- 1Eighth Clinical School, Guangzhou University of Chinese Medicine, Foshan, China
- 2Endocrinology Department, Foshan Hospital of Traditional Chinese Medicine, Foshan, China
Objective: Magnetic resonance imaging-guided focused ultrasound (MRgFUS) is a novel noninvasive treatment for drug-resistant Parkinson’s disease (PD) related tremor. This study aims to evaluate MRgFUS’s efficacy and safety in PD through a systematic review and meta-analysis, examining pre-and post-treatment MDS-UPDRSIII and/or CRST scores and associated adverse events.
Materials and methods: We conducted an extensive literature search across PubMed, Embase, Web of Science, and Cochrane Library databases, screening studies based on set criteria and analyzing MDS-UPDRSIII, CRST, and adverse events pre- and post-MRgFUS treatment.
Results: Out of 468 retrieved articles, 20 studies involving 258 patients, spanning 2014–2023, were included.17 studies indicated significant MDS-UPDRSIII score reductions post-MRgFUS treatment, while 3 showed significant CRST score declines. In the “on” medication state, pooled MDS-UPDRSIII scores at 1, 3, 6, and 12 months were 12.18 (95% CI: 5.83–18.52), 12.10 (95% CI: 8.22–15.97), 14.85 (95% CI: 9.28–20.41), and 20.65 (95% CI: 12.15–29.14) respectively. In the “off” state, scores were 11.45 (95% CI: −3.50-26.40), 14.71 (95% CI: 4.95–24.46), 21.52 (95% CI: 19.28–23.75), and 22.28 (95% CI: 15.26–29.30). Adverse events were typically mild and transient, with speech disturbances, ataxia, and sensory abnormalities being common post-operative neurological complications.
Conclusion: MRgFUS offers an effective and relatively safe treatment option for patients with drug-resistant PD-related tremor.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, No. CRD42023428332.
1 Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder, with the risk of onset increasing with age (1). The clinical manifestations of this disease include motor-related symptoms such as bradykinesia, rigidity, and tremor, as well as non-motor symptoms including impaired olfaction, cognitive disorders, and psychiatric disturbances (2). These symptoms significantly impact the quality of life of the patients, bringing immense psychological and medical burdens to their families (3).
The primary treatment strategy for PD is usually pharmacotherapy, which includes anticholinergic agents, dopaminergic receptor agonists and levodopa (4).These medications can help alleviating patients’ symptoms and improve their quality of life. For those who do not respond well to medications or experience significant side effects, surgical treatment becomes an alternative option. Currently, Deep Brain Stimulation (DBS) is the predominant surgical approach for treating PD, particularly interventions targeting the ventral intermediate nucleus (VIM), globus pallidus internus (GPI), and subthalamic nucleus (STN) (5, 6). This is suitable for advanced PD, when oral or transdermal treatments are no longer effective (7). However, despite the adjustable advantages of DBS, it is important to note that this method is invasive and quite costly, in addition to the risks associated with device implantation and electrical stimulation, which cannot be ignored (6, 8).
.Magnetic Resonance Imaging-guided Focused Ultrasound (MRgFUS) is a non-invasive neurosurgical technique that offers minimally invasive ablation, paving a new way for the treatment of PD-related tremor (9). This technique is characterized by its non-invasiveness, lack of radiation, and the absence of a need for anesthesia (10, 11). It works by thermally ablating specific brain regions (such as VIM and GPI) during the treatment process, forming a coagulative necrotic focus (12). Compared to traditional invasive surgeries, MRgFUS significantly reduces the risks of infection and cerebral hemorrhage (13). Studies have demonstrated its safety and efficacy in treating Essential Tremor (ET) (14, 15). However the safety and efficacy of MRgFUS in treating PD related tremor and in improving other PD symptoms need to be better elucidated. Currently, there is a relatively limited literature review on the efficacy and safety of MRgFUS in the treatment of Parkinson’s disease (16, 17). In terms of relevant reports for which meta-analysis was performed, only one article exists, and that article included only two papers (18). In contrast, our study employs a single-arm meta-analysis approach that encompasses a wider range of research literature and takes into account a longer span of years to provide a more comprehensive and in-depth analysis. This will help to fill the knowledge gap in the existing literature. Therefore, our research aims to systematically review relevant literature to assess the safety and efficacy of MRgFUS in treating drug-resistant PD-related tremor. Our study results may provide a scientific basis for the clinical application of MRgFUS in the treatment of drug-resistant PD-related tremor.
2 Methods and analysis
2.1 Purpose and registration
A systematic review and meta-analysis will be performed to synthesize the evidence and assess the efficacy and safety of MRgFUS for the treatment of drug-resistant PD-related tremor. This protocol is registered in PROSPERO (No. CRD42023428332). This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) statement (19).
2.2 Information sources and search strategies
A systematic search was conducted on August 6, 2023, using PubMed, Embase, Web of science, and Cochrane Library electronic databases for the keywords (“MRgFUS” or “HIFU” or “focused ultrasound”) AND (“Parkinson disease”).
Inclusion criteria: articles must report on the efficacy and/or safety of MRgFUS treatment in patients with PD. For efficacy: quantitative or qualitative data on major symptoms such as tremor, bradykinesia, and rigidity need to be included. For safety: need to include the incidence and detailed description of complications, adverse events associated with MRgFUS therapy; the intervention in the study was the administration of MRgFUS treatment to the study cases. Exclusion criteria: studies with less than 3 patients, case reports, experimental animal studies, reviews, conference abstracts, duplicate publications, or literature with missing data.
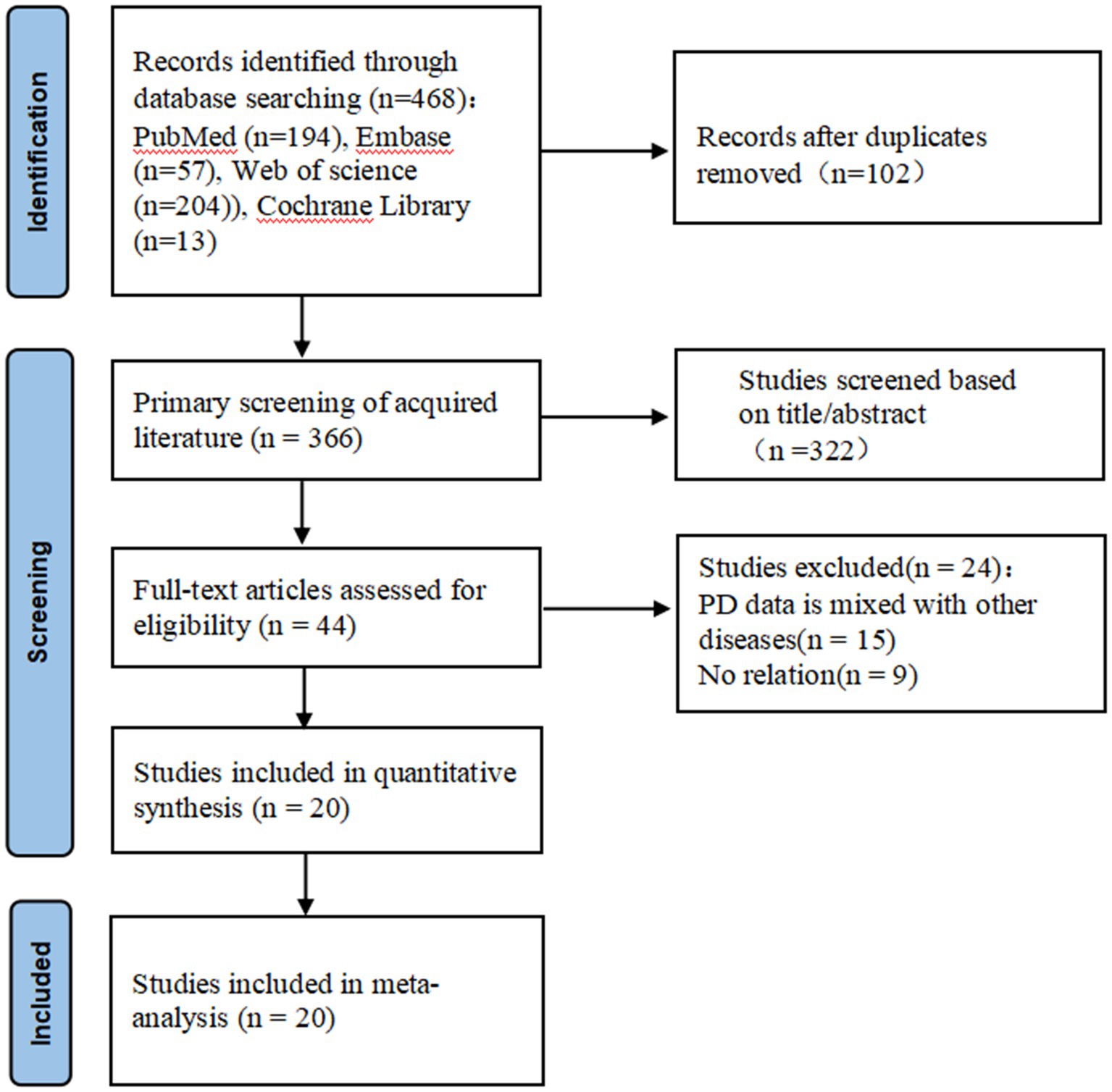
2.3 Data extraction and analysis
Two independent researchers undertook the literature search. After deduplicating with NoteExpress, abstracts and titles were preliminarily screened based on inclusion criteria. Relevant full-text articles were further assessed, and in cases of data overlap, only the most updated or comprehensive study was retained. Disparities between researchers were reconciled through consultation with a third expert. Subsequently, a collective in-depth analysis and data extraction from the selected studies were done, ensuring unanimous agreement on divergences. The selection process is illustrated in the PRISMA flow diagram (Figure 1). Essential details for each study, such as authorship, publication date, study design, sample demographics, PD duration, follow-up duration, efficacy metrics (including both on- and off-medication Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRSIII) scores, and/or pre- and post-treatment Clinical Rating Scale for Tremor (CRST) scores), recurrent events of tremor and postoperative adverse events, were systematically recorded. The MDS-UPDRSIII scale usually covers a comprehensive assessment of hand movements, upper extremity movements, and lower extremity movements. Increased patient scores in these areas usually reflect severe impairments in movement in patients with PD. The CRST score is primarily used to assess resting and locomotor tremor at different sites, as well as other symptoms associated with tremor. Higher MDS-UPDRSIII scores indicate more severe impairment of motor function in patients with PD, and higher CRST scores indicate more significant symptoms of resting and motor tremor in patients with PD.
2.4 Statistical analysis
Meta-analysis was performed using the R language (v4.2.2) meta-function package to meta-analyze the data, with measurements expressed as mean difference and standard deviation (MD ± SD), and dichotomous data expressed as proportions and 95% confidence intervals. Heterogeneity among the results of the included studies was tested with I2 and p-values, and if there was good statistical homogeneity among the studies (p > 0.1; I2 ≤ 50%), Meta-analysis was performed using a fixed-effects model; if there was statistical heterogeneity (p < 0.1; I2 > 50%), Meta-analysis could be performed using a random-effects model. The level of test for Meta was α = 0.05 with a statistically significant p < 0.05.
3 Results
3.1 Study characteristics
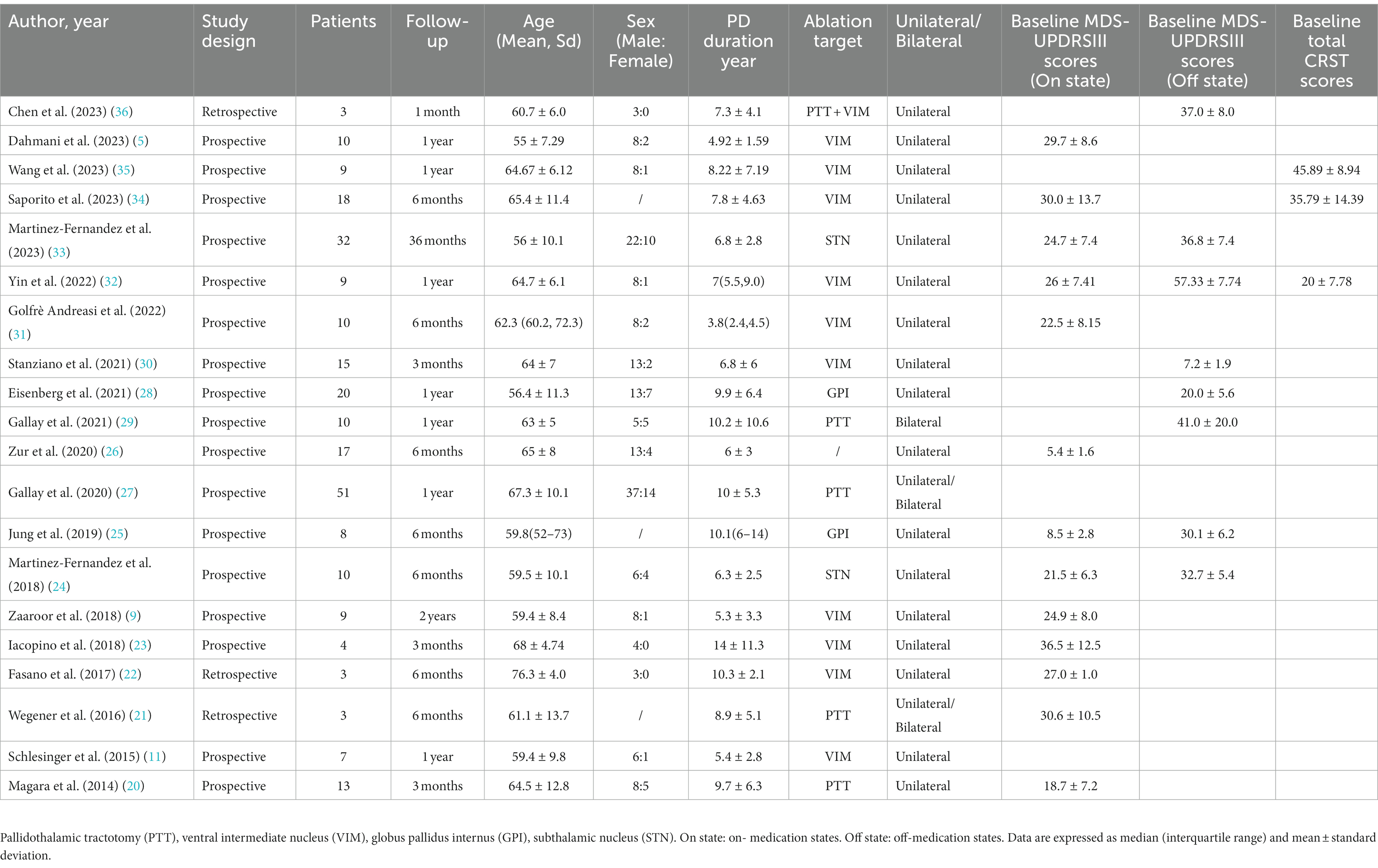
This meta-analysis included 20 studies published between 2014 and 2023 (5, 9, 11, 20–36), encompassing 258 patients. Among these, two were retrospective studies and 18 were prospective. Table 1 provides a detailed breakdown of the characteristics of each study. The participants were primarily middle-aged and elderly, with a majority being male. The follow-up duration varied across studies, ranging from as short as 1 month to as long as 3 years. Three studies (21, 27, 29) documented cases of bilateral pallidothalamic tractotomy (PTT)ablation, while the rest reported unilateral ablations (5, 9, 11, 20, 22–26, 28, 30–36). Regarding the surgical targets: 11 studies (5, 9, 11, 22, 23, 30–32, 34–36) selected the VIM nucleus; PTT was chosen as the target in 4 studies (20, 21, 27, 29); whereas STN (24, 33) and GPI (25, 28) were each selected in 2 studies.
3.2 Tremor scores
3.2.1 MDS-UPDRSIII scores (on-medication and off-medication states)
The mean MDS-UPDRSIII score for drug-resistant PD patients in the on-medication states on the treatment side at baseline was 27.77 ± 13.03. Five studies (5, 9, 25, 26, 36) involving 47 patients reported the mean MDS-UPDRSIII scores at 1 month from baseline to non-pharmacological status, which showed that the scores showed a high degree of heterogeneity (I2 = 96.9%, p < 0.05), and the pooled standard mean difference was 12.88 (95% CI:5.32–20.44).Four studies (20, 23, 25, 32) involving 34 patients reported mean MDS-UPDRS III scores at 3-month postoperative follow-up, showing a high degree of heterogeneity (I2 = 72.71%, p < 0.05), with a combined score of 12.10 (95% CI: 8.22–15.97). Ten studies (5, 9, 21, 22, 24–26, 31, 33, 34) involving 120 patients reported mean MDS-UPDRSIII scores at 6-month postoperative follow-up, showing a high degree of heterogeneity (I2 = 97.86%, p < 0.05), with a combined score of 14.85 (95% CI: 9.28–20.41). Three studies (5, 32, 33) concerning 51 patients and reporting the mean MDS-UPDRSIII scores at 1-year postoperative follow-up showed a high level of heterogeneity (I2 = 96.44%, p < 0.05), with a pooled result of 20.65 (95% CI: 12.15–29.14) (Figure 2).
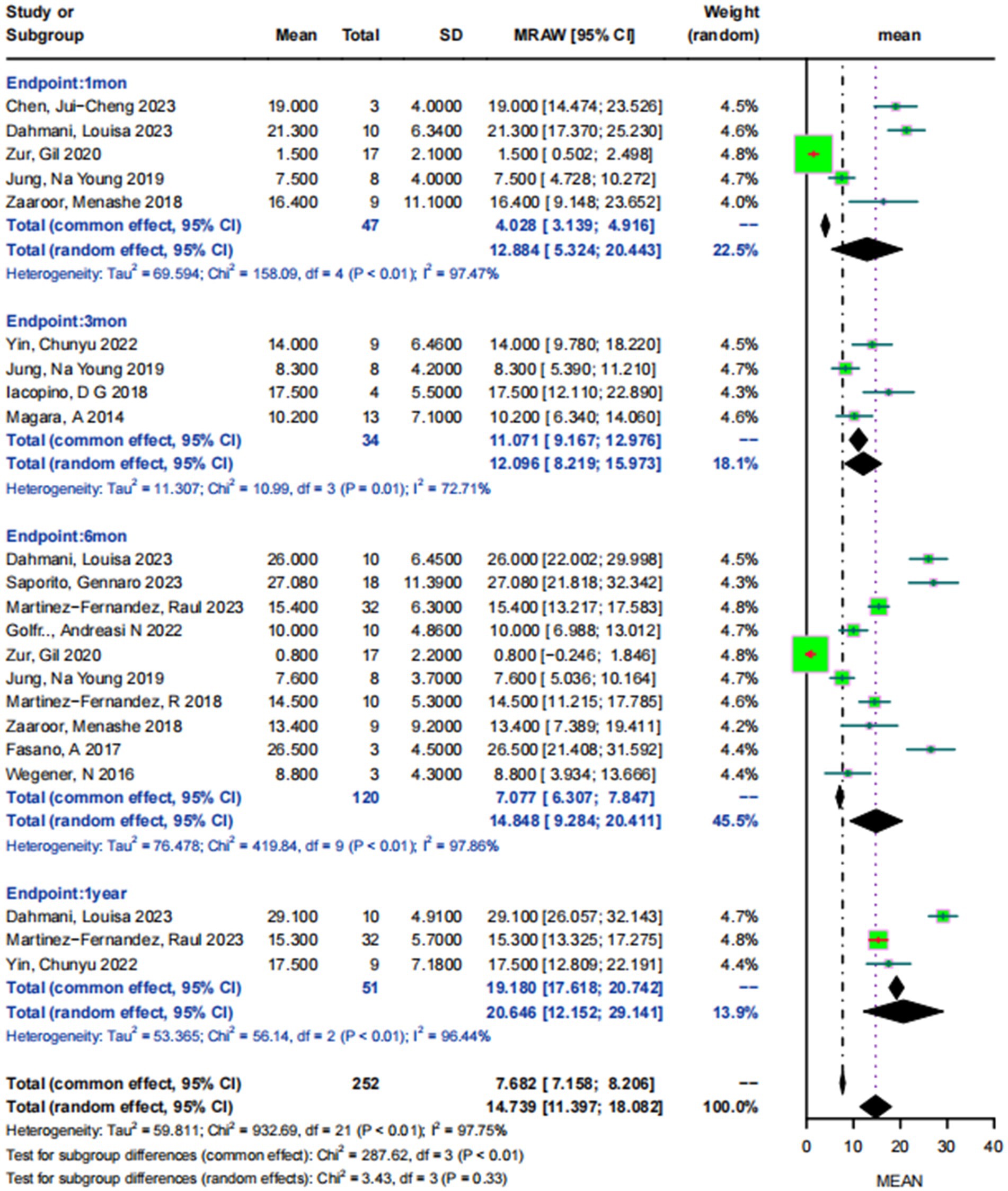
Figure 2. Forest plot of MDS-UPDRSIII scores at 1 month, 3 months, 6 months, and 1 year post-MRgFUS treatment in the on- medication states.
Mean MDS-UPDRSIII scores for patients with drug-resistant PD who were off-medication states on the treatment side at baseline were 31.65 ± 12.75. The 2 studies (25, 30) involving 23 patients reported the mean MDS-UPDRSIII scores at 1 month postoperatively, and their results showed a high degree of heterogeneity in the scores (I2 = 95.62%, p < 0.05), with a combined analysis of 11.45 95% CI:−3.50–26.40. Four studies (25, 28, 30, 32), involving 52 patients, reported mean MDS-UPDRSIII scores at 3-month postoperative follow-up exhibited a high degree of heterogeneity (I2 = 95.66%, p < 0.05), and 14.71 (95% CI:4.95–24.46) after combining. Three studies (24, 25, 33) involving 50 patients reported the mean MDS-UPDRSIII score at 6-month postoperative follow-up, showing a heterogeneity of scores of 0 (I2 = 0, p < 0.05) and a combined MDS-UPDRSIII score of 21.52 (95% CI:19.28–23.75). Three studies (29, 32, 33) involving 51 patients reported the mean MDS-UPDRSIII scores at 1-year postoperative follow-up, showing a high degree of heterogeneity (I2 = 73.54%, p < 0.05), with a combined result of 22.28 (95% CI: 15.26–29.30) (Figure 3).
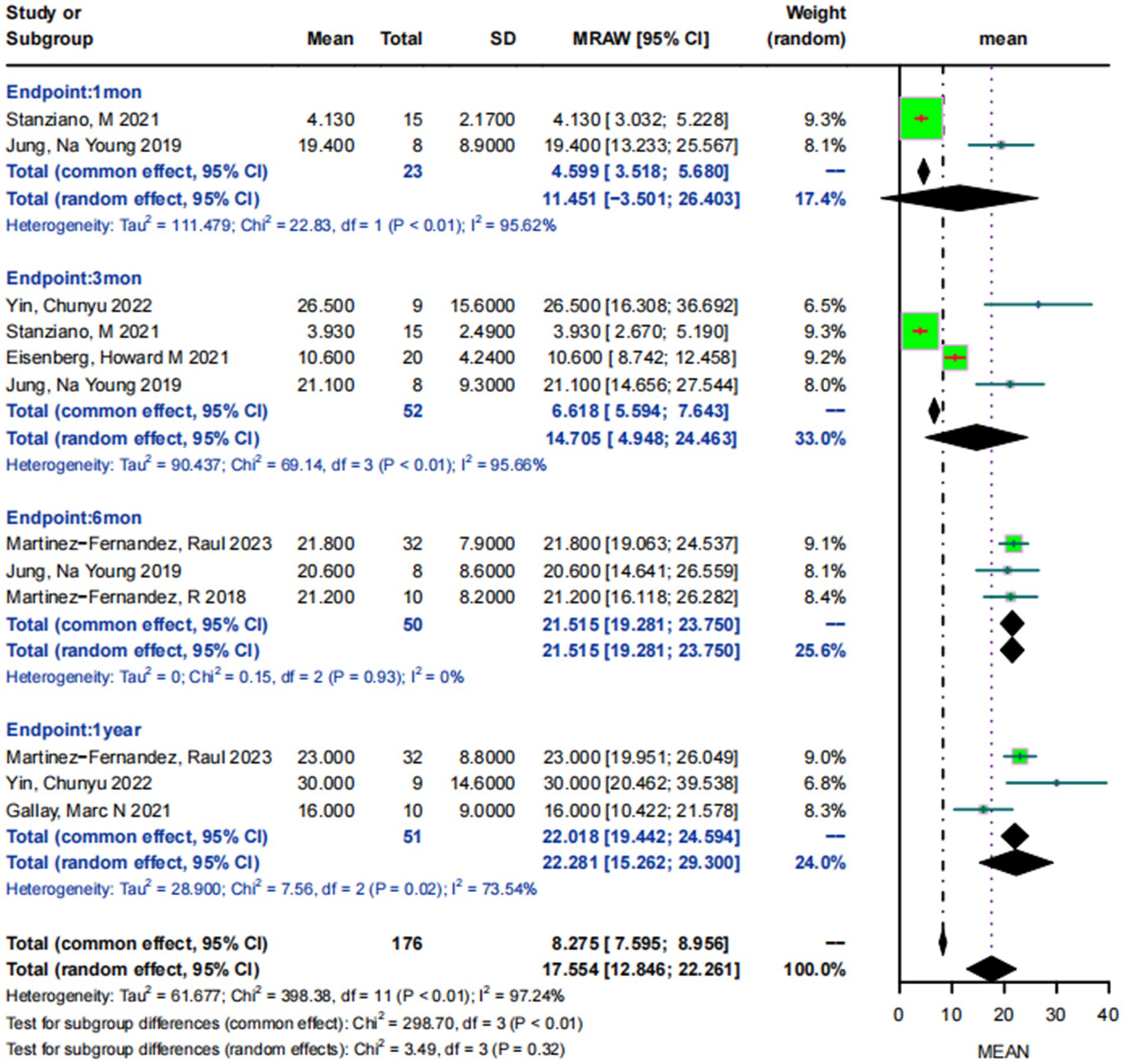
Figure 3. Forest plot of MDS-UPDRSIII scores at 1 month, 3 months, 6 months, and 1 year post-MRgFUS treatment in the off-medication states.
In both states, MRgFUS treatment effectively reduced the MDS-UPDRSIII scores, indicating its efficacy. Comparatively, the baseline score in the “off” medication state was higher, but the post-treatment reduction trend mirrored the “on” state, suggesting a potentially more pronounced effect in the “off” state. This could be attributed to the higher baseline score in the “off” state, offering more room for improvement. Over time, the therapeutic effect diminishes, possibly due to the progressive nature of the disease.
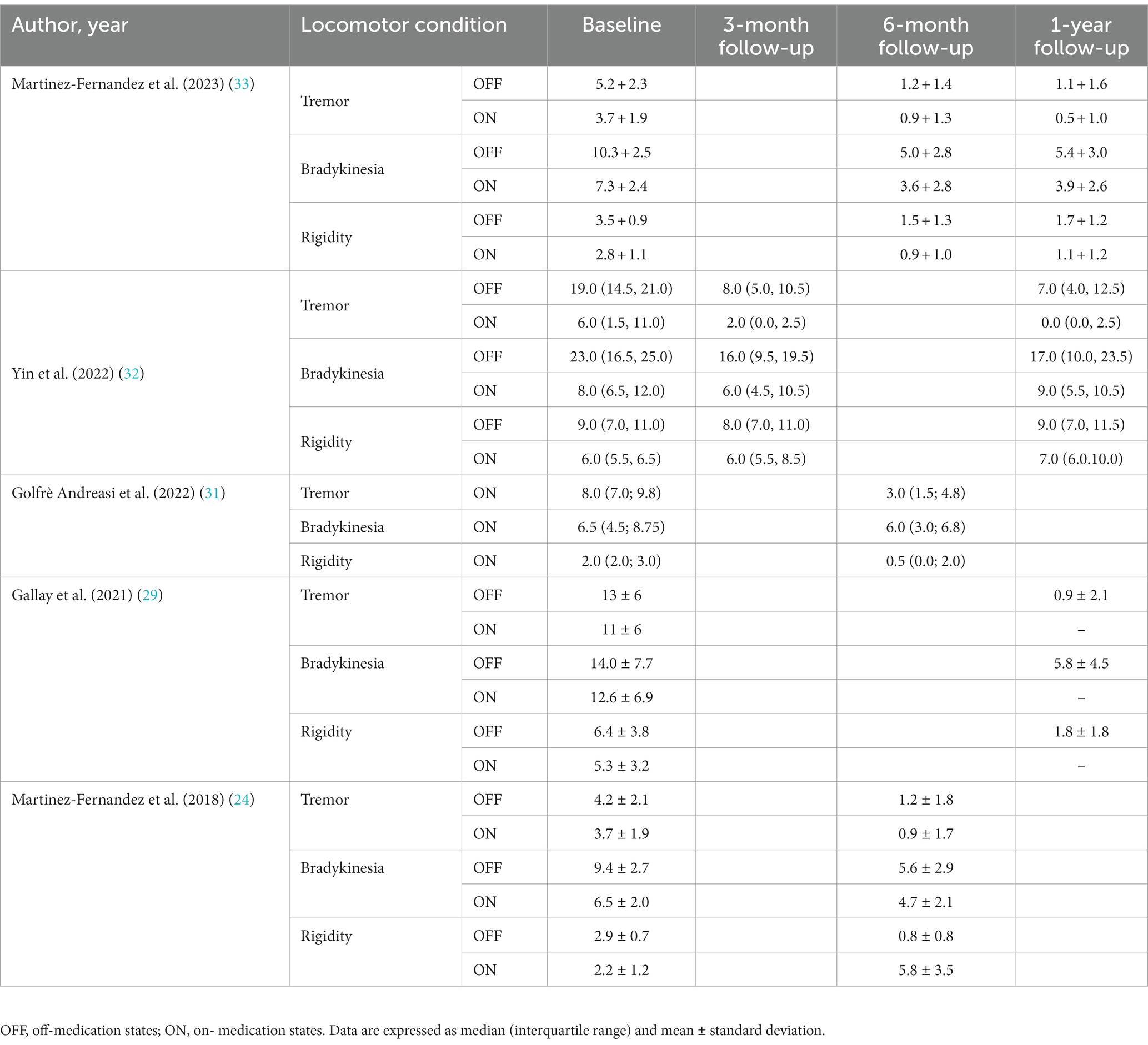
As can be seen from the Table 2, MRgFUS had a positive impact on the treatment of motor symptoms in drug-resistant PD patients, especially in terms of tremor and bradykinesia. It is important to note, however, that the treatment effect was relatively small in stiffness symptoms. These results emphasize the potential of MRgFUS treatment in improving different motor symptoms in patients with Parkinson’s disease, but also highlight the variability between different symptoms.
3.3 CRST scores
In three studies, total CRST scores were documented. Wang et al. (35) observed an initial score of 45.89 ± 8.94, which significantly reduced to 17.89 ± 11.92 1 year post-surgery. Saporito et al. (34) recorded a baseline score of 35.79 ± 14.39, which dropped to 23.03 ± 10.95 6 months postoperatively. Similarly, Yin et al. (32) registered an initial score of 20 ± 7.78, declining to 3.44 ± 2.83 1 year post-intervention. Due to the insufficiency of data, a meta-analysis on the aforementioned results could not be conducted.
3.4 Recurrent events of tremor
Eisenberg et al. (28) reported a recurrence of tremor in a PD patient at month 3 after GPI-targeted surgery. Zaaroor et al. (9) mentioned 2 patients experiencing tremor recurrence, one with significant recurrence within 3 months of undergoing the VIM procedure and the other with minor recurrence within 6 months. Schlesinger et al. (11) documented that 1 patient each experienced transient mild tremor recurrence at various time points after VIM surgery, including 1 week, 1 month, and 6 months. Magara et al. (20) noted that four patients experienced tremor recurrence within 3 months of PTT surgery.
3.5 Adverse events
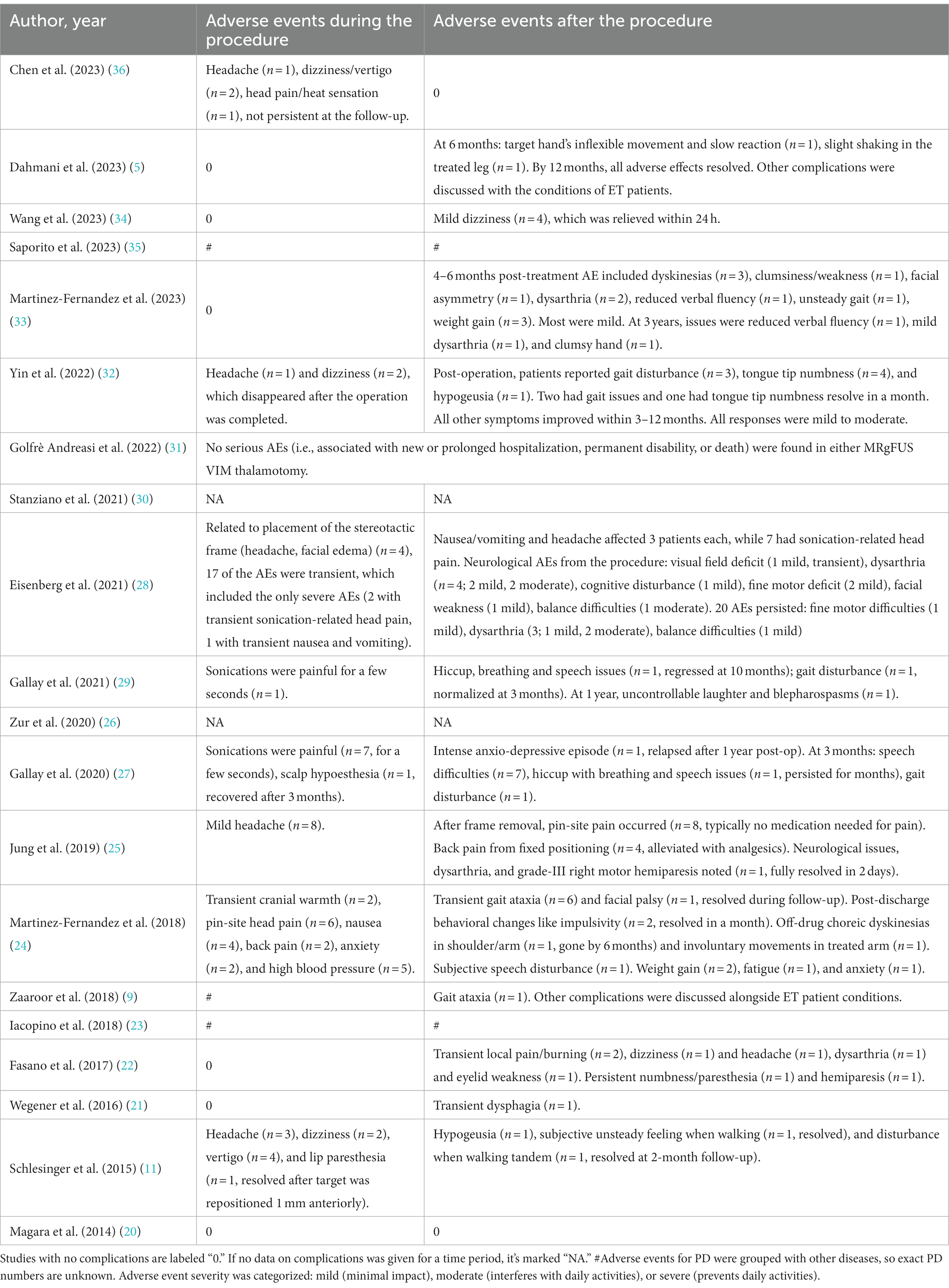
We have summarized the adverse events during and after surgery in the included studies (Table 3). Generally, the procedure was safe for these patients, with the majority of adverse events being mild and transient.
Adverse events from MRgFUS can be primarily categorized into two main types: neurological complications and side effects associated with MRI/ultrasound or the frame. Neurological complications can be further delineated into: sensory deficits (e.g., taste disturbances, sensory loss, visual field defects, paresthesia, numbness, or burning sensations, total of 20 cases), motor disturbances (e.g., facial or limb weakness, eyelid spasms, total of 12 cases), ataxia (e.g., unsteady gait, hand coordination difficulties, total of 18 cases), speech disorder (total of 18 cases), cognitive and emotional disturbances (e.g., anxiety, depression, fatigue, and behavioral changes, total of 10 cases), hypertension (total of 5 cases), and thalamotomy-related dizziness (n = 5) and headache (n = 4). Side effects related to MRI/ultrasound or the frame primarily included: headache (30 cases), dizziness (10 cases), head burning sensation (3 cases), facial swelling (4 cases), nausea and vomiting (total of 4 cases), pain induced by ultrasound (8 cases), and back pain (6 cases). Additionally, the studies reported instances of hiccupping, respiratory difficulties (2 cases), weight gain (5 cases), and swallowing difficulties (1 case).
Out of the total, 65 patients (representing 25.2%) experienced side effects associated with MRI/ultrasound or the frame, with headache and dizziness being the most common. These events usually subsided on their own within a few days without the need for specialized intervention. The most commonly reported neurological adverse events were sensory abnormalities, ataxia, and speech disorders, which generally improved within 3 months post-operation and had a minimal impact on patients’ daily lives. The severity of most adverse reactions ranged from mild to moderate. The only three severe adverse events reported were by Eisenberg et al. (28), which included two cases of transient headache related to ultrasound and one case of transient nausea and vomiting; neither of these met the United States Food and Drug Administration (FDA) definition for severe adverse reactions. In a study by Gallay et al. (29), one patient experienced uncontrollable laughter and eyelid spasms a year post-operation, and in another of their studies (27), a patient underwent a brief yet intense episode of anxiety and depression, which then recurred after more than a year post-operation. Fasano et al. (22) reported persistent side effects in two patients: numbness and hemiparesis accompanied by hemihypoesthesia. It remains uncertain whether these persistent adverse events will fade with extended follow-up.
3.6 Quality of the evidence
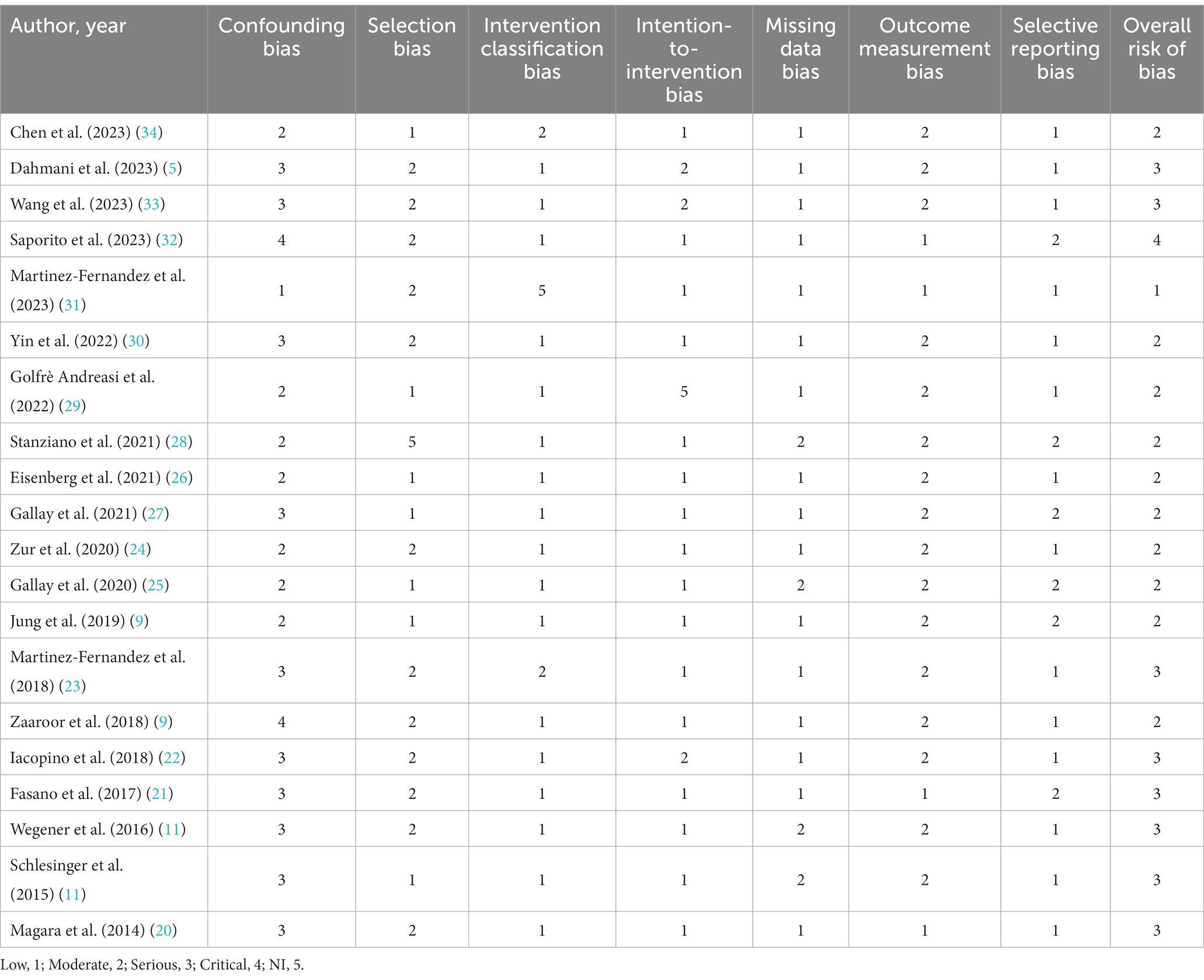
Two researchers independently evaluated the studies using the ROBINS-I scale (37). The included studies were assessed for potential biases in seven areas: confounding bias, selection of participants bias, intervention classification bias, intention to intervene deviation bias, missing data bias, outcome measurement bias, and selective reporting bias. These evaluations are presented in Table 4. In cases of disagreement, the issues were resolved through mutual consultation or determined through a discussion with a third party.
Of the 20 studies selected. A few studies had some quality issues, including potential confounders and selective reporting of risk. However, some studies performed relatively well in certain aspects, such as lower risk bias and better methodological quality. Overall, these studies provide preliminary information about MRgFUS treatment for drug-resistant PD-related tremor, but caution is needed in interpreting the results, especially in the presence of potential wind traps. Future studies should focus more on methodologic quality to further validate the efficacy and safety of this treatment.
4 Discussion
MRgFUS as a novel non-invasive intervention technique has gradually become a new option for treating medication-resistant PD patients. To our knowledge, this is the first comprehensive meta-analysis of the efficacy of MRgFUS in the treatment of PD. Overall, this study suggests that MRgFUS treatment for drug-resistant PD is both effective and safe.
In both states, MRgFUS significantly reduced the MDS-UPDRSIII scores. However, as time post-surgery progresses, scores tend to rise, suggesting a potential diminishing therapeutic effect, warranting further longitudinal studies. We recognize this and also consider that Parkinson’s disease is a progressive neurodegenerative disease. The progression of this disease may be an important reason for the rise in scores. Given this, MRgFUS’s capability for repeated treatments emerges as a distinct advantage. Since MRgFUS primarily targets symptoms unresponsive to medication, improvements during the drug-off state are particularly noteworthy for an accurate assessment of the surgical intervention’s benefits. Thus, notable symptom relief by MRgFUS during the off-medication states, given its critical role in patients’ daily challenges, represents a crucial therapeutic milestone. Nonetheless, enhancements in the on-medication states also epitomize the overall treatment efficacy. In line with previous reports, our data indicates that speech disorder, ataxia, and sensory abnormalities are the most common adverse events in the neurological system after MRgFUS treatment (16). Complications related to MRI/ultrasound or the frame are typically transient reactions during the treatment process, such as headaches, dizziness, nausea, vomiting, scalp numbness, or a burning sensation. Our findings reveal that over a quarter of patients experienced ultrasound-related complications, with headaches and dizziness being the most frequent. Additionally, the use of anesthetics is avoided during the MRgFUS ultrasound procedure, offering a safer treatment alternative for patients at high risk from general anesthesia. Compared to other therapeutic technologies, an advantage of MRgFUS is that most surgery-related complications can be detected in real-time during surgery. This allows physicians to mitigate or reverse most side effects by adjusting the initial treatment target.
A total of 4 different surgical targets were used in the 20 studies we reviewed, with VIM being the most commonly used surgical target. MRgFUS produces varying effects and potential complications across different targets. VIM therapy is commonly used to suppress tremor symptoms, but may be accompanied by sensory or motor impairment, resulting in sensory abnormalities, muscle weakness, or dyskinesia. Some patients may also experience pain after the procedure, which may require additional management. STN treatment has an ameliorating effect on major motor symptoms, such as rigidity, tremor, and bradykinesia, and also reduces the dose of levodopa-related treatments. However, the treatment may also lead to movement disorders and speech or cognitive problems. GPI treatment provides significant relief from almost all symptoms of drug-resistant PD, especially when accompanied by cognitive decline and mood disorders, but may also trigger motor deficits and language or cognitive dysfunction. PTT treatment produces positive results in dyskinesia and dystonia, but may result in abnormal sensations or increased pain after the procedure, as well as some temporary headaches or discomfort (38). It is important to note that complications of the MRgFUS procedure can vary greatly from patient to patient, and with improvements in surgical techniques, it has become possible to reduce the risk of complications. Future research should be directed toward exploring which target delivers the best results in MRgFUS therapy and whether there are adverse effects associated with target selection. In addition, MRgFUS single-target thalamotomy has not demonstrated significant efficacy in some of the motor symptoms of PD such as rigidity, bradykinesia, and gait disturbances, as well as in a number of non-motor symptoms such as cognitive deficits, affective problems, and sleep disorders. However, Chen et al. (36) have demonstrated that dual-targeted MRgFUS significantly reduced resting and locomotor tremor in drug-resistant PD. Future research directions should focus on exploring the potential benefits of MRgFUS for drug-resistant PD patients in terms of non-motor symptoms in order to improve the overall quality of life of patients.
Moreover, numerous studies have concurrently addressed the efficacy and safety of MRgFUS in treating both ET and drug-resistant PD. This conflation precluded their inclusion in our analysis, potentially affecting the comprehensiveness of our data. Given this backdrop, we advocate for more dedicated clinical studies focusing solely on drug-resistant PD, especially since the safety and efficacy of MRgFUS in treating ET have already been established.
We concluded that selection of appropriate patients for MRgFUS treatment is critical to ensuring the efficacy and safety of the treatment. Current selection criteria may be based primarily on patient history, disease stage, and ancillary tests. However, as our understanding of PD grows, there may be other biomarkers or neuropsychological assessment tools that can more accurately predict which patients are most likely to benefit from MRgFUS therapy. Considering that the duration of PD and lesions vary widely from patient to patient, a uniform treatment approach may not be appropriate for all patients. Therefore, new metrics with predictive value could help to individualize treatment. It is recommended to consider combining MRgFUS with other non-pharmacological treatments (e.g., cognitive rehabilitation, physical therapy, or DBS) to assess whether treatment effects can be further enhanced. In exploring this direction, we should focus on improving the overall quality of life of patients and advancing individualized treatment to ensure the best outcome for each patient.
4.1 Limitations
This study primarily relies on the MDS-UPDRSIII for assessing treatment outcomes. While it is a key tool for evaluating PD, the inclusion of other crucial indicators such as quality of life and mental state was limited by data availability, potentially hindering a comprehensive understanding of the MRgFUS treatment effects. In addition, most of our studies had limited sample sizes and short follow-up periods.
5 Conclusion
MRgFUS is a potential option for the treatment of drug-resistant PD-related tremor with satisfactory efficacy and safety. Speech disorders, ataxia and sensory abnormalities are the most common postoperative side effects, but the symptoms are mild and usually transient. However, because MRgFUS is a relatively new technique, follow-up data and randomized clinical trials are quite limited. More rigorous study designs, larger sample sizes, and longer follow-up times are needed in the future to further investigate the efficacy, safety, and durability of MRgFUS in the treatment of drug-resistant PD-related tremor in order to determine its long-term benefits in the management of drug-resistant PD-related tremor.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XT: Conceptualization, Writing – original draft. RH: Formal analysis, Software, Supervision, Writing – review & editing. PH: Formal analysis, Writing – review & editing. JY: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kalia, LV, and Lang, AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
2. Sveinbjornsdottir, S. The clinical symptoms of Parkinson's disease. J Neurochem. (2016) 139:318–24. doi: 10.1111/jnc.13691
3. Xiong, Y, Han, D, He, J, Zong, R, Bian, X, Duan, C, et al. Correlation of visual area with tremor improvement after MRgFUS thalamotomy in Parkinson's disease. J Neurosurg. (2022) 136:681–8. doi: 10.3171/2021.3.JNS204329
4. Giugni, JC, and Okun, MS. Treatment of advanced Parkinson's disease. Curr Opin Neurol. (2014) 27:450–60. doi: 10.1097/WCO.0000000000000118
5. Dahmani, L, Bai, Y, Li, M, Ren, J, Shen, L, Ma, J, et al. Focused ultrasound thalamotomy for tremor treatment impacts the cerebello-thalamo-cortical network. NPJ Parkinsons Dis. (2023) 9:90. doi: 10.1038/s41531-023-00543-8
6. Ko, TH, Lee, YH, Chan, L, Tsai, KW, Hong, CT, and Lo, WL. Magnetic resonance-guided focused ultrasound surgery for Parkinson's disease: a mini-review and comparison between deep brain stimulation. Parkinsonism Relat Disord. (2023) 111:105431. doi: 10.1016/j.parkreldis.2023.105431
7. Mansouri, A, Taslimi, S, Badhiwala, JH, Witiw, CD, Nassiri, F, Odekerken, V, et al. Deep brain stimulation for Parkinson's disease: meta-analysis of results of randomized trials at varying lengths of follow-up. J Neurosurg. (2018) 128:1199–213. doi: 10.3171/2016.11.JNS16715
8. Yeoh, TY, Manninen, P, Kalia, SK, and Venkatraghavan, L. Anesthesia considerations for patients with an implanted deep brain stimulator undergoing surgery: a review and update. Can J Anaesth. (2017) 64:308–19. doi: 10.1007/s12630-016-0794-8
9. Zaaroor, M, Sinai, A, Goldsher, D, Eran, A, Nassar, M, and Schlesinger, I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg. (2018) 128:202–10. doi: 10.3171/2016.10.JNS16758
10. Bond, AE, Shah, BB, Huss, DS, Dallapiazza, RF, Warren, A, Harrison, MB, et al. Safety and efficacy of focused ultrasound Thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol. (2017) 74:1412–8. doi: 10.1001/jamaneurol.2017.3098
11. Schlesinger, I, Eran, A, Sinai, A, Erikh, I, Nassar, M, Goldsher, D, et al. MRI guided focused ultrasound Thalamotomy for moderate-to-severe tremor in Parkinson's disease. Parkinsons Dis. (2015) 2015:219149. doi: 10.1155/2015/219149
12. Han, X, Huang, R, Meng, T, Yin, H, and Song, D. The roles of magnetic resonance-guided focused ultrasound in pain relief in patients with bone metastases: a systemic review and Meta-analysis. Front Oncol. (2021) 11:617295. doi: 10.3389/fonc.2021.617295
13. Moro, E, Volkmann, J, Konig, IR, Winkler, S, Hiller, A, Hassin-Baer, S, et al. Bilateral subthalamic stimulation in Parkin and PINK1 parkinsonism. Neurology. (2008) 70:1186–91. doi: 10.1212/01.wnl.0000307748.11216.03
14. Agrawal, M, Garg, K, Samala, R, Rajan, R, Naik, V, and Singh, M. Outcome and complications of MR guided focused ultrasound for essential tremor: a systematic review and Meta-analysis. Front Neurol. (2021) 12:654711. doi: 10.3389/fneur.2021.654711
15. Elias, WJ, Lipsman, N, Ondo, WG, Ghanouni, P, Kim, YG, Lee, W, et al. A randomized trial of focused ultrasound Thalamotomy for essential tremor. N Engl J Med. (2016) 375:730–9. doi: 10.1056/NEJMoa1600159
16. Xu, Y, He, Q, Wang, M, Gao, Y, Liu, X, Li, D, et al. Safety and efficacy of magnetic resonance imaging-guided focused ultrasound neurosurgery for Parkinson's disease: a systematic review. Neurosurg Rev. (2021) 44:115–27. doi: 10.1007/s10143-019-01216-y
17. Lennon, JC, and Hassan, I. Magnetic resonance-guided focused ultrasound for Parkinson's disease since ExAblate, 2016-2019: a systematic review. Neurol Sci. (2021) 42:553–63. doi: 10.1007/s10072-020-05020-1
18. Ge, Y, Wang, Z, Gu, F, Yang, X, Chen, Z, Dong, W, et al. Clinical application of magnetic resonance-guided focused ultrasound in Parkinson's disease: a meta-analysis of randomized clinical trials. Neurol Sci. (2021) 42:3595–604. doi: 10.1007/s10072-021-05443-4
19. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
20. Magara, A, Bühler, R, Moser, D, Kowalski, M, Pourtehrani, P, and Jeanmonod, D. First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. J Ther Ultrasound. (2014) 2:11. doi: 10.1186/2050-5736-2-11
21. Wegener, N, Kaegi, G, Bauer, R, Werner, B, Martin, E, Schreglmann, SR, et al. MR-guided high intensity focused ultrasound in Parkinson's disease: a series of 5 cases. Mov Disord. (2016) 31:S23. doi: 10.1002/mds.26688
22. Fasano, A, Llinas, M, Munhoz, RP, Hlasny, E, Kucharczyk, W, and Lozano, AM. MRI-guided focused ultrasound thalamotomy in non-ET Tremor Syndromes. Neurology. (2017) 89:771–5. doi: 10.1212/WNL.0000000000004268
23. Iacopino, DG, Gagliardo, C, Giugno, A, Giammalva, GR, Napoli, A, Maugeri, R, et al. Preliminary experience with a transcranial magnetic resonance-guided focused ultrasound surgery system integrated with a 1.5-T MRI unit in a series of patients with essential tremor and Parkinson's disease. Neurosurg Focus. (2018) 44:17614. doi: 10.3171/2017.11.FOCUS17614
24. Martinez-Fernandez, R, Rodriguez-Rojas, R, Del Alamo, M, Hernandez-Fernandez, F, Pineda-Pardo, JA, Dileone, M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. Lancet Neurol. (2018) 17:54–63. doi: 10.1016/S1474-4422(17)30403-9
25. Jung, NY, Park, CK, Kim, M, Lee, PH, Sohn, YH, and Chang, JW. The efficacy and limits of magnetic resonance-guided focused ultrasound pallidotomy for Parkinson's disease: a phase I clinical trial. J Neurosurg. (2019) 130:1853–61. doi: 10.3171/2018.2.JNS172514
26. Zur, G, Lesman-Segev, OH, Schlesinger, I, Goldsher, D, Sinai, A, Zaaroor, M, et al. Tremor relief and structural integrity after MRI-guided focused US Thalamotomy in tremor disorders. Radiology. (2020) 294:676–85. doi: 10.1148/radiol.2019191624
27. Gallay, MN, Moser, D, Rossi, F, Magara, AE, Strasser, M, Buehler, R, et al. MRgFUS Pallidothalamic Tractotomy for chronic therapy-resistant Parkinson's disease in 51 consecutive patients: single center experience. Front Surg. (2020) 6:76. doi: 10.3389/fsurg.2019.00076
28. Eisenberg, HM, Krishna, V, Elias, WJ, Cosgrove, GR, Gandhi, D, Aldrich, CE, et al. MR-guided focused ultrasound pallidotomy for Parkinson's disease: safety and feasibility. J Neurosurg. (2021) 135:792–8. doi: 10.3171/2020.6.JNS192773
29. Gallay, MN, Moser, D, Magara, AE, Haufler, F, and Jeanmonod, D. Bilateral MR-guided focused ultrasound Pallidothalamic Tractotomy for Parkinson's disease with 1-year follow-up. Front Neurol. (2021) 12:1153. doi: 10.3389/fneur.2021.601153
30. Stanziano, M, Golfrè Andreasi, N, Messina, G, Rinaldo, S, Palermo, S, Verri, M, et al. Resting state functional connectivity signatures of MRgFUS vim Thalamotomy in Parkinson's disease: a preliminary study. Front Neurol. (2021) 12:734. doi: 10.3389/fneur.2021.786734
31. Golfrè, AN, Cilia, R, Romito, LM, Bonvegna, S, Straccia, G, Elia, AE, et al. Magnetic resonance-guided focused ultrasound Thalamotomy may spare dopaminergic therapy in early-stage tremor-dominant Parkinson's disease: a pilot study. Mov Disord. (2022) 37:2289–95. doi: 10.1002/mds.29200
32. Yin, C, Zong, R, Song, G, Zhou, J, Pan, L, and Li, X. Comparison of motor scores between OFF and ON states in tremor-dominant Parkinson's disease after MRgFUS treatment. J Clin Med. (2022) 11:502. doi: 10.3390/jcm11154502
33. Martinez-Fernandez, R, Natera-Villalba, E, Manez Miro, JU, Rodriguez-Rojas, R, Marta Del Alamo, M, Angel Pineda-Pardo, J, et al. Prospective long-term follow-up of focused ultrasound unilateral Subthalamotomy for Parkinson disease. Neurology. (2023) 100:E1395–405. doi: 10.1212/WNL.0000000000206771
34. Saporito, G, Sucapane, P, Ornello, R, Cerone, D, Bruno, F, Splendiani, A, et al. Cognitive outcomes after focused ultrasound thalamotomy for tremor: results from the COGNIFUS (COGNitive in focused UltraSound) study. Parkinsonism Relat Disord. (2023) 106:105230. doi: 10.1016/j.parkreldis.2022.105230
35. Wang, X, Wang, S, Lin, J, Zhang, D, Lu, H, Xiong, Y, et al. Gray matter alterations in tremor-dominant Parkinson's disease after MRgFUS thalamotomy are correlated with tremor improvement: a pilot study. Quant Imaging Med Surg. (2023) 13:4415–28. doi: 10.21037/qims-22-1403
36. Chen, J, Lu, M, Chen, CM, and Tsai, C. Stepwise dual-target magnetic resonance-guided focused ultrasound in tremor-dominant Parkinson disease: a feasibility study. World Neurosurg. (2023) 171:E464–70. doi: 10.1016/J.WNEU.2022.12.049
37. Sterne, JA, Hernan, MA, Reeves, BC, Savovic, J, Berkman, ND, Viswanathan, M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
Keywords: MRgFUS, Parkinson’s disease, efficacy, safety, Meta-analysis
Citation: Tian X, Hu R, He P and Ye J (2023) Efficacy and safety of magnetic resonance-guided focused ultrasound for Parkinson’s disease: a systematic review and meta-analysis. Front. Neurol. 14:1301240. doi: 10.3389/fneur.2023.1301240
Edited by:
Francesca Pistoia, University of L’Aquila, ItalyReviewed by:
Antonio Suppa, Sapienza University of Rome, ItalyRoberto Cilia, IRCCS Carlo Besta Neurological Institute Foundation, Italy
Copyright © 2023 Tian, Hu, He and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhong Ye, MTM3MDMwNjY5MDNAMTYzLmNvbQ==
 Xiaona Tian
Xiaona Tian Rongrui Hu
Rongrui Hu Peicong He
Peicong He Jianhong Ye2*
Jianhong Ye2*