- 1South China Research Center for Acupuncture and Moxibustion, Medical College of Acu-Moxi and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Biostatistics, Yale School of Public Health, New Haven, CT, United States
- 3Center for Methods in Implementation and Prevention Science, Yale School of Public Health, New Haven, CT, United States
- 4Department of Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Objectives: To quantify the association of cigarette smoking, including cigarettes per day and quitting duration, with the risk of different types of stroke morbidity and mortality in the general population, and to clarify the shape of the dose-response relations.
Study Selection: Prospective cohort studies and reported on the association between smoking, quitting and the incidence or mortality of stroke were included.
Data Extraction and Synthesis: All available data were converted uniformly to odds ratios (ORs) and were pooled using random-effects meta-analysis with inverse variance weighting. A dose-response meta-analysis was performed to explore the quantitative relationship between different smoking characteristics and the risk of different pathologic types of stroke incidence.
Results: Twenty-five studies with 3,734,216 individuals were included. Compared to never smokers, the pooled ORs of stroke morbidity and mortality were 1.45 (1.24–1.70) and 1.44 (1.23–1.67) among ever smokers and 1.90 (1.55–2.34) and 1.70 (1.45–1.98) among current smokers. The risk of different pathologic types of stroke was also increased among ever and current smokers. There was a significant non-linear dose-response association between the number of cigarette smoking and the risk of stroke incidence. Comparing no smoking, the ORs for smoking five and 35 cigarettes per day were 1.44 (1.35–1.53) and 1.86 (1.71–2.02). Other pathologic types of stroke have a similar dose-response relationship. There was also non-linear dose-response association between the length of time since quitting and risk of stroke. The risk of stroke decreased significantly after quitting for 3 years [OR = 0.56 (0.42–0.74)].
Conclusion: The risk of different types of stroke among smokers is remarkably high. Our findings revealed a more detailed dose-response relationship and have important implications for developing smoking control strategies for stroke prevention.
Systematic Review Registration: https://inplasy.com/inplasy-2020-6-0062/, identifier: INPLASY202060062.
Introduction
Among 240 causes of death, stroke is the second leading cause of death and disability globally and one of the four largest contributors to disability-adjusted life years among neurological disorders (1, 2). According to the Global Burden of Disease findings in 2017, the global burden of stroke remains high, leading to 6.2 million deaths and 132.1 million disability-adjusted life-years (3). A key to reducing the global burden of stroke is renewed emphasis on stroke prevention.
Previous studies examining the association of smoking with stroke have yielded mixed findings. Smoking has been recognized as a preventable independent risk factor for stroke, with 12.4% of accidental stroke cases being attributable to current smoking behavior (4). Paradoxically, several recent studies have shown that smoking could be associated with a better early outcome in stroke patients, lower mortality rates or the same total mortality rates (5, 6). Some studies suggest that previous smoking behavior is associated with a lower clinical severity in patients with stroke (5, 7). Furthermore, smokers who received thrombolysis had a significantly greater drop in stroke severity scores from baseline than nonsmokers who received thrombolysis and lower mortality over 1 year (8, 9). However, other studies suggested that smoking was not associated with good functional outcomes after adjusting for covariates (10, 11). Given contradictory evidence in previous individual study results, additional integrative research efforts are required to reach a consensus.
Notably, the effect of smoking is closely related to its dose, and the association of different smoking characteristics with stroke warrants further investigation. A robust relationship between smoking dose and stroke can inform a decision model for doctors so that patients could possibly know how much less they need to smoke each day or how many years they need to quit smoking before experiencing noticeable health benefits. Particularly, a more precise quantification of the association between current and/or former smoking and stroke risk as well as the identification of a possible threshold for the effect remain to be determined. To date, only a few studies have examined the relationship between smoking dose and stroke and find mixed results. For example, one study used a linear model to evaluate the dose-response relationship between stroke risk and cigarette consumption (12). Other studies speculate that there is a substantial gap between this dose-response model and the actual risk of stroke (13).
Based on these existing results, we carried out a comprehensive systematic review of recent prospective cohort studies that reported the effects of smoking on the risk of different pathologic types of stroke. We evaluated the association between cigarette smoking and the risk of different pathologic types of stroke and estimate their dose-response relationship. By synthesizing evidence across studies and accounting for study heterogeneity, we characterize a more refined dose-response relationship that may have important implications for developing smoking control strategies for stroke.
Methods
Literature Search
For this meta-analysis, we systematically searched the PubMed, Embase, Books@Ovid, Journals@Ovid, Your Journals@Ovid, Joanna Briggs Institute EBP, ACP Journal Club, CCTR, CDSR, CCA, CLCMR, DARE, CLHTA, CLEED, AMED, Ovid Emcare, HAPI, HealthSTAR, and Ovid MEDLINE(R) databases for studies written in English and published prior to July 31, 2021.The search terms included words associated with stroke and the Cochrane Tobacco Addiction Group search strategy. The full search criteria are listed in Appendix 1. In addition, we manually searched for additional relevant articles in the reference lists of identified articles and other publications. This study follows PRISMA-IPD guidelines for individual-participant data reporting (Appendix 2). As a systematic review and meta-analysis, ethical approval was not necessary for this study. This study is registered with INPLASY (NO. INPLASY202060062).
Inclusion and Exclusion Criteria
Articles were included if they were a prospective cohort study and provided relative risks (RRs), ORs or hazard ratios (HRs) as well as 95% confidence intervals (CIs) for the association between cigarette smoking status and stroke. Cigarette smoking status describes the status at baseline, including never and ever smoking (ever smoking includes both former smokers and current smokers). Studies that involved participants who smoked different amounts of cigarettes or who reported different lengths of time since smoking cessation were also acceptable.
Studies were excluded if they set an inexact definition of stroke or included some disease endpoints other than stroke. Compared with spontaneous intracerebral hemorrhage, traumatic hemorrhages, such as subdural and epidural hematomas and other types of intracranial bleeding that are not caused by a vascular event but due to injury, have different mechanisms, courses and outcomes. With the rapid development of technology, there is no longer a clear timeline of diagnosis between stroke and transient ischemic attack (TIA). However, they are still two different diseases because TIA leaves no permanent neurological deficit. Therefore, we only included ischemic stroke (IS) and hemorrhagic stroke (HS) (including intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH)). Traumatic hemorrhage and TIA should not be characterized as stroke and were not included in this meta-analysis.
We refer to the definition of stroke incidence by the American Heart Association and the American Stroke Association in 2013 and ICD codes (14). In the case of duplicate reports from the same cohort, we included the most recent publication or the publication with the longest follow-up period. Two authors (JL and XT) independently evaluated the full texts to determine whether those articles should be incorporated into the analysis. Disagreements between the two authors were settled by consensus-based discussion with a third reviewer (LL).
Data Extraction and Quality Assessment
The follow data were extracted using a standard table: authors, year of publication, inclusion and exclusion criteria, sample size, study population (age, gender, countries and continents and whether the study patients suffer from cardiovascular disease or baseline disease or clinical information), definition of smoking, smoking status (current, former, never, dose of cigarette consumption, duration of smoking cessation), multivariate-adjusted OR, HR,or RR with 95% CIs of stroke for each smoking status category and follow-up time. This meta-analysis evaluated the correlation between cigarette smoking and the incidence or mortality of stroke, including different pathologic types of stroke, by pooling multivariate-adjusted ORs, RRs, and HRs. Multivariate adjustments were allowed to vary by study but must include age.
We evaluated the quality of the included studies using the Newcastle–Ottawa Scale (NOS) for cohort studies. Using this 9-point scale, high-quality studies were defined as a score of 7 or greater; moderate-quality studies were defined as 3–6 points; and low-quality studies were defined as below 3 points. If there were disagreements between the two authors (LW and SG) in the data extraction or quality assessment process, a third author (HW) was consulted for consensus.
Statistical Analysis
We conducted the meta-analysis using Review Manager v.5.3 software (Cochrane Collaboration, Oxford, UK). In our study, HRs and RRs converted to ORs; HRs were considered as RRs; RRs could be converted into ORs using the formula RR = OR/[(1-P0) + (P0 × OR)], in which P0 was the event incidence in the control group. We converted RRs into ORs directly when studies did not provide P0 because the incidence or mortality of stroke in the study population is always low (<10%) (15). Multivariate-adjusted ORs of stroke with cigarette smokers (former or current) vs. never cigarette smokers were pooled by random effects models, including incidence, mortality and different pathologic types. The I2 statistic and the Cochrane Q test were used to assess between-study heterogeneity. Subgroup analysis was performed to investigate the difference between current smokers and former smokers vs. never smokers.
We performed a dose-response analysis of cigarette smoking or quitting duration on stroke risk by Stata13.1 (Stata Corporation, College Station, TX, USA). The distribution of cases, person-years and the adjusted OR with 95% CI for at least three exposure categories were required. We chose the midpoint of the interval when cigarette number or quitting duration categories intervals were presented. When the upper level for the highest category was open-ended, the exposure doses were calculated as 1.5 times their exposure levels (16). A potential non-linear dose-response association was assessed by modeling the dose and quitting duration of cigarette smoking and was checked by restricted cubic splines with four knots at the 5th, 35th, 65th, and 95th percentiles of the distribution. To test for non-linearity, a likelihood ratio test (for nested models) was applied to compare the model with both the linear and the spline terms and the model with the linear term only (17). If the non-linear model does not provide a significantly better fit for the curve, a linear model will be considered instead to quantify the association of stroke risk and cigarette consumption.
To explore factors associated with study heterogeneity, we conducted univariable random effects meta-regression for each of the outcomes when there were at least ten studies available for analysis. We also conducted a post-hoc multiple regression analysis adjusting for risk of stroke at baseline, type gender, length of follow-up and country. To evaluate the robustness of the results, leave-one-out sensitivity analyses were conducted for the primary outcome. We also conducted sensitivity analysis by excluding non-high-quality studies based on NOS scores.
Results
Search Results
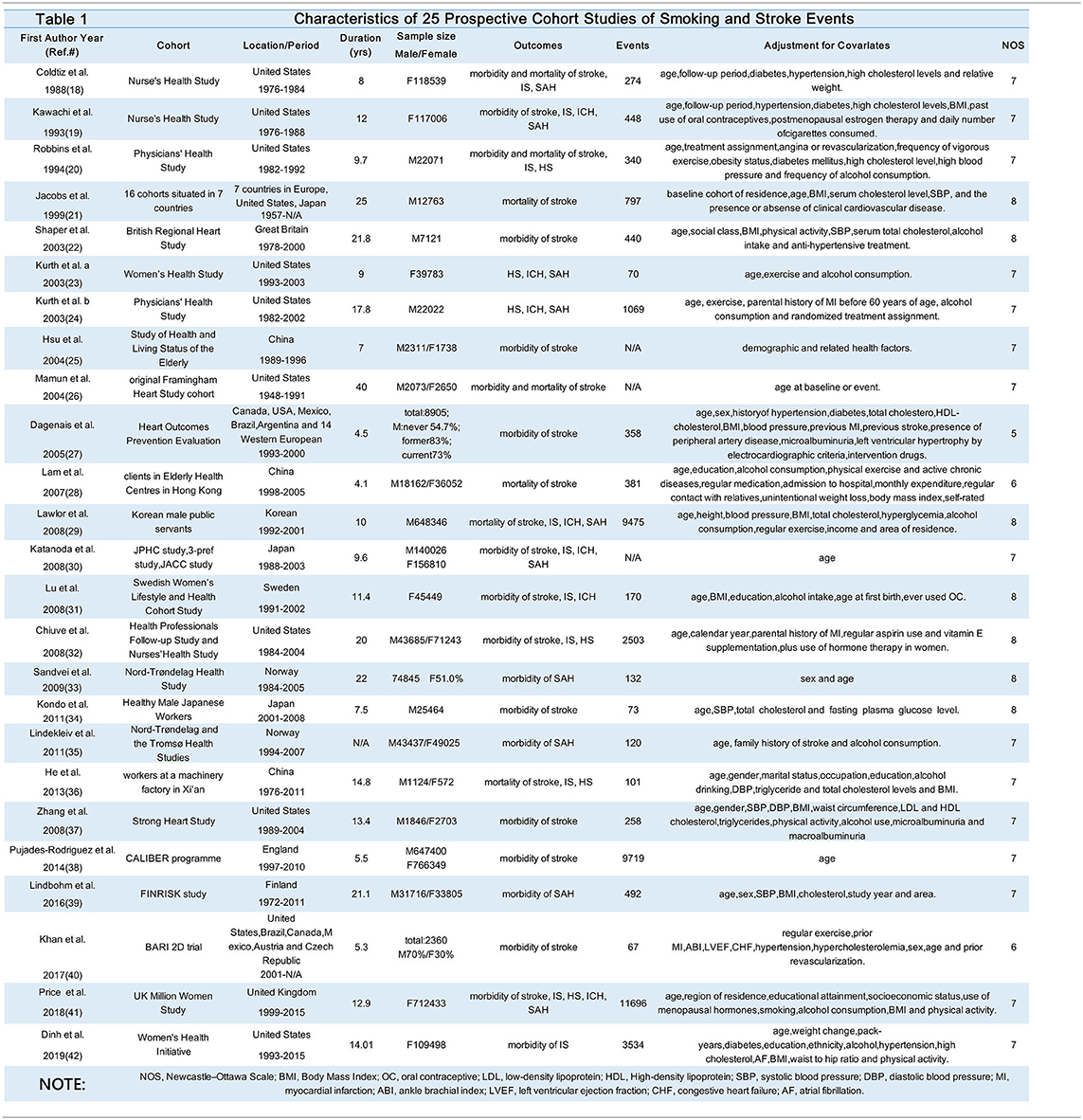
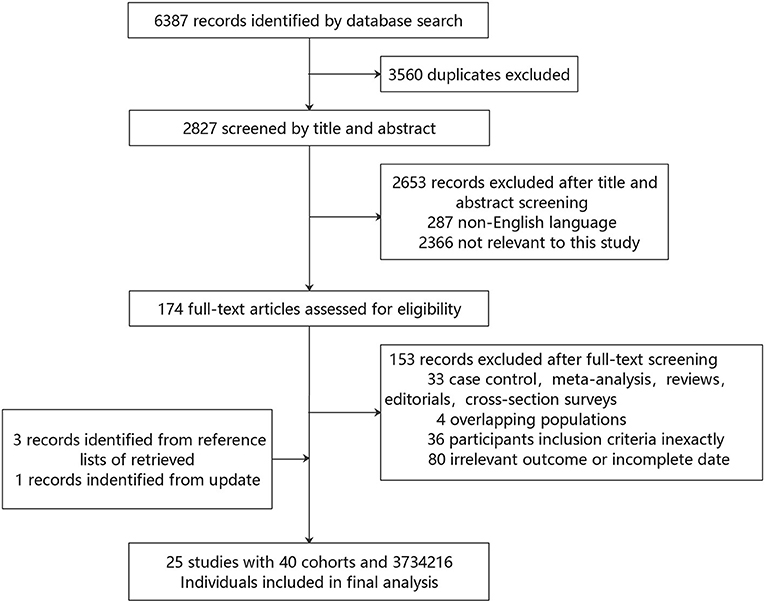
Finally, 25 studies (18–42) were included in the meta-analysis, encompassing more than 40,000 events of stroke (Table 1). The specific screening procedure is summarized in Figure 1. Studies were from geographically diverse settings (33 countries) and the majority of the studies (88%) were rated as high quality.
Meta-Analysis
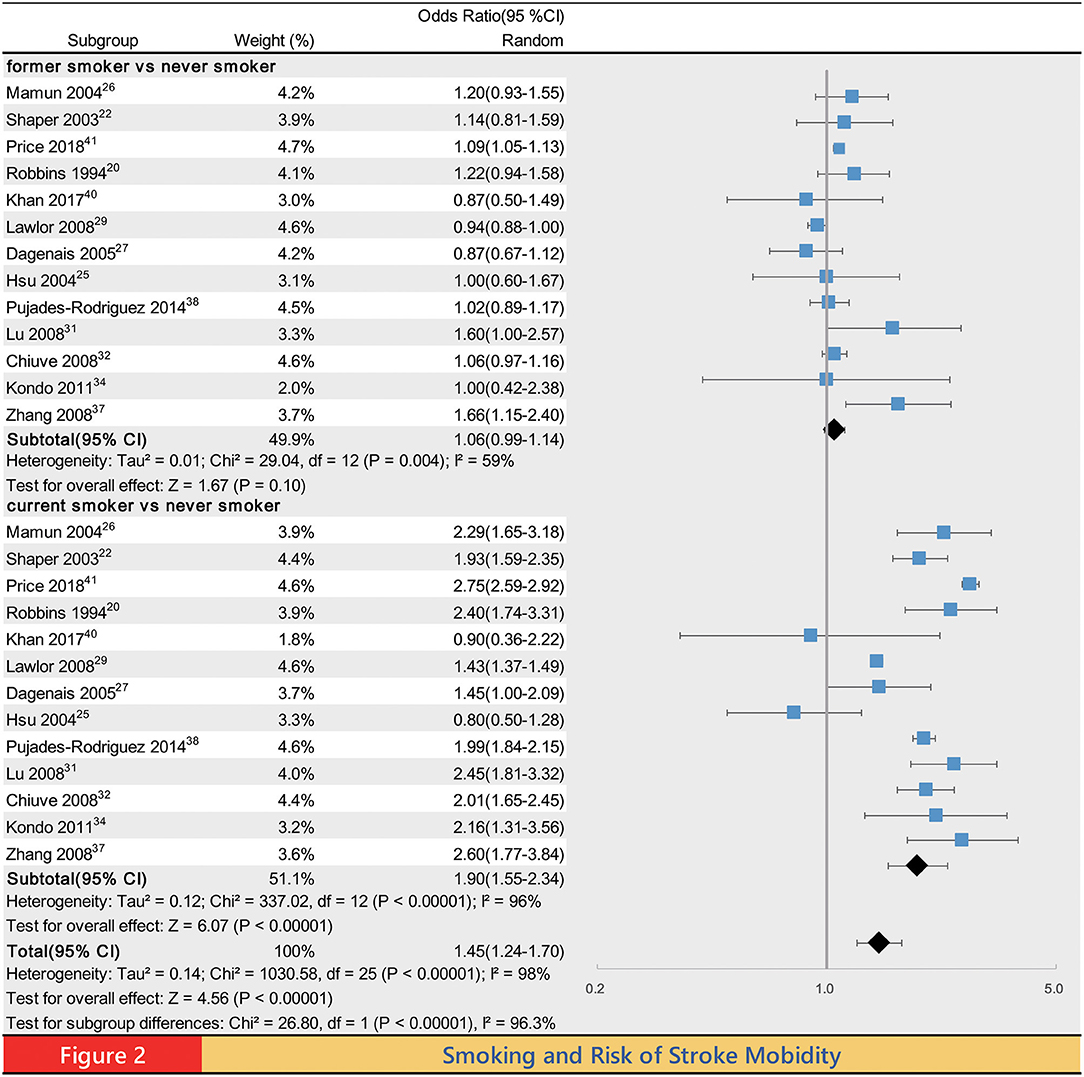
Meta-analysis of the association between smoking status and stroke incidence yielded a summary OR of 1.45 (95% CI 1.24–1.70, P < 0.00001) for ever smokers, 1.06 (0.99, 1.14, P = 0.10) for former smokers and 1.90 (1.55–2.34 P < 0.00001) for current smokers compared with never smokers (Figure 2). Compared with never smokers, the pooled ORs of stroke mortality for ever smokers, former smokers and current smokers were 1.44 (1.23–1.67, P < 0.00001), 1.10 (0.99–1.22, P = 0.09), and 1.70 (1.45–1.98, P < 0.00001), respectively (Supplementary Figure 1). Compared with never smoker, the pooled OR of IS incidence for ever smokers, former smokers and current smokers was 1.55 (1.26–1.91, P < 0.0001), 1.05 (1.00–1.11, P = 0.03), and 2.09 (1.74–2.50, P < 0.00001; Supplementary Figure 2); the pooled OR of HS incidence for ever smokers, former smokers and current smokers was 1.49 (1.06–2.11, P = 0.02), 1.01 (0.86–1.18, P = 0.90) and 2.58 (2.23–2.97, P < 0.00001; Supplementary Figure 3); the pooled OR of ICH incidence for ever smokers, former smokers and current smokers was 1.25 (1.03–1.50, P = 0.02), 0.97 (0.84–1.13, P = 0.73) and 1.61 (1.17–2.23, P = 0.004; Supplementary Figure 4); the pooled OR of SAH incidence for ever smokers, former smokers and current smokers was 2.13 (1.60–2.85, P < 0.00001), 1.23 (1.02–1.49, P = 0.03), and 3.39 (2.59–4.45, P < 0.00001), respectively. Although the incidence of SAH was the lowest in the above categories of stroke (3), SAH seems to be most affected by smoking (Supplementary Figure 5).
There were statistically significant differences in the incidence of stroke, IS, HS, ICH, SAH, and mortality of stroke between ever smokers and never smokers. Such a difference was also observed between former smokers and current smokers. As expected, current smokers had the highest risk for all of these outcomes. Except for the incidence of IS and SAH, the differences between former smokers and never smokers in the risk of the rest of the outcomes were not statistically significant.
Dose-Response Analysis
Visually, there was a significant non-linear dose-response association between the number of cigarettes per day (CPD) and risk of stroke incidence (P non-linearity < 0.001, Figure 3A); the OR showed a significantly increasing trend, especially as the number of CPD increased from one to ten. Compared to no smoking, the ORs for smoking five, ten, and 35 CPD were 1.44 (1.35–1.53), 1.63 (1.52–1.74), and 1.86 (1.71–2.02), respectively. Five CPD accounted for more than half of the additional risk from large doses of smoking (≥30 CPD). Smoking just ten CPD provides most of the risks of stroke associated with smoking. The OR of stroke incidence increased again when the number of CPD was more than 20. There was a similar non-linear dose-response association between CPD and risk of IS incidence (P non-linearity < 0.001, Figure 4A). For IS, compared to no smoking, the ORs of smoking 5, 10, and 35 CPD were 1.51 (1.39–1.63), 1.73 (1.59–1.90), and 2.04 (1.82–2.28) respectively. Similar to the stroke dose curve, smoking five CPD was associated with nearly half the additional risk from smoking in large doses (≥30 CPD).
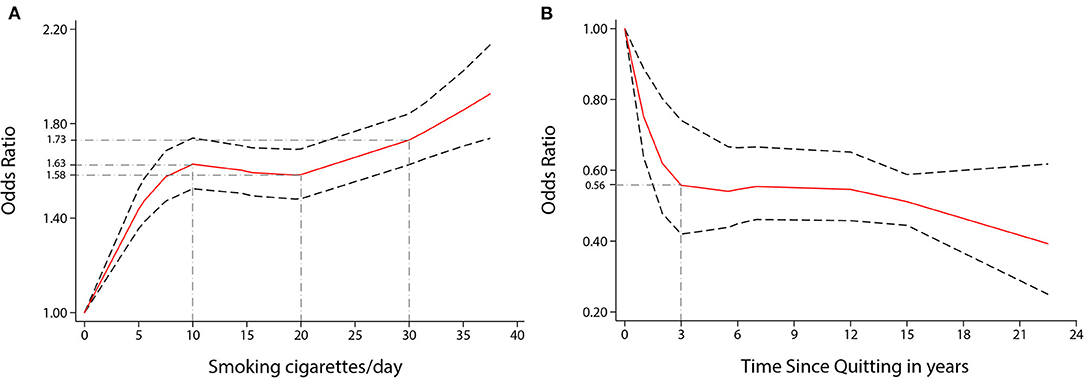
Figure 3. Non-linear dose-response analyses of smoking/quitting and risk of stroke in meta-analysis. (A) Association between CPD and risk of stroke incidence restricted cubic splines with four knots (0, 5, 15.5, 35 CPD) and 0 CPD as a reference. P non-linearity = 0.0000. (B) Association between the length of time since quitting and the risk of stroke incidence restricted cubic splines with four knots (0, 1, 6, 22.5 years) and quitting 0 years as a reference. P non-linearity = 0.0022. The solid line represents the estimated OR, and the dashed lines represent the 95% CI.
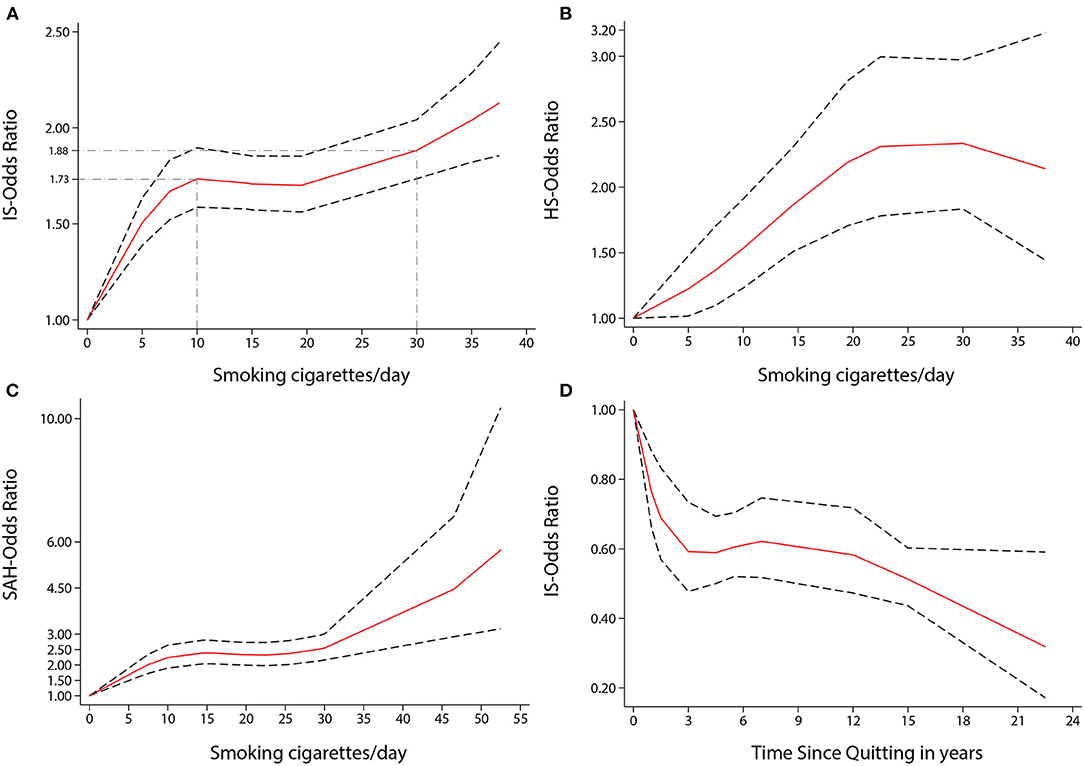
Figure 4. Non-linear dose-response analyses of smoking/quitting and risk of IS/HS/SAH in meta-analysis. (A) Association between dose of cigarette consumption and risk of Ischemic stroke incidence restricted cubic splines with 4 knots (0, 5, 15, 37.5 CPD) and 0 CPD as reference. P non-linearity = 0.0000. Solid line represents the estimated OR and the dashed lines represent the 95% CI. OR = 1.73, 95% CI 1.59–1.90, for 10 CPD; OR = 1.88, 95% CI 1.74–2.04, for 30 CPD. (B) Association between dose of cigarette consumption and risk of Hemorrhagic stroke incidence restricted cubic splines with 4 knots (0, 5, 19.5, 37.5 CPD) and 0 CPD as reference. P non-linearity = 0.0447. Solid line represents the estimated OR and the dashed lines represent the 95% CI. OR = 1.53, 95% CI 1.23–1.91, for 10 CPD; OR = 2.34, 95% CI 1.83–2.97, for 30 CPD. (C) Association between dose of cigarette consumption and risk of Subarachnoid Hemorrhage incidence restricted cubic splines with 4 knots (0, 6.5, 21, 49.5 CPD) and 0 CPD as reference. P non-linearity = 0.0002. Solid line represents the estimated OR and the dashed lines represent the 95% CI. OR = 2.24, 95% CI 1.90–2.64, for 10 CPD; OR = 2.55, 95% CI 2.16–3.01, for 30 CPD. (D) Association between the length of time since quitting and risk of Ischemic stroke incidence restricted cubic splines with 4 knots (0, 1, 5.5, 22.5 year) and quitting 0 year as reference. P non-linearity = 0.0022. Solid line represents the estimated OR and the dashed lines represent the 95% CI. OR = 0.59, 95% CI 0.48–0.73, for 3 year; OR = 0.51, 95% CI 0.44–0.60, for 15 year; OR = 0.32, 95% CI 0.17–0.59, for 22.5 year.
There was a non-linear dose-response relationship between the incidence of HS and SAH and CPD. The pooled OR of incidence of HS increased rapidly when the CPD ranged from 1 to 22 (P non-linearity < 0.05, Figure 4B). The ORs of smoking ten and 30 CPD were 1.53 (1.23–1.91) and 2.34 (1.83–2.97), respectively. The pooled OR of SAH incidence increased rapidly when the CPD ranged from 1 to 15 and when the CPD was more than 30 (P non-linearity < 0.001, Figure 4C). The ORs of smoking 10 and 30 CPD were 2.24 (1.90–2.64) and 2.55 (2.16–3.01), respectively.
There was also a non-linear dose-response relationship between the incidence of stroke and the length of time since quitting cigarette smoking (P non-linearity < 0.01, Figure 3B). Despite only quitting for 3 years, the risk of stroke decreased rapidly [OR = 0.56 (0.42–0.74)]. Furthermore, the longer people quit smoking, the lower their risk of stroke. Similar non-linear dose-response relationship between the risk of IS incidence and the length of time since quitting [P non-linearity < 0.001, Figure 4D, OR = 0.59 (0.48–0.73) for three years and OR = 0.32 (0.17–0.59), for 22.5 years]. There was a tendency of stronger risk reduction for longer quitting cigarette smoking.
Meta-Regression Analysis
We found no correlation between sex and the risk of stroke incidence (Univariable P = 0.265, Multiple P = 0.425). The continents from which people came had no correlation with the risk of stroke incidence (Univariable P = 0.374, Multiple P = 0.747). However, the follow-up time significantly modified the association between cigarette smoking and the risk of stroke incidence (Univariable P = 0.005, Multiple P = 0.013; Supplementary Table 1).
Sensitivity Analysis
Substantial heterogeneity was observed among studies of smoking and stroke risk. However, the results of sensitivity analyses suggested that removal of any individual study did not materially alter the pooled OR; therefore, the pooled results were not dominated by any single study outlier (Supplementary Figure 6). In addition, the pooled results of sensitivity analysis in morbidity and mortality of stroke by excluding non-high-quality studies were also similar to the main results (Supplementary Figures 7, 8).
Discussion
Summary of Results
We have shown that smokers, especially current smokers, have a significantly increased risk of total stroke and different types of stroke, such as IS, HS, ICH, and SAH. We also show a dose-response relationship between CPD and duration of cessation and risk of different pathologic types of stroke.
Through the results of the dose-response analysis, we can clearly see that smoking and quitting smoking change stroke risk in a way that is not a simple linear relationship. Smoking has a strong and sensitive impact on stroke risk. The risk of stroke rises rapidly even with just one more CPD. While the human body's repair and adjustment functions limit the damage caused by low and medium doses of cigarette consumption, the damage caused by smoking will exceed the capacity of the human repair function when the number of CPD exceeds 20. There is another conjecture that explains such an association: when the number of CPD exceeds 20, different damage mechanisms in the body appear or dominate, so the risk of stroke rises rapidly again.
According to the results of the dose-response analysis of quitting, we found that the risk of stroke and IS drop rapidly in the first three years of quitting. This means that the tendency to decrease thrombosis and cerebral perfusion and the negative effects on changes in hemodynamics function and thrombosis caused by smoking might be reversed by the third year. Atherosclerosis from smoking can also be repaired by the human themselves, but it takes more than ten years to show up in a reduction of the stroke risk, and the longer the time, the greater the effect. The risk difference between former and never smokers was not statistically significant in the incidence of stroke (p = 0.10), HS (p = 0.90), ICH (p = 0.73) or the mortality of stroke (p = 0.09) in the subgroup analysis. Therefore, the effects of smoking on the risk of stroke may be reversed by the body's strong repair ability.
Mechanism of Smoking and Stroke
The deleterious effect of cigarette smoke is related to a mixture of more than 7000 chemicals contributing to endothelial dysfunction, inflammation, dyslipidemia, vascular and hemodynamic function, and a prothrombotic state. This can cause atherosclerosis and increase the risk of thrombotic events. Decreased vasodilatation and diminished nitric oxide bioavailability were also observed in smokers (43). The effects of the above causes and mechanisms greatly increase the risk of cardiovascular disease. Cerebrovascular disease has a similar pathogenesis. Atherosclerosis formation, thrombosis and decreased cerebral perfusion increase the risk of stroke, especially IS. Kurth et al. (23) summarized that smoking increased the risk of SAH by promoting the presence, formation and rupture of aneurysms and increased the risk of ICH by damaging the structure of the arterial wall.
Study Strengths and Limitations
Our study used a nonlinear model to demonstrate a more realistic dose-response relationship, and facilitate a accurate understanding of the relationship between smoking dosage and risk of stroke. In addition, we further refined the dose-response relationship between each stroke type (IS, HS, ICH, and SAH) and the number of cigarettes smoked. We also dynamically analyzed the relationship between length of smoking cessation and risk reduction, which could provide clearer guidance and stronger confidence to quitters and potential quitters. Moreover, we limited the ICD codes of the included study and removed age restrictions for a more comprehensive analysis of stroke. Compared to previously published studies, our study included more detailed analyses to support our findings, which leads to new insights.
The most important point among these factors was that we observed a significant nonlinear dose-response association between CPD and quitting and the risk of each stroke type (IS, HS, ICH and SAH) incidence. Hackshaw et al. (12) used a log-linear variance weighted regression model to evaluate the dose-response relationship between stroke risk and cigarette consumption. They consider that smoking one CPD had 41% and 31% of the excess RR of men and women who smoked 20 CPD, respectively. However, we came up with inconsistent results. Although we agree that small CPD also poses a significant risk of stroke, smoking only five CPD led to more than half of the additional risk from 20 or 30 CPD, and ten CPD provided most of the risk of stroke associated with smoking. However, it is unreasonable that one cigarette brings approximately half of the excess risk of one pack of cigarettes. Their model may have exaggerated the risk of smoking one cigarette and 20 cigarettes according to our results. In addition, Oono et al. (44) also used a nonlinear dose-response relationship, but they only discussed the relationship between second-hand smoke and stroke.
It is also worth mentioning that part of the stroke cohort studies that did not use accurate stroke definitions and ICD codes, equated stroke directly with cerebrovascular diseases (ICD9, 430-438, ICD10, I60-I69). Some of them may also include TIA. All previous meta-analyses on smoking and stroke did not exclude research with inaccurate definitions. This led to an inaccurate number of stroke events and an overestimation of stroke risk. Our study set the inclusion criteria to bring our results closer to the real relationship between smoking and stroke risk.
Furthermore, Mons et al. (45) reported a lower risk of stroke from smoking. That was because they included older people over the age of 60. The reason why smoking has a less negative effect is that older people have a higher incidence and mortality of stroke. However, stroke should no longer be considered a disease of the elderly, and two-thirds of all strokes occur among persons <70 years of age reported by Global Burden Disease study (46). Krishnamurthi et al. (3) also found a concerning trend toward increased stroke burden in people aged 45–59 years old. Therefore, our inclusion criteria did not limit the age of participants, and our outcome applies to a wider range of populations.
This study has several limitations. Because some articles were excluded due to inexact definitions of stroke and ICD codes, the data related to smoking and outcomes for IS, ICH, and SAH mortality are sparse. We were unable to assess dose-response analysis between smoking, quitting and the mortality of different pathologic types of stroke.
In addition, heterogeneity across studies was high among the included studies and may be due to different study designs and characteristics of participants. For example, the sample size, follow-up time, multiple adjustments and definitions of never, former and current smokers varied widely from study to study. However, sensitivity analyses showed that any meta-analysis did not alter the pooled OR significant, and the pooled results were stable.
Policy Implications
Due to its high morbidity, mortality and disability rate, stroke has brought a heavy burden to modern society in different aspects. At present, the stroke population is still increasing, for example in China, and carrying out primary stroke prevention, such as reducing smoking, is the best way to prevent stroke (47). Therefore, the government and media need to publicize tobacco control in more detail and quantitatively to make it more effective. Health professionals and the public should realize that low-dose (five to ten CPD) cigarette consumption is associated with a high risk of stroke, and cessation for more than three years is associated with significant benefits. If people cannot quit smoking, they should be limited to five or fewer CPD to significantly reduce their risk of stroke. In addition, the government should also encourage and support the development of smoking cessation institutions. Moreover, smoke-free laws should be enforced in public places and indoors to reduce exposure to cigarette smoking.
Conclusion
Smoking will increase the risk of stroke with different pathologic types. There was a non-linear dose-response relationship between the amount of cigarette smoking and duration of cessation and stroke risk. Low-dose smoking can carry half or more of the additional risk from large doses of smoking. Quitting smoking for more than 3 years will deliver significant health benefits. Our findings provide a more detailed dose-response relationship and have important implications for developing smoking control strategies for stroke.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
LL developed the study concept. JL and XT did the literature search, article screening, data extraction, and statistical analyses. HW, LW, and SG evaluated the quality of studies. FL, CT, NX, and LL directed research and revised manuscripts. All authors were involved in drafting the manuscript.
Funding
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2020B1111100008 to LL), the Youth Scientific Research Training Project of GZUCM (2019QNPY02 to LL), the Young Top Talent Project of Scientific and Technological Innovation in Special Support Plan for Training High-level Talents in Guangdong (2017TQ04R627 to LL), the Youth Program of the National Natural Science Foundation of China (81904297 to LW), the Elite Youth Education Program of Guangzhou University of Chinese Medicine (QNYC20190106 to LW), and the Science and Technology Planning Project of Guangdong Province (2014B090902002 to NX). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.772373/full#supplementary-material
References
1. Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:439–58. doi: 10.1016/S1474-4422(19)30034-1
2. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
3. Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. (2020) 54:171–9. doi: 10.1159/000506396
4. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. (2016) 388:761–75. doi: 10.1016/S0140-6736(16)30506-2
5. Bejot Y, Jacquin A, Daubail B, Lainay C, Janoura S, Aboa-Eboule C, et al. Smoking status and severity of ischemic stroke. A population-based study. Eur Neurol. (2014) 71:59–64. doi: 10.1159/000355021
6. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male british doctors. BMJ. (2004) 328:1519. doi: 10.1136/bmj.38142.554479.AE
7. Bang OY, Park HY, Lee PH, Kim GM, Chung CS, Lee KH. Improved outcome after atherosclerotic stroke in male smoker. J Neurol Sci. (2007) 260:43–8. doi: 10.1016/j.jns.2007.04.002
8. Ovbiagele B, Saver JL. The smoking-thrombolysis paradox and acute ischemic stroke. Neurology. (2005) 65:293–5. doi: 10.1212/01.WNL.0000168163.72351.f3
9. Zidovetzki R, Chen P, Fisher M, Hofman FM, Faraci FM. Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C-associated pathway. Stroke. (1999) 30:651–5. doi: 10.1161/01.str.30.3.651
10. Nakamura K, Huxley R, Ansary-Moghaddam A, Woodward M. The hazards and benefits associated with smoking and smoking cessation in Asia: a meta-analysis of prospective studies. Tob Control. (2009) 18:345–53. doi: 10.1136/tc.2008.028795
11. Zhang P, Guo ZN, Sun X, Zhao Y, Yang Y. Meta-analysis of the smoker's paradox in acute ischemic stroke patients receiving intravenous thrombolysis or endovascular treatment. Nicotine Tob Res. (2019) 21:1181–8. doi: 10.1093/ntr/ntz094
12. Hackshaw A, Morris JK, Boniface S, Tang J, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. (2018) 360:j5855. doi: 10.1136/bmj.j5855
13. Cole TJ. Re: low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. (2018) 360:j5855.
14. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
15. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
16. Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ. (2013) 346:e8539. doi: 10.1136/bmj.e8539
17. Frank E, Harrell Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer (2015). doi: 10.1007/978-3-319-19425-7
18. Colditz GA, Bonita R, Stampfer MJ, Willett WC, Rosner B, Speizer FE, et al. Cigarette smoking and risk of stroke in middle-aged women. New Engl J Med. (1988) 318:937–41. doi: 10.1056/NEJM198804143181501
19. Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation and decreased risk of stroke in women. JAMA. (1993) 269:232–6. doi: 10.1001/jama.269.2.232
20. Robbins AS, Manson JE, Lee IM, Satterfield S, Hennekens CH. Cigarette smoking and stroke in a cohort of U.S. male physicians. Ann Intern Med. (1994) 120:458–62. doi: 10.7326/0003-4819-120-6-199403150-00002
21. Jacobs DRJ, Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A, et al. Cigarette smoking and mortality risk: twenty-five-year follow-up of the seven countries study. Arch Intern Med. (1999) 159:733–40. doi: 10.1001/archinte.159.7.733
22. Shaper AG, Wannamethee SG, Walker M. Pipe and cigar smoking and major cardiovascular events, cancer incidence and all-cause mortality in middle-aged british men. Int J Epidemiol. (2003) 32:802–8. doi: 10.1093/ije/dyg206
23. Kurth T, Kase CS, Berger K, Gaziano JM, Cook NR, Buring JE. Smoking and risk of hemorrhagic stroke in women. Stroke. (2003) 34:2792–5. doi: 10.1161/01.STR.0000100165.36466.95
24. Kurth T, Kase CS, Berger K, Schaeffner ES, Buring JE, Gaziano JM. Smoking and the risk of hemorrhagic stroke in men. Stroke. (2003) 34:1151–5. doi: 10.1161/01.STR.0000065200.93070.32
25. Hsu H, Pwu R. Too late to quit? Effect of smoking and smoking cessation on morbidity and mortality among the elderly in a longitudinal study. Kaohsiung J Med Sci. (2004) 20:484–91. doi: 10.1016/S1607-551X(09)70247-5
26. Mamun AA, Peeters A, Barendregt J, Willekens F, Nusselder W, Bonneux L, et al. Smoking decreases the duration of life lived with and without cardiovascular disease: a life course analysis of the Framingham heart study. Eur Heart J. (2004) 25:409–15. doi: 10.1016/j.ehj.2003.12.015
27. Dagenais GR, Yi Q, Lonn E, Sleight P, Ostergren J, Yusuf S, et al. Impact of cigarette smoking in high-risk patients participating in a clinical trial. A substudy from the heart outcomes prevention evaluation (HOPE) trial. Euro J Cardiovasc Prevent Rehabil. (2005) 12:75–81. doi: 10.1097/00149831-200502000-00012
28. Lam TH, Li ZB, Ho SY, Chan WM, Ho KS, Tham MK, et al. Smoking, quitting and mortality in an elderly cohort of 56,000 Hong Kong Chinese. Tob Control. (2007) 16:182–9. doi: 10.1136/tc.2006.019505
29. Lawlor DA, Song Y, Sung J, Ebrahim S, Smith GD. The association of smoking and cardiovascular disease in a population with low cholesterol levels: a study of 648,346 men from the Korean national health system prospective cohort study. Stroke. (2008) 39:760–7. doi: 10.1161/STROKEAHA.107.494823
30. Katanoda K, Marugame T, Saika K, Satoh H, Tajima K, Suzuki T, et al. Population attributable fraction of mortality associated with tobacco smoking in Japan: a pooled analysis of three large-scale cohort studies. J Epidemiol. (2008) 18:251–64. doi: 10.2188/jea.JE2007429
31. Lu M, Ye W, Adami H, Weiderpass E. Stroke incidence in women under 60 years of age related to alcohol intake and smoking habit. Cerebrovasc Dis. (2008) 25:517–25. doi: 10.1159/000131669
32. Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. (2008) 118:947–54. doi: 10.1161/CIRCULATIONAHA.108.781062
33. Sandvei MS, Romundstad PR, Muller TB, Vatten L, Vik A. Risk factors for aneurysmal subarachnoid hemorrhage in a prospective population study: the HUNT study in Norway. Stroke. (2009) 40:1958–62. doi: 10.1161/STROKEAHA.108.539544
34. Kondo T, Osugi S, Shimokata K, Honjo H, Morita Y, Maeda K, et al. Smoking and smoking cessation in relation to all-cause mortality and cardiovascular events in 25,464 healthy male Japanese workers. Circ J. (2011) 75:2885–92. doi: 10.1253/circj.CJ-11-0416
35. Lindekleiv H, Sandvei MS, Njolstad I, Lochen M, Romundstad PR, Vatten L, et al. Sex differences in risk factors for aneurysmal subarachnoid hemorrhage: a cohort study. Neurology. (2011) 76:637–43. doi: 10.1212/WNL.0b013e31820c30d3
36. He Y, Jiang B, Li LS, Li LS, Sun DL, Wu L, et al. Changes in smoking behavior and subsequent mortality risk during a 35-year follow-up of a cohort in Xi'an, China. Am J Epidemiol. (2014) 179:1060–70. doi: 10.1093/aje/kwu011
37. Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, et al. Incidence and risk factors for stroke in American Indians: the strong heart study. Circulation. (2008) 118:1577–84. doi: 10.1161/CIRCULATIONAHA.108.772285
38. Pujades-Rodriguez M, George J, Shah AD, Rapsomaniki E, Denaxas S, West R, et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1937360 people in England: lifetime risks and implications for risk prediction. Int J Epidemiol. (2015) 44:129–41. doi: 10.1093/ije/dyu218
39. Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Smoking, and risk for subarachnoid hemorrhage. Stroke. (2016) 47:1975–81. doi: 10.1161/STROKEAHA.116.012957
40. Khan AA, Chung MJ, Novak E, Mori Brooks M, Brown DL. The long-term risk of smoking in diabetic patients with stable ischemic heart disease treated with intensive medical therapy and lifestyle modification. Eur J Prev Cardiol. (2017) 24:1506–14. doi: 10.1177/2047487317711046
41. Price AJ, Wright FL, Green J, Balkwill A, Kan SW, Yang TO, et al. Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology. (2018) 90:e298–306. doi: 10.1212/WNL.0000000000004856
42. Dinh PC, Schrader LA, Svensson CJ, Margolis KL, Silver B, Luo J. Smoking cessation, weight gain, and risk of stroke among postmenopausal women. Prev Med. (2019) 118:184–90. doi: 10.1016/j.ypmed.2018.10.018
43. Ramotowski B, Gurbel PA, Tantry U, Budaj A. Smoking and cardiovascular diseases: paradox greater than expected? Pol Arch Intern Med. (2019) 129:700–6. doi: 10.20452/pamw.14931
44. Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health UK. (2011) 33:496–502. doi: 10.1093/pubmed/fdr025
45. Mons U, Muezzinler A, Gellert C, Schottker B, Abnet CC, Bobak M, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. (2015) 350:h1551. doi: 10.1136/bmj.h1551
46. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. (2017) 120:439–48. doi: 10.1161/CIRCRESAHA.116.308413
Keywords: stroke, cigarette smoking, dose-response, quantitative relationship, meta-analysis
Citation: Luo J, Tang X, Li F, Wen H, Wang L, Ge S, Tang C, Xu N and Lu L (2022) Cigarette Smoking and Risk of Different Pathologic Types of Stroke: A Systematic Review and Dose-Response Meta-Analysis. Front. Neurol. 12:772373. doi: 10.3389/fneur.2021.772373
Received: 09 September 2021; Accepted: 06 December 2021;
Published: 25 January 2022.
Edited by:
Vincent Thijs, University of Melbourne, AustraliaReviewed by:
Wen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaBettina Von Sarnowski, Universitätsmedizin Greifswald, Germany
Copyright © 2022 Luo, Tang, Li, Wen, Wang, Ge, Tang, Xu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Lu, bHVsaW1pbmdsZW9uQGd6dWNtLmVkdS5jbg==; Nenggui Xu, bmd4dTgwMThAZ3p1Y20uZWR1LmNu; Chunzhi Tang, am9yZGFuNjY0QGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jianyu Luo1†
Jianyu Luo1† Xiaorong Tang
Xiaorong Tang Fan Li
Fan Li Hao Wen
Hao Wen Liming Lu
Liming Lu