- 1Key Laboratory of Precision Nutrition and Food Quality, Department of Nutrition and Health, College of Food Sciences and Nutritional Engineering, China Agricultural University, Beijing, China
- 2Rongcheng Center for Disease Control and Prevention, Rongcheng, China
- 3Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, United States
- 4Graduate School at Shenzhen, Tsinghua University, Shenzhen, China
- 5Shenzhen Evergreen Medical Institute, Shenzhen, China
- 6Institute for Biomedicine, Anhui Medical University, Hefei, China
- 7College of Pharmacy, Jinan University, Guangzhou, China
- 8Department of Cardiology, Peking University First Hospital, Beijing, China
- 9Department of Preventive Medicine, School of Public Health & Management, Wenzhou Medical University, Wenzhou, China
- 10National Clinical Research Center for Kidney Disease, State Key Laboratory for Organ Failure Research, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China
Background: Vitamin K plays a role in preventing vascular calcification and may have a synergetic influence with vitamin D on cardiovascular health. However, whether this relationship applies to stroke, especially in a high-risk population of hypertensive individuals, remains unclear. The present study aims to study the joint association of low vitamin K1 and D status with first stroke in general hypertensive adults.
Methods: This study used a nested, case–control design with data from the China Stroke Primary Prevention Trial. The analysis included 604 first total stroke patients and 604 matched controls from a Chinese population with hypertension. Odds ratios (ORs) and 95% confidence intervals were calculated using conditional logistic regression.
Results: There was a non-linear negative association between plasma vitamin K1 and the risk of first total stroke or ischemic stroke in the enalapril-only group. Compared to participants in vitamin K1 quartile 1, a significantly lower risk of total stroke (OR = 0.58, 95% CI: 0.36, 0.91, P = 0.020) or ischemic stroke (OR = 0.34, 95% CI: 0.17, 0.63, P < 0.001) was found in participants in vitamin K1 quartile 2-4 in the enalapril-only group. When further divided into four subgroups by 25(OH)D and vitamin K1, a significantly higher risk of total stroke or ischemic stroke was observed in participants with both low vitamin K1 and 25(OH)D compared to those with both high vitamin K1 and 25(OH)D in the enalapril-only group. No increased risk was observed in the groups low in one vitamin only.
Conclusion: Low concentrations of both vitamin K1 and 25(OH)D were associated with increased risk of stroke.
Introduction
Cardiovascular disease (CVD) is the primary cause of morbidity in the world, leading to more than 17 million deaths per year, with about 6.7 million deaths attributed to stroke (1). Ischemic stroke (IS), caused by the blockage of blood vessels to the brain, accounts for about 80% of the stroke population (2). Stroke is a multifactorial disease that is related to modifiable risk factors such as hypertension, diabetes, and nutrient deficiencies (3). Vitamin K1 is a fat-soluble vitamin mainly obtained from green leafy vegetables and is required for the activation of hepatic coagulation factors. Recent studies have shown that vitamin K1 also plays an important role in bone (4) and cardiovascular health (5). For example, vitamin K is responsible for activating vitamin K-dependent Gla-proteins in extrahepatic tissues, such as matrix Gla-protein (MGP), which has the function of inhibiting vascular calcification (6).
Observational studies and randomized controlled trials have shown that low serum vitamin K1 is significantly associated with coronary artery calcium progression, especially in hypertensive individuals (7, 8). A meta-analysis showed that the presence and severity of coronary artery calcification was related to stroke events during medium and long-term follow-up (9). However, the protective role of vitamin K1 in stroke has not been well-validated in observational studies or clinical trials. A previous study found no association between high dephosphorylated-uncarboxylated Matrix Gla-Protein (dp-ucMGP) levels, reflecting poor vitamin K status, and increased stroke risk in the general population (10). Prior observational studies have also been unable to identify an association between dietary vitamin K1 intake and overall risk of ischemic stroke (11, 12). However, a Mendelian randomization study indicated that a genetic predisposition to higher circulating vitamin K1 levels is associated with an increased risk of large artery atherosclerotic stroke (13). Overall, significant research gaps remain regarding low vitamin K status and stroke, especially among high-risk populations, such as people with hypertension.
Accumulating epidemiological evidence has found that low 25-Hydroxyvitamin D [25(OH)D] levels, which is the general marker of vitamin D status, to be associated with CVD risk factors and increased CVD risk (14). Vitamin D has a potent role in the regulation of the renin–angiotensin aldosterone system, as well as anti-inflammatory, antioxidative (14). It has been reported that 25(OH)D level was inversely related to stroke risk, with a non-linear dose response relationship (15). Previous studies have shown that vitamins D and K have a synergistic effect on cardiovascular health (16, 17). As noted previously, prospective studies of the association between blood concentrations of vitamin K1 and subsequent stroke are sparse, and additionally, the question remains whether a joint association exists between vitamins D and K1, and stroke. This case-control study aimed to investigate the association between plasma vitamin K1 concentrations and the risk of stroke in patients with hypertension, taking into account the possible influence of vitamin D status on this association.
Materials and Methods
Study Design and Participants
This study population stems from the China Stroke Primary Prevention Trial (CSPPT) cohort of patients with hypertension. A detailed description of the CSPPT has been provided elsewhere (18). Briefly, CSPPT was a multi-community, randomized, double-blind clinical trial conducted from 2008 to 2013 with 20,702 adults in 32 communities in China. Eligible participants were men and women aged 45–75 years who had hypertension, defined as seated, resting systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or who had anti-hypertensive medication at baseline. The major exclusion criteria included history of physician-diagnosed stroke, myocardial infarction (MI), heart failure, post-coronary revascularization, and/or congenital heart disease, and/or current supplementation by folic acid, vitamin B12 or vitamin B6. Eligible participants were randomly assigned in a 1:1 ratio to two treatment group: enalapril-only group and enalapril-folic acid group. In the enalapril-only group, participants received a daily tablet containing 10 mg enalapril only. In the enalapril-folic acid group, participants received a daily tablet containing 10 mg enalapril and 0.8 mg folic acid. Concomitant use of other antihypertensive drugs (mainly calcium channel blockers or diuretics) was allowed during the trial periods, but not B-vitamins.
As shown in Supplementary Figure 1, after a median follow-up of 4.5 years, 637 patients had first stroke in the CSPPT. The present study used a 1:1 matched case–control design. Patients with first stroke were selected as cases (n = 635), excluding two cases that could not be matched to a control. Another 635 participants without stroke, matched by baseline age (±1 year), sex, treatment group and study site, served as controls. After excluding participants with missing 25(OH)D and vitamin K1 data, we obtained 604 stroke case-control pairs, of which 484 were ischemic stroke, 118 were hemorrhagic and 2 were undefined case-control pairs.
Measurement of Vitamin K1 and Vitamin D
A venous blood sample was obtained from each study participant at baseline after overnight fasting. Blood samples were centrifuged and stored at −80°C until analysis. The plasma concentrations of 25(OH)D and vitamin K1 were analyzed to assess the vitamin D and K status of participants. Plasma vitamin K1, 25(OH)D3 and 25(OH)D2 were measured by liquid chromatography with tandem quadrupole mass spectrometry (LC-MS/MS) in a commercial lab (Beijing DIAN Medical Laboratory, China). Total 25(OH)D was used in all analyses and was calculated as the sum of 25(OH)D3 and 25(OH)D2. Season-adjusted vitamin D levels were calculated by adding the residuals from a linear regression model of 25(OH)D by season of blood draw to the overall mean value.
Outcome Assessment
The stroke outcome in this study was a first non-fatal or fatal stroke (ischemic or hemorrhagic), excluding subarachnoid hemorrhage and silent stroke. All participants underwent brain CT and/or magnetic resonance imaging (MRI). The diagnosis of stroke and stroke subtypes was based on medical records and imaging data that were reviewed by at least two adjudicators who were senior stroke neurologists. Source data for all suspected stroke cases including medical records and imaging data as well as event report forms were submitted to the event adjudication committee for further verification. Stroke etiology in our study was identified by the ICD10 codes of diagnosis. The outcomes were total stroke (ICD9 430,431, 433,434 and 436 or ICD10 I60, I61, I63 and I64), ischemic stroke (ICD9 433 and 434 or ICD10 I63) and hemorrhagic stroke (ICD9 430 and 431 or ICD10 I60 and I61).
Data Collection and Assessment
Information on age, sex, body mass index (BMI), smoking status, alcohol consumption and other demographic factors, was collected using a standardized questionnaire. Current smokers were defined as smoking at least 1 cigarette per day or smoking >18 packs for the past 12 months; ex-smokers were those who had not smoked in at least 12 months before enrollment, and all others were defined as never smokers. Current alcohol drinkers were defined as individuals who consumed ≥3 drinks per week over the past year; former drinkers were those who had quit drinking for more than 1 year, and all others were defined as never alcohol drinkers (19, 20). Fasting venous blood samples were also collected at enrollment and at the exit visit. Seated blood pressure was measured by trained research staff after participants had rested for 10 min. Baseline plasma fasting glucose, serum fasting lipids, homocysteine and fasting serum total calcium (arsenazo-III method) levels were measured using automatic clinical analyzers (Beckman Coulter). Serum folate levels were measured in a commercial laboratory using a chemiluminescent immunoassay (New Industrial). The MTHFRC677T (rs1801133) polymorphism of methylenetetrahydrofolate reductase (MTHFR), the main regulatory enzyme for folate metabolism, was detected using the Taq Man assay.
Statistical Analysis
Normally distributed continuous variables were presented as mean ± standard deviation and compared using t-tests. Non-normally distributed continuous variables were presented as median (75th percentile−25th percentile) and compared using rank-sum tests. Categorical variables were presented as number (percentage) and were compared using chi-square tests. All covariates had <5% total missing data. Missing values of continuous variables were replaced by the median and missing values of categorical variables were replaced by a large proportion of values. Logarithmic transformation was performed to normalize the distribution of vitamin K1. Multivariate conditional logistic regression analysis was performed to evaluate the association between vitamin K1 and stroke. All of the potential confounders in the univariate analyses were included in a stepwise conditional logistic regression analysis as the adjustment variable-selection process. The inclusion criterion for the stepwise regression analysis was P = 0.3 and the elimination criterion was P = 0.2. The adjusted variables in the final model included: body mass index (BMI, kg/m2), baseline SBP (mmHg), time-averaged SBP and DBP, baseline fasting blood glucose (mmol/L), total cholesterol (mmol/L), triglycerides (mmol/L), serum folate (ng/mL), antihypertensive medication usage at baseline (yes vs. no), smoking status (ever vs. never) and antiplatelet drug usage at baseline (yes vs. no). Potential interactions were examined by including the interaction terms in the logistic regression models. A two tailed P < 0.05 was considered statistically significant in all analyses. R software, version 3.2.5 (http://www.R-project.org/) was used for all statistical analyses.
Results
Study Participants and Baseline Characteristics
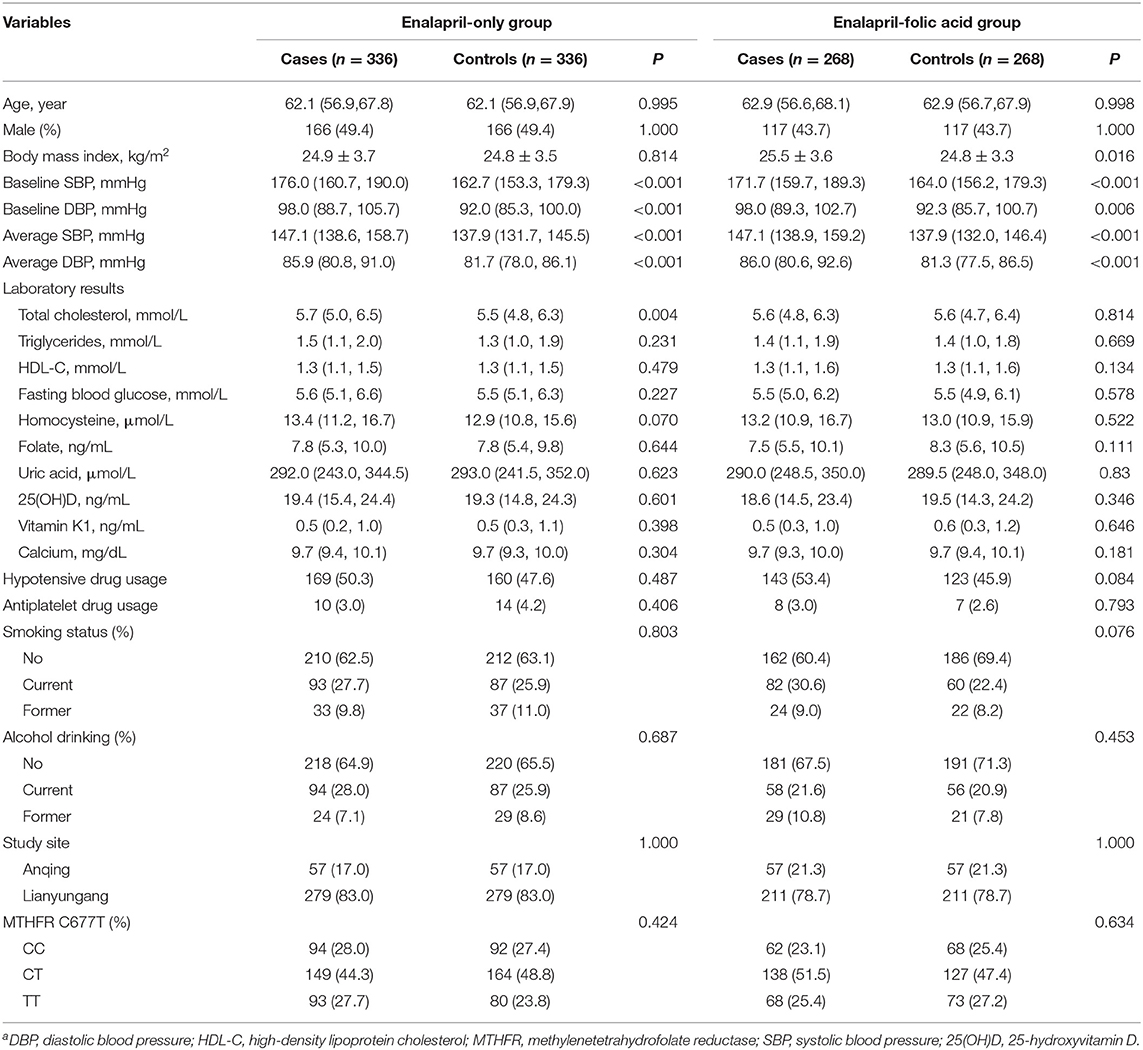
Individuals with missing vitamin K1 and/or D data and unpaired cases or controls were excluded from the analysis. The final analyses included 604 total stroke cases matched with 604 controls (Supplementary Figure 1). A total of 672 (55.6%) participants were in the enalapril-only group and 536 (44.4%) participants were in the enalapril-folic acid group. The mean age of the study population was 62.2 years, and 46.9% were male. Baseline characteristics of total participants by case-control status and by treatment group are presented in Table 1. In the enalapril-only group, stroke cases had high total cholesterol and high blood pressure at baseline and during follow-up. In the enalapril-folic acid group, stroke cases had high body mass index and high blood pressure at baseline and during follow-up.
Baseline Plasma Vitamin K1 and the Risk of First Stroke
Table 2 presents the results of the independent associations between vitamin K1 levels and the risk of stroke (total and subtypes) adjusted by potential confounding factors. For the total population, no significant results were seen between the risk of total stroke and its subtypes. The participants in the second, third and fourth quartiles of vitamin K1 showed a decreased odds of risk of ischemic stroke compared to those in quartile 1 in the unadjusted and adjusted models, although these associations did not reach statistical significance.
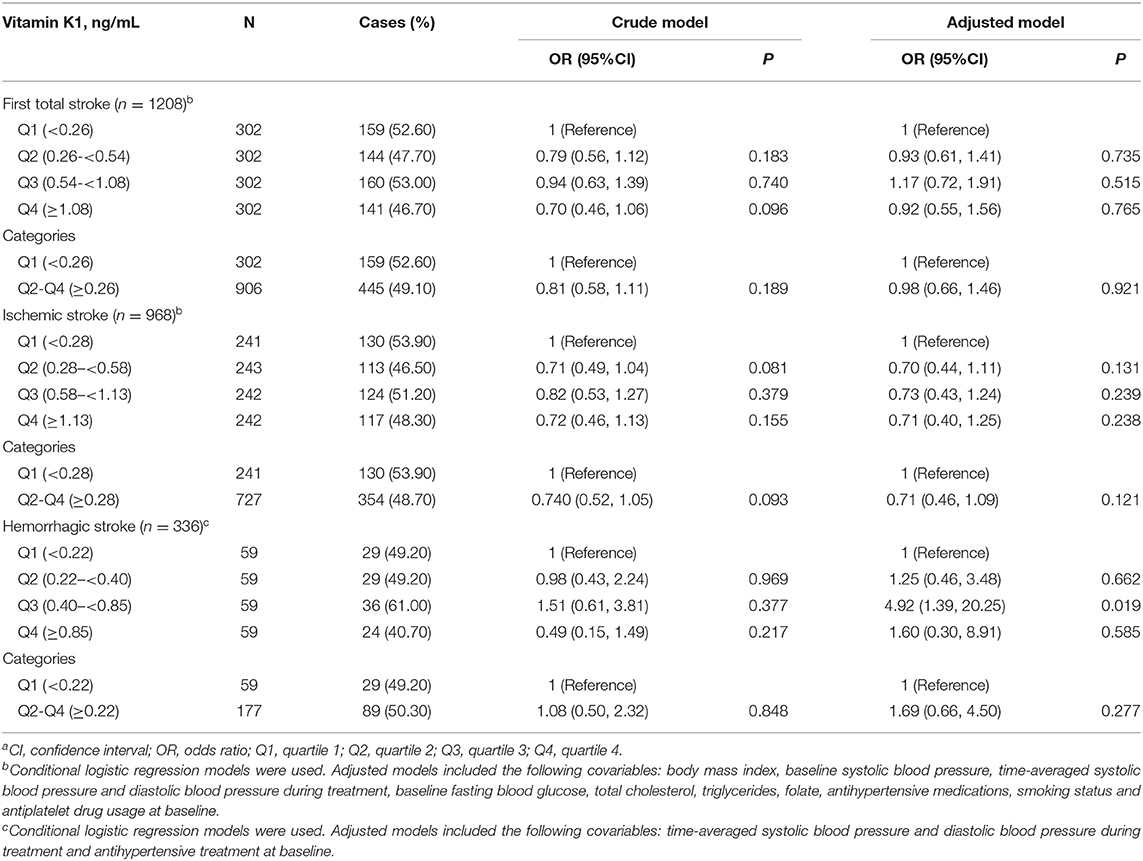
Table 2. The relationship of baseline plasma vitamin K1 with the risk of stroke (total and subtypes)a.
Subgroup analyses were performed based on the potential confounding factors (Supplementary Figures 2, 3). A significantly stronger inverse association between plasma vitamin K1 (quartile 2–4 vs. quartile 1) and total stroke risk or ischemic stroke risk was observed in the enalapril-only group, while the association was slightly positive for participants in the enalapril-folic acid group. The interaction was significant (P = 0.010 for total stroke and P = 0.024 for ischemic stroke). Moreover, a trend of lower risk of total stroke or ischemic stroke with higher concentrations of vitamin K1 was observed in participants with serum calcium levels ≥9.7 mg/dL but not in participants with low serum calcium levels. Tests for interaction were significant (P = 0.022 for total stroke and P = 0.046 for ischemic stroke).
Given that folic acid treatment might affect the association between vitamin K1 and stroke risk, the possible effect of treatment group on the vitamin K1—first stroke association was further investigated. Overall, there was a non-linear, negative association between plasma vitamin K1 and the risk of first total stroke or ischemic stroke in the enalapril-only group (Table 3). Compared to participants with vitamin K1 in quartile 1, a significantly lower risk of total stroke (OR = 0.58, 95% CI: 0.36, 0.91, P = 0.020) or ischemic stroke (OR = 0.34, 95% CI: 0.17, 0.63, P < 0.001) was found in participants with vitamin K1 in quartile 2–4 in the enalapril-only group. However, in the enalapril-folic acid group, a significantly higher first total stroke risk, but not ischemic stroke risk, was found in participants with vitamin K1 in quartile 2–4 (OR = 2.43; 95% CI: 1.31, 4.66, P = 0.006), compared to those with vitamin K1 in quartile 1. Furthermore, there was no significant association between plasma vitamin K1 and first hemorrhagic stroke in either treatment group.
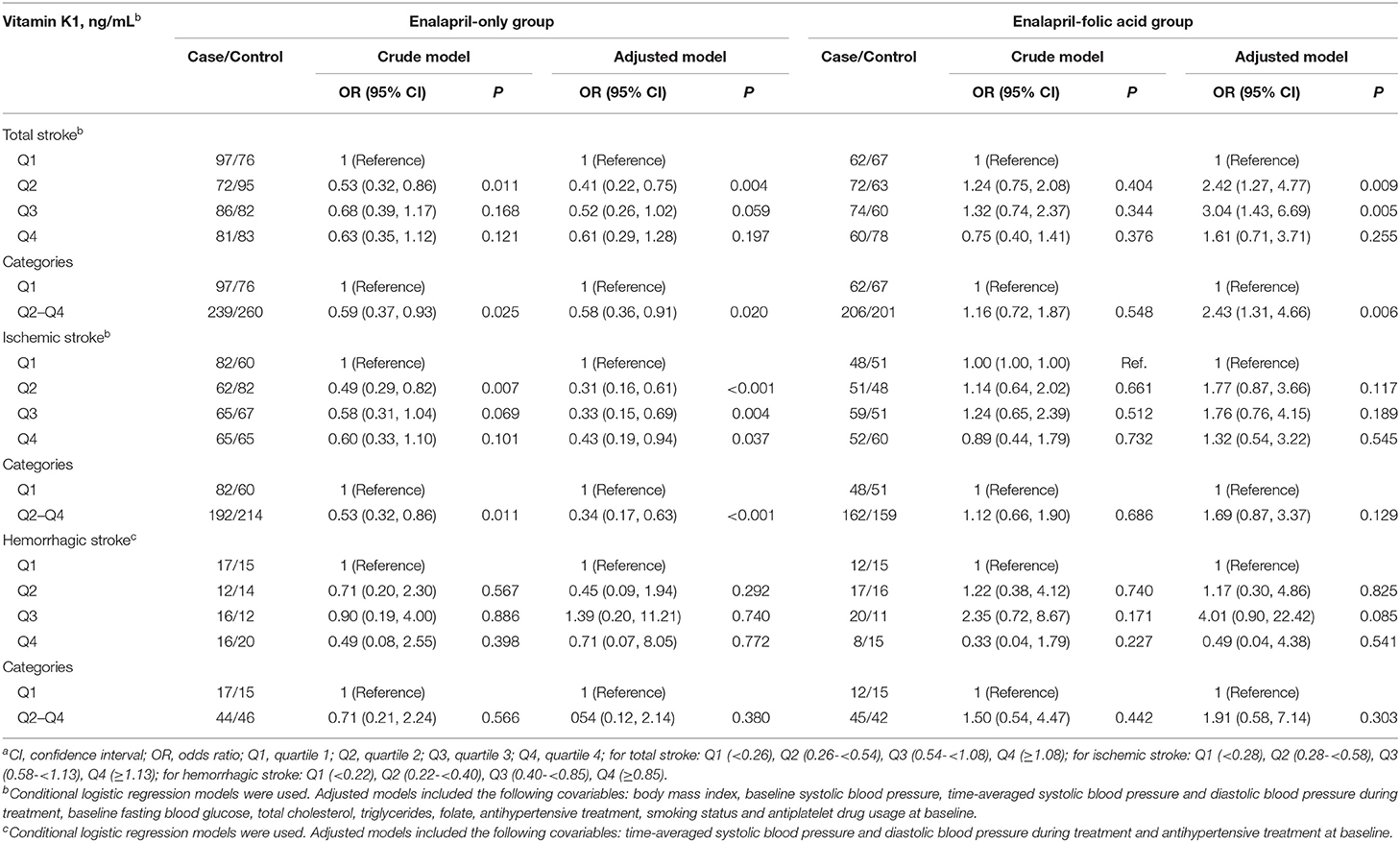
Table 3. Baseline plasma vitamin K1 and the risk of first stroke (total and subtypes) by treatment groupa.
Joint Associations Between Plasma Vitamin K1, 25(OH)D and the Risk of Stroke
Plasma 25(OH)D and vitamin K1 were divided into categorical variables using clinical cut points for 25(OH)D (20 ng/mL) and the first quartile value for vitamin K1. Using high levels of both plasma 25(OH)D and vitamin K1 as the reference, only the combination of low 25(OH)D and low vitamin K1 was associated with increased risk of total stroke (OR = 2.36, 95%CI: 1.17,4.90, P = 0.018) in the enalapril-only group (Table 4). The test of interaction was significant (P = 0.040). The combination of low 25(OH)D and low vitamin K1 was associated with increased risk of ischemic stroke (OR = 3.81, 95% CI: 1.70, 9.00, P = 0.002) in the enalapril-only group. The test of interaction was significant (P = 0.036). No increased risk was observed in the groups low in one vitamin only. In addition, no significant association between the combination of low 25(OH)D and low vitamin K1 with the risk of first total stroke or ischemic stroke was found in the enalapril-folic acid group. Moreover, the combination of low 25(OH)D and low vitamin K1 was not associated with the risk of hemorrhagic stroke, regardless of the group of treatment.
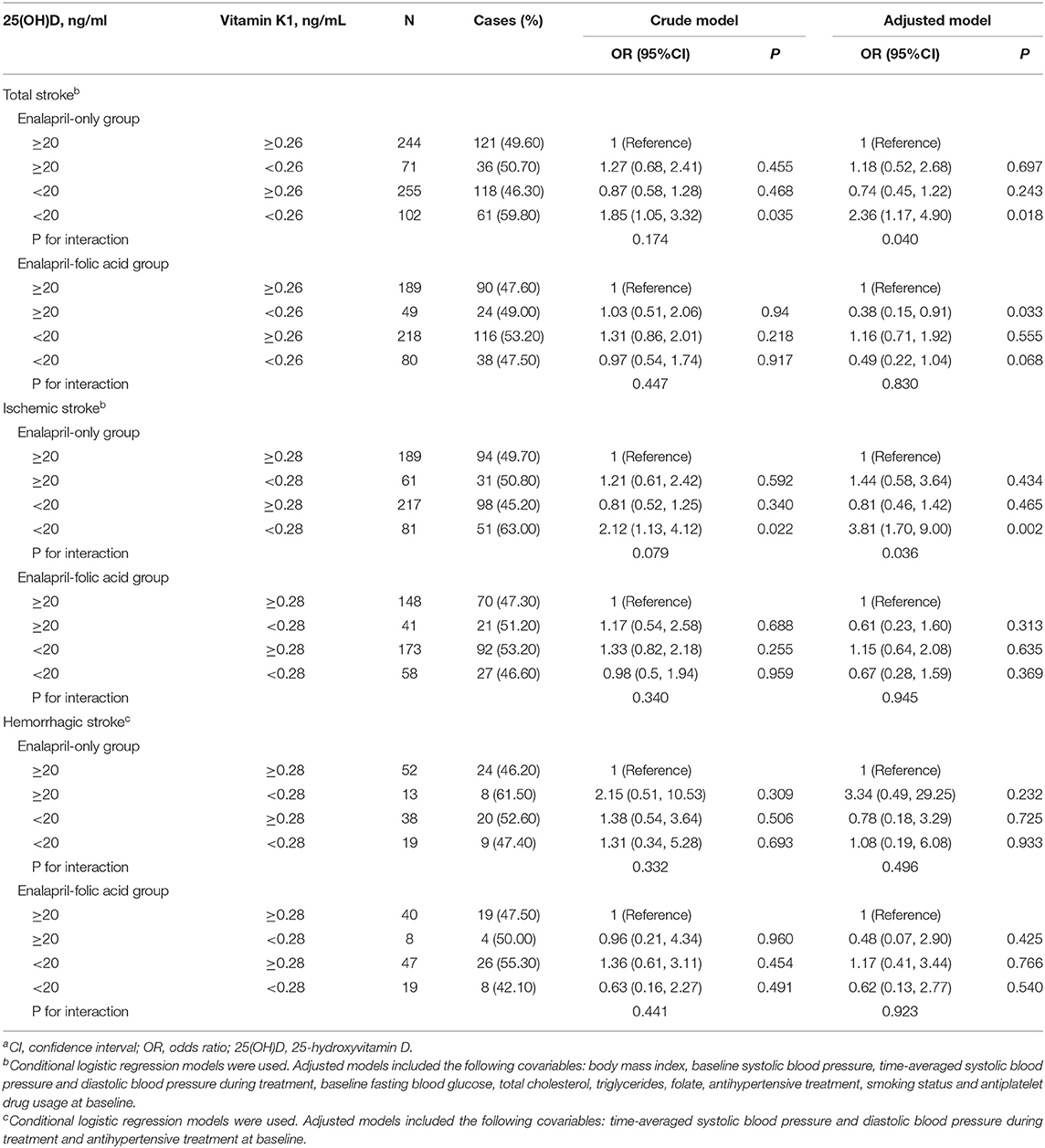
Table 4. The association of plasma vitamin K1 and 25 hydroxyvitamin D [25(OH)D] status with stroke (total and subtypes)a.
Discussion
In this population-based case-control study, we reported on the association of plasma vitamin K1 with stroke in hypertensive patients. There was a significant interactive effect of vitamin K1 and folic acid treatment on total stroke or ischemic stroke. A combination of low vitamin D and K status was statistically associated with increased risk of total stroke or ischemic stroke, especially in those participants in the enalapril-only group.
A large body of evidence supports the beneficial effects of vitamin K1 on musculoskeletal health as well as cardiovascular health. Low nutritional intake and bioavailability of vitamin K appears to be a plausible risk factor for stroke, via the importance of vitamin K in the maturation of MGP (one of the vitamin K–dependent proteins) as an inhibitor of tissue calcification (21). Two possible explanations for the results of our study are that the vitamin K concentrations were relatively low and participants were patients with hypertension. There is increasing evidence that the role of vitamin K in CVD may be particularly important in certain high-risk subgroups. Results from the Health, Aging, and Body Composition Study suggest that low plasma vitamin K1 is associated with a higher CVD risk in older adults treated for hypertension (22). Notably, treatment intervention in our study might be a significant modifier: a stronger association was found in participants treated with enalapril alone. Since adults with hypertension in our study were more likely to have lower serum folate than in US (medium 7.82 vs. 11.5 ng/mL) (23). Folic acid has a potent antioxidant and antithrombotic effect in the prevention of cardiovascular disease (24). Therefore, we hypothesize that folic acid treatment may have possibly attenuated the relationships between low vitamin K1 and high stroke risk. It has previously been reported that the combined use of enalapril and folic acid can significantly reduce the risk of first stroke compared with enalapril alone (18).
A meta-analysis reported an association between vitamin D deficiency and stroke (15). Researchers have also presented a potential physiological role of vitamin D in regulating vascular calcification (25). To our knowledge, the associations between vitamin D and K concentrations on stroke have only been studied in isolation. While prior studies have examined vitamins D and K together, they did not focus on stroke. Previous prospective studies have reported substantial interactions for the combined effect of insufficient vitamin D and K status in terms of blood pressure (26) and aortic stiffness (27). The first study of a double-blind placebo-controlled trial to explore the combined effect of vitamin D and K supplementation on cardiovascular health revealed that supplementation of vitamin K1, vitamin D, and minerals was superior to vitamin D and minerals alone in preventing a decrease of elastic properties of the carotid artery, over a 3-year follow-up (28). A recent, large, prospective cohort study in the Netherlands showed that the combined association of low vitamin D and K status with mortality risk was greater than the sum of low vitamin D and K status alone (29). Another clinical study also demonstrated that combined vitamin D and K deficiency is highly prevalent in kidney transplant recipients and is associated with increased mortality and graft failure risk compared with high vitamin D and K status (30). Our results corroborate the findings of this trial: combined low vitamin D and vitamin K status was associated with a higher risk of stroke compared to those with high vitamin D and K status.
We have not yet discovered the exact physiological mechanisms explaining the joint association between vitamin K, vitamin D and stroke. However, some potential overlaps in their action exist. Both vitamin D and K can stimulate the γ-carboxylase system of vitamin K–dependent proteins. Two of these proteins are osteocalcin and MGP, which play a role in cardiovascular health (31, 32). Osteocalcin has been shown to regulate bone mineralization and calcium homeostasis, with the potential to prevent calcium build-up in soft tissues, thereby preventing vascular calcification (33). Similarly, MGP limits calcium incorporation into the extracellular matrix of soft tissues, thus acting as a potent inhibitor of soft tissue calcification. The MGP gene contains a vitamin D (calcitriol) responsive element in its promoter region (34). Circulating vitamin D has been shown to upregulate MGP expression in vascular smooth muscle cells of rats (35). It has also been demonstrated that vitamin K promotes 1,25-dihydroxyvitamin D–stimulated osteocalcin accumulation and mineralization (36). In addition, vitamin D and K have prominent anti-inflammatory effects (37), which is a modifiable risk factor for cardiovascular and cerebrovascular diseases. Thus, vitamins K and D can mutually enhance each other's physiological roles and our results provide a plausible clinical correlate to these experimental findings.
The main strength of our study is that it was conducted on a large population-based cohort, with well-designed quality assurance and quality control throughout. We assessed the joint associations of 25(OH)D and vitamin K1 in relation to first stroke in a population free of baseline stroke through a 1:1 case-control matching design, minimizing potential bias and misclassification. Despite these advantages, our study has several limitations. First, our study faces the same limitations as all case-control studies. A large randomized interventional trial that comparts a placebo, each vitamin separately, and both vitamins combined, would fully elucidate the real clinical impact of vitamin K-D in terms of stroke benefit. Second, although we adjusted for multiple confounding factors, our results may have been influenced by other unknown factors that should be better accounted for in future studies. High vitamin K1 and D levels might reflect healthy dietary and lifestyle patterns, rather than the nutrient itself, however, we were not able to exclude the effects of dietary/lifestyle habits on the results, as these data were not available in our study. Third, given the specificity of our study population (elderly Chinese with hypertension), findings from this study cannot be readily generalized to other populations. Furthermore, since our study population only included Han Chinese, further confirmatory studies in other ethnic groups are needed. Finally, expression levels of MGP were not measured in our study and further studies are needed to determine whether vitamin K is associated with stroke through this pathway.
In conclusion, in the present study, we observed that the simultaneous presence of low vitamin K and D status was associated with a significantly higher risk of ischemic stroke in a hypertensive, population-based sample. We speculate that this phenomenon reflects an overlap in the pleiotropic functions of both vitamins, resulting in a synergistic magnification of disease risk when both are insufficient. Larger, prospective interventional studies are needed to verify these findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number: FWA00001263). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YW and HM analyzed the data and wrote the manuscript. BX, ZW, ZZ, GM, and HZ helped with copyediting. LL and PC audited the data. YS, JL, YZ, YH, BW, GT, XQ, and XX conducted research. YH and HG had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program [2016YFE0205400, 2018ZX09739010, and 2018ZX09301034003], the Department of Science and Technology of Guangdong Province [2020B121202010], the Science and Technology Planning Project of Guangzhou, China [201707020010], the Science, Technology and Innovation Committee of Shenzhen [GJHS20170314114526143 and JSGG20180703155802047], the Economic, Trade and Information Commission of Shenzhen Municipality [20170505161556110, 20170505160926390, and 201705051617070], and the 111 Project from the Education Ministry of China [No. B18053].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MH declared a past co-authorship with several of the authors YH and XQ to the handling editor.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge all of the study participants of this project. We wish to thank all members for their dedication and hard work and the participants who gave their precious time.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.881994/full#supplementary-material
References
1. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
2. Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. (2007) 6:149–61. doi: 10.1016/S1474-4422(07)70028-5
3. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. (2017) 120:472–95. doi: 10.1161/CIRCRESAHA.116.308398
4. Apalset EM, Gjesdal CG Eide GE, Tell GS. Intake of vitamin K1 and K2 and risk of hip fractures: the Hordaland Health Study. Bone. (2011) 49:990–5. doi: 10.1016/j.bone.2011.07.035
5. Chen HG, Sheng LT, Zhang YB, Cao AL, Lai YW, Kunutsor SK, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. (2019) 58:2191–205. doi: 10.1007/s00394-019-01998-3
6. Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. (2009) 203:489–93. doi: 10.1016/j.atherosclerosis.2008.07.010
7. Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, et al. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. (2013) 98:197–208. doi: 10.3945/ajcn.112.056101
8. Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. (2009) 89:1799–807. doi: 10.3945/ajcn.2008.27338
9. Chaikriangkrai K, Jhun HY, Palamaner Subash Shantha G, Bin Abdulhak A, Sigurdsson G, Nabi F, et al. Coronary artery calcium score as a predictor for incident stroke: Systematic review and meta-analysis. Int J Cardiol. (2017) 236:473–7. doi: 10.1016/j.ijcard.2017.01.132
10. Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, et al. Circulating desphospho-uncarboxylated matrix γ-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemost. (2014) 12:1028–34. doi: 10.1111/jth.12609
11. Erkkilä AT, Booth SL, Hu FB, Jacques PF, Lichtenstein AH. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis. (2007) 17:58–62. doi: 10.1016/j.numecd.2006.03.008
12. Vissers LE, Dalmeijer GW, Boer JM, Monique Verschuren WM, van der Schouw YT, Beulens JW. Intake of dietary phylloquinone and menaquinones and risk of stroke. J Am Heart Assoc. (2013) 2:e000455. doi: 10.1161/JAHA.113.000455
13. Larsson SC, Traylor M, Markus HS. Circulating vitamin K1 levels in relation to ischemic stroke and its subtypes: a mendelian randomization study. Nutrients. (2018) 10:1575. doi: 10.3390/nu10111575
14. Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. (2016) 13:404–17. doi: 10.1038/nrcardio.2016.73
15. Shi H, Chen H, Zhang Y, Li J, Fu K, Xue W, et al. 25-Hydroxyvitamin D level, vitamin D intake, and risk of stroke: a dose-response meta-analysis. Clin Nutr. (2020) 39:2025–34. doi: 10.1016/j.clnu.2019.08.029
16. Bolton-Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. (2007) 22:509–19. doi: 10.1359/jbmr.070116
17. Braam LA, Hoeks AP, Brouns F, Hamulyák K, Gerichhausen M J, Vermeer C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost. (2004) 91:373–80. doi: 10.1160/TH03-07-0423
18. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. (2015) 313:1325–35. doi: 10.1001/jama.2015.2274
19. Wu H, Wang B, Ban Q, Chen L, Yan D, Yu Y, et al. Association of total homocysteine with blood pressure in a general population of Chinese adults: a cross-sectional study in Jiangsu province, China. BMJ Open. (2018) 8:e021103. doi: 10.1136/bmjopen-2017-021103
20. Zhou Z, Li J, Yu Y, Li Y, Zhang Y, Liu L, et al. Effect of smoking and folate levels on the efficacy of folic acid therapy in prevention of stroke in hypertensive men. Stroke. (2018) 49:114–20. doi: 10.1161/STROKEAHA.117.018273
21. Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. (2012) 26:155–66. doi: 10.1016/j.blre.2012.03.002
22. Shea MK, Booth SL, Weiner DE, Brinkley TE, Kanaya AM, Murphy RA, et al. Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the health, aging, and body composition study (Health ABC). J Nutr. (2017) 147:888–95. doi: 10.3945/jn.117.249375
23. Nerurkar PV, Gandhi K, Chen JJ. Correlations between coffee consumption and metabolic phenotypes, plasma folate, and vitamin B12: NHANES 2003 to 2006. Nutrients. (2021) 13:1348. doi: 10.3390/nu13041348
24. Moens AL, Vrints CJ, Claeys MJ, Timmermans JP, Champion HC, Kass DA. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am J Physiol Heart Circ Physiol. (2008) 294:H1971–7. doi: 10.1152/ajpheart.91503.2007
25. Hou YC, Lu CL, Zheng CM, Chen RM, Lin YF, Liu WC, et al. Emerging role of vitamins D and K in modulating uremic vascular calcification: the aspect of passive calcification. Nutrients. (2019) 11:152. doi: 10.3390/nu11010152
26. van Ballegooijen AJ, Cepelis A, Visser M, Brouwer IA, van Schoor NM, Beulens JW. Joint association of low vitamin D and vitamin K status with blood pressure and hypertension. Hypertension. (2017) 69:1165–72. doi: 10.1161/HYPERTENSIONAHA.116.08869
27. Mayer O Jr, Seidlerová J, Wohlfahrt P, Filipovský J, Cífková R, Cerná V, et al. Synergistic effect of low K and D vitamin status on arterial stiffness in a general population. J Nutr Biochem. (2017) 46:83–9. doi: 10.1016/j.jnutbio.2017.04.010
28. Caluwe R, Pyfferoen L, De Boeck K, De Vriese AS. The effects of vitamin K supplementation and vitamin K antagonists on progression of vascular calcification: ongoing randomized controlled trials. Clin Kidney J. (2016) 9:273–9. doi: 10.1093/ckj/sfv146
29. van Ballegooijen AJ, Beulens JWJ, Kieneker LM, de Borst MH, Gansevoort RT, Kema IP, et al. Combined low vitamin D and K status amplifies mortality risk: a prospective study. Eur J Nutr. (2021) 60:1645–54. doi: 10.1007/s00394-020-02352-8
30. van Ballegooijen AJ, Beulens JWJ, Keyzer CA, Navis GJ, Berger SP, de Borst MH, et al. Joint association of vitamins D and K status with long-term outcomes in stable kidney transplant recipients. Nephrol Dial Transplant. (2020) 35:706–14. doi: 10.1093/ndt/gfy399
31. Kim KM, Lim S, Moon JH, Jin H, Jung KY, Shin CS, et al. Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone. (2016) 83:178–83. doi: 10.1016/j.bone.2015.11.008
32. Schurgers LJ, Cranenburg ECM, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. (2008) 100:593–603. doi: 10.1160/TH08-02-0087
33. Okura T, Kurata M, Enomoto D, Jotoku M, Nagao T, Desilva VR, et al. Undercarboxylated osteocalcin is a biomarker of carotid calcification in patients with essential hypertension. Kidney Blood Press Res. (2010) 33:66–71. doi: 10.1159/000289575
34. Fraser JD, Price PA. Induction of matrix Gla protein synthesis during prolonged 1,25-dihydroxyvitamin D3 treatment of osteosarcoma cells. Calcif Tissue Int. (1990) 46:270–9. doi: 10.1007/BF02555007
35. Farzaneh-Far A, Weissberg PL, Proudfoot D, Shanahan CM. Transcriptional regulation of matrix gla protein. Z Kardiol. (2001) 90(Suppl. 3:38–42). doi: 10.1007/s003920170040
36. Koshihara Y, Hoshi K, Ishibashi H, Shiraki M. Vitamin K2 promotes 1alpha,25(OH)2 vitamin D3-induced mineralization in human periosteal osteoblasts. Calcif Tissue Int. (1996) 59:466–73. doi: 10.1007/BF00369212
Keywords: vitamin K1, 25-hydroxyvitamin D, hypertensive adults, nested case-control study, first stroke
Citation: Wei Y, Ma H, Xu B, Wang Z, He Q, Liu L, Zhou Z, Song Y, Chen P, Li J, Zhang Y, Mao G, Wang B, Tang G, Qin X, Zhang H, Xu X, Huo Y and Guo H (2022) Joint Association of Low Vitamin K1 and D Status With First Stroke in General Hypertensive Adults: Results From the China Stroke Primary Prevention Trial (CSPPT). Front. Neurol. 13:881994. doi: 10.3389/fneur.2022.881994
Received: 08 March 2022; Accepted: 11 April 2022;
Published: 12 May 2022.
Edited by:
Cheng-Yang Hsieh, Sin-Lau Christian Hospital, TaiwanReviewed by:
Jun Ni, Peking Union Medical College Hospital (CAMS), ChinaMingli He, Affiliated Hospital of Jiangnan University, China
Nilay D. Solanki, Ramanbhai Patel College of Pharmacy, India
Veena Nayak, Manipal Academy of Higher Education, India
Copyright © 2022 Wei, Ma, Xu, Wang, He, Liu, Zhou, Song, Chen, Li, Zhang, Mao, Wang, Tang, Qin, Zhang, Xu, Huo and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiyuan Guo, Z3VvaHVpeXVhbkBjYXUuZWR1LmNu; Yong Huo, aHVveW9uZ0AyNjMubmV0LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Yaping Wei
Yaping Wei Hai Ma2†
Hai Ma2† Zhuo Wang
Zhuo Wang Lishun Liu
Lishun Liu Yun Song
Yun Song Yan Zhang
Yan Zhang Guangyun Mao
Guangyun Mao Xianhui Qin
Xianhui Qin Xiping Xu
Xiping Xu Yong Huo
Yong Huo Huiyuan Guo
Huiyuan Guo