- 1Department of Orthopaedics, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 2English for International Communication Program, International College, Chiang Mai Rajabhat University, Chiang Mai, Thailand
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 4Department of Rehabilitation Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 5Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Introduction: The Boston Carpal Tunnel Questionnaire (BCTQ) is a widely recommended patient-reported outcome measure to evaluate symptoms and functions in carpal tunnel syndrome (CTS) patients. We aimed to evaluate the translation and cross-cultural adaptation of the Thai version of the BCTQ (Thai BCTQ) and to investigate the psychometric properties including internal consistency, test-retest reliability, construct validity and responsiveness.
Methods: The Thai BCTQ was field tested with 15 healthy volunteers and 15 CTS patients to evaluate the item-objective congruence of each item. Following that, one hundred and twenty-four CTS patients were included for psychometric evaluation in this study. Internal consistency was assessed using Cronbach’s alpha. Test-retest reliability was examined using the intraclass correlation coefficient (ICC). To evaluate construct validity, Spearman’s rank correlation of the symptom severity scale (Thai BCTQ -S), the functional status scale (Thai BCTQ -F) and the subscales of the Thai MHQ were analyzed. Responsiveness was determined using the standardized response mean (SRM).
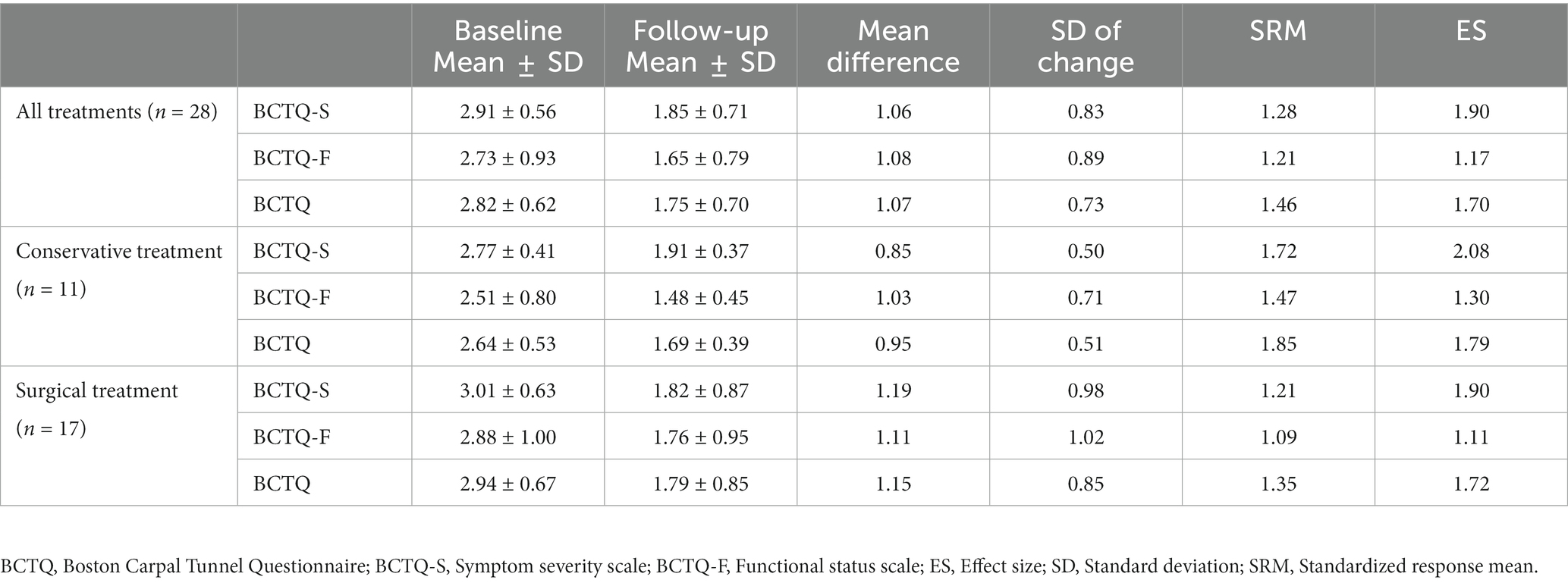
Results: Minor modification of the Thai version was made to better explain the term “tingling”. The Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ demonstrated adequate Cronbach’s alpha values (0.91-0.94) and good test-retest reliability (ICC=0.89-0.98). Regarding related dimensions, a strong correlation (r=0.67, P<0.008) was found between the Thai BCTQ-F and the Function subscale of Thai MHQ as well as between Thai BCTQ-F and the Activities of Daily Living subscale of the Thai MHQ (r=0.75, P<0.008). In unrelated dimensions, there was a relatively weak correlation between the Thai BCTQ-S and the Aesthetics subscale of the Thai MHQ (r=0.32, P=0.0116). The SRM of the Thai BCTQ was 1.46, indicating large responsiveness.
Discussion: The Thai BCTQ has adequate internal consistency in both the symptom and function scales as well as good construct validity and test-retest reliability indicating it is suitable for evaluating Thai CTS patients. This tool also has a high ability to detect clinically significant changes in symptoms and function over time after receiving conservative or surgical treatment.
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy causing pain, numbness, tingling and weakness of the hand (1, 2). The estimated prevalence of CTS based on diagnostic criteria is up to 14.4% of the general population (3). This condition is most commonly found in females with a peak incidence occurring between the age of 50 and 59 years (4). The suspected cause of CTS is compression of the median nerve at the carpal tunnel resulting from the occupying effect of oedema as well as tendon inflammation, hormonal changes, and manual activity leading to disturbance of the blood flow to the nerve (2). Previous meta-analyzes have demonstrated that diabetes mellitus, hypothyroidism, obesity and pregnancy increase the risk of CTS (5–8).
Currently, patient-reported outcome measures (PROMs) are the standard outcome measurements for evaluating the primary outcomes of clinical practice along with field research based on patients’ perceptions (9–11). Common PROMs used to evaluate clinical outcomes in the general hand and wrist region include the Disabilities of the Arm, Shoulder, and Hand (DASH), the Michigan Hand Outcomes Questionnaire (MHQ) and the Patient-Rated Wrist/Hand Evaluation (PRWHE) (12–17). For assessing disease-specific outcomes, the Boston Carpal Tunnel Questionnaire (BCTQ) is the recommended PROM for evaluating symptoms and function in CTS patients (12, 18). Previous studies have demonstrated that the original BCTQ provides good psychometric properties for assessing clinical outcomes in CTS patients (18, 19). This tool has been translated and cross culturally adapted into many language versions (20–34). Upatham et al. translated the BCTQ into Thai in 2006. However the psychometric property testing including reproducibility, construct validity and responsiveness in that version have not yet been evaluated (35).
The aims of this study were to evaluate the translation and cross-cultural adaptation of the Thai BCTQ and to investigate its psychometric properties including internal consistency, reproducibility, construct validity and responsiveness. We hypothesized that the scales of the Thai BCTQ are internally consistent, reproducible, valid and responsive to clinical change at a level comparable to versions in other languages.
Materials and methods
This study consisted of 2 processes: reviewing the translation and cross-cultural adaptation of Thai BCTQ and psychometric testing of the Thai BCTQ. The institutional research ethics committee approved this observational study (ORT-2563-07604). Written informed consent was received from all participants.
The Boston carpal tunnel questionnaire
The BCTQ is a disease-specific PROM for CTS patients. It contains 2 measurement scales: a symptom severity scale (BCTQ-S) and a functional status scale (BCTQ-F). The BCTQ-S uses 11 questions to evaluate the intensity and frequency of pain, numbness, weakness and loss of dexterity on a five-point scale ranging from 1 (no symptoms) to 5 (severe symptoms). The results are interpreted as the average scores of the 11 questions. The BCTQ-F has 8 questions to evaluate the level of difficulty in performing daily tasks, each rated on a five-point scale ranging from 1 (no difficulty) to 5 (cannot do at all due to hand or wrist symptoms). The results are interpreted as the average scores of the 8 questions (18).
Review of the previous translation and cross-cultural adaptation
A previous Thai version of the BCTQ had been translated and cross-culturally adapted to Thailand following the recommendations of Beaton et al. (35, 36). The process consisted of 5 stages: forward translation, synthesis, back translation and review by an expert committee and field testing (36). However, there had been no field testing of that version of the Thai BCTQ. We field tested the previous Thai BCTQ with 15 healthy volunteers and 15 patients who had been diagnosed with carpal tunnel syndrome based on guidelines for the process of cross-cultural adaptation of self-report measures (36). All participants were native Thai speakers who had the ability to read, write and understand the Thai language fluently. They were invited to evaluate all instructions and questionnaire items for item-objective congruence (IOC) and to provide suggestions. The IOC value is used to evaluate the content validity (37). Briefly, all participants will rate each item based on the degree to which they do or do not measure specific objectives listed by the test developer. The scoring system for each questionnaire item is +1 = clearly measuring, 0 = degree to which it measures the content area is unclear or − 1 = clearly not measuring. The IOC value for each item was calculated using the summation of scores from each participant divided by the number of total participants. The IOC value of 0.5 or more is considered satisfactory (38). If any item had an IOC value less than 0.5, that item was reconstructed and reevaluated until the IOC value was more than 0.5. Finally, the revised Thai BCTQ was translated to back into English and sent to the developers (18) for approval.
Psychometric testing of the Thai BCTQ
We prospectively enrolled Thai patients having carpal tunnel syndrome at the Orthopedic Outpatient Clinic of the University Hospital between December 2020 and July 2021. Inclusion criteria were patients who had been diagnosed with CTS according to the criteria of practice parameters for carpal tunnel syndrome (39). Any inconclusive diagnoses of CTS were confirmed via electrodiagnosis measurement evaluated by a Rehabilitation Medicine Board-qualified staff member. All patients were between 18 and 70 years old using Thai as their first language. The exclusion criteria were patients who had a musculoskeletal disorder above wrist level, a history of hand or wrist fracture, underlying rheumatoid arthritis, gout, hypothyroidism, active cervical radiculopathy, other concomitant nerve entrapments such as Guyon’s canal or cubital tunnel syndrome, concomitant hand or wrist disorders, e.g., stenosing tenosynovitis or osteoarthritis of the finger or hand at the time of enrollment, pregnancy and an active cerebral disorder or communication problems. We followed the COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) checklist in conducting this study (40).
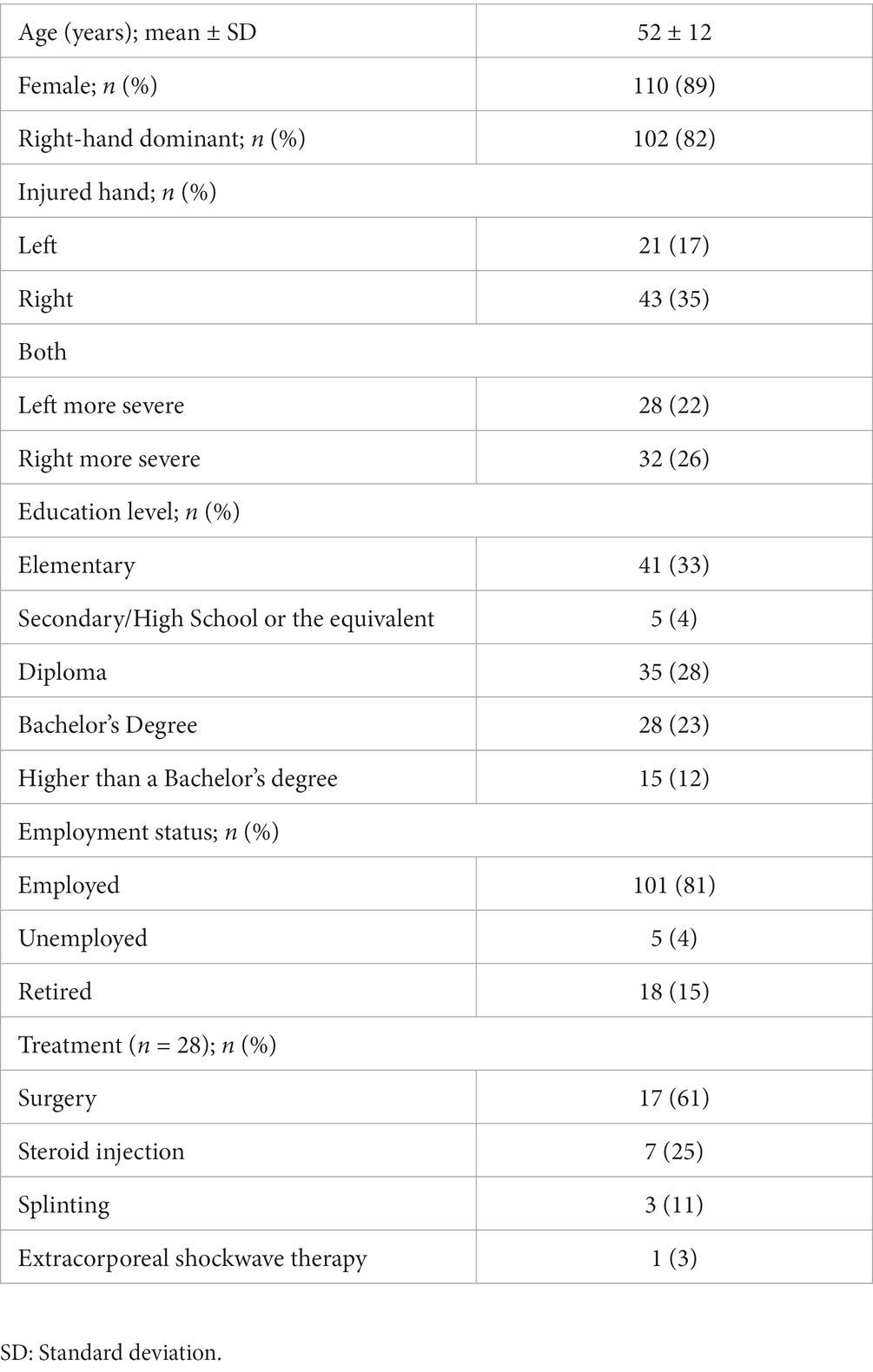
Patient demographic data recorded included age, sex, dominant hand, and injured hand. The psychometric properties of Thai BCTQ which were assessed included internal consistency, reproducibility, construct validity and responsiveness.
Internal consistency
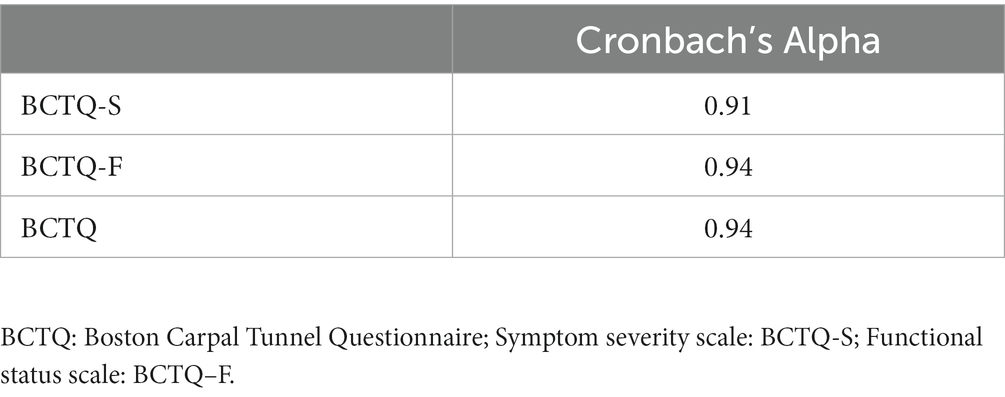
Internal consistency is defined as the degree of interrelatedness among items determining the same outcome. The internal consistency of the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ was assessed using Cronbach’s alpha. Cronbach’s alpha values range from 0 to 1, with higher scores representing greater interrelatedness between items. Adequate internal consistency is indicated by Cronbach’s alpha values of 0.70 or higher (41).
Test–retest reliability (reproducibility)
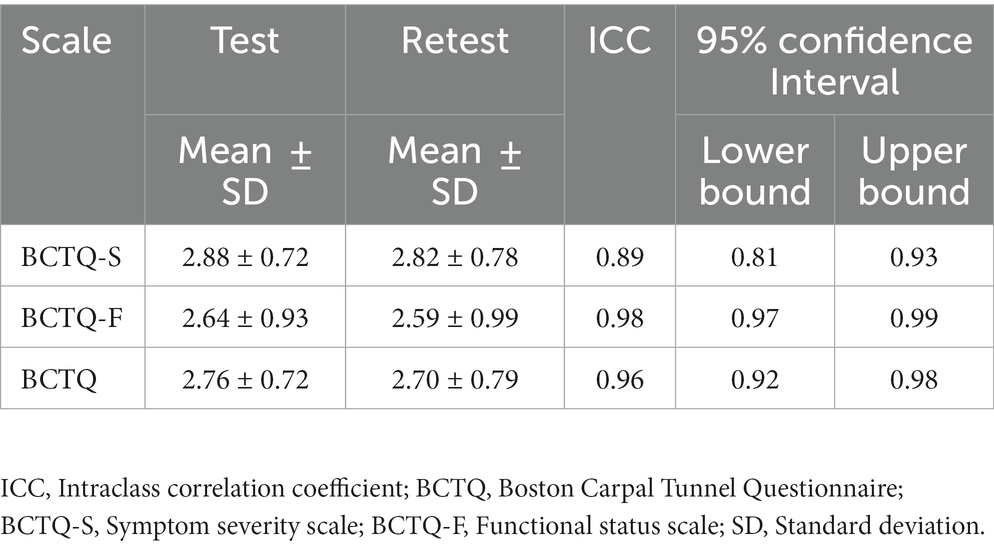
Test–retest reliability is the ability of measurements to obtain the same results in a stable individual (42). The recommended time between the initial and the repeat the measurement is 1 week to avoid recall and to ensure that clinically significant change has not occurred. The Thai BCTQ was administered to CTS patients who had failed conservative treatment and were scheduled for carpal tunnel release; a repeat administration was conducted with the same patients after a 7-day interval. The test–retest reliability of the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ were measured by intraclass correlation coefficient (ICC), which has a range of from 0 to 1 with ICC values higher than 0.7 indicating good reliability (42).
Construct validity
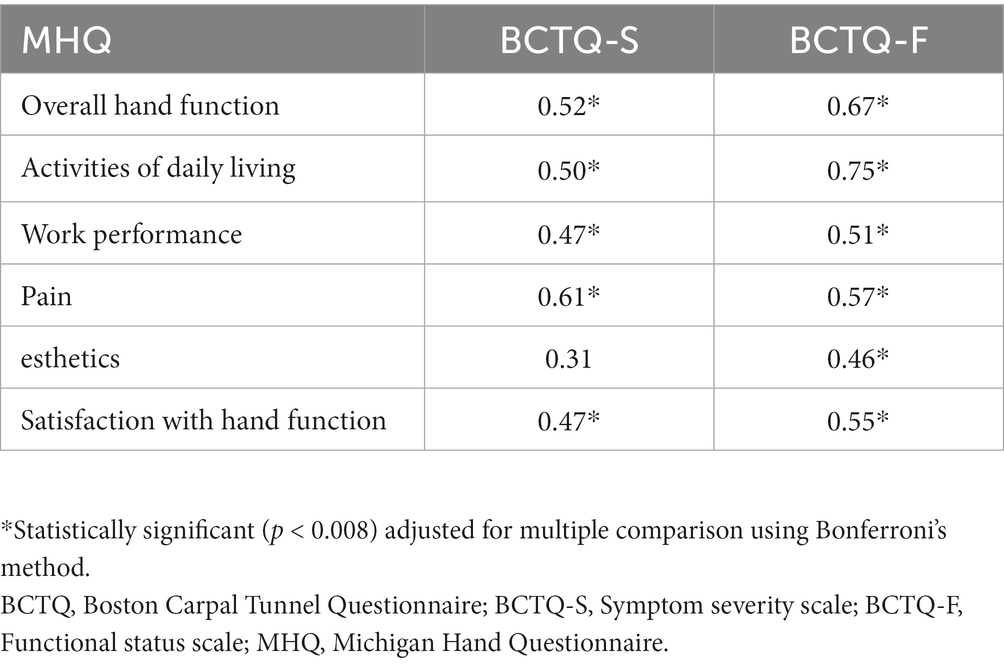
Construct validity is a measure of the association between PROMs and the standard instrument which used to evaluate the concepts being measured (42, 43). We used the Thai version of the Michigan Hand Outcomes Questionnaire (Thai MHQ) to assess the construct validity of the concepts being measured (44). We evaluated the correlation between the Thai BCTQ-S, the Thai BCTQ-F and the corresponding subscale of the Thai MHQ using Spearman’s rank correlation coefficient (r). We hypothesized that the same or related subscales should have a high correlation (the Thai BCTQ-S and the Pain subscale of the Thai MHQ, the Thai BCTQ-F and the Function subscale of the Thai MHQ), while unrelated subscales should show a weak correlation (the Thai BCTQ-S and the esthetics subscale of the Thai MHQ, Thai BCTQ-F and the esthetics subscale of the Thai MHQ). The level of correlation was rated as weak (r < 0.3), moderate (0.3 ≤ r ≤ 0.6), or strong (r > 0.6) (44).
Michigan hand outcomes questionnaire
The Michigan Hand Outcomes Questionnaire (MHQ) is a PROM which is widely used in evaluating hand and wrist regions. It has specific questions in each of 6 subscales including overall hand function (5 items each for the left and right hand), activities of daily living (5 items each for the left and right hand, 7 items for both hands); work performance (5 items); pain (5 items each for the left and right hand); esthetics (4 items each for the left and right hand); and satisfaction with hand function (6 items each for the left and right hand) (12, 13). The MHQ had been translated and cross-culturally adapted to Thai (Thai MHQ) and was shown to have adequate construct validity, reliability and responsiveness for Thai patients (44). Raw scores were converted to scaled scores with a range of 0 to 100. For the Pain subscale, higher scores indicate more pain. In other subscales, higher scores indicate better hand performance (13).
Responsiveness
Responsiveness is defined as the ability of PROMs to detect clinically significant changes over time (42). We evaluated the responsiveness of the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ by comparing the scores at baseline and at 12th weeks post-treatment using the standardized response mean (SRM) and effect size (ES) (45). SRM was analyzed as the observed mean change divided by the standard deviation of the observed change while ES was calculated as the observed mean change divided by the standard deviation of the baseline scores. SRM is the preferred value for comparing paired data measurements at different time points for the same patient. SRM and ES values of 0.8, 0.5, and 0.2 were considered to be large, moderate, and small, respectively (45).
Floor or ceiling effects
Floor or ceiling effects were considered to be present if more than 15% of patients reported the lowest or highest possible scores (46). The responsiveness of patients with the lowest or highest possible score is reduced since changes cannot be evaluated in these patients (42).
Statistical analysis
For demographic data, categorical variables are reported as frequencies and percentages. Continuous variables are reported as means and standard deviations. Statistical significance was set at p < 0.05. In multiple comparisons, the p-value was adjusted using Bonferroni’s method. A minimum sample size of 30 participants has been suggested by many statisticians for psychometric analyzes (47).
Results
Field testing
Minor cross-cultural adaptations were made to the previous Thai version of the Thai BCTQ-S. During field testing, participants were confused regarding the meaning of the Thai terms for “numbness” and “tingling” which resulted in the IOC of those items being below 0.5. The participants suggested expanding the explanation of the term “tingling.” The developers suggested adding “tingling (paresthesia).” With that change, upon re-evaluation the IOC value of the items which included this term increased to more than 0.5. All other items had an IOC > 0.5, an acceptable level. With this modification, the final Thai BCTQ was approved by the developers (Jeffrey N. Katz and Barry P. Simmons; Supplementary materials S1, S2).
Psychometric testing
Demographic data are shown in Table 1. One hundred and twenty-four patients were recruited. Most were female and right-handed. The average age was 52 years. Only 12% (15 CTS patients) were defined only on clinical grounds and not by nerve conduction studies. All 124 patients were able to complete the Thai BCTQ without major assistance.
Internal consistency and test–retest reliability
The internal consistency of the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ was assessed with 124 CTS patients (Table 2). All had adequate internal consistency ranging from 0.91 to 0.94. After excluding patients who did not participate in the follow-up retest after a week and patients who could not complete all the items, test–retest reliability was performed with 49 CTS patients. The results showed the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ had good reliability with ICC values between 0.89 and 0.98 (Table 3).
Construct validity
After excluding CTS patients who had missing data in the Thai BCTQ or the Thai MHQ, construct validity of the Thai BCTQ was evaluated in CTS 68 patients. The correlations of subscales for the Thai BCTQ and the Thai MHQ were compared (Table 4). For related dimensions, a strong correlation (r = 0.61, p < 0.008) was found between the Thai BCTQ-S and the Pain subscale of the Thai MHQ, indicating convergent validity. The relationship of the Thai BCTQ-F and the Function subscale of the Thai MHQ had a strong correlation (r = 0.67, p < 0.008) as was the case with the Thai BCTQ-F and the Activities of daily living subscale of the Thai MHQ (r = 0.75, p < 0.008). In the case of unrelated dimensions, there were relatively weak correlations between the Thai BCTQ-S and the esthetics subscale of the Thai MHQ (r = 0.32, p = 0.0116) and between the Thai BCTQ-F and the esthetics subscale of the Thai MHQ (r = 0.46, p < 0.008) indicating discriminant validity.
Responsiveness
Twenty-eight patients participated in the followed-up after receiving treatment and completed all items in the Thai BCTQ both before treatment and at follow-up. Eleven patients (39%) received conservative treatment and 17 patients (61%) underwent carpal tunnel release. The SRM and ES of the Thai BCTQ were 1.46 and 1.70, respectively, indicating large responsiveness (Table 5). Subgroup analyzes of SRM and ES of the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ in CTS patients who had conservative or surgical treatment also showed large responsiveness (Table 5).
Floor or ceiling effects
No floor or ceiling effects were found in the Thai BCTQ.
Discussion
The study assessed the psychometric properties of the Thai BCTQ and found that it demonstrated excellent reliability, construct validity and responsiveness. However, some vital points the authors would to address are as follows.
During the process of developing the Thai BCTQ, minor changes were made to clarify the term “tingling,” changing it to “tingling (paresthesia),” since in field testing most participants thought that “numbness” and “tingling” had the same meaning. Some minor modifications were made to the functional status scale in other versions (23, 24, 28). However, there were no major linguistic or cultural discrepancies among the different language versions.
In the evaluation of internal consistency and test–retest reliability, the Thai BCTQ-S, Thai BCTQ-F and Thai BCTQ showed good internal consistency and test–retest reliability with Cronbach’s Alpha and ICC values higher than 0.8. These results are in concordance with other versions (18, 20–34, 48).
In the exploration of construct validity, most publications demonstrated correlation between each scale of the BCTQ and other patient-reported outcome measures (PROMs) such as the Disability of the Arm, Shoulder, and Hand questionnaire (DASH), and the 36-item Short-Form Health Survey (SF-36), Pain Visual Analog Scale, EQ-5D and MHQ (20–22, 24, 25, 27, 29, 34, 48). In this study, we used the MHQ to evaluate the correlation of the subscales to each scale of the BCTQ since the MHQ is more specific in the evaluation of symptoms and function of the hand and wrist regions than DASH, SF-36 and EQ-5D. We found strong correlations between the BCTQ-F and the Function subscale of MHQ, BCTQ-F and Activities of daily living subscale of MHQ as well as the BCTQ-S and the Pain subscale in MHQ. Only weak correlations were found between the BCTQ-S and the esthetics subscale in MHQ and between BCTQ-F and the esthetics subscale in MHQ. These results are comparable to the Polish version of the BCTQ which was evaluated for construct validity using the correlation between the BCTQ and MHQ subscales (25).
The responsiveness of the Thai BCTQ-S, BCTQ-F and BCTQ demonstrated a large standardized response mean (SRM) and effect size (ES). In our cohort, large responsiveness was shown both in CTS patients who had conservative and those who had surgical treatment. Our results are similar to the Swedish, Dutch and Danish versions which showed large SRM and ES in both scales after surgical release (20, 30, 49). Some versions, such as the Korean, Chinese and Japanese, had large SRM and ES in the BCTQ-S and moderate SRM and ES in the BCTQ-F in CTS patients who had surgical release (22, 24, 34). In the comparison of responsiveness of CTS patients who had conservative treatment, another Korean version reported moderate ES in the BCTQ-S and BCTQ-F (48), while our study found a large ES in both. The differences in results might be a consequence of different treatments, e.g., all Korean patients received only steroid injections while CTS patients in our cohort received one of three treatments: steroid injection, splinting or extracorporeal shockwave therapy (48).
To the best of our knowledge, this study was the first to test the psychometric properties of the Thai BCTQ more comprehensively. This study, however, had some limitations. First, although the present study underwent a complete validation process for aspects of validity, reliability and responsiveness compared to other publications (21, 23, 25, 26, 29, 32, 33), a longitudinal study to evaluate the responsiveness of the Thai BCTQ over longer time periods may provide more details of the ability to detect clinical changes. Second, the sample size to evaluate the responsiveness of the Thai BCTQ was quite low. To evaluate the responsiveness of the Thai BCTQ, future research with adequate number of patients should be conducted to confirm our results. Third, the Thai BCTQ should be further evaluated according to item response theory, e.g., the Rasch measurement model, to provide additional information on both persons and item calibration (50).
Conclusion
The Thai BCTQ has excellent internal consistency in both the symptom and function scales as well as good construct validity and reproducibility for the evaluation of symptoms in Thai CTS patients. This tool also has a high ability to detect clinically significant changes over time following either conservative or surgical treatment. This Thai BCTQ is recommended for use as the standard PROM for clinicians and researchers for the evaluation of symptoms and functions of Thai CTS patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PA initiated conception and design of the study, performed the collection and acquisition of data, performed the data analysis with interpretation, wrote the manuscript, and responsible for critical revision. JN performed the collection, acquisition of data, and edited the manuscript. KS, ST, TW, and NW participated in data interpretation and manuscript editing. WM performed the data analysis, interpreted the data, and edited the manuscript. This study was supported by Faculty of Medicine, Chiang Mai University.
Acknowledgments
The authors are grateful to Dr. G. Lamar Robert, Ph.D. and Dr. Chongchit S. Robert, Ph.D., for editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1132218/full#supplementary-material
References
1. Dawson, DM. Entrapment neuropathies of the upper extremities. N Engl J Med. (1993) 329:2013–8. doi: 10.1056/NEJM199312303292707
2. Padua, L, Coraci, D, Erra, C, Pazzaglia, C, Paolasso, I, Loreti, C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. (2016) 15:1273–84. doi: 10.1016/S1474-4422(16)30231-9
3. Atroshi, I, Gummesson, C, Johnsson, R, Ornstein, E, Ranstam, J, and Rosen, I. Prevalence of carpal tunnel syndrome in a general population. JAMA. (1999) 282:153–8. doi: 10.1001/jama.282.2.153
4. Mondelli, M, Giannini, F, and Giacchi, M. Carpal tunnel syndrome incidence in a general population. Neurology. (2002) 58:289–94. doi: 10.1212/WNL.58.2.289
5. Shiri, R. Hypothyroidism and carpal tunnel syndrome: a meta-analysis. Muscle Nerve. (2014) 50:879–83. doi: 10.1002/mus.24453
6. Padua, L, Di Pasquale, A, Pazzaglia, C, Liotta, GA, Librante, A, and Mondelli, M. Systematic review of pregnancy-related carpal tunnel syndrome. Muscle Nerve. (2010) 42:697–702. doi: 10.1002/mus.21910
7. Pourmemari, MH, and Shiri, R. Diabetes as a risk factor for carpal tunnel syndrome: a systematic review and meta-analysis. Diabet Med. (2016) 33:10–6. doi: 10.1111/dme.12855
8. Shiri, R, Pourmemari, MH, Falah-Hassani, K, and Viikari-Juntura, E. The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev. (2015) 16:1094–104. doi: 10.1111/obr.12324
9. Schoneveld, K, Wittink, H, and Takken, T. Clinimetric evaluation of measurement tools used in hand therapy to assess activity and participation. J Hand Ther. (2009) 22:221–36. doi: 10.1016/j.jht.2008.11.005
10. Valdes, K, MacDermid, J, Algar, L, Connors, B, Cyr, LM, Dickmann, S, et al. Hand therapist use of patient report outcome (PRO) in practice: a survey study. J Hand Ther. (2014) 27:299–308. doi: 10.1016/j.jht.2014.07.001
11. Hoang-Kim, A, Pegreffi, F, Moroni, A, and Ladd, A. Measuring wrist and hand function: common scales and checklists. Injury. (2011) 42:253–8. doi: 10.1016/j.injury.2010.11.050
12. Smith, MV, Calfee, RP, Baumgarten, KM, Brophy, RH, and Wright, RW. Upper extremity-specific measures of disability and outcomes in orthopaedic surgery. J Bone Joint Surg Am. (2012) 94:277–85. doi: 10.2106/JBJS.J.01744
13. Chung, KC, Pillsbury, MS, Walters, MR, and Hayward, RA. Reliability and validity testing of the Michigan hand outcomes questionnaire. J Hand Surg Am. (1998) 23:575–87. doi: 10.1016/S0363-5023(98)80042-7
14. MacDermid, JC. Development of a scale for patient rating of wrist pain and disability. J Hand Ther. (1996) 9:178–83. doi: 10.1016/S0894-1130(96)80076-7
15. MacDermid, JC, and Tottenham, V. Responsiveness of the disability of the arm, shoulder, and hand (DASH) and patient-rated wrist/hand evaluation (PRWHE) in evaluating change after hand therapy. J Hand Ther. (2004) 17:18–23. doi: 10.1197/j.jht.2003.10.003
16. Hudak, PL, Amadio, PC, and Bombardier, C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The upper extremity collaborative group (UECG). Am J Ind Med. (1996) 29:602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L
17. Beaton, DE, Katz, JN, Fossel, AH, Wright, JG, Tarasuk, V, and Bombardier, C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the disabilities of the arm, shoulder and hand outcome measure in different regions of the upper extremity. J Hand Ther. (2001) 14:128–42. doi: 10.1016/S0894-1130(01)80043-0
18. Levine, DW, Simmons, BP, Koris, MJ, Daltroy, LH, Hohl, GG, Fossel, AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. (1993) 75:1585–92. doi: 10.2106/00004623-199311000-00002
19. Leite, JC, Jerosch-Herold, C, and Song, F. A systematic review of the psychometric properties of the Boston carpal tunnel questionnaire. BMC Musculoskelet Disord. (2006) 7:78. doi: 10.1186/1471-2474-7-78
20. Atroshi, I, Johnsson, R, and Sprinchorn, A. Self-administered outcome instrument in carpal tunnel syndrome. Reliability, validity and responsiveness evaluated in 102 patients. Acta Orthop Scand. (1998) 69:82–8. doi: 10.3109/17453679809002363
21. Sezgin, M, Incel, NA, Serhan, S, Camdeviren, H, As, I, and Erdogan, C. Assessment of symptom severity and functional status in patients with carpal tunnel syndrome: reliability and functionality of the Turkish version of the Boston questionnaire. Disabil Rehabil. (2006) 28:1281–6. doi: 10.1080/09638280600621469
22. Imaeda, T, Uchiyama, S, Toh, S, Wada, T, Okinaga, S, Sawaizumi, T, et al. Validation of the Japanese Society for Surgery of the hand version of the carpal tunnel syndrome instrument. J Orthop Sci. (2007) 12:14–21. doi: 10.1007/s00776-006-1087-9
23. Mody, GN, Anderson, GA, Thomas, BP, Pallapati, SC, Santoshi, JA, and Antonisamy, B. Carpal tunnel syndrome in Indian patients: use of modified questionnaires for assessment. J Hand Surg Eur. (2009) 34:671–8. doi: 10.1177/1753193409101469
24. Kim, JK, and Lim, HM. The Korean version of the carpal tunnel questionnaire. Cross cultural adaptation, reliability, validity and responsiveness. J Hand Surg Eur Vol. (2015) 40:200–5. doi: 10.1177/1753193414540083
25. Trybus, M, Koziej, M, Belka, M, Bednarek, M, and Banach, M. The polish version of the Boston carpal tunnel questionnaire: associations between patient-rated outcome measures and nerve conduction studies. J Plast Reconstr Aesthet Surg. (2019) 72:924–32. doi: 10.1016/j.bjps.2018.12.032
26. Ulbrichtova, R, Jakusova, V, Svihrova, V, Dvorstiakova, B, and Hudeckova, H. Validation of the Slovakian version of Boston carpal tunnel syndrome questionnaire (BCTSQ). Acta Med (Hradec Kralove). (2019) 62:105–8. doi: 10.14712/18059694.2019.133
27. Karabinov, V, Slavchev, SA, and Georgiev, GP. Translation and validation of the Bulgarian version of the Boston carpal tunnel questionnaire. Cureus. (2020) 12:e10901. doi: 10.7759/cureus.10901
28. Multanen, J, Ylinen, J, Karjalainen, T, Kautiainen, H, Repo, JP, and Hakkinen, A. Reliability and validity of the Finnish version of the Boston carpal tunnel questionnaire among surgically treated carpal tunnel syndrome patients. Scand J Surg. (2020) 109:343–50. doi: 10.1177/1457496919851607
29. Hamzeh, HH, and Alworikat, NA. Cross cultural adaptation, reliability and construct validity of the Boston carpal tunnel questionnaire in standard Arabic language. Disabil Rehabil. (2021) 43:430–5. doi: 10.1080/09638288.2019.1629651
30. Mosegaard, SB, Stilling, M, Breddam, M, and Hansen, TB. Measurement properties of the Danish version of the Boston carpal tunnel questionnaire. JPRAS Open. (2021) 29:17–25. doi: 10.1016/j.jpra.2021.03.007
31. Oteo-Alvaro, A, Marin, MT, Matas, JA, and Vaquero, J. Spanish validation of the Boston carpal tunnel questionnaire. Med Clin (Barc). (2016) 146:247–53. doi: 10.1016/j.medcli.2015.10.013
32. Bougea, A, Zambelis, T, Voskou, P, Katsika, PZ, Tzavara, C, Kokotis, P, et al. Reliability and validation of the Greek version of the Boston carpal tunnel questionnaire. Hand (N Y). (2018) 13:593–9. doi: 10.1177/1558944717725379
33. Hassankhani, GG, Moradi, A, Birjandinejad, A, Vahedi, E, Kachooei, AR, and Ebrahimzadeh, MH. Translation and validation of the Persian version the Boston carpal tunnel syndrome questionnaire. Arch Bone Joint Surg. (2018) 6:71–7.
34. Lue, YJ, Lu, YM, Lin, GT, and Liu, YF. Validation of the Chinese version of the Boston carpal tunnel questionnaire. J Occup Rehabil. (2014) 24:139–45. doi: 10.1007/s10926-013-9438-9
35. Upatham, S, and Kumnerddee, W. Reliability of Thai version Boston questionnaire. J Med Assoc Thail. (2008) 91:1250–6.
36. Beaton, DE, Bombardier, C, Guillemin, F, and Ferraz, MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). (2000) 25:3186–91. doi: 10.1097/00007632-200012150-00014
37. Rovinelli, RJ, and Hambleton, RK. On the use of content specialists in the assessment of criterion-referenced test item validity. Dutch J Educ Res. (1977) 2:49–60.
38. Tongprasert, S, Rapipong, J, and Buntragulpoontawee, M. The cross-cultural adaptation of the DASH questionnaire in Thai (DASH-TH). J Hand Ther. (2014) 27:49–54. doi: 10.1016/j.jht.2013.08.020
39. Practice Parameter for Carpal Tunnel Syndrome (Summary Statement). Report of the quality standards Subcommittee of the American Academy of neurology. Neurology. (1993) 43:2406–9. doi: 10.1212/WNL.43.11.2406
40. Mokkink, LB, Terwee, CB, Patrick, DL, Alonso, J, Stratford, PW, Knol, DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. (2010) 19:539–49. doi: 10.1007/s11136-010-9606-8
41. Cronbach, LJ. A case study of the split-half reliability coefficient. J Educ Psychol. (1946) 37:473–80. doi: 10.1037/h0054328
42. Terwee, CB, Bot, SD, De Boer, MR, Van der Windt, DA, Knol, DL, Dekker, J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. (2007) 60:34–42. doi: 10.1016/j.jclinepi.2006.03.012
43. Kirshner, B, and Guyatt, G. A methodological framework for assessing health indices. J Chronic Dis. (1985) 38:27–36. doi: 10.1016/0021-9681(85)90005-0
44. Atthakomol, P, Manosroi, W, Sanguanrungsirikul, S, Punoppamas, S, Benjachaya, S, Tongprasert, S, et al. A Thai version of the Michigan hand questionnaire (Thai MHQ): an investigation of the psychometric properties. Health Qual Life Outcomes. (2020) 18:313. doi: 10.1186/s12955-020-01548-0
45. Stratford, PW, Binkley, JM, and Riddle, DL. Health status measures: strategies and analytic methods for assessing change scores. Phys Ther. (1996) 76:1109–23. doi: 10.1093/ptj/76.10.1109
46. McHorney, CA, and Tarlov, AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. (1995) 4:293–307. doi: 10.1007/BF01593882
47. Onwuegbuzie, AJ, and Collins, KM. A typology of mixed methods sampling designs in social science research. Qual Rep. (2007) 12:281–316.
48. Park, DJ, Kang, JH, Lee, JW, Lee, KE, Wen, L, Kim, TJ, et al. Cross-cultural adaptation of the Korean version of the Boston carpal tunnel questionnaire: its clinical evaluation in patients with carpal tunnel syndrome following local corticosteroid injection. J Korean Med Sci. (2013) 28:1095–9. doi: 10.3346/jkms.2013.28.7.1095
49. De Kleermaeker, F, Levels, M, Verhagen, WIM, and Meulstee, J. Validation of the Dutch version of the Boston carpal tunnel questionnaire. Front Neurol. (2019) 10:1154. doi: 10.3389/fneur.2019.01154
Keywords: Thai, Boston carpal tunnel questionnaire, responsiveness, field testing, validity, reliability
Citation: Atthakomol P, Nudchapong J, Sangseekaew K, Manosroi W, Tongprasert S, Wongpakaran T and Wongpakaran N (2023) Field testing and psychometric properties of Thai version of the Boston carpal tunnel questionnaire. Front. Neurol. 14:1132218. doi: 10.3389/fneur.2023.1132218
Edited by:
Rossella Tupler, University of Modena and Reggio Emilia, ItalyReviewed by:
Nienke Mendelaar, Erasmus Medical Center, NetherlandsMatteo Tagliapietra, Azienda Ospedaliera Universitaria Integrata, Italy
Copyright © 2023 Atthakomol, Nudchapong, Sangseekaew, Manosroi, Tongprasert, Wongpakaran and Wongpakaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pichitchai Atthakomol, cC5hdHRoYWtvbW9sQGdtYWlsLmNvbQ==
 Pichitchai Atthakomol
Pichitchai Atthakomol Jirawat Nudchapong1
Jirawat Nudchapong1 Worapaka Manosroi
Worapaka Manosroi Siam Tongprasert
Siam Tongprasert Tinakon Wongpakaran
Tinakon Wongpakaran