- 1Henan Key Laboratory of Child Brain Injury and Henan Pediatric Clinical Research Center, Third Affiliated Hospital and Institute of Neuroscience, Zhengzhou University, Zhengzhou, China
- 2Department of Neonatology, Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Center for Perinatal Medicine and Health, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 4Department of Women’s and Children’s Health, Karolinska Institute, Stockholm, Sweden
- 5Center for Brain Repair and Rehabilitation, Institute of Neuroscience and Physiology, University of Gothenburg, Goteborg, Sweden
Objective: We investigated the association between bronchopulmonary dysplasia (BPD) and 3 years death or neurodevelopmental impairment (NDI) in very preterm infants without severe brain injury.
Method: Our prospective cohort study recruited preterm infants who were born prior to 32 weeks of gestational age and survived in the neonatal intensive care unit until 36 weeks of corrected age. Upon reaching 3 years of age, each infant was assessed for death or NDI such as cerebral palsy, cognitive deficit, hearing loss, and blindness. Correlations between BPD and death or NDI were determined using multiple logistic regression analyses adjusted for confounding factors.
Result: A total of 1,417 infants without severe brain injury who survived until 36 weeks of corrected age were initially enrolled in the study. Over the study period, 201 infants were lost to follow-up and 5 infants were excluded. Our final dataset, therefore, included 1,211 infants, of which 17 died after 36 weeks of corrected age and 1,194 were followed up to 3 years of age. Among these infants, 337 (27.8%) developed BPD. Interestingly, by 3 years of age, BPD was demonstrated to be independently associated with death or NDI, with an adjusted odds ratio of 1.935 (95% confidence interval: 1.292–2.899, p = 0.001), in preterm infants without severe neonatal brain injury.
Conclusion: Our findings indicate that BPD is strongly associated with death or NDI in preterm infants without severe neonatal brain injury at 3 years of age. Further research is needed to understand the mechanisms linking the development of BPD with death or NDI and whether appropriate treatment of BPD may ameliorate or prevent the development of neurological complications.
Introduction
Prematurity remains the leading cause of neonatal mortality and associated morbidities in very preterm infants (1). Although advances in neonatal medical care have significantly reduced newborn mortality rates, surviving infants still face substantial risks of neonatal morbidities and the development of long-term neurological disabilities (2, 3). While severe neonatal brain injuries, such as cystic periventricular leukomalacia (cPVL) and grade III-IV intraventricular hemorrhage (IVH), are widely recognized as contributors to adverse neurodevelopmental outcomes, they have not been observed in the majority (82.3%, 93/113) of preterm infants who later develop neurological disabilities (4). The etiology of neurodevelopmental disorders in preterm infants is, therefore, both multifactorial and poorly understood.
Preterm infants with undeveloped lungs often require supplemental oxygen therapy, which can, through hypoxia-induced oxidative stress and inflammation, contribute to the development of bronchopulmonary dysplasia (BPD) (5). Bronchopulmonary dysplasia is characterized by impaired lung function due to a reduction in alveoli and abnormal pulmonary vascular development. This can lead to short- and long-term respiratory complications that can, in turn, further exacerbate existing lung damage, resulting in recurrent wheezing episodes and even death. Among these complications, respiratory infections, particularly viral infections, are the most common cause of hospital readmission in preschool-age infants with BPD and the frequency of these infections may be attributed to the immaturity of humoral and adaptive immunity in these infants.
Interestingly, a significant increase in mortality and neurological disabilities, including retinopathy of prematurity (ROP) and abnormal brain development, among preterm infants with BPD has been demonstrated in numerous studies (6–8). A systematic review of 11 studies has reported a significant association between BPD and cerebral palsy (OR, 2.10; 95% CI: 1.57, 2.82), and this relationship is linked to the severity of BPD (9). This connection may be attributed to the brain injury resulting from recurrent episodes of hypoxemia and chronic inflammation in preterm infants affected by BPD. Furthermore, a retrospective study encompassing infants devoid of IVH and those with low-grade IVH has provided evidence that BPD might be linked to adverse neurodevelopmental outcomes, even in preterm infants without severe brain injury. Nevertheless, the precise nature of this association remains somewhat unclear due to the research limitations (10). Therefore, we, here, have conducted a prospective cohort study across two centers to further investigate the association between BPD and 3 years death or NDI in preterm infants without severe neonatal brain injury. We hypothesized that BPD could elevate the risk of adverse outcomes at 3 years in very preterm infants without severe brain injury.
Methods
Study design and population
This prospective cohort study recruited preterm infants with a gestational age of less than 32 weeks and who were admitted to the Third Affiliated Hospital and Children’s Hospital of Zhengzhou University between July 2015 and December 2019. BPD was defined as the continued requirement of supplemental oxygen at 36 weeks of corrected age, and BPD severity was classified in accordance with Jensen et al.’s criteria (11) by assessing the mode of respiratory support at 36 weeks of corrected age. This classification comprises Grade 1, which includes the use of nasal cannula at flow rates ≤2 L/min; Grade 2, encompassing nasal cannula at flow rates >2 L/min or non-invasive positive airway pressure; and Grade 3, involving the need for invasive mechanical ventilation. Infants who died before 36 weeks of corrected age, experienced severe brain injury (as defined below), transferred to other hospitals, had missing medical records, as well as presented with congenital cranial malformations and/or genetic or metabolic diseases, were excluded from the study. Children who developed brain injury conditions such as encephalitis, brain trauma, and epilepsy, during the follow-up period were also excluded from final analyses. This study was reviewed and approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. Written informed consent was obtained from the participants’ legal guardian.
Data collection in infants
In infants, cranial ultrasonography examinations were conducted within 3 days after birth, at 7 days, and then weekly until either death or discharge. Cerebral magnetic resonance imaging (MRI) was conducted at approximately 40 weeks of corrected age to assess the presence and severity of any potential brain injury. Severe brain injury in neonates was diagnosed by the presence of conditions such as cPVL and grade III-IV IVH, which were assessed through head ultrasound (HUS) and magnetic resonance imaging (MRI) techniques (12). The following clinical data were collected by professional neonatologists: neonatal characteristics (gestational age, birth weight, sex, small for gestational age (SGA), 5 min Apgar <4, cesarean section, twins/multiple births), maternal characteristics (pregnancy hypertension, maternal age ≥ 35 years, fetal distress, placental abruption, gestational diabetes, premature rupture of membranes, antenatal steroids), neonatal treatment and morbidities (mechanical ventilation >7 days, postnatal steroids, respiratory distress syndrome (RDS), sepsis, severe anemia, necrotizing enterocolitis (NEC), ROP, as well as the education level and monthly income of the parent(s).
Respiratory distress syndrome denoted a progressive dyspnea of preterm infants after birth (13). The diagnosis of sepsis included blood culture-confirmed sepsis and clinical sepsis. Clinical sepsis referred to the presence of severe clinical symptoms of infection requiring anti-infective therapy but with negative blood cultures (14). Severe anemia was diagnosed based on hemoglobin concentrations, respiratory status, and age (15). Retinopathy of prematurity was diagnosed according to international criteria (16). Necrotizing enterocolitis was categorized as stage II or III based on Bell’s staging criteria (17). Infants with invasive ventilator dependence beyond the first week were administered postnatal steroids, specifically, a low-dose dexamethasone regimen (18).
Follow-up assessments and study outcomes
Regular follow-up assessments of all infants were conducted at intervals of at least 3 months following hospital discharge to monitor neurodevelopment and growth until children reached 3 years of age. Death after 36 weeks of corrected age, suffering from respiratory and neurological diseases, and readmissions due to respiratory diseases were recorded during the follow-up period. The mental development index (MDI) and motor functions at 3 years of age were evaluated according to the Bayley Scales of Infant Development II by experienced pediatric neurologists. Audiometric and visual evaluations were performed by skilled professionals at hearing screening centers and ophthalmology department. The neurologists and examiners conducting these evaluations were blinded to the treatment history of the infants.
To evaluate the association between death or NDI and the development of BPD in infants, we defined death or NDI as either death after 36 weeks of corrected age or the emergence of NDI at 3 years of age. NDI was characterized by the presence of one or more of the following conditions among the survivors: cerebral palsy (CP), MDI < 70, hearing loss, and blindness. Cerebral palsy was defined as a group of non-progressive impairments affecting the development of movement and posture, and any type and severity of CP were involved in our study (19). The MDI was based on the Bayley Scales of Infant Development II, with a score below 70 indicating severe impairment of neurodevelopment (20). Hearing impairment was defined as a partial or total loss of hearing (21). Blindness was defined as a best-corrected visual acuity worse than 20/200 (21).
Statistical analysis
Data analysis was conducted using SPSS, version 23.0. Clinical characteristics were compared using Chi-squared tests, Fisher’s exact tests, or Kruskal-Wallis tests as appropriate. The rates of death or NDI among different groups were compared using chi-square tests or Fisher’s exact tests. Univariate analysis was conducted to understand the impact of BPD on death or NDI. A multivariable logistic regression analysis was conducted to account for the influence of potential confounding factors on death or NDI. Subgroup analysis was performed using the Mantel–Haenszel test. A value of p < 0.05 indicated statistical significance.
Results
Population characteristics
A total of 1,787 infants with a gestational age of less than 32 weeks were initially enrolled in this study. Among them, 1,417 infants survived until 36 weeks of corrected age without developing exclusionary criteria such as the development of severe brain injury (Figure 1). During the follow-up period, 201 infants were lost to follow-up at 3 years of age and 5 infants developed exclusionary criteria (1 with brain trauma, 3 with encephalitis, and 1 with epilepsy). Thus, a total of 1,211 infants were included in the final analyses, of which 17 died after 36 weeks of corrected age and 1,194 were followed up to 3 years of age (Figure 1). Out of the 1,211 infants included in the study, 337 (27.8%) of them developed BPD. Within this group of 337 infants with BPD, 75 (22.3%) were categorized as having Grade 2 & 3 BPD. All comprehensive neonatal characteristics, maternal characteristics, and neonatal morbidities are summarized in Table 1.

Figure 1. Study schematic. The flow chart showing the number of infants who were screened for analysis and followed up to 3 years of age.

Table 1. Clinical characteristics between BPD and non-BPD preterm infants without severe brain injury.
Infants with BPD and those without BPD were observed to share similar maternal and neonatal characteristics such as small for gestational age (SGA), cesarean section births, twin/multiple births, pregnancy hypertension, maternal age ≥ 35 years, fetal distress, placental abruption, gestational diabetes, premature rupture of membranes, antenatal steroids, NEC, as well as the education level and monthly income of the parent(s). However, when compared to infants without BPD, infants with BPD exhibited significantly lower gestational age (p = 0.000), birth weight (p = 0.000), as well as higher rates of being male (p = 0.009), 5-min Apgar score < 4 (p = 0.001), needing mechanical ventilation exceeding 7 days (p = 0.000), postnatal steroids treatment (p = 0.000), and developing RDS (p = 0.029), sepsis (p = 0.000), severe anemia (p = 0.000), and ROP (p = 0.011) (Table 1).
During the follow-up period until 3 years of age, infants with BPD exhibited a significantly higher rate of recurrent hospitalization due to respiratory infections compared to infants without BPD (82 out of 322, 25.5% vs. 95 out of 872, 10.9%, p = 0.000). Further, 6 infants (1.9%) with BPD and 3 (0.9%) without BPD developed asthma, and 36 infants (11.2%) with BPD and 43 (4.9%) without BPD received neurodevelopmental rehabilitation training.
The impact of BPD on death or NDI of preterm infants at 3 years of age
Univariate analysis revealed that, by 3 years of age, infants with BPD exhibited significantly higher rates of CP (4.3% vs. 0.7%, p = 0.000), MDI < 70 (10.2% vs. 6.4%, p = 0.025), NDI (15.5% vs. 8.3%, p = 0.000), death (4.5% vs. 0.2%, p = 0.000), and death or NDI (19.3% vs. 8.5%, p = 0.000) compared to infants without BPD. However, observations of deafness (1.2% vs. 1.1%, p = 1.000) and blindness (1.9% vs. 0.5%, p = 0.045) were similar between the two infant groups (Table 2).
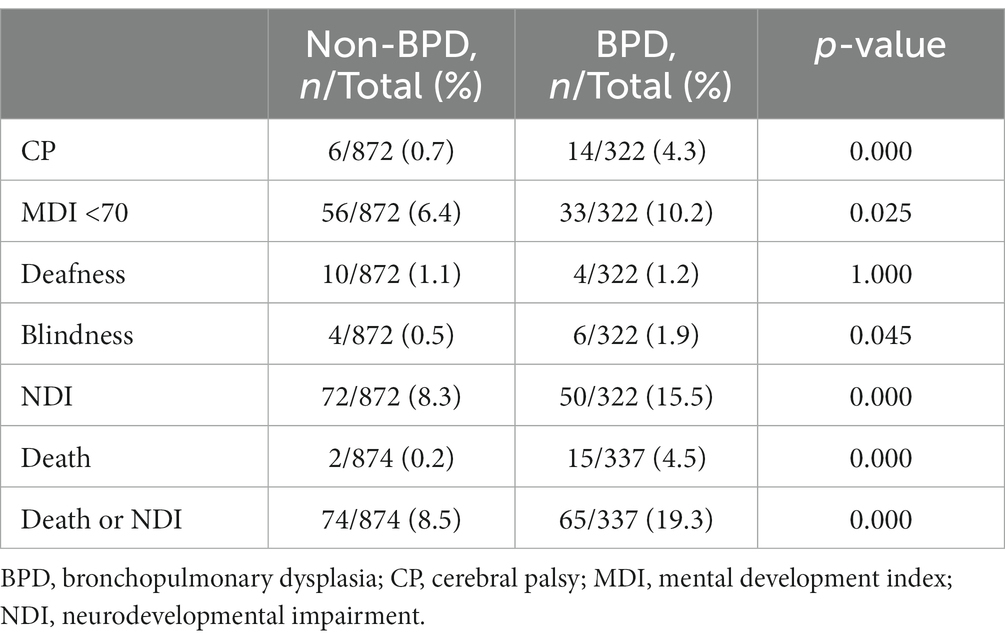
Table 2. Unadjusted death or NDI at 3 years of age between BPD and non-BPD preterm infants without severe brain injury.
Subsequently, we performed a multivariable logistic regression analysis to account for potential confounding variables, including gestational age, birth weight, sex, duration of mechanical ventilation exceeding 7 days, sepsis, severe anemia, and postnatal steroids, in order to assess their impact on death or NDI. Notably, even after adjusting for these confounding factors, the results demonstrated that BPD maintained a significant association with an elevated risk of adverse outcomes in preterm infants who did not exhibit severe brain injury and reached the age of 3 [adjusted odds ratio (aOR) = 1.935, 95% confidence interval (CI): 1.292–2.899, p = 0.001]. Comprehensive details of the results from both univariate and multivariable logistic regression analyses are presented in Tables 2, 3, respectively.
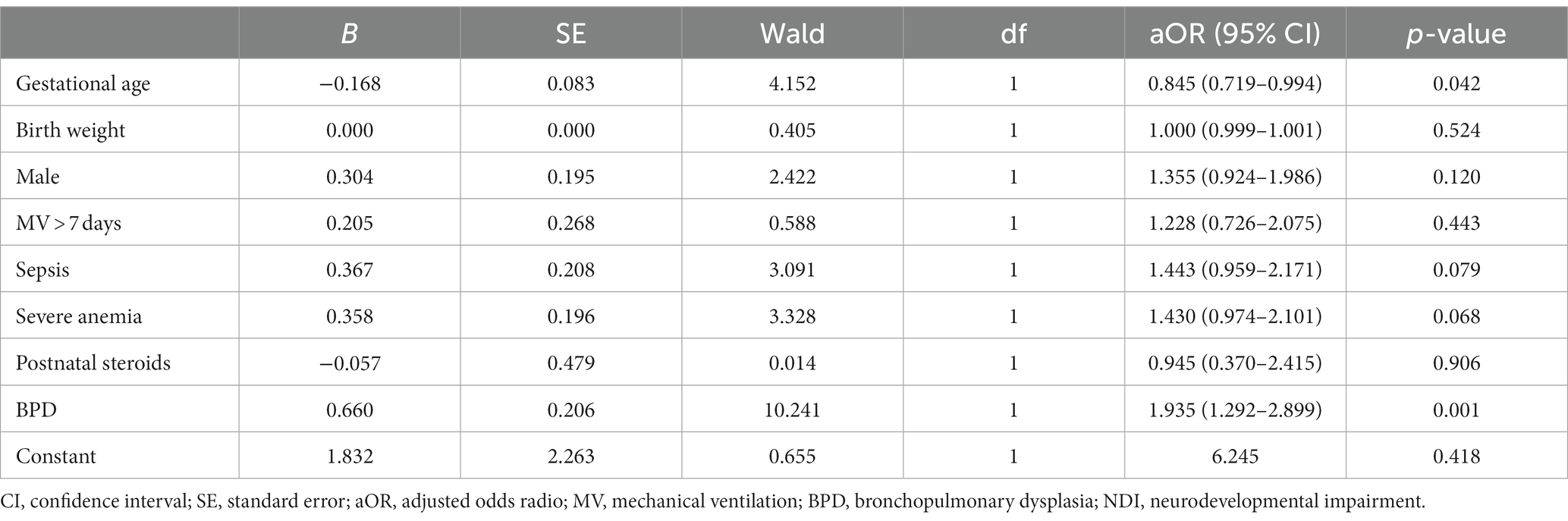
Table 3. Multivariable logistic regression analysis on the impact of BPD on death or NDI in preterm infants without severe brain injury at 3 years of age.
To further elucidate the effect of BPD severity on death or NDI, we conducted a multivariable logistic regression analysis based on BPD severity. This analysis indicated that Grade 2 & 3 BPD was significantly associated with adverse outcomes (aOR = 3.407, 95% CI: 1.923–6.037, p = 0.000), whereas Grade 1 BPD exhibited a less pronounced association (aOR = 1.549, 95% CI: 0.988–2.429, p = 0.057) (Supplementary Table 1).
Effect of gestational age, birth weight, and sex on the association of BPD with death or NDI
Subgroup analyses were conducted based on three strata of gestational age (<28 weeks, 28–29/6 weeks, and 30–32 weeks), three strata of birth weight (<1,000 g and 1,000–1,499 g, and ≥ 1,500 g), and two strata of sex (female and male). The results showed that BPD significantly increased the incidence of death or NDI in preterm infants across two gestational age strata (<28 weeks and 28–29/6 weeks), two birth weight strata (<1,000 g and 1,000–1,499 g), and in both sex strata (male and female). However, all the interactions were not statistically significant (p > 0.05) (Table 4), thus suggesting that gestational age, birth weight, and sex do not have a significant interactive effect on the impact of BPD on death or NDI in preterm infants without severe brain injury. The results from subgroup analyses are presented in Table 4.
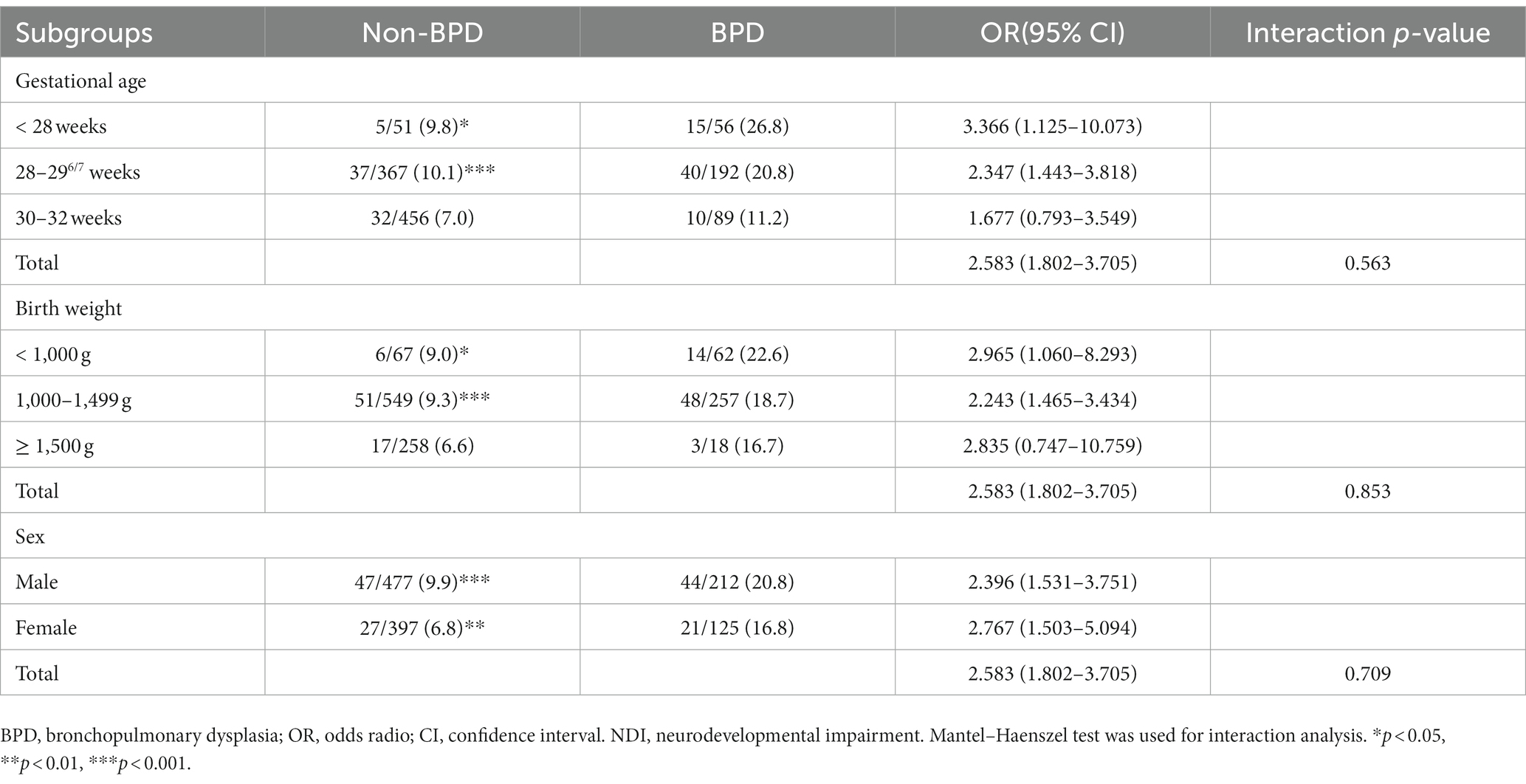
Table 4. Subgroup interaction analysis on the impact of BPD on death or NDI in preterm infants without severe brain injury at 3 years of age.
Discussion
A comprehensive understanding of the etiology of neurodevelopmental disorders in preterm infants is necessary to improve the survival rates and long-term quality of life of these infants. Here, using a large prospective cohort, we demonstrate that BPD in very preterm infants without severe neonatal brain injury is independently associated with an increased risk of death or NDI by 3 years of age.
BPD, as a chronic pulmonary disease, is associated with several neonatal morbidities, such as ROP and abnormal brain development, in infants (22, 23), but the mechanisms underlying this association are unclear (24). Several studies have suggested that lung-brain injury interactions may involve complex processes such as inflammation, oxidative stress, neurodegeneration, vascular formation, and structural remodeling (25–27). Indeed, a shared pathway between lung and brain injuries has been implicated by the observation that hyperoxia-induced injuries elicit similar oxidative stress responses in the immature lung and brain of infants (5). Further, prolonged exposure to oxygen can trigger lung inflammation and disrupt lung development, as well as contribute to abnormal white matter development in infants with immature brains due to the inflammatory activation of oligodendrocytes (25, 28). Exposure to hyperoxic conditions may also increase the production of reactive oxygen species, resulting in damage to immature organs (29). Importantly, hyperoxia-induced oxidative stress and inflammation in the lungs of preterm infants can lead to the development of BPD (30). Thus, infants with BPD experience both impaired lung growth and an increased risk of neurodevelopmental abnormalities (31, 32). Conversely, it is possible that abnormal brain development occurs as a secondary injury process resulting from abnormal lung development via the lung-brain axis. Children with BPD exhibit persistent structural abnormalities in their lungs (e.g., abnormal vessel distribution, reduced vessel densities, and alveolar simplification) and respiratory dysfunction (33), which are associated with altered white matter development and may exert long-term negative effects on the neurodevelopment of infants with immature brains (34). Overall, our findings show that BPD is a predictive factor for death or NDI in preterm infants, but additional studies are needed to discern the exact mechanisms and pathways mediating the interactions between lung and brain abnormalities/injuries in this vulnerable population.
Retinopathy of prematurity is a common occurrence in extremely preterm infants with BPD and has been linked to neurological disability (35, 36). Accordingly, our findings showed a higher incidence of severe ROP in infants with BPD compared to those without BPD. The development of ROP involves several complex factors that include relative hyperoxia, which damages the immature retina in infants with BPD (37). Further, concurrent inflammation in the lungs and eyes of infants receiving oxygen support may potentially contribute to the development of both BPD and ROP (38). To reduce the occurrence of ROP and improve neurological outcomes, it is crucial to monitor oxygen levels in infants with BPD using innovative technologies and methods.
Several factors may contribute to the association between BPD in preterm infants and the development of death or NDI later in life. For instance, following hospital discharge, infants with BPD face a heightened risk of rehospitalization due to factors such as compromised nutrition, growth and developmental abnormalities, weakened immune system, and heightened susceptibility to respiratory viral infections (39, 40), the latter of which was observed in our study population. These risks may contribute to worsened neurological outcomes as well as increased mortality and healthcare costs. Interestingly, our findings revealed that, while the probability of death or NDI was significantly higher in male infants as well as those with lower gestational age and birth weight, the impact of BPD on death or NDI was consistent across different gestational ages, birth weights, and sex. This indicates that the association between BPD and death or NDI is not influenced by gestational age, birth weight, and sex. Together, these findings suggest that prioritizing both home care, including nutritional support, and hospital-based interventions for infants diagnosed with BPD are essential to mitigate the risk of developing death or NDI later in life.
It is important to note the limitations of our study. First, the follow-up period only extended until the age of 3, which prevented the identification of poor neurological outcomes that may emerge later in life (41). Consequently, future efforts should focus on extending the follow-up period, potentially up to school age or beyond, to better understand the impact of BPD on the long-term neurological development of preterm infants. Second, we did not collect information regarding the initiation and duration of home oxygen therapy, which is a common at-home treatment for promoting the development and growth of preterm infants with BPD (42). Therefore, our data do not account for any differences in home treatment.
Conclusion
Our findings demonstrate that BPD in very preterm infants without severe brain injury is strongly associated with increased risk of mortality and neurological disabilities at 3 years of age. This identifies an urgent need to monitor the neurological development of preterm infants with BPD as well as develop treatment strategies that can help prevent or mitigate death or NDI in preterm infants with BPD. To address these challenges, further research is warranted to investigate the underlying pathophysiological mechanisms that connect lung injury and neurological disability in very preterm infants.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
WL: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization. YW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. CZha: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. YX: Conceptualization, Formal analysis, Methodology, Validati on, Visualization, Writing – review & editing. FX: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. XW: Conceptualization, Formal analysis, Methodology, Supervision, Visualization, Writing – review & editing. CZhu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program from the Ministry of Science and Technology of the People’s Republic of China (2022YFC2704801), the National Natural Science Foundation of China (U21A20347), the Swedish Research Council (2022-01019), Swedish Governmental grants to scientists working in health care (ALFGBG-965197), and a grant of the Henan Medical Science and Technique Foundation (LHGJ20190350 and SBGJ202301009).
Acknowledgments
We would like to thank all the infants and their parents who participated in this study and the neurologists for performing the neurodevelopmental assessment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1292372/full#supplementary-material
References
1. Perin, J, Mulick, A, Yeung, D, Villavicencio, F, Lopez, G, Strong, KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. (2022) 6:106–15. doi: 10.1016/S2352-4642(21)00311-4
2. Thebaud, B, Goss, KN, Laughon, M, Whitsett, JA, Abman, SH, Steinhorn, RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. (2019) 5:78. doi: 10.1038/s41572-019-0127-7
3. Ophelders, D, Gussenhoven, R, Klein, L, Jellema, RK, Westerlaken, RJJ, Hutten, MC, et al. Preterm brain injury, antenatal triggers, and therapeutics: timing is key. Cells. (2020) 9:1871. doi: 10.3390/cells9081871
4. Wang, Y, Song, J, Zhang, X, Kang, W, Li, W, Yue, Y, et al. The impact of different degrees of intraventricular hemorrhage on mortality and neurological outcomes in very preterm infants: a prospective cohort study. Front Neurol. (2022) 13:853417. doi: 10.3389/fneur.2022.853417
5. Obst, S, Herz, J, Alejandre Alcazar, MA, Endesfelder, S, Mobius, MA, Rudiger, M, et al. Perinatal hyperoxia and developmental consequences on the lung-brain axis. Oxidative Med Cell Longev. (2022) 2022:1–17. doi: 10.1155/2022/5784146
6. Martin, M, Smith, L, Hofheimer, JA, McGowan, EC, O'Shea, TM, Pastyrnak, S, et al. Bronchopulmonary dysplasia and neurobehavioural outcomes at birth and 2 years in infants born before 30 weeks. Arch Dis Child Fetal Neonatal Ed. (2023) 108:142–8. doi: 10.1136/archdischild-2021-323405
7. DeMauro, SB . Neurodevelopmental outcomes of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. (2021) 56:3509–17. doi: 10.1002/ppul.25381
8. Piris Borregas, S, Torres Valdivieso, MJ, Martín-Arriscado, C, de la Cruz, BJ, Sierra García, P, and Pallás Alonso, CR. Model that predicted death or disabilities in premature infants was valid at seven years of age. Acta Paediatr. (2019) 108:1245–9. doi: 10.1111/apa.14679
9. Gou, X, Yang, L, Pan, L, and Xiao, D. Association between bronchopulmonary dysplasia and cerebral palsy in children: a meta-analysis. BMJ Open. (2018) 8:e020735. doi: 10.1136/bmjopen-2017-020735
10. Bae, SP, Shin, SH, Yoon, YM, Kim, EK, and Kim, HS. Association of severe retinopathy of prematurity and bronchopulmonary dysplasia with adverse neurodevelopmental outcomes in preterm infants without severe brain injury. Brain Sci. (2021) 11:699. doi: 10.3390/brainsci11060699
11. Jensen, EA, Dysart, K, Gantz, MG, McDonald, S, Bamat, NA, Keszler, M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200:751–9. doi: 10.1164/rccm.201812-2348OC
12. Kuint, J, Lerner-Geva, L, Chodick, G, Boyko, V, Shalev, V, Reichman, B, et al. Type of re-hospitalization and association with neonatal morbidities in infants of very low birth weight. Neonatology. (2019) 115:292–300. doi: 10.1159/000495702
13. Sweet, DG, Carnielli, VP, Greisen, G, Hallman, M, Klebermass-Schrehof, K, Ozek, E, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. (2023) 120:3–23. doi: 10.1159/000528914
14. Dong, H, Zhang, L, Li, B, Li, J, Chen, Y, Richard, SA, et al. Screening inflammatory protein biomarkers on premature infants with necrotizing enterocolitis. Inflamm Res. (2023) 72:757–68. doi: 10.1007/s00011-023-01702-6
15. Song, J, Dong, H, Xu, F, Wang, Y, Li, W, Jue, Z, et al. The association of severe anemia, red blood cell transfusion and necrotizing enterocolitis in neonates. PLoS One. (2021) 16:e0254810. doi: 10.1371/journal.pone.0254810
16. Sun, H, Song, J, Kang, W, Wang, Y, Sun, X, Zhou, C, et al. Effect of early prophylactic low-dose recombinant human erythropoietin on retinopathy of prematurity in very preterm infants. J Transl Med. (2020) 18:397. doi: 10.1186/s12967-020-02562-y
17. Wang, Y, Song, J, Sun, H, Xu, F, Li, K, Nie, C, et al. Erythropoietin prevents necrotizing enterocolitis in very preterm infants: a randomized controlled trial. J Transl Med. (2020) 18:308. doi: 10.1186/s12967-020-02459-w
18. Doyle, LW, Davis, PG, Morley, CJ, McPhee, A, Carlin, JB, and Investigators, DS. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. (2006) 117:75–83. doi: 10.1542/peds.2004-2843
19. Xu, Y, Li, Y, Richard, SA, Sun, Y, and Zhu, C. Genetic pathways in cerebral palsy: a review of the implications for precision diagnosis and understanding disease mechanisms. Neural Regen Res. (2024) 19:673-5374.385855. doi: 10.4103/1673-5374.385855
20. Lowe, JR, Erickson, SJ, Schrader, R, and Duncan, AF. Comparison of the Bayley II mental developmental index and the Bayley III cognitive scale: are we measuring the same thing? Acta Paediatr. (2012) 101:e55–8. doi: 10.1111/j.1651-2227.2011.02517.x
21. Alzahrani, M, Tabet, P, and Saliba, I. Pediatric hearing loss: common causes, diagnosis and therapeutic approach. Minerva Pediatr. (2015) 67:75–90.
22. Singh, JK, Wymore, EM, Wagner, BD, Thevarajah, TS, Jung, JL, Kinsella, JP, et al. Relationship between severe bronchopulmonary dysplasia and severe retinopathy of prematurity in premature newborns. J AAPOS. (2019) 23:209.e1–4. doi: 10.1016/j.jaapos.2019.02.008
23. Grelli, KN, Keller, RL, Rogers, EE, Partridge, JC, Xu, D, Barkovich, AJ, et al. Bronchopulmonary dysplasia precursors influence risk of white matter injury and adverse neurodevelopmental outcome in preterm infants. Pediatr Res. (2021) 90:359–65. doi: 10.1038/s41390-020-01162-2
24. Ziaka, M, and Exadaktylos, A. Brain-lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care. (2021) 25:358. doi: 10.1186/s13054-021-03778-0
25. Dapaah-Siakwan, F, Zambrano, R, Luo, S, Duncan, MR, Kerr, N, Donda, K, et al. Caspase-1 inhibition attenuates hyperoxia-induced lung and brain injury in neonatal mice. Am J Respir Cell Mol Biol. (2019) 61:341–54. doi: 10.1165/rcmb.2018-0192OC
26. Thomas, JM, Sudhadevi, T, Basa, P, Ha, AW, Natarajan, V, and Harijith, A. The role of sphingolipid signaling in oxidative lung injury and pathogenesis of bronchopulmonary dysplasia. Int J Mol Sci. (2022) 23:1254. doi: 10.3390/ijms23031254
27. Ali, A, Zambrano, R, Duncan, MR, Chen, S, Luo, S, Yuan, H, et al. Hyperoxia-activated circulating extracellular vesicles induce lung and brain injury in neonatal rats. Sci Rep. (2021) 11:8791. doi: 10.1038/s41598-021-87706-w
28. Ritter, J, Schmitz, T, Chew, LJ, Buhrer, C, Mobius, W, Zonouzi, M, et al. Neonatal hyperoxia exposure disrupts axon-oligodendrocyte integrity in the subcortical white matter. J Neurosci. (2013) 33:8990–9002. doi: 10.1523/JNEUROSCI.5528-12.2013
29. Shim, SY, and Kim, HS. Oxidative stress and the antioxidant enzyme system in the developing brain. Korean J Pediatr. (2013) 56:107–11. doi: 10.3345/kjp.2013.56.3.107
30. Baker, CD, and Abman, SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology. (2015) 107:344–51. doi: 10.1159/000381129
31. Trittmann, JK, Nelin, LD, and Klebanoff, MA. Bronchopulmonary dysplasia and neurodevelopmental outcome in extremely preterm neonates. Eur J Pediatr. (2013) 172:1173–80. doi: 10.1007/s00431-013-2016-5
32. Decollogne, L, Epiard, C, Chevallier, M, Ego, A, Alin, L, and Debillon, T. Neurodevelopmental impairment at 2 years of age in children born before 29 weeks' gestation with bronchopulmonary dysplasia. Arch Pediatr. (2021) 28:23–8. doi: 10.1016/j.arcped.2020.10.012
33. Cheong, JLY, and Doyle, LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. (2018) 42:478–84. doi: 10.1053/j.semperi.2018.09.013
34. Morken, TS, Nyman, AK, Sandvig, I, Torp, SH, Skranes, J, Goa, PE, et al. Brain development after neonatal intermittent hyperoxia-hypoxia in the rat studied by longitudinal mri and immunohistochemistry. PLoS One. (2013) 8:e84109. doi: 10.1371/journal.pone.0084109
35. Glass, TJA, Chau, V, Gardiner, J, Foong, J, Vinall, J, Zwicker, JG, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F532–7. doi: 10.1136/archdischild-2016-312533
36. Podraza, W, Michalczuk, B, Jezierska, K, Domek, H, Kordek, A, Loniewska, B, et al. Correlation of retinopathy of prematurity with bronchopulmonary dysplasia. Open Med. (2018) 13:67–73. doi: 10.1515/med-2018-0012
37. Richter, AE, Bos, AF, Huiskamp, EA, and Kooi, EMW. Postnatal cerebral hyperoxia is associated with an increased risk of severe retinopathy of prematurity. Neonatology. (2019) 116:356–62. doi: 10.1159/000501859
38. Wickramasinghe, LC, van Wijngaarden, P, Tsantikos, E, and Hibbs, ML. The immunological link between neonatal lung and eye disease. Clin Transl Immunol. (2021) 10:e1322. doi: 10.1002/cti2.1322
39. Bauer, SE, Vanderpool, CPB, Ren, C, and Cristea, AI. Nutrition and growth in infants with established bronchopulmonary dysplasia. Pediatr Pulmonol. (2021) 56:3557–62. doi: 10.1002/ppul.25638
40. Townsi, N, Laing, IA, Hall, GL, and Simpson, SJ. The impact of respiratory viruses on lung health after preterm birth. Eur Clin Respir J. (2018) 5:1487214. doi: 10.1080/20018525.2018.1487214
41. Radic, JA, Vincer, M, and McNeely, PD. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of nova scotia from 1993 to 2010. J Neurosurg Pediatr. (2015) 15:580–8. doi: 10.3171/2014.11.Peds14364
Keywords: bronchopulmonary dysplasia, preterm infant, brain injury, neurological disability, neurodevelopmental impairment
Citation: Li W, Wang Y, Song J, Zhang C, Xu Y, Xu F, Wang X and Zhu C (2023) Association between bronchopulmonary dysplasia and death or neurodevelopmental impairment at 3 years in preterm infants without severe brain injury. Front. Neurol. 14:1292372. doi: 10.3389/fneur.2023.1292372
Edited by:
Jonathan Michael Davis, Tufts University, United StatesReviewed by:
Elisabeth McGowan, Women & Infants Hospital of Rhode Island, United StatesPaige Church, Beth Israel Deaconess Medical Center, Harvard Medical School, United States
Copyright © 2023 Li, Wang, Song, Zhang, Xu, Xu, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changlian Zhu, Y2hhbmdsaWFuLnpodUBuZXVyby5ndS5zZQ==; emh1Y0B6enUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Wenli Li
Wenli Li Yong Wang
Yong Wang Juan Song
Juan Song Chen Zhang
Chen Zhang Yiran Xu
Yiran Xu Falin Xu1,2
Falin Xu1,2 Xiaoyang Wang
Xiaoyang Wang Changlian Zhu
Changlian Zhu