- 1Sydney Brain Centre, The Ingham Institute for Applied Medical Research, Liverpool, NSW, Australia
- 2South Western Sydney Clinical School, University of New South Wales, Sydney, NSW, Australia
- 3Melbourne Brain Centre at Royal Melbourne Hospital, Melbourne, VIC, Australia
- 4Department of Medicine, University of Melbourne, Melbourne, VIC, Australia
- 5Allied Health Research Unit, St Vincent's Health Network Sydney, Sydney, NSW, Australia
- 6Faculty of Health Sciences, Australian Catholic University, North Sydney, NSW, Australia
- 7Department of Neurology and Neurophysiology, Liverpool Hospital, Sydney, NSW, Australia
- 8Queensland Department of Agriculture and Fisheries, Brisbane, QLD, Australia
- 9Apollo Medical Imaging Technology Pty Ltd., Melbourne, VIC, Australia
Background: Hemorrhagic transformation (HT) following reperfusion therapies for acute ischaemic stroke often predicts a poor prognosis. This systematic review and meta-analysis aims to identify risk factors for HT, and how these vary with hyperacute treatment [intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT)].
Methods: Electronic databases PubMed and EMBASE were used to search relevant studies. Pooled odds ratio (OR) with 95% confidence interval (CI) were estimated.
Results: A total of 120 studies were included. Atrial fibrillation and NIHSS score were common predictors for any intracerebral hemorrhage (ICH) after reperfusion therapies (both IVT and EVT), while a hyperdense artery sign (OR = 2.605, 95% CI 1.212–5.599, I2 = 0.0%) and number of thrombectomy passes (OR = 1.151, 95% CI 1.041–1.272, I2 = 54.3%) were predictors of any ICH after IVT and EVT, respectively. Common predictors for symptomatic ICH (sICH) after reperfusion therapies were age and serum glucose level. Atrial fibrillation (OR = 3.867, 95% CI 1.970–7.591, I2 = 29.1%), NIHSS score (OR = 1.082, 95% CI 1.060–1.105, I2 = 54.5%) and onset-to-treatment time (OR = 1.003, 95% CI 1.001–1.005, I2 = 0.0%) were predictors of sICH after IVT. Alberta Stroke Program Early CT score (ASPECTS) (OR = 0.686, 95% CI 0.565–0.833, I2 =77.6%) and number of thrombectomy passes (OR = 1.374, 95% CI 1.012–1.866, I2 = 86.4%) were predictors of sICH after EVT.
Conclusion: Several predictors of ICH were identified, which varied by treatment type. Studies based on larger and multi-center data sets should be prioritized to confirm the results.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=268927, identifier: CRD42021268927.
1. Introduction
Stroke is a leading cause of death and disability in Australia and around the world, with one in four people affected by stroke in their lifetime (1). Reperfusion therapies, including intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT), can significantly improve patient outcomes (2) but are associated with complications, of which the most devastating is haemorrhagic transformation (HT). Haemorrhagic transformation after cerebral infarction is reported to occur in between 3.2 and 43.3% of strokes (3), and often results in a poorer prognosis. Aetiologically HT is a multifactorial phenomenon, and the ability to accurately predict the development of HT after reperfusion therapies has great potential to guide clinical decision making in order to maximize benefits and minimize harm.
Previous studies have identified many risk factors for HT, including (but not limited to) atrial fibrillation, higher baseline National Institute of Health Stroke Scale (NIHSS) score, advanced age, longer time from stroke onset to treatment (OTT), and lower baseline Alberta Stroke Program Early CT score (ASPECTS). Although a wide range of HT risk factors have been reported, findings have often been contradictory. For example, number of stent retriever passes at EVT has been variably reported to predict HT in comparable single-center cohorts (4–6), highlighting the heterogeneity of the evidence base.
Intravenous tissue plasminogen activator (tPA) improves outcome following ischaemic stroke when administered to appropriately selected patients up to 9 h after symptom onset (7–9). Endovascular thrombectomy (also known as mechanical thrombectomy), used either alone or in combination with IVT, has shown substantial benefit in patients with large vessel occlusion (10–14). Emerging evidence suggests that risk factors for HT vary considerably depending on the reperfusion treatment employed. In particular, higher rates of sICH have been reported following EVT (15), with certain imaging characteristics (occlusion site, ASPECTS) predicting HT in this setting (6, 16–19). Both individually and in combination, such predictors which are readily available in the hyperacute setting, have potential to guide clinical decision-making and prognostication.
This study reviews our current understanding of prognostic factors for HT in different treatment settings (IVT and EVT, respectively). Specifically, we aim to answer the review questions: (1) What are the baseline risk factors of haemorrhagic transformation after endovascular thrombectomy? (2) What are the baseline risk factors of haemorrhagic transformation after intravenous thrombolysis? (3) Is there any differences in risk factors of haemorrhagic transformation between endovascular thrombectomy and intravenous thrombolysis?
2. Methods
2.1. Search strategy
Electronic databases PubMed and EMBASE were used to identify relevant studies. The reference lists of eligible studies and systematic reviews were also checked and hand searching completed to find any additional relevant studies. The following search terms including their synonyms and available MeSH terms were used to retrieve relevant studies: Acute Ischemic Stroke, Hemorrhagic Transformation, Endovascular Thrombectomy, Intravenous Thrombolysis. The key search terms were combined using the Boolean operator “and” and “or” to retrieve the search results. Databases were searched from inception to August 2021.
2.2. Eligibility criteria
To be eligible for inclusion, studies were required to meet the following criteria: (1) Full-text publications in English. (2) Patients were diagnosed with acute ischaemic stroke. (3) Patient cohort aged 18 years old and over. (4) HT confirmed by CT/MRI scan within 48 hours after treatment. (5) Study included at least 50 patients. (6) Clinical or imaging data was measured prior to or during reperfusion treatment. (7) Treatment type of enrolled patients were either IVT or EVT, or bridging therapy (IVT plus EVT). (8) Predictors of HT were based on multivariate analysis and expressed as odds ratio (OR) with 95% CI.
2.3. Study screening and data extraction
Studies returned from the search results were screened using three steps. First, duplicate studies from across different databases were removed. Second, titles and abstracts of the search results were screened to check for eligibility by two independent reviewers (JS and CL), with disagreements resolved by discussion, and with a third reviewer (LC) if necessary. Finally, eligible full texts were screened by the same independent reviewers (JS and CL), with disagreements being resolved by discussion, and with a third reviewer if necessary.
For data extraction, two reviewers extracted the data independently using a predefined data extraction spreadsheet. Data were extracted from the selected studies guided by the CHARMS checklist (20), including authors and years, published journal, study type (randomized controlled trial or observational cohort), a single center or multi-center study, baseline characteristics of participants such as age, gender, onset-to-treatment time, NIHSS score, definitions of reported intracerebral hemorrhage (ICH) and the number of patients with HT, the HT confirmed timing after treatment, treatment type (intravenous therapy or endovascular therapy), risk factors identified and their type (continuous or categorized), regions and sample size. For performance measurements, odds ratio and 95% Confidence Interval (CI) and confounding variables adjusted in the multivariate analysis were extracted for prognostic factor studies.
2.4. Quality assessment
Two reviewers independently performed risk of bias assessments of the included studies. The two reviewers resolved any disagreements via discussion among themselves and with a third reviewer if required, until a consensus was reached.
To assess risk of bias in the included studies, the Quality In Prognosis Studies (QIPS) tool was used to evaluate validity and bias across six domains: participation, attrition, prognostic factor measurement, confounding measurement, and account, outcome measurement, and analysis and reporting (21).
2.5. Statistical analysis
Combined hemorrhagic transformation rates with 95% CIs were computed for symptomatic ICH (sICH) and any ICH, respectively. A meta-analysis of risk factors using extracted OR with 95% CI from individual studies was conducted if the risk factor was reported in a minimum of two studies. Odds ratio is an appropriate measure for categorical outcomes (22) and is a preferable report measure in meta-analysis on outcome prediction models (23). As well as odds ratio was the most prevalent measure reported in the included studies, we only extracted the odds ratio that was adjusted for confounding factors, which is preferable to analyses based on summary statistics according to Cochrane guidelines (24).
The I2 test was used to evaluate heterogeneity among included studies (25). For I2 statistic, 25, 50, and 75% were the threshold for low, moderate, and high heterogeneity. The τ2 was used to estimate the variance of the distribution of true effect sizes (26), and the confidence intervals around τ2 were calculated to quantify the uncertainty of heterogeneity (27). Prediction intervals were calculated to estimate the effect sizes of future studies based on present evidence (28). A random-effects model was used to analyse the data, regardless of heterogeneity. Begg's funnel plots were used to test potential publication bias for those results with number of studies > 10. Sensitivity analysis was conducted by removing included studies one by one to detect the influence of individual studies on the estimate of the overall effect. All statistical analyses were conducted with Stata software package (V.13.1; Stata, College Station, Texas, USA) and R 4.1.2 (R Foundation), with a p-value of p < 0.05 considered statistically significant.
3. Results
3.1. Literature search and study characteristics
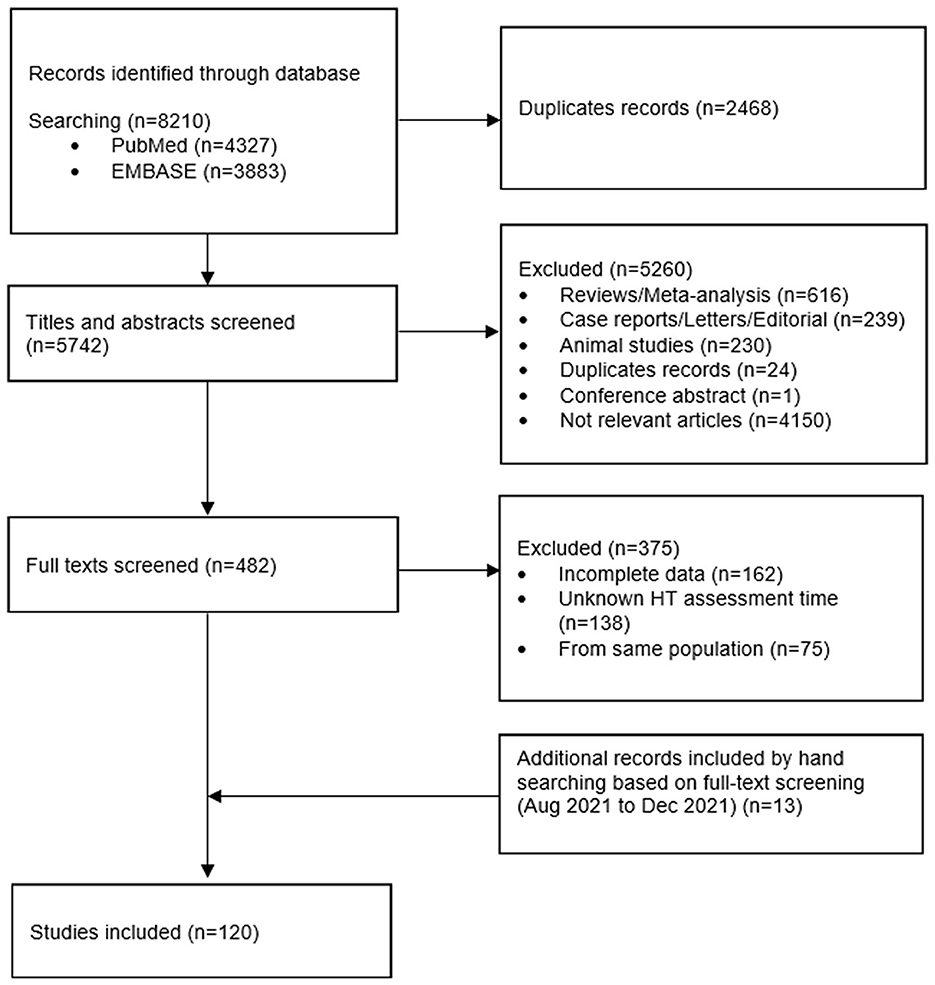
Literature search and screening processes are shown in Figure 1. Initially the search result included 5,742 articles after removing duplicates. After title and abstract screening, 482 articles remained. After full-text screening, 107 studies were included based on the search results, and another 13 relevant studies were identified via manual searching. In total, 120 studies (5, 6, 16–19, 29–142) were included in the meta-analysis.
Among the 120 included studies, 67 enrolled patients who were treated with IVT and 53 enrolled patients who were treated with EVT. Table 1 shows the characteristics of the included studies. The number of participants ranged from 71 (44) to 88,094 (55), with a total median sample size of 414 (Interquartile Range: 204.5–1,125). Further information on the characteristics of the included studies is summarized in Supplementary Table (“General characteristics”).
The general study quality was good, with a lack of reporting in the “Study confounding” domain in ~48% (58 out of 120) of the included studies. The results of quality assessment for each study are presented in Supplementary Table (“Quality assessment – QUIPS”) and Supplementary Figures 1, 2 (143).
3.2. Event rates of ICH and sICH
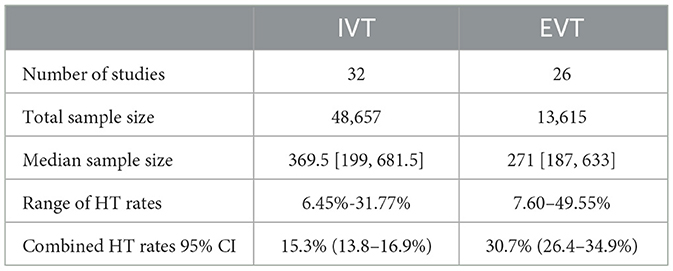
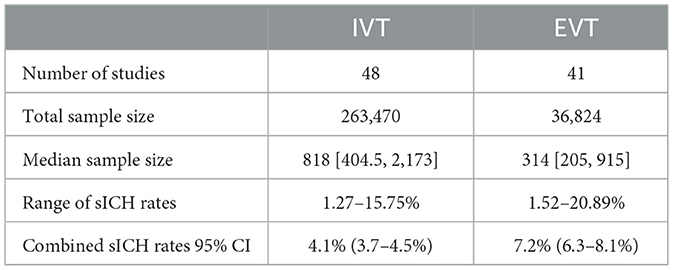
Tables 2, 3 show the event rates of any ICH and sICH per treatment type. In total, there were 32 IVT-based studies and 26 EVT-based studies that reported any ICH rates. Among the reported studies, any ICH rate ranged from 6.45% (106) to 49.55% (65), with a combined any ICH rate of 22.0% (95% CI 20.0–24.1%). The number of studies reporting sICH rates was 48 for IVT and 41 for EVT respectively. The sICH rate ranged from 1.27% (55) to 20.89% (129) with a combined sICH rate of 5.2% (95% CI 4.8–5.6%). Four main sICH criteria were applied in the included studies: the Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS-MOST) criteria (144), the European Cooperative Acute Stroke Study (ECASS) criteria (145), the National Institute of Neurological Diseases and Stroke (NINDS) criteria (7) and the Heidelberg Bleeding Classification (HBC) (146). The proportion of studies using each sICH criteria is shown in Supplementary Figure 3. In cases of multiple sICH criteria, SITS-MOST criteria were used to calculate the sICH rate; if SITS-MOST criteria were not reported, ECASS criteria were used.
3.3. HT risk factors
In total, over 100 distinct risk factors were reported in the 113 prognostic factor studies. Since many risk factors were only reported in a single study, the meta-analysis included 24 risk factors that contributed to any ICH, and 32 risk factors that contributed to sICH. A summary of reported risk factors in the included study is shown in Supplementary Table (“Study results”).
3.4. Meta-analysis of risk factors related to ICH
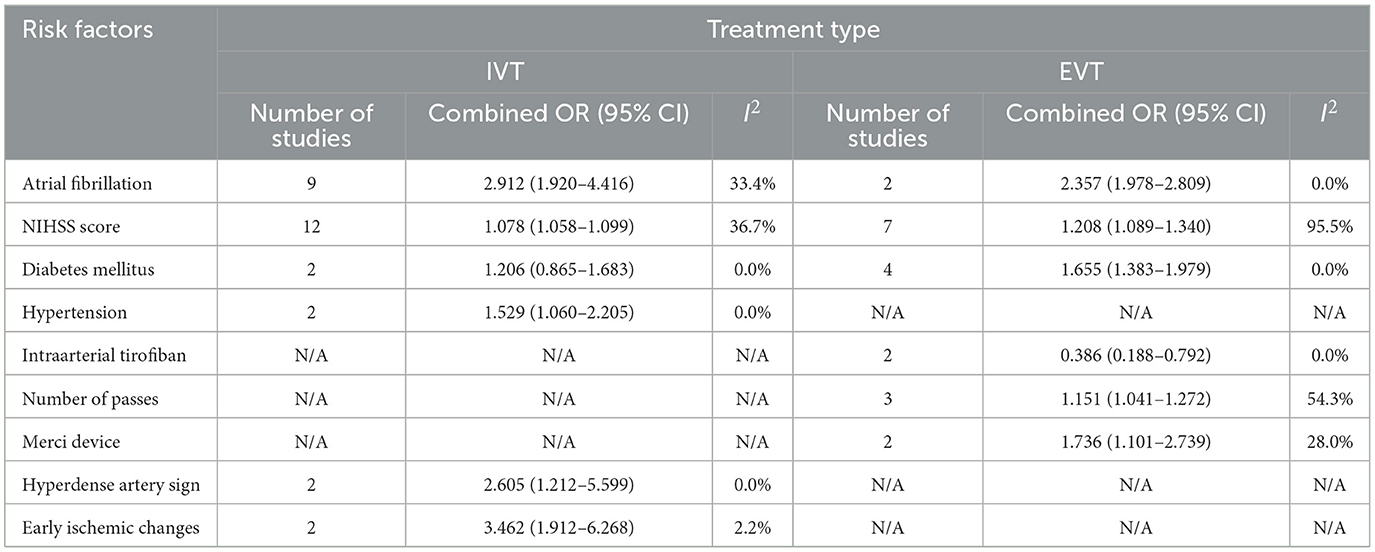
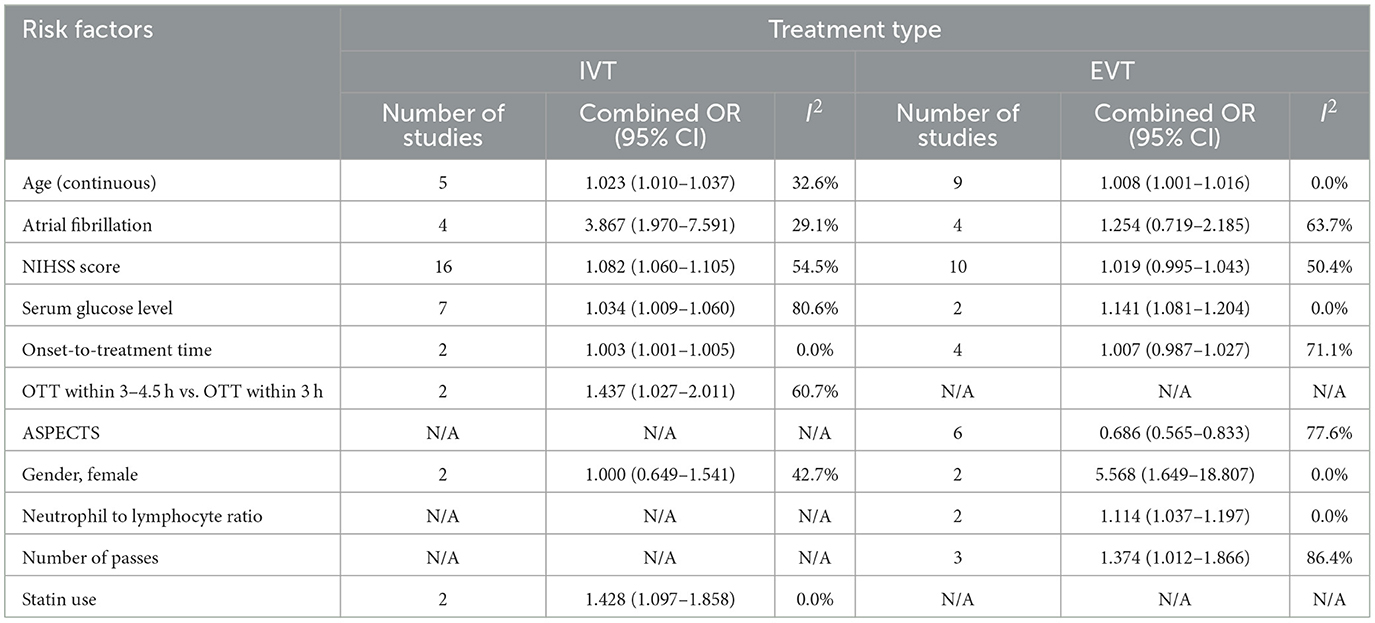
Figures 2, 3 show forest plots of risk factors for any ICH (147). A combined total of 16 risk factors for any ICH after IVT and 14 risk factors for any ICH after EVT were included in the meta-analysis. Meta-analysis showed that early ischemic changes, atrial fibrillation, hyperdense artery sign, hypertension and NIHSS score were predictors for any ICH after IVT, while atrial fibrillation, use of the Merci Device, diabetes mellitus, NIHSS score and number of thrombectomy passes were predictors for any ICH after EVT. Intraarterial tirofiban was associated with a lower risk of any ICH after EVT. Table 4 lists predictors for any ICH.
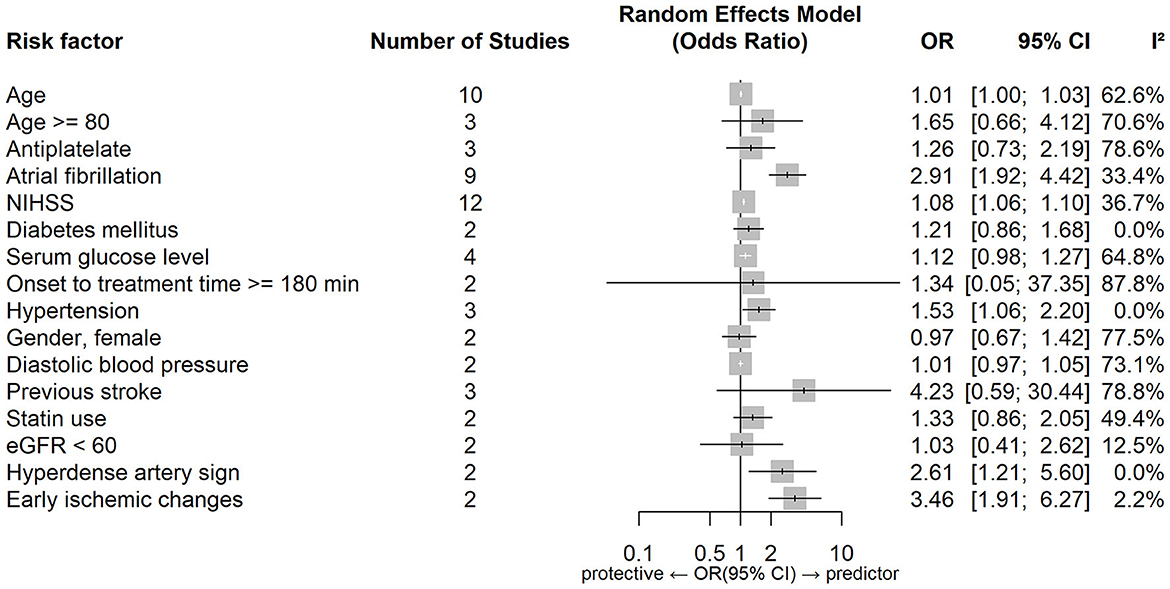
Figure 2. Forest plot of predictors for any ICH after IVT. OR, Odd Ratio; CI, Confidence Interval; NIHSS, National Institute of Health Stroke Scale; eGFR, estimated Glomerular Filtration Rate.
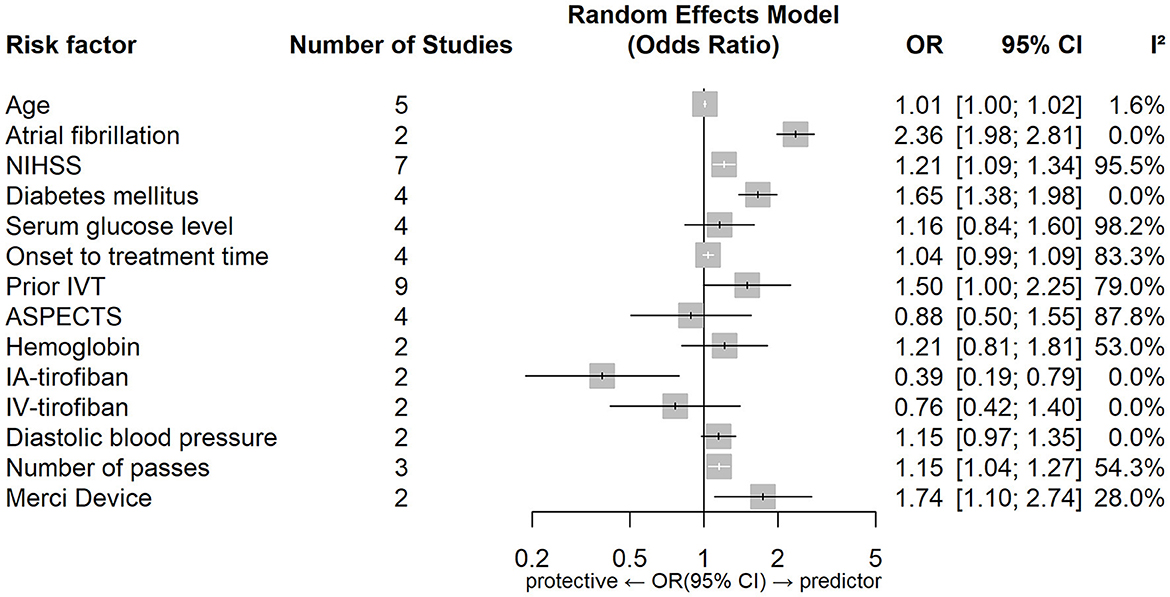
Figure 3. Forest plot of predictors for any ICH after EVT. OR, Odd Ratio; CI, Confidence Interval; NIHSS, National Institute of Health Stroke Scale; IVT, Intravenous Thrombolysis; ASPECTS, Alberta Stroke Program Early CT Score; IA, Intraarterial; IV, Intravenous.
3.5. Meta-analysis of risk factors related to sICH
Figures 4, 5 show forest plots of risk factors for sICH. A combined total of 19 risk factors for sICH after IVT and 22 risk factors for sICH after EVT were included in the meta-analysis. Meta-analysis showed that atrial fibrillation, OTT within 3–4.5 h VS OTT within 3 h, statin use, NIHSS score, serum glucose level, age and onset-to-treatment time were predictors of sICH after IVT, while female gender, number of thrombectomy passes, serum glucose level, neutrophil to lymphocyte ratio, age and lower ASPECTS were predictors of sICH after EVT. Table 5 lists predictors for sICH.
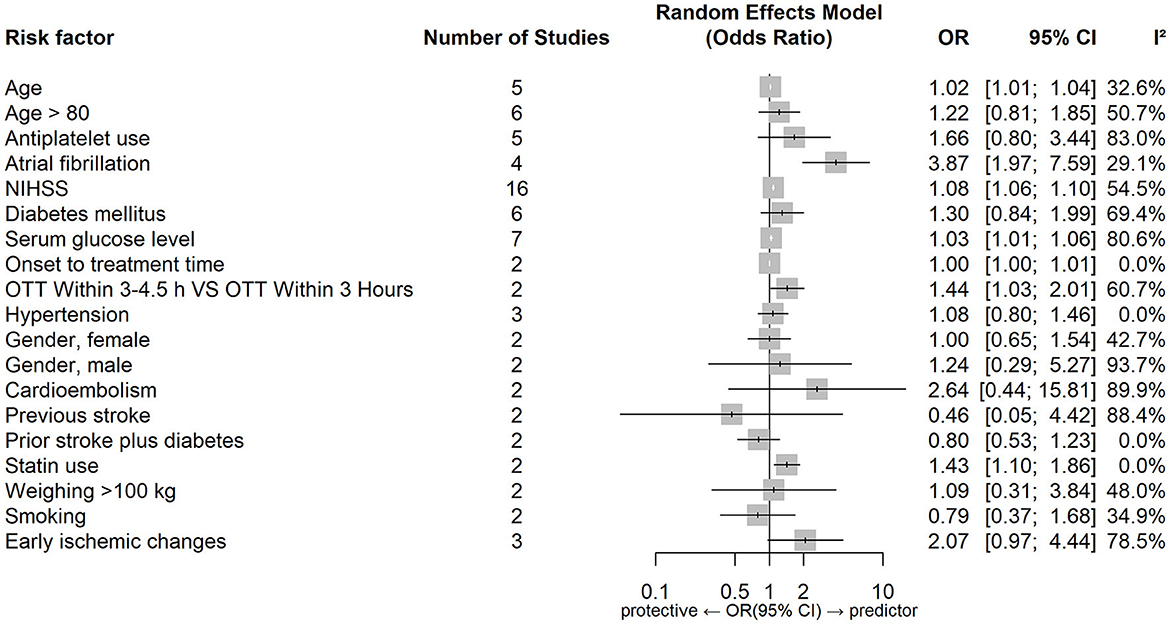
Figure 4. Forest plot of predictors for sICH after IVT. OR, Odd Ratio; CI, Confidence Interval; NIHSS, National Institute of Health Stroke Scale; OTT, Onset to Treatment Time.
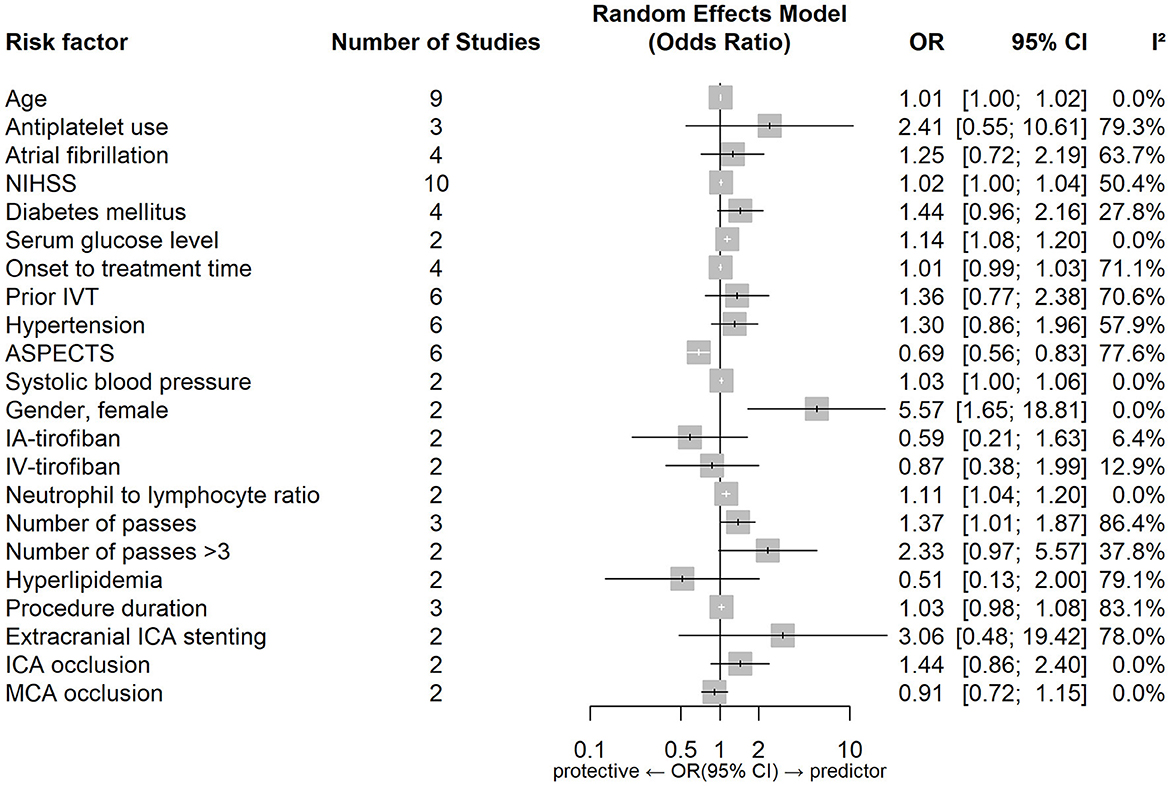
Figure 5. Forest plot of predictors for sICH after EVT. OR, Odd Ratio; CI, Confidence Interval; NIHSS, National Institute of Health Stroke Scale; IVT, Intravenous Thrombolysis; ASPECTS, Alberta Stroke Program Early CT Score; IA, Intraarterial; IV, Intravenous; ICA, Internal Carotid Artery; MCA, Middle Cerebral Artery.
3.6. Sensitivity analysis
In order to assess whether any particular study had a disproportionate influence on the meta-analysis results, heterogeneity assessment was done for the results with number of studies ≥3 and I2 ≥50%. For results where the confidence interval around τ2 did not contain zero, a further sensitivity analysis was done by removing one study at a time. Three results showed a statistically significant association between prior IVT and any ICH after EVT, but with very high heterogeneity (I2 ranged from 74–81%). The upper limit of the sensitivity analysis showed a statistically significant association between previous stroke and any ICH after IVT, with low-to-moderate heterogeneity but a very wide confidence interval (OR = 13.06, 95% CI 1.08–157.97, I2 = 46%). The upper limit of the sensitivity analysis also showed a statistically significant association between antiplatelet use and sICH after IVT with low heterogeneity (OR = 2.17, 95% CI 1.50–3.14, I2 = 0.0%), and also between antiplatelet use and sICH after EVT with high heterogeneity and a wide confidence interval (OR = 4.17, 95% CI 1.00–17.45, I2 = 83%). The upper limit of the sensitivity analysis also showed statistically significant association between early ischemic changes and sICH after IVT, with low to moderate heterogeneity (OR = 2.98, 95% CI 1.37–6.49, I2 = 44%). A summary of heterogeneity assessment and sensitivity analysis is shown in Supplementary Table (“Heterogeneity assessment” and “Sensitivity analysis”).
3.7. Assessment of publication bias
Funnel plots were used to assess publication bias for NIHSS score as a predictor of any ICH after IVT (number of studies N = 12) and sICH after IVT (number of studies N = 16). No evidence of publication bias was found for NIHSS score predicting any ICH after IVT (Egger's test, P = 0.185). However, there was evidence of significant small study bias for NIHSS score predicting sICH after IVT (Egger's test, intercept = 1.796, t = 4.48, P < 0.001), although one particular study (105) had a significant influence on this statistic.
4. Discussion
We performed a systematic review and meta-analysis to identify risk factors for HT after reperfusion therapies for acute ischaemic stroke. Although many factors have previously been reported as predictors of HT, findings are derived from widely varying studies.
4.1. Disparities in HT rates
The combined rates of both any ICH and sICH after EVT (30.7 and 7.2%) and IVT (15.3 and 4.1%) in our study were lower than those reported in previous work (35% for any ICH and 8% for sICH after EVT and 6.5% for sICH after IVT) (148, 149). As well as having a larger sample size, our analysis also standardized the definition of ICH across all included studies that reported multiple sICH criteria using SITS-MOST criteria. The incidence of HT was generally lower when these criteria were applied (150). In contrast, Hao et al. (148) study did not standardize the identification of ICH, and only 5% of their included studies used SITS-MOST criteria (vs. 18.4% in our study). Tsivgoulis et al. (149) review had a relatively small sample size (N = 12 vs. N ranging from 63 to 1,643 in other included studies), with only one study using SITS-MOST criteria.
Our analysis found that the combined rate of any ICH and sICH were lower in patients treated with IVT, in comparison to those treated with EVT. This result is consistent with previous reports (15). Several factors are thought to be responsible for higher HT rates after EVT. First, EVT studies by their nature include only patients with large vessel occlusion (LVO) stroke, who usually have worse stroke severity with higher NIHSS scores (151–153) and larger areas of involved tissue. Resulting ASPECTS scores are generally lower, and indeed this factor was found to independently predict sICH after EVT in our study. Second, in a finding corroborated by our analysis for the Merci device, the use of thrombectomy devices themselves have several implications for HT risk that center on vessel abrasion, including those arising from the type of device used and the number of passes required to achieve satisfactory reperfusion. Third, EVT is more commonly performed in patients with atrial fibrillation (18, 44, 96, 154) and with longer onset-to-treatment times (17, 68, 96), and uses additional antiplatelet agents during the procedure (112). Finally, other procedure-related factors such as the use of general anesthesia (155), distal embolization and extracranial stenting (16) may also contribute to a higher HT rate after EVT. These factors were not identified in our meta-analysis, due to a lack of relevant studies.
4.2. Predictors of any ICH
Common predictors for any ICH after reperfusion therapy (both IVT and EVT) were atrial fibrillation and a higher NIHSS score. While these two factors also predicted sICH after IVT they did not do so after EVT, however the relevant data set for EVT was limited by moderate-to-high heterogeneity. Hypertension, a hyperdense artery sign, and early ischemic change on non-contrast CT were predictors of any ICH after IVT and diabetes mellitus, number of thrombectomy passes and use of the Merci device were predictors of any ICH after EVT. Intriguingly, intraarterial tirofiban was found to be protective for any ICH after EVT.
Hyperdense artery sign and early ischemic changes were found to be predictors of any ICH after IVT. There were insufficient studies included in the meta-analysis that examined the same predictors for EVT. Hyperdense artery sign is associated with a higher clot burden and cardioembolic stroke, both of which predict a potentially larger area of infarcted brain tissue and a diminished response to thrombolysis (156–158). Early ischemic changes (including hypodensity and swelling/effacement) indicate the presence of brain oedema arising from prolonged hypoperfusion, and possibly the development of irreversible injury (159). These imaging features have been shown to predict HT, in particular where a significant portion (>33%) of the involved vascular territory is affected (160). Both hyperdense artery sign and early ischemic changes were imaging-based predictors derived from non-contrast CT, the most widely studied (and quantitative) stroke imaging modality.
Number of thrombectomy passes and use of the Merci device were predictors of any ICH after EVT, although the former result was subject to significant heterogeneity (prediction interval 0.4183–3.1667). Successive thrombectomy passes are thought to damage the arterial intima and weaken the vessel wall, causing micro-perforations at the time of device deployment/retraction (96) and so increasing the likelihood of HT (161). Use of the Merci device may increase vessel injury, vasospasm, or arterial dissection (96).
The effect of intraarterial tirofiban and intravenous tirofiban on HT risk varied in a key report (162), promoting us to regard route of administration of tirofiban as an independent variable. Surprisingly we found that intraarterial tirofiban was protective for any ICH after EVT. Among previous studies only Sun et al. (112) concluded that intraarterial tirofiban significantly decreased the odds of any ICH, with others either reporting contradictory or inconclusive findings (79, 163, 164). The contradictory findings may be explained by different rates of adjunctive IVT in these studies (increased ICH risks with adjunctive IVT). Sun et al. (162) study had a relatively small sample size (N = 195), and selection bias was introduced because use of tirofiban was administered at the neuro-interventional specialists' discretion. This is likely to have led to the exclusion of patients with larger infarct sizes who were at higher risk of subsequent ICH (165, 166). In addition, in patients receiving tirofiban it is possible that more stringent post-procedural blood pressure management may have been pursued, and the use of antiplatelet and anticoagulant therapies may have been more aggressively rationalized to reduce the risk of ICH (164). Notably Sun et al. conclusions were specific to patients with stroke due to large artery atherosclerosis, and did not reach significance for cardioembolism. Zhao et al. (164), who specifically recruited patients with cardioembolic stroke, concluded that intraarterial tirofiban was not protective for any ICH after EVT. Taken together these findings suggest that tirofiban's effect may be specific to both route of administration and stroke etiology.
4.3. Predictors of sICH
Common predictors for sICH after reperfusion therapy were higher age and a higher serum glucose level. Atrial fibrillation, a higher NIHSS score, longer onset-to-treatment time and statin use were predictors of sICH after IVT, while lower ASPECTS, female gender, higher neutrophil-to-lymphocyte ratio and number of thrombectomy passes were predictors of sICH after EVT.
Lower APSECTS (indicating larger stroke volumes) were found to independently predict sICH after EVT in our study. As described above a hyperglycaemic environment can impair cell metabolism and reduce vasoreactivity, which may disrupt the blood brain barrier integrity and increase the permeability, leading to the development of HT (5, 44, 101, 139, 167).
OTT within 3–4.5 h VS OTT within 3 h and statin use were found only to be the predictors of sICH after IVT, with insufficient studies to examine these associations for EVT. Although statin use was found to be predictive of sICH after IVT this finding was derived from two studies, with several others reporting no association between statin use and sICH (168–171).
Atrial fibrillation, a higher NIHSS score and longer onset-to-treatment time were found to be predictors of sICH after IVT but not after EVT. This result was also confirmed by the heterogeneity assessment, indicating there are different predictors of sICH after each treatment type.
A higher neutrophil-to-lymphocyte ratio was found only to predict sICH after EVT, there were insufficient results for IVT to examine the same factor. Neutrophil-to-lymphocyte ratio is a biomarker of systemic inflammation. Higher neutrophils lead to increased release of MMP-9 (matrix metalloproteinase-9) and disruption of neurovascular units and blood brain barrier integrity, increasing the risk of sICH (172–174).
4.4. Assessment of heterogeneity and publication bias
In this study, heterogeneity assessment and sensitivity analysis were done to explore the robustness of the results. Heterogeneity assessment was performed for 25 results and a further sensitivity analysis was done for 11 results, of which six differed from the original findings. The robustness of the meta-analysis was therefore generally good.
The between-study heterogeneity observed could be explained by several factors. First, different study designs with different inclusion criteria resulted in large variations in patient characteristics including stroke severity, average age, onset-to-treatment time, disease history and stroke etiology. There was also significant variation in the number and the ethnicity of enrolled patients. Second, definitions of the same risk factors were not standardized across different studies, and in some studies were ambiguous or not clearly stated. For example, there was no uniform method of defining hyperdense artery sign: a clot with a Hounsfield unit ratio of 1.1 indicated a hyperdense artery sign in one study (175), while in another a ratio of 1.5 was used to exclude a hyperdense artery sign (176). Furthermore, for drug related risk factors, different studies used different medication regimes with regard to type, dose, and timing of administration. For example, while prior antiplatelet use was identified as a predictor in multiple studies, the specific drug varied despite the fact that different agents are recognized to variably affect HT (177). Previous work has also demonstrated that different doses of tirofiban could have different effects on HT (163). Third, measurement bias, especially for biomarkers, is likely to have been a factor. A previous review reported that the definition of hyperglycaemia varied from study to study, and the measurement methods used included both random and fasting serum levels (178). Fourth, as previously mentioned, four different kinds of sICH criteria were used across the included studies, which is likely to have caused the rates of sICH identified to vary significantly. Lastly, studies went to different lengths to adjust for confounders in their multivariate analyses, or made no adjustments at all.
Finally, one of our two assessments for publication bias (NIHSS score predicting sICH after IVT) showed significant evidence of small study bias, possibly because we did not include abstracts or search the gray literature. As a result, some negative studies may have been omitted. However, the asymmetry of the funnel plots can also be caused by between-study heterogeneity (179), and heterogeneity assessment showed that the result of NIHSS score predicting sICH after IVT had moderate to high heterogeneity (I2 = 54.5%).
4.5. Strengths and limitations
This study is the first to compare risk factors for HT following different treatment types (IVT vs. EVT), an approach which has the potential to guide patient selection and clinical decision-making. It is also the first study to systematically review risk factors for sICH after IVT and the second to systematically review risk factors of HT after EVT (148). Although several systematic reviews have examined risk factors for HT after IVT (165, 180, 181), they did not differentiate “any ICH” from sICH. Several studies were also limited by the use of geographically restricted (often Chinese) patient groups. Compared to any ICH, sICH is more likely to predict a poor prognosis, making any study that identifies predictors of sICH of particular clinical relevance.
Our study has several limitations. First, we were unable to conduct a meta-analysis incorporating all reported risk factors because many were only reported in single studies. We also did not analyze risk factors for HT based on radiological criteria (hemorrhagic infarction and parenchymal hematoma) due to limited studies reported relevant information in our included studies. However, a systematic review (182) investigating predictors for different radiological degrees of HT had similar findings with this study. Second, there were large disparities in HT rates (6.45 to 49.55% in any ICH), indicating substantial differences of methodology across different incorporated studies. This possibly renders the meta-analysis results were very unstable and questions whether the pooled studies were homogenous. Although we performed heterogeneity assessment and sensitivity analysis to examine the robustness of the meta-analysis results, the meta-analysis results still need to be interpreted carefully. Third, we did not account for measures made to minimize the risk and extent of HT such as tight blood pressure and glucose control post procedure. Fourth, we did not differentiate treatment type of thrombectomy only and bridging therapy when analyzing the combined HT rates and predictors for HT. Fifth, we did not perform subgroup analysis by removing studies with a high risk of bias. However, results of sensitivity analysis demonstrated the general robustness of the meta-analysis findings and identified specific finding that needed to be interpreted with caution. Sixth, we only searched two electronic databases for the literature search. Nevertheless, initial search results returned nearly six thousand non-duplicated studies and additional manual searching was done to mitigate this potential risk of bias. Lastly, we did not search the gray (unpublished) literature in order to mitigate the risk of publication bias.
4.6. Implications for clinical practice
When treating patients with EVT, neuro-interventionalists should consider the impact of multiple retrieval attempts/device passes and be mindful of their choice of thrombectomy device, as both were found to be predictors of any ICH in this study. In addition, patients with atrial fibrillation, a higher NIHSS score, higher age, or a higher serum glucose level should be considered in the highest risk category for HT in the hours and stays after hyperacute management, regardless of treatment.
4.7. Recommendations for future research
EVT studies are generally more recent than IVT studies (respectively 64.2 and 20.9% were published after 2020). EVT studies are also fewer in number (N = 53 vs. N = 67) and are generally smaller in size (median sample size 305 compared to 488). Meanwhile 27 studies proposed imaging-based predictors for HT, with 20 published after 2018. Improved imaging technology in recent years, particularly the advent of CT perfusion, have shown great potential to enhance patient selection by more accurately characterizing infarct core and penumbra. For example, CT perfusion-based predictors including those measuring infarct core volume (19, 53, 183, 184) and blood-brain-barrier permeability (6, 142, 185–188) haven been reported in previous work, and novel CT perfusion-based parameters such as net water uptake (189) continue to emerge. These techniques have largely emerged in tandem with EVT and indeed have enabled its application in the extended therapeutic window, meaning that the majority of published randomized trial data charactering HT has EVT as a focus. Conversely, data for IVT is in the main derived from trials using CT/CTA [although CTP-directed thrombolysis is an emerging evidence-based treatment approach (190)]. Furthermore, use of Tenecteplase, a newer thrombolytic agent with improved ease of use and a potentially more favorable safety profile, has been less widely studied. Core areas for future studies therefore include (a) novel imaging predictors of HT (particularly those using CT perfusion, given its use in the hyperacute setting) and (b) HT rates/characteristics after the administration of Tenecteplase (with and without EVT).
Both the use of multiple criteria for sICH and substantial variation in the timing of follow-up imaging introduced significant heterogeneity into the meta-analysis. Future studies should be harmonized to incorporate the use of SITS-MOST criteria to characterize HT in scans not performed more than 48 h after hyperacute therapy. These two simple steps would ameliorate much of the variability we found.
5. Conclusion
Hemorrhagic transformation is one of the most devastating complications of reperfusion therapy for patients with acute ischaemic stroke. This meta-analysis identified several predictors for HT, including atrial fibrillation, a higher NIHSS score, higher age, a higher serum glucose level number of thrombectomy passes, and lower ASPECTS. Key predictors for HT in the published literature, identified here, will form the basis for future studies. However, given the large disparities and heterogeneity across the included studies, the meta-analysis results need to be interpreted with caution, and studies based on larger and multi-center data sets should be prioritized to confirm the results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JS and CL screened abstracts and titles of potentially relevant studies, screened the full-text papers, and extracted data and assessed the quality independently. JS performed all statistical analysis, drafted the manuscript, and made critical revisions to the manuscript. LC contributed to the design of the study and made critical revisions to the manuscript. MP and AB conceived the study and made critical revisions to the manuscript. LL, CB, and XL critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
QY was employed by Apollo Medical Imaging Technology Pty Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1079205/full#supplementary-material
Supplementary Figure 1. Risk of bias QUIPS summary.
Supplementary Figure 2. Risk of bias QUIPS traffic light.
Supplementary Figure 3. The proportions of sICH definitions in included studies.
References
1. Global R. Country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
2. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American heart association/American stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2015) 46:3020–35. doi: 10.1161/STR.0000000000000074
3. Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, et al. Hemorrhagic transformation in acute ischemic stroke: the mast-e study. Stroke. (1999) 30:1326–32. doi: 10.1161/01.STR.30.7.1326
4. Hassan AE, Kotta H, Shariff U, Preston L, Tekle W, Qureshi A. There Is No Association between the number of stent retriever passes and the incidence of hemorrhagic transformation for patients undergoing mechanical thrombectomy. Front Neurol. (2019) 10:818. doi: 10.3389/fneur.2019.00818
5. Zhang X, Xie Y, Wang H, Yang D, Jiang T, Yuan K, et al. Symptomatic intracranial hemorrhage after mechanical thrombectomy in Chinese ischemic stroke patients: the Asian Score. Stroke. (2020) 3:2690–6. doi: 10.1161/STROKEAHA.120.030173
6. Yu X, Pan J, Zhao X, Hou Q, Liu B. Predicting hemorrhagic transformation after thrombectomy in acute ischemic stroke: a multimodal score of the regional pial collateral. Neuroradiology. (2022) 64:493–502. doi: 10.1007/s00234-021-02795-8
7. Tissue Plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
8. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr., American Heart Association Stroke C. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American heart association. Am Stroke Assoc Stroke. (2009) 40:2945–8. doi: 10.1161/STROKEAHA.109.192535
9. Jauch EC, Saver JL, Adams HP Jr., Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association. Am Stroke Assoc Stroke. (2013) 44:870–947. doi: 10.1161/STR.0b013e318284056a
10. Berkhemer OA, Fransen PSS, Beumer D, Van Den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
11. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 h after symptom onset in ischemic stroke. N Eng J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
12. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Eng J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
13. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
14. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European stroke organisation (Eso)—European society for minimally invasive neurological therapy (Esmint) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by stroke alliance for Europe (safe). Eur Stroke J. (2019) 4:6–12. doi: 10.1177/2396987319832140
15. Mokin M, Kass-Hout T, Kass-Hout O, Dumont TM, Kan P, Snyder KV, et al. Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: a systematic review of clinical outcomes. Stroke. (2012) 43:2362–8. doi: 10.1161/STROKEAHA.112.655621
16. Bracco S, Zanoni M, Casseri T, Castellano D, Cioni S, Vallone IM, et al. Endovascular treatment of acute ischemic stroke due to tandem lesions of the anterior cerebral circulation: a multicentric italian observational study. Radiol Med. (2021) 126:804–17. doi: 10.1007/s11547-020-01331-7
17. Constant Dit Beaufils P, Labreuche J, Fahed R, Piotin M, Blanc R, Redjem H, et al. Prognosis and risk factors associated with asymptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke: a prospective multicenter cohort study. Eur J Neurol. (2021) 28:229–37. doi: 10.1111/ene.14539
18. Enomoto Y, Yoshimura S, Egashira Y, Yamagami H, Sakai N. The risk of intracranial hemorrhage in japanese patients with acute large vessel occlusion; subanalysis of the rescue-Japan registry. J Stroke Cerebrovasc Dis. (2016) 25:1076–80. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.022
19. Neuberger U, Kickingereder P, Schönenberger S, Schieber S, Ringleb PA, Bendszus M, et al. Risk factors of intracranial hemorrhage after mechanical thrombectomy of anterior circulation. Ischemic Stroke. (2019) 3:461-9. doi: 10.1007/s00234-019-02180-6
20. Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the charms checklist. PLoS Med. (2014) 11:e1001744. doi: 10.1371/journal.pmed.1001744
21. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
22. Khan S. Meta-Analysis of Odds Ratio. Meta-Analysis: Methods for Health and Experimental Studies. Singapore: Springer Singapore (2020). p. 87–118. doi: 10.1007/978-981-15-5032-4_5
23. van den Berg T, Heymans MW, Leone SS, Vergouw D, Hayden JA, Verhagen AP, et al. Overview of data-synthesis in systematic reviews of studies on outcome prediction models. BMC Med Res Methodol. (2013) 13:1–10. doi: 10.1186/1471-2288-13-42
24. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions.: John Wiley & Sons (2019). doi: 10.1002/9781119536604
25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
27. Jackson D. Confidence intervals for the between-study variance in random effects meta-analysis using generalised cochran heterogeneity statistics. Res Synth Methods. (2013) 4:220–9. doi: 10.1002/jrsm.1081
28. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. (2016) 6:e010247. doi: 10.1136/bmjopen-2015-010247
29. Agrawal V, Rai B, Fellows J, McCullough PA. In-hospital outcomes with thrombolytic therapy in patients with renal dysfunction presenting with acute ischaemic stroke. Nephrol Dial Transplantat. (2010) 25:1150–7. doi: 10.1093/ndt/gfp619
30. Ahmed N, Davalos A, Eriksson N, Ford GA, Glahn J, Hennerici M, et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the safe implementation of treatments in stroke international stroke thrombolysis register (Sits-Istr). Arch Neurol. (2010) 67:1123–30. doi: 10.1001/archneurol.2010.210
31. Ahmed N, Kellert L, Lees KR, Mikulik R, Tatlisumak T, Toni D. Results of intravenous thrombolysis within 45 to 6 hours and updated results within 3 to 45 hours of onset of acute ischemic stroke recorded in the safe implementation of treatment in stroke international stroke thrombolysis register (Sits-Istr): an observational study. JAMA Neurol. (2013) 70:837–44. doi: 10.1001/jamaneurol.2013.406
32. Ahmed N, Lees KR, Ringleb PA, Bladin C, Collas D, Toni D, et al. Outcome after stroke thrombolysis in patients >80 years treated within 3 h vs. >3–45 h. Neurology. (2017) 89:1561–8. doi: 10.1212/WNL.0000000000004499
33. Ahmed N, Mazya M, Nunes AP, Moreira T, Ollikainen JP, Escudero-Martinez I, et al. Safety and outcomes of thrombectomy in ischemic stroke with vs. without intravenous thrombolysis. Neurology. (2021) 04:12327. doi: 10.1212/WNL.0000000000012327
34. de Lecinana MA, Fuentes B, Masjuan J, Simal P, Diaz-Otero F, Reig G, et al. Thrombolytic Therapy for Acute Ischemic Stroke after Recent Transient Ischemic Attack. Int J Stroke. (2012) 7:213–8. doi: 10.1111/j.1747-4949.2011.00690.x
35. Altersberger VL, Kellert L, Al Sultan AS, Martinez-Majander N, Hametner C, Eskandari A, et al. Effect of haemoglobin levels on outcome in intravenous thrombolysis-treated stroke patients. Eur Stroke J. (2020) 5:138–47. doi: 10.1177/2396987319889468
36. Anadani M, Lapergue B, Blanc R, Kyheng M, Labreuche J, Machaa MB, et al. Admission blood pressure and outcome of endovascular therapy: secondary analysis of aster trial. J Stroke Cerebrovasc Dis. (2020) 29:105347. doi: 10.1016/j.jstrokecerebrovasdis.2020.105347
37. Anadani M, Spiotta A, Alawieh A, Turjman F, Piotin M, Steglich-Arnholm H, et al. Effect of extracranial lesion severity on outcome of endovascular thrombectomy in patients with anterior circulation tandem occlusion: analysis of the titan registry. J Neurointerv Surg. (2019) 11:970–4. doi: 10.1136/neurintsurg-2018-014629
38. Anadani M, Spiotta AM, Alawieh A, Turjman F, Piotin M, Haussen DC, et al. Emergent carotid stenting plus thrombectomy after thrombolysis in tandem strokes: analysis of the titan registry. Stroke. (2019) 50:2250–2. doi: 10.1161/STROKEAHA.118.024733
39. Aoki S, Hosomi N, Sueda Y, Kono T, Takamatsu K, Ohyama H, et al. Multicenter study of intravenous recombinant tissue plasminogen activator infusion around Hiroshima, Japan: the Hiroshima acute stroke retrospective and prospective registry study. J Stroke Cerebrovasc Dis. (2015) 24:2747–53. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.005
40. Ariës MJ, Uyttenboogaart M, Vroomen PC, De Keyser J, Luijckx GJ. Tpa treatment for acute ischaemic stroke in patients with leukoaraiosis. Eur J Neurol. (2010) 17:866–70. doi: 10.1111/j.1468-1331.2010.02963.x
41. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. (2011) 42:2235–9. doi: 10.1161/STROKEAHA.110.604603
42. Benali F, Hinsenveld WH, van der Leij C, Roozenbeek B, van de Graaf RA, Staals J, et al. Effect of heparinized flush concentration on safety and efficacy during endovascular thrombectomy for acute ischemic stroke: results from the Mr clean registry. Cardiovasc Intervent Radiol. (2021) 44:750–5. doi: 10.1007/s00270-020-02726-9
43. Branscheidt M, Schneider J, Michel P, Eskioglou E, Kaegi G, Stark R, et al. No impact of body mass index on outcome in stroke patients treated with Iv thrombolysis Bmi and Iv thrombolysis outcome. PLoS ONE. (2016) 11:4413. doi: 10.1371/journal.pone.0164413
44. Cao R, Ye G, Wang R, Xu L, Jiang Y, Wang G, et al. Collateral vessels on 4d Cta as a predictor of hemorrhage transformation after endovascular treatments in patients with acute ischemic stroke: a single-center study. Front Neurol. (2020) 11:60. doi: 10.3389/fneur.2020.00060
45. Cappellari M, Pracucci G, Forlivesi S, Saia V, Limbucci N, Nencini P, et al. Direct thrombectomy for stroke in the presence of absolute exclusion criteria for thrombolysis. J Neurol. (2020) 267:3731–40. doi: 10.1007/s00415-020-10098-w
46. Cappellari M, Saia V, Pracucci G, Sallustio F, Gandini R, Nappini S, et al. Functional and radiological outcomes after bridging therapy versus direct thrombectomy in stroke patients with unknown onset: bridging therapy vs. direct thrombectomy in unknown onset stroke patients with 10-point aspects. Eur J Neurol. (2021) 28:209–19. doi: 10.1111/ene.14529
47. Chalumeau V, Blanc R, Redjem H, Ciccio G, Smajda S, Desilles JP, et al. Anterior cerebral artery embolism during thrombectomy increases disability and mortality. J Neurointerv Surg. (2018) 10:1057–62. doi: 10.1136/neurintsurg-2018-013793
48. Chang A, Llinas EJ, Chen K, Llinas RH, Marsh EB. Shorter intensive care unit stays? The majority of post-intravenous Tpa (tissue-type plasminogen activator) symptomatic hemorrhages occur within 12 h of treatment. Stroke. (2018) 49:1521–4. doi: 10.1161/STROKEAHA.118.021398
49. Chao TH, Lin TC, Shieh Y, Chang TY, Hung KL, Liu CH, et al. Intracerebral hemorrhage after thrombolytic therapy in acute ischemic stroke patients with renal dysfunction. Eur Neurol. (2013) 70:316–21. doi: 10.1159/000353296
50. Chen S, Lu X, Zhang W, Han Z, Yang W, Huang X, et al. Does prior antiplatelet treatment increase the risk of hemorrhagic transformation and unfavorable outcome on day 90 after intravenous thrombolysis in acute ischemic stroke patients? J Stroke Cerebrovasc Dis. (2016) 25:1366–70. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.038
51. Chen Z, Sun Y, Zhang Y, He Y, Chen H, Su Y. Low Tsh level predicts a poor clinical outcome in patients with anterior circulation ischemic stroke after endovascular thrombectomy. Neurol Sci. (2020) 41:1821–8. doi: 10.1007/s10072-020-04281-0
52. Cheng Z, Huang X, Muse FM, Xia L, Zhan Z, Lin X, et al. Low serum magnesium levels are associated with hemorrhagic transformation after thrombolysis in acute ischemic stroke. Front Neurol. (2020) 11:962. doi: 10.3389/fneur.2020.00962
53. Cheripelli BK, Huang X, Macisaac R, Muir KW. Interaction of recanalization, intracerebral hemorrhage, and cerebral edema after intravenous thrombolysis. Stroke. (2016) 47:1761–7. doi: 10.1161/STROKEAHA.116.013142
54. Cocho D, Borrell M, Marti-Fabregas J, Montaner J, Castellanos M, Bravo Y, et al. Pretreatment hemostatic markers of symptomatic intracerebral hemorrhage in patients treated with tissue plasminogen activator. Stroke. (2006) 37:996–9. doi: 10.1161/01.STR.0000206461.71624.50
55. Cooray C, Karlinski M, Kobayashi A, Ringleb P, Kõrv J, Macleod MJ, et al. Safety and early outcomes after intravenous thrombolysis in acute ischemic stroke patients with prestroke disability. Int J Stroke. (2021) 16:710–8. doi: 10.1177/1747493020954605
56. Coutinho JM, Liebeskind DS, Slater LA, Nogueira RG, Clark W, Davalos A, et al. Combined intravenous thrombolysis and thrombectomy vs. thrombectomy alone for acute ischemic stroke: a pooled analysis of the swift and star studies. JAMA Neurol. (2017) 74:268–74. doi: 10.1001/jamaneurol.2016.5374
57. Couture M, Marnat G, Griffier R, Gariel F, Olindo S, Renou P, et al. Antiplatelet therapy increases symptomatic ich risk after thrombolysis and thrombectomy. Acta Neurol Scand. (2021). doi: 10.1111/ane.13468
58. Cucchiara B, Kasner SE, Tanne D, Levine SR, Demchuk A, Messe SR, et al. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the stroke-acute ischemic nxy treatment (Saint) I and Saint Ii trials. Stroke. (2009) 40:3067–72. doi: 10.1161/STROKEAHA.109.554386
59. Curtze S, Haapaniemi E, Melkas S, Mustanoja S, Putaala J, Sairanen T, et al. White matter lesions double the risk of post-thrombolytic intracerebral hemorrhage. Stroke. (2015) 46:2149–55. doi: 10.1161/STROKEAHA.115.009318
60. Desilles JP, Rouchaud A, Labreuche J, Meseguer E, Laissy JP, Serfaty JM, et al. Blood-brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology. (2013) 80:844–51. doi: 10.1212/WNL.0b013e31828406de
61. Dharmasaroja PA, Muengtaweepongsa S, Pattaraarchachai J, Dharmasaroja P. Intracerebral hemorrhage following intravenous thrombolysis in thai patients with acute ischemic stroke. J Clin Neurosci. (2012) 19:799–803. doi: 10.1016/j.jocn.2011.08.035
62. Diedler J, Ahmed N, Glahn J, Grond M, Lorenzano S, Brozman M, et al. Is the maximum dose of 90 mg alteplase sufficient for patients with ischemic stroke weighing >100 Kg? Stroke. (2011) 42:1615–20. doi: 10.1161/STROKEAHA.110.603514
63. Dorado L, Castano C, Millan M, Aleu A, De La Ossa NP, Gomis M, et al. Hemorrhagic risk of emergent endovascular treatment plus stenting in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:1326–31. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.006
64. Dorado L, Millan M, De La Ossa NP, Guerrero C, Gomis M, Lopez-Cancio E, et al. Influence of antiplatelet pre-treatment on the risk of intracranial haemorrhage in acute ischaemic stroke after intravenous thrombolysis. Eur J Neurol. (2010) 17:301–6. doi: 10.1111/j.1468-1331.2009.02843.x
65. Du M, Li S, Huang X, Zhang S, Bai Y, Yan B, et al. Intravenous thrombolysis before thrombectomy may increase the incidence of intracranial hemorrhage intreating carotid T occlusion. J Stroke Cerebrovasc Dis. (2021) 30:5473. doi: 10.1016/j.jstrokecerebrovasdis.2020.105473
66. Ehrlich ME, Liang L, Xu H, Kosinski AS, Hernandez AF, Schwamm LH, et al. Intravenous tissue-type plasminogen activator in acute ischemic stroke patients with history of stroke plus diabetes mellitus. Stroke. (2019) 50:1497–503. doi: 10.1161/STROKEAHA.118.024172
67. Engelter ST, Soinne L, Ringleb P, Sarikaya H, Bordet R, Berrouschot J, et al. Iv thrombolysis and statins. Neurology. (2011) 77:888–95. doi: 10.1212/WNL.0b013e31822c9135
68. Fernandez Menendez S, Murias Quintana E, Vega Valdes P, Morales Deza E, Lopez-Cancio E, Benavente Fernandez L, et al. Efficacy and safety of endovascular treatment in acute tandem carotid occlusions: analysis of a single-center cohort. Cerebrovasc Dis Extra. (2020) 10:50–8. doi: 10.1159/000507919
69. Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 min. Circulation. (2011) 123:750–8. doi: 10.1161/CIRCULATIONAHA.110.974675
70. Ford GA, Ahmed N, Azevedo E, Grond M, Larrue V, Lindsberg PJ, et al. Intravenous alteplase for stroke in those older than 80 years old. Stroke (2010) 41:2568–74. doi: 10.1161/STROKEAHA.110.581884
71. Fuentes B, Martínez-Sánchez P, Alonso de. Leciñana M, Simal P, Reig G, Díaz-Otero F, et al. Diabetes and previous stroke: hazards for intravenous thrombolysis? Eur J Neurol. (2012) 19:587–93. doi: 10.1111/j.1468-1331.2011.03576.x
72. Goyal N, Tsivgoulis G, Chang JJ, Malhotra K, Pandhi A, Ishfaq MF, et al. Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. (2018) 49:1985–7. doi: 10.1161/STROKEAHA.118.021477
73. Guo Y, Yan S, Zhang S, Zhang X, Chen Q, Liu K, et al. Lower serum calcium level is associated with hemorrhagic transformation after thrombolysis. Stroke. (2015) 46:1359–61. doi: 10.1161/STROKEAHA.115.008992
74. Hebert S, Clavel P, Maier B, Mizutani K, Delvoye F, Lapergue B, et al. Benefits and safety of periprocedural heparin during thrombectomy in patients contra-indicated for alteplase. J Stroke Cerebrovasc Dis. (2020) 29:5052. doi: 10.1016/j.jstrokecerebrovasdis.2020.105052
75. Hsieh CY, Lin HJ, Sung SF, Hsieh HC, Lai EC, Chen CH. Is renal dysfunction associated with adverse stroke outcome after thrombolytic therapy? Cerebrovasc Dis. (2014) 37:51–6. doi: 10.1159/000356348
76. Huang Q, Gu M, Zhou J, Jiang T, Shi H, Chen X, et al. Endovascular treatment of acute ischemic stroke due to anterior circulation large vessel occlusion beyond 6 h: a real-world study in China. BMC Neurol. (2021) 21:22. doi: 10.1186/s12883-021-02122-x
77. Huang X, Cai Q, Xiao L, Gu M, Liu Y, Zhou Z, et al. Influence of procedure time on outcome and hemorrhagic transformation in stroke patients undergoing thrombectomy. J Neurol. (2019) 266:2560–70. doi: 10.1007/s00415-019-09451-5
78. Huo X, Raynald A, Jing J, Wang A, Mo D, Gao F, et al. Safety and efficacy of oral antiplatelet for patients who had acute ischaemic stroke undergoing endovascular therapy. Stroke Vasc Neurol. (2021) 6:230–7. doi: 10.1136/svn-2020-000466
79. Jang SH, Sohn SI, Park H, Lee SJ, Kim YW, Hong JM, et al. The safety of intra-arterial tirofiban during endovascular therapy after intravenous thrombolysis. Am J Neuroradiol. (2021) 42:1633–7. doi: 10.3174/ajnr.A7203
80. Jiang S, Fei A, Peng Y, Zhang J, Lu YR, Wang HR, et al. Predictors of outcome and hemorrhage in patients undergoing endovascular therapy with solitaire stent for acute ischemic stroke. PLoS ONE. (2015) 10:4452. doi: 10.1371/journal.pone.0144452
81. Jian Y. zhao L, Li T, Zhang L, Sun M, Dang M, et al. Bilirubin: a novel predictor of hemorrhagic transformation and symptomatic intracranial hemorrhage after mechanical thrombectomy. Neurol Sci. (2020) 41:903–9. doi: 10.1007/s10072-019-04182-x
82. Juceviciute N, Mikuzis P, Balnyte R. Absolute blood eosinophil count could be a potential biomarker for predicting haemorrhagic transformation after intravenous thrombolysis for acute ischaemic stroke. BMC Neurol. (2019) 19:6. doi: 10.1186/s12883-019-1359-6
83. Koga M, Shiokawa Y, Nakagawara J, Furui E, Kimura K, Yamagami H, et al. Low-dose intravenous recombinant tissue-type plasminogen activator therapy for patients with stroke outside european indications: stroke acute management with urgent risk-factor assessment and improvement (Samurai) Rtpa registry. Stroke J Cereb Circ. (2011) 29:1176. doi: 10.1161/STROKEAHA.111.631176
84. Kuo KH, Chang FC, Lai YJ, Pan YJ. Hyperdense artery sign, clot characteristics, and response to intravenous thrombolysis in han chinese people with acute large arterial infarction. J Stroke Cerebrovasc Dis. (2016) 25:695–701. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.031
85. Kurmann R, Engelter ST, Michel P, Luft AR, Wegener S, Branscheidt M, et al. Impact of smoking on clinical outcome and recanalization after intravenous thrombolysis for stroke: multicenter cohort study. Stroke. (2018) 49:1170–5. doi: 10.1161/STROKEAHA.117.017976
86. Lee C, Na JU, Lee JH, Han SK, Choi PC, Lee YH, et al. Characteristics of blood tests in patients with acute cerebral infarction who developed symptomatic intracranial hemorrhage after intravenous administration of recombinant tissue plasminogen activator. Clin Exp Emerg Med. (2019) 6:160–8. doi: 10.15441/ceem.18.056
87. Lee JI, Gliem M, Gerdes G, Turowski B, Kaschner M, Kraus B, et al. Safety of bridging antiplatelet therapy with the gpiib-iiia inhibitor tirofiban after emergency stenting in stroke. PLoS ONE. (2017) 12:218. doi: 10.1371/journal.pone.0190218
88. Lee YB, Yoon W, Lee YY, Kim SK, Baek BH, Kim JT, et al. Predictors and impact of hemorrhagic transformations after endovascular thrombectomy in patients with acute large vessel occlusions. J Neurointervent Surg. (2019) 11:469–73. doi: 10.1136/neurintsurg-2018-014080
89. Lin TC, Lin YK, Chen CI, Chan L, Chi NF, Yuan RY, et al. Serum lipid level is not associated with symptomatic intracerebral hemorrhage after intravenous thrombolysis for acute ischemic stroke. PeerJ. (2018) 2018:6021. doi: 10.7717/peerj.6021
90. Lin X, Cao Y, Yan J, Zhang Z, Ye Z, Huang X, et al. Risk factors for early intracerebral hemorrhage after intravenous thrombolysis with alteplase. J Atheroscler Thromb. (2020) 27:1176–82. doi: 10.5551/jat.49783
91. Liu M, Pan Y, Zhou L, Wang Y. Predictors of post-thrombolysis symptomatic intracranial hemorrhage in Chinese patients with acute ischemic stroke. PLoS ONE. (2017) 12:464. doi: 10.1371/journal.pone.0184646
92. Luo Y, Chen J, Yan XL, Jin H, Sun X, Guo ZN, et al. Association of non-traditional lipid parameters with hemorrhagic transformation and clinical outcome after thrombolysis in ischemic stroke patients. Curr Neurovasc Res. (2020) 17:736–44. doi: 10.2174/1567202617999210101223129
93. Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. (2015) 85:1408–16. doi: 10.1212/WNL.0000000000002029
94. Maros ME, Brekenfeld C, Broocks G, Leischner H, McDonough R, Deb-Chatterji M, et al. Number of retrieval attempts rather than procedure time is associated with risk of symptomatic intracranial hemorrhage. Stroke. (2021) 3:1580–8. doi: 10.1161/STROKEAHA.120.031242
95. Mori E, Minematsu K, Nakagawara J, Yamaguchi T. Factors predicting outcome in stroke patients treated with 06 Mg/Kg alteplase: evidence from the Japan alteplase clinical trial (J-Act). J Stroke Cerebrovasc Dis. (2011) 20:517–22. doi: 10.1016/j.jstrokecerebrovasdis.2010.04.001
96. Nogueira RG, Gupta R, Jovin TG, Levy EI, Liebeskind DS, Zaidat OO, et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: a multicenter retrospective analysis of 1,122 patients. J Neurointerv Surg. (2015) 7:16–21. doi: 10.1136/neurintsurg-2013-010743
97. Nogueira RG, Mohammaden MH, Haussen DC, Budzik RF, Gupta R, Krajina A, et al. Endovascular therapy in the distal neurovascular territory: results of a large prospective registry. J Neurointerv Surg. (2020) 3:6851. doi: 10.1136/neurintsurg-2020-016851
98. Pan X, Zhou F, Shen R, Zhu Y, Arima H, Yang J, et al. Influence of renal function on stroke outcome after mechanical thrombectomy: a prospective cohort study. BMC Neurol. (2020) 20:5. doi: 10.1186/s12883-020-01720-5
99. Papanagiotou P, Haussen DC, Turjman F, Labreuche J, Piotin M, Kastrup A, et al. Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC. (2018) 11:1290–9. doi: 10.1016/j.jcin.2018.05.036
100. Pikija S, Sztriha LK, Killer-Oberpfalzer M, Weymayr F, Hecker C, Ramesmayer C, et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflamm. (2018) 15:2. doi: 10.1186/s12974-018-1359-2
101. Pundik S, McWilliams-Dunnigan L, Blackham KL, Kirchner HL, Sundararajan S, Sunshine JL, et al. Older age does not increase risk of hemorrhagic complications after intravenous and/or intra-arterial thrombolysis for acute stroke. J Stroke Cerebrovasc Dis. (2008) 17:266–72. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.003
102. Qiu M, Fang M, Liu X. Low free triiodothyronine levels predict symptomatic intracranial hemorrhage and worse short-term outcome of thrombolysis in patients with acute ischemia stroke. Medicine. (2017) 96:8539. doi: 10.1097/MD.0000000000008539
103. Ramos-Araque ME, Chavarria-Miranda A, Gomez-Vicente B, Lopez-Cancio Martinez E, Castanon Apilanez M, Castellanos M, et al. Oral anticoagulation and risk of symptomatic hemorrhagic transformation in stroke patients treated with mechanical thrombectomy: data from the nordictus registry. Front Neurol. (2020) 11:4251. doi: 10.3389/fneur.2020.594251
104. Robinson TG, Wang X, Arima H, Bath PM, Billot L, Broderick JP, et al. Low vs. standard-dose alteplase in patients on prior antiplatelet therapy: the enchanted trial (enhanced control of hypertension and thrombolysis stroke study). Stroke. (2017) 48:1877–83. doi: 10.1161/STROKEAHA.116.016274
105. Rocco A, Heuschmann PU, Schellinger PD, Kohrmann M, Diedler J, Sykora M, et al. Glycosylated hemoglobin A1 predicts risk for symptomatic hemorrhage after thrombolysis for acute stroke. Stroke. (2013) 44:2134–8. doi: 10.1161/STROKEAHA.111.675918
106. Sadeghi-Hokmabadi E, Baş DF, Farhoudi M, Taheraghdam A, Savadi Oskouei D, Yazdchi M, et al. Renal dysfunction is an independent risk factor for poor outcome in acute ischemic stroke patients treated with intravenous thrombolysis: a new cutoff value. Stroke Res Treat. (2017) 2017:2371956. doi: 10.1155/2017/2371956
107. Sarikaya H, Arnold M, Engelter ST, Lyrer PA, Mattle HP, Michel P, et al. Outcome of intravenous thrombolysis in stroke patients weighing over 100 kg. Cerebrovasc Dis. (2011) 32:201–6. doi: 10.1159/000328813
108. Scott PA, Frederiksen SM, Kalbfleisch JD, Xu Z, Meurer WJ, Caveney AF, et al. Safety of intravenous thrombolytic use in four emergency departments without acute stroke teams. Acad Emerg Med. (2010) 17:1062–71. doi: 10.1111/j.1553-2712.2010.00868.x
109. Seners P, Perrin C, Lapergue B, Henon H, Debiais S, Sablot D, et al. Bridging therapy or Iv thrombolysis in minor stroke with large vessel occlusion. Annals Neurol. (2020) 88:160–9. doi: 10.1002/ana.25756
110. Sobolewski P, Brola W, Stoinski J, Szczuchniak W, Fudala M, Hatalska-Zerebiec R, et al. Intravenous thrombolysis in patients aged more than 80 years in the three rural hospitals in southeast poland: an observational study. Geriat Gerontol Int. (2014) 14:689–94. doi: 10.1111/ggi.12135
111. Sorensen SB, Barazangi N, Chen C, Wong C, Grosvenor D, Rose J, et al. Generalized safety and efficacy of simplified intravenous thrombolysis treatment (Smart) criteria in acute ischemic stroke: the multi smart study. J Stroke Cerebrovasc Dis. (2016) 25:1110–8. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.016
112. Sun C, Chen X, Huang C, Li X, Shan Y, Zou Y, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol. (2019) 10:1100. doi: 10.3389/fneur.2019.01100
113. Sylaja PN, Cote R, Buchan AM, Hill MD. Thrombolysis in patients older than 80 years with acute ischaemic stroke: canadian alteplase for stroke effectiveness study. J Neurol Neurosurg Psychiatry. (2006) 77:826–9. doi: 10.1136/jnnp.2005.086595
114. Sztriha LK, Manawadu D, Jarosz J, Keep J, Kalra L. Safety and clinical outcome of thrombolysis in ischaemic stroke using a perfusion Ct mismatch between 3 and 6 h. PLoS ONE. (2011) 6:e25796. doi: 10.1371/journal.pone.0025796
115. Tong X, Bauer CT, Jia B, Zhang X, Huo X, Luo G, et al. Current status of aspiration thrombectomy for acute stroke patients in china: data from angel-act registry. Therap Adv Neurol Disord. (2021) 14:7715. doi: 10.1177/17562864211007715
116. Tsivgoulis G, Frey JL, Flaster M, Sharma VK, Lao AY, Hoover SL, et al. Pre-tissue plasminogen activator blood pressure levels and risk of symptomatic intracerebral hemorrhage. Stroke. (2009) 40:3631–4. doi: 10.1161/STROKEAHA.109.564096
117. Tsivgoulis G, Goyal N, Kerro A, Katsanos AH, Krishnan R, Malhotra K, et al. Dual antiplatelet therapy pre-treatment in Iv thrombolysis for acute ischemic stroke. Neurology. (2018) 91:e1067–e76. doi: 10.1212/WNL.0000000000006168
118. Tutuncu S, Ziegler AM, Scheitz JF, Slowinski T, Rocco A, Endres M, et al. Severe renal impairment is associated with symptomatic intracerebral hemorrhage after thrombolysis for ischemic stroke. Stroke. (2013) 44:3217–9. doi: 10.1161/STROKEAHA.113.002859
119. Vaclavik D, Vilionskis A, Jatuzis D, Karlinski MA, Gdovinova Z, Korv J, et al. Clinical outcome of cardioembolic stroke treated by intravenous thrombolysis. Acta Neurol Scand. (2018) 137:347–55. doi: 10.1111/ane.12880
120. Vergouwen MD, Casaubon LK, Swartz RH, Fang J, Stamplecoski M, Kapral MK, et al. Subtherapeutic warfarin is not associated with increased hemorrhage rates in ischemic strokes treated with tissue plasminogen activator. Stroke. (2011) 42:1041–5. doi: 10.1161/STROKEAHA.110.599183
121. Xu X, Li C, Wan T, Gu X, Zhu W, Hao J, et al. Risk factors for hemorrhagic transformation after intravenous thrombolysis in acute cerebral infarction: a retrospective single-center study. World Neurosurg. (2017) 101:155–60. doi: 10.1016/j.wneu.2017.01.091
122. Yang X, Li C, Li J, Hou D, Luo Y, Zhang S, et al. Insulin resistance is significantly related with worse clinical outcomes in non-diabetic acute ischemic stroke patients treated with intravenous thrombolysis. J Stroke Cerebrovasc Dis. (2021) 30:5526. doi: 10.1016/j.jstrokecerebrovasdis.2020.105526
123. Zerna C, Siepmann T, Barlinn K, Kepplinger J, Pallesen LP, Puetz V, et al. Association of time on outcome after intravenous thrombolysis in the elderly in a telestroke network. J Telemed Telecare. (2016) 22:18–24. doi: 10.1177/1357633X15585241
124. Zhong CS, Beharry J, Salazar D, Smith K, Withington S, Campbell BCV, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke. (2021) 5:1087–90. doi: 10.1161/STROKEAHA.120.030859
125. Zhu X, Wang N, Lin H, Zhang P, Chen L, Zhang M, et al. Safety and efficacy of intravenous thrombolytic therapy in patients with acute posterior circulation stroke: a single-center study. J Stroke Cerebrovasc Dis. (2020) 29:4537. doi: 10.1016/j.jstrokecerebrovasdis.2019.104537
126. Zou M, Churilov L, He A, Campbell B, Davis SM, Yan B. Hyperdense middle cerebral artery sign is associated with increased risk of hemorrhagic transformation after intravenous thrombolysis for patients with acute ischaemic stroke. J Clin Neurosci. (2013) 20:984–7. doi: 10.1016/j.jocn.2012.10.013
127. Hassan AE, Ringheanu VM, Preston L, Tekle WG, Qureshi AI. Acute intracranial stenting with mechanical thrombectomy is safe and efficacious in patients diagnosed with underlying intracranial atherosclerotic disease. Intervent Neuroradiol. (2022) 28:419–25. doi: 10.1177/15910199211039403
128. Akbik F, Alawieh A, Dimisko L, Howard BM, Cawley CM, Tong FC, et al. Bridging thrombolysis in atrial fibrillation stroke is associated with increased hemorrhagic complications without improved outcomes. J Neurointerv Surg. (2022) 14:979–84. doi: 10.1136/neurintsurg-2021-017954
129. Cai L, Yu X, Yu J, Xu J, Xu L, Ling C, et al. Can tirofiban improve the outcome of patients with acute ischemic stroke: a propensity score matching analysis. Frontiers in neurology. (2021) 12. doi: 10.3389/fneur.2021.688019
130. Schlemm L, Braemswig TB, Boutitie F, Vynckier J, Jensen M, Galinovic I, et al. Cerebral microbleeds and treatment effect of intravenous thrombolysis in acute stroke: an analysis of the wake-up randomized clinical trial. Neurology. (2022) 98:e302–e14. doi: 10.1212/WNL.0000000000013055
131. Shen Y, Li D, Tang B, Cao Q, Hou Z, Xu L. Factors Associated with symptomatic intracranial haemorrhage after intravenous thrombolysis in severe white matter lesions: a retrospective analysis. Postgrad Med J. (2021). doi: 10.1136/postgradmedj-2021-140886
132. Lin C, Pan H, Qiao Y, Huang P, Su J, Liu J. Fibrinogen level combined with platelet count for predicting hemorrhagic transformation in acute ischemic stroke patients treated with mechanical thrombectomy. Front Neurol. (2021) 5:1508. doi: 10.3389/fneur.2021.716020
133. Mowla A, Razavi S-M, Lail NS, Mohammadi P, Shirani P, Kavak KS, et al. Hyperdense middle cerebral artery sign and response to combination of mechanical thrombectomy plus intravenous thrombolysis in acute stroke patients. J Neurol Sci. (2021) 429:117618. doi: 10.1016/j.jns.2021.117618
134. Bala F, Bricout N, Nouri N, Cordonnier C, Henon H, Casolla B. Safety and outcomes of endovascular treatment in patients with very severe acute ischemic stroke. J Neurol. (2022) 269:2493–502. doi: 10.1007/s00415-021-10807-z
135. Merlino G, Pez S, Gigli GL, Sponza M, Lorenzut S, Surcinelli A, et al. Stress hyperglycemia in patients with acute ischemic stroke due to large vessel occlusion undergoing mechanical thrombectomy. Front Neurol. (2021) 3:1701. doi: 10.3389/fneur.2021.725002
136. Happi Ngankou E, Gory B, Marnat G, Richard S, Bourcier R, Sibon I, et al. Thrombectomy Complications in Large Vessel Occlusions: Incidence, Predictors, and Clinical Impact in the Etis Registry. Stroke (2021). 52:e764–8. doi: 10.1161/STROKEAHA.121.034865
137. Cheng Z, Zhan Z, Huang X, Xia L, Xu T, Han Z. Troponin elevation on admission along with dynamic changes and their association with hemorrhagic transformation after thrombolysis. Front Aging Neurosci. (2021) 3:684. doi: 10.3389/fnagi.2021.758678
138. Feng X, Ye G, Cao R, Qi P, Lu J, Chen J, et al. Identification of predictors for hemorrhagic transformation in patients with acute ischemic stroke after endovascular therapy using the decision tree model. Clin Interv Aging. (2020) 15:1611–24. doi: 10.2147/CIA.S257931
139. Guo H, Xu W, Zhang X, Zhang S, Dai Z, Li S, et al. A nomogram to predict symptomatic intracranial hemorrhage after intravenous thrombolysis in chinese patients. Neuropsychiatr Dis Treat. (2021) 17:2183–90. doi: 10.2147/NDT.S320574
140. Wu Y, Chen H, Liu X, Cai X, Kong Y, Wang H, et al. A new nomogram for individualized prediction of the probability of hemorrhagic transformation after intravenous thrombolysis for ischemic stroke patients. BMC Neurol. (2020) 20:22. doi: 10.1186/s12883-020-02002-w
141. Zhou Z, Yin X, Niu Q, Liang S, Mu C, Zhang Y. Risk factors and a nomogram for predicting intracranial hemorrhage in stroke patients undergoing thrombolysis. Neuropsychiatr Dis Treat. (2020) 16:1189–97. doi: 10.2147/NDT.S250648
142. Zhang XX, Yao FR, Zhu JH, Chen ZG, Shen YP, Qiao YN, et al. Nomogram to predict haemorrhagic transformation after stroke thrombolysis: a combined brain imaging and clinical study. Clin Radiol. (2022) 77:e92–e8. doi: 10.1016/j.crad.2021.09.017
143. McGuinness LA, Higgins JP. Risk-of-bias visualization (Robvis): an R Package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12:55–61. doi: 10.1002/jrsm.1411
144. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (Sits-Most): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
145. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (Ecass Ii). Second European-Australasian acute stroke study investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
146. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
147. Harrer M, Cuijpers P, Furukawa T, Ebert DD. Dmetar: Companion R Package for the Guide'doing Meta-Analysis in R'. R Package Version 00. (2019) 9000.
148. Hao Z, Yang C, Xiang L, Wu B, Liu M. Risk Factors for intracranial hemorrhage after mechanical thrombectomy: a systematic review and meta-analysis. Expert Rev Neurother. (2019) 19:927–35. doi: 10.1080/14737175.2019.1632191
149. Tsivgoulis G, Katsanos AH, Zand R, Sharma VK, Kohrmann M, Giannopoulos S, et al. Antiplatelet pretreatment and outcomes in intravenous thrombolysis for stroke: a systematic review and meta-analysis. J Neurol. (2017) 264:1227–35. doi: 10.1007/s00415-017-8520-1
150. Seet RCS, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis. (2012) 34:106–14. doi: 10.1159/000339675
151. Monteiro A, Khan S, Waqas M, Dossani RH, Ruggiero N, Siddiqi NM, et al. Mechanical thrombectomy versus intravenous alteplase alone in acute isolated posterior cerebral artery occlusion: a systematic review. J Neurointerv Surg. (2022) 14:564–7. doi: 10.1136/neurintsurg-2021-018017
152. Waqas M, Kuo CC, Dossani RH, Monteiro A, Baig AA, Alkhaldi M, et al. Mechanical thrombectomy vs. intravenous thrombolysis for distal large-vessel occlusion: a systematic review and meta-analysis of observational studies. Neurosurg Focus. (2021) 51:E5. doi: 10.3171/2021.4.FOCUS21139
153. Muir KW, Ford GA, Messow C-M, Ford I, Murray A, Clifton A, et al. Endovascular therapy for acute ischaemic stroke: the pragmatic ischaemic stroke thrombectomy evaluation (Piste) randomised, controlled trial. J Neurol Neurosurg Psychiatry. (2017) 88:38–44. doi: 10.1136/jnnp-2016-314117
154. Smaal JA, de Ridder IR, Heshmatollah A, van Zwam WH, Dippel D, Majoie CB, et al. Effect of atrial fibrillation on endovascular thrombectomy for acute ischemic stroke. A meta-analysis of individual patient data from six randomised trials: results from the hermes collaboration. Eur Stroke J. (2020) 5:245–51. doi: 10.1177/2396987320923447
155. Cappellari M, Pracucci G, Forlivesi S, Saia V, Nappini S, Nencini P, et al. General anesthesia versus conscious sedation and local anesthesia during thrombectomy for acute ischemic stroke. Stroke. (2020) 45:2036–44. doi: 10.1161/STROKEAHA.120.032094
156. Paliwal PR, Ahmad A, Shen L, Yeo LL, Loh PK, Ng KW, et al. Persistence of hyperdense middle cerebral artery sign on follow-up ct scan after intravenous thrombolysis is associated with poor outcome. Cerebrovasc Diseases. (2012) 33:446–52. doi: 10.1159/000336863
157. Kim SK, Baek BH, Lee YY, Yoon W. Clinical implications of Ct hyperdense artery sign in patients with acute middle cerebral artery occlusion in the era of modern mechanical thrombectomy. J Neurol. (2017) 264:2450–6. doi: 10.1007/s00415-017-8655-0
158. Agarwal P, Kumar S, Hariharan S, Eshkar N, Verro P, Cohen B, et al. Hyperdense middle cerebral artery sign: can it be used to select intra-arterial versus intravenous thrombolysis in acute ischemic stroke? Cerebrovasc Dis. (2004) 17:182–90. doi: 10.1159/000075789
159. Grond M, Von Kummer R, Sobesky J, Schmulling S, Rudolf J, Terstegge K, et al. Early X-ray hypoattenuation of brain parenchyma indicates extended critical hypoperfusion in acute stroke. Stroke. (2000) 31:133–9. doi: 10.1161/01.STR.31.1.133
160. Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. (2001) 32:1079–84. doi: 10.1161/01.STR.32.5.1079
161. Abraham P, Pannell JS, Santiago-Dieppa DR, Cheung V, Steinberg J, Wali A, et al. Vessel wall signal enhancement on 3-T Mri in acute stroke patients after stent retriever thrombectomy. Neurosurg Focus. (2017) 42:E20. doi: 10.3171/2017.1.FOCUS16492
162. Yang J, Wu Y, Gao X, Bivard A, Levi CR, Parsons MW, et al. Intraarterial vs. intravenous tirofiban as an adjunct to endovascular thrombectomy for acute ischemic stroke. Stroke. (2020) 51:2925–33. doi: 10.1161/STROKEAHA.120.029994
163. Wu Y, Yin C, Yang J, Jiang L, Parsons MW, Lin L. Endovascular thrombectomy: tirofiban increases bleeding risk in acute stroke patients. Stroke. (2018) 49:2783–5. doi: 10.1161/STROKEAHA.118.022919
164. Zhao W, Xu J, Li S, Liu G, Wu L, Li C, et al. Low-dose tirofiban is associated with reduced in-hospital mortality in cardioembolic stroke patients treated with endovascular thrombectomy. J Neurol Sci. (2021) 427:7539. doi: 10.1016/j.jns.2021.117539
165. Wen L, Zhang S, Wan K, Zhang H, Zhang X. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving intravenous thrombolysis: a meta-analysis. Medicine. (2020) 99:e18995. doi: 10.1097/MD.0000000000018995
166. Montalvo M, Mistry E, Chang AD, Yakhkind A, Dakay K, Azher I, et al. Predicting symptomatic intracranial haemorrhage after mechanical thrombectomy: the tag score. J Neurol Neurosurg Psychiatry. (2019) 90:1370–4. doi: 10.1136/jnnp-2019-321184
167. Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Ann Neurol. (2011) 70:583–90. doi: 10.1002/ana.22538
168. Geng J, Song Y, Mu Z, Xu Q, Shi G, Sun Y, et al. Early use of statin in patients treated with alteplase for acute ischemic stroke. Acta Neurochir Suppl. (2016) 121:269–75. doi: 10.1007/978-3-319-18497-5_47
169. Mowla A, Shah H, Lail NS, Vaughn CB, Shirani P, Sawyer RN. Statins use and outcome of acute ischemic stroke patients after systemic thrombolysis. Cerebrovas Dis. (2020) 49:503–8. doi: 10.1159/000510095
170. Miedema I, Uyttenboogaart M, Koopman K, De Keyser J, Luijckx GJ. statin use and functional outcome after tissue plasminogen activator treatment in acute ischaemic stroke. Cerebrovas Dis. (2010) 29:263–7. doi: 10.1159/000275500
171. Bruning T, Al-Khaled M. Do statins reduce the mortality rate in stroke patients treated with systemic thrombolysis in a 5-year single-center study? Neural Regene Res. (2021) 16:1807–12. doi: 10.4103/1673-5374.306088
172. Switonska M, Piekus-Slomka N, Slomka A, Sokal P, Zekanowska E, Lattanzi S. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in ischemic stroke patients undergoing revascularization. Brain Sci. (2020) 10:1–9. doi: 10.3390/brainsci10110771
173. Gökhan S, Ozhasenekler A, Durgun HM, Akil E, Ustündag M, Orak M. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic Attack. Age. (2013) 67:11.3.
174. Hermann DM, Kleinschnitz C, Gunzer M. Implications of polymorphonuclear neutrophils for ischemic stroke and intracerebral hemorrhage: predictive value, pathophysiological consequences and utility as therapeutic target. J Neuroimmunol. (2018) 321:138–43. doi: 10.1016/j.jneuroim.2018.04.015
175. Puig J, Pedraza S, Demchuk A. Daunis-I-Estadella J, Termes H, Blasco G, et al. Quantification of thrombus hounsfield units on noncontrast ct predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. Ame J Neuroradiol. (2012) 33:90–6. doi: 10.3174/ajnr.A2878
176. Niesten J, van der Schaaf I, Biessels G, van Otterloo A, van Seeters T, Horsch A, et al. Relationship between thrombus attenuation and different stroke subtypes. Neuroradiology. (2013) 55:1071–9. doi: 10.1007/s00234-013-1217-y
177. Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MM, et al. Outcomes of thrombolytic therapy for acute ischemic stroke in chinese patients: the Taiwan thrombolytic therapy for acute ischemic stroke (Ttt-Ais) study. Stroke. (2010) 41:885–90. doi: 10.1161/STROKEAHA.109.575605
178. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
179. Page MJ, Sterne JA, Higgins JP, Egger M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods. (2021) 12:248–59. doi: 10.1002/jrsm.1468
180. Guo Y, Yang Y, Zhou M, He L. Risk factors of haemorrhagic transformation for acute ischaemic stroke in chinese patients receiving intravenous recombinant tissue plasminogen activator: a systematic review and meta-analysis. Stroke and Vascular Neurology. (2018) 3:203–8. doi: 10.1136/svn-2018-000141
181. Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. (2012) 43:2904–9. doi: 10.1161/STROKEAHA.112.665331
182. Honig A, Percy J, Sepehry AA, Gomez AG, Field TS, Benavente OR. hemorrhagic transformation in acute ischemic stroke: a quantitative systematic review. J Clin Med. (2022) 11:1162. doi: 10.3390/jcm11051162
183. Kim BK, Kim B, You SH. Clinical relevance of computed tomography perfusion-estimated infarct volume in acute ischemic stroke patients within the 6-h therapeutic time window. Cerebrovasc Dis. (2022) 51:438–46. doi: 10.1159/000519901
184. Campbell BC, Christensen S, Parsons MW, Churilov L, Desmond PM, Barber PA, et al. Advanced imaging improves prediction of hemorrhage after stroke thrombolysis. Ann Neurol. (2013) 73:510–9. doi: 10.1002/ana.23837
185. Bivard A, Kleinig T, Churilov L, Levi C, Lin L, Cheng X, et al. Permeability measures predict hemorrhagic transformation after ischemic stroke. Ann Neurol. (2020) 88:466–76. doi: 10.1002/ana.25785
186. Yen P, Cobb A, Shankar JJ. Does computed tomography permeability predict hemorrhagic transformation after ischemic stroke? World J Radiol. (2016) 8:594–9. doi: 10.4329/wjr.v8.i6.594
187. Kim T, Koo J, Kim SH, Song IU, Chung SW, Lee KS. Blood-brain barrier permeability assessed by perfusion computed tomography predicts hemorrhagic transformation in acute reperfusion therapy. Neurol Sci. (2018) 39:1579–84. doi: 10.1007/s10072-018-3468-1
188. Hom J, Dankbaar JW, Soares BP, Schneider T, Cheng SC, Bredno J, et al. Blood-brain barrier permeability assessed by perfusion ct predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. (2011) 32:41–8. doi: 10.3174/ajnr.A2244
189. Nawabi J, Elsayed S, Scholz H, Kemmling A, Meyer L, Kniep H, et al. Interaction effect of baseline serum glucose and early ischemic water uptake on the risk of secondary hemorrhage after ischemic stroke. Front Neurol. (2021) 12:193. doi: 10.3389/fneur.2021.690193
Keywords: stroke, risk factor, intracranial hemorrhage, hemorrhagic transformation, reperfusion therapy, intravenous thrombolysis, endovascular thrombectomy
Citation: Sun J, Lam C, Christie L, Blair C, Li X, Werdiger F, Yang Q, Bivard A, Lin L and Parsons M (2023) Risk factors of hemorrhagic transformation in acute ischaemic stroke: A systematic review and meta-analysis. Front. Neurol. 14:1079205. doi: 10.3389/fneur.2023.1079205
Received: 25 October 2022; Accepted: 31 January 2023;
Published: 20 February 2023.
Edited by:
Bin Qiu, Yale University, United StatesReviewed by:
Klearchos Psychogios, Metropolitan Hospital, GreeceSimona Lattanzi, Marche Polytechnic University, Italy
Asaf Honig, University of British Columbia, Canada
Francisco Moniche, Virgen del Rocío University Hospital, Spain
Copyright © 2023 Sun, Lam, Christie, Blair, Li, Werdiger, Yang, Bivard, Lin and Parsons. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longting Lin,  bG9uZ3RpbmcubGluQHVuc3cuZWR1LmF1; Mark Parsons,
bG9uZ3RpbmcubGluQHVuc3cuZWR1LmF1; Mark Parsons,  bWFyay5wYXJzb25zQHVuc3cuZWR1LmF1
bWFyay5wYXJzb25zQHVuc3cuZWR1LmF1
 Jiacheng Sun
Jiacheng Sun Christina Lam3,4
Christina Lam3,4 Lauren Christie
Lauren Christie Christopher Blair
Christopher Blair Freda Werdiger
Freda Werdiger Qing Yang
Qing Yang Andrew Bivard
Andrew Bivard Longting Lin
Longting Lin Mark Parsons
Mark Parsons