- 1Functional Neurosurgery Department, National Children's Health Center of China, Beijing Children's Hospital, Capital Medical University, Beijing, China
- 2Key Laboratory of Major Diseases in Children, Ministry of Education, Beijing, China
- 3Neurology Department, National Children's Health Center of China, Beijing Children's Hospital, Capital Medical University, Beijing, China
- 4Neurosurgery Department, Affiliated, Jining Medical College, Jining, China
- 5Neurosurgery Department, People's Liberation Army (PLA) General Hospital, Beijing, China
Objective: To analyze the interictal discharge (IID) patterns on pre-operative scalp electroencephalogram (EEG) and compare the changes in IID patterns after removal of epileptogenic tubers in preschool children with tuberous sclerosis complex (TSC)-related epilepsy.
Methods: Thirty-five preschool children who underwent resective surgery for TSC-related epilepsy were enrolled retrospectively, and their EEG data collected before surgery to 3 years after surgery were analyzed.
Results: Twenty-three (65.7%) patients were seizure-free post-operatively at 1-year follow-up, and 37–40% of post-operative patients rendered non-IID on scalp EEGs, and patients with focal IIDs or generalized IID patterns on pre-operative EEG presented a high percentage of normal post-operative scalp EEGs. IID patterns on pre-operative scalp EEGs did not influence the outcomes of post-operative seizure controls, while patients with non-IID and focal IID on post-operative EEGs were likely to achieve post-operative seizure freedom. Patients with new focal IIDs presented a significantly lower percentage of seizure freedom than those without new focal IIDs on post-operative EEGs at 3-year follow-up.
Conclusion: Over 1/3 children with TSC presented normal scalp EEGs after resective epileptsy surgery. Patients with post-operative seizure freedom were more likely to have non-IIDs on post-operative EEGs. New focal IIDs were negative factors for seizure freedom at the 3-year follow-up.
Highlights
- The pre-operative interictal discharge patterns on scalp EEGs had no obvious effect on post-operative seizure control in TSC-related epilepsy.
- The interictal discharge patterns on post-operative scalp EEGs were consistent in TSC patients who underwent resective epileptic surgery.
- In 37–40% of post-operative patients, interictal discharge on scalp EEGs were not present.
- Patients with post-operative seizure freedom were more likely to have absent interictal discharge on post-operative scalp EEG.
Introduction
Approximately 90% of patients with tuberous sclerosis complex (TSC) suffer from epilepsy, which are often medically resistant and can present with multiple seizure types (1–3). Epilepsy surgery, especially resective surgery, is the most efficient approach for patients with TSC-related intractable epilepsy, and 68–75% of patients with TSC present post-operative seizure freedom and a worthwhile reduction (>90%) in seizure frequency after resective surgery (4–7).
Due to an autosomal dominant epilepsy with multiple tubers in most patients with TSC-related epilepsy, the biggest questions to the resective surgery for TSC are whether the numbers and volumes of cortical tubers, and the number of epileptogenic tubers will increase with age, and whether new epileptogenic tubers will appear after removal of epileptogenic tubers, besides the difficulty in localizing the epileptogenic tuber(s). It has been reported that the number and relative volume of cortical tubers will not change after 1 year of age in TSC patients (8), and consistent location of interictal epileptiform activity on scalp electroencephalographs (EEGs) indicated the relative stability of epileptogenic tubers in patients with TSC-related epilepsy (9).
As previously reported, total resection of the actual seizure-onset zone does not always lead to seizure freedom in focal seizures. In some cases, additional post-surgical recordings suggest that areas adjacent to the resection trigger epileptic seizures. These observations led to the concept of potential seizure-onset zones (10). Therefore, surgical resections did not result in seizure freedom either because of incomplete resection of the actual seizure-onset zone or incomplete resection of the potential seizure-onset zone (10). Weiner et al. reported a three-stage operation in patients with TSC-related epilepsy (11). They conserved subdural intracranial electrodes for several days after the first resective surgery and found that the margins of the resection and other tubers could induce seizures. However, there is no long-term study on whether new epileptogenic tubers will present and provoke seizures after resective surgery. Hence, the long-term IID patterns on post-operative EEG were analyzed to study whether there were new epileptogenic tubers after the removal of epileptogenic tubers.
Methods
Patients
Patients were enrolled retrospectively according to the following criteria: patients who were no more than 6-year-old; subjects who underwent resective surgery from January 2015 to June 2020 in the comprehensive epilepsy centers of our hospitals; subjects who had been diagnosed with TSC according to the revised diagnostic criteria of Northrup et al. (12); and patients who had pre-operative scalp EEG recordings and post-operative scalp EEG recording at 2-year follow-up. The study was approved by the ethics committee of the Fourth Medical Center of the PLA General Hospital (Permit No. 2019KY004-HS001).
Pre-operative Evaluation
Non-invasive pre-operative evaluations included neurological physical examinations, high-resolution magnetic resonance imaging (MRI), long-term video EEG recordings, interictal positron emission tomography (PET), and Gesell development quotient tests. MRI scans included 3.0T routine axial T1- and T2-weighted, diffusion-weighted, and sagittal T1-weighted imaging and 2-mm thickness/zero interval axial and coronal T2-flair imaging. Each scalp EEG recording had to include more than three habitual seizures. MRI-PET co-registration was performed for each patient. Stereo-EEGs were recorded to detect epileptogenic cortex tubers when the predominant cortex tuber on MRI or the areas with focal ictal symptoms were discrepancies with the region with the focal scalp EEG (7, 13). The epileptogenic tuber was defined as the first tuber with initial rhythmic discharge on scalp EEG or stereo-EEG before a clinical seizure attack.
Pre-operative and Post-operative Scalp EEG Recording and Analysis
Scalp EEGs were made with 21-channel recordings with electrode positions according to the 10–20 system. Digital recordings of EEG traces were available for assessment. All scalp EEGs were recorded for no <24 h. Two observers (L.Y and S.C) reviewed all the EEGs. Both observers were blinded to the previous reports and recording time of EEG, information about patients' name and seizure semiology or frequency, and MRI findings. A final consensus reading was performed to identify the location of the epileptiform abnormalities. Consistency was defined as the presence of interictal epileptiform activity at the same location in all EEGs reviewed. If the assessment did not correspond to the original assessment, a third observer (S.L) reviewed the recordings. The results of interictal EEG were divided into four groups: non-interictal discharge (IID) (without interictal epileptiform discharges, IIDs) (Figure 1B,B3), focal IID (unique unilateral focal IID with or without generalized IID) (Figures 1A,A1,A2, 2), multifocal IID (two or more independent focal IID or unilateral IID with or without generalized IID) (Figures 1C,C1,C2, 2), and generalized IID (any bilaterally synchronous and symmetric pattern IID without focal IIDs, but it can be in a restricted field) (14) (Figure 1D,D1,D2). For the post-operative interictal EEG patterns, focal/multifocal IID was subdivided into focal/multi-focal IID-E (new IID located elsewhere to region with pre-operative IIDs) a focal/multi-focal IID-N (continuous IID located near the resected region), multi-focal IID-EN (new IID located elsewhere to region with pre-operative IIDs and continuous IID located near the resected region), multi-focal/focal IID (focal/multi-focal IID located regions with pre-operative multiple focal IID but the resected region), and non-IID (Figure 2).
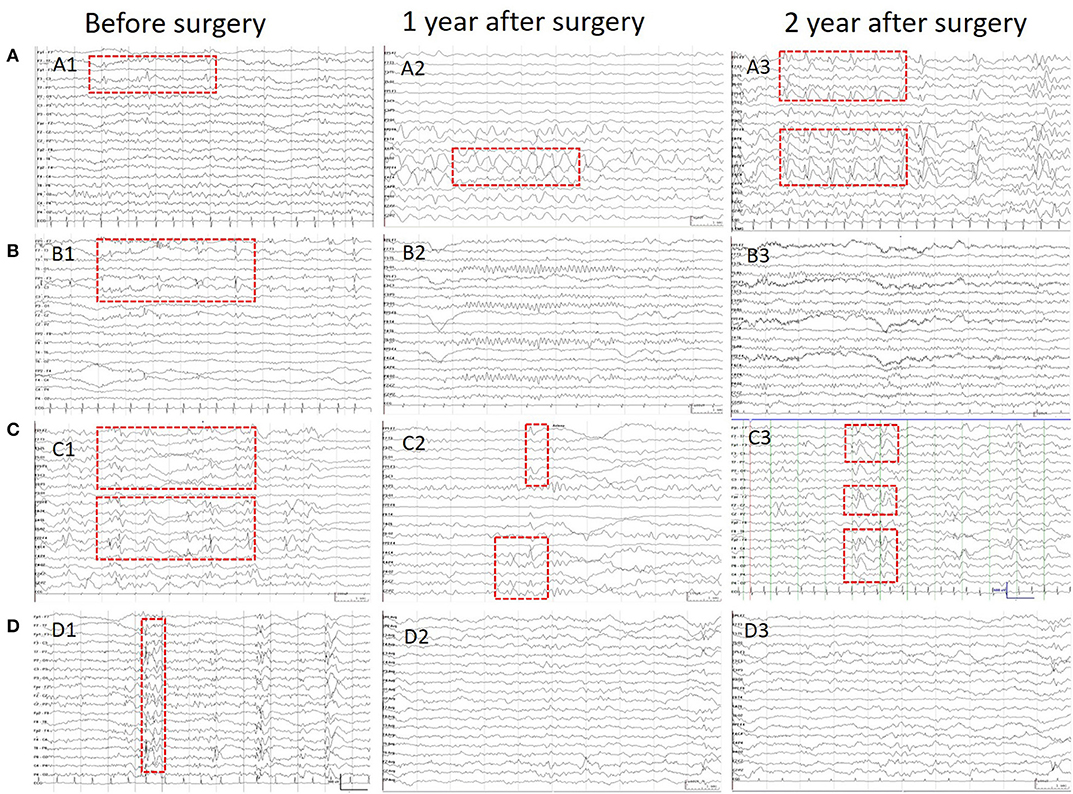
Figure 1. Patients' IIDs patterns on EEG before and after surgeries. This figure shows the different IID patterns of four cases on pre-operative and post-operative scalp EEGs. (A) Shows the focal IIDs on the left frontal areas on pre-operative EEG (A1), focal IIDs and slow waves on the right frontal areas on EEG in 1 year after surgery (A2), and multifocal IIDs on the EEG in 2 years after surgery. (B) Shows the focal IIDs on pre-operative EEGs (B1), and non-IIDs on EEGs in 1- (B2) and 2-year after surgery (B3). (C) Shows the multifocal IIDs on pre-operative EEGs (C1), and multifocal IIDs on EEGs in 1- (C2) and 2-year (C3) after surgery. (D) Shows the generalized IIDs on pre-operative EEGs (D1), and non-IIDs on EEGs in 1- (D2) and 2- year (D3) after surgery.
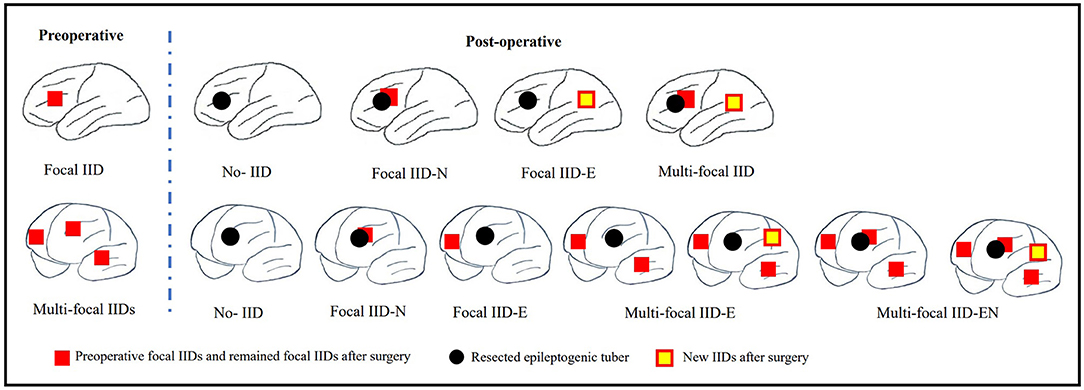
Figure 2. Schematic diagram of pre-operative focal IID and multi-focal IID patterns and their post-operative IID patterns. The top row of this figure shows the pre-operative focal IID pattern and its post-operative patterns, including non-IID, focal IID-N (continuous IID located near the resected region), focal IID-E (new IID located elsewhere to the resected region), and multifocal IID-EN (new IID located elsewhere to the resected region and continuous IID located near the resected region). The bottom row of this figure shows the pre-operative multiple focal IID pattern and its post-operative patterns, including non-IID, focal IID-N, focal IID-E (continuous IID located elsewhere to resected region), multifocal IID-E (continuous IID or continuous IID and new IID located elsewhere to regions with pre-operative IIDs), multi-focal IID-EN (continuous IID or new IID located elsewhere to regions with pre-operative IID, and continuous IID located near the resected region).
Surgical Methods and Post-operative Medicine Treatment
The patient underwent lobectomy and/or tuberectomy. Tuberectomy was used for the epileptogenic tuber within or near the eloquent area. Lobectomy was performed for large epileptogenic tubers or multiple epileptogenic tubers in the anterior temporal lobe, frontal pole, or occipital pole. Multiple tuberectomies or lobectomy combined with tuberectomy were considered when multiple epileptogenic tubers or epileptogenic and propagating tubers could not be removed by a single lobectomy. Pre-operative and post-operative medicine treatment was provided to all patients with 2–4 types of optimal anti-seizure medications.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 26.0; SPSS, Inc., Chicago, IL, USA). The outcomes were described as percentages, means, and standard deviations. McNemar-Bowker's test was used to analyze influence of the IIDs patterns on pre-operative EEG on the outcome of IIDs patterns on post-operative EEG. Generalized estimating equation (GEE) was used to analyze the influences factors on the post-operative seizure control of the three time points of follow-up. Chi-square and Fisher's exact tests were performed for univariate analyses of categorical variables. When the two-tailed error probability “p” was < 0.05, the outcome was considered significant.
Results
Patients
Fifty-three preschool children (0–6 years old) with TSC-related intractable epilepsy underwent epilepsy surgery in our hospital from January 2015 to December 2019, and 35 of them, who had pre-operative and post-operative EEG recordings and surgical outcomes, were enrolled in this retrospective study. There were 11 (31.4%) girls and 24 (68.6%) boys. The average age at surgery was 3.51 [SD = 1.69, range (0.7–6.0), medium: 3.0, interquartile range (2.0–5.0)] years, the average age at first unprovoked seizure was 0.90 [SD = 1.13, range (0.0–5.0), medium 0.6, interquartile range (0.3–1.0)] years, and the pre-operative history of seizure ranged from 0.7 to 5.7 [mean = 2.55, SD = 1.50, medium 2.2, interquartile range (1.2–3.5)] years. Stereo-EEGs were performed on 11 (31.4%) of the 35 children.
Surgery Approach and Post-operative Seizure Freedom
The surgical approach consisted of 15 tuberectomies, 9 lobectomies, and 11 multiple tuberectomies or lobectomies combined with tuberectomies. All 35 patients completed 1- and 2-year follow-up, and 25 (71.4%) and 23 (65.7%) of them had post-operative seizure freedom. Eighteen (60%) out of the 30 patients who completed the 3-year follow-up had post-operative seizure freedom.
Pre-operative and Post-operative IIDs
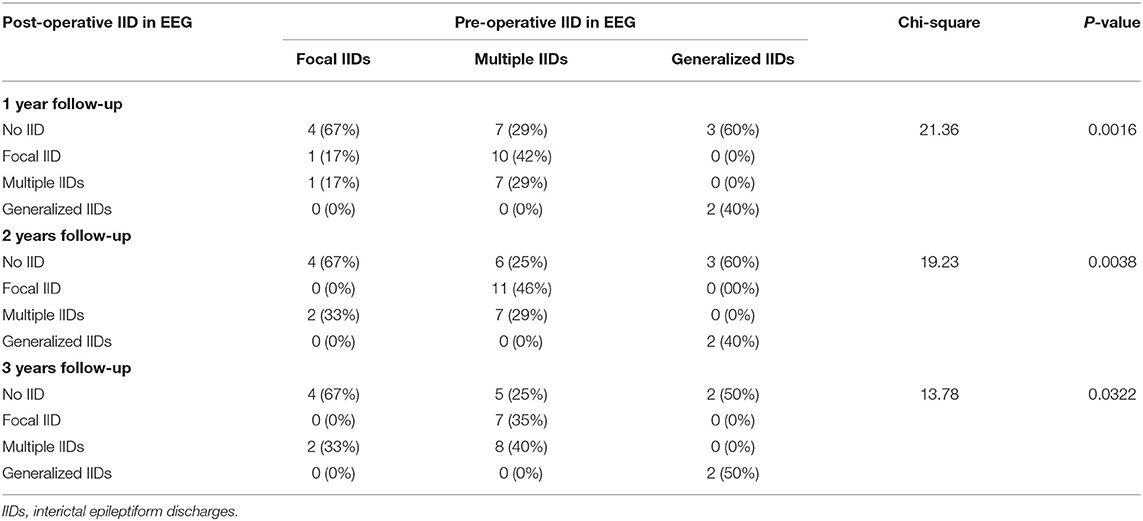
All pre-operative scalp EEGs indicated IIDs. All patients had scalp EEGs 1-year (40% without IID) and 2 years (37.1% without IID) after surgery, respectively, and 30 (36.7% without IID) children had post-operative scalp EEGs at the 3-year follow-up. No significant difference was found in the percentage of non-IID on scalp EEGs in the different post-operative periods (p = 0.8929). Eleven patients did not present with IIDs on post-operative scalp EEGs at all three follow-ups, and one case (3%) with focal IID on scalp EEG at 1-year follow-up reached normal EEG at 2-year follow-up, and two patients with normal EEGs at 1-year follow-up presented with focal or multi-focal IIDs at 2- and 3-year follow-up (Figure 3). There were significant differences in the outcomes of IID patterns on post-operative scalp EEGs at 1- (p = 0.0016), 2- (p = 0.0038) or 3-year (p = 0.0322) follow-up among patients with different IID patterns on pre-operative scalp EEG (Table 1; Figure 3).
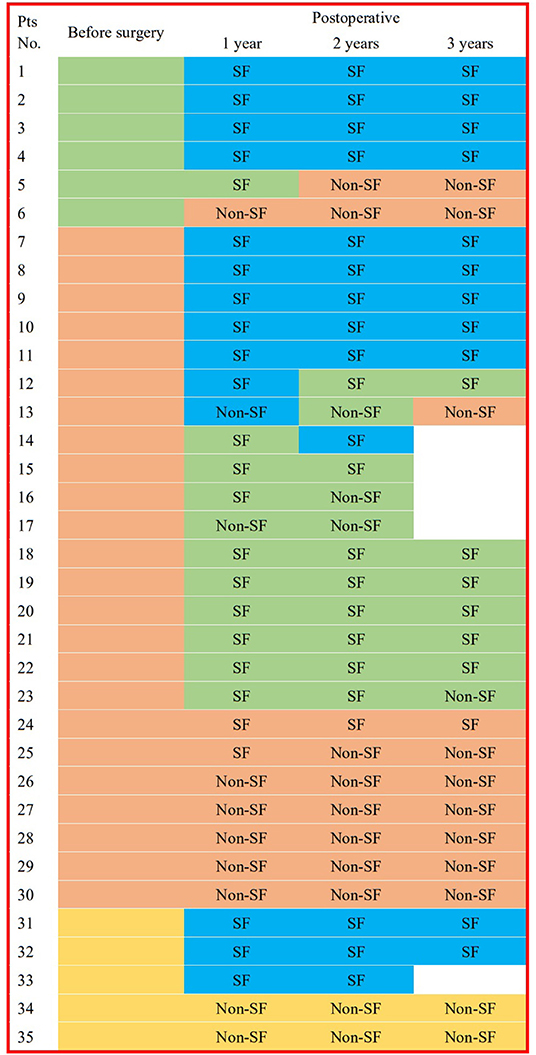
Figure 3. IID patterns on scalp EEGs in different times for each patient. This figure shows the interictal epileptiform discharge (IID) patterns from 3 years before the surgery to 3 years after surgery in every patient. Blue box for EEG without IID; green box for focal IIDs; orange box for multiple focal IIDs, and golden box for generalized IIDs. pts, patients; pre-op, pre-operative; post-operative, post-operative; SF, seizure freedom.
Patients' Characteristics and Post-operative Seizure Freedom
The influence of age at surgery, age at first seizure, seizure type at onset, pre-operative history, and number of resected tubers on the post-operative seizure control was not found. However, patients with pre-operative seizure history <2 years presented statistically significant higher percentage of post-operative of seizure free than those with seizure history longer than 2 years (OR = 6.387, 95%CI: 1.174–34.746, p = 0.032) (Table 2).
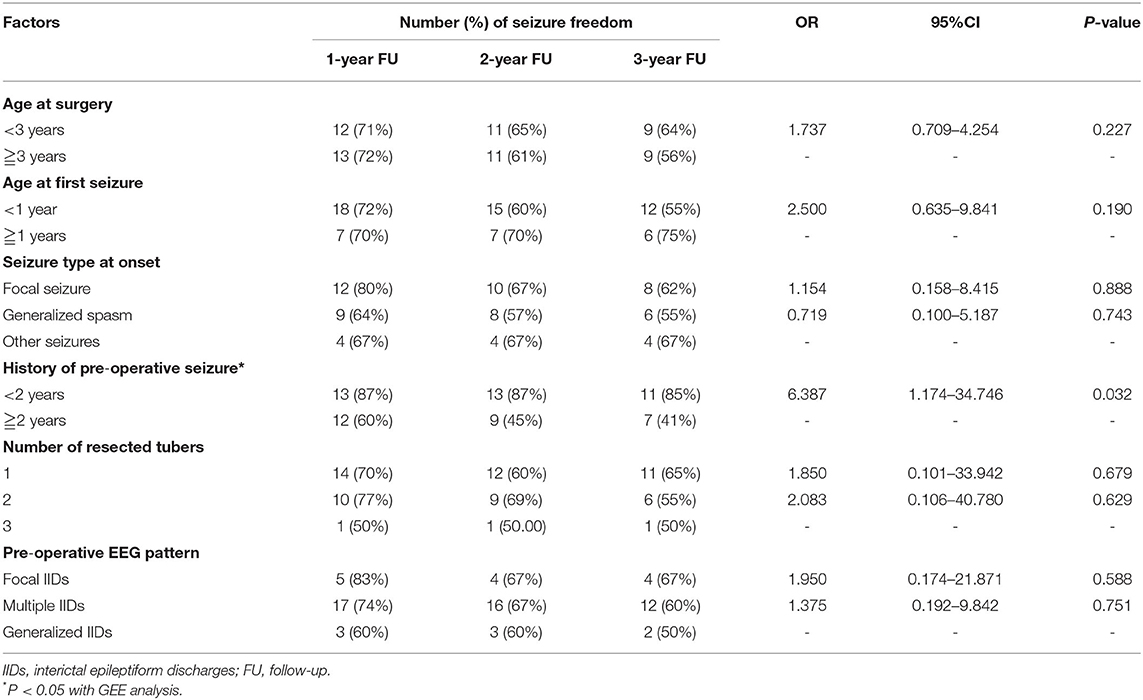
Table 2. Relationship of pre-operative influence factors and number of resected tuber and post-operative seizure freedom.
IIDs Patterns and Post-operative Seizure Freedom
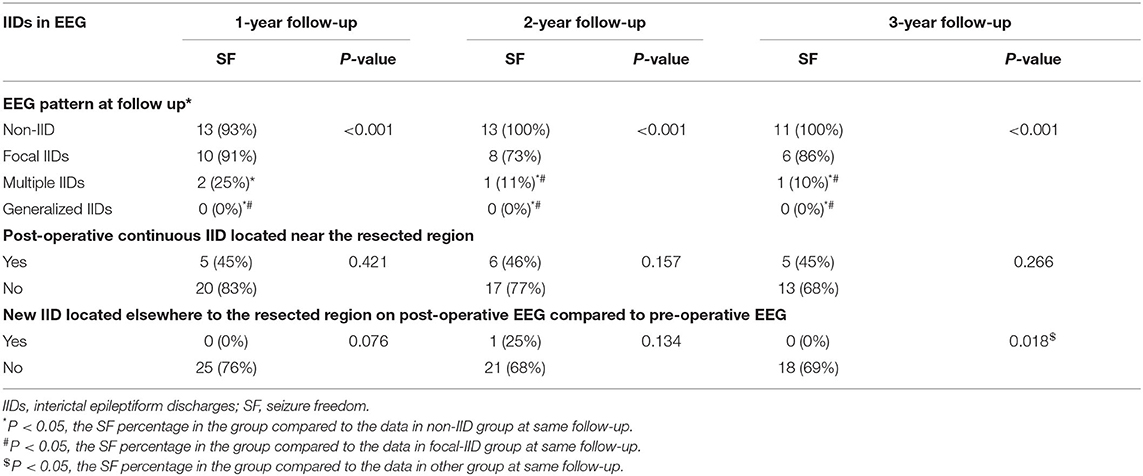
IID patterns on scalp EEGs before surgery did not affect the outcomes of post-operative seizure control (p > 0.05, Table 2). Significant differences were found in patients' seizure controls at 1-, 2-, and 3-year follow-up among patients with different IID patterns on scalp EEGs at the same time after surgery (p < 0.001, Table 3). Non-IIDs and focal IIDs on post-operative EEGs indicated the highest percentage of seizure freedom (Table 3).
IIDs in Areas of Resected Tubers and New Finding of Focal IIDs After Surgery
There were 21 patients who underwent a single area resection (six lobectomies and 15 tuberectomies), and 14 patients underwent multiple area resections (three lobectomies combined tuberectomies, and 11 multiple tuberectomies). In the resected areas, 25 (71.4%), 22 (62.8%), and 19 (63.3%) patients' scalp EEGs did not have IIDs in the resected areas at 1-, 2-, and 3-year follow-up, respectively (Table 3), and no significant difference was found among the percentages of patients without IIDs in the resected area among the three follow-ups (Table 3). No significant difference was found in post-operative seizure control between patients with IIDs in the resected area and those without IIDs in the resected area (Table 3).
Compared to all pre-operative scalp EEG results before surgery in each patient, there were two (5.7%), four (11.4%), and four (13.3%) patients with new focal IIDs on scalp EEGs at 1–3 years of follow-ups, respectively, and there was no significant difference in the percentage of new focal IIDs among the three follow-ups (p = 0.5588). However, significant difference was found in patients' seizure controls at 3-year follow-up between patients with or without new focal IID on post-operative EEGs (p = 0.018, Table 3).
Discussion
To the best of our knowledge, this is the first study to examine post-operative scalp EEGs in patients with TSC-related epilepsy after resective surgery. Although ictal scalp EEG and intracranial EEG have been used to localize the onset zone of seizures, interictal EEG is usually used to define the irritation area (2, 6, 7, 15, 16). However, some studies have confirmed that scalp EEG can also be a biomarker significantly associated with an underlying cortical pathology and aid in localizing suspicious brain regions (2, 17–20). Furthermore, interictal EEG patterns have been reported to be associated with post-operative seizure control in patients with TSC-related epilepsy (4, 21). Because over 70% of patients reach post-operative seizure freedom at the 1-year follow-up and 2–4 h of scalp video-EEG is routinely used for post-operative EEG examination, ictal EEG cannot be recorded in most patients at follow-up. Moreover, intracranial EEG is not suitable for post-operative follow-up. Therefore, interictal epileptiform discharge on scalp EEG is one of the best tools to observe long-term EEG changes after removal of the epileptogenic tubers in TSC patients.
The outcomes in this cohort show that patients with multifocal or generalized IIDs, besides focal IIDs, on pre-operative scalp EEG can also present non-IIDs on post-operative EEGs at 1–3 years of follow-up, which proves that focal epileptogenic tubers could lead to multifocal or generalized IIDs. Furthermore, patients with pre-operative multifocal or generalized IIDs do not have a low percentage of post-operative seizure freedom compared to those with pre-operative focal IID, which indicates that pre-operative IID patterns have no obvious effect on post-operative seizure control in carefully selected patients and comprehensive pre-operative evaluations. Due to the lower myelinization of the brain and less organized brain networks (22), young children with focal onset zone can presence diffuse interictal activity pre-operatively.
The maximal brain growth rate occurs around birth, and the brain is ~95% of the size of the adult brain by 6 years of age. The bulk of this early growth comes from a variety of sources, including increases in synapses and dendrites, as well as myelination (23). Therefore, it may be the most obvious period of EEG discharge pattern changes. Nevertheless, 6–12% of patients presented new focal IIDs in 1–3 years of follow-up after removal of epileptogenic tubers compared to pre-operative IID patterns on scalp EEG, and 37–40% patients did not have obvious IIDs on post-operative scalp EEG, including 11 patients without IID for 3 years. Furthermore, obvious correlations in IIDs patterns in post-operative EEG were found among 1-, 2- and 3-year follow-up, which indicated stability of IIDs patterns in post-operative EEG. Concurrently, 60% of post-operative patients achieved seizure freedom for 3 years. Therefore, we first showed that the epileptogenic tubers in patients with TSC-related epilepsy were relatively stable, and reconfirmed that not all tubers were independent epileptogenic tubers.
Continuous IIDs near the resected tubers can also be found in 29–37% of scalp EEGs in post-operative patients. The reasons for the continuous IID may include incomplete removal of tubers, propagation from IID in other tubers, and hyperexcitability of the cortex near the epileptogenic tubers. However, the presence of continuous IID did not affect the post-operative seizure freedom, which indicated that the incomplete removal of tubers should not be the main reason.
This study has some limitations. First, not all patients had all the 3-year EEG results before and after surgery due to the limitations of a retrospective study. Second, the sample of enrolled subjects was not large because of the low incidence of TSC-related epilepsy and the limited number of patients who underwent resective surgery.
In conclusion, pre-operative IID patterns had no obvious effect on post-operative seizure control after comprehensive pre-operative evaluations, while patients with focal IIDs or generalized IID patterns on pre-operative EEG presented a high percentage of normal post-operative scalp EEGs. In 37–40% of post-operative patients, non-IID on scalp EEGs was shown, and patients with post-operative seizure freedom were more likely to have non-IID on post-operative EEGs. New focal IIDs were negative factors for seizure freedom at the 3-year follow-up. The IID patterns on post-operative scalp EEGs were consistent, and epileptogenic tubers in patients with TSC-related epilepsy were relatively stable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Fourth Medical Center of the PLA General Hospital. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SL: conceptualization, methodology, writing-original draft preparation, writing-reviewing and editing, funding acquisition, and investigation. LY and YW: methodology, writing-original draft preparation, and investigation. SZ and JZ: subjects collections and investigation. TL: investigation and data analysis. SC and GZ: supervision and writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Nature and Science Foundation of China (82071448, SL) and Beijing Nature and Science Foundation of China (7202045, SL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to show gratitude to the patients and their families for their long-term cooperation. We also appreciate the contribution provided by the following people: Dr. S. Y. Cai in Center for Clinical Epidemiology and Evidence-Based Medicine, Beijing Children's Hospital, and Mrs. N. Liu, X. Y. Shang and all technicians in the Neurophysiologic Laboratory of Neurosurgery Department of the Fourth Medical Center, PLA General Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.868633/full#supplementary-material
References
1. Cui J, Yu X, Liang S, Zhang S, Hu X. First five generations Chinese family of tuberous scleroses complex due to a new mutation of the TSC1 gene. J Clin Neurosci. (2018) 54:39–44. doi: 10.1016/j.jocn.2018.05.007
2. Gupta A, de Bruyn G, Tousseyn S, Krishnan B, Lagae L, Agarwal N, et al. Epilepsy and neurodevelopmental comorbidities in tuberous sclerosis complex: a natural history study. Pediatr Neurol. (2020) 106:10–6. doi: 10.1016/j.pediatrneurol.2019.12.016
3. Liang S, Li A, Zhao M, Jiang H, Yu X, Meng X, et al. Epilepsy surgery in tuberous sclerosis complex: emphasis on surgical candidate and neuropsychology. Epilepsia. (2010) 51:2316–21. doi: 10.1111/j.1528-1167.2010.02669.x
4. Fallah A, Guyatt GH, Snead III OC, Ebrahim S, Ibrahim GM, Mansouri A, et al. Predictors of seizure outcomes in children with tuberous sclerosis complex and intractable epilepsy undergoing resective epilepsy surgery: an individual participant data meta analysis. PLoS ONE. (2013) 8:e53565. doi: 10.1371/journal.pone.0053565
5. Jansen FE, Van Huffelen AC, Algra A, van Nieuwenhuizen O. Epilepsy surgery in tuberous sclerosis: a systematic review. Epilepsia. (2017) 48:1477–84. doi: 10.1111/j.1528-1167.2007.01117.x
6. Liang S, Zhang J, Yang Z, Zhang S, Cui Z, Cui J, et al. Long-term outcomes of epilepsy surgery in tuberous sclerosis complex. J Neurol. (2017) 264:1146–54. doi: 10.1007/s00415-017-8507-y
7. Liu S, Yu T, Guan Y, Zhang K, Ding P, Chen L, et al. Resective epilepsy surgery in tuberoussclerosis complex: a nationwide multicentre retrospective study from China. Brain. (2020) 143:570–81. doi: 10.1093/brain/awz411
8. Rovira A, Ruiz-Falcó ML, García-Esparza E, López-Laso E, Macaya A, Málaga I, et al. Recommendations for the radiological diagnosis and follow-up of neuropathological abnormalities associated with tuberous sclerosis complex. J Neuro-oncol. (2014) 118:205–23. doi: 10.1007/s11060-014-1429-y
9. Jansen JE, van Huffelen AC, Bourez-Swart M, van Nieuwenhuizen O. Consistent localization of interictal epileptiform activity on EEGs of patients with tuberous sclerosis complex. Epilepsia. (2005) 46:415–9. doi: 10.1111/j.0013-9580.2005.31704.x
10. Lüders H, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord. (2006) 8:S1–9.
11. Weiner HL, Carlson C, Ridgway EB, Zaroff CM, Miles D, LaJoie J, et al. Epilepsy surgery in young children with tuberous sclerosis: results of a novel approach. Pediatrics. (2006) 117:1494–502. doi: 10.1542/peds.2005-1206
12. Northrup H, Aronow ME, Bebin EM, Bissler J, Darling TN, de Vries PJ, et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr Neurol. (2021) 123:50–66. doi: 10.1016/j.pediatrneurol.2021.07.011
13. China Association Against Epilepsy. Chinese experts consensus on surgical treatment in patients with tuberous sclerosis complex-related epilepsy. Zhongguo Dang Dai Er Ke Za Zhi. (2019) 29:735–42. doi: 10.7499/j.issn.1008-8830.2019.08.001
14. Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, et al. American clinical neurophysiology society's standardized critical care EEG terminology: 2021. Version J Clin Neurophysiol. (2021) 38:1–29. doi: 10.1097/WNP.0000000000000806
15. Lagarde S, Bonini F, McGonigal A, Chauvel P, Gavaret M, Scavarda D, et al. Seizure-onset patterns in focal cortical dysplasia and neurodevelopmental tumors: Relationship with surgical prognosis and neuropathologic subtypes. Epilepsia. (2016) 57:1426–35. doi: 10.1111/epi.13464
16. Yu X, Ding D, Yuan L, Zhang J, Liang S, Zhang S, et al. Cortico-cortical evoked potentials in children with tuberous sclerosis complex using stereo-electroencephalography. Front Neurol. (2019) 10:1093. doi: 10.3389/fneur.2019.01093
17. Dash GK, Rathore C, Jeyaraj MK, Wattamwar P, Sarma SP, Radhakrishnan K. Interictal regional paroxysmal fast activity on scalp EEG is common in patients with underlying gliosis. Clin Neurophysiol. (2018) 129:946–51. doi: 10.1016/j.clinph.2018.02.007
18. Epitashvili N, San Antonio-Arce V, Brandt A, Schulze-Bonhage A. Scalp electroencephalographic biomarkers in epilepsy patients with focal cortical dysplasia. Ann Neurol. (2018) 84:564–75. doi: 10.1002/ana.25322
19. Jayalakshmi S, Dhondji M, Vooturi S, Patil A, Vadapalli R. Inter-ictal EEG patterns in malformations of cortical development and epilepsy. Clin Neurol Neurosurg. (2020) 196:106022. doi: 10.1016/j.clineuro.2020.106022
20. Madhavan D, Schaffer S, Yankovsky A, Arzimanoglou A, Renaldo F, Zaroff CM, et al. Surgical outcome in tuberous sclerosis complex: a multicenter survey. Epilepsia. (2007) 48:1625–8. doi: 10.1111/j.1528-1167.2007.01112.x
21. Noachtar S, Bilgin Ö, Rémi J, Chang N, Midi I, Vollmar C, et al. Interictal regional polyspikes in noninvasive EEG suggest cortical dysplasia as etiology of focal epilepsies. Epilepsia. (2008) 49:1011–7. doi: 10.1111/j.1528-1167.2008.01583.x
22. Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A. (2010) 107:19067–72. doi: 10.1073/pnas.1009073107
Keywords: epilepsy, epileptogenic tuber, interictal discharge (IID), scalp electroencephalographs (EEGs), tuberous sclerosis complex (TSC)
Citation: Yuan L, Wang Y, Cheng S, Zhang J, Zhang S, Liu T, Zhang G and Liang S (2022) Interictal Discharge Pattern in Preschool-Aged Children With Tuberous Sclerosis Complex Before and After Resective Epilepsy Surgery. Front. Neurol. 13:868633. doi: 10.3389/fneur.2022.868633
Received: 03 February 2022; Accepted: 10 May 2022;
Published: 31 May 2022.
Edited by:
Kette D. Valente, University of São Paulo, BrazilReviewed by:
Alexander Gregory Weil, University of Montreal, CanadaMartha Feucht, Medical University of Vienna, Austria
Bernardo dos Santos, University of São Paulo, Brazil
Copyright © 2022 Yuan, Wang, Cheng, Zhang, Zhang, Liu, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuli Liang, bGlhbmdzbF8zMDRAc2luYS5jb20=; Guojun Zhang, emdqNjIwNTFAMTYzLmNvbQ==
†These authors share first authorship
 Liu Yuan1,2†
Liu Yuan1,2† Yangshuo Wang
Yangshuo Wang Shuli Liang
Shuli Liang