- 1Tianjin Key Laboratory of Exercise Physiology and Sports Medicine, Institute of Sport, Exercise & Health, Tianjin University of Sport, Tianjin, China
- 2Beijing Xiaotangshan Hospital, Beijing, China
- 3Department of Rehabilitation, Lianyungang Hospital of Traditional Chinese Medicine, Lianyungang, China
Background: Although some studies have shown that exercise has a good effect on improving the cardiopulmonary function of stroke patients, it still needs to be determined which exercise method does this more effectively. We, therefore, aimed to evaluate the effectiveness of different exercise methods in improving cardiovascular function in stroke patients through a network meta-analysis (NMA), providing a basis to select the best treatment plan for stroke patients.
Methods: We systematically searched CNKI, WanFang, VIP, CBM, PubMed, Embase, Web of Science, and The Cochrane Library databases from establishment to 30 April 2023. Randomized controlled trials (RCTS) on exercise improving cardiopulmonary function in stroke patients were included, and we screened the included articles and extracted the relevant data. RevMan (version 5.4) and Stata (version 17.0) were used for data analysis.
Results: We included 35 RCTs and a total of 2,008 subjects. Intervention measures included high-intensity interval training (HIIT), aerobic training (AT), resistance training (RT), combined aerobic and resistance exercise (CE), and conventional therapy (CT). In the network meta-analysis, the surface under the cumulative ranking area (SUCRA) ranking result indicated that HIIT improved peak oxygen uptake (VO2peak) and 6 mins walking distance (6MWD) optimally, with rankings of HIIT (100.0%) > CE (70.5%) > AT (50.2%) > RT (27.7%) > CT (1.6%), and HIIT (90.9%) > RT (60.6%) > AT (48.9%) > RT (48.1%) > CT (1.5%), respectively. The SUCRA ranking result showed that CE improved systolic blood pressure (SBP) and diastolic blood pressure (DBP) optimally, with rankings of CE (82.1%) > HIIT (49.8%) > AT (35.3%) > CT (32.8%), and CE (86.7%) > AT (45.0%) > HIIT (39.5%) > CT (28.8%), respectively.
Conclusion: We showed that exercise can effectively improve the cardiopulmonary function of stroke patients. HIIT was the most effective in improving VO2peak and 6MWD in stroke patients. CE was the most effective in improving SBP and DBP in stroke patients. However, due to the limitations of existing clinical studies and evidence, larger sample size, multi-center, and high-quality RCTs are needed to verify the above conclusions in the future.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier [CRD42023436773].
1 Introduction
Stroke, also known as a cerebrovascular accident, is an acute cerebrovascular disease characterized by focal neurological deficits caused by various vascular causes (such as ischemia or hemorrhage) (1). The absolute number of incident strokes globally increased by 70.0% from 1990 to 2019, whereas prevalent strokes increased by 85.0% and deaths from stroke increased by 43.0% (2). Stroke has become the second leading cause of death globally after ischemic heart disease (3). The common functional disorders in stroke patients include motor, sensory, cardiopulmonary, speech, and swallowing (4).
Stroke patients typically exhibit varying degrees of impaired cardiopulmonary function. Peak aerobic capacity (VO2peak) is the highest level of oxygen consumption (VO2) attained during a graded exercise test (5). VO2peak levels in stroke patients may drop 8–22 mL/kg/min, approximately 53% compared to the average age and sex-matched population (6). VO2peak levels required for independent living in healthy people is 15–18 mL/kg/min (7), and very low VO2peak levels after stroke may prohibit patients from performing higher levels of ADL and limit the sustainability of lower levels of ADL (8). In addition, maintaining cardiovascular health is essential to reduce the risk of recurrent stroke (9). Therefore, improving the cardiopulmonary function of stroke patients as soon as possible has important clinical significance for functional recovery and quality of life improvement.
After the stroke, exercise is an essential component in reducing the risk of future cardiovascular events and stroke recurrence (10), and there is increasing evidence that exercise has substantial benefits in improving cardiopulmonary function and musculoskeletal health in stroke patients. The Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders recommend individualized exercise rehabilitation training for stroke survivors to improve cardiopulmonary function (Class I recommendation, Level B evidence) (11). The current exercise methods applied to stroke patients mainly include high-intensity interval training (HIIT), aerobic training (AT), resistance training (RT), and combined aerobic and resistance exercise (CE). HIIT is an efficient method of exercise that involves performing a high-intensity workout in a short period and actively recovering or resting during exercise (12). AT refers to the exercise carried out by the body with sufficient oxygen supply, mainly focused on aerobic metabolism (13). RT is an active movement of muscles relying on their strength to overcome external resistance (14), whereas CE refers to the combination of aerobic exercise and strength training. Conventional therapy (CT) refers to routine treatment and care, and the patient does not perform any regular exercise.
Scholars worldwide have explored different interventions, but the most effective and safe interventions to improve the cardiopulmonary function of stroke patients have yet to be concluded. Pairwise meta-analysis uses CT as the control, which cannot compare the treatment effects of multiple interventions. Network meta-analysis (NMA) was developed from the pairwise meta-analysis, from comparing two standard treatment factors to comparing numerous treatment factors simultaneously. The primary function of NMA is to evaluate and rank multiple interventions simultaneously (15). Therefore, we aimed to use NMA to assess and compare the effects of different exercise methods on improving cardiopulmonary function in stroke patients to provide sufficient evidence for future clinical practice.
2 Materials and methods
2.1 Study enrollment and reporting
This study was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (16). PRISMA extension statements were used to ensure that all aspects of methods and results were reported (17). The protocol is registered in PROSPERO (registration number: CRD42023436773).
2.2 Search strategy
Two authors separately searched for randomized controlled trials (RCTs) regarding exercise improving cardiopulmonary function in stroke patients from the China National Knowledge Infrastructure (CNKI), WanFang Knowledge Service Platform (WanFang), Chinese Scientific Journals Database (VIP), Chinese Biomedical Literature Service System (CBM), PubMed, Embase, Web of Science, and The Cochrane Library databases. The retrieval period started from the establishment of the database to 30 April 2023. By combining medical subject headings with free words using Boolean logic operators, we integrated the following terms for a comprehensive search: “stroke,” “apoplexy,” “hemiplegia,” “cerebrovascular disease,” “cerebral infarction,” “cerebral hemorrhage,” “sport,” “exercise,” “train,” “physical activity,” “resistance exercise,” “aerobic exercise,” “high-intensity interval training,” “random,” “randomized controlled trial,” and “RCT.” In addition, we manually screened the list of references in the relevant meta-analysis and reviews to minimize the omission of literature that meets the inclusion criteria. Taking PubMed search as an example, the details of the search strategy are shown in Supplementary Table S1.
2.3 Selection and exclusion criteria
The inclusion criteria were formulated according to the principles of Population, Intervention, Comparison, Outcome, and Study design (PICOS) (18). Eligible studies had to meet the following criteria: (1) population: adult stroke patients with stable vital signs, no cognitive impairment and movement contraindication, and with the consent of the patient and his family members; (2) intervention: HIIT, AT, RT, and CE; (3) comparison: the control group only received CT or any of the above interventions; (4) outcome: in the included article, at least one of the following results must be reported: peak oxygen uptake (VO2peak), 6 min walking distance (6MWD), systolic blood pressure (SBP), and diastolic blood pressure (DBP); (5) study design: randomized controlled trial. The exclusion criteria were as follows: (1) studies that do not specify the type of exercise intervention; (2) studies with unclear descriptions of participant age; (3) conference articles, reviews, dissertations, and non-RCTS (e.g., case reports, observational studies, cross-sectional studies, and studies without a control group); (4) studies with more patients withdrawing midway; (5) studies that could not be downloaded; and (6) studies with incomplete outcome data and contacting the authors three times without response.
2.4 Study selection
Two authors (CW and YX) independently screened the article using EndNote X9 software. If there was any disagreement during the process, the decision was made through consultation or jointly with the third author (LZ). During article screening, we first used the duplicate check function of the software to eliminate any of the same articles. The title, abstract, and body of the literature were then read sequentially, and those that did not meet the inclusion criteria were eliminated. In case of missing important information, we contacted the corresponding authors of the literature by email or other means to ensure the completeness of the data.
2.5 Data extraction and quality assessment
Two authors (WF and ZL) independently reviewed all the articles and extracted the data. The extracted data includes basic publication information (first author’s name and country of origin), participant characteristics (age and sample size), intervention characteristics (type, intensity, duration, and period), and outcome measures (VO2peak, 6MWD, SBP, and DBP) at baseline and last observation, to observe their change scores. When there were disagreements during data extraction, the third author (MY) was involved in the discussion and decision-making. Two authors (WF and ZL) used the Cochrane Risk of Bias Tool to evaluate the included article in the following aspects: (I) random sequence generation; (ii) allocation concealment; (iii) blinding of participants and personnel; (iv) blinding of outcome assessment; (v) incomplete outcome data; (vi) selective reporting; (vii) other bias (19). The risk assessment was divided into three levels: “low risk,” “high risk,” and “unclear.” The evaluation process was carried out by two authors independently, and if there were any disputes in the process, the third author (MY) was consulted and a decision made together.
2.6 Statistical analysis
Odds ratio for binary variables and mean difference (MD) for continuous variable were used as the effect indicators, and the 95% confidence interval (CI) was provided for each effect size. For continuous variable indicators, we calculated the difference before and after treatment and the standard deviation according to the method provided in 16.1.3.2 of Cochrane Handbook 5.0.2 for statistical analysis. We used RevMan (version 5.4) for pairwise meta-analysis. The p-value of the chi-square test and the I2 index from the heterogeneity test were used to express the level of statistical heterogeneity. Different effect models were selected according to the level of heterogeneity of the test data. When the level of heterogeneity was low (p ≥ 0.1, I2 ≤ 50%), we selected the fixed effect model for analysis. Otherwise, a random effect model (p < 0.1, I2 > 50%) was used (20).
We used Stata (version 17.0) for all statistical analysis and various charts, such as network meta-analysis diagrams of eligible comparisons, the surface under the cumulative ranking area (SUCRA), funnel plot of publication bias, and so on (21). When there are closed loops between interventions, we first need to assess global inconsistency. When p > 0.05, the inconsistent model was not significant, and the consistent model was selected (22). We used a node-splitting approach to assess local inconsistency (23). At the same time, it is also necessary to evaluate the loop inconsistency and calculate the inconsistency factors (IF) and 95% confidence interval (CI) for each closed loop. If the lower limit of 95% CI included or was close to 0, the consistency between the direct comparison results and the indirect comparison results was good; otherwise, the closed loop was considered to have obvious inconsistency. If no closed loop was formed between the interventions, the consistency model was used for analysis directly. Intervention outcomes were ranked using the SUCRA. The closer SUCRA was to 100%, the better the effect of the intervention. Finally, the publication bias of the included articles was evaluated by drawing the funnel plot of publication bias and Egger’s test. Publication bias was indicated when there was asymmetry in the funnel plot of publication bias and p < 0.05 in Egger’s test (24).
3 Results
3.1 Study identification and selection
We strictly searched the above eight databases according to the inclusion and exclusion criteria and preliminarily obtained 8,692 articles. After eliminating duplicates, 6,297 articles remained. By reading the titles and abstracts of the articles, those that did not meet the inclusion criteria were excluded, leaving 226 articles. By reading the full text, we excluded a further 191 articles, including non-RCTs (n = 91), articles with unrelated intervention (n = 17), articles with irrelevant outcomes (n = 58), articles with unavailable full text (n = 14), and articles with incomplete data (n = 11). Ultimately, 35 articles met our study requirements (Figure 1).
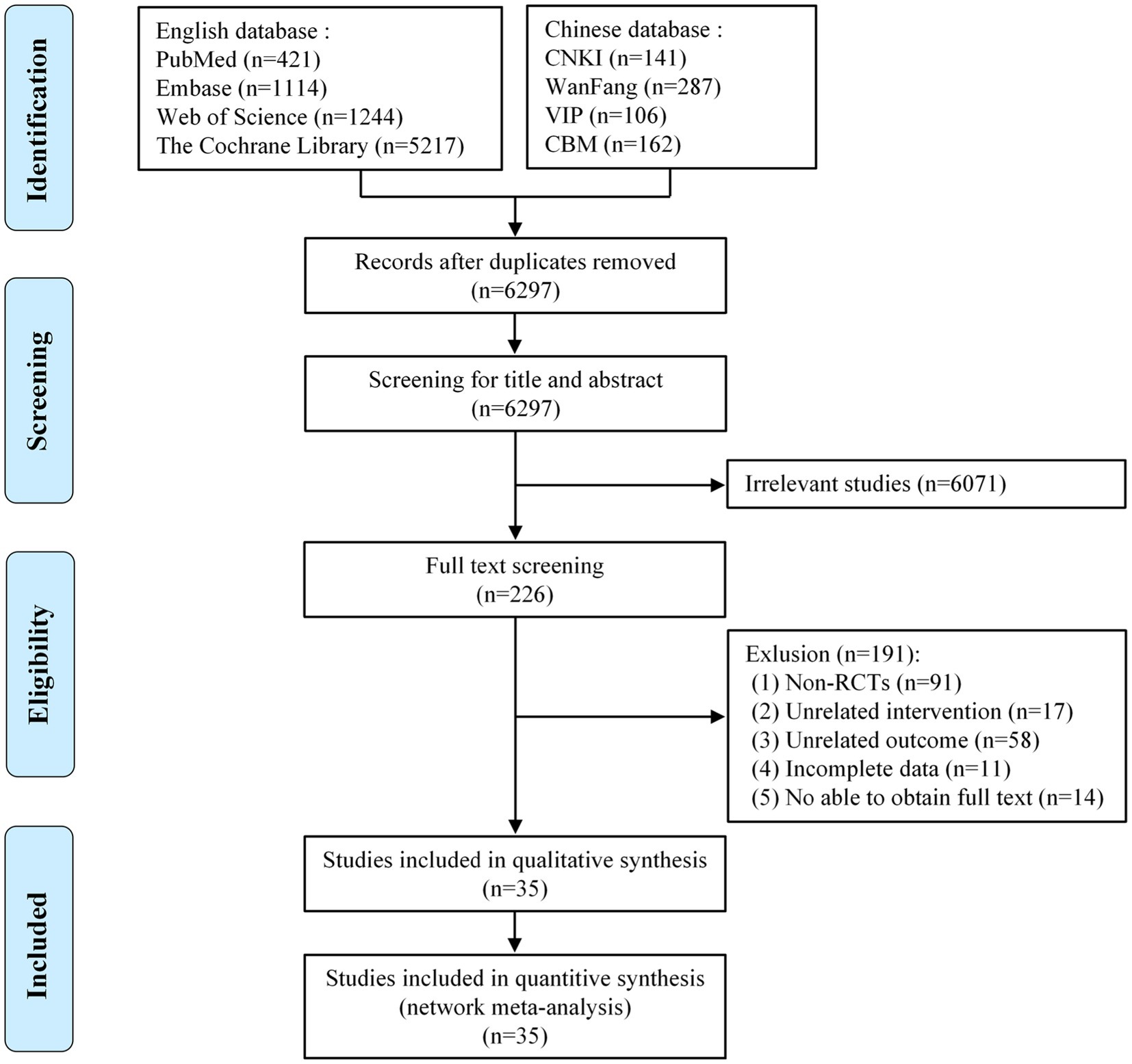
Figure 1. Flow diagram of eligible studies selection process. CNKI, China national knowledge infrastructure; WanFang, WanFang knowledge service platform; VIP, Chinese scientific journals database; CBM, Chinese biomedical literature service system; n, number of publications.
3.2 Characteristics of the included studies
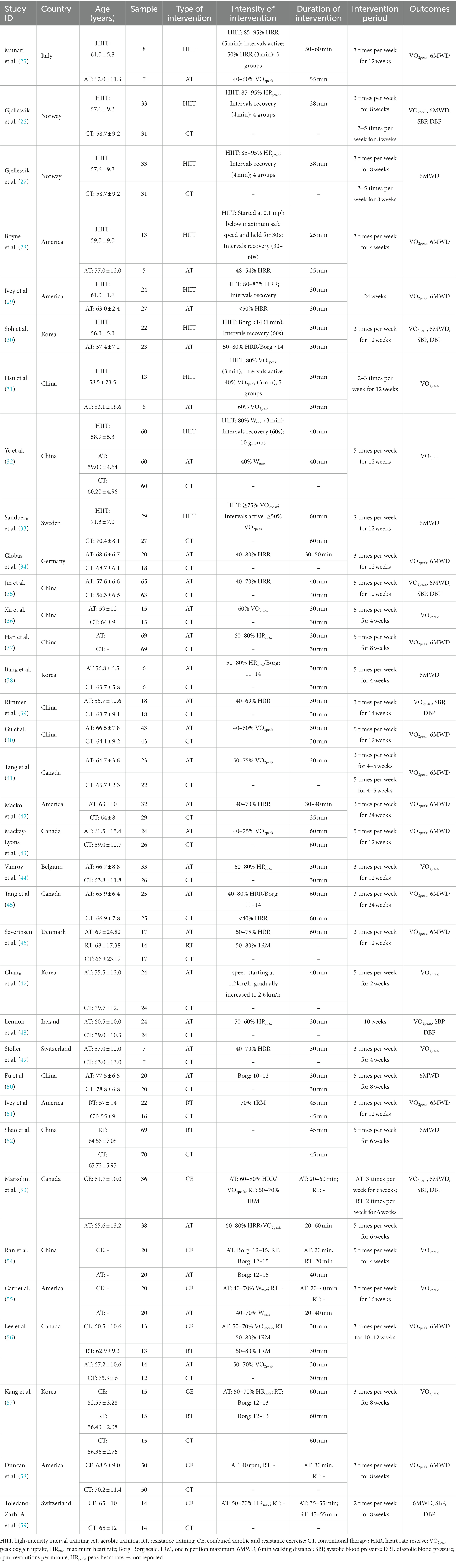
We finally included 35 RCTs with 1,075 patients in the intervention group and 933 patients in the control group, ranging in age from 55 to 78 years. The RCTs were from China (n = 9), the United States (n = 7), Canada (n = 4), South Korea (n = 4), Norway (n = 2), Ireland (n = 1), Denmark (n = 1), Switzerland (n = 1), Sweden (n = 1), Australia (n = 1), Italy (n = 1), Belgium (n = 1), Germany (n = 1), and Israel (n = 1). Among the 35 articles, one was a four-arm trial, three were three-arm trials, and 31 articles were two-arm trials. Twenty-nine articles used VO2peak as the outcome measure; 21 used 6MWD as the outcome measure; and seven used SBP and DBP as the outcome measure. Table 1 shows the key characteristics of the patients and interventions included in this study.
3.3 Quality evaluation
All 35 articles included were RCTs. Twenty-one articles reported random sequence generation, rated as a low risk of bias; 14 did not adequately report how randomization was performed and were rated as uncertain risk of bias; 12 described allocation concealment and were rated as having a low risk of bias; 22 did not fully report blinding of researchers and subjects, rated as an uncertain risk of bias; 13 did not blind the investigators and subjects and were rated as a high risk of bias; and eight articles described the blinding of outcome measures and were rated as having a low risk of bias. None of the remaining articles were reported and rated as having an uncertain risk of bias. All 35 articles showed good data integrity and did not report the study results selectively. Furthermore, all articles did not describe any other bias. Figure 2 shows the details of the bias risk assessment results.
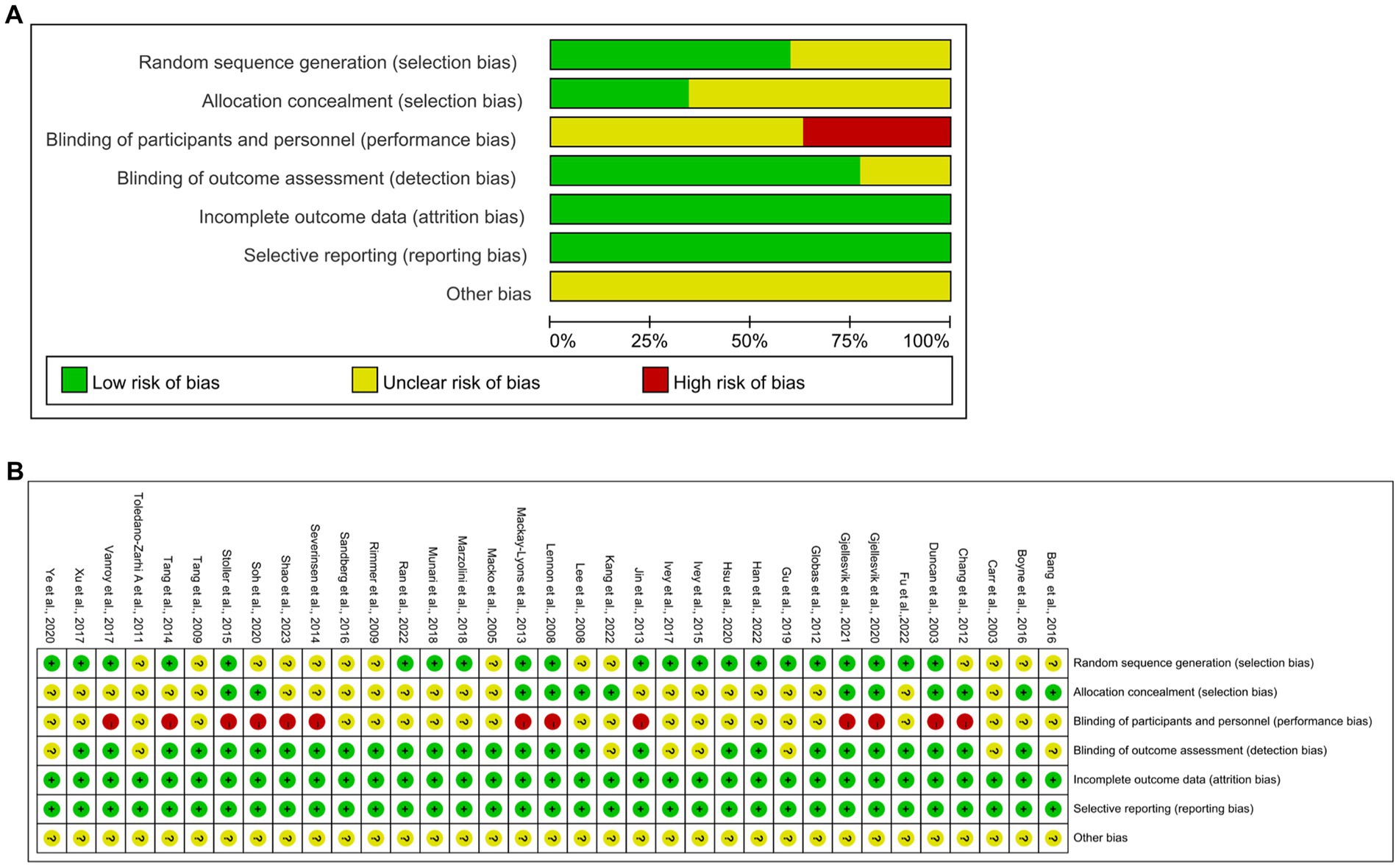
Figure 2. Quality assessment of selected studies by the cochrane risk of bias tool. (A) Risk of bias graph: review authors judgments about each risk of bias item presents as percentages across all included studies. (B) Risk of bias summary: review authors judgements about each risk of bias item for each included study.
3.4 Pairwise meta-analysis
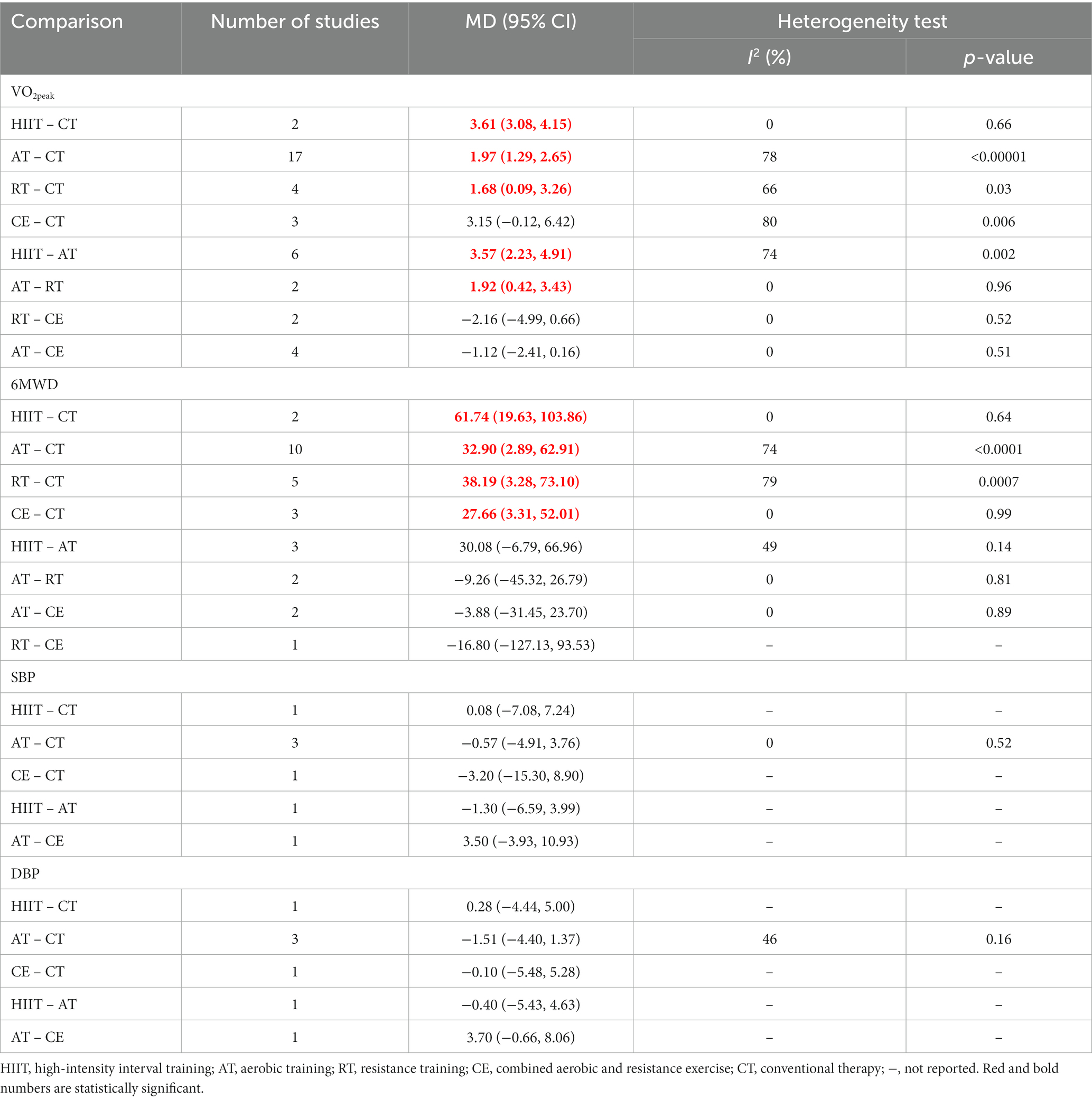
In this study, we used a pairwise meta-analysis to comprehensively compare two interventions. We carried out eight pairwise meta-analyses to compare VO2peak, 8 to compare 6MWD, 5 to compare SBP, and 5 to compare DBP, respectively, which can be summarily seen in Table 2. The detailed forest plots of the pairwise meta-analysis results were shown in Supplementary Figures S1–S4.
3.5 Network analysis results
3.5.1 VO2peak
VO2peak was reported in 29 articles involving five interventions: HIIT, AT, RT, CE, and CT with a total of 1,534 patients. Figure 3A shows the NMA diagrams of eligible comparisons, and the blue dots represent different interventions. The size of the dots represents the sample size; the straight line between two dots represents a direct comparison between two various interventions; and the thicker the solid line indicates the more significant number of studies in that pairwise comparison.
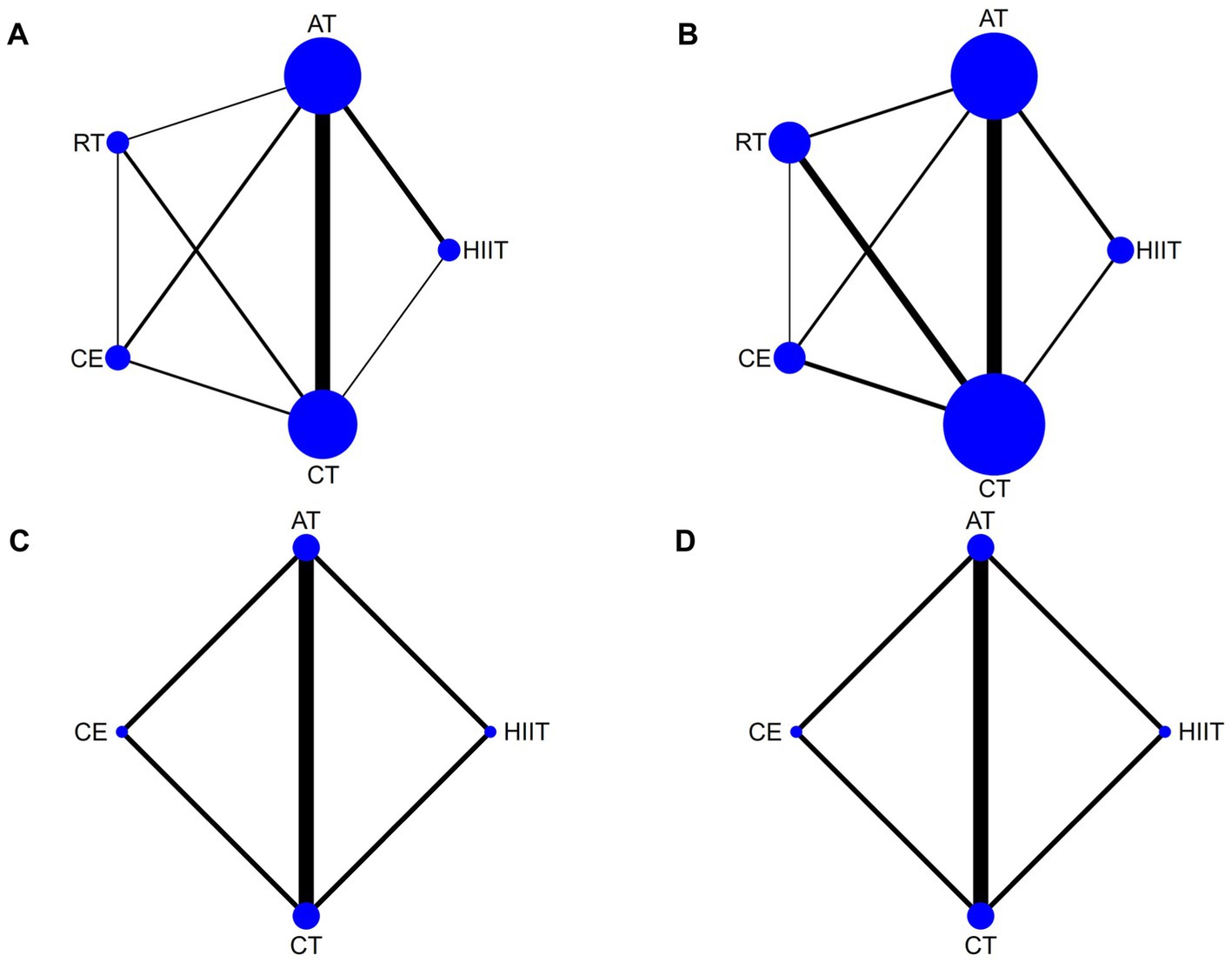
Figure 3. Network meta-analysis diagrams of eligible comparisons. Width of the lines is proportional to the number of trial. Size of every circle is proportional to the number of randomly assigned participants (sample size). (A) VO2peak; (B) 6WMD; (C) SBP; (D) DBP. HIIT, high-intensity interval training; AT, aerobic training; RT, resistance training; CE, combined aerobic and resistance exercise; CT, conventional therapy.
The inconsistency model evaluated global inconsistency, which showed p = 0.069 (>0.05) (Supplementary Figure S5A). The inconsistency test was not significant, so we used the consistency model. We used the node-splitting approach to assess local inconsistency and only measured p < 0.05 for HIIT compared with CT (Supplementary Table S2). Nine closed loops were formed for the five interventions, and we assessed loop inconsistency for all closed loops. The results showed that all the 95% CI included 0, and all the IF were close to 0, indicating that the statistical results of NMA were highly credible (Supplementary Figure S6A).
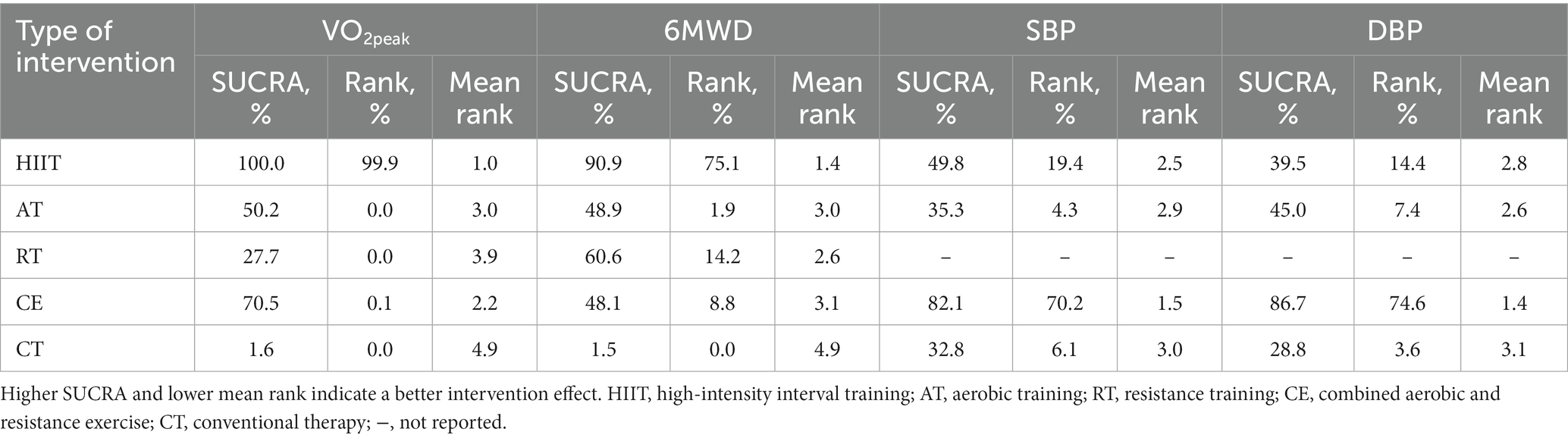
The NMA results showed that VO2peak generated a total of 10 pairwise comparisons. HIIT significantly improved VO2peak compared to CE (MD = 2.55, 95% CI [0.96, 4.19]), AT (MD = 3.29, 95% CI [2.21, 4.37]), and RT (MD = 4.12, 95% CI [2.41, 5.83]). Compared with CT, HIIT (MD = 5.15, 95% CI [3.97, 6.32]), CE (MD = 2.59, 95% CI [1.31, 3.88]), and AT (MD = 1.86, 95% CI [1.22, 2.49]) significantly improved VO2peak in stroke patients. There was no statistically significant difference between the other two interventions (p > 0.05) (Figure 4A). Table 3 showed the SUCRA ranking for all interventions. According to the analysis, HIIT (SUCRA, 100.0%) may be the most effective intervention to improve VO2peak in stroke patients, followed by CE (SUCRA, 70.5%), AT (SUCRA, 50.2%), RT (SUCRA, 27.7%), and CT (SUCRA, 1.6%).
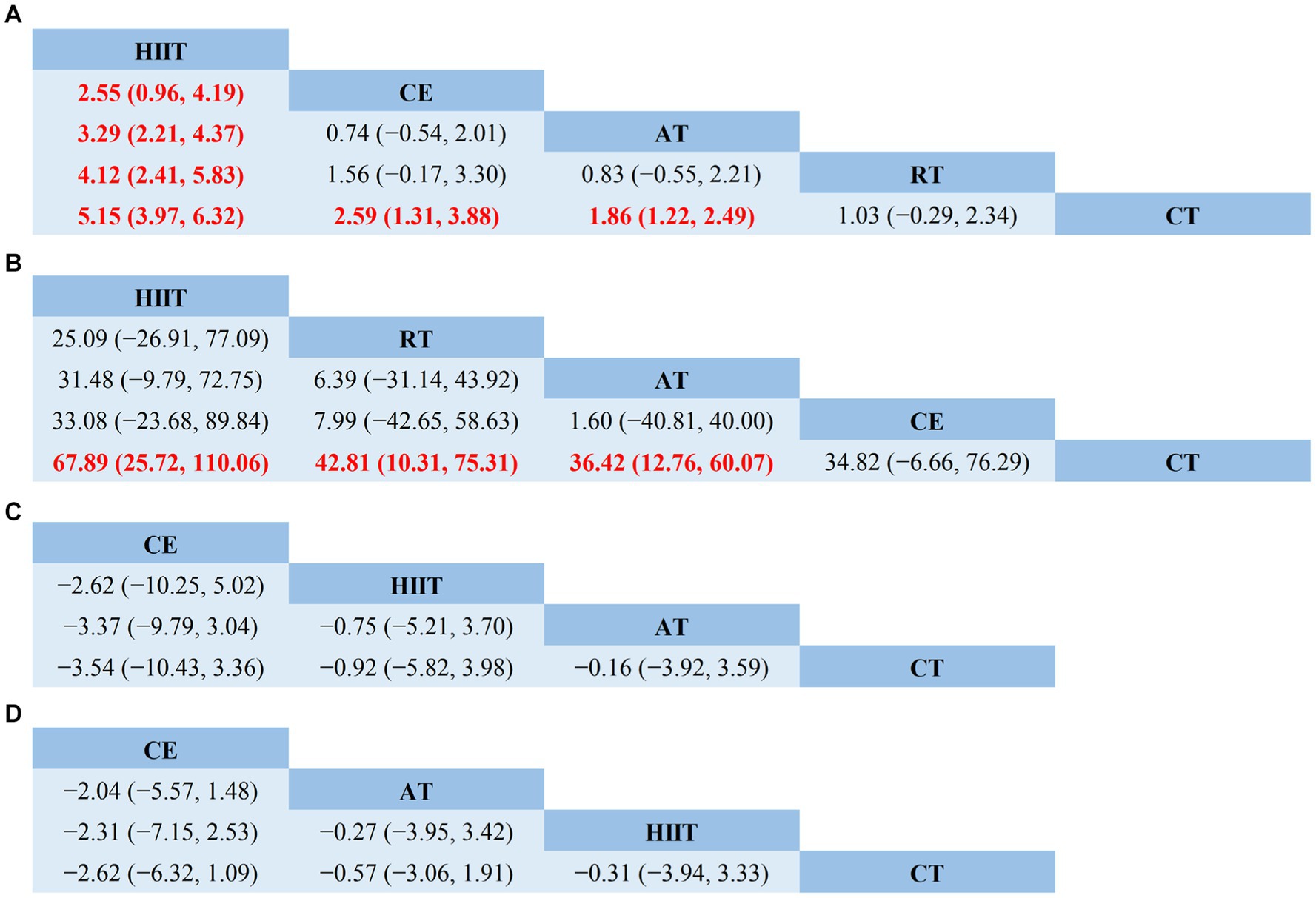
Figure 4. Network meta-analysis of head-to-head comparisons. (A) VO2peak; (B) 6WMD; (C) SBP; (D) DBP. Red and bold numbers are statistically significant. HIIT, high-intensity interval training; AT, aerobic training; RT, resistance training; CE, combined aerobic and resistance exercise; CT, conventional therapy.
3.5.2 6MWD
6MWD was reported in 21 articles involving five interventions: HIIT, AT, RT, CE, and CT, with a total of 1,156 patients. Figure 3B shows the NMA diagrams of eligible comparisons. The inconsistency model evaluated global inconsistency, which showed p = 0.6592 (>0.05) (Supplementary Figure S5B). The inconsistency test was not significant, so the consistency model was used.
The node-splitting approach was used to evaluate local inconsistency. The measured p-values were all >0.05, indicating good local consistency (Supplementary Table S3). Nine closed loops were formed for the five interventions, and we assessed loop inconsistency for all closed loops. The results showed that all the 95% CI included 0, and all the IF were close to 0, indicating that the statistical results of NMA were highly credible (Supplementary Figure S6B). The NMA results showed that 6MWD generated a total of 10 pairwise comparisons. Compared with CT, HIIT (MD = 67.89, 95% CI [25.72, 110.06]), RT (MD = 42.81, 95% CI [110.31, 75.31]), and AT (MD = 36.42, 95% CI [12.76, 60.07]) can significantly improve 6MWD in stroke patients. There was no statistically significant difference between the other two interventions (p > 0.05) (Figure 4B). Table 3 showed the SUCRA ranking for all interventions. According to the results of the SUCRA analysis, HIIT (SUCRA, 90.9%) may be the most effective intervention to improve 6MWD in stroke patients, followed by RT (SUCRA, 60.6%), AT (SUCRA, 48.9%), CE (SUCRA, 48.1%), and CT (SUCRA, 1.5%).
3.5.3 Systolic blood pressure
SBP was reported in seven articles involving four interventions: HIIT, AT, CE, and CT with a total of 394 patients. Figure 3C shows the NMA diagrams of eligible comparisons. The inconsistency model evaluated global inconsistency, which showed p = 0.9259 (>0.05) (Supplementary Figure S5C). The inconsistency test was not significant, so the consistency model was used.
The node-splitting approach was used to evaluate local inconsistency. The measured p-values were all >0.05, indicating good local consistency (Supplementary Table S4). Three closed loops were formed for the four interventions, and we assessed loop inconsistency for all closed loops. The results showed that all the 95% CI included 0, and all the IF were close to 0, indicating that the statistical results of NMA were highly credible (Supplementary Figure S6C). The NMA results showed that SBP generated a total of six pairwise comparisons and there was no statistically significant difference between the pairwise comparisons (p > 0.05) (Figure 4C). Table 3 showed the SUCRA ranking for all interventions. According to the results of the SUCRA analysis, CE (SUCRA, 82.1%) may be the most effective intervention to improve SBP in stroke patients, followed by HIIT (SUCRA, 49.8%), AT (SUCRA, 35.3%), and CT (SUCRA, 32.8%).
3.5.4 Diastolic blood pressure
DBP was reported in seven articles involving four interventions: HIIT, AT, CE, and CT with a total of 394 patients. Figure 3D shows the NMA diagrams of eligible comparisons. The inconsistency model evaluated global inconsistency, which showed p = 0.5571 (>0.05) (Supplementary Figure S5D). The inconsistency test was not significant, so the consistency model was used.
The node-splitting approach was used to evaluate local inconsistency. The measured p-values were all greater than 0.05, indicating good local consistency (Supplementary Table S5). Three closed loops were formed for the four interventions, and we assessed loop inconsistency for all closed loops. The results showed that all the 95% CI included 0, and all the IF were close to 0, indicating that the statistical results of NMA were highly credible (Supplementary Figure S6D). The NMA results showed that SBP generated a total of six pairwise comparisons, and there was no statistically significant difference between the pairwise comparisons (p > 0.05) (Figure 4D). Table 3 showed the SUCRA ranking for all interventions. According to the results of the SUCRA analysis, CE (SUCRA, 86.7%) may be the most effective intervention to improve DBP in stroke patients, followed by AT (SUCRA, 45.0%), HIIT (SUCRA, 39.5%), and CT (SUCRA, 28.8%).
3.6 Publication bias
We evaluated publication bias for VO2peak, 6MWD, SBP, and DBP using the funnel plot of publication bias (Figure 5) and Egger’s test. The colored dots in the funnel plot of publication bias represent pairwise comparisons between two different interventions. The greater the number of dots, the greater the number of pairwise comparisons. The dots in our funnel plot of publication bias were generally symmetrically distributed and concentrated at the top of the funnel. However, a few dots were allocated on the outside of the funnel in Figure 5A, and a few dots were distributed below and outside the funnel in Figure 5B, indicating a possible publication bias. In addition, we used Egger’s test for secondary validation of publication bias. The results showed VO2peak (Egger’s test p = 0.819) (Supplementary Figure S7A), 6MWD (Egger’s test p = 0.384) (Supplementary Figure S7B), SBP (Egger’s test p = 0.268) (Supplementary Figure S7C), and DBP (Egger’s test p = 0.812) (Supplementary Figure S7D), indicating that there was no publication bias in this study.
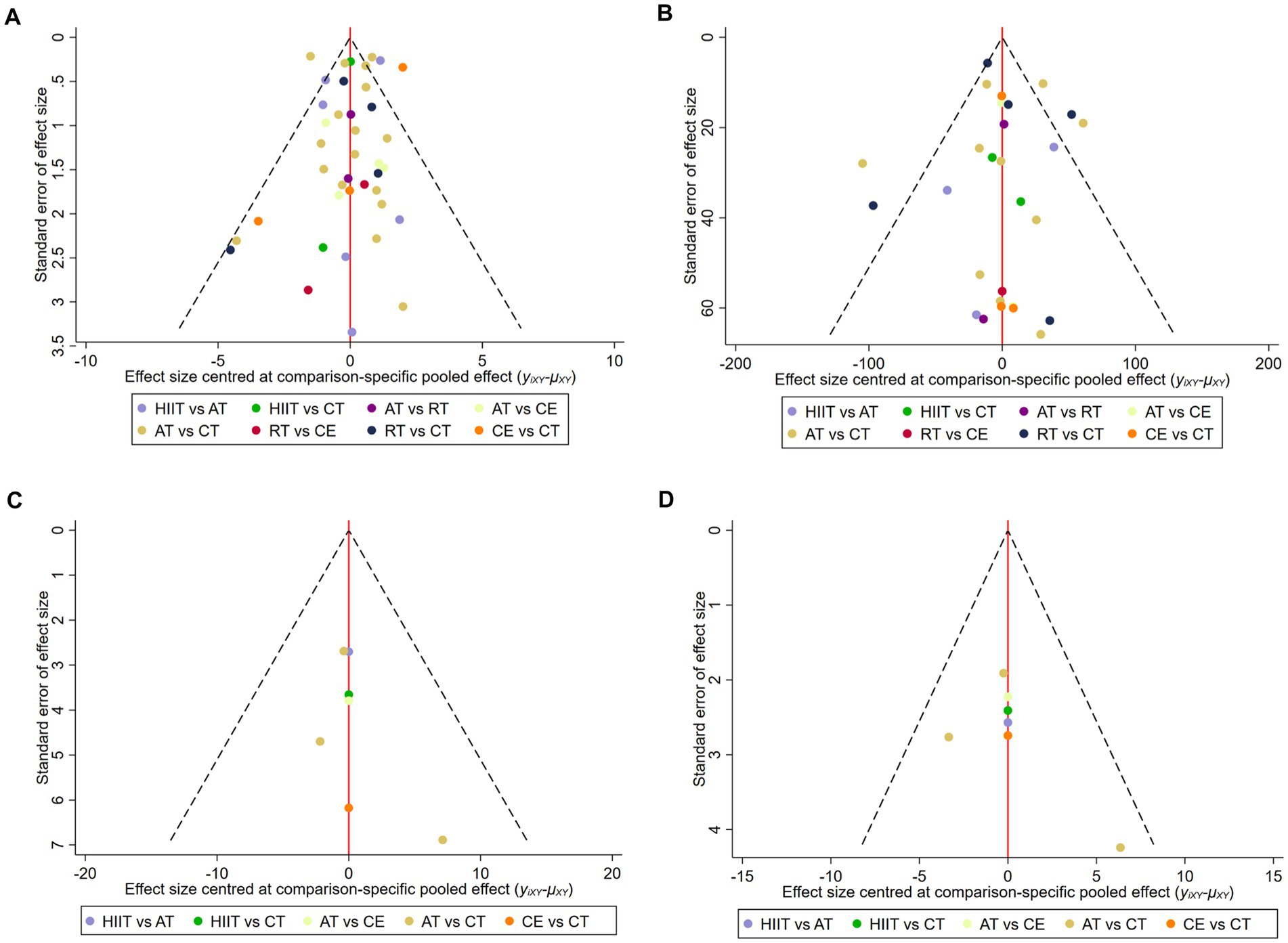
Figure 5. Funnel plot of publication bias. (A) VO2peak; (B) 6WMD; (C) SBP; (D) DBP. HIIT, high-intensity interval training; AT, aerobic training; RT, resistance training; CE, combined aerobic and resistance exercise; CT, conventional therapy.
3.7 Safety assessment of exercise training
In most of the included studies, investigators strictly implemented safety measures to ensure patient safety. Fifteen studies reported no adverse events during the exercise intervention period. Nine studies reported adverse events during the intervention period (Supplementary Table S6), involving 60 patients and four interventions (HIIT, AT, RT, and CT). The main adverse events included falls, fractures after falls, pneumonia, seizures, recurrent stroke, lower limb deep vein thrombosis, joint or muscle pain, dizziness, unstable blood pressure, brain tumors, concussions, aortic aneurysms, hernias, traumatic bleeding, and death. No adverse events were reported in the remaining 11 studies. Because there was no significant difference in the incidence of adverse events between the intervention group and the control group (p > 0.05), we believe exercise intervention is feasible for stroke patients. Still, it should be noted that exercise intervention must be carried out under the guidance and supervision of professionals.
4 Discussion
After stroke, the decline of nervous system function, prolonged bed rest, and decreased exercise will seriously affect the cardiopulmonary circulation system of patients. Girard et al. (60) found that stroke patients were inactive for 21 to 80% of their time during inpatient rehabilitation. In addition, it is difficult for patients to achieve 40% HRR in routine rehabilitation treatment. Conventional rehabilitation therapy is not sufficient to produce the effect of cardiopulmonary training, which hinders the recovery of functional independence of patients and may increase the risk of future stroke and other cardiovascular events. Therefore, for stroke patients, additional exercise to improve cardiopulmonary function is critical and necessary throughout the rehabilitation process. Our NMA evaluated the relative effectiveness of different exercise methods in improving VO2peak, 6MWD, SBP, and DBP in stroke patients by analyzing data from RCTs. This NMA was based on 35 studies, including a total of 2,008 patients. Twenty-nine RCTs assessed the effectiveness of four exercise methods and CT in improving VO2peak in stroke patients; 21 RCTs assessed the effects of four exercise methods and CT on 6MWD; seven RCTs assessed the effects of three exercise methods and CT on SBP and DBP. To our knowledge, this is the first NMA to compare the effects of different exercise methods on improving cardiopulmonary function in stroke patients. The NMA showed that HIIT was the most effective in improving VO2peak, followed by CE. HIIT was the most effective in improving 6MWD, followed by RT. CE was the most effective in improving SBP and DBP in stroke patients. HIIT and AT also improved blood pressure in stroke patients to varying degrees.
In published studies, VO2peak is one of the most commonly used measures to assess cardiopulmonary function in stroke patients, is the gold standard to evaluate an individual’s cardiopulmonary fitness, and is negatively correlated with cardiovascular risk and all-cause mortality (61). 6MWD, which refers to the maximum distance a participant can walk in 6 min, is also a commonly used indicator to assess an individual’s aerobic capacity and exercise endurance and is reliable and valid for evaluating cardiopulmonary function in stroke patients (62). This study shows that HIIT is the most potential exercise method to improve VO2peak and 6MWD in stroke patients. We summarized the following four advantages of HIIT. First, HIIT can improve the cardiopulmonary function of stroke patients by increasing the stroke volume, myocardial contractility, and the number and volume of mitochondria in cells (63). Second, the exercise intensity of HIIT is high enough to stimulate both aerobic and anaerobic metabolic areas, so that the plasma volume and red blood cell volume are increased, and the venous return is improved. Ultimately, the stroke volume of the subjects increased (64, 65), the blood flow resistance decreased (66), the cardiopulmonary function was improved, and the effect was sustainable (67). Third, decreased neuromuscular recruitment after stroke can lead to decreased skeletal muscle oxidative capacity. HIIT can increase neuromuscular recruitment, reduce the proportion of fast-twitch (type II) muscle fibers, and change the corresponding aerobic oxidation substrates to increase aerobic endurance. Therefore, HIIT can reduce exercise fatigue in stroke patients (68, 69). Fourth, HIIT has significant advantages in terms of time efficiency. Meanwhile, the personalization of HIIT may enhance the enjoyment of exercise, thereby enhancing patients’ adherence to exercise (70).
Although HIIT is a highly effective exercise modality, the optimal exercise intensity, frequency, and duration are still controversial. Crozier et al. (69) recommended that the duration of high-intensity exercise should range from 30 s to 4 min, the interval recovery phase should range from 30 s to 3 min, and the total duration of a single intervention should be 25 to 30 min, which provides a valuable reference for the formulation of individualized plans for HIIT. Meanwhile, the recovery time after the end of HIIT is also critical. Because evidence suggests that older adults (mean age, 63.0 ± 3.4 years) train at least 3 days intervals to reduce the risk of fatigue and achieve optimal recovery (71). Therefore, HIIT can be performed twice a week at the beginning of training and then gradually increase the frequency of exercise as tolerated. In clinical practice, clinicians or rehabilitation therapists have the flexibility to formulate the best exercise prescription according to the specific condition of patients and the above-recommended programs.
In addition, whether HIIT increases the risk of acute cardiovascular events is still uncertain. Rognmo et al. (72) found the following in their analysis of 4,846 patients with coronary artery disease who underwent HIIT and MICT, “Of the total exercise time of 175,820 h, one fatal cardiac arrest was reported during MICT (129,456 h of exercise), and two nonfatal cardiac arrests were reported during HIIT (46,364 h of exercise), with no myocardial infarction.” The results showed that the risk of cardiovascular events was low for both types of exercise, but HIIT produced significant cardiovascular adaptations. Wewege et al. (73) conducted a systematic review study on the safety of HIIT in patients with cardiovascular disease, which included 23 studies involving 1,117 patients. Among the 23 studies, 14 used the classical 4 × 4 min long interval protocol for HIIT, and the rest lasted from 30 s to 3 min. The systematic review reported one adverse event for every 3,417 HIIT sessions (2,227 training hours); one adverse event occurred every 7,134 MICT sessions (5,606 training hours). There was no difference in the risk of adverse events between the two exercise methods. We, therefore, conclude that HIIT can be used as an additional option to traditional aerobic exercise to improve cardiopulmonary function in stroke patients with stable clinical symptoms, recent regular exercise, correct exercise risk screening before exercise intervention, and motor function monitoring during exercise. Finally, it should be noted that few patients with severe stroke or more comorbidities were included in these studies. Therefore, the existing research results cannot be directly generalized to all stroke patients, and cardiopulmonary rehabilitation programs for stroke patients with severe or more comorbidities should be further explored in the future.
We also assessed two indirect measures of cardiopulmonary function (SBP and DBP). Hypertension is the most important modifiable risk factor for stroke, and about 64% of stroke patients have a history of hypertension before onset (74). When SBP is >115 mmHg or DBP >75 mmHg, the likelihood of cardiovascular events increases with blood pressure (75). The risk of fatal cardiovascular events doubles with each increase in SBP (20 mmHg) or DBP (10 mmHg) (76). Therefore, optimizing the management of blood pressure is of great significance to improve the prognosis of stroke. Taking antihypertensive drugs to rapidly lower blood pressure, even to lower levels within the hypertensive range, can affect patients to varying degrees. Therefore, blood pressure management through exercise is undoubtedly a suitable rehabilitation method for stroke patients. CE is known internationally as “concurrent training” or “concurrent strength and endurance training” (77). Wilson et al. (78) believe that concurrent training is a method to obtain strength, muscle hypertrophy, and muscle endurance in the same training phase. Davis et al. (79) suggest that concurrent training can maintain physical strength levels, improve endurance and other essential physical qualities, and be more beneficial than traditional rehabilitation training. This NMA showed that CE was the most effective in improving SBP and DBP in stroke patients, and HIIT and AT also improved blood pressure in stroke patients to varying degrees. However, there was no significant difference in the effect of each intervention on blood pressure improvement (p > 0.05). Although exercise positively affects blood pressure and health status in patients with hypertension, excessively vigorous exercise in the short term may increase the risk of adverse events (80). To safely and effectively improve blood pressure in stroke patients, CE with moderate intensity (AT: 40–60% HRR, RT: 50–70% 1RM), 3 days per week for 20 weeks should be prioritized, as this exercise program has the best intervention effect (81). However, it should be noted that managing blood pressure in stroke patients is complicated due to the variability of the etiology and hemodynamics caused by stroke. Therefore, when exercise is used to reduce the blood pressure of stroke patients in clinical treatment, it should be combined with the specific condition of the patient, and the blood pressure should be controlled reasonably after a comprehensive evaluation.
Our study has some strengths. Firstly, 35 articles and 2,008 adult stroke survivors were included, indicating a large sample size. Secondly, the interventions included four methods of exercise and CT, and the effects of the interventions were evaluated by four outcome measures. Then, to ensure a good level of evidence, we strictly followed inclusion and exclusion criteria to ensure that only RCTs were included. Finally, our study is the first NMA to compare the effects of different exercise methods on improving cardiopulmonary function in stroke patients, providing a preliminary basis for further detailed research in this area. Our study also has some limitations that should be considered. First, the intensity, duration, frequency, and period of exercise interventions in the included studies were not consistent, and the types of exercise were also different, including treadmill exercise, power cycling exercise, and weight-bearing walking exercise, which may limit the results of the study. Second, the ages of the included patients are slightly different, and some data indicators will be affected by age, which will affect the quality of the article. Further subgroup analysis based on age is needed in the future. Third, the number of articles using RT as an intervention is small. At the same time, the limited amount of articles on improving SBP and DBP may reduce the reliability of the conclusions. Fourth, some studies did not describe random sequence generation and allocation concealment, which may cause certain selection biases. Finally, adverse events may not be strictly reported in the included RCTs. Therefore, the safety of exercise intervention needs to be further studied.
5 Conclusion
In this NMA, no single exercise method was optimal for all outcome indicators. Different exercise methods have distinct advantages in improving cardiopulmonary function in stroke patients. HIIT was more effective than other exercise methods in improving VO2peak and 6MWD. CE was the most effective in improving DBP and SBP. Still, it is worth noting that the number of articles included in the latter two outcome measures is limited, and the conclusions still need to be further verified. At the same time, due to the limitations of existing clinical studies and evidence, larger sample size, multi-center, and high-quality RCTs are needed to verify the above conclusions in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
CW: Writing – original draft. YX: Writing – original draft. LZ: Data curation, Software, Writing – review & editing. WF: Data curation, Software, Writing – review & editing. ZL: Data curation, Software, Writing – review & editing. MY: Methodology, Writing – review & editing. LW: Project administration, Supervision, Writing – review & editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Beijing Hospitals Authority Youth Programme (code: QML20212201).
Acknowledgments
We are sincerely grateful to all members of our team and reviewers for their valuable comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1288032/full#supplementary-material
References
1. Sacco, RL , Kasner, SE , Broderick, JP , Caplan, LR , Connors, JJB , and Culebras, A . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
2. Lindsay, MP , Norrving, B , Sacco, RL , Brainin, M , Hacke, W , and Martins, S . World stroke organization (WSO): global stroke fact sheet 2019. Int J Stroke. (2019) 14:806–17. doi: 10.1177/1747493019881353
3. Benjamin, EJ , Blaha, MJ , Chiuve, SE , Cushman, M , and Das, SR . Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
4. Jiang, B . Analysis of the epidemiological characteristics of post-stroke complications and suggestions for the optimization ofGrass-roots management in grass-roots management system. Chin Gen Pract. (2021) 24:1445–53. doi: 10.12114/j.issn.1007-9572.2021.00.420
5. Beltz, NM , Gibson, AL , Janot, JM , Kravitz, L , Mermier, CM , and Dalleck, LC . Graded exercise testing protocols for the determination of VO2max: historical perspectives, Progress, and future considerations. J Sports Med. (2016) 2016:3968393–12. doi: 10.1155/2016/3968393
6. Smith, AC , Saunders, DH , and Mead, G . Cardiorespiratory fitness after stroke: a systematic review. Int J Stroke. (2012) 7:499–510. doi: 10.1111/j.1747-4949.2012.00791.x
7. Shephard, RJ . Maximal oxygen intake and independence in old age. Br J Sports Med. (2009) 43:342–6. doi: 10.1136/bjsm.2007.044800
8. Ivey, FM , Hafer-Macko, CE , and Macko, RF . Exercise rehabilitation after stroke. NeuroRx. (2006) 3:439–50. doi: 10.1016/j.nurx.2006.07.011
9. Pase, MP , Beiser, A , Enserro, D , Xanthakis, V , Aparicio, H , and Satizabal, CL . Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke. (2016) 47:1201–6. doi: 10.1161/STROKEAHA.115.012608
10. Billinger, SA , Arena, R , Bernhardt, J , Eng, JJ , Franklin, BA , and Johnson, CM . Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2532–53. doi: 10.1161/STR.0000000000000022
11. Zhang, T , Zhao, J , Li, X , Bai, Y , Wang, B , and Qu, Y . Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of stroke rehabilitation. Stroke Vasc Neurol. 5:250–9. doi: 10.1136/svn-2019-000321
12. Rodrigues, L , Moncion, K , Eng, JJ , Noguchi, KS , Wiley, E , and de Las, HB . Intensity matters: protocol for a randomized controlled trial exercise intervention for individuals with chronic stroke. Trials. (2022) 23:442. doi: 10.1186/s13063-022-06359-w
13. Mersy, DJ . Health benefits of aerobic exercise. Postgrad Med. (1991) 90:103–12. doi: 10.1080/00325481.1991.11700983
14. Howley, ET . Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. (2001) 33:S364. doi: 10.1097/00005768-200106001-00005
15. Rouse, B , Chaimani, A , and Li, T . Network meta-analysis: an introduction for clinicians. Intern Emerg Med. (2017) 12:103–11. doi: 10.1007/s11739-016-1583-7
16. Page, MJ , McKenzie, JE , Bossuyt, PM , Boutron, I , Hoffmann, TC , and Mulrow, CD . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Welch, V , Petticrew, M , Petkovic, J , Moher, D , Waters, E , and White, H . Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. Int J Equity Health. (2015) 14:92. doi: 10.1186/s12939-015-0219-2
18. Hutton, B , Salanti, G , Caldwell, DM , Chaimani, A , Schmid, CH , and Cameron, C . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
19. Savović, J , Weeks, L , Sterne, JAC , Turner, L , Altman, DG , and Moher, D . Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. (2014) 3:37. doi: 10.1186/2046-4053-3-37
20. Cumpston, M , Li, T , Page, MJ , Chandler, J , Welch, VA , and Higgins, JP . Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
21. Shim, S , Yoon, B-H , Shin, I-S , and Bae, J-M . Network meta-analysis: application and practice using Stata. Epidemiol Health. (2017) 39:e2017047. doi: 10.4178/epih.e2017047
22. White, IR , Barrett, JK , Jackson, D , and Higgins, JPT . Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. (2012) 3:111–25. doi: 10.1002/jrsm.1045
23. Dias, S , Welton, NJ , Caldwell, DM , and Ades, AE . Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
24. Fleiss, JL . The statistical basis of meta-analysis. Stat Methods Med Res. (1993) 2:121–45. doi: 10.1177/096228029300200202
25. Munari, D , Pedrinolla, A , Smania, N , Picelli, A , Gandolfi, M , and Saltuari, L . High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med. (2018) 54:408–18. doi: 10.23736/S1973-9087.16.04224-6
26. Gjellesvik, TI , Becker, F , Tjønna, AE , Indredavik, B , Nilsen, H , and Brurok, B . Effects of high-intensity interval training after stroke (the HIIT-stroke study): a multicenter randomized controlled trial. Arch Phys Med Rehabil. (2020) 101:939–47. doi: 10.1016/j.apmr.2020.02.006
27. Gjellesvik, TI , Becker, F , Tjønna, AE , Indredavik, B , Lundgaard, E , and Solbakken, H . Effects of high-intensity interval training after stroke (the HIIT stroke study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. (2021) 102:1683–91. doi: 10.1016/j.apmr.2021.05.008
28. Boyne, P , Dunning, K , Carl, D , Gerson, M , Khoury, J , and Rockwell, B . High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther. (2016) 96:1533–44. doi: 10.2522/ptj.20150277
29. Ivey, FM , Stookey, AD , Hafer-Macko, CE , Ryan, AS , and Macko, RF . Higher treadmill training intensity to address functional aerobic impairment after stroke. J Stroke Cerebrovasc Dis. (2015) 24:2539–46. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.002
30. Soh, S-H , Joo, MC , Yun, NR , and Kim, M-S . Randomized controlled trial of the lateral push-off skater exercise for high-intensity interval training vs conventional treadmill training. Arch Phys Med Rehabil. (2020) 101:187–95. doi: 10.1016/j.apmr.2019.08.480
31. Hsu, C-C , Fu, T-C , Huang, S-C , Chen, CP-C , and Wang, J-S . Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: a randomized controlled trial. Ann Phys Rehabil Med. (2021) 64:101385. doi: 10.1016/j.rehab.2020.03.010
32. Ye, RJ , Sun, LS , Zhang, Q , and Wang, K . Influence of aerobic exercise intensity on cardiac function and exercise tolerance in patients with stroke complicated coronary heart disease. Chin J Cardiovasc Rehabil Med. (2020) 29:536–40. doi: 10.3969/j.ISSN.1008-0074.2020.05.03
33. Sandberg, K , Kleist, M , Falk, L , and Enthoven, P . Effects of twice-weekly intense aerobic exercise in early subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2016) 97:1244–53. doi: 10.1016/j.apmr.2016.01.030
34. Globas, C , Becker, C , Cerny, J , Lam, JM , Lindemann, U , and Forrester, LW . Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair. (2012) 26:85–95. doi: 10.1177/1545968311418675
35. Jin, H , Jiang, Y , Wei, Q , Chen, L , and Ma, G . Effects of aerobic cycling training on cardiovascular fitness and heart rate recovery in patients with chronic stroke. NeuroRehabilitation. (2013) 32:327–35. doi: 10.3233/NRE-130852
36. Xu, Q , Pan, Y , Yang, XH , Ma, D , Xiao, YQ , and Wu, Q . Effect of aerobic exercise combined with routine rehabilitation therapy oncardiopulmonary exercise function and rehabilitation efficacy in stroke patientswith hemiplegia. Chin J Cerebrovasc Dis. (2017) 14:465–9. doi: 10.3969/j.issn.1672-5921.2017.09.004
37. Han, KY , Liu, GL , SuLW, TZQ , and Zhang, H . Effects of intelligent aerobic bicycle training on ischemic stroke patients at different disease courses. Chin J Rehabil Theory Pract. (2022) 28:822–7. doi: 10.3969/j.issn.1006-9771.2022.07.013
38. Bang, D-H , and Son, Y-L . Effect of intensive aerobic exercise on respiratory capacity and walking ability with chronic stroke patients: a randomized controlled pilot trial. J Phys Ther Sci. (2016) 28:2381–4. doi: 10.1589/jpts.28.2381
39. Rimmer, JH , Rauworth, AE , Wang, EC , Nicola, TL , and Hill, B . A preliminary study to examine the effects of aerobic and therapeutic (nonaerobic) exercise on cardiorespiratory fitness and coronary risk reduction in stroke survivors. Arch Phys Med Rehabil. (2009) 90:407–12. doi: 10.1016/j.apmr.2008.07.032
40. Gu, L , Wu, L , and Sun, XG . Quantitative evaluation of effects of aerobic training on cardiopulmonary function in the elderly patients with stroke hemiplegia. Pract Geriatr. (2019) 33:149–52. doi: 10.3969/j.issn.1003-9198.2019.02.011
41. Tang, A , Sibley, KM , Thomas, SG , Bayley, MT , Richardson, D , and McIlroy, WE . Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabil Neural Repair. (2009) 23:398–406. doi: 10.1177/1545968308326426
42. Macko, RF , Ivey, FM , Forrester, LW , Hanley, D , Sorkin, JD , and Katzel, LI . Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. (2005) 36:2206–11. doi: 10.1161/01.STR.0000181076.91805.89
43. Mackay-Lyons, M , McDonald, A , Matheson, J , Eskes, G , and Klus, M-A . Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabil Neural Repair. (2013) 27:644–53. doi: 10.1177/1545968313484809
44. Vanroy, C , Feys, H , Swinnen, A , Vanlandewijck, Y , Truijen, S , and Vissers, D . Effectiveness of active cycling in subacute stroke rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. (2017) 98:1576–1585.e5. doi: 10.1016/j.apmr.2017.02.004
45. Tang, A , Eng, JJ , Krassioukov, AV , Madden, KM , Mohammadi, A , and Tsang, MYC . Exercise-induced changes in cardiovascular function after stroke: a randomized controlled trial. Int J Stroke. (2014) 9:883–9. doi: 10.1111/ijs.12156
46. Severinsen, K , Jakobsen, JK , Pedersen, AR , Overgaard, K , and Andersen, H . Effects of resistance training and aerobic training on ambulation in chronic stroke. Am J Phys Med Rehabil. (2014) 93:29–42. doi: 10.1097/PHM.0b013e3182a518e1
47. Chang, WH , Kim, MS , Huh, JP , Lee, PKW , and Kim, Y-H . Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: a randomized controlled study. Neurorehabil Neural Repair. (2012) 26:318–24. doi: 10.1177/1545968311408916
48. Lennon, O , Carey, A , Gaffney, N , Stephenson, J , and Blake, C . A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil. (2008) 22:125–33. doi: 10.1177/0269215507081580
49. Stoller, O , de Bruin, ED , Schindelholz, M , Schuster-Amft, C , and de Bie, RA . Efficacy of feedback-controlled robotics-assisted treadmill exercise to improve cardiovascular fitness early after stroke: a randomized controlled pilot trial. J Neurol Phys Ther. (2015) 39:156–65. doi: 10.1097/NPT.0000000000000095
50. Fu, CH , Chen, M , Li, XT , Xiao, QQ , Hu, K , and Kong, FC . Effects of resistance training on respiratory function, exercise endurance and negative emotion in stroke patients at chronic stage. Chin J of. Stroke. (2022) 17:573. doi: 10.3969/j.issn.1673-5765.2022.06.003
51. Ivey, FM , Prior, SJ , Hafer-Macko, CE , Katzel, LI , Macko, RF , and Ryan, AS . Strength training for skeletal muscle endurance after stroke. J Stroke Cerebrovasc Dis. (2017) 26:787–94. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.018
52. Shao, C , Wang, Y , Gou, H , Xiao, H , and Chen, T . Strength training of the nonhemiplegic side promotes motor function recovery in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2023) 104:188–94. doi: 10.1016/j.apmr.2022.09.012
53. Marzolini, S , Brooks, D , Oh, P , Jagroop, D , MacIntosh, BJ , and Anderson, ND . Aerobic with resistance training or aerobic training alone poststroke: a secondary analysis from a randomized clinical trial. Neurorehabil Neural Repair. (2018) 32:209–22. doi: 10.1177/1545968318765692
54. Ran, LF , Ye, ZQ , Guo, JH , Liu, XX , and Wang, N . Effect of isokinetic muscle strength training combined with aerobic exercise on cardiopulmonary function and lower limb movement function in stroke patients. Chin J Conva Med. (2022) 31:1298–302. doi: 10.13517/j.cnki.ccm.2022.12.017
55. Carr, M , and Jones, J . Physiological effects of exercise on stroke survivors. Top Stroke Rehabil. (2003) 9:57–64. doi: 10.1310/0J2K-MDNX-1Q0L-8LX6
56. Lee, M-J , Kilbreath, SL , Singh, MF , Zeman, B , Lord, SR , and Raymond, J . Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise-controlled study. J Am Geriatr Soc. (2008) 56:976–85. doi: 10.1111/j.1532-5415.2008.01707.x
57. Kang, D , Park, J , Jeong, I , and Eun, S-D . Comparing the effects of multicomponent exercise with or without power training on the cardiorespiratory fitness, physical function, and muscular strength of patients with stroke: a randomized controlled trial. J Sports Med Phys Fitness. (2022) 62:722–31. doi: 10.23736/S0022-4707.21.12306-0
58. Duncan, P , Studenski, S , Richards, L , Gollub, S , Lai, SM , and Reker, D . Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. (2003) 34:2173–80. doi: 10.1161/01.STR.0000083699.95351.F2
59. Toledano-Zarhi, A , Tanne, D , Carmeli, E , and Katz-Leurer, M . Feasibility, safety and efficacy of an early aerobic rehabilitation program for patients after minor ischemic stroke: a pilot randomized controlled trial. NeuroRehabilitation. (2011) 28:85–90. doi: 10.3233/NRE-2011-0636
60. Girard, V , Bellavance-Tremblay, H , Gaudet-Drouin, G , Lessard, G , Dupont, M , and Gagnon, M-A . Cardiorespiratory strain during stroke rehabilitation: are patients trained enough? A systematic review. Ann Phys Rehabil Med. (2021) 64:101443. doi: 10.1016/j.rehab.2020.09.007
61. Loe, H , Nes, BM , and Wisløff, U . Predicting VO2peak from submaximal- and peak exercise models: the HUNT 3 fitness study. Norway PLoS One. (2016) 11:e0144873. doi: 10.1371/journal.pone.0144873
62. Luo, L , Meng, H , Wang, Z , Zhu, S , Yuan, S , and Wang, Y . Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2020) 63:59–68. doi: 10.1016/j.rehab.2019.07.006
63. Calverley, TA , Ogoh, S , Marley, CJ , Steggall, M , Marchi, N , and Brassard, P . HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol. (2020) 598:2513–30. doi: 10.1113/JP275021
64. Montero, D , Diaz-Cañestro, C , and Lundby, C . Endurance training and V˙O2max: role of maximal cardiac output and oxygen extraction. Med Sci Sports Exerc. (2015) 47:2024–33. doi: 10.1249/MSS.0000000000000640
65. Ross, R , Blair, SN , Arena, R , Church, TS , Després, J-P , and Franklin, BA . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. (2016) 134:e653–99. doi: 10.1161/CIR.0000000000000461
66. Tomczak, CR , Thompson, RB , Paterson, I , Schulte, F , and Cheng-Baron, J . Effect of acute high-intensity interval exercise on postexercise biventricular function in mild heart failure. J Appl Physiol. (2011) 110:398–406. doi: 10.1152/japplphysiol.01114.2010
67. Fu, T-C , Wang, C-H , Lin, P-S , Hsu, C-C , Cherng, W-J , and Huang, S-C . Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. (2013) 167:41–50. doi: 10.1016/j.ijcard.2011.11.086
68. Tan, R , Nederveen, JP , Gillen, JB , Joanisse, S , Parise, G , and Tarnopolsky, MA . Skeletal muscle fiber-type-specific changes in markers of capillary and mitochondrial content after low-volume interval training in overweight women. Physiol Rep. (2018) 6:e13597. doi: 10.14814/phy2.13597
69. Crozier, J , Roig, M , Eng, JJ , MacKay-Lyons, M , Fung, J , and Ploughman, M . High-intensity interval training after stroke: an opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neurorehabil Neural Repair. (2018) 32:543–56. doi: 10.1177/1545968318766663
70. Biddle, SJH , and Batterham, AM . High-intensity interval exercise training for public health: a big HIT or shall we HIT it on the head? Int J Behav Nutr Phys Act. (2015) 12:95. doi: 10.1186/s12966-015-0254-9
71. Rognmo, Ø , Moholdt, T , Bakken, H , Hole, T , Mølstad, P , and Myhr, NE . Cardiovascular risk of high-versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. (2012) 126:1436–40. doi: 10.1161/CIRCULATIONAHA.112.123117
72. Herbert, P , Grace, FM , and Sculthorpe, NF . Exercising caution: prolonged recovery from a single session of high-intensity interval training in older men. J Am Geriatr Soc. (2015) 63:817–8. doi: 10.1111/jgs.13365
73. Wewege, MA , Ahn, D , Yu, J , Liou, K , and Keech, A . High-intensity interval training for patients with cardiovascular disease-is it safe? A systematic review. J Am Heart Assoc. (2018) 7:e009305. doi: 10.1161/JAHA.118.009305
74. Murphy, SJX , and Werring, DJ . Stroke: causes and clinical features. Medicine. (2020) 48:561–6. doi: 10.1016/j.mpmed.2020.06.002
75. Adab, P , Cheng, KK , Jiang, CQ , Zhang, WS , and Lam, TH . Age-specific relevance of usual blood pressure to vascular mortality. Lancet. (2003) 361:1391. doi: 10.1016/s0140-6736(03)13063-2
76. Lewington, S , Clarke, R , Qizilbash, N , Peto, R , and Collins, R . Prospective studies collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360:1903–13. doi: 10.1016/s0140-6736(02)11911-8
77. Zang, W , Fang, M , He, H , Mu, L , Zheng, X , Shu, H, et al. Comparative efficacy of exercise modalities for cardiopulmonary function in hemodialysis patients: a systematic review and network meta-analysis. Front Public Health. (2022) 10:1040704. doi: 10.3389/fpubh.2022.1040704
78. Wilson, JM , Marin, PJ , Rhea, MR , Wilson, SMC , Loenneke, JP , and Anderson, JC . Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. (2012) 26:2293–307. doi: 10.1519/JSC.0b013e31823a3e2d
79. Davis, WJ , Wood, DT , Andrews, RG , Elkind, LM , and Davis, WB . Concurrent training enhances athletes’ strength, muscle endurance, and other measures. J Strength Cond Res. (2008) 22:1487–502. doi: 10.1519/JSC.0b013e3181739f08
80. Lu, Z , Xu, Y , Song, Y , Bíró, I , and Gu, Y . A mixed comparisons of different intensities and types of physical exercise in patients with diseases related to oxidative stress: a systematic review and network meta-analysis. Front Physiol. (2021) 12:700055. doi: 10.3389/fphys.2021.700055
Keywords: stroke, exercise, cardiopulmonary function, randomized controlled trials, network meta-analysis
Citation: Wang C, Xu Y, Zhang L, Fan W, Liu Z, Yong M and Wu L (2024) Comparative efficacy of different exercise methods to improve cardiopulmonary function in stroke patients: a network meta-analysis of randomized controlled trials. Front. Neurol. 15:1288032. doi: 10.3389/fneur.2024.1288032
Edited by:
Raffaele Ornello, University of L’Aquila, ItalyCopyright © 2024 Wang, Xu, Zhang, Fan, Liu, Yong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjin Yong, Mzc0OTU2ODkzQHFxLmNvbQ==; Liang Wu, MTk3Mnd1bGlhbmdAc2luYS5jb20=
†These authors have contributed equally to this work
 Chengshuo Wang
Chengshuo Wang Yanan Xu2†
Yanan Xu2†