- 1Department of Neurosurgery, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Cardiology, PLA Rocket Force Characteristic Medical Center, Beijing, China
Intracerebral hemorrhage (ICH) accounts for ~15% of all strokes and is associated with high mortality and disability rates. The systemic inflammation response index (SIRI) is a novel systemic inflammatory marker based on peripheral neutrophil, monocyte, and lymphocyte counts. This study aimed to evaluate the prognostic significance of admission SIRI in patients with spontaneous ICH and compare its predictive ability with that of the neutrophil-to-lymphocyte ratio (NLR). This retrospective study was conducted based on a prospectively collected database of patients with ICH between June 2016 and January 2019. Propensity score matching (PSM) was conducted to adjust for potential imbalances in the clinical parameters. A total of 403 patients were included in the original cohort. The optimal SIRI cut-off value was 2.76. After 1:1 PSM based on potential confounding variables, a new cohort containing 262 patients was established for further analysis. In the original cohort, SIRI served as an independent predictor of 3-month functional outcome [odds ratio (OR), 1.302; 95% CI, 1.120–1.512; p = 0.001] and 1-month mortality (OR, 1.072; 95% CI, 1.020–1.126; p = 0.006), while NLR was independently associated with only 3-month functional outcomes (OR, 1.051; 95% CI, 1.004–1.100; p = 0.031) and not 1-month mortality. The same applied to the PSM cohort. Receiver operating characteristic analyses and predictive models indicated that in most instances, SIRI was superior to NLR and their components in predicting the outcomes of patients with ICH. Our study found that SIRI is determined to be an independent predictive indicator for ICH patients in 3-month functional outcomes and 1-month mortality. The prognostic predictive ability of SIRI was stronger than that of NLR.
Introduction
Intracerebral hemorrhage (ICH) is a life-threatening condition with a high mortality and disability rate and occurs due to spontaneous bleeding into the brain parenchyma, involving the ventricles and subarachnoid spaces in extreme circumstances. ICH accounts for ~15% of all strokes (1). In terms of ICH, 75–85% of cases originate from the spontaneous rupture of small vessels damaged. by chronic hypertension or amyloid angiopathy (2). The incidence of ICH is higher in male and elderly patients. Rapid CT after onset can be used to recognize almost all forms of acute ICH and help make optimal medical decisions within the shortest time. The global burden of ICH mainly results from inadequate management of chronic hypertension and other modifiable risk factors (3)
Growing evidence has indicated that inflammatory responses participate in the pathophysiological processes of brain injury after ICH, and inflammation is one of the crucial contributors to ICH-induced secondary brain injury (4). Leukocytes play an important role in immune response, cell migration, perihematomal edema formation, blood–brain barrier (BBB) integrity, and cell death after ICH (5, 6). Accumulating data have demonstrated that increased blood leukocyte count is associated with more serious disease and worse outcomes in ischemic and hemorrhagic strokes (7). Neutrophil-to-lymphocyte ratio (NLR), based on the coexistence of lymphopenia and leukocytosis in the initial inflammatory response, may be a useful peripheral biomarker for predicting the prognosis of stroke (8). Other peripheral inflammatory biomarkers, whose prognostic ability in ICH patients has also been confirmed, are systemic immune-inflammation index, NLR, and platelet-to-lymphocyte ratio (9, 10). Systemic inflammatory response syndrome, which is defined based on the changes in leukocyte and vital signs, is also associated with outcomes (11, 12).
The systemic inflammation response index (SIRI) is a novel systemic inflammatory marker based on peripheral neutrophil, monocyte, and lymphocyte counts. In previous studies, SIRI was found to be an independent prognostic indicator in various tumors (13–15). Therefore, this study aimed to evaluate the prognostic significance of admission SIRI in patients with spontaneous ICH and compare its prognostic ability with that of NLR.
Materials and Methods
Study Design
This retrospective study was conducted based on a prospectively collected database of ICH patients at the Department of Neurosurgery of West China Hospital, Sichuan University between June 2016 and January 2019. All patients in this cohort were managed according to the latest guidelines for stroke, and their baseline clinical data were retrieved from the electronic medical record system of the West China Hospital (16).
The exclusion criteria were as follows: (1) age <18 years; (2) incomplete baseline clinical data; (3) ICH caused by a tumor, aneurysm, or arteriovenous malformation; (4) absence of CT angiography and follow-up CT within 24 h of admission; (5) a history of infectious diseases, cancers, rheumatic diseases, blood system diseases, or other diseases which evidently affect peripheral blood cells; (6) loss to follow-up.
Clinical Parameter Assessment
Clinical variables were retrieved from the electronic medical record system, including the following variables: (1) demographics: age of onset and sex; (2) clinical history: history of hypertension, diabetes mellitus, smoking, alcohol abuse, and stroke; (3) admission conditions: Glasgow Coma Scale (GCS) score, admission systolic blood pressure, diastolic blood pressure, and duration from onset to hospitalization; (4) ICH imaging characteristics: hematoma volume, location of hematoma, presence of intraventricular hematoma, and hematoma expansion (HE); (5) treatment; (6) routine blood tests. Notably, routine blood tests were conducted immediately after admission. SIRI was defined as neutrophil count × monocyte count/lymphocyte count, and NLR was defined as neutrophil count/lymphocyte count.
Patients were followed up every month after admission. The primary outcomes were 3-month functional outcomes and 1-month mortality rate. The modified Rankin Scale (mRS) was used to evaluate patients' functional outcomes at each follow-up. Patients who had been discharged were followed up by telephone. Good outcome was defined as an mRS score of 0–2, while a poor outcome was defined as an mRS score of 3–6 (17).
The volume of parenchymal hematoma was calculated on the initial CT scans using 3D Slicer (http://www.slicer.org), and manual segmentation was applied by two independent neurosurgeons (18). HE was defined as hematoma enlargement ≥6 ml or ≥33% within 24 h (19). Surgical interventions mainly included hematoma evacuation with craniotomy and external ventricular drainage.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 22.0; IBM, Armonk, NY, USA) and R software (version 3.6.1). Continuous variables are presented as mean ± SD or median with interquartile range, while categorical variables are presented as frequency and percentage. Categorical variables were compared using the χ2 or Fisher's exact test. Continuous variables that conformed to the normal distribution were compared using Student's t-test; otherwise, the Mann–Whitney U-test was employed. Logistic regression analyses were used to determine the influence of risk factors on outcomes in patients with ICH. Variables with p < 0.1 in univariate analysis were included in backward stepwise multivariate logistic regression. Receiver operating characteristic (ROC) analysis was conducted to assess the accuracy of the SIRI, NLR, and other markers for outcomes. The optimal cut-off value of SIRI was determined by calculating the maximum Youden index using ROC. DeLong's test was employed to compare the areas under the curve (AUC). Predictive models for outcomes were constituted by independent predictive indicators in multivariate logistic regression; Harrell's concordance index (C-index) and Akaike information criterion (AIC) were used to assess the predictive accuracy and model-fitting of predictive models, respectively. Higher C-index indicated better predictive accuracy, and lower AICs indicated superior model-fitting (20, 21). A two-sided p < 0.05 was considered statistically significant. Propensity score matching (PSM) was conducted to adjust for an imbalance of clinical parameters with a p-value of <0.1 in univariate analysis. These patients were matched 1:1 using the nearest-neighbor algorithm with a caliper width of 0.2 and without replacement.
Ethics
This study was approved by the Ethical Committee of Sichuan University (2013NO52) and conducted following the principles of the Declaration of Helsinki. All patients and their authorized trustees were informed and provided signed informed consent to use their clinical data for research purposes.
Results
Baseline Clinical Characteristics
As shown in Figure 1, a total of 403 patients were included in the original cohort. The optimal cut-off value of SIRI was determined to be 2.76 in ROC analysis. Among the 403 patients, 189 patients had SIRI <2.76 and 214 had SIRI ≥2.76. After 1:1 PSM based on potential confounding variables, a new cohort containing 262 patients was established for further analysis.
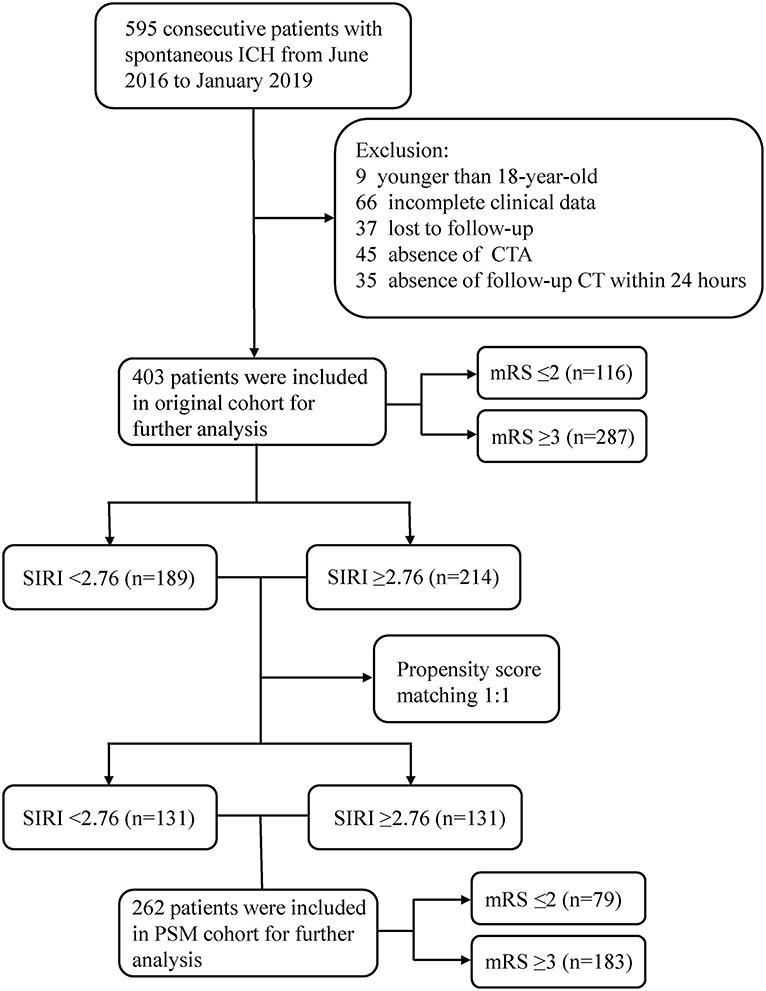
Figure 1. Flow chart of the current study. ICH, intracerebral hemorrhage; CTA, computed tomography angiography; mRS, modified Rankin Scale; SIRI, systemic inflammation response index; PSM, propensity score matching.
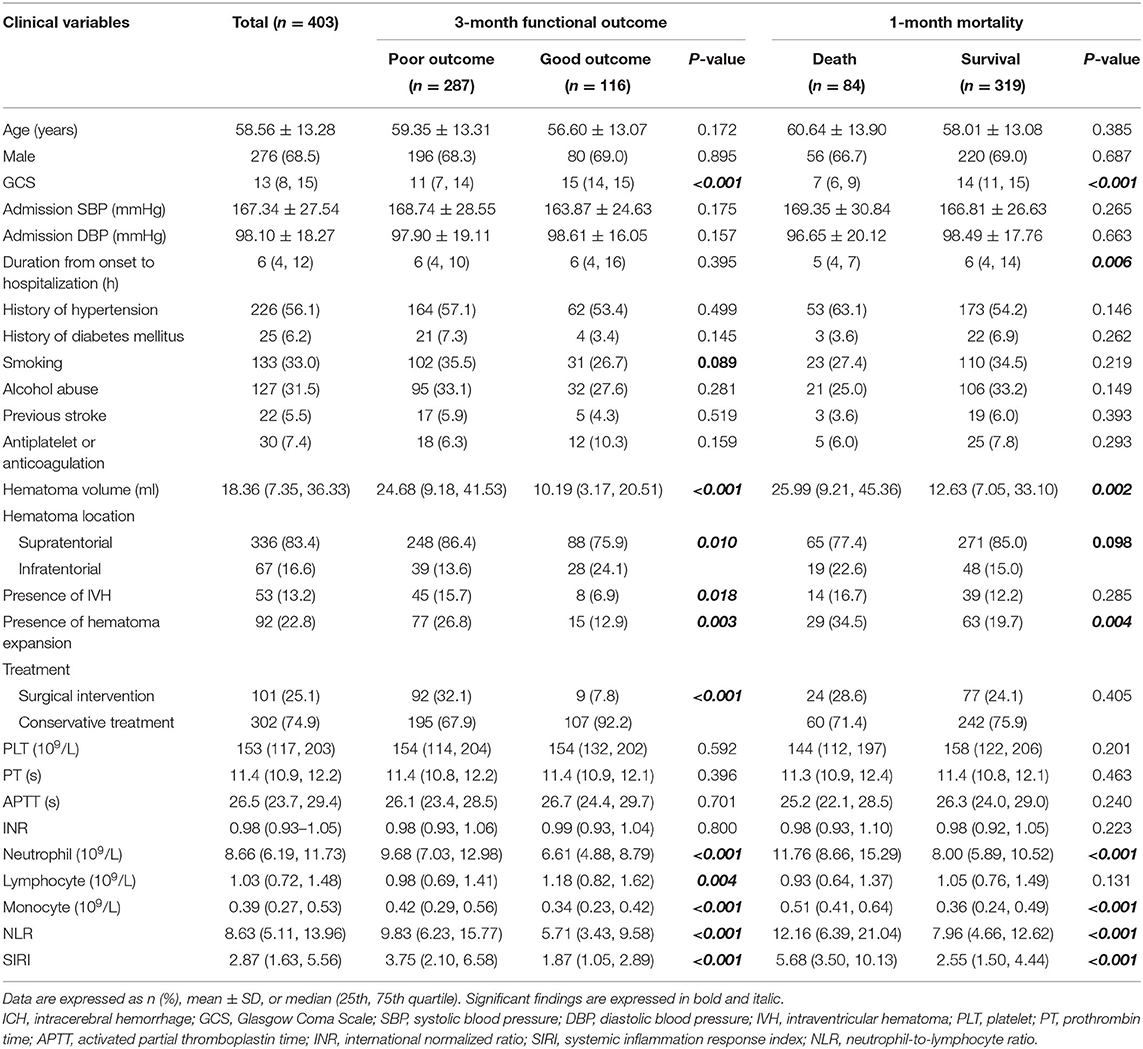
In the original cohort (Table 1), 287 patients had poor outcomes at 3 months with mRS score ≥3, while 116 patients had good outcomes with mRS score ≤ 2. Clinical variables including GCS score (p < 0.001), hematoma volume (p < 0.001), hematoma location (p = 0.010), presence of IVH (p = 0.018), presence of HE (p = 0.003), and treatment method (p < 0.001) were associated with 3-month functional outcomes. Meanwhile, neutrophil count (p < 0.001), lymphocyte count (p = 0.004), monocyte count (p < 0.001), NLR (p < 0.001), and SIRI (p < 0.001) were also associated with 3-month functional outcomes. Regarding 1-month mortality, 84 patients died within 30 days of admission, and 319 patients survived the first month. Among the clinical variables, GCS score (p < 0.001), duration from onset to hospitalization (p = 0.006), hematoma volume (p = 0.002), and presence of HE (p = 0.004) were associated with 1-month mortality. Peripheral blood markers, including neutrophil count (p < 0.001), monocyte count (p < 0.001), NLR (p < 0.001), and SIRI (p < 0.001), were significantly associated with 1-month mortality, whereas lymphocyte count (p = 0.131) was not associated with 1-month mortality.
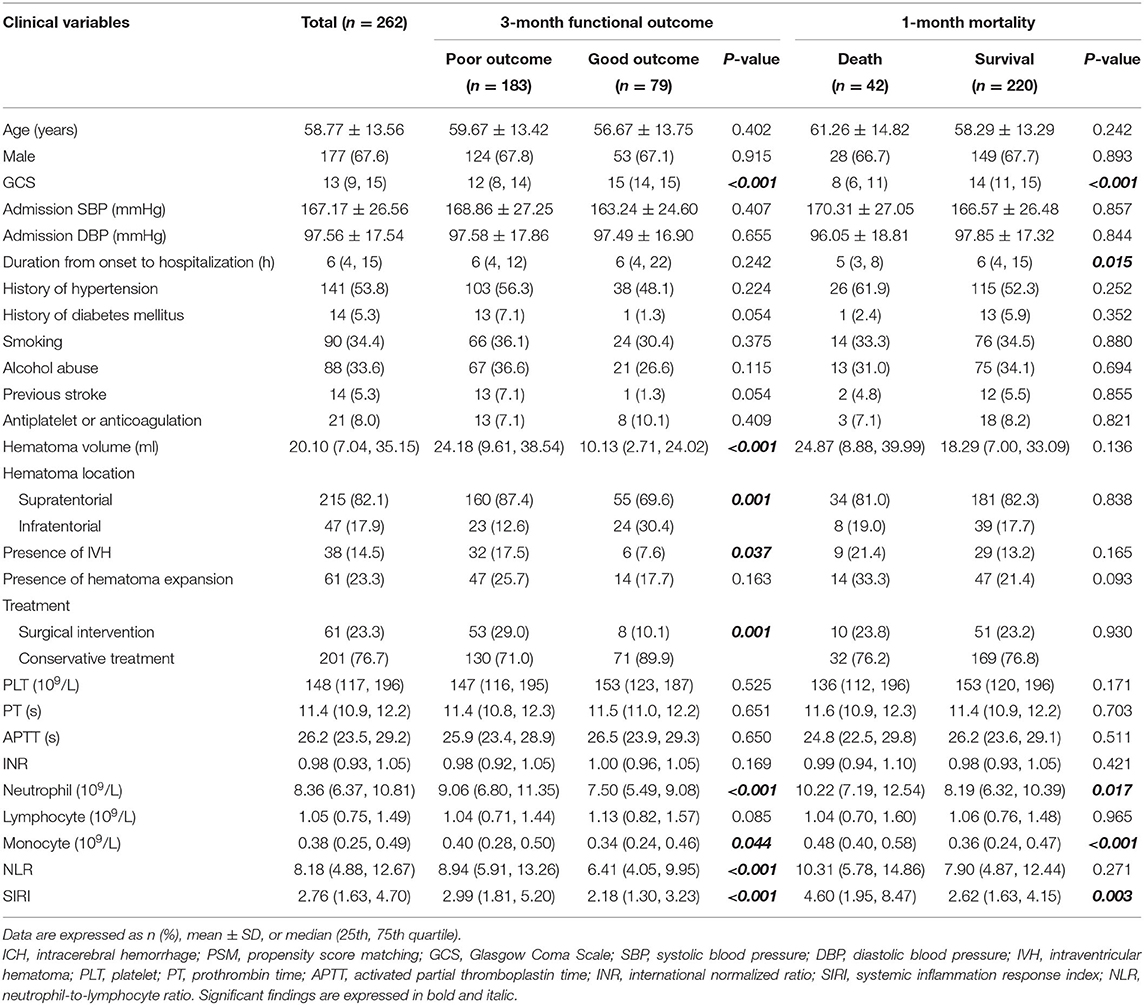
The clinical characteristics of the PSM cohort are listed in Table 2, with a lower GCS score (p < 0.001), larger hematoma volume (p < 0.001), presence of IVH (p = 0.037), supratentorial hematoma (p = 0.001), and surgical interventions (p = 0.001) associated with unfavorable outcomes at 3 months after admission. In the group with 1-month mortality, a lower GCS score (p < 0.001) and shorter duration from onset to hospitalization (p = 0.015) were directly related to death. Higher neutrophil count, monocyte count, and SIRI were associated with unfavorable outcomes in both groups. Higher NLR was significantly related to poor 3-month functional outcomes (p < 0.001) but not 1-month mortality (p = 0.271).
Association of SIRI With Outcomes
In the original cohort, multivariate logistic analysis (Table 3) revealed that the following factors all served as independent predictors for 3-month functional outcomes, including GCS score [odds ratio (OR), 0.686; 95% CI 0.606–0.776; p < 0.001), hematoma volume (OR, 1.022; 95% CI 1.002–1.042; p = 0.027), hematoma location (OR, 2.452; 95% CI 1.100–5.467; p = 0.028), treatment method (OR, 2.455; 95% CI 1.044–5.773; p = 0.040), lymphocyte count (OR, 0.469; 95% CI 0.290–0.758; p = 0.002), monocyte count (OR, 28.642; 95% CI 4.427–185.296; p < 0.001), NLR (OR, 1.051; 95% CI 1.004–1.100; p = 0.031), and SIRI (OR, 1.302; 95% CI 1.120–1.512; p = 0.001). As for 1-month mortality, GCS score (OR, 0.700; 95% CI 0.637–0.769; p < 0.001), hematoma volume (OR, 1.012; 95% CI 1.000–1.025; p = 0.046), monocyte count (OR, 3.734; 95% CI 1.283–10.869; p = 0.016), and SIRI (OR, 1.072; 95% CI 1.020–1.126; p = 0.006) were independent risk factors, but not NLR (OR, 1.021; 95% CI 0.987–1.057; p = 0.225).
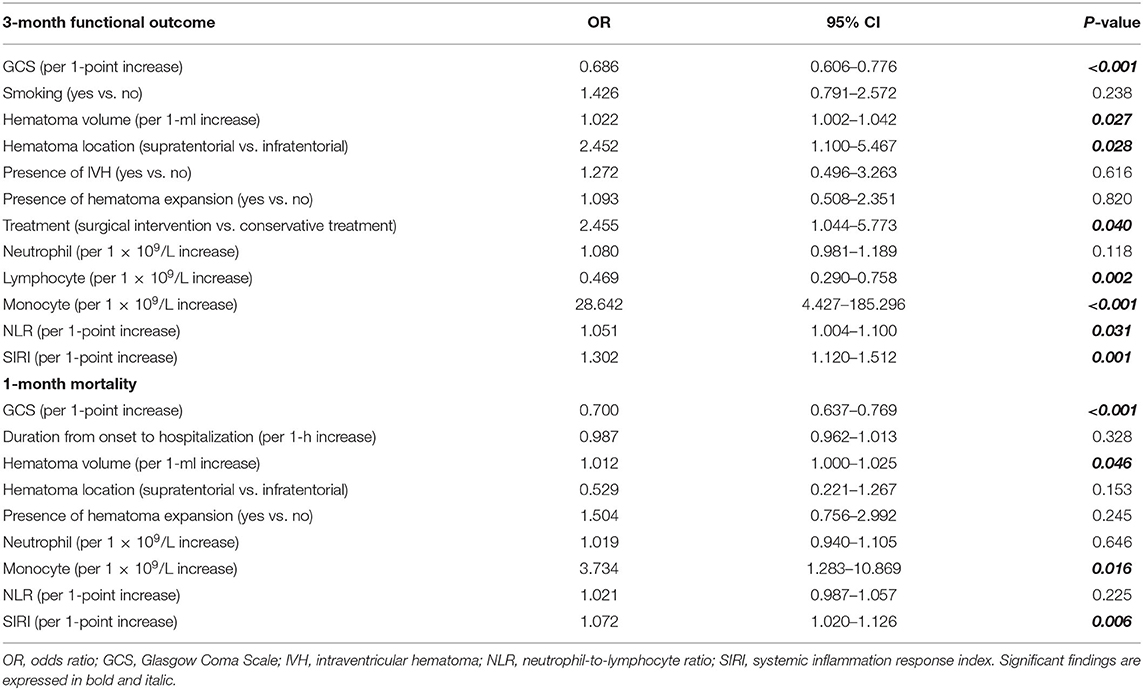
Table 3. Multivariate logistic regression of included clinical variables for 3-month functional outcome and 1-month mortality in original cohort.
As shown in Table 4, in the PSM cohort, GCS score (OR, 0.611; 95% CI 0.508–0.736; p < 0.001), hematoma volume (OR, 1.034; 95% CI 1.012–1.057; p = 0.002), neutrophil count (OR, 1.143; 95% CI 1.030–1.269; p = 0.012), NLR (OR, 1.076; 95% CI 1.016–1.139; p = 0.012), and SIRI (OR, 1.312; 95% CI 1.096–1.571; p = 0.003) were independently associated with 3-month functional outcomes. GCS score (OR, 0.684; 95% CI 0.607–0.770; p < 0.001), monocyte count (OR, 35.970; 95% CI, 4.490–288.130; p = 0.001), and SIRI (OR, 1.153; 95% CI 1.050–1.265; p = 0.003) were independently related to 1-month mortality. NLR was not included in the multivariate logistic analysis because the p-value in the univariate analysis did not match the criteria.
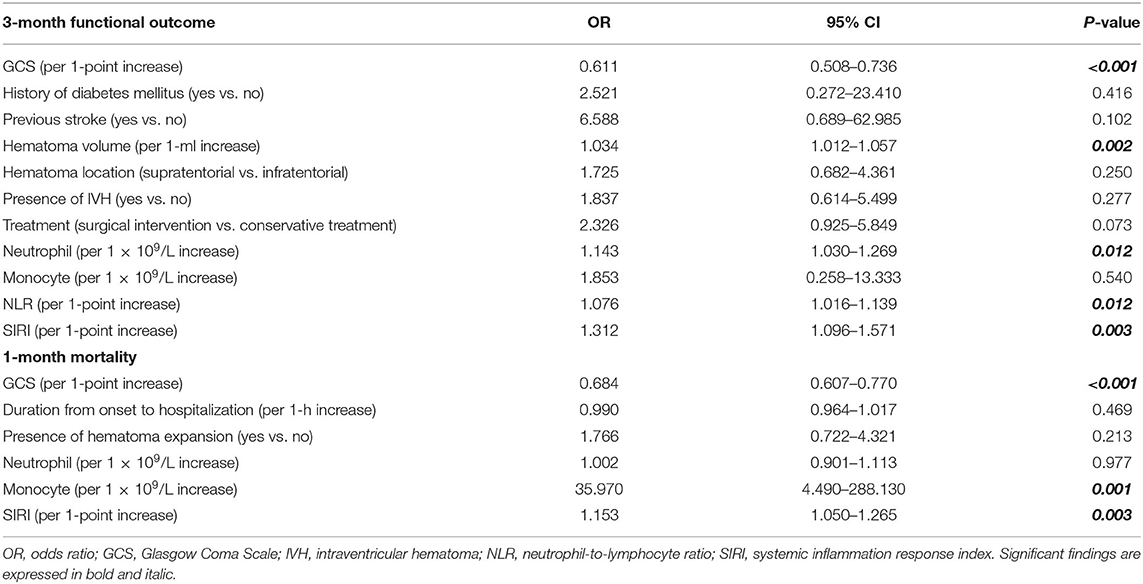
Table 4. Multivariate logistic regression of included clinical variables for 3-month functional outcome and 1-month mortality in PSM cohort.
Predictive Ability of SIRI and NLR in Outcomes
ROC analysis was employed to determine and compare the predictive ability of SIRI and NLR in 3-month functional outcomes and 1-month mortality in patients with ICH (Figure 2, Supplementary Figure 1). In the original cohort, SIRI had a stronger predictive ability than NLR in 3-month functional outcome (Figure 2A, AUC 0.748 vs. 0.698; DeLong's test, Z = 2.35, p = 0.019) and 1-month mortality (Figure 2B, AUC 0.745 vs. 0.656; DeLong's test, Z = 4.73, p < 0.001). The same applied to the PSM cohort, where the predictive ability of SIRI was also better than that of NLR in 1-month mortality (Figure 2D, AUC 0.644 vs. 0.554; DeLong's test, Z = 3.14, p = 0.002). In 3-month functional outcome, although predictive ability of SIRI was superior to NLR, there was no statistical difference (Figure 2C, AUC 0.653 vs. 0.636; DeLong's test, Z = 0.60, p = 0.550).
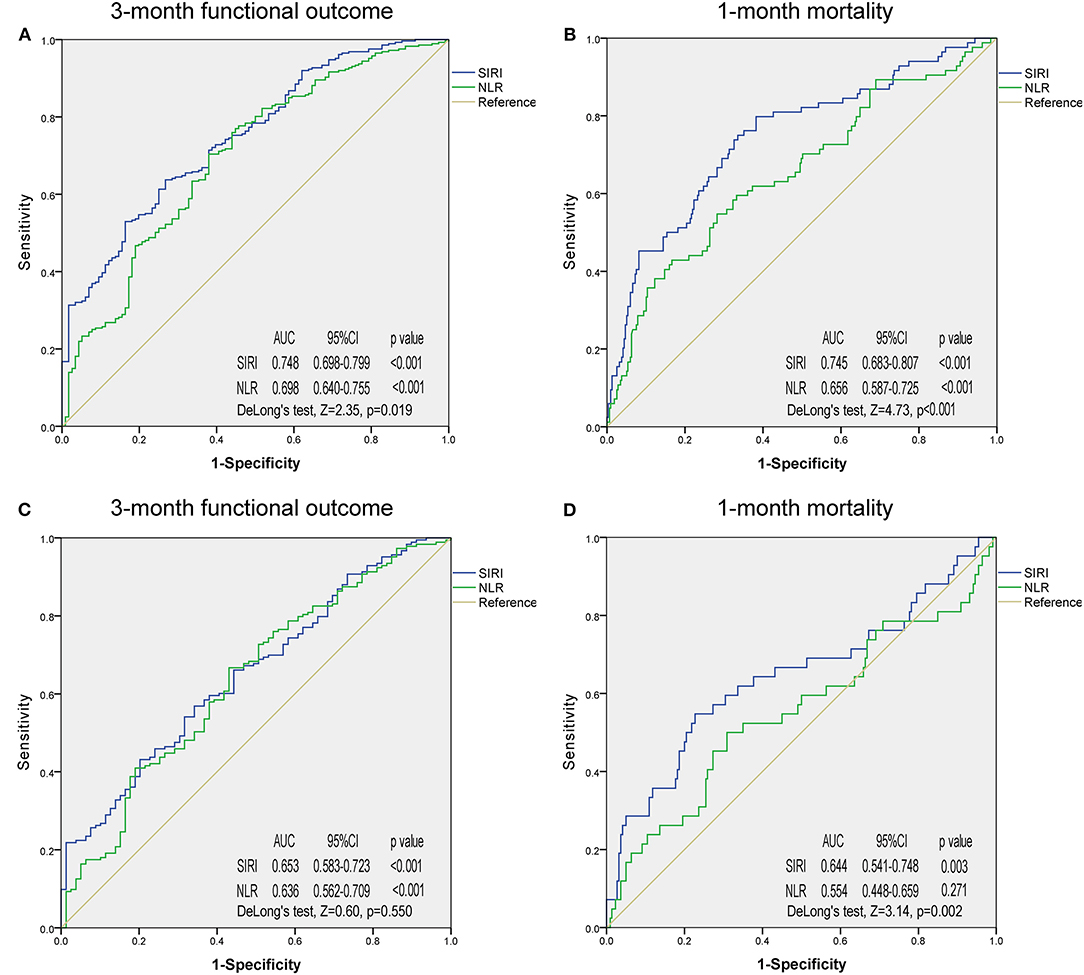
Figure 2. Receiver operating characteristic curves of systemic inflammation response index and neutrophil-to-lymphocyte ratio for predicting 3-month functional outcome and 1-month mortality in the original cohort (A,B) and propensity score matching cohort (C,D). SIRI, systemic inflammation response index; NLR, neutrophil-to-lymphocyte ratio; AUC, area under the curve.
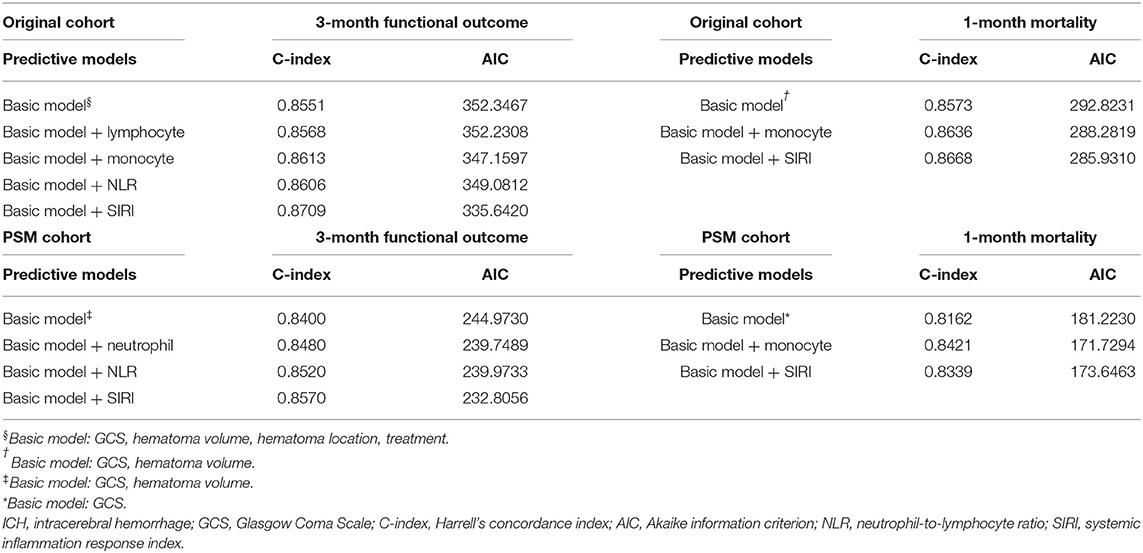
Predictive models were conducted to further evaluate the predictive accuracy of the aforementioned markers (Table 5). Basic models consisted of independent predictive indicators other than peripheral blood markers. The results indicated that basic models with SIRI had highest C-index and lowest AIC in 3-month functional outcome in both original and PSM cohort, indicating the best predictive accuracy and model-fitting. With regard to 1-month mortality, the basic model with SIRI was superior to that with monocytes in the original cohort, but the result was opposite in PSM cohort.
Discussion
In recent years, with the improvement of quality of life and medical conditions, excellent medical treatments, including medication and surgery, have been provided, which have a potent and direct impact on ICH morbidity and mortality (16). Multidisciplinary collaborations, such as between imaging, pathology, physiology, and neurosurgery, are needed to understand this condition and its underlying mechanism. In this study, we focused on the prognostic role of systemic inflammation biomarkers in peripheral blood in patients with spontaneous ICH.
Secondary damage due to ICH in the brain parenchyma induced by inflammatory cells and inflammatory cascades plays a crucial role in disease progression, thus affecting outcomes. Local inflammation adjacent to the primary injury could not be evaluated or measured directly, whereas systemic inflammation might reflect local inflammation in the peripheral blood system to some extent. Damage-associated molecular patterns, which are released by injured or dying neurons and cytokines during early injury, can gain access to the systemic circulation through the broken BBB or cerebrospinal fluid drainage system (22). In animal models of ischemic stroke, immuno-dysregulation after ischemic stroke includes upregulation of systemic inflammatory response. In animal models, a large ICH volume results in decreased leukocytes and lymphocytes and increased monocytes (7). In the same, higher leukocyte counts have been associated to hematoma growth and early neurological deterioration (8). Relevant evidence indicated that the peripheral cellular immune system changed dramatically in the immediate aftermath of ICH (23). Therefore, changes in specific inflammatory markers in the peripheral blood are an indicator of the severity of the primary injury, theoretically. In oncology, inflammatory markers from peripheral blood are used to predict tumor progression and prognosis (24).
We have introduced a novel systemic inflammatory marker SIRI in our study, which was first reported in pancreatic cancer in 2016 (25). Since SIRI and NLR have a great similarity in their components, their predictive abilities in prognosis are compared in this study. NLR has been widely used as an effective indicator and monitor in various diseases, but not limited to tumors, rheumatic diseases, cardiovascular diseases, and infectious diseases (26–29). It is a very sensitive but less specific hematologic parameter to measure stress, intensity of infection/inflammation, and severity of illness of various origin (30). It has also been determined to play a strong predictive role in prognosis for ICH and subarachnoid hemorrhage patients in previous studies (31, 32). Similar to most related studies, the results of this study indicate that NLR is an independent risk factor for 3-month functional outcomes measured by the mRS. Compared with NLR, SIRI is mainly reported in the field of cancer. Recent researches about SIRI in aneurysmal subarachnoid hemorrhage showed that higher level of SIRI served as an independent indicator of unfavorable clinical outcomes (33, 34). In our research, SIRI was superior to NLR in predicting 3-month functional outcomes and has significant advantages in predicting 1-month mortality. However, NLR did not serve as an independent risk factor for 1-month mortality in ICH patients in our study.
Monocytes are mononuclear myeloid cells that originate from the bone marrow and circulate within the bloodstream (35). Like neutrophils, monocyte recruitment in circulation and injured tissues is a key feature of inflammation (36). A previous study has shown that a higher monocyte count on admission is an independent predictor of HE (37). In a study by Walsh et al., absolute monocyte count was independently associated with 30-day case fatality in 240 adult ICH patients, which is consistent with their previous study and our current study (38, 39). In a previous study by Mackey et al., elevated monocyte count was also an independent risk factor for 30-day case fatality (40). In the current study, we also found that monocyte count also served as independent prognostic predictors in 3-month functional outcome and 1-month mortality in the original cohort, and presented an excellent predictive ability in 1-month mortality in the PSM cohort. In consideration of the prognostic ability of monocytes in ICH patients, this could partly explain why the combination of monocyte and NLR gains predictive ability in outcomes.
From another perspective, stability of prognostic capacity in single component including neutrophil, lymphocyte, and monocyte was inferior to SIRI according to the results from multivariate analysis, ROC analyses, and predictive models. In sum, the ability to mirror the extent of inflammation corresponds to the ability to predict prognosis. For peripheral blood-relevant inflammatory markers, diversity compound modes are worth trying and easily realized, which might improve the predictive ability in specific diseases.
In fact, inflammation was not only a prognostic indicator for ICH patients but also a crucial therapeutic target based on the theory that cellular and molecular components of inflammation are involved in post-hemorrhagic secondary brain injury (41). Although the progression of developing specific therapeutic targets remains challenging, markers such as NOD-like receptor family, pyrin domain-containing 3 (NLRP3), C–C chemokine receptor type 1 (CCR1), and Toll-like receptor 4 (TLR4) are proven effective in intervening the progression of ICH-related inflammation (42–44).
There are several limitations to this study. First, follow-up blood tests at each follow-up time point were absent in this study due to incomplete baseline clinical data. For various reasons, it was inconvenient and difficult for some patients to have blood tests regularly, especially when they were not in the hospital. Second, the sample size was not large enough to be divided into training and validation cohorts for further verification. Third, more complicated and comprehensive prognostic patterns are needed to evaluate the prognosis of ICH patients in various aspects, including cognitive function and quality of life. Fourth, not all patients were admitted to hospital within 24 h after onset. Although these patients were in the minority, this might induce unknown bias in laboratory results. Fifth, some occult infections cannot be diagnosed at an early stage by using clinical and laboratory criteria; this might also create bias. Finally, this analysis was conducted in a single institution; therefore, the results should be verified using multi-center data.
Conclusion
To our knowledge, this is the first study focusing on the prognostic significance of admission SIRI in patients with spontaneous ICH. In this study, SIRI was determined to be an independent predictive indicator for ICH patients in both 3-month functional outcomes and 1-month mortality. Furthermore, its prognostic predictive ability is better than that of NLR. In the near future, multi-center collabora tion is needed to further verify the results and illuminate the underlying mechanism.
Data Availability Statement
The datasets for this study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JZ and JL: study design. JL and YY: data acquisition and writing—original draft. JL, XL, and JZ: statistical analysis. JL and ZY: result interpretation. JZ and HL: writing—review and editing. JZ: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81801186), the Science and Technology Department of Sichuan Province (grant number 2020YFQ0009), and the Outstanding Subject Development 135 Project of West China Hospital, Sichuan University (grant number ZY2016102).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Master Zheng for his assistance. Thanks for the great support of our ICH team. We pay tribute to all medical staff working on the front line. Also, we would like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.718032/full#supplementary-material
Supplementary Figure 1. Receiver operating characteristic curves of inflammatory markers for predicting 3-month functional outcome and 1-month mortality in the original cohort (A,B) and propensity score matching cohort (C,D). SIRI, systemic inflammation response index; NLR, neutrophil-to-lymphocyte ratio; AUC, area under the curve.
Abbreviations
ICH, intracerebral hemorrhage; BBB, blood–brain barrier; NLR, neutrophil-to-lymphocyte ratio; SIRI, systemic inflammation response index; GCS, Glasgow Coma Scale; mRS, modified Rankin Scale; HE, hematoma expansion; ROC, receiver operating characteristic; PSM, propensity score matching; OR, odds ratio; AUC, area under the curve.
References
1. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. (2001) 344:1450–60. doi: 10.1056/NEJM200105103441907
2. Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. (1988) 19:547–54. doi: 10.1161/01.STR.19.5.547
3. Schrag M, Kirshner H. Management of intracerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol. (2020) 75:1819–31. doi: 10.1016/j.jacc.2019.10.066
4. Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. (2015) 6:4–8. doi: 10.1007/s12975-014-0384-4
5. Mracsko E, Javidi E, Na SY, Kahn A, Liesz A, Veltkamp R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. (2014) 45:2107–14. doi: 10.1161/STROKEAHA.114.005801
6. Fu Y, Liu Q, Anrather J, Shi F-D. Immune interventions in stroke. Nat Rev Neurol. (2015) 11:524–35. doi: 10.1038/nrneurol.2015.144
7. Saand AR, Yu F, Chen J, Chou SH. Systemic inflammation in hemorrhagic strokes - a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. (2019) 39:959–88. doi: 10.1177/0271678X19841443
8. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. (2016) 47:1654–7. doi: 10.1161/STROKEAHA.116.013627
9. Trifan G, Testai FD. Systemic Immune-Inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2020) 29:105057. doi: 10.1016/j.jstrokecerebrovasdis.2020.105057
10. Zhang W, Shen Y. Platelet-to-lymphocyte ratio as a new predictive index of neurological outcomes in patients with acute intracranial hemorrhage: a retrospective study. Med Sci Monit. (2018) 24:4413–20. doi: 10.12659/MSM.910845
11. Hagen M, Sembill JA, Sprügel MI, Gerner ST, MadŽar D, Lücking H, et al. Systemic inflammatory response syndrome and long-term outcome after intracerebral hemorrhage. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e588. doi: 10.1212/NXI.0000000000000588
12. Boehme AK, Hays AN, Kicielinski KP, Arora K, Kapoor N, Lyerly MJ, et al. Systemic inflammatory response syndrome and outcomes in intracerebral hemorrhage. Neurocrit Care. (2016) 25:133–40. doi: 10.1007/s12028-016-0255-9
13. Li S, Lan X, Gao H, Li Z, Chen L, Wang W, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. (2017) 143:2455–68. doi: 10.1007/s00432-017-2506-3
14. Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2018) 65:503–10. doi: 10.1016/j.intimp.2018.10.002
15. Xie QK, Chen P, Hu WM, Sun P, He WZ, Jiang C, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. (2018) 16:273. doi: 10.1186/s12967-018-1638-9
16. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
17. Broderick JP, Adeoye O, Elm J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke. (2017) 48:2007–12. doi: 10.1161/STROKEAHA.117.017866
18. Xu X, Chen X, Zhang J, Zheng Y, Sun G, Yu X, et al. Comparison of the Tada formula with software slicer: precise and low-cost method for volume assessment of intracerebral hematoma. Stroke. (2014) 45:3433–5. doi: 10.1161/STROKEAHA.114.007095
19. Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. (1997) 28:1–5. doi: 10.1161/01.STR.28.1.1
20. Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. (2012) 17:228–43. doi: 10.1037/a0027127
21. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
22. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
23. Jiang C, Wang Y, Hu Q, Shou J, Zhu L, Tian N, et al. Immune changes in peripheral blood and hematoma of patients with intracerebral hemorrhage. FASEB J. (2020) 34:2774–91. doi: 10.1096/fj.201902478R
24. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
25. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
26. Lee HN, Kim YK, Kim GT, Ahn E, So MW, Sohn DH, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-α agents in patients with rheumatoid arthritis: a retrospective chart review analysis. Korean J Intern Med. (2019) 39:859–68. doi: 10.1007/s00296-019-04276-x
27. Angkananard T, Anothaisintawee T. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. (2018) 2018:2703518. doi: 10.1155/2018/2703518
28. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
29. Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. (2020) 38:641–7. doi: 10.1016/j.ajem.2019.10.023
30. Zahorec R, Hulin I, Zahorec P. Rationale use of Neutrophil-to-lymphocyte ratio for early diagnosis and stratification of COVID-19. Bratisl Lek Listy. (2020) 121:466–70. doi: 10.4149/BLL_2020_077
31. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. (2019) 10:137–45. doi: 10.1007/s12975-018-0649-4
32. Al-Mufti F, Amuluru K, Damodara N, Dodson V, Roh D, Agarwal S, et al. Admission neutrophil-lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurointerv Surg. (2019) 11:1135–40. doi: 10.1136/neurintsurg-2019-014759
33. Yun S, Yi HJ, Lee DH, Sung JH. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. (2021) 30:105861. doi: 10.1016/j.jstrokecerebrovasdis.2021.105861
34. Zhang P, Li Y, Zhang H, Wang X, Dong L, Yan Z, et al. Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br J Neurosurg. (2020). doi: 10.1080/02688697.2020.1831438. [Epub ahead of print].
35. Mitchell AJ, Roediger B, Weninger W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. (2014) 291:22–31. doi: 10.1016/j.cellimm.2014.05.010
36. Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. (2017) 37:35–42. doi: 10.1161/ATVBAHA.116.308198
37. Morotti A, Phuah CL, Anderson CD, Jessel MJ, Schwab K, Ayres AM, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke. (2016) 47:1473–8. doi: 10.1161/STROKEAHA.116.013176
38. Walsh KB, Sekar P, Langefeld CD, Moomaw CJ, Elkind MS, Boehme AK, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke. (2015) 46:2302–4. doi: 10.1161/STROKEAHA.115.009880
39. Adeoye O, Walsh K, Woo JG, Haverbusch M, Moomaw CJ, Broderick JP, et al. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2014) 23:e107–1. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.006
40. Mackey J, Blatsioris AD, Saha C, Moser EAS, Carter RJL, Cohen-Gadol AA, et al. Higher monocyte count is associated with 30-day case fatality in intracerebral hemorrhage. Neurocrit Care. (2021) 34:456–64. doi: 10.1007/s12028-020-01040-z
41. Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke. (2020) 22:29–46. doi: 10.5853/jos.2019.02236
42. Yan J, Zuo G, Sherchan P, Huang L, Ocak U, Xu W, et al. CCR1 activation promotes neuroinflammation through CCR1/TPR1/ERK1/2 signaling pathway after intracerebral hemorrhage in mice. Neurotherapeutics. (2020) 17:1170–83. doi: 10.1007/s13311-019-00821-5
43. Fang H, Wang PF, Zhou Y, Wang YC, Yang QW. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation. (2013) 10:27. doi: 10.1186/1742-2094-10-27
Keywords: systemic inflammation response index, neutrophil to lymphocyte ratio, intracerebral hemorrhage, prognosis, propensity score matching
Citation: Li J, Yuan Y, Liao X, Yu Z, Li H and Zheng J (2021) Prognostic Significance of Admission Systemic Inflammation Response Index in Patients With Spontaneous Intracerebral Hemorrhage: A Propensity Score Matching Analysis. Front. Neurol. 12:718032. doi: 10.3389/fneur.2021.718032
Received: 31 May 2021; Accepted: 13 August 2021;
Published: 24 September 2021.
Edited by:
Robert G. Kowalski, University of Colorado, United StatesReviewed by:
João Pinho, University Hospital RWTH Aachen, GermanyYanlin Zhang, Second Affiliated Hospital of Soochow University, China
Kara Melmed, New York University, United States
Copyright © 2021 Li, Yuan, Liao, Yu, Li and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zheng, emhlbmdqdW4wODMyMUBxcS5jb20=
 Junhong Li
Junhong Li Yunbo Yuan1
Yunbo Yuan1