- 1Department of Neurological Surgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Neurology, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Neurosurgery, The Second People's Hospital, Quzhou, China
Objective: To evaluate the efficacy and safety of tirofiban for patients with acute ischemic stroke (AIS), especially posterior circulation stroke (PCS).
Methods: We enrolled consecutive patients with AIS who suffered large artery occlusion (LAO) and underwent mechanical thrombectomy (MT) between January 2016 and May 2020. Patients were divided into two groups according to whether tirofiban was used during MT. The primary efficacy outcome was a favorable functional outcome, defined as a modified Rankin Scale (mRS) score of 0–2 at 3 months. The safety outcomes were the rate of mortality at 3 months and the presence of intracranial hemorrhage (ICH) and symptomatic intracranial hemorrhage (sICH). Cohorts were balanced using 1:1 propensity score matching (PSM). Subgroup analysis was further performed to compare the efficacy and safety of tirofiban between the anterior circulation stroke (ACS) and PCS groups.
Results: A total of 292 patients were eligible for this study and divided into the tirofiban group (n = 51) and the no-tirofiban group (n = 241). In the propensity-score-matched cohort, the tirofiban group had a higher rate of favorable outcomes than the no-tirofiban group (49.0 vs. 25.5%, p = 0.014), and the mortality at 3 months showed a greater downward trend in the tirofiban group than the no-tirofiban group (15.6 vs. 33.3% p = 0.064). The risk of sICH and ICH was the same between the tirofiban and control groups (17.6 vs. 27.4% p = 0.236, 31.3 vs. 45.1% p = 0.154, respectively). Tirofiban use was predictive of favorable outcomes [adjusted odds ratio (aOR) = 2.87, 95% confidence interval (CI) 1.52–6.44, p = 0.043] after multiple logistic regression analysis. Subgroup analysis revealed that tirofiban use was significantly associated with favorable outcomes in ACS (aOR = 3.66, 95% CI 1.24–5.22, p = 0.019) but not in PCS (aOR = 1.12, 95% CI 0.47–7.52, p = 0.570).
Conclusion: We demonstrated that tirofiban may be associated with improving favorable outcome for the AIS patients who underwent MT, without increasing ICH or sICH. Furthermore, our results indicated that for PCS patients tirofiban may not be associated with favorable outcome, and more comprehensive randomized controlled trials are needed to confirm this finding.
Introduction
A number of randomized clinical trials have shown the benefit of mechanical thrombectomy (MT) in the treatment of acute ischemic stroke (AIS) that is due to large-vessel occlusion (LVO) (1, 2). However, this endovascular recanalization approach may lead to endothelial injury, plaque rupture, and subsequent platelet activation, leading to early re-occlusion and poor prognosis. Tirofiban is a non-peptide selective glycoprotein (GP) IIb/IIIa receptor inhibitor that reversibly inhibits fibrinogen-dependent platelet aggregation and subsequent formation of thrombi, which contribute to the major atherosclerotic complications in the progression of AIS (3).
Clinical trials in patients with AIS initially demonstrated the safety and efficacy of tirofiban as an adjunct to MT for AIS patients. However, the results of these trials have been controversial; some studies have shown that tirofiban does not improve prognosis and may even increase intracranial hemorrhage (ICH) and mortality. Therefore, more data are needed to confirm further the benefits and risks of tirofiban. A series of follow-up studies have reported a number of clinically valuable findings about the tirofiban regimen following MT, including the specific dose (4), injection method (5), and patient selection according to etiology (6). However, the specific indications and patient selection are still under debate. Considering the different clinical characteristics of posterior circulation stroke (PCS) and anterior circulation stroke (ACS) (7), as well as the different postoperative prognoses of patients with MT between the two groups (8), there is a hypothesis that tirofiban may have inconsistent risks and benefits in PCS and ACS. At present, there are some studies about the benefit of MT in PCS patients and the administration of tirofiban following MT (9, 10), but there are few studies exploring tirofiban in patients with PCS.
The main aim of this study was to test the safety and efficacy of tirofiban following MT in AIS patients. Propensity score matching (PSM) was used to match tirofiban- and no-tirofiban-treated patients for potential confounders. Regression analysis after PSM was performed to identify independent associations with the outcomes. The secondary aims were to compare the risks and benefits of tirofiban following MT between PCS and ACS.
Methods
Patient Selection
In this retrospective study, a total of 292 patients who underwent MT secondary to large artery occlusion (LAO) between January 2015 and May 2019 were enrolled. The inclusion criteria were as follows: (1) patients with AIS secondary to LAO confirmed by computed tomographic angiography (CTA), (2) patients with neurological deficits with a National Institutes of Health Stroke Scale (NIHSS) score of 6 or higher at presentation, (3) patients who were 18 years of age or older, and (4) patients who underwent MT.
The exclusion criteria were as follows: (1) cerebral hemorrhage confirmed by computed tomography; (2) platelet count <100 × 109/L, blood glucose concentration <2.8 or >22.0 mmol/L, and severe hepatic or renal dysfunction; and (3) incomplete data or loss to follow-up. This study was approved by the Ethics Committee of Zhejiang University School of Medicine Second Affiliated Hospital.
Procedures
All enrolled patients were treated according to current guidelines for AIS and underwent MT employed second-generation stent-retriever devices (Solitaire AB/FR, Covidien/ev3, Irvine, CA; Trevo Proview, Stryker, CA). Alternative rescue therapies, including balloon angioplasty and rescuing stent, were determined by the operators during the procedure based on the characteristics of the lesion and access. Following thrombectomy, all patients underwent postprocedural computed tomography (CT) within 12–24 h, and the presence of hemorrhage on CT was determined and scored by a blinded neuroradiologist.
Tirofiban was considered for application in the following situations: (1) rescue treatment with emergency stenting and balloon angioplasty for residual artery stenosis or failed thrombectomy, (2) successful mechanical recanalization with ≥3 passes with stent retriever, (3) severe in situ atherosclerosis with high risk of early re-occlusion, and (4) other recanalization refractory conditions and presumed endothelial damage. Tirofiban was continuously given at a rate of 8 μg/kg·h after an intravenous bolus of 10 μg/kg if there was no evidence of ICH on immediate head CT after MT. Twenty-four hours later, dual antiplatelet therapy was given after ICH was ruled out by another head CT.
Baseline Assessment and Outcome Measures
Data were extracted through a retrospective review of patient charts, procedure notes, image data, and follow-up notes. Baseline characteristics were collected, including age, sex, presenting NIHSS score, Alberta Stroke Program Early Computed Tomography Score (ASPECTS) or posterior circulation ASPECTS (pc-ASPECTS), comorbidities (diabetes, hypertension, atrial fibrillation, hyperlipidemia, and a history of prior stroke), antiplatelet drug and anticoagulation drug use, and coagulation function indicators. Procedural variables included time from symptom onset to groin puncture, time from symptom onset to reperfusion, tissue plasminogen activator (t-PA) use, retrieval times ≥3, rescue therapy including balloon angioplasty and rescuing stent, and Thrombolysis in Cerebral Infarction (TICI) grading. A TICI grade better than 2b was defined as successful recanalization. The arterial occlusion site was recorded as ACS and PCS. The stroke etiology was classified according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria: large artery atherosclerosis (LAA), cardioembolism (CE), and stroke of other determined or undetermined causes.
The primary outcome measure was functional outcome, which was measured by the modified Rankin Scale (mRS) at 3 months. A favorable outcome was defined as an mRS score of 0–2. The scores were collected by a stroke neurologist during routine follow-up visits at 90 days (±14) after stroke for the majority of patients. Telephone discussions with patients or their families were used to obtain information. ICH was considered present when head CT revealed a region consistent with newly developed blood extravasation. Correspondingly, symptomatic intracranial hemorrhage (sICH) was defined as any hemorrhage with neurological deterioration, indicated by an NIHSS score of ≥4 points above the baseline value, or as any hemorrhage leading to mortality. Two investigators, who were blinded to all clinical information, independently reviewed the CT and magnetic resonance imaging (MRI) images to determine the presence of ICH or sICH.
Statistical Analysis
Statistical analyses were performed using SPSS V.25 for the majority of the data. Patient variables were analyzed using descriptive statistics and univariate comparisons. Comparisons were performed using the t-test for continuous measures, for non-continuous variables, and χ2 test for categorical measures. All tests were two sided, and an α < 0.05 was considered significant.
PSM was performed with R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) to ensure an even distribution of possible confounders between the two groups. A 1:1 matched analysis using nearest-neighbor matching with a caliper distance of 0.1 without replacement was performed based on the estimated propensity score of each patient. After matching patient characteristics, these were analyzed again to confirm successful matching. Multivariate logistic regression analysis was used to assess the odds ratio (OR) and corresponding 95% confidence interval (CI) to explore whether tirofiban can independently affect favorable clinical outcomes (mRS scores 0–2) and safety outcomes, including ICH, sICH, and mortality at 3 months.
Results
Patient Characteristics
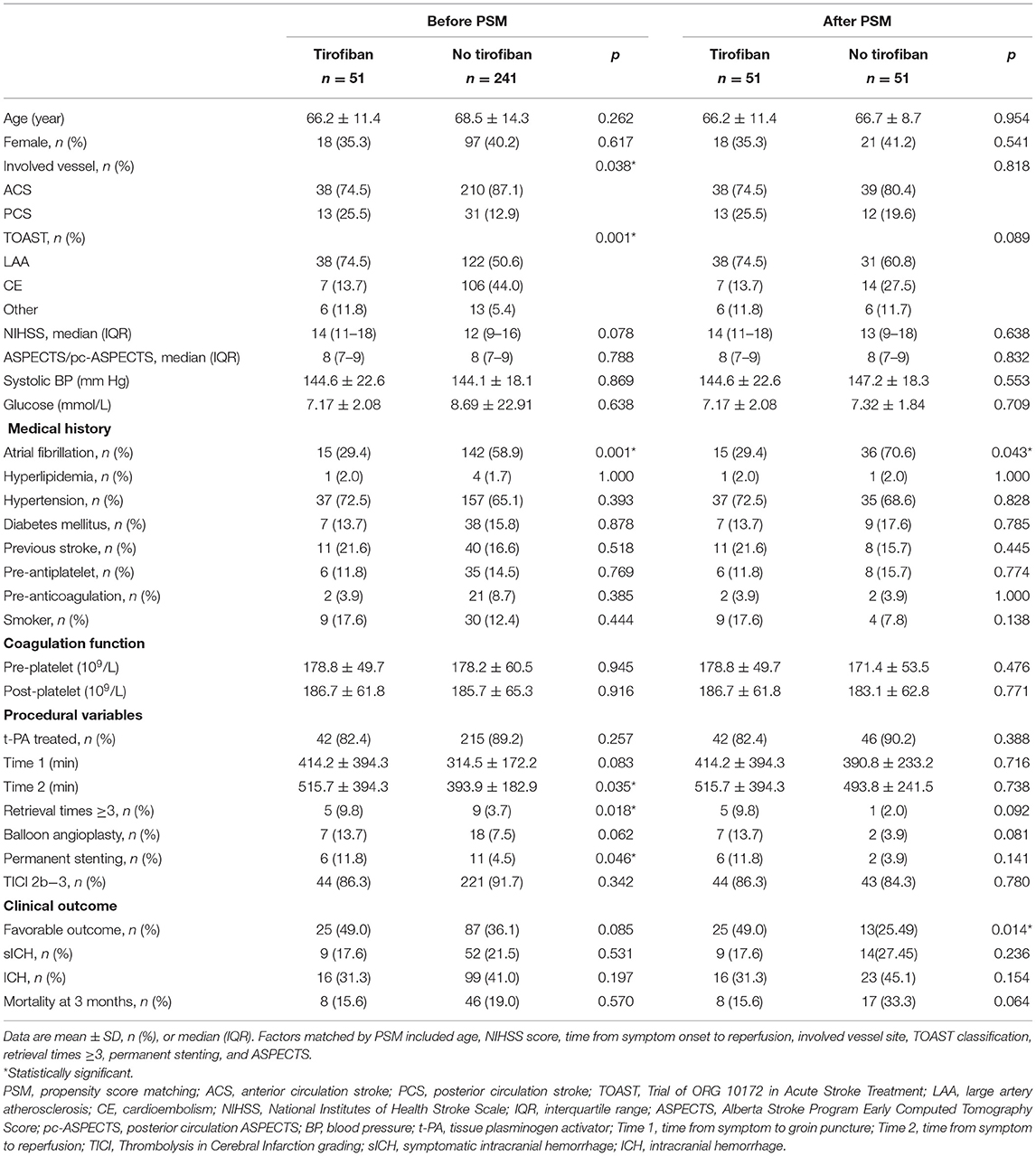
A total of 292 patients were eligible for this study. The baseline characteristics and outcomes of the patients are presented in Table 1 and were compared between the tirofiban group (n = 51) and the no-tirofiban group (n = 241). There was no significant between-group difference with respect to age, sex, or NIHSS score at presentation (p > 0.05). Coagulation function was assessed by preoperative and postoperative coagulation indicators, including prothrombin time (PT), activated partial thromboplastin time (APTT), and platelet count, which were similar between the two groups. PCS was more common in the tirofiban group (25.5 vs. 12.9%, p = 0.038). LAA was the cause of stroke in 74.5% of tirofiban patients, compared with 50.6% in the no-tirofiban group (p < 0.001). In contrast, CE was lower in the tirofiban group (29.4 vs. 58.9%, p < 0.01). Other medical histories and comorbidities showed no between-group differences.
The analysis of procedural variables showed that ~88.1% of patients received t-PA, without a significant difference between the two groups. The mean times from symptom onset to recanalization were comparable (414.2 ± 394.3 vs. 314.6 ± 172.2, p > 0.05), but the time from symptom onset to reperfusion in the tirofiban group was longer (515.78 ± 394.34 vs. 393.97 ± 182.95, p = 0.035). Furthermore, the patients with tirofiban were more often to accept the rescue therapies including balloon angioplasty (13.7 vs. 7.5%, p = 0.062) and permanent stenting (11.8 vs. 4.5%, p = 0.046) and undergo MT with retrieval times ≥3 (9.8 vs. 3.7%, p = 0.018). The overall rate of recanalization was 90.8%, and it was not significantly different between the two groups.
Safety and Efficacy Outcomes
The efficacy and safety outcome measures were not significantly different. The rates of favorable outcomes (mRS 0–2) were 49.0 and 36.1%, respectively, in the tirofiban and no-tirofiban groups, but p > 0.05. The overall mortality at 3 months was 18.50% across both groups and was slightly, but not significantly, lower in the tirofiban group than in the no-tirofiban group (15.6 vs. 19.1%, p > 0.05). Procedure-related complications in the tirofiban group, including ICH (31.4 vs. 41.1%, p = 0.197) and sICH (17.65 vs. 21.58%, p = 0.531), did not occur more frequently than in the no-tirofiban group.
Variables in the PSM were selected based on previous univariate analysis, including age, NIHSS score, time from symptom onset to reperfusion, involved vessel site TOAST classification, and ASPECTS. Finally, 51 cases were successfully matched, and the standard deviation indicated that the matching effect was satisfactory. After PSM, the characteristics of the two groups were relatively the same after matching (Table 1). After PSM, tirofiban significantly improved the rates of favorable outcomes in the tirofiban and no-tirofiban groups (49.0 vs. 25.5%, p = 0.014) (Figure 1). No difference was found in mortality and the rates of ICH and sICH (p > 0.05).
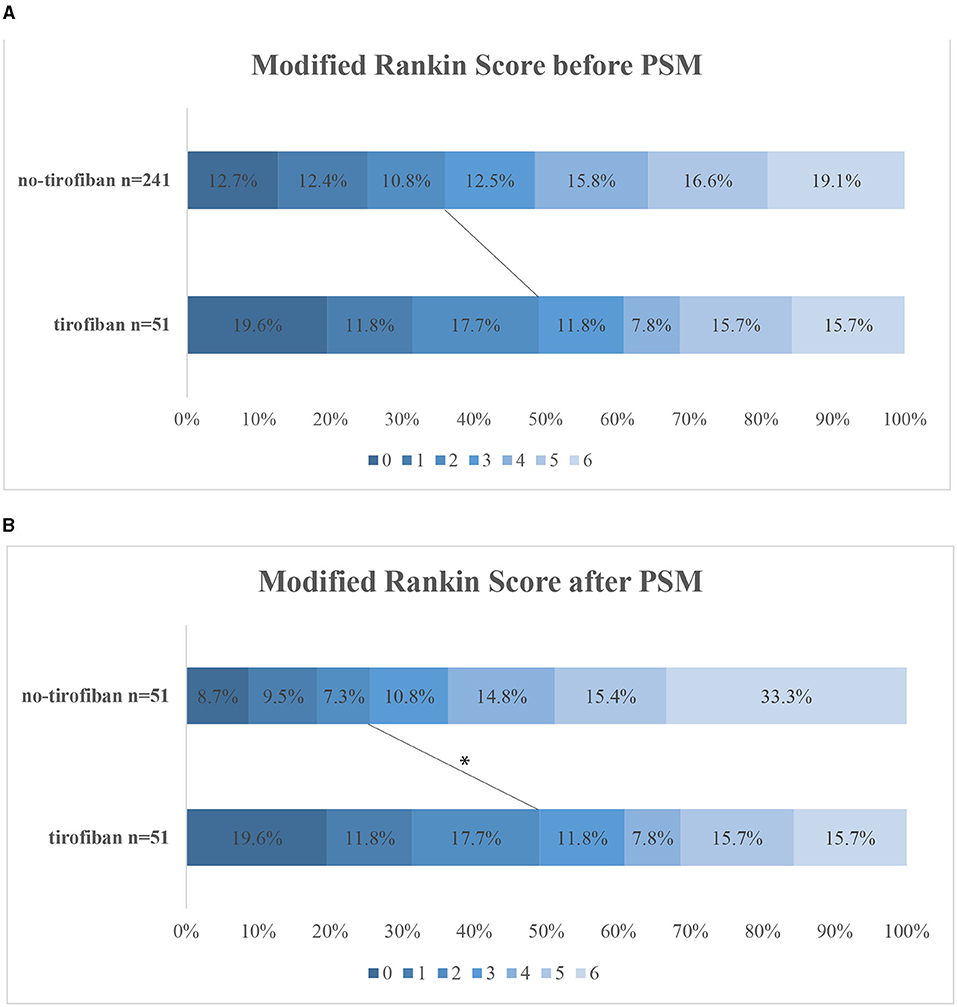
Figure 1. Distribution of mRS at 3 months categories before PSM (A) and after PSM (B). The lines indicate differences in favorable outcome (mRS 0–2) between groups. *p < 0.05. mRS, modified Rankin Scale; PSM, propensity score matching.
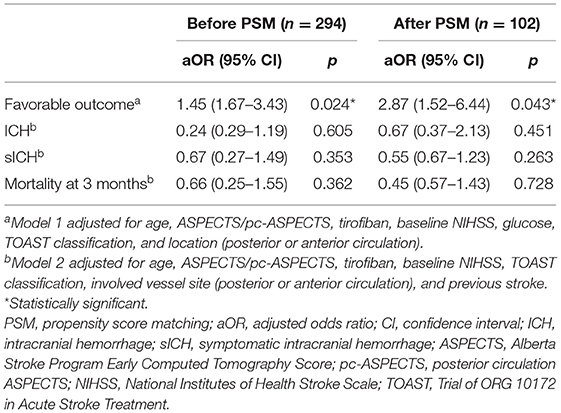
Stepwise regression was performed to identify the factors associated with the safety and efficacy outcomes (Supplementary Table 1). In the overall patients, tirofiban tended to improve the rates of favorable outcomes independently [adjusted odds ratio (aOR) = 1.45, 95% CI 1.67–3.43, p = 0.024]. Furthermore, tirofiban did not show any association with the incidences of ICH, sICH, or mortality (p = 0.605, 0.353, and 0.362, respectively).
The results remained stable after PSM for potential confounders. The use of tirofiban showed an independent association with better outcomes (aOR = 2.87, 95% CI 1.52–6.44, p = 0.043). Tirofiban was not associated with a risk of ICH (aOR = 0.67, 95% CI 0.37–2.13, p = 0.451), sICH (aOR = 0.55, 95% CI 0.67–1.23, p = 0.263), or mortality at 3 months (aOR = 0.45, 95% CI 0.57–1.43, p = 0.728). And the multivariate regression models were adjusted, respectively, for the favorable outcome and safety outcome (Table 2).
Effects on ACS and PCS
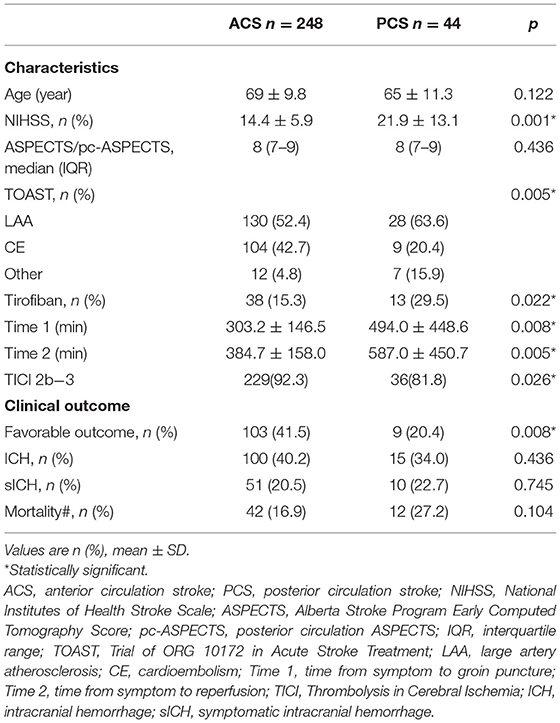
Based on the above regression analysis results, we performed a subgroup analysis according to location to explore further the association between tirofiban and favorable outcomes. First, the main characteristics and clinical outcomes of patients with ACS and PCS were compared with univariate analysis, and there were many differences between the two groups (Table 3). Patients with PCS had higher NIHSS scores on admission than those with ACS (21.9 ± 13.1 vs. 14.4 ± 5.9, p = 0.001), a longer time from symptom onset to groin puncture (494.0 ± 448.6 vs. 303.2 ± 146.5, p = 0.008), and a longer time from symptom onset to reperfusion (587.0 ± 450.7 vs. 384.7 ± 158.0, p = 0.005). A total of 63.6% of patients with PCS had LAA stroke, compared with 52.4% of patients with ACS (p = 0.005). Relatedly, tirofiban administration was more common in PCS patients (29.5 vs. 15.3%, p = 0.001). In terms of comparison with the outcomes of the two groups, patients in the PCS group had significantly worse favorable clinical outcomes (20.4 vs. 41.5%, p = 0.008). There were no significant differences in ICH, sICH, or risk of death between the two groups.
A multivariate analysis was performed to adjust the confounders and to identify the use of tirofiban as the independent predictor of favorable outcomes in the overall group (aOR = 1.45, 95% CI 1.67–3.43, p = 0.024). In the ACS patients, tirofiban was associated with favorable outcomes (aOR = 3.66, 95% CI 1.24–5.22, p = 0.019); however, such an association was not observed in PCS patients (aOR = 1.12, 95% CI 0.47–7.52, p = 0.570). Detailed information on the regression coefficients and p-values is presented in Supplementary Table 2. The comparison between subgroups is more intuitively reflected in Figure 2.
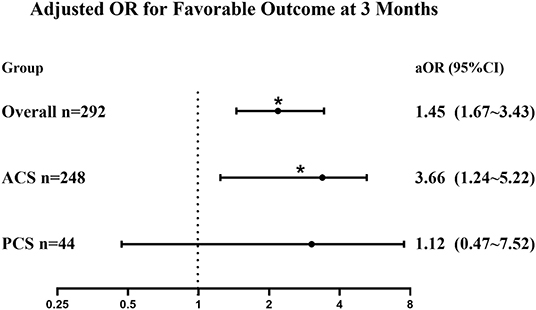
Figure 2. Forest plot of adjusted OR for the association between tirofiban administration and favorable outcomes (mRS 0–2) in patients with posterior circulation stroke (PCS) and anterior circulation stroke (ACS). Adjusted for age, ASPECTS/pc-ASPECTS, tirofiban, baseline NIHSS, glucose, TOAST classification, and location (posterior or anterior circulation). aOR, adjusted odds ratio; mRS, modified Rankin Scale; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; pc-ASPECTS, posterior circulation ASPECTS; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of ORG 10172 in Acute Stroke Treatment. *p < 0.05.
Discussion
In this study, we evaluated the safety and efficacy of tirofiban as an adjuvant therapy for MT in AIS patients. The main findings of our study are as follows: (1) more patients in the tirofiban group had favorable clinical outcomes after PSM matching, and ICH and mortality did not differ between the two groups; (2) multivariate regression analysis demonstrated that tirofiban was positively associated with favorable clinical outcomes but not with ICH, mortality, or other safety indicators; and (3) tirofiban was associated with increasing favorable clinical outcomes in patients with ACS but not in patients with PCS.
The purpose of therapy for AIS patients is to achieve rapid cerebral vascular recanalization to restore cerebral blood flow (10), and MT has become the first-line treatment for LVO (11). However, many patients who achieve rapid vascular recanalization by MT are still at high risk of acute reocclusion, especially LAA patients (12, 13). The potential cause may be in situ atherosclerotic occlusion, local platelet aggregation, and broken plaques (14). LAA strokes are the most common stroke type in China, so it is urgent to find ways to prevent reocclusion (15).
Tirofiban is a relatively short-acting and reversible GP IIb/IIIa receptor inhibitor that inhibits fibrinogen binding to platelets, effectively preventing platelet aggregation and secondary thrombosis (16). There have been a number of clinical trials demonstrating the safety and efficacy of tirofiban administration in AIS patients undergoing MT, but with controversial results. Most studies have demonstrated that tirofiban has great clinical application prospects in MT, and tirofiban has proved to be an independent predictor of favorable outcomes (17–19). A multicenter retrospective cohort confirmed that the safety of tirofiban was not associated with ICH or mortality (20). In contrast, other studies have shown that tirofiban does not improve clinical outcomes (21) and may be associated with an increased risk of fatal ICH (22, 23). The reasons for the controversial results are complex, including the heterogeneity of experiments, relatively small sample sizes, patient selection bias, and different tirofiban application regimens (5, 24). In our study, tirofiban was continuously given at a rate of 8 μg/kg·h after an intravenous bolus of 10 μg/kg if there was no evidence of ICH. The dose of tirofiban was not consistent in previous studies, but it did not vary extensively. And the total amount of tirofiban infusion was low and only varied from 0.5 to 2.0 mg in most centers (3). Nevertheless, the specific dose may affect the results especially for the risk of ICH, and further research about the dose is needed.
In this study, the use of tirofiban did not lead to significantly more benefits before PSM. We found the patients in the tirofiban group were more often with rescuer therapies, repeated attempts of thrombectomy, and longer time from symptom onset to reperfusion, which were associated with difficulty of vascular recanalization. Additionally, in our study tirofiban was more selectively adopted for patients with LAA stroke rather than CE stroke (74.5 vs. 13.7%, p = 0.01). This patient selection bias may explain the lower rate of recanalization in the tirofiban group (86.3 vs. 91.7%, p = 0.342). After the correction of these confounding factors by PSM, the rate of recanalization has become similar in the matched cohort, and the use of tirofiban significantly improved the rate of favorable outcomes in AIS patients.
A number of studies have noted the differences between ACS and PCS in clinical characteristics, treatment, and prognosis (25). PCS accounts for 5–19% of AIS patients (26, 27). A large clinical trial including 90,484 patients treated with t-PA demonstrated that PCS was associated with worse outcomes (28). Some studies have suggested that MT for PCS is a safe and feasible treatment option (29). MT is widely used in PCS clinically, and our study results showed that more PCS patients than ACS patients were treated with tirofiban (29.5 vs. 15.3%). However, few studies have focused on whether tirofiban administration in MT has consistent safety and efficacy between ACS and PCS. Subgroup analysis was performed, and we found that PCS patients had an obviously worse prognosis than ACS patients (20.4 vs. 41.5%, respectively), and they also had a higher NIHSS score at admission and a longer time from symptom onset to reperfusion. Further analysis by logistic regression illustrated that tirofiban was associated with increasing favorable clinical outcomes in patients with ACS but not in patients with PCS. However, Alawieh et al. thought that patients with PCS benefit equally from tirofiban administration compared with ACS (30). Given the limited sample size, our results only indicated that the effect of tirofiban may be modified by the occlusion sites and the PCS patients seemed to benefit less. Whether tirofiban treatment can affect efficacy outcomes of PCS patients treated with MT requires future research.
There are also the limitations in this study. First, this experiment included only patients in the past 5 years from a single center, which reduced the changes and differences in perioperative patient care procedures but also resulted in a small sample size. Additionally, this is a retrospective study with inevitable patient selection bias. Even though we used advanced statistical methods, including PSM and multivariate adjustment, we cannot correct residual or unmeasured confounding. Furthermore, the balloon angioplasty and stenting are more commonly used in tirofiban groups, which may exaggerate the effect of tirofiban. Considering these limitations, these results should be analyzed with more caution, and larger multicenter data will be required to study this effect further.
Conclusion
We demonstrated that tirofiban may be associated with improving favorable outcome for the AIS patients who underwent MT, without increasing ICH or sICH. Furthermore, our results indicated that for PCS patients tirofiban may not be associated with favorable outcome, and more comprehensive randomized controlled trials are needed to confirm this finding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
LC, LX, and JX collected the data and drafted the manuscript. JY, CL, and XY analyzed the data and performed all statistical analyses. ML, CY, and CQ conceived the study and made critical revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 81701152 and 81971097), Natural Science Foundation of Zhejiang Province (LY19H090022), and Medical and Health Science and Technology Program of Zhejiang Province (2016KYA199).
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 81701152 and 81971097) and Natural Science Foundation of Zhejiang Province (LY19H090022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.688019/full#supplementary-material
References
1. Martins SO, Mont'Alverne F, Rebello LC, Abud DG, Silva GS, Lima FO, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. (2020) 382:2316–26. doi: 10.1056/NEJMoa2000120
2. Saver J, Goyal M, Bonafe A, Diener H, Levy E, Pereira V, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
3. Yang M, Huo X, Miao Z, Wang Y. Platelet Glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs. (2019) 79:515–29. doi: 10.1007/s40265-019-01078-0
4. Zhao W, Che R, Shang S, Wu C, Li C, Wu L, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. (2017) 48:3289–94. doi: 10.1161/STROKEAHA.117.019193
5. Yang J, Wu Y, Gao X, Bivard A, Levi C, Parsons M, et al. Intraarterial versus intravenous tirofiban as an adjunct to endovascular thrombectomy for acute ischemic stroke. Stroke. (2020) 51:2925–33. doi: 10.1161/STROKEAHA.120.029994
6. Sun B, Shi Z, Pu J, Yang S, Wang H, Yang D, et al. Effects of mechanical thrombectomy for acute stroke patients with etiology of large artery atherosclerosis. J Neurol Sci. (2019) 396:178–83. doi: 10.1016/j.jns.2018.10.017
7. Kim JT, Park MS, Choi KH, Kim BJ, Han MK, Park TH, et al. Clinical outcomes of posterior versus anterior circulation infarction with low national institutes of health stroke scale scores. Stroke. (2017) 48:55–62. doi: 10.1161/STROKEAHA.116.013432
8. Caplan L, Wityk R, Glass T, Tapia J, Pazdera L, Chang H, et al. New England medical center posterior circulation registry. Ann Neurol. (2004) 56:389–98. doi: 10.1002/ana.20204
9. Weber R, Minnerup J, Nordmeyer H, Eyding J, Krogias C, Hadisurya J, et al. Thrombectomy in posterior circulation stroke: differences in procedures and outcome compared to anterior circulation stroke in the prospective multicentre REVASK registry. Eur J Neurol. (2019) 26:299–305. doi: 10.1111/ene.13809
10. Berkhemer O, Fransen P, Beumer D, van den Berg L, Lingsma H, Yoo A, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
11. Yajima H, Kanaya H, Ogino M, Ueki K, Kim P. Middle meningeal artery embolization for chronic subdural hematoma with high risk of recurrence: a single institution experience. Clin Neurol Neurosurg. (2020) 197:106097. doi: 10.1016/j.clineuro.2020.106097
12. Kang D, Yoon W, Kim S, Baek B, Lee Y, Kim Y, et al. Endovascular treatment for emergent large vessel occlusion due to severe intracranial atherosclerotic stenosis. J neurosurg. (2018) 130:1−8. doi: 10.3171/2018.1.JNS172350
13. Kim Y, Sohn S, Yoo J, Hong J, Kim C, Kang D, et al. Local tirofiban infusion for remnant stenosis in large vessel occlusion: tirofiban ASSIST study. BMC Neurol. (2020) 20:284. doi: 10.1186/s12883-020-01864-4
14. Mosimann P, Kaesmacher J, Gautschi D, Bellwald S, Panos L, Piechowiak E, et al. Predictors of unexpected early reocclusion after successful mechanical thrombectomy in acute ischemic stroke patients. Stroke. (2018) 49:2643–51. doi: 10.1161/STROKEAHA.118.021685
15. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687 adults. Circulation. (2017) 135:759–71. doi: 10.1161/CIRCULATIONAHA.116.025250
16. Sun X, Zhang H, Tong X, Gao F, Ma G, Miao Z. Effects of periprocedural tirofiban vs. oral antiplatelet drug therapy on posterior circulation infarction in patients with acute intracranial atherosclerosis-related vertebrobasilar artery occlusion. Front Neurol. (2020) 11:254. doi: 10.3389/fneur.2020.00254
17. Yang M, Huo X, Gao F, Wang A, Ma N, Shi H, et al. Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol. (2020) 27:1056–61. doi: 10.1111/ene.14170
18. Yi HJ, Sung JH, Lee DH. Safety and efficacy of intra-arterial tirofiban injection during mechanical thrombectomy for large artery occlusion. Curr Neurovasc Res. (2019) 16:416–24. doi: 10.2174/1567202616666191023154956
19. Huo X, Yang M, Ma N, Gao F, Mo D, Li X, et al. Safety and efficacy of tirofiban during mechanical thrombectomy for stroke patients with preceding intravenous thrombolysis. Clin Interv Aging. (2020) 15:1241–8. doi: 10.2147/CIA.S238769
20. Zhao L, Jian Y, Li T, Wang H, Lei Z, Sun M, et al. The safety and efficiency of tirofiban in acute ischemic stroke patients treated with mechanical thrombectomy: a multicenter retrospective cohort study. Biochem Res Int. (2020) 2020:5656173. doi: 10.1155/2020/5656173
21. Pan X, Zheng D, Zheng Y, Chan P, Lin Y, Zou J, et al. Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur J Neurol. (2019) 26:1105–10. doi: 10.1111/ene.13946
22. Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. (2013) 44:1453–5. doi: 10.1161/STROKEAHA.111.000502
23. Wu Y, Yin C, Yang J, Jiang L, Parsons M, Lin L. Endovascular thrombectomy: tirofiban increases bleeding risk in acute stroke patients. Stroke. (2018) 49:2783–5. doi: 10.1161/STROKEAHA.118.022919
24. Goyal M, Menon B, van Zwam W, Dippel D, Mitchell P, Demchuk A, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
25. Dornák T, Král M, Sedláčková Z, Šanák D, Cecháková E, Divišová P, et al. Predictors for intracranial hemorrhage following intravenous thrombolysis in posterior circulation stroke. Transl Stroke Res. (2018) 9:582–8. doi: 10.1007/s12975-018-0608-0
26. Frid P, Drake M, Giese AK, Wasselius J, Schirmer MD, Donahue KL, et al. Detailed phenotyping of posterior vs. anterior circulation ischemic stroke: a multi-center MRI study. J Neurol. (2020) 267:649–58. doi: 10.1007/s00415-019-09613-5
27. Sarraj A, Medrek S, Albright K, Martin-Schild S, Bibars W, Vahidy F, et al. Posterior circulation stroke is associated with prolonged door-to-needle time. Int J Stroke. (2013) 10:672–8. doi: 10.1111/j.1747-4949.2012.00952.x
28. Sommer P, Posekany A, Serles W, Marko M, Scharer S, Fertl E, et al. Is functional outcome different in posterior and anterior circulation stroke? Stroke. (2018) 49:2728–32. doi: 10.1161/STROKEAHA.118.021785
29. Meyer L, Stracke C, Jungi N, Wallocha M, Broocks G, Sporns P, et al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol. (2021) 78:434–44. doi: 10.1001/jamaneurol.2021.0001
30. Alawieh A, Vargas J, Turner RD, Turk AS, Chaudry MI, Lena J, et al. Equivalent favorable outcomes possible after thrombectomy for posterior circulation large vessel occlusion compared with the anterior circulation: the MUSC experience. J NeuroInterv Surg. (2018) 10:735–40. doi: 10.1136/neurintsurg-2017-013420
Keywords: tirofiban, acute ischemic stroke, propensity score matching, mechanical thrombectomy, posterior circulation stroke
Citation: Cai L, Yu X, Yu J, Xu J, Xu L, Ling C, Lou M, Yu C and Qian C (2021) Can Tirofiban Improve the Outcome of Patients With Acute Ischemic Stroke: A Propensity Score Matching Analysis. Front. Neurol. 12:688019. doi: 10.3389/fneur.2021.688019
Received: 30 March 2021; Accepted: 28 July 2021;
Published: 13 September 2021.
Edited by:
QinJian Sun, Shandong University, ChinaReviewed by:
Shashvat M. Desai, University of Pittsburgh, United StatesKristen Sanfilippo, Washington University School of Medicine in St. Louis, United States
Copyright © 2021 Cai, Yu, Yu, Xu, Xu, Ling, Lou, Yu and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Yu, WWM2NjEwNzBAMTYzLmNvbQ==; Cong Qian, Y29uZ3FpYW5Aemp1LmVkdS5jbg==
 Lingxin Cai1
Lingxin Cai1 Jun Yu
Jun Yu Cong Qian
Cong Qian