- Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China
Background and purpose: Previous studies have stimulated debates on low-dose alteplase administration in acute ischemic stroke (AIS) among the Asian population. We sought to evaluate the safety and efficacy of low-dose alteplase in Chinese patients with AIS using a real-world registry.
Methods: We analyzed data from the Shanghai Stroke Service System. Patients receiving alteplase intravenous thrombolysis within 4.5 hours were included. These patients were divided into the low-dose alteplase group (0.55–0.65 mg/kg) and the standard-dose alteplase group (0.85–0.95 mg/kg). Baseline imbalances were adjusted by using the propensity score matching. The primary outcome was mortality or disability, which was defined as the modified Rankin scale (mRS) score ranging from 2 to 6 at discharge. The secondary outcomes were in-hospital mortality, symptomatic intracranial hemorrhage (sICH) and functional independence (mRS score 0–2).
Results: From January 2019 to December 2020, a total of 1,334 patients were enrolled and 368 (27.6%) were treated with low-dose alteplase. The median age of the patients was 71 years, and 38.8% were female. Our study showed that the low-dose group had significantly higher rates of death or disability (adjusted odds ratio (aOR) = 1.49, 95% confidence interval (CI) [1.12, 1.98]) and less functional independence (aOR = 0.71, 95%CI [0.52, 0.97]) than the standard-dose group. There was no significant difference in sICH or in-hospital mortality between the standard-dose and low-dose alteplase groups.
Conclusions: Low-dose alteplase was related to a poor functional outcome without lowering the risk of sICH, compared with standard-dose alteplase for AIS patients in China.
1. Introduction
Intravenous thrombolysis using alteplase (0.9 mg/kg) for acute ischemic stroke (AIS) within 4.5 hours of onset has been proven effective (1, 2). Despite the solid evidence on the efficacy of alteplase, a higher risk of symptomatic intracranial hemorrhage (sICH) after thrombolysis still cannot be ignored. Hence, some Japanese trials compared two alteplase dosages of low dose (0.6 mg/kg) and standard dose (0.9 mg/kg) in AIS patients and indicated that a low dose of alteplase might be more suitable for Asian people (3, 4). Furthermore, the low-dose alteplase treatment has been strongly recommended for Japanese AIS patients within 4.5 hours by Japan Stroke Society Guidelines (5).
However, some studies in China suggested that the standard dosage might have better clinical outcomes than the low-dose alteplase without increasing the incidence of sICH (6, 7). The ENhanced Control of Hypertension And Thrombolysis strokE study (ENCHANTED) trial, as the only randomized controlled trial (RCT), has reported a lower incidence of sICH in the low-dose alteplase group but not achieved non-inferiority of low-dose to standard-dose alteplase for death or disability at 90 days (8). In our previous pool-data analysis, the low dosage was still considerable based on several regional databases (9).
Due to the high cost of a new RCT and the narrow gap expected to be found between dosages, we tried to investigate whether low-dose alteplase can bring better safety and a comparable functional outcome than standard dosage in Chinese patients with AIS using our real-world database.
2. Materials and methods
2.1. Study population
All data of this study were obtained from the Shanghai Stroke Service System (4S) registry, which was designed as a regional stroke network tracking real-time data on stroke care performance in Shanghai metropolitan area. The protocol of the 4S network has been reported previously and this study was approved by Institutional Review Board in Huashan hospital, with the waiver of consent as no identifying information was collected (10).
At all stroke centers in Shanghai, patients 18 years or older diagnosed with ICD-10 stroke codes (I63, I61, and G45) at discharge were registered in the 4S database. The eligibility criteria included: (1) aged 18 years or older; (2) had a clinical diagnosis of ischemic stroke with ICD-10 codes I63 at discharge; (3) received thrombolysis treatment within 4.5 hours of symptom onset; (4) no definite indication nor contraindication for either dose of alteplase. Patients with incomplete medical records were excluded. Based on the dosage of alteplase they used, the eligible patients were divided into low-dose alteplase group (0.55–0.65 mg/kg) and standard-dose alteplase group (0.85–0.95 mg/kg).
2.2. Data collection
Each stroke center in Shanghai used the electronic medical record with the web-based collection tools to document the stroke care procedures and automatically extract all medical data (10). Data of eligible AIS patients receiving thrombolysis with alteplase were analyzed in our study.
2.3. Outcomes
The primary functional outcome was death or disability, defined as modified Rankin scale (mRS) score 2-6 at discharge. The secondary functional outcome included in-hospital mortality, sICH, functional independence (mRS score 0–2) and distribution of scores on the mRS (ordinal shift analysis). The definition of sICH was Heidelberg Bleeding Classification (11).
2.4. Data analysis
Continuous data were expressed as mean (standard deviation) or median (interquartile range), and categorical data were described as frequency and percentage. Wilcoxon rank-sum test was used for continuous outcomes. All categorical variables were tested by Pearson χ2 test or Fisher's exact test separately.
Propensity score matching (PSM) was used to adjust baseline imbalances. The propensity score was estimated with the multivariable logistic regression model, with the IV-alteplase dose (standard dosage and low dosage) as the dependent variable and all baseline characteristics in Table 1 as covariates. Patients with low-dose therapy were matched 1:4 to those with standard-dose therapy according to the propensity score, with replacement, using the nearest neighbor matching with a 0.2 caliper (propensity score-matched cohort). Bias reduction after PSM was evaluated using standardized mean differences in covariate means. We performed a sensitivity analysis to assess the robustness of the results with a 1:1 matched design.
Logistic regression was used to compare the functional outcome between the standard- and low-dose alteplase groups. When comparing the outcomes, cumulative incidences with a 95% confidence interval (CI) were presented and adjusted by patient features, including age, sex, history of ischemic stroke, hypertension, diabetes, dyslipidemia, atrial fibrillation (AF), myocardial infarction, baseline National Institutes of Health Stroke Scale (NIHSS) score, time from onset to treatment, premorbid modified Rankin scale (mRS) score and TOAST classification. The functional outcomes in subgroups were divided based on demographic variables (sex, age ≤ 70 vs. >70 years), TOAST classification, clinical severity (baseline NIHSS scores ≤ 10 vs. > 10), time from onset to treatment (≤ 3 vs > 3 hours) and premorbid mRS (0–1 vs. 2–5). All tests were 2-sided and a p-value < 0.05 was considered statistically significant. Data analyses were conducted with Stata/SE 15.0.
3. Results
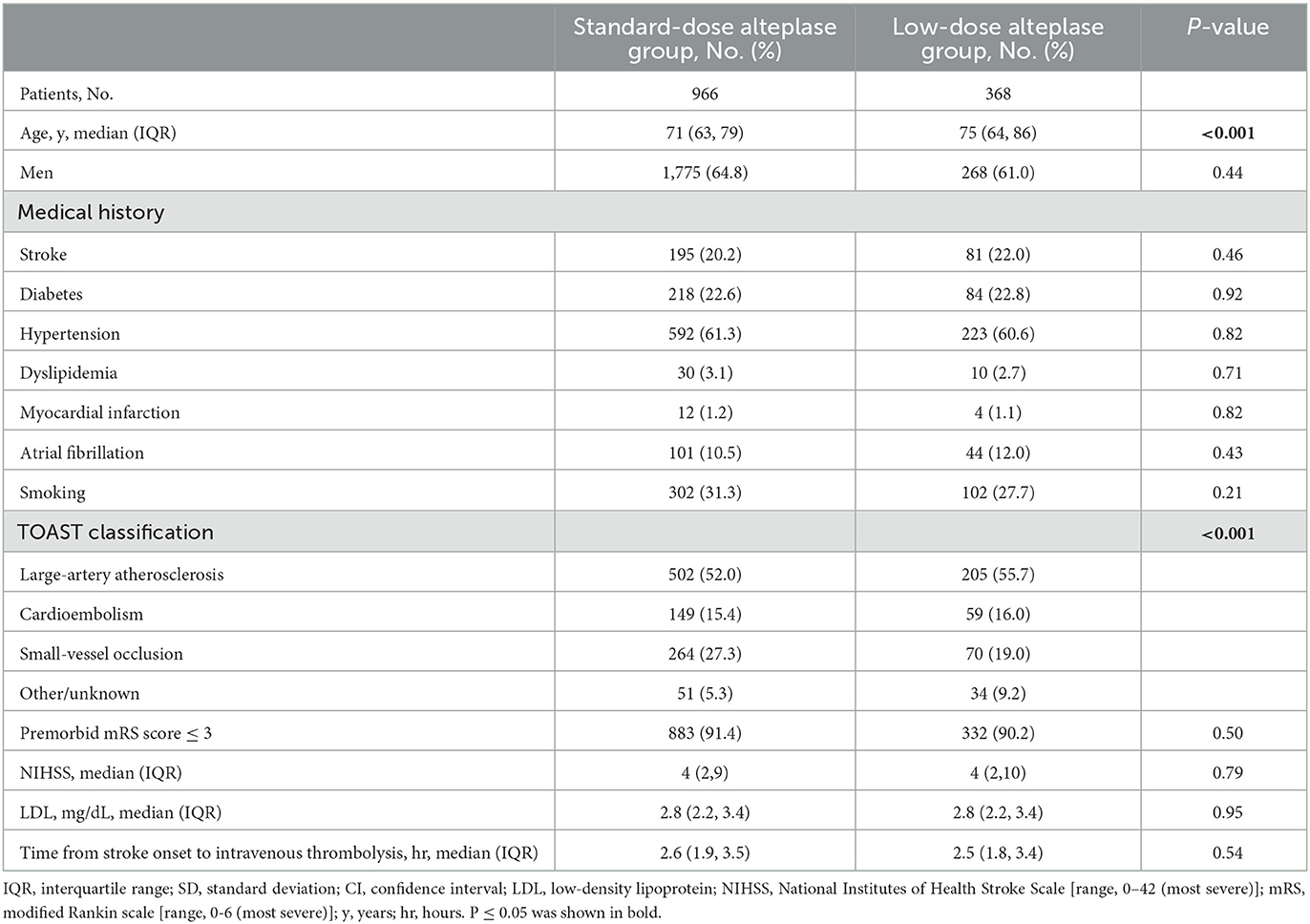
From Jan 2019 to Dec 2020, a total of 4995 patients received thrombolysis with alteplase. After excluding the cases with missing data (ie, the un-noted dosage of alteplase, treatment extended to the 4.5-hours window, absence of the mRS score or the NIHSS score at discharge), 3,179 patients were included in the study. The flowchart was shown in Figure 1. Baseline characteristics were shown in Supplementary Table 1. The patients in the low-dose group were older than those in the standard-dose group. Also, the low-dose group had a higher proportion of patients with a history of stroke, AF, or disability. After adjustment of PSM, 1,334 AIS patients were included in the final analysis. Among them, 368 (27.8%) patients received low-dose alteplase. The median age of the patients was 71 years, and 38.8% were female. The baseline characteristics in both groups after PSM were presented in Table 1. After PSM, the baseline characteristics were balanced except for age and TOAST classification; the absolute standardized differences were basically within an acceptable margin of 0.1 (Supplementary Table 2).
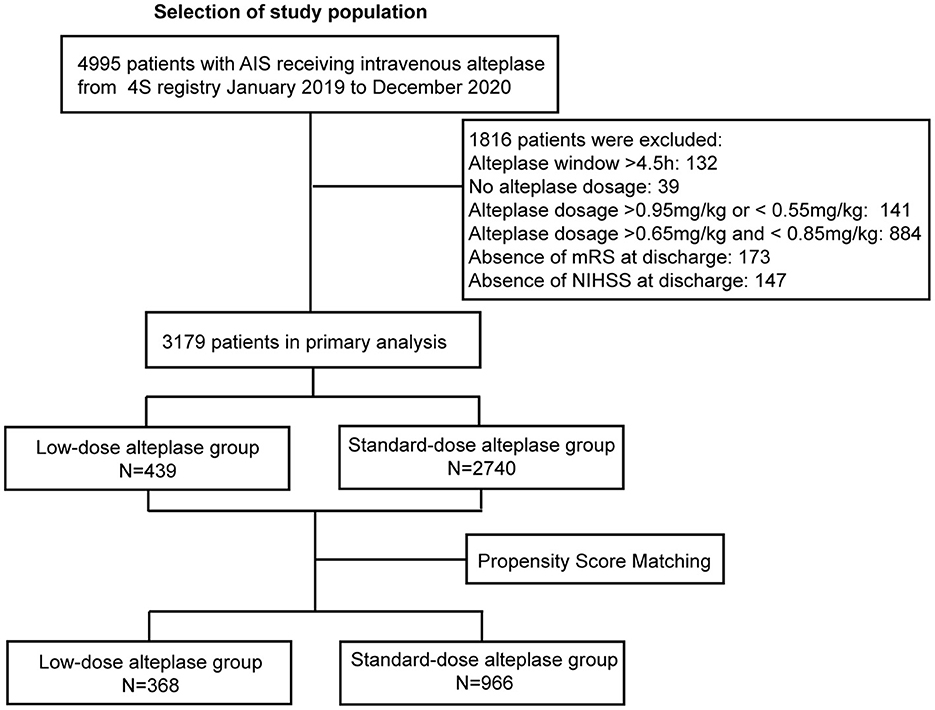
Figure 1. Study flow chart and numbers of eligible patients in each group. 4S, Shanghai Stroke Service System; AIS, acute ischemic stroke; NIHSS, National Institutes of Health Stroke Scale [range, 0–42 (most severe)]; mRS, modified Rankin scale [range, 0–6 (most severe)].
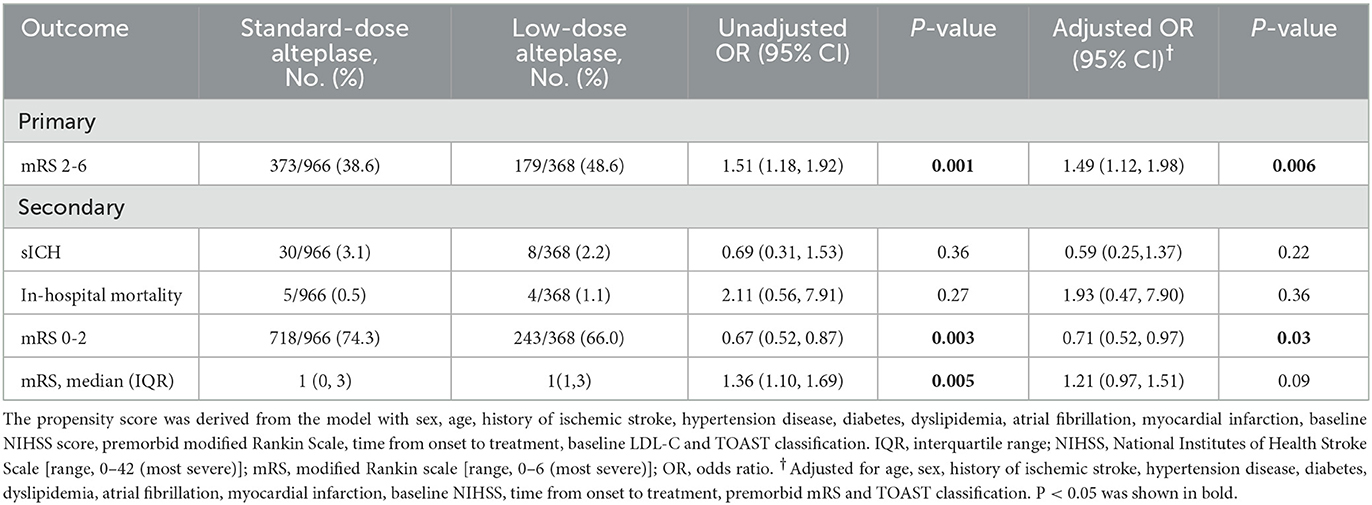
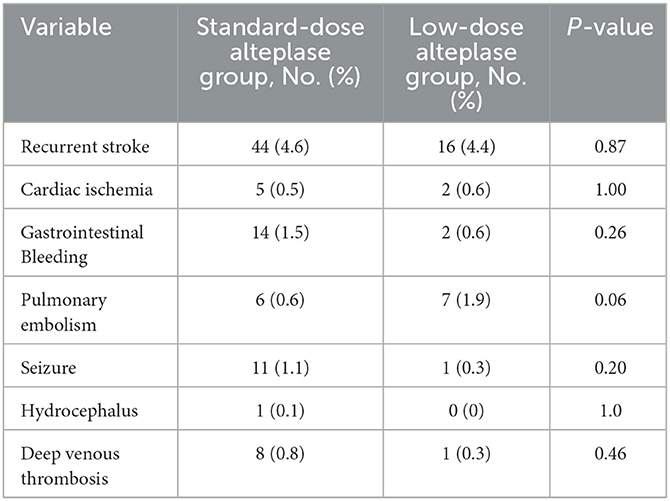
The primary and secondary outcomes were summarized in Table 2. The low-dose group showed its association with a higher rate of mortality or disability (adjusted odds ratio (aOR) = 1.49, 95%CI [1.12, 1.98], p = 0.006) than the standard-dose group. Treatment with low-dose alteplase also resulted in a lower rate of mRS score 0-2 than that with standard-dose alteplase (aOR = 0.71, 95% CI [0.52, 0.97], p = 0.03). The results were similar to the univariate regression model. The distribution of mRS scores at discharge was shown in Figure 2. The common OR across the mRS scores between the low- and standard-dose alteplase was 1.36 (95% CI [1.10, 1.69]) in the univariate analysis, but without statistical significance after adjustment of baseline characteristics. In addition, no significant difference was found in the risk of in-hospital mortality or sICH for two groups. The sensitivity analysis for a 1:1 matched design showed generally similar results (Supplementary Table 3). Other serious adverse events during the hospitalization according to assigned treatment were presented in Table 3. There was a trend toward a higher incidence of pulmonary embolism in the low-dose alteplase group.

Figure 2. Distribution of Modified Rankin Scale Scores on discharge by low-dose vs. standard-dose alteplase in propensity score matching dataset.
We performed subgroup analyses by age, gender, stroke subtypes, stroke severity, time from onset to treatment, and premorbid mRS score (Figure 3). All characteristics did not modify the treatment effect, except for stroke severity (p = 0.02). The patients with baseline NIHSS score ≤ 10 benefited more from the standard-dose alteplase therapy (OR = 1.56, 95%CI [1.16, 2.11], p = 0.003).
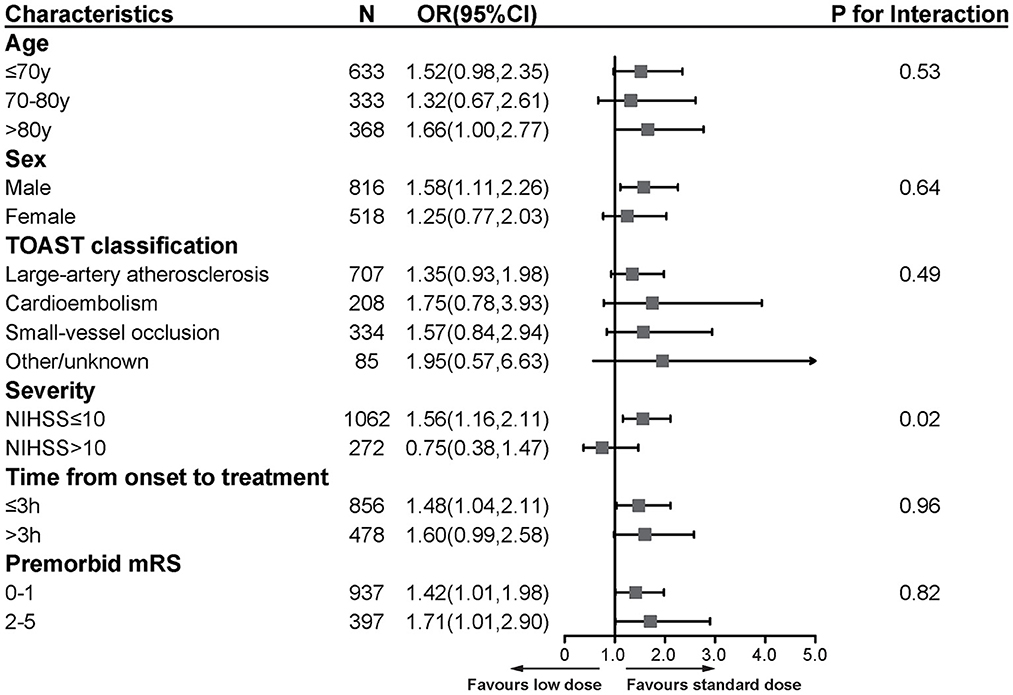
Figure 3. Subgroup analyses of primary functional outcome between low-dose and standard-dose group in propensity score matching dataset. The forest plot shows the difference in the primary functional outcome across all subgroups. The primary functional outcome was death or disability at discharge, defined by scores of 2 to 6 on the mRS [range, 0 (no symptoms) to 6 (death)]. The OR was calculated by using logistic regression, taking the following variables into account: age, sex, medical history (ischemic stroke, hypertension disease, diabetes, dyslipidemia, atrial fibrillation, myocardial infarction), baseline NIHSS, time from onset to treatment, premorbid score and TOAST classification. NIHSS, National Institutes of Health Stroke Scale [range, 0–42 (most severe)]; mRS, modified Rankin scale [range, 0–6 (most severe)]; OR, odds ratio.
4. Discussion
Our study demonstrated that patients receiving low-dose alteplase were associated with a higher risk of death or disability than those receiving standard-dose alteplase. Moreover, patients treated with low-dose alteplase were not associated with a lower sICH rate, and tend to have a higher rate of pulmonary embolism. Mild and moderate stroke patients with a baseline NIHSS score ≤ 10 may have better outcomes when treated with standard-dose alteplase.
Despite that a dose of 0.9 mg/kg alteplase is widely recommended by most guidelines for AIS patients (12–14), the high risks of sICH after thrombolysis with alteplase cannot be ignored, particularly among Asians (15). The J-ACT trial showed that the rate of mRS 0-1 at 90 days and the incidence of sICH in Japanese AIS patients receiving 0.6 mg/kg alteplase were comparable to 0.9 mg/kg alteplase treatment of the NINDS trial (1, 4). The results of several non-randomized trials in Japan subsequently showed the similar efficacy and safety outcome of low dosage thrombolysis with alteplase when compared with the standard dosage (3, 16, 17). In 2016, the ENCHANTED trial, as the only RCT involving 3310 AIS patients (63% Asian), aimed to compare low-dose with standard-dose alteplase. Although the trial failed to achieve the non-inferiority of low-dose as compared to standard-dose alteplase concerning the outcome of death or disability at 90 days (8), it also did not prove the superiority of standard-dose alteplase. There were significantly fewer sICH and trends toward the lower mortality rate with low-dose alteplase. Therefore, low-dose alteplase performed well in the safety outcome, which provided an alternative approach for both doctors and patients to make the personalized decision for the individual patient with AIS.
Compared with the ENCHANTED trial, our study achieved a similar efficacy outcome. What is new in our study, clinical physicians were more likely to administrate low dosage due to elder age and combined with AF. Our study found no association between alteplase dosage and the rate of sICH or mortality, which was different from the ENCHANTED trial. However, our findings were consistent with the results of TIMS-China (6, 7). An Asian stroke registry study also found no association between alteplase dosage and sICH risk (9). Our results might be the reflection of the real-world or false-negative error, attributed to the confounding bias of the observational study.
In subgroup analyses, we found that patients with mild-moderate stroke (NIHSS ≤ 10) in the standard-dose group were more likely to show a favorable outcome. However, the significant heterogeneity of stroke severity might be caused by a small sample of patients with a baseline NIHSS > 10. Our previous study showed that in mild stroke patients (NIHSS ≤ 4), there was no difference in the sICH risk and clinical improvement between both dosages (18). Therefore, more studies on the effectiveness and safety of low-dose thrombolysis for patients with different stroke severity are still needed.
The ENCHANTED trial raised the concern of the high risk of sICH in standard-dose alteplase treatment. Considering the bleeding-prone constitution of the Asian population, a secondary analysis of the ENCHANTED trial showed similar efficacy and safety outcomes of both doses of alteplase in both Asians and non-Asians (19). Also, the clinical use of low-dose alteplase in AIS patients with older age and other high-bleeding risks remained uncertain. Our subgroup study suggested that low-dose alteplase might be related to unfavorable functional outcomes even in patients older than 80.
Our study had several limitations. Firstly, our research population was limited to the Shanghai metropolitan. The management of risk factors and other demographic characteristics may not extend to that of other regions in China. Secondly, it was an observational retrospective study based on a registry database, the confounding bias cannot be ignored even with PSM. Thirdly, the relatively small sample size of the low-dose group might have impacted the statistical results. Fourthly, our study lacked mortality and functional outcomes in the long-term follow-up. Considering these limitations, further well-designed studies for patients with specialized characteristics are needed to provide more reference to medical practice.
5. Conclusions
Our observational study indicated that Chinese AIS patients receiving low-dose alteplase might be associated with an unfavorable functional outcome without lowering the risk of sICH. Our findings from the real-world dataset might provide more evidence to support standard-dose alteplase regimen for AIS patients in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Institutional Review Board in Huashan Hospital, with the waiver of consent as no identifying information was collected.
Author contributions
JX, XC, YX, YW, and SC performed the analysis. JX, XC, and YX drafted the manuscript. QD, YD, and KF revised the manuscript. YD and KF concepted this study, supervised the analysis, and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Young Elite Scientist Sponsorship Program by Chinese Association Science and Technology (YESS20170270) and Special Project of Clinical Research in Health Industry of Shanghai Municipal Health Commission [201940377]. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We thank all participating hospitals and relevant clinicians from the Shanghai Stroke Service System: Ailian Du, Shanghai Tong Ren Hospital; Bin Zhang, Shanghai Fengxian District Central Hospital; Canxing Yuan, Longhua Hospital Shanghai University of Traditional Chinese Medicine; Changde Wang, Shanghai TCM-Integrated Hospital; Chunmei Hu, Shanghai Baoshan Hospital; Danhong Wu, Shanghai FIfth People's Hospital Affiliated to Fudan University; Dongya Huang, Shanghai East Hospital Tongji University; Gang Xue, Shanghai Fengcheng Hospital; Gangming Xi, Shanghai Xuhui Hospital; Guojun Luo, Jinshan Branch of Shanghai Sixth People's Hospital; Guoyi Li, Putuo District Central Hospital of Shanghai; Hengbin Zu, Jinshan Hospital of Fudan University; Jiandao Yang, Shanghai Construction Group Hospital; Jianhua Xu, Jiading Central Hospital Shanghai University of Medicine and Health Sciences; Jianhua Zhuang, Shanghai Changzheng Hospital; Jianren Liu, Ninth People's Hospital Shanghai Jiao Tong University School of Medicine; Jing Zhao, Shanghai Central Hospital of Minhang District; Jun Liu, Shanghai Ruijin Hospital Shanghai Jiao Tong University school of Medicine; Junhui She, Junhui She, Shanghai Shi Bei Hospital; Langfeng Shi, Jingan District Centre Hospital; Li Wang, Shanghai Nanxiang Hospital; Lihong Huang, Shanghai Jingan District Zhabei Central Hospital; Mei Jiang, Shanghai Pudong Gongli Hospital; Min Yu, Shanghai Seventh People's Hospital; Ningjing Huang, Shanghai Municipal Hospital of Traditional Chinese Medicine; Ping Zhong, Shanghai Shidong Hospital; Qiang Dong, Huashan Hospital Affiliated to Fudan University; Qilin Fang, Zhongshan Wusong Hospital; Qinghua Li, Shanghai Pudong Hospital; Qingke Bai, Shanghai Pudong New Area People's Hospital; Wenshi Wei, Huadong Hospital Affiliated to Fudan University; Xiaofei Yu, Xiaofei Yu, Shuguang Hospital Shanghai University of Traditional Chinese Medicine; Xiaohong Liu, Shanghai Putuo District People's Hospital; Xiaoyun Xu, Shanghai Pudong Zhoupu Hospital; Xin wang, Shanghai Zhongshan Hospital; Xu Chen, Shanghai Eighth People's Hospital; Xueyuan Liu, Tenth People's Hospital of Tongji University; Yan Han, Yue Yang Hospital of Shanghai University of Medicine and Health Sciences; Yangtai Guan, Shanghai Renji Hospital Shanghai Jiao Tong University school of Medicine; Ying Xu, Renhe Hospital of Baoshan District; Yingchun Zhao, Shanghai Song Jiang District Central Hospital; Yong Bi, Shanghai Fourth People's Hospital; Yuhui Wang, Shanghai Punan Hospital; Yuncheng Wu, Shanghai General Hospital Affiliated to Shanghai Jiao Tong University school of Medicine; Yunhua Yue, Shanghai Yangpu District Central Hospital; Yuwu Zhao, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University; Zhenguo Liu, Shanghai Xinhua Hospital Affiliated to Shanghai Jiao Tong University school of Medicine; Zhiyu Nie, Shanghai Tongji Hospital of Tongji University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1120547/full#supplementary-material
Abbreviations
4S, Shanghai Stroke Service System; AF, atrial fibrillation; AIS, acute ischemic stroke; aOR, adjusted odds ratio; CI, confidence interval; ENCHANTED, The ENhanced Control of Hypertension And Thrombolysis strokE study; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; PSM, Propensity score matching; RCT, randomized controlled trial; sICH, symptomatic intracranial hemorrhage.
References
1. Tissue plasminogen activator for acute ischemic stroke. J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
2. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischemic stroke. J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
3. Toyoda K, Koga M, Naganuma M, Shiokawa Y, Nakagawara J, Furui E, et al. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke. (2009) 40:3591–5. doi: 10.1161/STROKEAHA.109.562991
4. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan alteplase clinical trial (J-ACT). Stroke. (2006) 37:1810–5. doi: 10.1161/01.STR.0000227191.01792.e3
5. Miyamoto S, Ogasawara K, Kuroda S, Itabashi R, Toyoda K, Itoh Y, et al. Japan stroke society guideline 2021 for the treatment of stroke. Int J Stroke. (2022) 17:1039–49. doi: 10.1177/17474930221090347
6. Liao X, Wang Y, Pan Y, Wang C, Zhao X, Wang DZ, et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke. (2014) 45:2354–8. doi: 10.1161/STROKEAHA.114.005989
7. Liu M, Pan Y, Zhou L, Wang Y. Low-dose rt-PA may not decrease the incidence of symptomatic intracranial haemorrhage in patients with high risk of symptomatic intracranial haemorrhage. Neurol Res. (2019) 41:473–9. doi: 10.1080/01616412.2019.1580454
8. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. (2016) 374:2313–23. doi: 10.1056/NEJMoa1515510
9. Wang X, Li J, Moullaali TJ, Lee KJ, Kim BJ, Bae HJ, et al. Low-dose versus standard-dose alteplase in acute ischemic stroke in Asian stroke registries: an individual patient data pooling study. Int J Stroke. (2019) 14:670–7. doi: 10.1177/1747493019858777
10. Dong Y, Fang K, Wang X, Chen S, Liu X, Zhao Y, et al. The network of Shanghai Stroke Service System (4S): A public health-care web-based database using automatic extraction of electronic medical records. Int J Stroke. (2018) 13:539–44. doi: 10.1177/1747493018765492
11. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
12. Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2013) 44:870–947. doi: 10.1161/STR.0b013e318284056a
13. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
14. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:1–97. doi: 10.1177/2396987321989865
15. Ueshima S, Matsuo O. The differences in thrombolytic effects of administrated recombinant t-PA between Japanese and Caucasians. Thromb Haemost. (2002) 87:544–6. doi: 10.1055/s-0037-1613042
16. Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan alteplase clinical trial II (J-ACT II). Stroke. (2010) 41:461–5. doi: 10.1161/STROKEAHA.109.573477
17. Nakagawara J, Minematsu K, Okada Y, Tanahashi N, Nagahiro S, Mori E, et al. Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-marketing alteplase registration study (J-MARS). Stroke. (2010) 41:1984–9. doi: 10.1161/STROKEAHA.110.589606
18. Dong Y, Han Y, Shen H, Wang Y, Ma F, Li H, et al. Who may benefit from lower dosages of intravenous tissue plasminogen activator? Results from a cluster data analysis. Stroke Vascular Neurol. (2020) 5:348–52. doi: 10.1136/svn-2020-000388
Keywords: ischemic stroke, thrombolysis, acute stroke therapy, recombinant tissue plasminogen activator, symptomatic intracranial hemorrhage
Citation: Xu J, Chen X, Xie Y, Wang Y, Chen S, Dong Q, Dong Y and Fang K (2023) Low-dose vs. standard-dose alteplase for Chinese patients with acute ischemic stroke: A propensity score analysis. Front. Neurol. 14:1120547. doi: 10.3389/fneur.2023.1120547
Received: 10 December 2022; Accepted: 01 February 2023;
Published: 21 February 2023.
Edited by:
Heling Chu, Shanghai Jiao Tong University, ChinaReviewed by:
Ryosuke Doijiri, Iwate Prefectural Central Hospital, JapanLongfei Wu, Xuanwu Hospital, Capital Medical University, China
Copyright © 2023 Xu, Chen, Xie, Wang, Chen, Dong, Dong and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Fang,  ZmFuZ2t1bkBodWFzaGFuLm9yZy5jbg==; Yi Dong,
ZmFuZ2t1bkBodWFzaGFuLm9yZy5jbg==; Yi Dong,  ZHJkb25neWlAeWVhaC5uZXQ=
ZHJkb25neWlAeWVhaC5uZXQ=
†These authors have contributed equally to this work and share first authorship
 Jiawen Xu
Jiawen Xu Xi Chen
Xi Chen Yanan Xie†
Yanan Xie† Yi Dong
Yi Dong