- 1Department of Rehabilitation Medicine, National Rehabilitation Center, Ministry of Health and Welfare, Seoul, South Korea
- 2Translational Research Center for Rehabilitation Robots, National Rehabilitation Center, Ministry of Health and Welfare, Seoul, South Korea
Prior studies examining predictors of favorable clinical outcomes after upper limb robot-assisted therapy (RT) have many shortcomings. Therefore, the aim of this study was to identify meaningful predictors and a prediction model for clinically significant motor improvement in upper limb impairment after RT for each stroke phase. This retrospective, single-center study enrolled patients with stroke who received RT using InMotion2 along with conventional therapy (CT) from January 2015 to September 2019. Demographic characteristics, clinical measures, and robotic kinematic measures were evaluated. The primary outcome measure was the Fugl-Meyer Assessment-Upper Extremity (FMA-UE) and we classified patients with improvement more than the minimal clinically important difference as responders for each stroke phase. Univariable and multivariable logistic regression analyses were performed to assess the relationship between potential predictors and RT responders and determine meaningful predictors. Subsequently, meaningful predictors were included in the final prediction model. One hundred forty-four patients were enrolled. The Hand Movement Scale and time since onset were significant predictors of clinically significant improvement in upper limb impairment (P = 0.045 and 0.043, respectively), as represented by the FMA-UE score after RT along with CT, in patients with subacute stroke. These variables were also meaningful predictors with borderline statistical significance in patients with chronic stroke (P = 0.076 and 0.066, respectively). Better hand movement and a shorter time since onset can be used as realistic predictors of clinically significant motor improvement in upper limb impairment after RT with InMotion2 alongside CT in patients with subacute and chronic stroke. This information may help healthcare professionals discern optimal patients for RT and accurately inform patients and caregivers about outcomes of RT.
Introduction
Upper extremity dysfunction commonly occurs after a stroke, affecting ~80% of people with acute stroke and 50% of people with chronic stroke. It negatively affects activities of daily living as well as social activities (1, 2). Therefore, improving upper extremity function is a primary therapeutic goal in stroke rehabilitation (3). Several systematic reviews suggest that repetitive, task-specific, and intensive therapy may result in motor improvement after stroke (4, 5). Robotic systems can provide more consistent, intensive, and repetitive training without fatigue, along with task-specific training by easily applying new constraints to optimize the required movement pattern, as compared to conventional therapy (CT) (6). Recent systematic reviews on robot-assisted therapy (RT) of the upper limb after stroke have reported that a more meaningful clinical outcome is obtained with RT than with CT (7, 8).
Identifying the predictors of a favorable clinical outcome after RT is imperative. It could help healthcare professionals to identify those patients who are best suited for RT and to accurately guide patients and caregivers about the outcomes of RT. It would also improve the cost efficiency of RT, which is currently steep.
Several studies have been conducted to identify predictors so that favorable outcomes with upper limb RT can be ensured among patients with stroke. Hsieh et al. (9) enrolled 55 patients with stroke who had undergone RT using the Bi-Manu-Track (Reha-Stim, Berlin, Germany) and found that the Box and Block Test score and female sex could predict favorable outcomes in the Fugl-Meyer Assessment-Upper Extremity (FMA-UE) and Motor Activity Log scores. The same researchers conducted a secondary analysis by enrolling 66 patients with stroke using the cohort data generated in the aforementioned study (10). Spasticity of the upper extremity and kinematic measures were added to the potential predictors analyzed in the previous study, and lessened flexor synergy and spasticity were found to be predictors of a favorable Wolf Motor Function Test result. Franceschini et al. (11) demonstrated that the Box and Block Test score, FMA-UE score, and Motricity Index (MI) upper limb could predict a favorable post-RT Modified Barthel Index using data from 60 patients with stroke who had undergone RT using InMotion2 (Interactive Motion Technologies, Watertown, MA, USA). Duret et al. (12) enrolled 46 patients with stroke who had undergone RT using InMotion2 and demonstrated that the time since onset and Fugl-Meyer Assessment (FMA) shoulder/elbow score were predictors of a favorable post-RT FMA shoulder/elbow score. Although these two variables could predict improvement after RT, they could not predict improvement more than the minimal clinically important difference (MCID), which is the minimal effect that has clinical relevance in patient management (13).
Many studies have been conducted to identify predictors; however, they had several limitations, such as inconsistently identified predictors, inadequate number of subjects, limited numbers of analyzed potential predictors, and unclearly distinguished stroke phases, despite the difference in recovery depending on the stroke phase. No predictor identified thus far can predict an improvement more than the MCID in the FMA-UE, the main tool used to assess impairment. We hypothesized that some predictors may have the potential to predict improvement more than the MCID of FMA-UE after RT, and these predictors may vary depending on the phase of the stroke. Therefore, the aim of the present study was to identify meaningful predictors and a prediction model for clinically significant motor improvement in cases of upper limb impairment after RT for each stroke phase.
Methods
Study Design and Setting
This retrospective, single-center study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (14). The Ethics Committee of the Institutional Review Board of the National Rehabilitation Center in South Korea approved this study (approval number, NRC-2019-04-030) and waived the requirement for informed consent because of the retrospective design.
From January 2015 to September 2019, patients with stroke who were admitted to the National Rehabilitation Center in South Korea, and who received RT using InMotion2, were enrolled in this study. The inclusion criteria were a definite diagnosis of unilateral stroke, as evidenced by computed tomography, magnetic resonance imaging, or medical records; a time since onset of ≥7 days for a first-ever stroke; and age ≥19 years. The exclusion criteria were as follows: neurological disorders other than stroke that can cause motor deficits, e.g., Parkinson disease, spinal cord injury, Guillain-Barré syndrome, traumatic brain injury, brain tumor, hypoxic brain injury, cerebral palsy, and peripheral neuropathy; spasticity in the elbow joint with a Modified Ashworth Scale (MAS) grade >3; severe upper extremity pain that could interfere with RT (Numeric Rating Scale score ≥5); upper extremity fracture within 3 months; uncontrolled severe medical conditions; a history of non-invasive brain stimulation; RT for <20 sessions; and incomplete medical records.
The patients' data were sourced from the electronic medical records in the database of our health care institute. The demographic, clinical, and robotic records were extracted. The patients' records were de-identified before analysis. The principal investigator (JH) conceived and designed the study, and an occupational therapist (SY) collected the data. Investigators (JJ and JH) performed data curation and statistical analysis and wrote and edited the paper.
We analyzed patients with stroke according to time since onset, which was classified as subacute phase (time since onset of ≥7 and <180 days) and chronic phase (time since onset of ≥180 days) (15).
Intervention and Apparatus
Each patient participated in a total of 20 sessions of RT using InMotion2; patients underwent one 30-min RT session per day, 5 days a week for 4 weeks. InMotion2, which has been proven efficient and safe for patients with subacute and chronic stroke (16, 17), is a two-degrees-of-freedom end-effector type robotic device that provides shoulder-elbow flexion/extension training in the horizontal plane. In the seated position with the trunk restrained by a five-point seatbelt to minimize compensatory movement and with the forearm supported by a forearm cradle, each patient performed goal-directed reaching movements in the gravity-compensated horizontal plane. The patients were instructed to move the handle from the center target to each of eight peripheral targets positioned 45 degrees apart in circular arrangements, and the position of the handle was marked on the screen for real-time visual feedback. All the patients also received CT, according to the standardized rehabilitative protocol, involving range of motion exercises, strengthening exercises for the affected upper extremity, and activities of daily living training.
Potential Predictors
To identify meaningful predictors, we included variables known to be related to outcome after therapeutic intervention (18, 19) and those suspected of clinical relevance, but not yet confirmed. Demographic characteristics [age, sex, time since onset, stroke subtype, stroke lesion (cortical, subcortical, or combined cortical and subcortical), and hemiplegic side], clinical measures [FMA-UE score, MI, Medical Research Council Scale for Muscle Strength (MRC) score, MAS grade at the elbow flexor muscle of the hemiplegic side, Hand Movement Scale (HMS), and Brunnstrom Recovery Stage (BRS)], and robotic kinematic measures [smoothness, reach error (RE), path error (PE), and independence] were selected for analysis.
The assessments of FMA-UE, MI, MAS, smoothness, RE, PE, and independence were conducted by experienced occupational therapists before the first RT session and after the last session. The evaluations of MRC-shoulder flexion, extension, abduction, and adduction; MRC-elbow flexion and extension; MRC-wrist flexion and extension; MRC-finger flexion and extension; HMS; and BRS were performed at admission.
Clinical Measures
The FMA-UE is a quantitative measure of motor impairment following a stroke and consists of 33 items rated on a three-point scale (maximum score, 66), with higher scores indicating less severe impairment (20). The scale is composed of sub-scores: 36 for the shoulder/elbow (FMA-A), 10 for the wrist (FMA-B), 14 for the hand (FMA-C), and 6 for coordination (FMA-D). These can be distributed into sub-scores of 42 for the proximal unit of the shoulder/elbow and coordination (FMA-Prox) and 24 for the distal unit of the wrist and hand (FMA-Dist).
The MI is based on the ability to move the upper extremity segment through a range of motion and to resist the force. The MI-upper limb consists of three domains (pinch grasp, elbow flexion, and shoulder abduction). Each domain is scored between 0 and 33, and the total upper limb score (maximum score, 100) is calculated by adding one to the sum of the three domain scores (21).
The MRC score ranges from 0 to 5, with higher scores representing greater muscle strength (22). The MRC-upper extremity score was calculated by summing the MRC-shoulder, MRC-elbow, MRC-wrist, and MRC-finger scores, whereas the MRC-shoulder score was calculated by adding the MRC-shoulder flexion, extension, abduction, and adduction scores. The MRC-elbow, MRC-wrist, and MRC-finger scores were each calculated as the sum of the MRC-elbow flexion and extension, MRC-wrist flexion and extension, and MRC-finger flexion and extension scores, respectively.
The MAS measures spasticity, with a higher grade indicating higher spasticity (23). The MAS spasticity grades of 1+, 2, 3, and 4 were converted to 2, 3, 4, and 5, respectively, while grade 1 remained the same.
The HMS ranges from 1 to 6 and evaluates the ability to perform hand movements of different degrees of difficulty, with a higher number representing better hand movement (24).
The BRS ranges from 1 to 6 and describes the stereotypical stages of motor recovery, starting with flaccidity to full recovery of motor function (25). The BRS consists of different parts; the two parts concerning the upper arm (BRS-upper arm) and the hand (BRS-hand) were used herein.
Robotic Kinematic Measures
Robotic kinematic measures (e.g., smoothness, RE, PE, and independence) were used as potential predictors. Assessments of kinematic measures consist of point-to-point reaching movements and circle drawing movements (26). The point-to-point reaching movement assessment was used to calculate smoothness, RE, and PE, while the circle drawing assessment was conducted to calculate independence. Smoothness was calculated as the mean of the speed divided by the peak speed and is expressed as a value ranging from 0 to 1, where a value closer to 1 indicates better control of movement speed (27). RE and PE represent the ability to move accurately along a straight path toward the center of targets and toward targets, respectively. RE was calculated as the normalized summed difference of the end of the reach from the center of the target with respect to time. PE was calculated as the normalization of the summed deviations from the desired straight path and the participant's actual path from one point to another with respect to time. RE and PE are expressed as a value ranging from 0 to 1, with a value closer to 0 indicating better performance (28). Independence was calculated as the ratio between the major and minor axes of the ellipse that best represents the path drawn by the hand during the circle drawing assessment. Values range from 0 to 1, where values closer to 1 represent fitting ellipses that are closer to a circle, and indicate better coordination of shoulder and elbow movements (29).
Outcome Measure
Since RT focuses upon upper limb impairment, we chose the FMA-UE as the primary outcome measure and calculated the difference in the FMA-UE score before and after RT (ΔFMA-UE). We considered 9 and 5.25 as the MCID for patients with subacute and chronic stroke, respectively (30, 31). As such, patients with subacute stroke who had a ΔFMA-UE value ≥9 and patients with chronic stroke who had a ΔFMA-UE value ≥5.25 were classified as responders in this study. Those with values below the aforementioned were classified as non-responders.
Statistical Analysis
The sample size calculation estimated that 58 subjects would provide 80% power with 5% α and an odds ratio of 2.5 (power analysis using logistic regression according to the guidelines of Lipsey & Wilson and G Power 3.1.9.7 software) (32).
Continuous variables are presented as means and standard deviations, and categorical variables are presented as numbers and percentages. The normal distribution of continuous variables was assessed using the Kolmogorov-Smirnov test. Meaningful predictors were determined using univariable and multivariable logistic regression analyses (33). We performed univariable logistic regression analyses to assess the relationship between potential predictors and the outcome measure, and extracted variables for which the P-value was <0.25 (34). These variables were further tested for correlations among variables using the Pearson or Spearman correlation test depending on the distribution (normal or not). We excluded variables that had a high correlation (|R| > 0.7) (35) and a low odds ratio. To prevent overfitting, we calculated outcome events per predictor variable (EPV) using the number of selected variables. It is recommended that the EPV should be at least 10:1 (36). Next, multivariable stepwise logistic regression analysis was used to determine meaningful predictors. Subsequently, meaningful predictors with a significance level of <0.05 were included in the final prediction model. The goodness-of-fit of the final model and each meaningful predictor was tested with the Hosmer-Lemeshow test. Finally, receiver operating characteristic curves were used to assess the predictive capacity of the developed prediction model and to determine the most reliable cut-off score of each meaningful predictor in relation to responders of RT. Herein, 95% confidence intervals (CIs) are reported for the area under the receiver operating characteristic curves (AUCs). A P-value < 0.05 was considered reflective of statistical significance. Statistical analyses were conducted using the IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
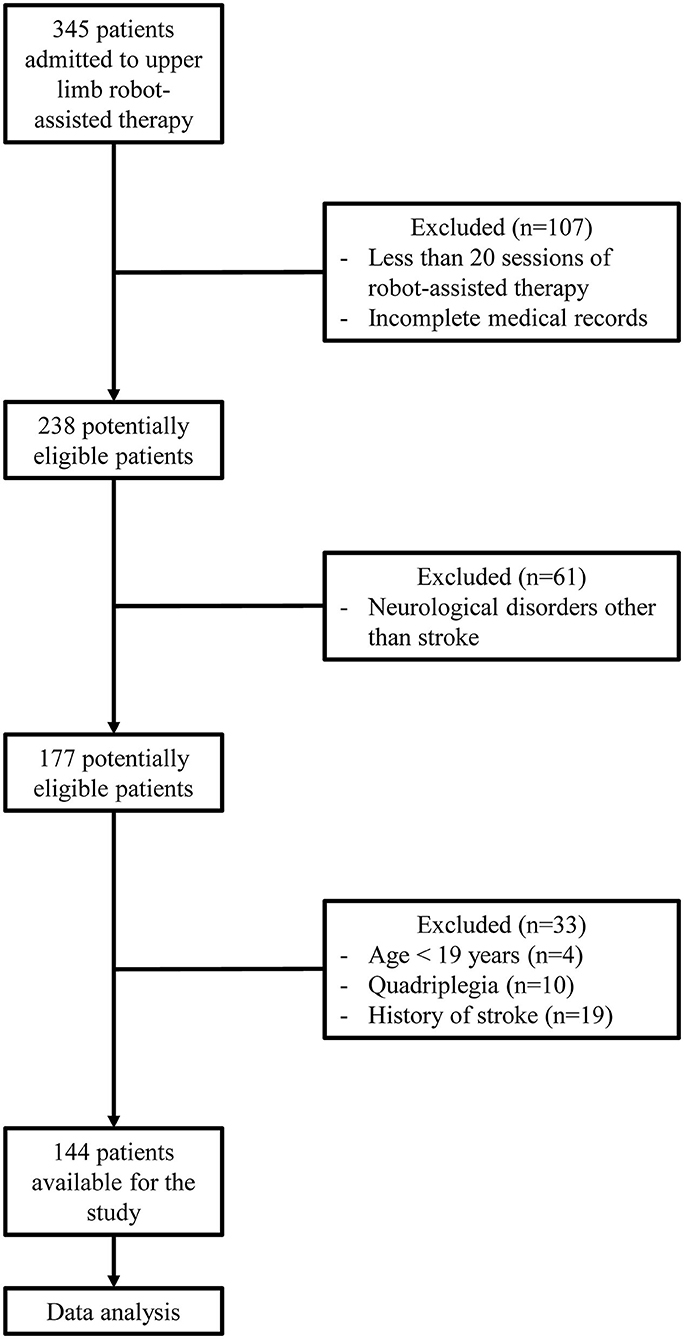
Three hundred forty-five patients underwent RT using InMotion2 between January 2015 and September 2019. Among them, 107 were excluded because of termination of the RT due to medical abnormalities, pain, decreased patient motivation, unexpected discharge, or the absence of evaluation following RT and incomplete medical records. Upon exclusion of 61 patients who underwent RT for a diagnosis other than stroke, 4 patients who were <19 years old, 10 quadriplegic patients, and 19 patients who had a history of stroke, a total of 144 patients were enrolled (Figure 1).
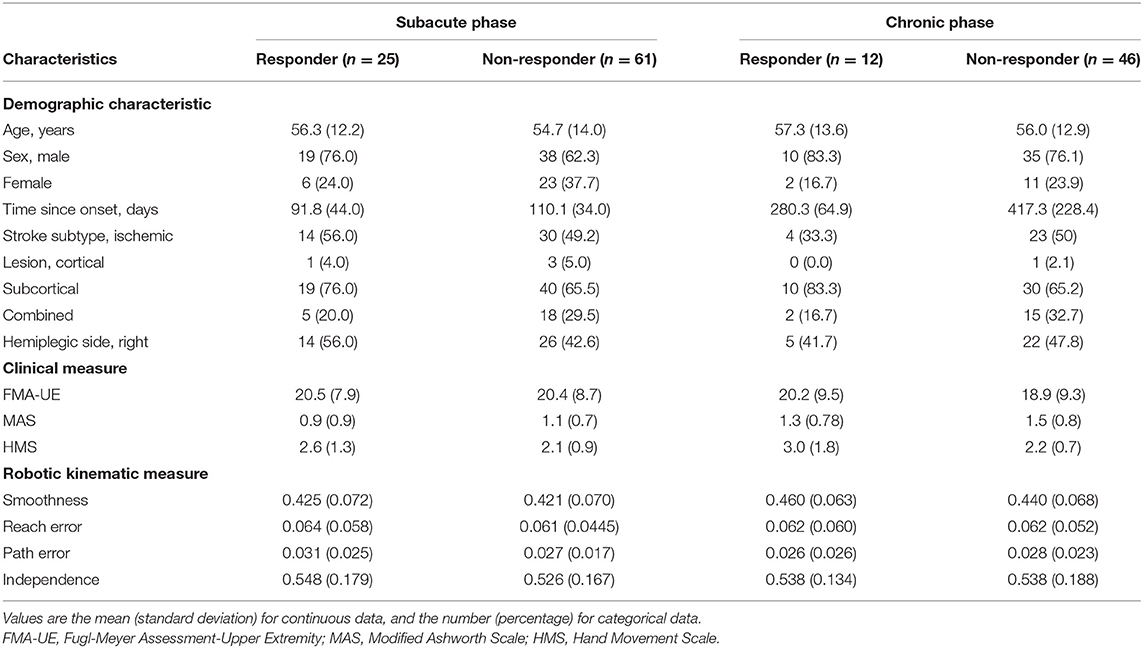
Overall, there were 86 patients with subacute stroke and 58 with chronic stroke. Among patients with subacute stroke, there were 25 responders and 61 non-responders. Among those with chronic stroke, there were 12 responders and 46 non-responders. The characteristics of responders and non-responders by stroke phase are shown in Table 1.
Potential and Meaningful Predictors
Subacute Phase
Variables identified through univariable logistic regression analysis of the relationship between potential predictors and responders of RT with a P-value < 0.25 were sex; time since onset; FMA-C score; MRC-wrist flexion, MRC-wrist extension, MRC-finger extension, and MRC-wrist scores; MAS grade; HMS; and BRS-hand (Supplementary Table 1). Among these variables, a high correlation was demonstrated between MRC-wrist flexion and MRC-wrist extension scores, MRC-wrist flexion and MRC-finger extension scores, MRC-wrist flexion and MRC-wrist scores, MRC-wrist extension and MRC-finger extension scores, MRC-wrist extension and MRC-wrist scores, and between the MRC-finger extension score and the HMS. We excluded the MRC-wrist flexion and MRC-wrist scores that had a low odds ratio. However, if an MRC-finger extension score had a low odds ratio, it was not excluded, as it was presumed to be a major potential predictor. Eight potential predictors were selected and the EPV was >10 (EPV = 10.75). Multivariable stepwise logistic regression analysis of selected potential predictors followed by application of a backward elimination procedure revealed the time since onset and HMS as significantly meaningful predictors (Table 2).
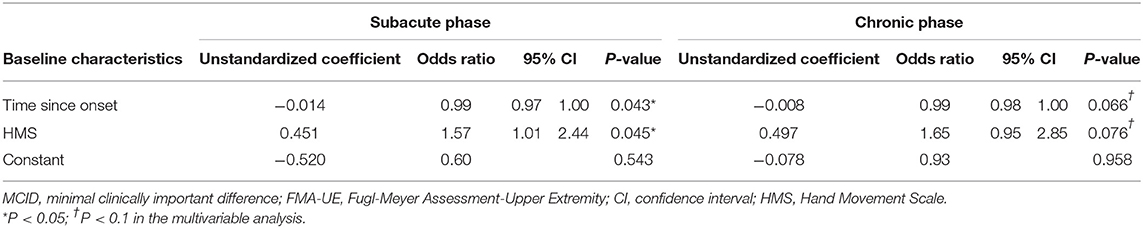
Table 2. Multivariable analyses using the MCID of the FMA-UE as the outcome measure according to stroke phase.
Chronic Phase
In the univariable logistic regression analysis of the relationship between potential predictors and responders of RT, the variables with a P-value < 0.25 were time since onset; MI-upper limb, MRC-wrist extension, MRC-finger flexion, MRC-finger extension, and MRC-finger scores; HMS; and BRS-hand (Supplementary Table 1). Among these variables, a high correlation was demonstrated between the MRC-finger flexion and MRC-finger scores, and between the MRC-finger extension and MRC-finger scores. The MRC-finger scores that had a low odds ratio were excluded. Seven potential predictors were finally selected. The EPV was <10, but was in line with the recommended range of ≥5–9 EPV (EPV = 8.3) (37). Multivariable stepwise logistic regression was conducted on the selected potential predictors, and the time since onset and HMS were identified as meaningful predictors using the backward elimination procedure (Table 2).
Final Prediction Model and Meaningful Predictor Cut-Off Score
In the final prediction model, the time since onset and HMS were included in each subacute and chronic stroke model. Below are the final logistic regression equations.
Both models showed a good fit (Hosmer-Lemeshow test, P > 0.05), and the corresponding AUC values were calculated and plotted as receiver operating characteristic curves (Figure 2). AUC values with 95% CIs were 0.658 (95% CI, 0.520–0.797) for the subacute phase model and 0.739 (95% CI, 0.606–0.872) for the chronic phase model.
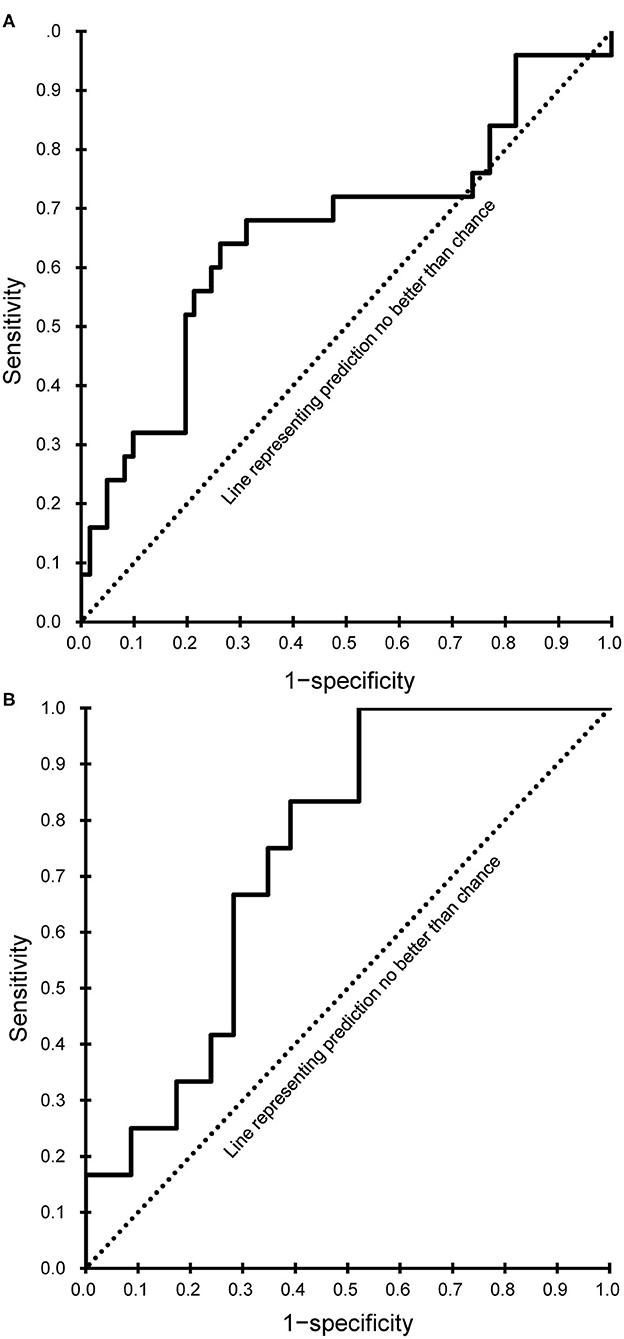
Figure 2. Receiver operating characteristic curves of the final prediction model. (A) Subacute phase. (B) Chronic phase.
Every meaningful predictor showed a good fit (Hosmer-Lemeshow test, P > 0.05). The sensitivity and specificity for the cut-off score of the meaningful predictors were calculated and plotted as receiver operating characteristic curves (Figure 3) for patients with subacute and chronic stroke. Corresponding AUC values with 95% CIs are shown in Table 3.
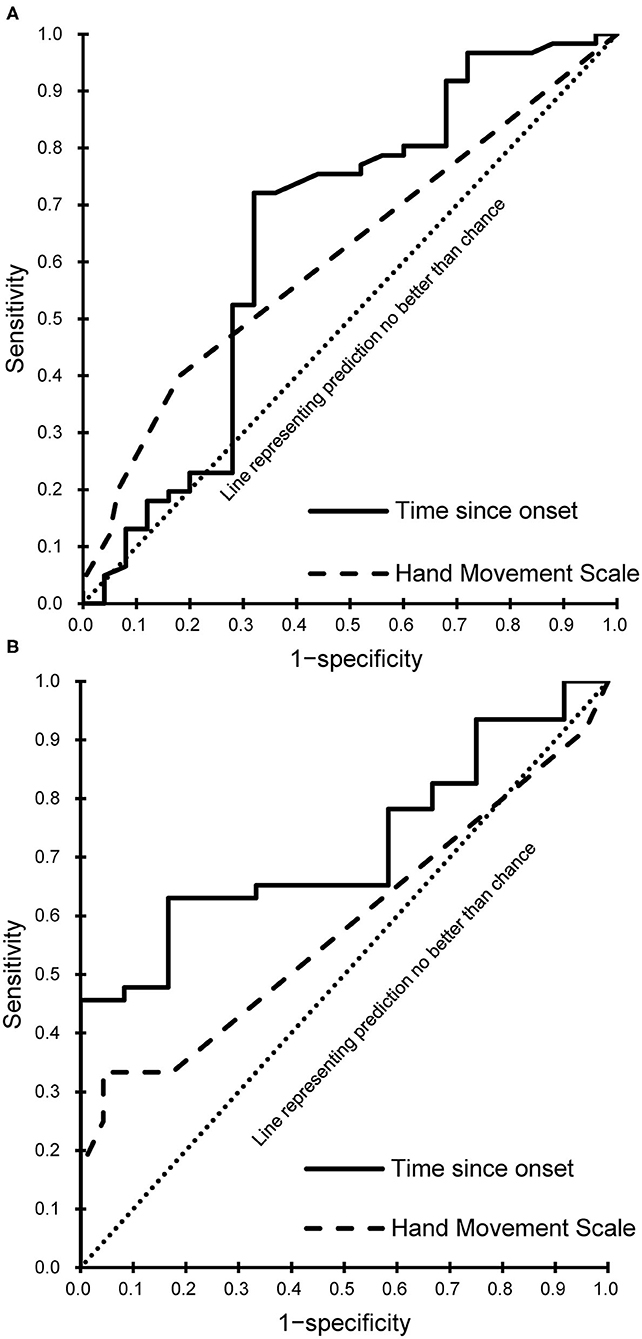
Figure 3. Receiver operating characteristic curves of the meaningful predictors. (A) Subacute phase. (B) Chronic phase.
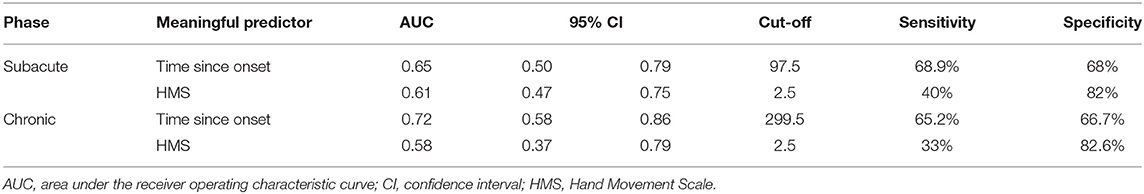
Table 3. The sensitivity and specificity for the cut-off score of the meaningful predictors according to stroke phase.
Discussion
This study demonstrated that the HMS and time since onset were significant predictors for clinically significant motor improvement in upper limb impairment, as represented by the FMA-UE score after RT with InMotion2 alongside CT in patients with subacute stroke. Similarly, the HMS and time since onset were meaningful predictors with borderline statistical significance in patients with chronic stroke.
This study demonstrated that the HMS is a meaningful predictor among patients with subacute and chronic stroke. A baseline HMS that exceeds 2.5, i.e., ≥3 was indicative of a favorable outcome post-RT. An HMS of 3 indicates possible active flexion and extension of all fingers in synergy. Active finger extension has been revealed as an indicator of better recovery of arm function in patients with stroke in multiple studies. Fritz et al. (38) confirmed that active finger extension could predict recovery following constraint-induced movement therapy. Additionally, Smania et al. (39) demonstrated that an MRC-finger extension score >3 could be a predictor of the subacute and chronic stroke phase recovery, and that an HMS >3 could predict recovery in the chronic phase; these results are supportive of the findings of our study. The HMS had a low sensitivity but a high specificity in the present study. Therefore, healthcare professionals can perform HMS when determining the beginning of RT in patients with a subacute or chronic stroke. In cases where the HMS score is ≤2, it can be explained to the patients or caregivers that it is difficult to expect the complete therapeutic effect of RT. This may lead to increased cost efficiency for RT, and the efficient use of hospital resources. The HMS is also easy to perform. For these reasons, the HMS is likely to be a suitable and convenient criterion for responders of RT.
The outcomes of this study are consistent with those of several prior studies showing that baseline dexterity is a major predictor of post-RT upper limb recovery. Hsieh et al. (9) and Huang et al. (10) demonstrated that the Box and Block Test score in patients with chronic stroke was a predictor of motor and functional outcomes following RT, whereas Franceschini et al. (11) confirmed that the Box and Block Test score was a predictor of post-RT functional outcome in patients with subacute stroke. Baseline hand movement, not baseline proximal upper limb function, predicts a favorable outcome; this may be explained by the fact that distal upper limb function is mostly represented unilaterally in the brain, whereas proximal upper limb function is represented bilaterally. Therefore, preservation of hand movement is more related to the degree of sparing of corticospinal pathways than it is to proximal upper limb function and represents a higher recovery potential (40, 41).
Herein, the time since onset was likewise identified as a meaningful predictor of a favorable outcome following RT. Undergoing RT at a shorter time since onset was more effective, specifically before 97.5 days since onset and 299.5 days since onset for patients with subacute and chronic stroke, respectively. Previous studies have confirmed that earlier intervention can predict favorable post-intervention outcomes in such cases. Duret et al. (12) and Mazzoleni et al. (42) suggested that early administration of RT could provide greater functional improvement. Paolucci et al. (18) demonstrated that CT was more effective in patients for whom it was initiated soon after stroke onset, compared to CT in those for whom it was initiated later. The predictive capability of the time since onset may not be surprising, because a shorter time after stroke may be associated with a greater potential for recovery, possibly improving the response to RT. Although stroke recovery is heterogeneous and the long-term effects of stroke are determined by the site and size of the initial stroke lesion, almost all stroke recovery follows a logarithmic pattern time course; in many stroke patients, motor recovery is almost complete after 8 to 12 weeks (1, 43). The time period of 97.5 days since onset that we identified is in line with the results of these prior studies. Moreover, the administration of RT within 299.5 days since onset in patients with chronic stroke was promising for significant recovery, albeit to a lesser degree than that observed for patients in the subacute phase. There is a growing body of evidence supporting the argument that the potential for neuroplasticity and adaptation continues and that motor function improves over time in chronic stroke (16, 44).
The MCID of the FMA-UE has been established 5.25 for patients with chronic stroke (31). For subacute stroke, we selected an FMA-UE score of 9 as the MCID (30). Although another study found an MCID of 4 for patients with subacute stroke (45), we chose 9 because motor recovery in the subacute phase is better than that in the chronic phase. Additionally, mean time since onset in our population was closer to that of Narayan Arya et al. (30) than that of Lundquist et al. (45).
Interestingly, the predictors found in responders of RT among patients with subacute and chronic stroke, were HMS and time since onset. Although both predictors were statistically significant for patients with subacute stroke, they had borderline statistical significance for patients with chronic stroke. This can be explained by combining the characteristics of the two variables. As demonstrated earlier, although a high HMS demonstrates a high potential for recovery due to relatively well-preserved corticospinal pathways following a stroke, RT may have not been as effective in the chronic phase as it was in the subacute phase, and other factors, such as muscle atrophy, fatigue, and pain, may have had a greater effect than the neural substrate related to neural plasticity.
No robotic kinematic measure examined in this study was able to predict responders of RT. This finding is supported by the study of Duret et al. (12), in which predictors of a favorable motor outcome in patients with subacute stroke were identified. However, robotic kinematic measures were unable to predict favorable post-RT outcomes because the measures currently being used are insufficient. Schwarz et al. (46) conducted a systematic review on the kinematic assessment of upper limb movements and demonstrated that the reliability, correlation with the FMA-UE score, and ability to detect longitudinal changes of the kinematic measures used were low. However, Krebs et al. (47) reported that a standard clinical outcome measure and significant correlation was observed when kinematic and kinetic measures were included simultaneously. As such, if an upgraded standardized kinematic measure or kinematic and kinetic measure is developed, additional research using it as a potential predictor may be needed.
This study has several limitations. First, as this was a retrospective study, potential confounding factors that could have affected the clinical outcomes were not accounted for, and because patients of just one rehabilitation hospital were studied, there may have been selection bias. Nonetheless, given that a relatively standardized rehabilitation therapy was conducted, and as all study subjects were patients admitted to the same rehabilitation hospital, environmental factors were minimized. Additionally, this study was conducted with a sufficient number of patients with subacute and chronic stroke. Second, it is difficult to say whether the identified predictors solely predicted favorable post-RT outcomes, as CT was administered along with RT. However, considering that CT and RT were administered to all patients and that RT is rarely administered without CT, the outcomes of this study can be used as realistic predictors. Third, early and late subacute phases were not divided despite the chances of the influence of these phases on recovery and outcome. Fourth, aside from the chronic phase prediction model and the time since onset in the chronic phase, the AUC values of the remaining prediction model and meaningful predictors were <0.7, indicating insufficient discrimination ability. Lastly, other kinematic measures such as movement duration, peak velocity and peak acceleration known to be related to outcome after RT (48), and neuropsychological impairments such as aphasia and neglect known to be related to post-stroke motor recovery (49, 50), psychosocial, and emotional factors, which may have affected the outcome, were not included as potential predictors. Therefore, controlled, prospective, and multicenter studies including a more comprehensive set of potential predictors are required to validate and improve our results in the future.
Conclusions
Better hand movement and a shorter time since onset can realistically serve to predict clinically significant motor improvement in upper limb impairment after RT with InMotion2 alongside CT, in patients with subacute and chronic stroke, whereas other demographic characteristics and robotic kinematic measures cannot predict responders of RT. These findings may assist healthcare professionals in discerning optimal patients for RT and in accurately informing patients and caregivers about the outcomes of RT.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the Institutional Review Board of the National Rehabilitation Center in South Korea. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JJL and J-HS contributed to conception and design of the study, performed the statistical analysis, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Translational Research Center for Rehabilitation Robots, Korea National Rehabilitation Center, Ministry of Health and Welfare, Republic of Korea (grant numbers NRCTR-IN19001, NRCTR-IN20001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Sun Young Jeon (Department of Rehabilitation Medicine, National Rehabilitation Center, Republic of Korea) for collecting the data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.668923/full#supplementary-material
References
1. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. (2011) 377:1693–702. doi: 10.1016/S0140-6736(11)60325-5
2. Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb. Stroke. (2003) 34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD
3. Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. (2014) 2014:CD010820. doi: 10.1002/14651858.CD010820.pub2
4. Lincoln NB, Parry RH, Vass CD. Randomized, controlled trial to evaluate increased intensity of physiotherapy treatment of arm function after stroke. Stroke. (1999) 30:573–9. doi: 10.1161/01.STR.30.3.573
5. Feys H, De Weerdt W, Verbeke G, Steck GC, Capiau C, Kiekens C, et al. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow-up study of a randomized trial. Stroke. (2004) 35:924–9. doi: 10.1161/01.STR.0000121645.44752.f7
6. Masiero S, Poli P, Rosati G, Zanotto D, Iosa M, Paolucci S, et al. The value of robotic systems in stroke rehabilitation. Exp Rev Med Dev. (2014) 11:187–98. doi: 10.1586/17434440.2014.882766
7. Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. (2015) 2015:CD006876. doi: 10.1002/14651858.CD006876.pub4
8. Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EEH, Meskers CGM, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair. (2017) 31:107–21. doi: 10.1177/1545968316666957
9. Hsieh Y, Lin K, Wu C, Lien H, Chen J, Chen C, et al. Predicting clinically significant changes in motor and functional outcomes after robot-assisted stroke rehabilitation. Arch Phys Med Rehabil. (2014) 95:316–21. doi: 10.1016/j.apmr.2013.09.018
10. Huang P-C, Hsieh Y-W, Wang C-M, Wu C-Y, Huang S-C, Lin K-C. Predictors of motor, daily function, and quality-of-life improvements after upper-extremity robot-assisted rehabilitation in stroke. Am J Occup Ther. (2014) 68:325. doi: 10.5014/ajot.2014.010546
11. Franceschini M, Goffredo M, Pournajaf S, Paravati S, Agosti M, De Pisi F, et al. Predictors of activities of daily living outcomes after upper limb robot-assisted therapy in subacute stroke patients. PLoS ONE. (2018) 13:e0193235. doi: 10.1371/journal.pone.0193235
12. Duret C, Pila O, Grosmaire A-G, Koeppel T. Can robot-based measurements improve prediction of motor performance after robot-assisted upper-limb rehabilitation in patients with moderate-to-severe sub-acute stroke? RNN. (2019) 37:119–29. doi: 10.3233/RNN-180892
13. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. (2007) 4:e297. doi: 10.1097/EDE.0b013e3181577511
14. Make B. How can we assess outcomes of clinical trials: the MCID approach. J Chron Obstruct Pulm Dis. (2007) 4:191–4. doi: 10.1080/15412550701471231
15. Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. (2017) 12:444–50. doi: 10.1177/1747493017711816
16. Fasoli SE, Krebs HI, Stein J, Frontera WR, Hughes R, Hogan N. Robotic therapy for chronic motor impairments after stroke: follow-up results. Arch Phys Med Rehabil. (2004) 85:1106–11. doi: 10.1016/j.apmr.2003.11.028
17. Sale P, Franceschini M, Mazzoleni S, Palma E, Agosti M, Posteraro F. Effects of upper limb robot-assisted therapy on motor recovery in subacute stroke patients. J NeuroEng Rehabil. (2014) 11:104. doi: 10.1186/1743-0003-11-104
18. Paolucci S, Antonucci G, Grasso MG, Morelli D, Troisi E, Coiro P, et al. Early versus delayed inpatient stroke rehabilitation: a matched comparison conducted in Italy. Arch Phys Med Rehabil. (2000) 81:695–700. doi: 10.1016/S0003-9993(00)90095-9
19. Stinear CM, Smith M-C, Byblow WD. Prediction tools for stroke rehabilitation. Stroke. (2019) 50:3314–22. doi: 10.1161/STROKEAHA.119.025696
20. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. (2002) 16:232–40. doi: 10.1177/154596802401105171
21. Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry. (1990) 53:576–9. doi: 10.1136/jnnp.53.7.576
22. Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty‘s Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with Aids to the Examination of the Peripheral Nervous System. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain. (2010) 133:2838–44. doi: 10.1093/brain/awq270
23. Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. (1987) 67:206–7. doi: 10.1093/ptj/67.2.206
24. Katrak P, Bowring G, Conroy P, Chilvers M, Poulos R, McNeil D. Predicting upper limb recovery after stroke: the place of early shoulder and hand movement. Arch Phys Med Rehabil. (1998) 79:758–61. doi: 10.1016/S0003-9993(98)90352-5
25. Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. (1966) 46:357–75. doi: 10.1093/ptj/46.4.357
26. Bosecker C, Dipietro L, Volpe B, Igo Krebs H. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabil Neural Repair. (2010) 24:62–9. doi: 10.1177/1545968309343214
27. Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, et al. Movement smoothness changes during stroke recovery. J Neurosci. (2002) 22:8297–304. doi: 10.1523/JNEUROSCI.22-18-08297.2002
28. Mazzoleni S, Posteraro F, Filippi M, Forte F, Micera S, Dario P, et al. Biomechanical assessment of reaching movements in post-stroke patients during a robot-aided rehabilitation. Appl Bionics Biomech. (2011) 8:39–54. doi: 10.1155/2011/298926
29. Dipietro L, Krebs HI, Fasoli SE, Volpe BT, Stein J, Bever C, et al. Changing motor synergies in chronic stroke. J Neurophysiol. (2007) 98:757–68. doi: 10.1152/jn.01295.2006
30. Narayan Arya K, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil. (2011) 18:599–610. doi: 10.1310/tsr18s01-599
31. Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. (2012) 92:791–8. doi: 10.2522/ptj.20110009
32. Lipsey M, Hurley S. “Design sensitivity: statistical power for applied experimental research,” In: Bickman L. editor. The Sage Handbook of Applied Social Research Methods. Thousand Oaks: SAGE (2008). p.44–76.
33. Opheim A, Danielsson A, Alt Murphy M, Persson HC, Sunnerhagen KS. Early prediction of long-term upper limb spasticity after stroke. Neurology. (2015) 85:873–80. doi: 10.1212/WNL.0000000000001908
34. Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression, 3rd ed. New York: John Wiley & Sons (2013).
36. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. (1996) 49:1373–9. doi: 10.1016/S0895-4356(96)00236-3
37. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. (2007) 165:710–8. doi: 10.1093/aje/kwk052
38. Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke. (2005) 36:1172–7. doi: 10.1161/01.STR.0000165922.96430.d0
39. Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, et al. Active finger extension. Stroke. (2007) 38:1088–90. doi: 10.1161/01.STR.0000258077.88064.a3
40. Newton JM, Ward NS, Parker GJM, Deichmann R, Alexander DC, Friston KJ, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain. (2006) 129:1844–58. doi: 10.1093/brain/awl106
41. Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. (2006) 130:170–80. doi: 10.1093/brain/awl333
42. Mazzoleni S, Duret C, Grosmaire AG, Battini E. Combining upper limb robotic rehabilitation with other therapeutic approaches after stroke: current status, rationale, and challenges. BioMed Res Int. (2017) 2017:1–11. doi: 10.1155/2017/8905637
43. Skilbeck CE, Wade DT, Hewer RL, Wood VA. Recovery after stroke. J Neurol Neurosurg Psychiatry. (1983) 46:5–8. doi: 10.1136/jnnp.46.1.5
44. Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Arch Phys Med Rehabil. (2006) 87:36–42. doi: 10.1016/j.apmr.2006.09.005
45. Lundquist CB, Maribo T. The Fugl–Meyer assessment of the upper extremity: reliability, responsiveness and validity of the Danish version. Disabil Rehabil. (2017) 39:934–9. doi: 10.3109/09638288.2016.1163422
46. Schwarz A, Kanzler CM, Lambercy O, Luft AR, Veerbeek JM. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke. (2019) 50:718–27. doi: 10.1161/STROKEAHA.118.023531
47. Krebs HI, Krams M, Agrafiotis DK, DiBernardo A, Chavez JC, Littman GS, et al. Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke. (2014) 45:200–4. doi: 10.1161/STROKEAHA.113.002296
48. Wright ZA, Majeed YA, Patton JL, Huang FC. Key components of mechanical work predict outcomes in robotic stroke therapy. J NeuroEngineering Rehabil. (2020) 17:53. doi: 10.1186/s12984-020-00672-8
49. Anderlini D, Wallis G, Marinovic W. Language as a predictor of motor recovery: the case for a more global approach to stroke rehabilitation. Neurorehabil Neural Repair. (2019) 33:167–78. doi: 10.1177/1545968319829454
Keywords: robotics, upper extremity, minimal clinically important difference, prognosis, rehabilitation, stroke
Citation: Lee JJ and Shin J-H (2021) Predicting Clinically Significant Improvement After Robot-Assisted Upper Limb Rehabilitation in Subacute and Chronic Stroke. Front. Neurol. 12:668923. doi: 10.3389/fneur.2021.668923
Received: 17 February 2021; Accepted: 01 June 2021;
Published: 01 July 2021.
Edited by:
Grazia Fernanda Spitoni, Sapienza University of Rome, ItalyReviewed by:
Silvia Sterzi, Campus Bio-Medico University, ItalySung-Hwa Ko, Pusan National University Yangsan Hospital, South Korea
Laura Piccardi, Sapienza University of Rome, Italy
Copyright © 2021 Lee and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joon-Ho Shin, YXNmcmVlbHlhc0BnbWFpbC5jb20=
 Jae Joon Lee
Jae Joon Lee Joon-Ho Shin
Joon-Ho Shin