DALYs number, prevalence and DALYs per 100,000 population (crude rate) and age-standardized prevalence and DALYs rate from 1990 to 2017; (2) global total and gender-specific prevalence and DALYs rate by age group in 2017; (3) gender-specific age-standardized prevalence and DALYs rate in 21 GBD regions in 1990 and 2017; (4) DALYs number and age-standardized DALYs rate in 195 countries and territories in 2017. Ethics approval and informed consent were not required for this study because of public accessibility to the data.
Socioeconomic Status
The socio-demographic index (SDI) is a composite indicator of development status, containing total fertility rate, educational attainment, and lag-distributed income. The SDI varies from 0 to 1 strongly correlated with health outcomes, with a higher value indicating a higher level of socioeconomic development (22–24). Countries were categorized into five groups by their overall development status level according to the 2017 SDI values (25): high SDI (>0.81), high-middle SDI (0.70–0.81), middle SDI (0.61–0.70), low-middle SDI (0.46–0.60), and low SDI (<0.46).
Health Inequalities
The Gini coefficient and the concentration index (CI) were used to quantify the magnitude of health inequalities in this study. Based on the Lorenz Curve, the Gini coefficient is a widely used measure of the extent of inequality (26, 27). The Gini coefficient ranges from 0 to 1. A region with perfect equality will have a value of 0, while a region with perfect inequality will have a value of 1 (28). The Gini coefficient is calculated by the age-standardized DALY rates owing to epilepsy from 195 countries to explore the trends in between-country health inequality from 1990 to 2017. The CI, derived from the concentration curve, is commonly used as an index to measure the socioeconomic-related inequalities (29). The CI is calculated by national age-standardized DALY rates and the corresponding SDI to measure the degree of health inequality associated with socioeconomic conditions. The CI ranges from −1 to 1. A value of 0 for CI means the absence of inequality related to socioeconomic characteristics. A positive (negative) value of the CI indicates that the epilepsy burden is more concentrated in countries with high (low) levels of socioeconomic development. The Gini coefficient was calculated by the INEQQERR module while the CI by the CONCINDC module by using STATA 15 (StataCorp, College Station, Texas, USA).
Statistical Analyses
All statistics were presented as values with 95% uncertainty intervals (UIs). The Wilcoxon signed rank test is a non-parametric statistical hypothesis test when comparing two related samples, matched samples, or repeated measurements on a single sample to assess whether their population mean ranks differ. The Wilcoxon signed rank test should be used if the differences between pairs of data are non-normally distributed (30). The Pearson correlation coefficient measures the relative strength of the linear relationship between two variables. Pearson's r ranges from −1 to 1. An r of −1 indicates a perfect negative linear relationship, an r of 0 indicates no linear relationship, and an r of 1 indicates a perfect positive linear relationship between variables (31). The paired Wilcoxon signed rank test was utilized to compare gender differences in global age-standardized prevalence and DALY rates for each SDI-based country group. Association of gender difference (male minus female) and gender ratio (male to female) in age-standardized DALY rates with SDI was tested by Pearson correlation and linear regression analyses. All the analyses were conducted with IBM SPSS 23.0 Statistical software and Prism Software (version 8; GraphPad). A p < 0.05 was considered statistically significant.
Results
Global Trends and Gender Disparity of Epilepsy Burden
The all-age prevalence number due to epilepsy increased by 60.6%, from 17.0 (95% UI: 13.0–21.5) million in 1990 to 27.3 (95% UI: 21.6–33.4) million in 2017 (Figure 1A). After controlling for the effect of population and age structure, age-standardized prevalence rate of epilepsy rose by 13.6%, from 316.0 (95% UI: 244.4–399.3) per 100,000 population in 1990 to 359.1 (95% UI: 283.8–441.4) per 100,000 population in 2017 (Figure 1B). Similarly, the all-age DALYs number owing to epilepsy increased from 13.0 (95% UI: 10.3–15.9) million in 1990 to 14.8 (95% UI: 11.4–19.0) million in 2017, with a rise of 13.8% (Figure 1C). After controlling for population and age structure, a similar decline was observed in the age-standardized DALY rate between 1990 and 2017 (Figure 1D). Age-standardized DALY rate fell by 16.1% from 233.5 (95% UI: 186.4–285.9) per 100,000 population in 1990 to 195.8 (95% UI: 151.4–251.8) per 100,000 population in 2017. Among 21 GBD regions, despite the substantial decline in DALY rates (Figure 2B; Supplementary Table 1), a slight rise in the age-standardized prevalence rate of epilepsy was observed for both sexes from 1990 to 2017 (Figure 2A).

Figure 1. Global total and gender-specific burden of epilepsy by year and age. (A) All-age prevalence numbers from 1990 to 2017. (B) Age-standardized prevalence rates per 100,000 population from 1990 to 2017. (C) All-age DALY numbers from 1990 to 2017. (D) Age-standardized DALY rates per 100,000 population from 1990 to 2017. (E) Age-specific prevalence rate in 2017. (F) Age-specific DALY rate in 2017. DALYs, disability-adjusted life-years.
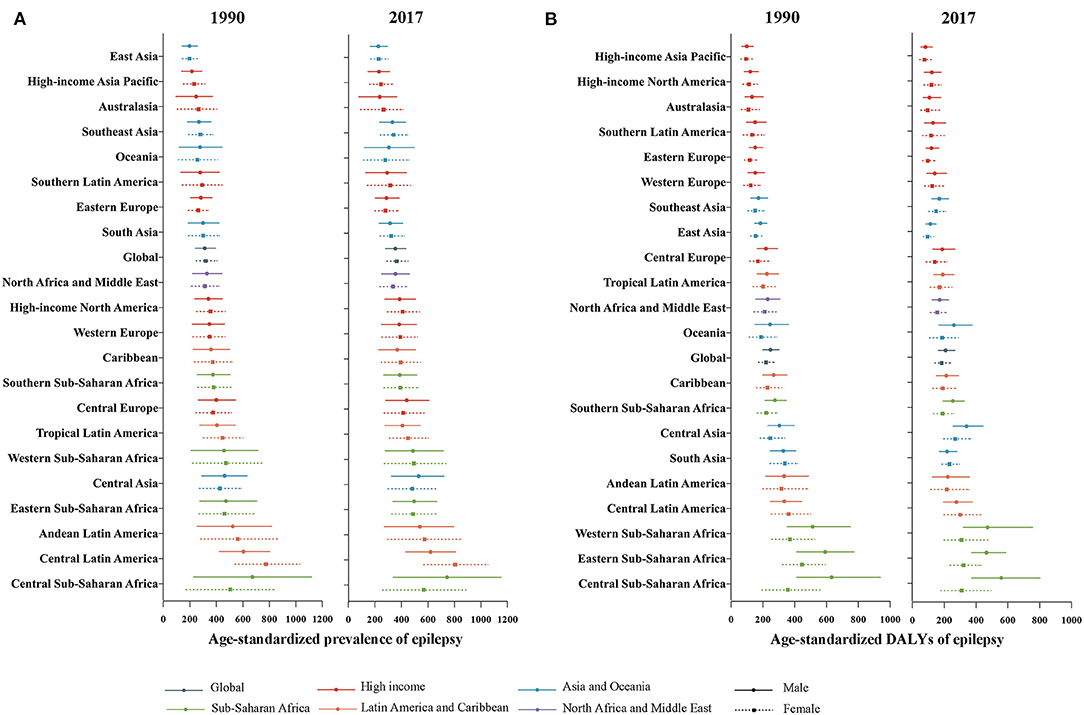
Figure 2. Global gender-specific burden of epilepsy by 21 GBD regions in 1990 and 2017. (A) Age-standardized prevalence rate. (B) Age-standardized DALYs rate. The Y-axis order is arranged according to the level of the age-standardized prevalence and DALYs rate of males. DALYs, disability-adjusted life-years.
As shown in Figure 1, gender disparities existed in global burden of epilepsy since 1990, in the aspect of absolute number (p < 0.001), crude rates (p < 0.001), and age-standardized rates (p < 0.001) of DALYs. The total DALY numbers were 7.9 (95% UI: 6.2–10.1) million in men and 6.8 (95% UI: 5.2–9.0) million in women in 2017. Similarly, male subjects had a higher epilepsy burden in the crude DALY rates (206.4 DALYs per 100,000 men vs. 180.7 DALYs per 100,000 women) and age-standardized DALY rates (208.1 DALYs per 100,000 men vs. 183.5 DALYs per 100,000 women) than females in 2017. In Figures 2A,B, the greatest gender gap in DALY rate was found in Central Sub-Saharan Africa (male 631.7 vs. female 356.3 in 1990, male 558.3 vs. female 309.7 in 2017), followed by Eastern Sub-Saharan Africa and Western Sub-Saharan Africa regions.
Global Gender-Specific Epilepsy Burden by Age
Age-specific prevalence rates of epilepsy reached a peak in the 95+ age group [817.2 (95% UI: 717.7–744.6] (Figure 1E). Three peaks [1–4: 244.7 (95% UI: 195.2–313.0), 15–19: 237.3 (95% UI: 178.0–309.3), and 85–89 years: 221.2 (95% UI: 159.6–304.5)] and a trough [50–54 years: 148.5 (95% UI: 116.3–191.0)] were observed on the age-specific DALY rate in 2017 (Figure 1F). Higher age-specific DALY rates were observed in males at all age groups (p < 0.01).
Geographic Variation and Socioeconomic Disparity in Epilepsy Burden
Among the 195 countries and territories analyzed in the GBD 2017 study, there was considerable geographic variation in the epilepsy burden worldwide (Figure 3). The epilepsy burden concentrated in many Asian and African countries with a large population and low socioeconomic status. As shown in Figure 3A, DALYs number was greatest in India [2,984,664.7 (95% UI: 2,409,842.9–3,681,587.1)], followed by China, Nigeria, Pakistan, and United Status. Notably, the highest rate of age-standardized DALYs was concentrated in the Central African Republic [525.1 (95% UI: 291.2–832.7) per 100,000 population], followed by several African countries such as Eritrea, Angola, Mozambique, and Congo (Figure 3B). The lowest age-standardized DALY rate was concentrated in developed countries in Asia and Europe, such as Japan [68.0 (95% UI: 42.2–107.0) per 100,000 population], Singapore, Spain, Switzerland, and Sweden.
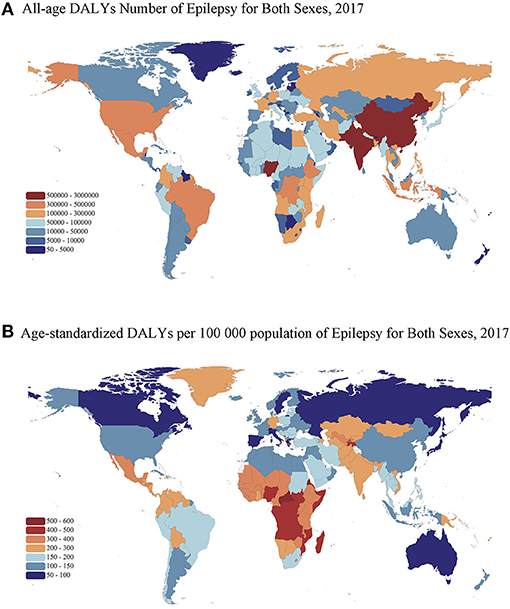
Figure 3. Maps of all-age DALY number and age-standardized DALYs rate of epilepsy in 2017 for both sexes. (A) All-age DALY number. (B) Age-standardized DALY rate per 100,000 population. DALYs, disability-adjusted life-years. The boundaries shown and the designations used on the maps do not imply the expression of any opinion of the authors.
Figure 4 shows the time trends of the Gini coefficient and CI. The Gini coefficients of epilepsy across countries decreased from 0.273 in 1995 to 0.259 in 2017 for the age-standardized DALY rate (Figure 4A), indicating that the between-country disparity of the epilepsy burden was declining. The negative values of the CI indicated that epilepsy burden was more concentrated in countries with lower socioeconomic development (Figure 4B).
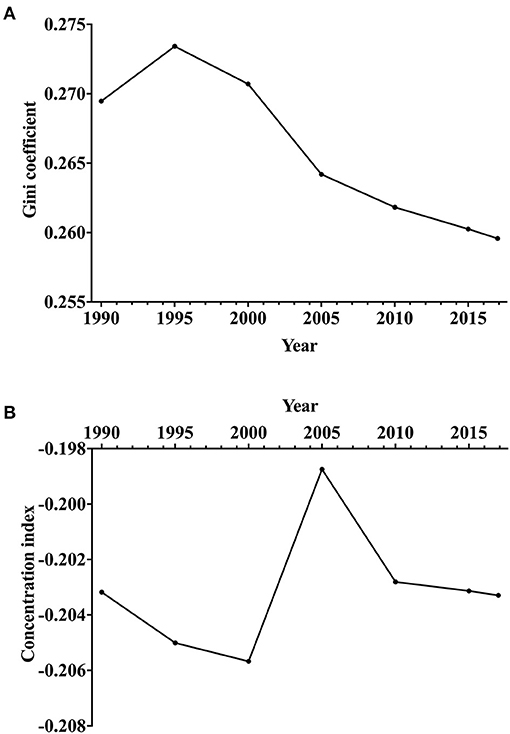
Figure 4. Trends of socioeconomic disparity in epilepsy burden in terms of age-standardized DALY rate across countries from 1990 to 2017. (A) Gini coefficient. (B) Concentration index. DALYs, disability-adjusted life-years.
The socio-demographic index (SDI) data in 2017 were available for 195 countries and territories, including 35 in the low SDI group, 41 in the low-middle SDI group, 40 in the middle SDI group, 41 in the high-middle SDI group, and 38 in the high SDI group. In Figures 5A,B, each bubble represents a country or a territory. The area of the bubble indicates the absolute burden number in the different SDI regions. The Y coordinate represents the age-standardized burden rate. The middle SDI and low-middle SDI regions suffered a higher burden in terms of prevalence numbers and age-standardized prevalence rates (Figure 5A), whereas the greatest DALY numbers and age-standardized DALY rates were located in the low SDI and low-middle regions (Figure 5B).
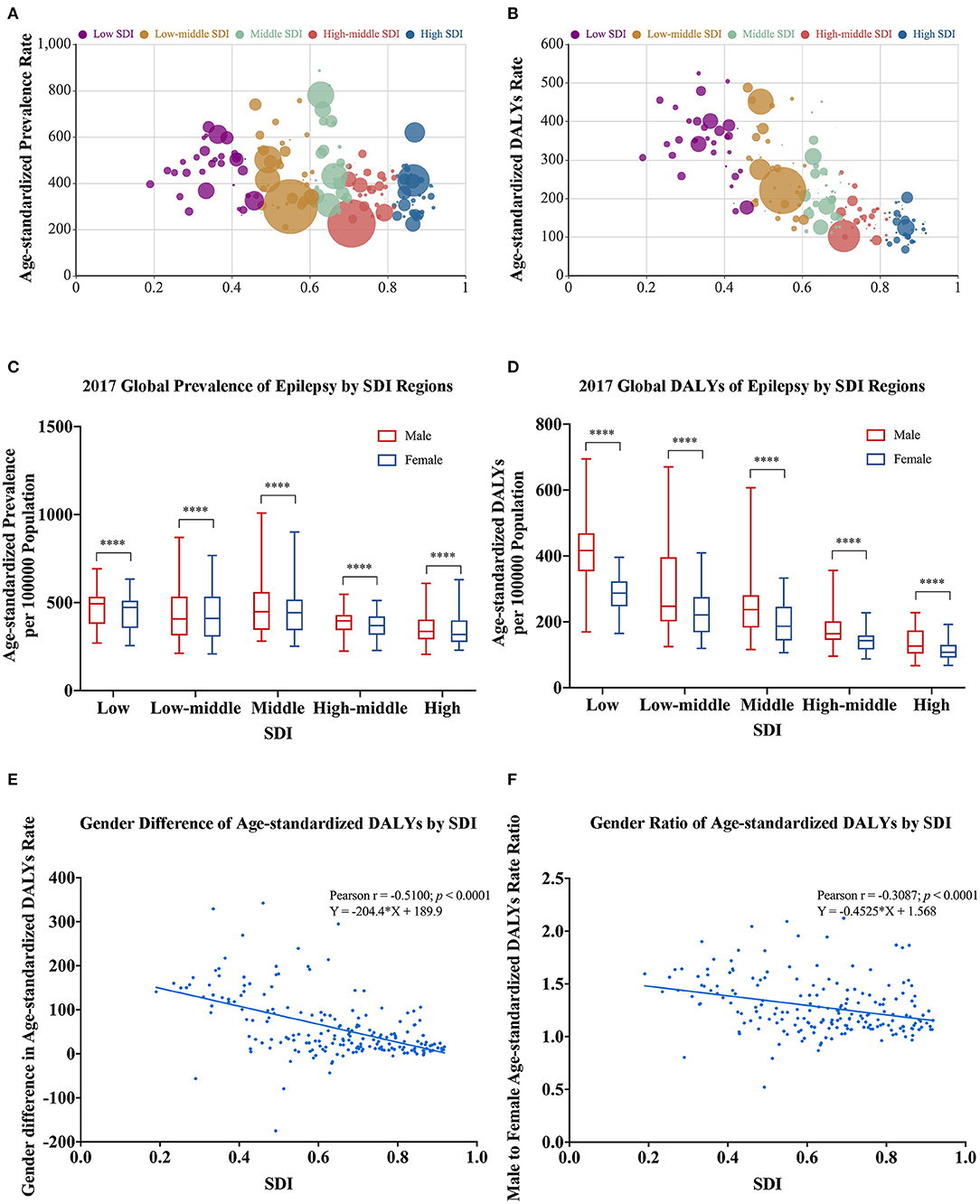
Figure 5. Gender-specific burden of epilepsy in different SDI regions in 2017. (A) All-age prevalence number and age-standardized prevalence rate (the area of the bubble represents the prevalence number). (B) All-age DALY number and age-standardized DALY rate (the area of the bubble represents the DALYs number). (C) Age-standardized prevalence rate by sex in different SDI groups. (D) Age-standardized DALY rate by sex in different SDI groups. (E) Association of gender difference (male minus female) in age-standardized DALY rates with SDI. (F) Association of gender ratio (male to female) in age-standardized DALY rates with SDI. DALYs, disability-adjusted life-years; SDI, socio-demographic index. *For difference between sexes; ****p < 0.0001, paired Wilcoxon signed rank test.
Males had higher age-standardized prevalence (p < 0.0001) and DALY rates (p < 0.0001) than females in all five SDI regions in 2017 (Figures 5C,D). Pearson correlation (r = −0.5100, p < 0.001) and linear regression analysis (Y = −204.4 × X + 189.9) indicated that gender differences (male minus female) in age-standardized DALY rates and SDI had a negative correlation (Figure 5E). Similarly, gender ratios (male to female) in age-standardized DALY rate were negatively associated with SDI in Pearson correlation (r = −0.3087, p < 0.001) and linear regression analysis (Y = −0.4525 × X + 1.568) (Figure 5F). Both sets of analyses showed that gender differences in epilepsy DALY rates were greater in countries with lower SDI.
Discussion
Epilepsy has been a common public concern with a worldwide distribution. Our analysis of the GBD 2017 study presented that the global burden of epilepsy declined in terms of age-standardized DALYs over the past decades. Epilepsy caused a higher burden on adolescents and old people, especially in males. Countries with lower socioeconomic status and underdeveloped regions tended to have higher epilepsy burden and greater gender gap.
Epilepsy accounts for a significant proportion of the world's disease burden, with an increasing incidence in LMICs (49–215 per 1,000,000 people per year) (32–35). In this study, epilepsy caused 14.8 million DALYs, which accounted for 0.59% of total global DALYs in the GBD 2017 study. The DALYs numbers of epilepsy were greatest in India and China. The increasing burden in our results, as measured by the absolute number of DALYs, might be partly due to rapidly aging and growing population, rising life expectancy, and more risk factors (e.g., infections, birth injury, trauma, and stroke) (19, 23). A high burden of epilepsy has also been demonstrated in previous studies of specific regions [South Africa (7), China (8), and southeast Nigeria (36)] by using the DALY metrics. The number of patients with epilepsy is expected to rise further, as more than 5 million new cases are diagnosed each year (32). These findings necessitate more health-care approaches of government for the management of epilepsy.
Despite a steadily growing tendency in the DALY number of epilepsies, a reduction was observed in crude DALY rate and age-standardized DALY rate between 1990 and 2017. This is supported by a systematic analysis for the GBD 2016 study (37), which found a significant reduction in the mortality and DALY rates in patients with epilepsy globally from 1990 to 2016. Similarly, the age-standardized DALYs of all neurological disorders had an overall decrease between 1990 and 2015 (9). Factors contributing to the health improvement include continuing progress in treatment conditions, advanced medical technology, public awareness of epilepsy, and measures on epilepsy prevention by the government (6, 37).
As shown in Figure 1F, after controlling for the effect of population, the DALY rate of epilepsy in 2017 had three peaks in 1–4, 15–19, and 85–89 years old in 2017. These findings are partially consistent with those of several previous studies (9, 38). Epilepsy has a bimodal distribution according to age with peaks in the youngest individuals and the elderly (9, 23). The higher incidence of epilepsy in Europe occurs at 0–1 years old (39). The incidence of epilepsy in resource-rich countries is highest in the first few months of life, particularly in the immediate postnatal period, which falls significantly after the 1st year of life (40). The peak of DALY rate in young infants was probably due to perinatal hypoxia and trauma, metabolic disturbances, congenital malformations of the brain, and infection (19, 41). Inadequate perinatal care and high premature mortality are possible reasons for higher burden of epilepsy in infants. Perinatal and post-infective encephalopathy, cortical dysplasia, and hippocampal sclerosis account for the most severe symptomatic epilepsies in children (42). Sillanpää et al. (43) found a 21.7-fold risk of occurrence of a disability in children with epilepsy compared with controls.
It is worth noting that the DALY rate also reaches a peak in the adolescents in our findings. Similarly, the prevalence rate of epilepsy had a peak during adolescence in Figure 1E. A GBD study of epilepsy indicated that the years of life lost (YLLs) of idiopathic epilepsy peaked at age under 5 years and at age of 15–19 years (37). The probable etiology or risk factor for epilepsy depends on the age of the patient and the type of epilepsy (44). Myoclonic and absence seizures often occur between 5 and 15 years old. Two thirds of the cases with epilepsy were classified as idiopathic or cryptogenic, which is usually seen in the adolescents (45). In adolescents, idiopathic epilepsies account for the majority of cases, although trauma and infection play a role. Most idiopathic epilepsy syndromes have complex inheritance, probably because of interacting genetic and environmental factors (46). Furthermore, Infants and adolescents with epilepsy have difficulty in access to diagnosis and health care. Stigmatization and poor acceptability of epilepsy impact on the quality of life and long-term outcomes of infants and adolescents with epilepsy (47).
Moreover, the most common causes in the elderly are idiopathic epilepsy, trauma, head injury, alcohol abuse, brain tumors, and cerebrovascular disease (46). Epilepsy is associated with a number of age-related and aging-related diseases, such as Alzheimer's disease, dementias, stroke, and vascular and metabolic disorders (23).
Our study revealed that gender disparities in epilepsy have existed at a global level since 1990. In our study, significantly higher prevalence rates (p < 0.0001) and DALY rates (p < 0.0001) were found among males in different SDI regions. Pearson correlation and linear regression analysis indicated that the greater sexual differences in epilepsy DALY rates appeared in countries with lower SDI. Our findings provide data support for the gender research of epilepsy. Several studies indicated that the incidence of epilepsy was slightly higher in men than in women (11, 48–50). Steroid hormones might be the potential mechanism of gender differences in epilepsy (49). However, we have not found the specific biological basis of sex disparity in epilepsy in the previous literature (1, 11, 49), which needs further studying. It has been suggested that males are more susceptible to injury-induced seizures than females (49). Males have a higher lifetime risk of suffering from epilepsy, and this might be owing to men's occupation and their exposure to risk factors, such as head trauma and alcohol use. Additionally, because of the stigma and low family economic status in rural areas, females tend to have a lower consultation rate (10). Another survey (32) suggested that women were more likely to conceal the symptoms of epilepsy for sociocultural reasons in India.
More importantly, our findings pointed out that the burden of epilepsy is higher in many developing regions with low SDI and low-middle SDI, such as Sub-Saharan Africa, Latin America, and Central and South Asia. The negative CI indicated that countries with low levels of socioeconomic development had a higher epilepsy burden. Epilepsy has a significant economic impact on health-care needs, premature death, and disruption of work or education for individuals and their families (4, 51). A review of studies estimating the cost of epilepsy reported that the direct and indirect cost per person per year ranged from US$ 1,736 to 5,848 and 2,037 to 8,587, respectively (4). Several systematic studies (32, 37, 52) have reported that there was an interconnection between epilepsy and poverty. The lower socioeconomic status was related to poor health services, poor awareness of medical care, and infrequent outpatient clinic visits. The association between lower socioeconomic status with a higher burden of epilepsy in our study is in accordance with a review on epilepsy in Asia (6). The median numbers of neurologists in Asia, a region with great differences in economic development, were 0.03 and 2.96 per 100,000 population in low-income countries and high-income countries, respectively. The high treatment gap, which can be caused by inadequate health care response to epilepsy and unaffordable drug treatment, resulted in difficulties in epilepsy management in low-income regions. Moreover, the research showed that the epilepsy burden of epilepsy-related premature mortality is higher in LMICs than a burden in HICs (53). Lack of access to medical facilities and preventable risk factors (drowning, head injuries, and burns) in low-income countries have promoted the occurrence of epilepsy.
Poor knowledge of epilepsy and less education also affect the burden of epilepsy. People in rural areas of Asia and Africa tended to have more negative attitudes toward epilepsy than those in developed countries (54, 55). Besides, patients with active epilepsy living in low-income and middle-income countries receive no timely and effective treatment because the stigma and discrimination are difficult to overcome (56). The stigma can impact the quality of life and work for patients and their families. The burden in countries with low socioeconomic status can be reduced by further education about epilepsy and improved treatments, including antiepileptic drugs (AEDs).
Encouragingly, the analysis of the Gini coefficient indicated that the health disparity was shrinking worldwide. Comprehensive epilepsy care programs played an important role in this decrease. Relevant projects in many countries, such as China (57), India (58), and Brazil (59) have been carried out to reduce the burden of epilepsy and improve access to health-care services (11). Furthermore, the steady rise of CI between 2000 and 2005 may also be associated with the progress in health from the World Health Organization (WHO) Program. Since 1997, the Global Campaign Against Epilepsy was formed by the WHO, the International League against Epilepsy (ILAE), and the International Bureau for Epilepsy (IBE) (17). Between 2000 and 2003, WHO regional declarations on epilepsy were adopted to encourage country cooperation on reducing the epilepsy treatment gap.
To our knowledge, this study has provided a comprehensive assessment of the global burden of epilepsy at a regional level by using systematic and reliable GBD measures. Compared with the previous studies on the global burden of epilepsy, our study highlights and analyzes the gender and socioeconomic disparities of global burden of epilepsy concretely. We discussed not only the gender disparity by year, age, and region, but also the relationship between gender difference and ratio in the global burden of epilepsy with SDI. This is a more specific perspective of discovering the gender disparity of global burden of epilepsy. This study could help the decision-makers to pay more attention to gender and socioeconomic-related disparities in epilepsy burden. The long-term patterns in gender and socioeconomic-related disparities in epilepsy burden could be analyzed by the annual updates of the GBD study.
However, our study has several limitations. First, our research is subject to the GBD 2017 study and therefore has the same GBD methodological disadvantages, which have been described previously (21, 23, 24, 33). Although the GBD 2017 study provided estimates through hierarchical models by collecting data from representative population-based studies, the quality and availability of data are still limited. Therefore, the data sources and statistical assumptions may lead to bias in the included literature. The selection and publication bias may have contributed to the rise of CI between 2000 and 2005. Second, most epidemiological research on epilepsy has been done in high-income regions. There is an urgent need for more research in low SDI regions to raise awareness of epilepsy. Third, the impact of risk factors and specific classification of epilepsy on the epilepsy burden could be explored in a further research.
Conclusion
The global epilepsy burden in terms of DALY rates had substantial declines in the past decades. However, continuous growth in DALY numbers indicates high costs of health care in the future owing to the aging population. Males, especially those who live in less developed countries, suffer a heavier burden of epilepsy than females. A higher burden of epilepsy has been found in countries with lower SDI. These findings could draw the attention to the gender difference and geographical distribution in global epilepsy burden and help to formulate health policies, especially in developing countries, to reduce the gender gap and burden of epilepsy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YH, YS, and QD: literature search and data collection. YH, YS, QD, YD, and CS: analysis and interpretation. YH, YS, QD, YD, CS, SW, MD, and YX: drafting of manuscript. All authors: approved the final version.
Funding
This study was supported by the Natural Science Foundation of China (no. 81900853) and the China Postdoctoral Science Fund (2019M652107).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the Global Burden of Disease Study collaborators for their work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.643450/full#supplementary-material
References
1. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet (London, England). (2019) 393:689–701. doi: 10.1016/S0140-6736(18)32596-0
2. Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. (2005) 46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x
3. Ostendorf AP, Gedela S. Effect of epilepsy on families, communities, and society. Semin Pediatr Neurol. (2017) 24:340–7. doi: 10.1016/j.spen.2017.10.007
4. Allers K, Essue BM, Hackett ML, Muhunthan J, Anderson CS, Pickles K, et al. The economic impact of epilepsy: a systematic review. BMC Neurol. (2015) 15:245. doi: 10.1186/s12883-015-0494-y
5. Fiest KM, Birbeck GL, Jacoby A, Jette N. Stigma in epilepsy. Curr Neurol Neurosci Rep. (2014) 14:444. doi: 10.1007/s11910-014-0444-x
6. Trinka E, Kwan P, Lee B, Dash A. Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia. (2019) 60(Suppl 1):7–21. doi: 10.1111/epi.14458
7. Wagner RG, Ibinda F, Tollman S, Lindholm L, Newton CR, Bertram MY. Differing methods and definitions influence DALY estimates: using population-based data to calculate the burden of convulsive epilepsy in rural South Africa. PLoS One. (2015) 10:e0145300. doi: 10.1371/journal.pone.0145300
8. Ding D, Hong Z, Wang WZ, Wu JZ, de Boer HM, Prilipko L, et al. Assessing the disease burden due to epilepsy by disability adjusted life year in rural China. Epilepsia. (2006) 47:2032–7. doi: 10.1111/j.1528-1167.2006.00802.x
9. GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. (2017) 16:877–97. doi: 10.1016/S1474-4422(17)30299-5
10. Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. (2009) 85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003
11. Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. (2005) 46:956–60. doi: 10.1111/j.1528-1167.2005.51204.x
12. Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of epilepsy in the Parsi community of Bombay. Epilepsia. (1988) 29:111–5. doi: 10.1111/j.1528-1157.1988.tb04405.x
13. Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia. (2002) 43 Suppl 6:21–5. doi: 10.1046/j.1528-1157.43.s.6.11.x
14. Espinosa-Jovel C, Toledano R, Aledo-Serrano Á, García-Morales I, Gil-Nagel A. Epidemiological profile of epilepsy in low income populations. Seizure. (2018) 56:67–72. doi: 10.1016/j.seizure.2018.02.002
15. Heaney DC, MacDonald BK, Everitt A, Stevenson S, Leonardi GS, Wilkinson P, et al. Socioeconomic variation in incidence of epilepsy: prospective community based study in south east England. BMJ. (2002) 325:1013–6. doi: 10.1136/bmj.325.7371.1013
16. Hesdorffer DC, Tian H, Anand K, Hauser WA, Ludvigsson P, Olafsson E, et al. Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia. (2005) 46:1297–303. doi: 10.1111/j.1528-1167.2005.10705.x
17. World Health Organization. Epilepsy. (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/epilepsy
18. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. (2010) 51:883–90. doi: 10.1111/j.1528-1167.2009.02481.x
19. Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet (London, England). (2012) 380:1193–201. doi: 10.1016/S0140-6736(12)61381-6
20. Begley CE, Beghi E. The economic cost of epilepsy: a review of the literature. Epilepsia. (2002) 43(Suppl 4):3–9. doi: 10.1046/j.1528-1157.43.s.4.2.x
21. GBD 2017 SDG Collaborators. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). (2018) 392:2091–138. doi: 10.1016/S0140-6736(18)32281-5
22. GBD 2017 Population and Fertility Collaborators. Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). (2018) 392:1995–2051. doi: 10.1016/S0140-6736(18)32278-5
23. Beghi E, Giussani G. Aging and the epidemiology of epilepsy. Neuroepidemiology. (2018) 51:216–23. doi: 10.1159/000493484
24. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7
25. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
26. Lou L, Wang J, Xu P, Ye X, Ye J. Socioeconomic disparity in global burden of cataract: an analysis for 2013 with time trends since 1990. Am J Ophthalmol. (2017) 180:91–6. doi: 10.1016/j.ajo.2017.04.008
27. Skaftun EK, Verguet S, Norheim OF, Johansson KA. Geographic health inequalities in Norway: a Gini analysis of cross-county differences in mortality from 1980 to 2014. Int J Equity Health. (2018) 17:64. doi: 10.1186/s12939-018-0771-7
28. Jin J, Wang J, Ma X, Wang Y, Li R. Equality of medical health resource allocation in China based on the Gini Coefficient Method. Iran J Public Health. (2015) 44:445–57.
29. Costa-Font J, Hernández-Quevedo C. Measuring inequalities in health: what do we know? What do we need to know? Health Policy. (2012) 106:195–206. doi: 10.1016/j.healthpol.2012.04.007
30. Rosner B, Glynn RJ, Lee ML. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics. (2006) 62:185–92. doi: 10.1111/j.1541-0420.2005.00389.x
31. Hazra A, Gogtay N. Biostatistics Series Module 6: correlation and linear regression. Indian J Dermatol. (2016) 61:593–601. doi: 10.4103/0019-5154.193662
32. Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. (2017) 88:296–303. doi: 10.1212/WNL.0000000000003509
33. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). (2018) 392:1859–922. doi: 10.1016/S0140-6736(18)32335-3
34. Beghi E. The Epidemiology of Epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
35. Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. (2011) 77:1005–12. doi: 10.1212/WNL.0b013e31822cfc90
36. Ughasoro MD, Onwujekwe OE, Ojinnaka NC. Determining the disability adjusted life years lost to childhood and adolescence epilepsy in southeast Nigeria: an exploratory study. Epilepsy Res. (2016) 125:37–41. doi: 10.1016/j.eplepsyres.2016.05.006
37. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:357–75. doi: 10.1016/S1474-4422(18)30454-X
38. Olafsson E, Hauser WA. Prevalence of epilepsy in rural Iceland: a population-based study. Epilepsia. (1999) 40:1529–34. doi: 10.1111/j.1528-1157.1999.tb02036.x
39. Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe - a systematic review. Eur J Neurol. (2005) 12:245–53. doi: 10.1111/j.1468-1331.2004.00992.x
40. Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935-1984. Epilepsia. (1995) 36:327–33. doi: 10.1111/j.1528-1157.1995.tb01005.x
41. Glass HC, Shellhaas RA, Tsuchida TN, Chang T, Wusthoff CJ, Chu CJ, et al. Seizures in Preterm Neonates: a Multicenter Observational Cohort Study. Pediatric neurology. (2017) 72:19–24. doi: 10.1016/j.pediatrneurol.2017.04.016
42. Guerrini R. Epilepsy in children. Lancet (London, England). (2006) 367:499–524. doi: 10.1016/S0140-6736(06)68182-8
43. Sillanpää M. Epilepsy in children: prevalence, disability, and handicap. Epilepsia. (1992) 33:444–9. doi: 10.1111/j.1528-1157.1992.tb01689.x
44. Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet (London, England). (1990) 336:1267–71. doi: 10.1016/0140-6736(90)92960-P
45. Kwan P, Sander JW. The natural history of epilepsy: an epidemiological view. J Neurol Neurosurg Psychiatry. (2004) 75:1376–81. doi: 10.1136/jnnp.2004.045690
46. Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: contributions of the Rochester epidemiology project. Mayo Clinic Proc. (1996) 71:570–5. doi: 10.4065/71.6.570
47. Ackermann S, Le Roux S, Wilmshurst JM. Epidemiology of children with epilepsy at a tertiary referral centre in South Africa. Seizure. (2019) 70:82–9. doi: 10.1016/j.seizure.2019.06.018
48. Kim DW, Lee SY, Chung SE, Cheong HK, Jung KY. Clinical characteristics of patients with treated epilepsy in Korea: a nationwide epidemiologic study. Epilepsia. (2014) 55:67–75. doi: 10.1111/epi.12469
49. Reddy DS. The neuroendocrine basis of sex differences in epilepsy. Pharmacol Biochem Behav. (2017) 152:97–104. doi: 10.1016/j.pbb.2016.07.002
50. Sayehmiri K, Tavan H, Sayehmiri F, Mohammadi I, Carson KV. Prevalence of epilepsy in iran: a meta-analysis and systematic review. Iran J Child Neurol. (2014) 8:9–17. doi: 10.2147/PPA.S76563
51. Wibecan L, Fink G, Tshering L, Bruno V, Patenaude B, Nirola DK, et al. The economic burden of epilepsy in Bhutan. Trop Med Int Health. (2018) 23:342–58. doi: 10.1111/tmi.13035
52. Beghi E, Hesdorffer D. Prevalence of epilepsy–an unknown quantity. Epilepsia. (2014) 55:963–7. doi: 10.1111/epi.12579
53. Levira F, Thurman DJ, Sander JW, Hauser WA, Hesdorffer DC, Masanja H, et al. Premature mortality of epilepsy in low- and middle-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. (2017) 58:6–16. doi: 10.1111/epi.13603
54. Tiamkao S, Sawanyawisuth K, Singhpoo K, Ariyanuchitkul S, Ngamroop R. Differences of knowledge, attitudes, and behaviors towards epilepsy between populations in municipal and nonmunicipal areas. Psychol Res Behav Manag. (2013) 6:111–6. doi: 10.2147/PRBM.S50842
55. Ryu HU, Lee SA, Eom S, Kim HD. Perceived stigma in Korean adolescents with epilepsy: effects of knowledge about epilepsy and maternal perception of stigma. Seizure. (2015) 24:38–43. doi: 10.1016/j.seizure.2014.11.010
56. Saxena S, Li S. Defeating epilepsy: a global public health commitment. Epilepsia Open. (2017) 2:153–5. doi: 10.1002/epi4.12010
57. Wang W, Wu J, Dai X, Ma G, Yang B, Wang T, et al. Global campaign against epilepsy: assessment of a demonstration project in rural China. Bull World Health Organ. (2008) 86:964–9. doi: 10.2471/BLT.07.047050
58. Nizamie SH, Akthar S, Banerjee I, Goyal N. Health care delivery model in epilepsy to reduce treatment gap: World Health Organization study from a rural tribal population of India. Epilepsy Res. (2009) 84:146–52. doi: 10.1016/j.eplepsyres.2009.01.008
Keywords: global burden of disease, epilepsy, time trend, gender disparity, socioeconomic disparity
Citation: Hu Y, Shan Y, Du Q, Ding Y, Shen C, Wang S, Ding M and Xu Y (2021) Gender and Socioeconomic Disparities in Global Burden of Epilepsy: An Analysis of Time Trends From 1990 to 2017. Front. Neurol. 12:643450. doi: 10.3389/fneur.2021.643450
Received: 18 December 2020; Accepted: 11 March 2021;
Published: 16 April 2021.
Edited by:
Luiz Eduardo Betting, São Paulo State University, BrazilReviewed by:
Kheng Seang Lim, University of Malaya, MalaysiaAndrew T. Olagunju, McMaster University, Canada
Copyright © 2021 Hu, Shan, Du, Ding, Shen, Wang, Ding and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meiping Ding, bWVpcGluZ2RAMTYzLmNvbQ==; Yufeng Xu, eHV5dWZlbmcwMjE2QHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Yin Hu1†
Yin Hu1† Yi Shan
Yi Shan Qiang Du
Qiang Du Yao Ding
Yao Ding Chunhong Shen
Chunhong Shen Shuang Wang
Shuang Wang Yufeng Xu
Yufeng Xu