- 1Department of Quality Management, Children's Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 2Department of Neurology, Children's Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 3Key Laboratory of Environmental Medicine Engineering, Department of Epidemiology and Health Statistics, School of Public Health, Southeast University, Nanjing, Jiangsu, China
- 4Department of Neurology, Shenzhen Children’s Hospital, Shenzhen, Guangdong, China
Objective: To compare the efficacy and safety of inpatient and outpatient initiation ketogenic diet (KD) protocol of pediatric refractory epilepsy.
Methods: Eligible children with refractory epilepsy were randomly assigned to receive KD with inpatient and outpatient initiation. The generalized estimation equation (GEE) model was used to analyze the longitudinal variables of seizure reduction, ketone body, weight, height, body mass index (BMI), and BMI Z-score at different follow-up times between the two groups.
Results: Between January 2013 and December 2021, 78 and 112 patients were assigned to outpatient and inpatient KD initiation groups, respectively. There were no statistical differences between the two groups based on baseline demographics and clinical characteristics (all Ps > 0.05). The GEE model indicated that the rate of reduction of seizures≥50% in the outpatient initiation group was higher than that of the inpatient initiation group (p = 0.049). A negative correlation was observed between the seizure reduction and blood ketone body at 1, 6, and 12 months (all Ps < 0.05). There were no significant differences in height, weight, BMI, and BMI Z-score between the two groups over the 12-month period by the GEE models (all Ps > 0.05). Adverse events were reported by 31 patients (43.05%) in the outpatient KD initiation group and 46 patients (42.20%) in the inpatient KD initiation group, but these differences were not statistically significant (p = 0.909).
Conclusion: Our study shows that outpatient KD initiation is a safe and effective treatment for children with refractory epilepsy.
Introduction
Children and adolescents with epilepsy are at increased risk for poor long-term intellectual and psychosocial outcomes, along with a poor health-related quality of life (1). Epilepsy accounts for over 13 million disability-adjusted life years (DALYs) and is responsible for more than 0.5% of the global burden of disease (2). Although many underlying disease mechanisms can lead to epilepsy, the cause of the disease is still unknown in about 50% of cases globally (3). Structural, genetic, brain injury, vascular causes, and central nervous system infection are the most frequently identified risk factors (4), head injury, hypoxic–ischemic encephalopathy, and infections of the brain are significant risk factors for childhood epilepsy (5, 6). It is estimated that 49–139 per 100,000 people are diagnosed with epilepsy each year (7) and the point prevalence of active epilepsy was 6.38 per 1,000 population (8). The number of people with epilepsy is expected to increase further.
In China, it is estimated that 10 million people have epilepsy, but only around a third of them receive appropriate or adequate treatment (9). Antiepileptic drugs (AEDs) are the main approach to epilepsy treatment and achieve seizure freedom (10). However, they have not substantially altered the overall seizure-free outcomes, the failure to achieve sustained seizure freedom with adequate uses of two AEDs was defined as refractory epilepsy (11, 12). Currently, several non-pharmacologic interventions can be used to treat refractory epilepsy, such as metabolic therapy, brain stimulation therapy, and complementary therapy (13). The ketogenic diet (KD) is a diet that has been used since the early 1920s to control seizures in patients (14). The KD is very high in fats and extremely low in carbohydrates and protein, which promises to decrease seizure frequency significantly for patients with refractory epilepsy (15). Moreover, compared to AEDs and other treatments, the KD is inexpensive, fairly easy to implement, and the use of KD for anti-epileptics is increasing globally.
The KD is usually initiated during hospitalization, and patients are closely monitored under expert guidance, especially in cases of infants and children with severe co-morbidities (16). The 2019 coronavirus disease (COVID-19) pandemic and the associated lock-down measures have certainly affected children with epilepsy, particularly those who are candidates for KD (17), as a result, inpatient KD initiation has been sharply restricted (18). Therefore, continuing a specific diet during the COVID-19 pandemic is expected to be difficult in some aspects (19). Outpatient KD initiation that will result in easier implementation, shorter hospital stays, and reduced medical and family costs would have clinical and economic advantages (20). Some epilepsy centers are now beginning the KD by using an outpatient initiation protocol, however, this is not a general routine in many centers, and most KD centers still follow an inpatient protocol (21). There was no evidence to prove that inpatient initiation of the ketogenic diet was superior to outpatient initiation concerning long-term seizure control or cognitive improvement (22). This study aimed to compare the efficacy and safety of inpatient and outpatient initiation KD protocol in children with refractory epilepsy and to evaluate the differences in blood ketone body, weight, height, and BMI in children between the inpatient and outpatient initiation of the KD.
Materials and methods
Study design and participants
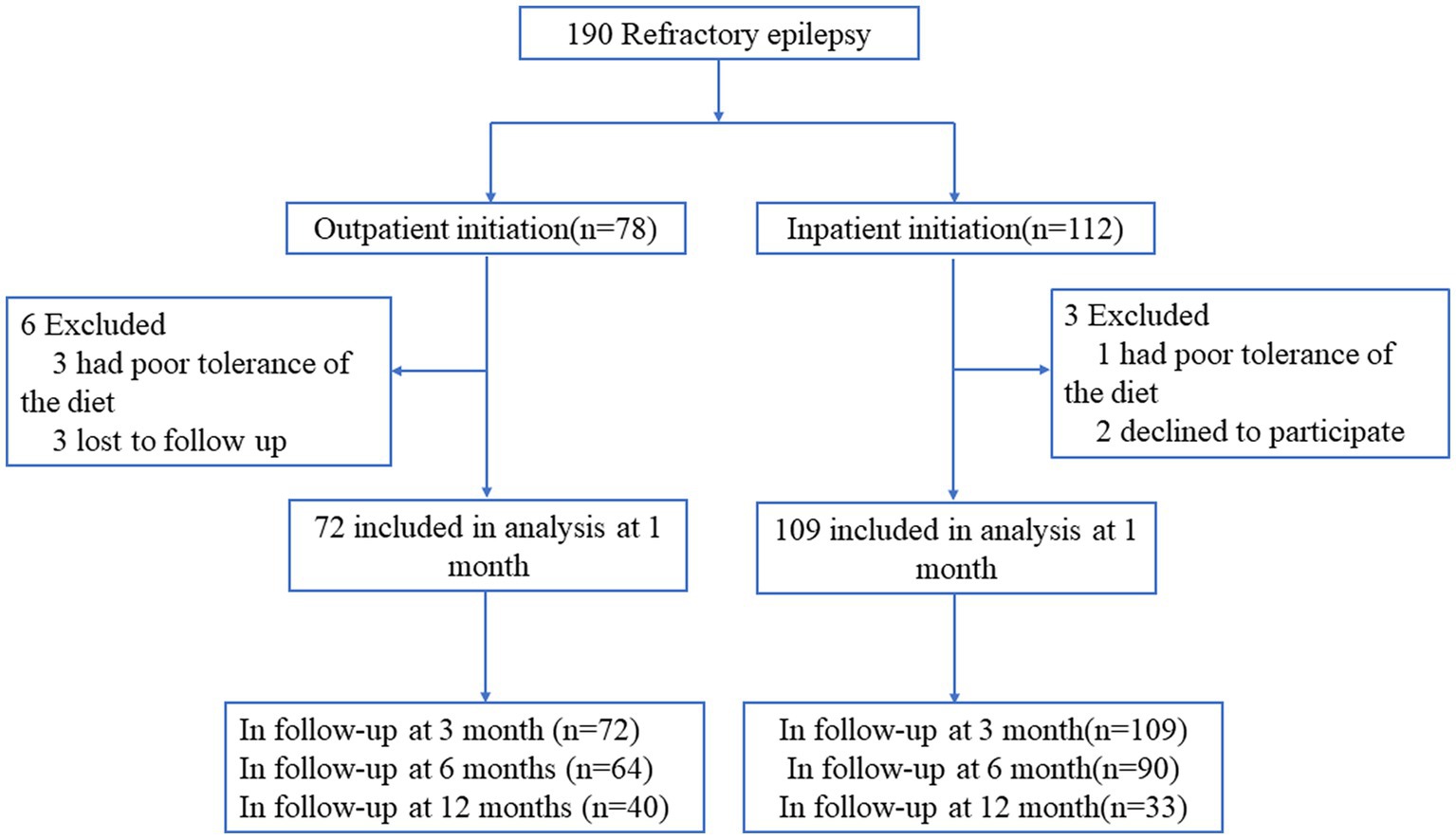
From January 2013 to December 2021, children with refractory epilepsy in the epilepsy center of Children’s Hospital of Nanjing Medical University were enrolled and underwent a ketogenic diet program. Inclusion criteria: the patients ≤16 years old; the patients have tried at least two AEDs; the patients have at least three countable seizures per week; the parents are willing to include their children in the study after written and verbal information; and they were motivated to and capable of adhering to the ketogenic diet. Exclusion criteria: previous treatment with the KD; the patient’s seizures are under acceptable control; with known or suspected inborn errors of metabolism in which KD is contraindicated; changes in the AED type or dose in 1 month before the inclusion; participating in any other epilepsy-related study. We used computer-generated random numbers to randomize patients to receive KD treatment by inpatient initiation or outpatient initiation (in a ratio of 1.5:1). Of 190 children with refractory epilepsy, 112 patients were assigned to the inpatient KD initiation group, and 78 patients were assigned to the outpatient KD initiation group. Detail of participants’ flow including all study exclusions and withdrawals are shown in Figure 1.
KD procedures
Our KD management group consists of physicians, nutritionists, and nurses. A gradual-initiation, non-fasting classic KD treatment protocol was used, which was started at a 1:1 ratio (lipids: nonlipids). Lipid content was gradually increased to 2:1, 2.5:1, 3:1, and 4:1 (23).
Inpatient KD initiation was admitted inpatients and closely monitored for the internal environment and any acute adverse effects during the first week in the hospital. Outpatient KD initiation included 1-day case admission for baseline measurements and parents were educated. All patients were monitored by the medical team regularly to look out for side effects, to ensure nutritional needs are being met, and to assess the diet’s effect on seizure control.
After admission, the KD management group educated the children’s families to prepare the KD at home depending on the child’s regular waking and eating schedule. The data on the children’s seizures and diet (seizure time, manifestation, frequency, duration, KD conditions, and adverse reactions) were recorded by their families. The length of KD treatment was 12 months.
Outcome
The primary clinical outcome was a ≥ 50% reduction in seizure frequency (24) after the introduction of KD. The percentage of patients achieving ≥50% reduction in seizure frequency was assessed at 1, 3, 6, and 12 months of KD treatment.
Body weight was measured to the nearest 0.1 kg using a calibrated electronic scale with participants in light clothing and without shoes. Height was measured to the nearest 0.1 cm with participants without shoes. BMI was computed as kg/m2. Blood ketone body concentrations were measured using an electrochemical capillary blood monitor device with the corresponding individually foil-wrapped test strips for Beta-hydroxybutyrate (BHB).
Adverse events
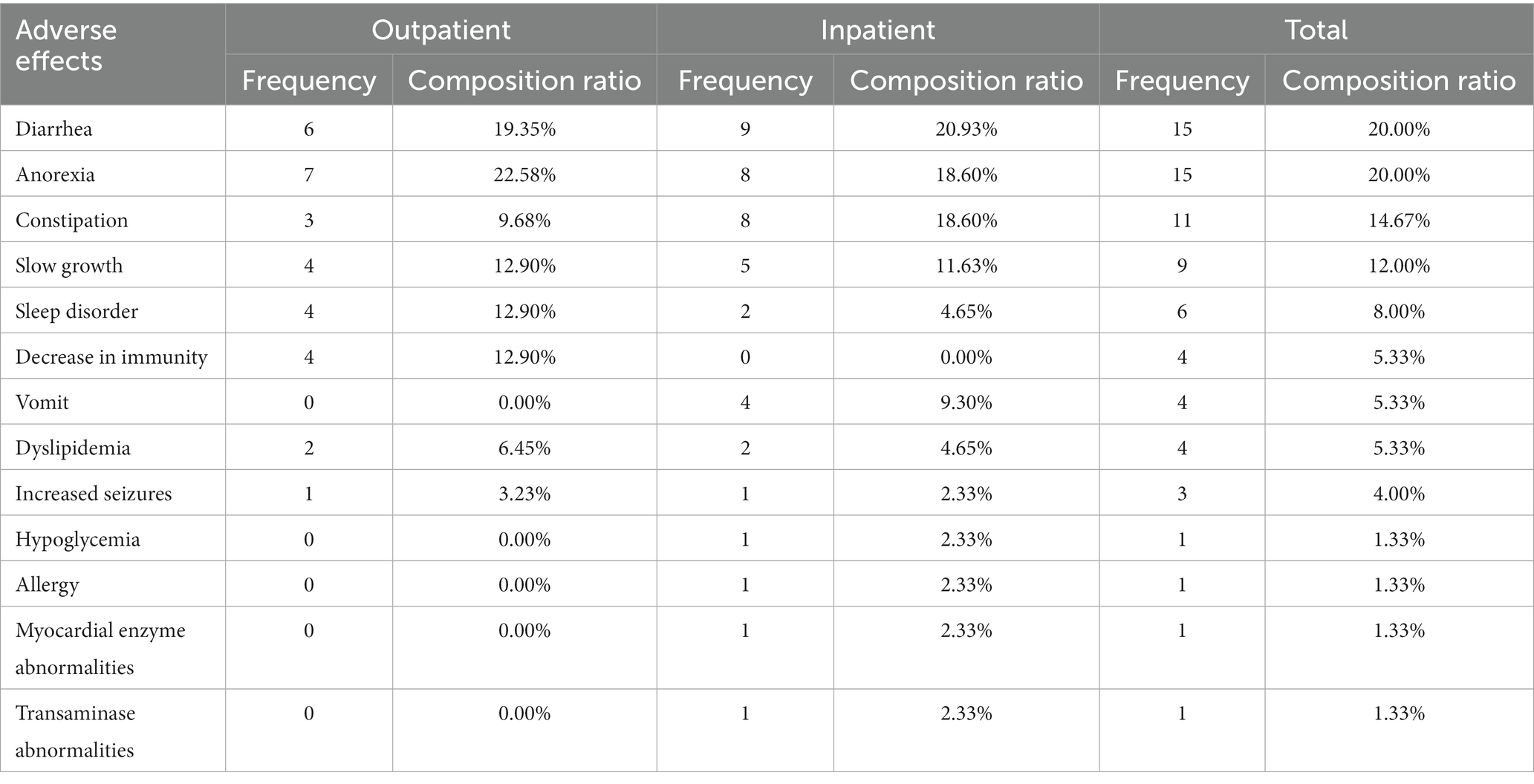
Adverse effects were evaluated using parental questionnaires, including diarrhea, vomiting, anorexia, constipation, decrease in immunity, slow growth, and so on. Participants responded with “yes” or “no,” depending on the presence of symptoms. All adverse effects were listed in Supplementary Table S1.
Statistical analysis
R version 4.1.0 was used for data cleaning and statistical analyses. Data are presented as mean ± standard deviation or frequency (%) as appropriate. The variables between the two groups were compared using independent samples t and Pearson’s Chi-square tests. The generalized estimation equation (GEE) model was used to analyze the longitudinal variables of seizure reduction, ketone body, weight, height, BMI, and BMI Z-score at different follow-up times. The significance level was considered when p < 0.05.
Ethics approval
This study was reviewed and approved by the Human Research Ethics Committee of the Children’s Hospital of Nanjing Medical University (approval ID: 201312030). The patients were recruited during a multi-center study initiated by Jianxiang Liao’s team at Shenzhen Children’s Hospital (Clinical trial registration: ChiCTR-IIR-16008342). All participating subjects received written detailed information and signed consent forms for the interview and the processing of sensitive personal data by their parents. The procedure of our study was performed following the principles stated in the Declaration of Helsinki.
Results
The demographic and clinical characteristics at baseline
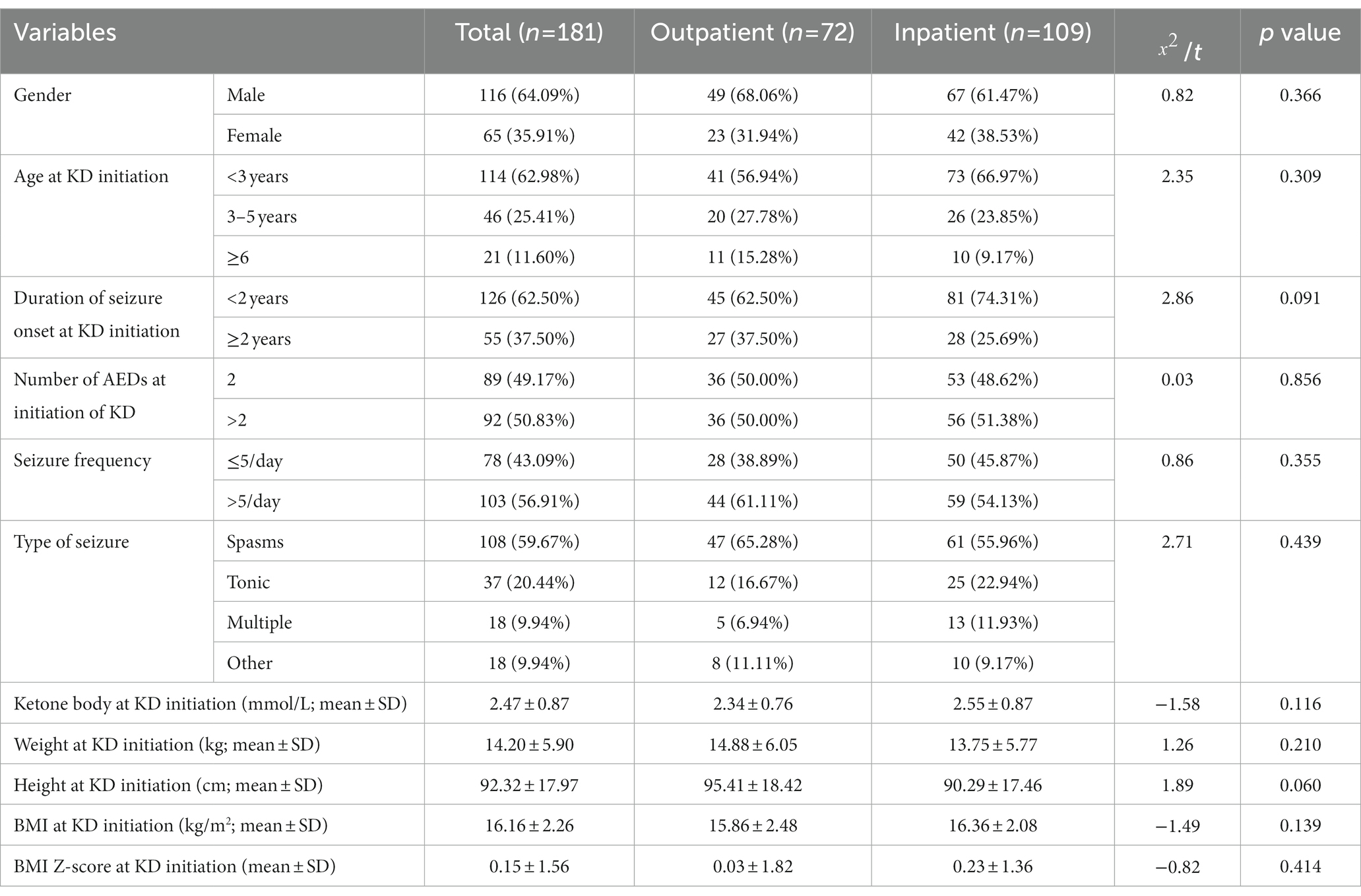
Between January 2013 and December 2021, a total of 190 patients were included in the study, among them, 78 patients were assigned to the outpatient KD initiation group and 112 patients were assigned to the inpatient KD initiation group. Nine patients withdrew before 1 month follow-up, six from the outpatient initiation group and three patients from the inpatient initiation group. Thus leaving 181 patients for intention-to-treat analysis including 109 in the inpatient initiation group and 72 in the outpatient initiation group. Of the 181 patients who remained in the study groups, 116 were males and 65 were females, and 114 patients were younger than 3 years old. Baseline seizure frequency was more than 5 times/day in 123 children, 50.83% were on three or more AEDs, and spasms was the main seizure type (59.67%, 108/181), followed by tonic (20.44%, 37/181), multiple (9.94%, 18/181), and other (9.94%, 18/181).The average blood ketone body, weight, height, BMI, and BMI Z-score at KD initiation of the study groups were 2.47 ± 0.87 mmol/L, 14.20 ± 5.90 kg, 92.32 ± 17.97 cm, 16.16 ± 2.26 kg/m2, and 0.15 ± 1.56, respectively (Table 1). Baseline demographics and clinical characteristics were comparable between the two groups (Table 1). There were no statistical differences between the two groups based on gender, age seizure type, duration of seizure onset, number of AEDs, seizure frequency, type of seizure, weight, height, BMI, and BMI Z-score at KD initiation (all Ps > 0.05). The proportion of patients completing ≥12 months of treatment was higher in the outpatient KD initiation group [40 (55.56%)] than in patients in the inpatient initiation [33 (30.28%); p < 0.001].
Comparison of the efficacy in the different groups and follow-up times based on the GEE model
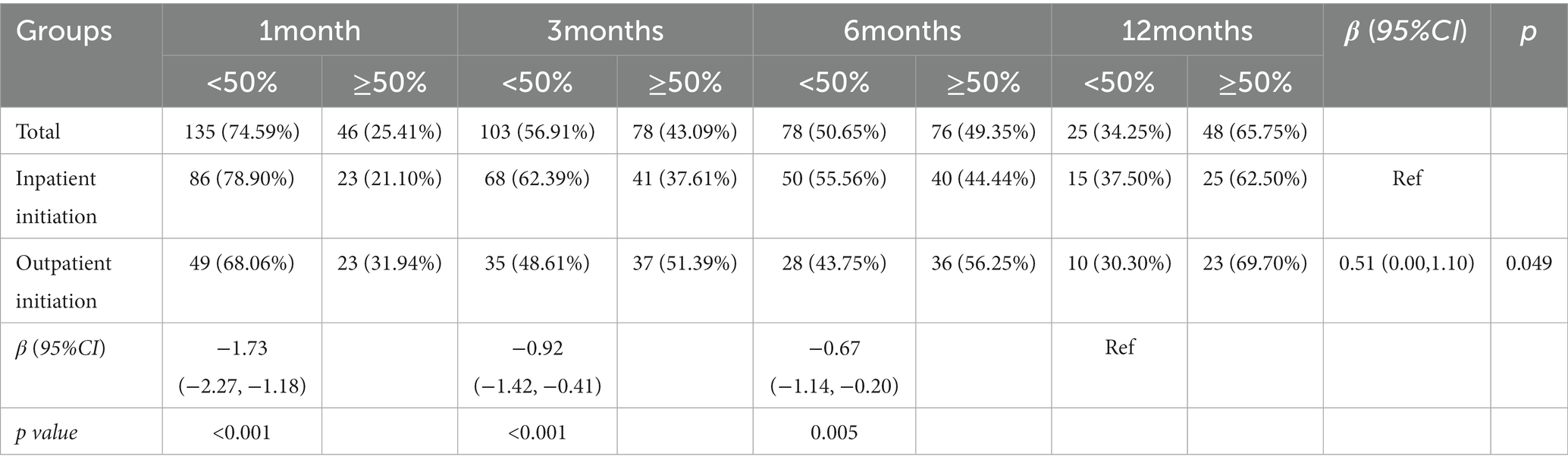
In patients completing 1-month (n = 181), 3 months (n = 181), 6 months (n = 152), and 12 months (n = 72) of KD treatment, the rate of seizure reduction ≥50% was 25.41, 43.09, 49.35, and 65.75%, respectively. For patients in the outpatient initiation group, the rate of seizure reduction ≥50% was 31.94, 51.39, 56.25, and 69.70%, at 1, 3, 6, and 12 months, respectively. The GEE model indicated that the rate of seizure reduction ≥50% in the outpatient initiation group was higher than that of the inpatient initiation group (p = 0.049). Compared with 12 months, the rate of seizure reduction ≥50% was lower at 1, 3, and 6 months (all Ps < 0.01; Table 2).
Comparison of the blood ketone body in the different groups and follow up times based on the GEE
No differences were observed at the beginning of KD initiation between inpatient and outpatient groups for blood ketone body concentration (Table 1). The GEE model indicated that after 12 months of KD treatment, blood ketone body concentrations of patients were higher in the inpatient initiation group than in the outpatient initiation group (p = 0.066). Compared with the baseline, there was no significant difference in blood ketone body concentrations at 1, 3, 6, and 12 months (all Ps > 0.05; Table 3).
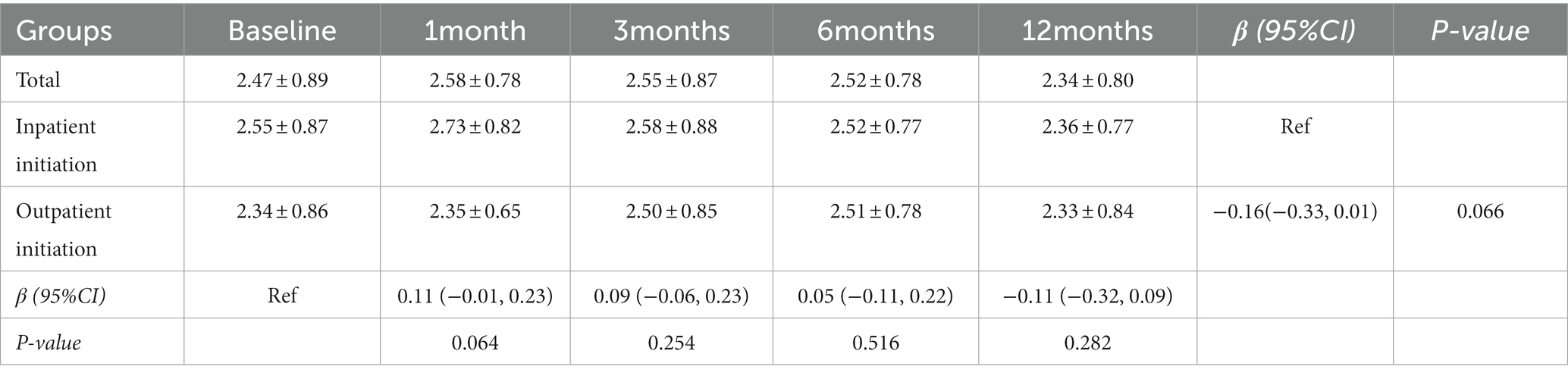
Table 3. Comparison of the blood ketone body in the different groups and follow-up times based on the GEE.
The relation between ketone body and the rate of seizure reduction ≥50%
It is apparent from Table 4 that there is a relation between the rate of seizure reduction ≥50% and blood ketone body level. Spearman’s rank correlation established a negative correlation between the rate of seizure reduction ≥50% and blood ketone body level at 1, 6, and 12 months (all Ps < 0.05).
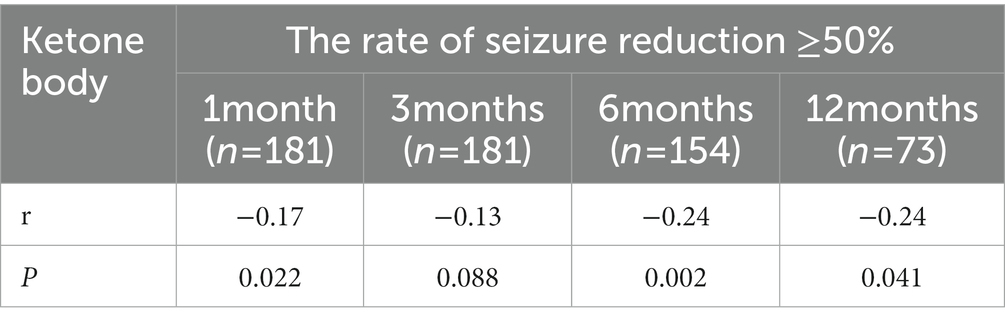
Table 4. The relation between ketone body and the rate of seizure reduction ≥50% at different follow up time.
Comparison of the impact of the KD on growth in children between inpatient and outpatient KD initiation groups
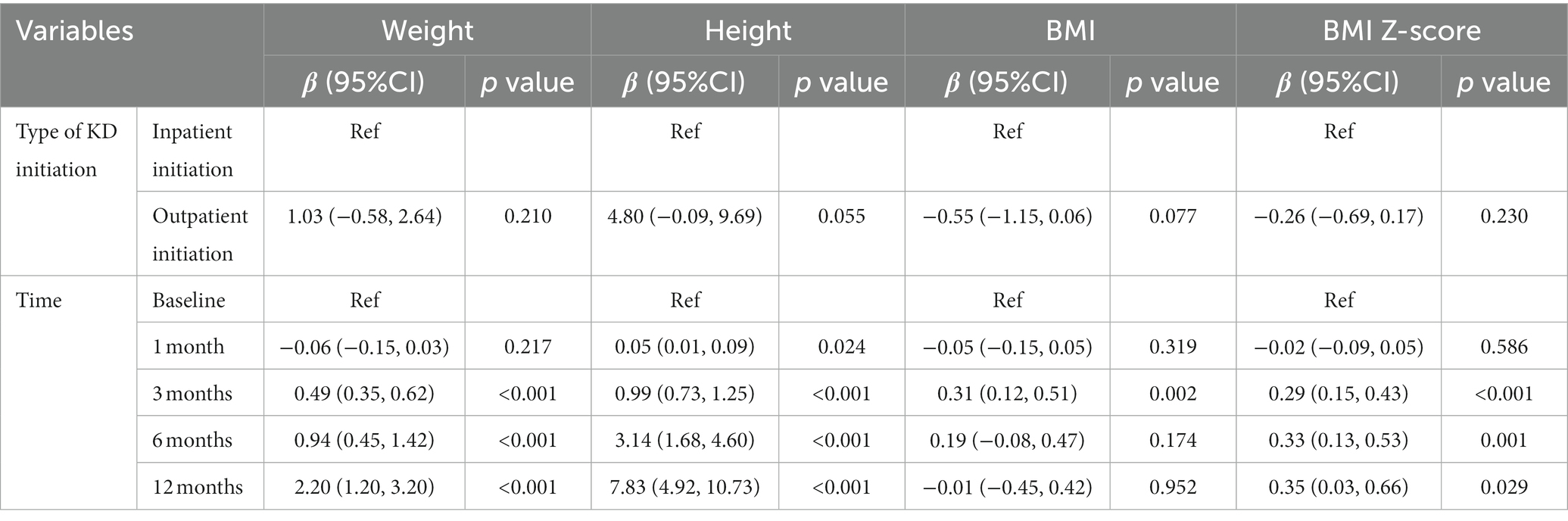
Baseline weight, height, BMI, and BMI Z-score were not significantly different between different KD initiation groups (Table 1), the changes in the growth of patients at different follow-up times were shown in Supplementary Table S2 and Figure 2. Table 5 shows the results of the GEE model, there were no significant differences in height, weight, BMI, and BMI Z-score between the inpatient KD initiation group and outpatient KD initiation group over the 12-month period by the GEE models (all Ps > 0.05). The GEE models also revealed that the mean weight was significantly higher at 3, 6, and 12 months than at the baseline (all Ps < 0.001). Compared to the baseline, the mean height was significantly higher at 1, 3, 6, and 12 months (all Ps < 0.05). For BMI, a significant increase in BMI was observed at the second follow-up (3 months) than the baseline (p = 0.002). BMI-z score was significantly increased after the intervention at 3, 6, and 12 months than the baseline (all Ps < 0.05).
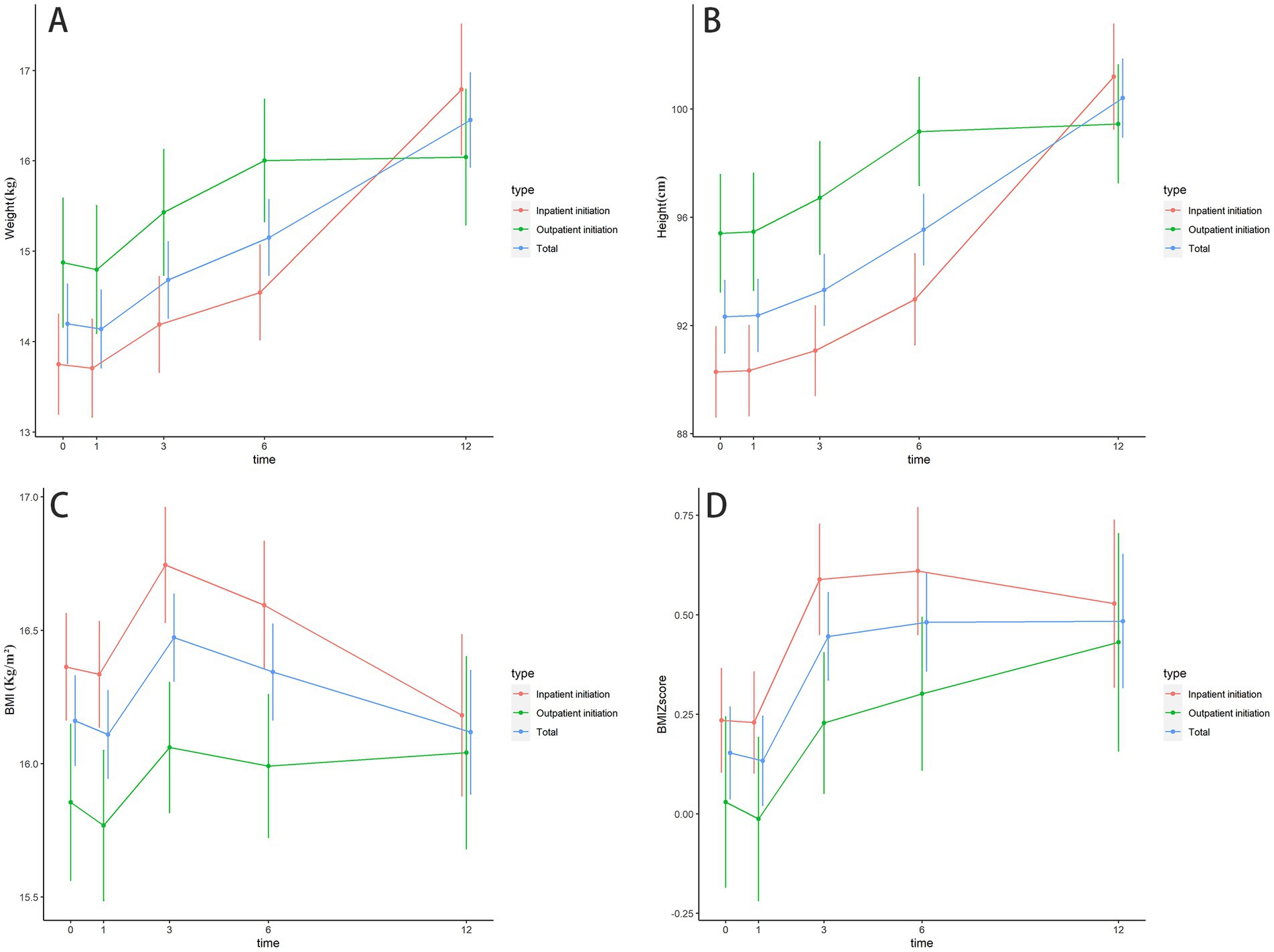
Figure 2. The changes in the growth of patients at different follow up times. (A) The changes in the weight of patients at different follow up times; (B) The changes in height of patients at different follow up times; (C) The changes in BMI of patients at different follow up times; (D) The changes in BMI Z-score of patients at different follow up times.
Adverse effects
Adverse events were reported by 31 patients (43.05%) in the outpatient KD initiation group, and 46 patients (42.20%) in the inpatient KD initiation group, and no statistical differences (p = 0.909) in the incidence of AE were found between the two groups (Table 6). Serious adverse events were not reported in the two groups. The most common adverse events were diarrhea [outpatient group, 6 (19.35%); inpatient group, 9 (20.93%)], anorexia [outpatient group, 7 (22.58%); inpatient group, 8 (18.60%)], constipation [outpatient group, 3 (9.68%); inpatient group, 8 (18.60%)], slow growth [outpatient group, 4 (12.90%); inpatient group, 5 (11.63%)], and sleep disorder [outpatient group, 4 (12.90%); inpatient group, 4 (4.65%); Table 6].
Discussion
Ketogenic diet is an ideal therapeutic tool for people with epilepsy and in particular for children with refractory epilepsy (25). More than 80% of the centers still routinely begin the classic KD in the hospital, to better follow the children, promptly operate the necessary adjustments, and train the parents to be able to properly manage epilepsy at home (16). Initiation of the KD in an inpatient setting is costly and often involves a considerable social and economic burden for parents and children, which caused barriers for patients to gain access to the KD (14). Since the outbreak of COVID-19 in early 2020, people’s life, study, work, and medical treatment were seriously affected (26). Due to the measure of city-wide lockdowns, hospital admissions fell dramatically (27), people with epilepsy and their caregivers reported an overall increase in seizures, with difficulties accessing medical care, particularly medications, investigations, information, and self-management (28). Thus, the inpatient initiation of KD for epilepsy has been severely impacted, highlighting the importance of outpatient initiation of KD.
Previous studies suggested that outpatient KD initiation is no worse than inpatient initiation in terms of effectiveness (16, 29), a significantly better response was observed in the outpatient KD initiation group in the treatment of refractory epilepsy in our study. On the other hand, we found the 12-month retention rate of the outpatient KD initiation group was higher than that of the inpatient KD initiation group. Moreover, there was no statistical difference in the comparison results in terms of blood ketone value, growth, and side effects. Our findings support the initiation of a ketogenic diet in outpatient settings. Telemedicine use among healthcare providers and patients was increased because of the COVID-19 pandemic (28, 30), and it has been helping healthcare workers to assess, diagnose, monitor, treat, and educate patients. With the help of telemedicine, we can greatly promote the popularization of outpatient initiation of KD, and facilitate the two-way interaction between patients and the management team of the ketogenic diet.
While consuming a ketogenic diet, ketone bodies were generated in the mitochondrial matrix of liver cells (31), which have long been thought to have a direct antiseizure effect but have not been proven (32). Ketone bodies include BHB, acetoacetic acid, and acetone. For clinical purposes, blood BHB testing is widely used for assessing ketone bodies (33). Previous studies have found inconsistent results on the relationship between blood BHB and seizure control (32, 34–39). In our study, we found that the blood BHB was increased at the beginning of KD initiation consistence with a similar previous study (34). We also observed a negative correlation between the blood ketone body and the reduction in seizure frequency at 1, 6, and 12 months, however, the ultimate validation of their role in the clinical setting has yet to be firmly demonstrated (38).
A KD is known to lead weight loss and is considered to be metabolically healthy (40). Several reports have indicated poor linear growth in children and very young children grow poorly on the diet (41, 42). However, our findings were opposite to existing literature, and a growth deficit was identified in only 5% (9/181) of the study participants, this ratio is significantly lower than previous study (43, 44). The sample size of our study is larger than similar studies (43, 44), so our findings are accurate and representative. Taken together, our findings demonstrate that growth retardation may occur in a minority of patients treated with KD.
Our data indicated adverse effects of the KD were mild, vomiting, constipation, and diarrhea were reported commonly. Because a gradual-initiation KD was used in our study, lipid content was gradually increased, this gradual process can avoid metabolic acidosis (45), and give children enough time to tolerate the ketogenic diet, resulting in fewer adverse effects (46). Although it was not sure whether these adverse effects are actually due to the implemented KD or perhaps an underlying disorder, KD is required to be performed under careful medical supervision. The family should also be informed about how to recognize the symptoms of adverse effects early. Most of the side effects were tolerable after adjustment of the KD and symptomatic treatment (47).
Conclusion
Our study shows that outpatient KD initiation is a safe and effective treatment for children with refractory epilepsy. There was no statistical difference in the comparison results in terms of blood ketone body, growth, and side effects between different initiation of KD, and therefore, outpatient initiation of KD should be encouraged.
Limitations
Our study also has some limitations that need to be addressed. First, the sample size is relatively small, and the simple randomization may have resulted in an unequal number of participants among groups. However, we compared the baseline data of the two groups, and there was no statistical difference. Second, we did not conduct an adherence survey of those patients who discontinued ketogenic diet intervention during the follow-up period. Third, the missing data in the follow-up coul have biased our results, even though we used the GEE model to handle missing values in longitudinal data.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by the Human Research Ethics Committee of the Children’s Hospital of Nanjing Medical University (approval ID: 201312030). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
WL: writing-original draft. XH: methodology. WG: formal analysis. CL: investigation. FT: software. LD: resources. XL: data curation. JL: supervision. HG: project administration. GZ: writing-review and editing. CW: conceptualization and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The study was financed by the Medical Scientific Research Project of Jiangsu Provincial Health Commission (ZD2022053) and China Postdoctoral Science Foundation (2022M721682).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1146349/full#supplementary-material
References
1. Dwivedi, R, Ramanujam, B, Chandra, PS, Sapra, S, Gulati, S, Kalaivani, M, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. (2017) 377:1639–47. doi: 10.1056/NEJMoa1615335
2. Singh, G, and Sander, JW. The global burden of epilepsy report: implications for low- and middle-income countries. Epilepsy Behav. (2020) 105:106949. doi: 10.1016/j.yebeh.2020.106949
3. Beghi, E, Giussani, G, Nichols, E, Abd-Allah, F, Abdela, J, and Abdelalim, A. Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:357–75. doi: 10.1016/S1474-4422(18)30454-X
4. Vezzani, A, Fujinami, RS, White, HS, Preux, PM, Blümcke, I, Sander, JW, et al. Infections, inflammation and epilepsy. Acta Neuropathol. (2016) 131:211–34. doi: 10.1007/s00401-015-1481-5
5. Attumalil, TV, Sundaram, A, Varghese, VO, Vijayakumar, K, and Kunju, PA. Risk factors of childhood epilepsy in Kerala. Ann Indian Acad Neurol. (2011) 14:283–6. doi: 10.4103/0972-2327.91950
6. Vlachy, J, Jo, M, Li, Q, Ayer, T, Keskinocak, P, Swann, J, et al. Risk factors for seizures among young children monitored with continuous electroencephalography in intensive care unit: a retrospective study. Front Pediatr. (2018) 6:303. doi: 10.3389/fped.2018.00303
7. WHO (2022). Epilepsy [Online]. Available at: https://www.who.int/news-room/fact-sheets/detail/epilepsy
8. Fiest, KM, Sauro, KM, Wiebe, S, Patten, SB, Kwon, CS, Dykeman, J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. (2017) 88:296–303. doi: 10.1212/WNL.0000000000003509
9. Ding, D, Zhou, D, Sander, JW, Wang, W, Li, S, and Hong, Z. Epilepsy in China: major progress in the past two decades. Lancet Neurol. (2021) 20:316–26. doi: 10.1016/S1474-4422(21)00023-5
10. Perucca, P, Scheffer, IE, and Kiley, M. The management of epilepsy in children and adults. Med J Aust. (2018) 208:226–33. doi: 10.5694/mja17.00951
11. Kwan, P, Arzimanoglou, A, Berg, AT, Brodie, MJ, Allen Hauser, W, Mathern, G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. (2010) 51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x
12. Kwan, P, and Brodie, MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. (2010) 9:27–9. doi: 10.1016/S1474-4422(09)70304-7
13. Alqahtani, F, Imran, I, Pervaiz, H, Ashraf, W, Perveen, N, Rasool, MF, et al. Non-pharmacological interventions for intractable epilepsy. Saudi Pharm J. (2020) 28:951–62. doi: 10.1016/j.jsps.2020.06.016
14. Kossoff, EH, Zupec-Kania, BA, Auvin, S, Ballaban-Gil, KR, Christina Bergqvist, AG, Blackford, R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the international ketogenic diet study group. Epilepsia Open. (2018) 3:175–92. doi: 10.1002/epi4.12225
15. Ułamek-Kozioł, M, Czuczwar, SJ, Januszewski, S, and Pluta, R. Ketogenic Diet and Epilepsy. Nutrients. (2019) 11:2510. doi: 10.3390/nu11102510
16. Van Der Louw, E, Olieman, J, Poley, MJ, Wesstein, T, Vehmeijer, F, Catsman-Berrevoets, C, et al. Outpatient initiation of the ketogenic diet in children with pharmacoresistant epilepsy: an effectiveness, safety and economic perspective. Eur J Paediatr Neurol. (2019) 23:740–8. doi: 10.1016/j.ejpn.2019.06.001
17. Armeno, M, Caballero, E, Verini, A, Reyes, G, Galarza, N, Cresta, A, et al. Telemedicine- versus outpatient-based initiation and management of ketogenic diet therapy in children with drug-resistant epilepsy during the COVID-19 pandemic. Seizure. (2022) 98:37–43. doi: 10.1016/j.seizure.2022.03.023
18. Wirrell, EC, Grinspan, ZM, Knupp, KG, Jiang, Y, Hammeed, B, Mytinger, JR, et al. Care delivery for children with epilepsy during the COVID-19 pandemic: an international survey of clinicians. J Child Neurol. (2020) 35:924–33. doi: 10.1177/0883073820940189
19. Kuroda, N, and Fujimoto, A. Considerations for continuing diet therapy in patients with epilepsy during the COVID-19 pandemic: a scoping review. Epilepsy Behav Rep. (2021) 16:100498. doi: 10.1016/j.ebr.2021.100498
20. Craiu, DC. Outpatient initiation of the ketogenic diet. Eur J Paediatr Neurol. (2019) 23:672–3. doi: 10.1016/j.ejpn.2019.09.007
21. Lin, A, Turner, Z, Doerrer, SC, Stanfield, A, and Kossoff, EH. Complications during ketogenic diet initiation: prevalence, treatment, and influence on seizure outcomes. Pediatr Neurol. (2017) 68:35–9. doi: 10.1016/j.pediatrneurol.2017.01.007
22. Vaisleib, I, Buchhalter, JR, and Zupanc, ML. Ketogenic diet: outpatient initiation, without fluid, or caloric restrictions. Pediatr Neurol. (2004) 31:198–202. doi: 10.1016/j.pediatrneurol.2004.03.007
23. Sondhi, V, Agarwala, A, Pandey, RM, Chakrabarty, B, Jauhari, P, Lodha, R, et al. Efficacy of ketogenic diet, modified Atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: a randomized clinical trial. JAMA Pediatr. (2020) 174:944–51. doi: 10.1001/jamapediatrics.2020.2282
24. Klein, P, Johnson, ME, Schiemann, J, and Whitesides, J. Time to onset of sustained ≥50% responder status in patients with focal (partial-onset) seizures in three phase III studies of adjunctive brivaracetam treatment. Epilepsia. (2017) 58:e21–5. doi: 10.1111/epi.13631
25. Zarnowska, IM. Therapeutic use of the ketogenic diet in refractory epilepsy: what we know and what still needs to be learned. Nutrients. (2020) 12:2616. doi: 10.3390/nu12092616
26. Moynihan, R, Sanders, S, Michaleff, ZA, Scott, AM, Clark, J, To, EJ, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. (2021) 11:e045343. doi: 10.1136/bmjopen-2020-045343
27. Birkmeyer, JD, Barnato, A, Birkmeyer, N, Bessler, R, and Skinner, J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. (2020) 39:2010–7. doi: 10.1377/hlthaff.2020.00980
28. Cross, JH, Kwon, CS, Asadi-Pooya, AA, Balagura, G, Gómez-Iglesias, P, Guekht, A, et al. Epilepsy care during the COVID-19 pandemic. Epilepsia. (2021) 62:2322–32. doi: 10.1111/epi.17045
29. Armeno, M, Verini, A, Caballero, E, Cresta, A, Valenzuela, GR, and Caraballo, R. Long-term effectiveness and adverse effects of ketogenic diet therapy in infants with drug-resistant epilepsy treated at a single center in Argentina. Epilepsy Res. (2021) 178:106793. doi: 10.1016/j.eplepsyres.2021.106793
30. Bukstein, DA, Eghrari-Sabet, J, Hart, M, Hill, T, Parikh, P, and Winders, TA. COVID-19 pandemic impact on telehealth use and perceptions for atopic and respiratory disease: survey results. Allergy Asthma Proc. (2022) 43:194–201. doi: 10.2500/aap.2022.43.220019
31. Vidali, S, Aminzadeh, S, Lambert, B, Rutherford, T, Sperl, W, Kofler, B, et al. Mitochondria: the ketogenic diet--a metabolism-based therapy. Int J Biochem Cell Biol. (2015) 63:55–9. doi: 10.1016/j.biocel.2015.01.022
32. Poff, AM, Rho, JM, and D'agostino, DP. Ketone Administration for Seizure Disorders: history and rationale for ketone esters and metabolic alternatives. Front Neurosci. (2019) 13:1041. doi: 10.3389/fnins.2019.01041
33. Musa-Veloso, K, Rarama, E, Comeau, F, Curtis, R, and Cunnane, S. Epilepsy and the ketogenic diet: assessment of ketosis in children using breath acetone. Pediatr Res. (2002) 52:443–8. doi: 10.1203/00006450-200209000-00023
34. Buchhalter, JR, D'alfonso, S, Connolly, M, Fung, E, Michoulas, A, Sinasac, D, et al. The relationship between d-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open. (2017) 2:317–21. doi: 10.1002/epi4.12058
35. Gilbert, DL, Pyzik, PL, and Freeman, JM. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J Child Neurol. (2000) 15:787–90. doi: 10.1177/088307380001501203
36. Si, J, Wang, Y, Xu, J, and Wang, J. Antiepileptic effects of exogenous β-hydroxybutyrate on kainic acid-induced epilepsy. Exp Ther Med. (2020) 20:1. doi: 10.3892/etm.2020.9307
37. Simeone, TA, Simeone, KA, and Rho, JM. Ketone Bodies as Anti-Seizure Agents. Neurochem Res. (2017) 42:2011–8. doi: 10.1007/s11064-017-2253-5
38. Simeone, TA, Simeone, KA, Stafstrom, CE, and Rho, JM. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology. (2018) 133:233–41. doi: 10.1016/j.neuropharm.2018.01.011
39. Van Delft, R, Lambrechts, D, Verschuure, P, Hulsman, J, and Majoie, M. Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure. (2010) 19:36–9. doi: 10.1016/j.seizure.2009.10.009
40. Grandl, G, Straub, L, Rudigier, C, Arnold, M, Wueest, S, Konrad, D, et al. Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J Physiol. (2018) 596:4597–609. doi: 10.1113/JP275173
41. Alaei, M, Ghazavi, MR, Mahvelati, F, Karimzadeh, P, Shiva, MR, and Tonekaboni, SH. The effect of the ketogenic diet on the growth and biochemical parameters of the children with resistant epilepsy. Iran J Child Neurol. (2010) 3:41–4.
42. Vining, EP, Pyzik, P, Mcgrogan, J, Hladky, H, Anand, A, Kriegler, S, et al. Growth of children on the ketogenic diet. Dev Med Child Neurol. (2002) 44:796–802. doi: 10.1111/j.1469-8749.2002.tb00769.x
43. Ferraris, C, Guglielmetti, M, Pasca, L, De Giorgis, V, Ferraro, OE, Brambilla, I, et al. Impact of the ketogenic diet on linear growth in children: a single-center retrospective analysis of 34 cases. Nutrients. (2019) 11:1442. doi: 10.3390/nu11071442
44. Wibisono, C, Rowe, N, Beavis, E, Kepreotes, H, Mackie, FE, Lawson, JA, et al. Ten-year single-center experience of the ketogenic diet: factors influencing efficacy, tolerability, and compliance. J Pediatr. (2015) 166:1030–1036.e1. doi: 10.1016/j.jpeds.2014.12.018
45. Bjurulf, B, Magnus, P, Hallböök, T, and Strømme, P. Potassium citrate and metabolic acidosis in children with epilepsy on the ketogenic diet: a prospective controlled study. Dev Med Child Neurol. (2020) 62:57–61. doi: 10.1111/dmcn.14393
46. Bergqvist, AG, Schall, JI, Gallagher, PR, Cnaan, A, and Stallings, VA. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia. (2005) 46:1810–9. doi: 10.1111/j.1528-1167.2005.00282.x
Keywords: refractory epilepsy, ketogenic diet, pediatric, generalized estimating equations, inpatient initiation, outpatient initiation
Citation: Li W, Hao X, Gu W, Liang C, Tu F, Ding L, Lu X, Liao J, Guo H, Zheng G and Wu C (2023) Analysis of the efficacy and safety of inpatient and outpatient initiation of KD for the treatment of pediatric refractory epilepsy using generalized estimating equations. Front. Neurol. 14:1146349. doi: 10.3389/fneur.2023.1146349
Edited by:
Fernando Cendes, State University of Campinas, BrazilReviewed by:
Yaodong Zhang, Children's Hospital Affiliated to Zhengzhou University, ChinaSifat Sharmin, The University of Melbourne, Australia
Copyright © 2023 Li, Hao, Gu, Liang, Tu, Ding, Lu, Liao, Guo, Zheng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunfeng Wu, Y2h1bmZlbmdAMjAwOC5zaW5hLmNvbQ==
†These authors have contributed equally to this work
 Wei Li
Wei Li Xiaoyan Hao1†
Xiaoyan Hao1† Fulai Tu
Fulai Tu