- 1Department of Pharmacy, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Pharmacy, The People's Hospital of Xin Tai City, Taian, China
- 3Stem Cell Clinical Institute, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
A 14-year-old child was diagnosed with complex regional pain syndrome (CRPS) after bromhidrosis surgery. She experienced a stinging, knife-like, and intermittent attack pain, accompanied by numbness of both upper limbs and limited movements. Ultrasound-guided radiofrequency surgery on the peripheral nerve did not reduce pain. Then, gabapentin 300 mg three times a day and 2% lidocaine by local subcutaneous injection once a day for 3 days were administrated. After the local subcutaneous injection of lidocaine, the pain was significantly relieved, and the pain induced by skin touch at the scar disappeared. No pain recurred after the 1-month follow-up. An evidence-based literature review showed that local or systemic intravenous lidocaine was used to reduce adult CRPS symptoms but less has been reported in children. In our case, a local subcutaneous injection of 2% lidocaine in a child for CRPS treatment was reported to be effective in relieving complex local pain with favorable outcomes. Though further high-quality randomized controlled trials are needed to investigate the effects and safety of local subcutaneous lidocaine injection on pain relief in children with CRPS, it could still provide a relatively safe and effective adjuvant therapy for minor patients.
1. Introduction
Complex regional pain syndrome (CRPS) is usually secondary to primary trauma and may be a syndrome of severe intractable, polytropic pain following fracture, postoperative or unintentional minor trauma, characterized by malnutrition and dysfunction (1). The fourth edition of the Diagnosis and Treatment Guidelines for CRPS in 2013 pointed out that the main clinical features are acute pain inconsistent with the original trauma, and clinical manifestations are pain, edema, erythema, hyperthermia, hypofunction, and so on. There are two typical types of sympathetic pain disorders: CRPS type I, which has no nerve damage, and CRPS type II, which has definite nerve damage and typical neuropathic pain features (2). The incidence of CRPS was 5–25 per 1,00,000, the ratio of male to female was 1:2-3, and the ratio of upper limb to lower limb was 2:1 (3), but the pathological pathogenesis of CRPS was still unknown.
The primary treatment of CRPS is multimodal pain management, which includes bisphosphonates, glucocorticoids, non-steroidal anti-inflammatory drugs, tricyclic antidepressants, antiepileptics, NMDA receptor antagonists, and drugs such as calcitonin and Botox. In addition, there are also other options such as psychological therapy, physical therapy, surgery, and interventional therapy. Minimally invasive interventional therapy mainly includes sympathetic nerve block, spinal cord electrical stimulation, and ultrasound-guided pulsed radiofrequency. Surgical treatment mainly includes lesion of dorsal root of spinal cord and neurolytic sympathetic procedures (1, 2).
We present a unique case of a child detailing the use of local subcutaneous lidocaine injection, which is poorly documented in the literature. An evidence-based literature review was conducted to help us scientifically analyze and grasp the safety and effectiveness of analgesic drugs.
2. Case presentation
A 14-year-old child (168 cm, 58 kg) had been diagnosed with complex regional pain syndrome (CRPS) on 28 October 2022. Two months after a bromhidrosis surgery, the patient developed axillary pain with numbness in both upper limbs. The pain site was the axillary region, radiating to both upper limbs with pain numbness and limited movements. The numerical rating scale (NRS) was 8 points. The patient reported pain as stinging, knife-like, and intermittent attacks, with a frequency of 6–8 episodes per day, each lasting 10–20 min. The pain was worse at night than during the day, occasionally interfering with sleep. The child had no significant weight loss and denied a history of chronic medical conditions such as high blood pressure.
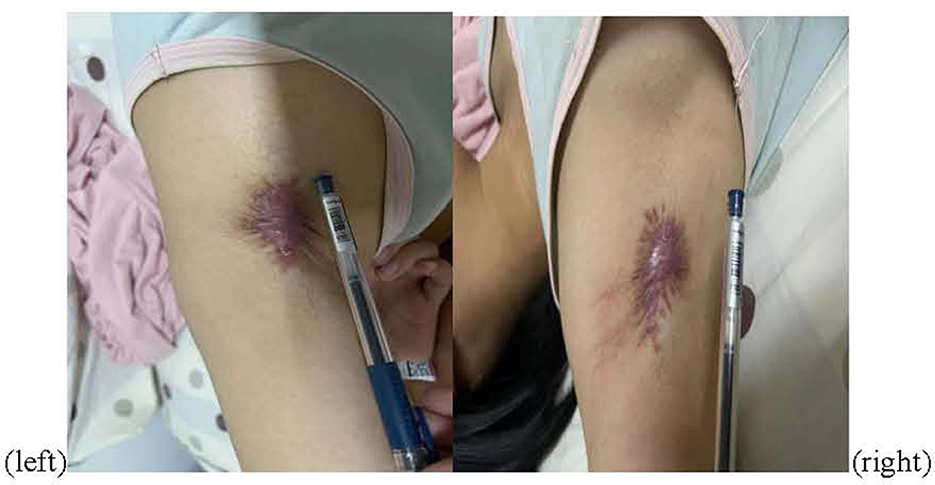
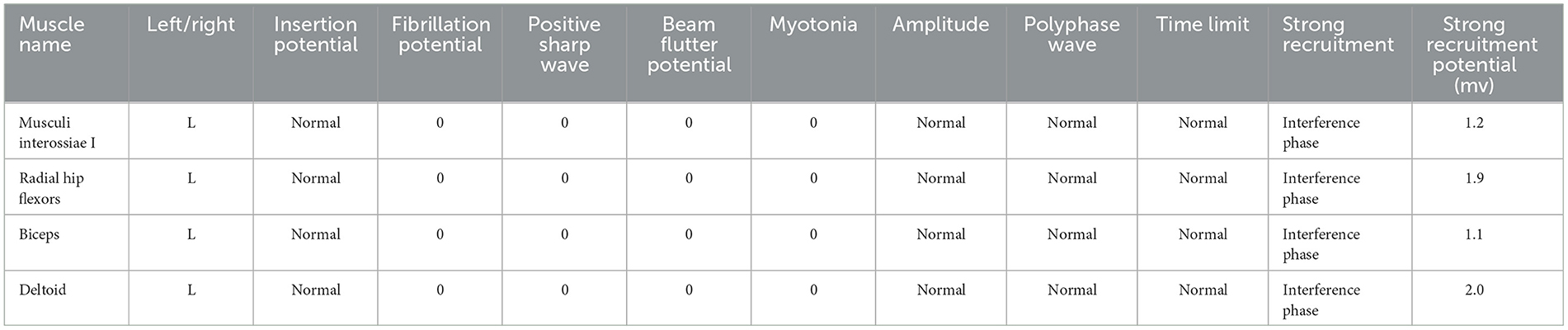
The patient received one local injection of scar softening needle on 14 October 2022 and then applied mupirocin ointment and human epidermal growth factor gel to the scar. The color of the scar deepened and the contracture improved, but the pain did not improve significantly. Physical examination revealed bilateral axillary surgical scars of 5 * 2 cm (Figure 1), peripheral skin contracture, local tenderness (++), limited movement of both upper limbs, abductor lift of 120°, forward flexion lift of 150°, back extension test to the medial margin of scapula, random contact of both upper limbs on the ulnar side caused pain, and no atrophy of the skin, tissues, and muscles of both upper limbs. The ulnar touch induced pain in both upper limbs (+), severe pain in the left armpit, numbness in the bilateral axilla of both upper limbs, and contracture of the hands and feet during the attack. The electromyography-evoked potential report showed no significant abnormalities in nerve motor conduction, sensory conduction, F-wave, or muscles (Table 1). Bilateral axillary ultrasound, blood, urine, and stool tests were normal. The patient met the Budapest diagnostic criteria for CRPS established by Harden in 2007 (4).
3. Therapeutic intervention and results
The patient was admitted to the hospital on 30th October and was given oral ibuprofen. Flurbiprofen ester gel ointment was applied to her right armpit for pain relief. On 1st November, radiofrequency surgery was performed on the left periaxillary nerve under ultrasound guidance, and 1 ml anti-inflammatory analgesic solution (2% lidocaine l ml +0.9% sodium chloride solution 2 ml + dexamethasone 5 mg) was given. The patient reported unbearable pain during the surgery. On the 1st day after the surgery, the patient reported increased pain in the left axilla, with no significant improvement in pain duration and attack frequency. The NRS score was 9 points. The patient was given gabapentin 300 mg, three times a day. However, after taking gabapentin, the child experienced dizziness and drowsiness. Considering that gabapentin was highly correlated with adverse reactions, the patient refused to continue taking gabapentin. After a comprehensive evaluation by clinical pharmacists and doctors, the patient was given a 3 ml local subcutaneous injection of 2% lidocaine. On 5th November, the patient reported significant relief in bilateral axillary pain, and the NRS score decreased from 8 to 3 points at the onset of pain. No adverse reactions were observed during the administration. The patient was discharged immediately. After 1 month of discharge, the patient was followed up by the clinical pharmacist, and the frequency and duration of the pain were significantly relieved. The pain disappeared completely with the subsequent application of lidocaine gel patches.
4. Discussion
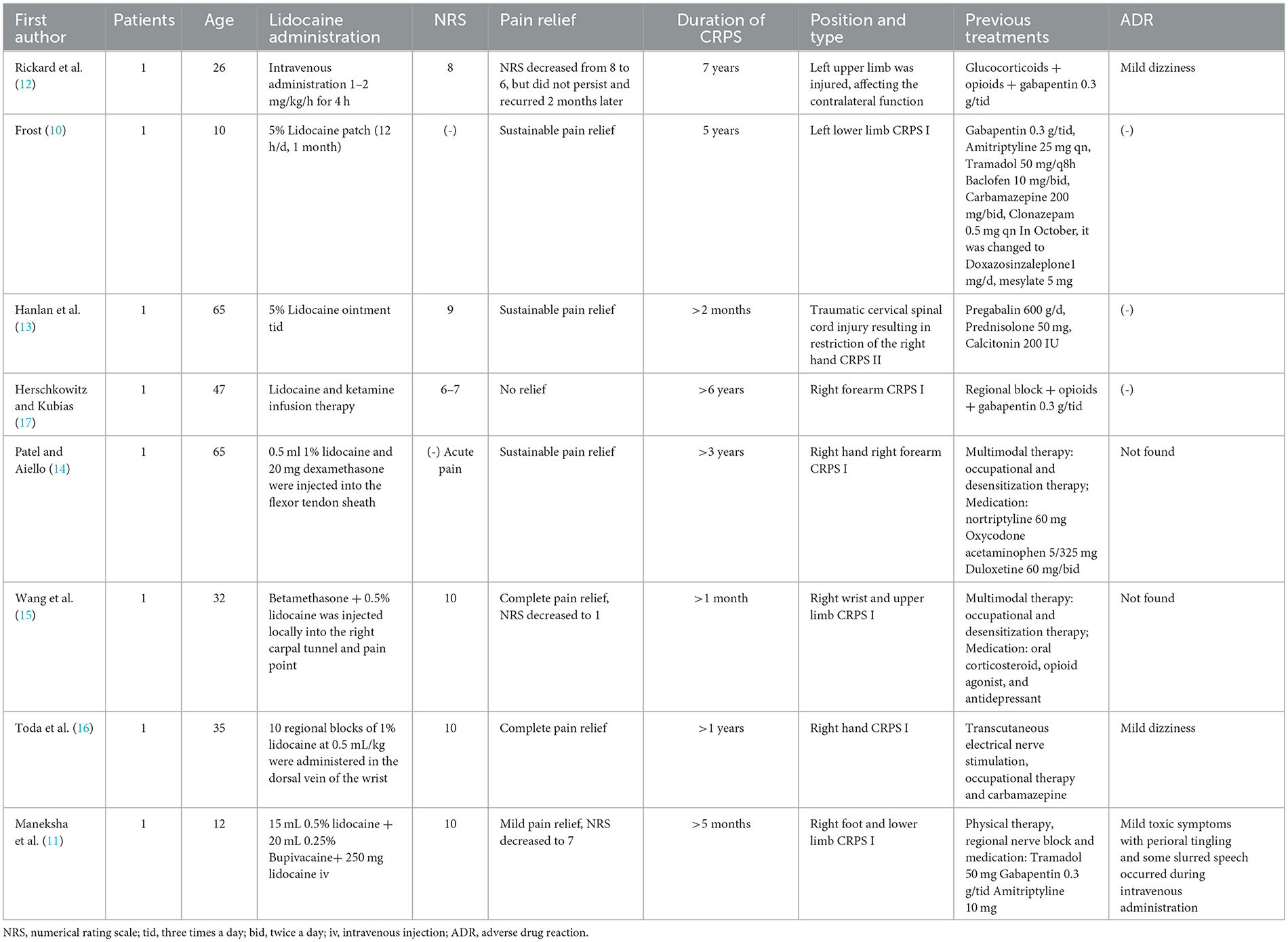
Lidocaine was used as an adjunct to CRPS, and local subcutaneous injection was rarely published (5–9). To date and to the best of our knowledge, only eight cases of CRPS with different lidocaine administration methods were retrieved (Table 2), of which six were adults and two were children The history of CRPS was more than 1 month, and the longest duration was 7 years. The pain was mainly concentrated in the upper and lower limbs and affected the patients' normal life. Of the two children, one received a 5% lidocaine local patch (10) and the other received a local block of 0.5% lidocaine 15 mL and 0.25% bupivacaine 20 mL, followed by an intravenous infusion of 250 mg lidocaine (11). Among the six adult patients, a 26-year-old patient was given intravenous medication at 1–2mg/kg/h for 4 h (12), one patient was given 5% lidocaine ointment three times a day (13), and four patients were given a local block therapy with combination medication (11, 14–16). Except for one patient who received a systemic infusion of lidocaine and ketamine without any pain relief (17), all the other patients achieved varying degrees of pain relief, of which two patients did not achieve sustained pain relief (11, 12).
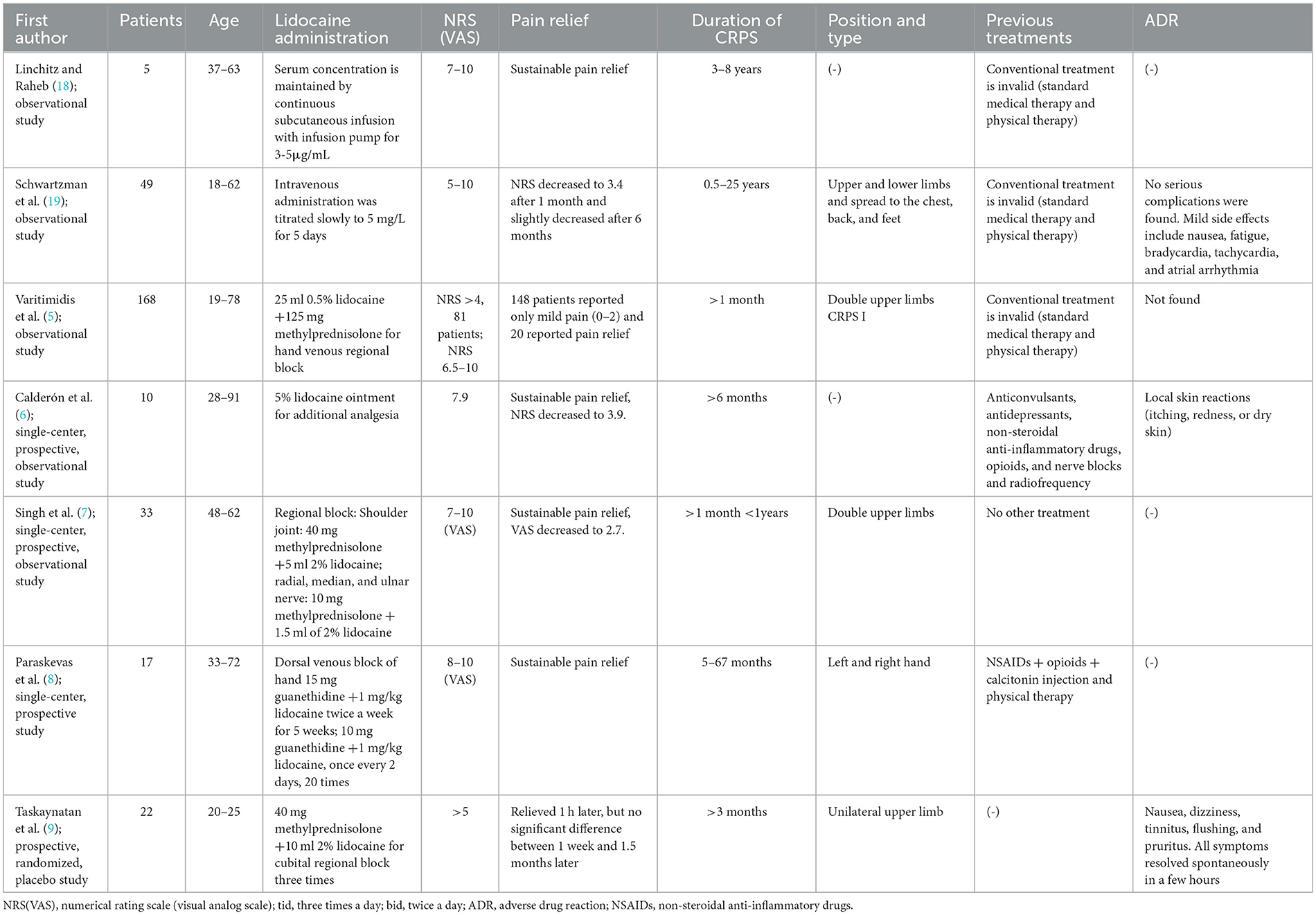
In addition, we retrieved seven clinical trials of lidocaine for CRPS (Table 3), including three observational studies and four prospective studies. Intravenous lidocaine was administered in two clinical trials, one sustained subcutaneous infusion of lidocaine 3–5μg/ml (18), and one intravenous infusion of lidocaine 5mg/L for 5 days (19). There were three trials of lidocaine combined with other agents for regional block (5, 7, 8), and one lidocaine patch as an additional analgesic (6). With the exception of 49 patients, mentioned in a study by Schwartzman et al. (19), who relapsed after 6 months of pain relief, all patients in these trials achieved significant pain relief and no recurrence. Moreover, the duration of remission was not associated with mean lidocaine levels or highest measured lidocaine levels but was significantly associated with baseline pain intensity. There was a randomized controlled double-blind trial of lidocaine for regional block. Taskaynatan et al. found that in 22 patients with CRPS, 2% lidocaine combined with 40 mg methylprednisolone did not provide lasting pain relief and only relieved pain for 1 h (9).
In terms of safety, we found that high concentrations of systemic lidocaine were more likely to cause adverse effects, including nausea, dizziness, fatigue, bradycardia, tachycardia, and atrial arrhythmias (Table 3) (12, 19). The adverse reactions in patients using lidocaine patches were mild, mainly skin reactions, which could be recovered after drug withdrawal (6). Patients who received lidocaine regional block had few adverse reactions, mainly mild dizziness (9, 11, 16).
In our case, the patient was a 14-year-old child who developed CRPS in both arms after surgery with increased pain following peripheral nerve radiofrequency therapy similar to the case reported by Frost et al. in (10). The patient experienced significant adverse reactions after taking gabapentin. The pain was significantly relieved 3 days after a local subcutaneous injection of 2% lidocaine, and no adverse reactions were observed during the administration.
Based on the above findings, a 5% lidocaine patch appears to have a significant analgesic effect on CRPS. Intravenous lidocaine could provide temporary relief, but it does not last long term. The usual dose of lidocaine for local block is 0.5–2%, and the pain relief is obvious, which is similar to the case we reported. It is suggested that topical lidocaine might relieve the pain of CRPS better and has a favorable safety. However, these clinical trials have been conducted on adults, and there have been few studies on adolescents.
By reviewing the literature, we attempted to analyze and speculate the reasons why local subcutaneous injection of lidocaine could relieve the pain of CRPS. A large number of ion channels related to pain regulation, such as voltage-gated sodium channel, potassium channel, and calcium channel, are distributed in the central and peripheral nervous system, which are closely related to pain perception (20, 21). Lidocaine is a selective Na+ channel blocker with strong membrane stabilization. It can selectively block the inward flow of Na+, block the K+ channel, prevent depolarization of damaged and dysfunctional nerves that are implicated in chronic pain (20, 21), increase the cellular efflux of glutamic acid and potassium, decrease excitability, and diminish neuronal transmission of pain signals, achieving the purpose of pain treatment (21, 22). Subcutaneous injection of lidocaine creates a high drug content in the spinal ganglia through the intradermal nerve terminal receptor pathway, severing the ring of pain stimulation, and thereby relieving pain (23).
In summary, we reported a case of a child treated with a local subcutaneous injection of 2% lidocaine for CRPS pain. At present, there is still no specific treatment for juvenile CRPS at home and abroad. There are also few clinical studies and systematic analyses on CRPS. Through a review of evidence-based literature, we concluded that local administration or local block used to treat CRPS pain in adults provided more pain relief than systemic administration of lidocaine. Our case and review of the literature suggested that local subcutaneous injection of lidocaine might be a convenient and effective treatment for CRPS in children. However, due to the small number of clinical cases collected and the lack of rigorous randomized controlled trials, the safety of CRPS in the treatment of adolescents remains uncertain. Therefore, further high-quality randomized controlled studies are needed to investigate the effect of local subcutaneous injection of lidocaine on pain relief in children with CRPS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Shandong Provincial Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW and HT contributed to the conception and design of the study and proofread all drafts. YS wrote the first draft of the manuscript. ZL wrote sections of the manuscript. HT provided guidance on the article. All authors contributed to the revision of the article, read, and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82102372) and the Natural Science Foundation of Shandong Province (ZR2020MC060 and ZR2020MH399).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harden RN, McCabe CS, Goebel A, Massey M, Suvar T, Grieve S, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 5th edition. Pain Med. (2022) 23:S1–S53. doi: 10.1093/pm/pnac046
2. Mangnus TJP, Bharwani KD, Dirckx M, Huygen FJPM. From a symptom-based to a mechanism-based pharmacotherapeutic treatment in complex regional pain syndrome. Drugs. (2022) 82:511–31. doi: 10.1007/s40265-022-01685-4
3. Field J. Complex regional pain syndrome: a review. J Hand Surg Eur Vol. (2013) 38:616–26. doi: 10.1177/1753193412471021
4. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. (2007) 8:326–31. doi: 10.1111/j.1526-4637.2006.00169.x
5. Varitimidis SE, Papatheodorou LK, Dailiana ZH, Poultsides L, Malizos KN. Complex regional pain syndrome type I as a consequence of trauma or surgery to upper extremity: management with intravenous regional anaesthesia, using lidocaine and methyloprednisolone. J Hand Surg Eur Vol. (2011) 36:771–7. doi: 10.1177/1753193411413852
6. Calderón E, Calderón-Seoane ME, García-Hernández R, Torres LM. 5% Lidocaine-medicated plaster for the treatment of chronic peripheral neuropathic pain: complex regional pain syndrome and other neuropathic conditions. J Pain Res. (2016) 9:763–70. doi: 10.2147/JPR.S113517
7. Singh NJ, Khanna M, Gupta A, Haldar P. Effectiveness of peripheral median, radial, and ulnar nerve block at wrist along with intra-articular steroid injection in shoulder joint in management of complex regional pain syndrome of upper limb: 1-week follow-up study. Neurol India. (2022) 70:1064–8. doi: 10.4103/0028-3886.349618
8. Paraskevas KI, Michaloglou AA, Briana DD, Samara M. Treatment of complex regional pain syndrome type I of the hand with a series of intravenous regional sympathetic blocks with guanethidine and lidocaine. Clin Rheumatol. (2006) 25:687–93. doi: 10.1007/s10067-005-0122-0
9. Taskaynatan MA, Ozgul A, Tan AK, Dincer K, Kalyon TA. Bier block with methylprednisolone and lidocaine in CRPS type I: a randomized, double-blinded, placebo-controlled study. Reg Anesth Pain Med. (2004) 29:408–12. doi: 10.1016/j.rapm.2004.05.007
10. Frost SG. Treatment of complex regional pain syndrome type 1 in a pediatric patient using the lidocaine patch 5%: a case report. Curr Ther Res Clin Exp. (2003) 64:626–9. doi: 10.1016/j.curtheres.2003.09.010
11. Maneksha FR, Mirza H, Poppers PJ. Complex regional pain syndrome (CRPS) with resistance to local anesthetic block: a case report. J Clin Anesth. (2000) 12:67–71. doi: 10.1016/s0952-8180(99)00126-9
12. Rickard JP, Kish T. Systemic intravenous lidocaine for the treatment of complex regional pain syndrome: a case report and literature review. Am J Ther. (2016) 23:e1266–1269. doi: 10.1097/MJT.0000000000000345
13. Hanlan AKL, Mah-Jones D, Mills PB. Early adjunct treatment with topical lidocaine results in improved pain and function in a patient with complex regional pain syndrome. Pain Physician. (2014) 17:E629–635.
14. Patel M, Aiello M. Successful treatment of acute worsening complex regional pain syndrome in affected dominant right-hand from secondary pathology of new onset third and fourth digit trigger finger. Case reports. Plast Surg Hand Surg. (2022) 9:123–5. doi: 10.1080/23320885.2022.2063871
15. Wang LK, Chen HP, Chang PJ, Kang FC, Tsai YC. Axillary brachial plexus block with patient controlled analgesia for complex regional pain syndrome type I: a case report. Reg Anesth Pain Med. (2001) 26:68–71. doi: 10.1053/rapm.2001.9879
16. Toda K, Muneshige H, Asou T. Intravenous regional block with lidocaine for treatment of complex regional pain syndrome. Clin J Pain. (2006) 22:222–4. doi: 10.1097/01.ajp.0000169666.17159.8f
17. Herschkowitz D, Kubias J. Wireless peripheral nerve stimulation for complex regional pain syndrome type I of the upper extremity: a case illustration introducing a novel technology. Scand J Pain. (2018) 18:555–60. doi: 10.1515/sjpain-2018-0014
18. Linchitz RM, Raheb JC. Subcutaneous infusion of lidocaine provides effective pain relief for CRPS patients. Clin J Pain. (1999) 15:67–72. doi: 10.1097/00002508-199903000-00010
19. Schwartzman RJ, Patel M, Grothusen JR, Alexander GM. Efficacy of 5-day continuous lidocaine infusion for the treatment of refractory complex regional pain syndrome. Pain Med. (2009) 10:401–12. doi: 10.1111/j.1526-4637.2009.00573.x
20. Zagon A. Innervation of serotonergic medullary raphe neurons from cells of the rostral ventrolateral medulla in rats. Neuroscience. (1993) 55:849–67. doi: 10.1016/0306-4522(93)90446-m
21. Kosharskyy B, Almonte W, Shaparin N, Pappagallo M, Smith H. Intravenous infusions in chronic pain management. Pain Physician. (2013) 16:231–49.
22. Tully J, Jung JW, Patel A, Tukan A, Kandula S, Doan A, et al. Utilization of intravenous lidocaine infusion for the treatment of refractory chronic pain. Anesth Pain Med. (2020) 10:e112290. doi: 10.5812/aapm.112290
Keywords: lidocaine, local subcutaneous injection, complex regional pain syndrome, analgesic therapy, case report
Citation: Su Y, Li Z, Wang Q and Tang H (2023) Local subcutaneous lidocaine injection for the treatment of complex regional pain syndrome: a case report and literature review. Front. Neurol. 14:1232199. doi: 10.3389/fneur.2023.1232199
Received: 31 May 2023; Accepted: 24 July 2023;
Published: 14 August 2023.
Edited by:
Giovanni Meola, University of Milan, ItalyReviewed by:
Hai Zhang, Shanghai First Maternity and Infant Hospital, ChinaJuan Wu, Western Theater General Hospital, China
Copyright © 2023 Su, Li, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Wang, d2FuZ3FpYW5fOTE4NkAxNjMuY29t; Hui Tang, dGFuZ2h1aTExMTBAMTYzLmNvbQ==
 Yaping Su
Yaping Su Zhenyu Li1
Zhenyu Li1 Qian Wang
Qian Wang Hui Tang
Hui Tang