- 1Suzhou Dushu Lake Hospital, Suzhou, Jiangsu, China
- 2School of Public Health, Soochow University Medical College, Suzhou, Jiangsu, China
Background and purpose: Perforator artery disease (PAD) is an important subtype of ischemic stroke. The risk factors affecting the prognosis of patients with PAD are unclear. This study aimed to investigate the risk factors affecting the unfavorable prognosis of PAD.
Methods: Patients with PAD were enrolled from Dushu Lake Hospital Affiliated to Soochow University and diagnosed as stroke with PAD during the period from September 2021 to July 2023 and followed up with a modified Rankin Scale (mRS) after 90 days, defining the mRS of 0–2 as a group with favorable prognosis, and 3–6 as a group with unfavorable functional outcome. Logistic regression was used to identify predictors for PAD. Multiple logistic regression analysis and receiver operating characteristics (ROC) were used to identify predictors of unfavorable prognosis.
Results: Of the 181 enrolled patients, 48 (26.5%) were identified with unfavorable prognosis. On multivariate analysis, increased age (OR = 1.076, 95% CI: 1.012 ~ 1.144, p = 0.019), higher National Institutes of Health Stroke Scale (NIHSS) score at admission (OR = 2.930, 95% CI: 1. 905 ~ 4.508, p < 0.001), and increased neutrophil-to-lymphocyte ratio (NLR) (OR = 3.028, 95% CI: 1.615 ~ 5.675, p = 0.001) were independent risk factors for unfavorable prognosis in patients with PAD, and the area under the receiver operating characteristic curve was 0.590, 0.905, and 0.798, and the multi-factor diagnostic model (Model 2) showed reliable diagnostic specificity and sensitivity (area under the curve = 0.956, p < 0.001, specificity 0.805, sensitivity 0.958, accuracy 0.845).
Conclusion: Increased baseline NLR and NIHSS score and aging may be independent risk factors for unfavorable prognosis of patients with PAD. NLR can be used as a potential biological indicator to predict the prognosis of stroke with PAD.
Introduction
Perforator artery disease (PAD), which accounts for approximately one-quarter of all ischemic strokes, was defined as an acute isolated infarct in the clinically relevant territory of perforator arteries such as the basal ganglia region and brainstem (1). According to the CISS typology, the probable pathogenesis of PAD includes atherosclerosis at the proximal segment of the penetrating arteries and lipohyalinotic degeneration of arterioles (2, 3). The diagnostic criteria include the following: (1) acute isolated infarct in the clinically relevant territory of one penetrating artery, regardless of the size of the infarct; (2) no evidence of atherosclerotic plaque (detected by HR-MRI) or any degree of stenosis in the parent artery (detected by TCD, MRA, CTA, or DSA); (3) with evidence of vulnerable plaques or stenosis ≥50% in ipsilateral proximal intracranial or extracranial large arteries, or cardiac disease that has a potential for embolism is classified in UE (multiple etiology); (4) other possible causes have been excluded. Due to different anatomical structures, patients with PAD also differ from those with large artery atherosclerosis in terms of pathogenesis, clinical features, and prognosis (4). Previous studies have shown that patients with PAD are prone to early neurological deterioration (END) and progressive movement disorders (PMD) (5, 6), but the risk factors affecting the prognosis of patients with PAD are unclear. Studies have suggested that neutrophil-to-lymphocyte ratio (NLR) affects post-thrombolysis early neurological outcomes in patients with large-vessel occlusive stroke (7, 8), but the effect on patients with PAD is rarely reported. Using the data from Dushu Lake Hospital affiliated to Soochow University, we analyzed the potential predictors for unfavorable outcomes in patients with PAD, as well as the correlation between NLR, an indicator of inflammation, and the prognosis of PAD and its clinical application value.
Materials and methods
Study participants
We retrospectively analyzed acute ischemic stroke patients with PAD who were hospitalized in Dushu Lake Hospital affiliated to Soochow University during the period from September 2021 to July 2023. Inclusion criteria: (1) meet the diagnostic criteria of the 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke (9), magnetic resonance imaging (MRI) clearly defined intracranial new-onset single infarct lesion, which was compatible with the patient’s clinical symptoms and signs. As for etiological cause, perforator artery occlusion was considered according to the CISS classification; (2) first onset of the disease, within 72 h from onset to admission; and (3) age ≥ 18 years. Exclusion criteria: (1) patients with previous cerebral infarction, cerebral hemorrhage, or brain tumor; (2) patients with combined acute and chronic infectious diseases, autoimmune diseases, hematological disorders, severe cardiac, hepatic, renal diseases, and malignant tumors; (3) patients taking immunosuppressant, hormone, antibiotic, and antiplatelet aggregation drugs in the last 6 months; and (4) patients with surgical procedures in the last 6 months. The flowchart in Figure 1A shows the process of the selection of participants.
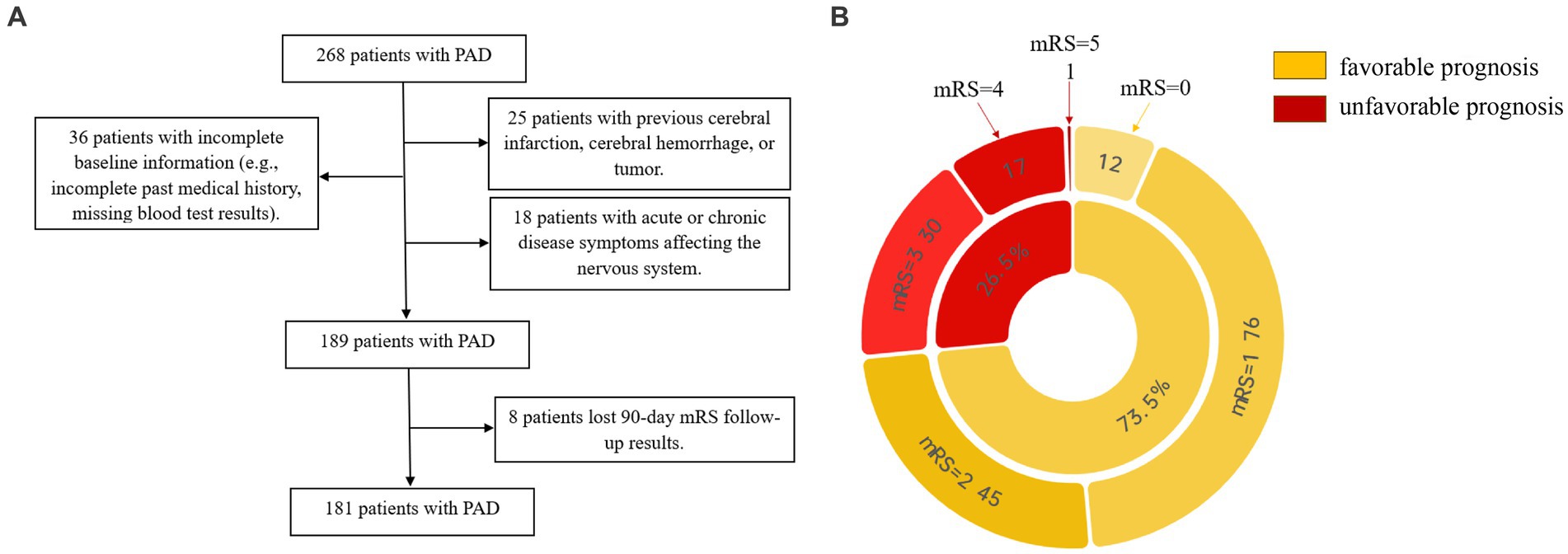
Figure 1. (A) Shows the process of the selection of participants. (B) Shows the distribution of prognostic subgroups according to mRS.
Data collection
All the participants underwent standard assessments of demographic characteristics (age, sex, and body mass index [BMI]), vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, current smoking, and current drinking), clinical assessment (blood pressure, treatment with IV thrombolysis, and NIHSS score), lesion location, and laboratory data. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured and recorded immediately after admission. Computed tomography, magnetic resonance, electrocardiogram, echocardiography, carotid ultrasonography, and transcranial Doppler were performed to assess the lesion location and confirm the stroke subtype. Laboratory data included total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), homocysteine (HCY), total leukocyte counts (WBC), neutrophil counts (NE), lymphocyte counts (LYM), and neutrophil-to-lymphocyte ratio (NLR). We conducted a follow-up observation using a 90-day modified Rankin Scale (mRS) (10) and further made the definition of the mRS of 0–2 as the favorable prognosis group, and 3–6 as the unfavorable prognosis group. The pie diagram in Figure 1B shows the distribution of prognostic subgroups according to mRS.
According to the intracranial arteries originating from the blood-supplying arteries at the site of the lesion, they were divided into anterior and posterior circulation groups: anterior circulation: an intracranial segment of the internal carotid artery, middle cerebral artery, anterior cerebral artery, ophthalmic artery, and anterior choroidal artery; and posterior circulation: posterior cerebral artery, basilar artery, and intracranial segment of the vertebral artery (11).
Statistical analysis
Categorical variables were expressed as n (%), and continuous variables were expressed as means (standard deviation, SD) or medians (interquartile range, IQR). Continuous numerical variables were tested for normality using the Shapiro–Wilk test; if they did not conform to normal distribution, they were expressed as medians (quartiles). Comparison of quantitative data of multiple groups was analyzed using the one-way ANOVA, and the Kruskal–Wallis test was used for those who did not meet the conditions of the one-way ANOVA; comparison of normal qualitative data was tested using the chi-square test. Multifactorial analysis was analyzed using the logistic regression model, and p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 26.0 (IBM, Armonk, NY) and Origin 2022.
Results
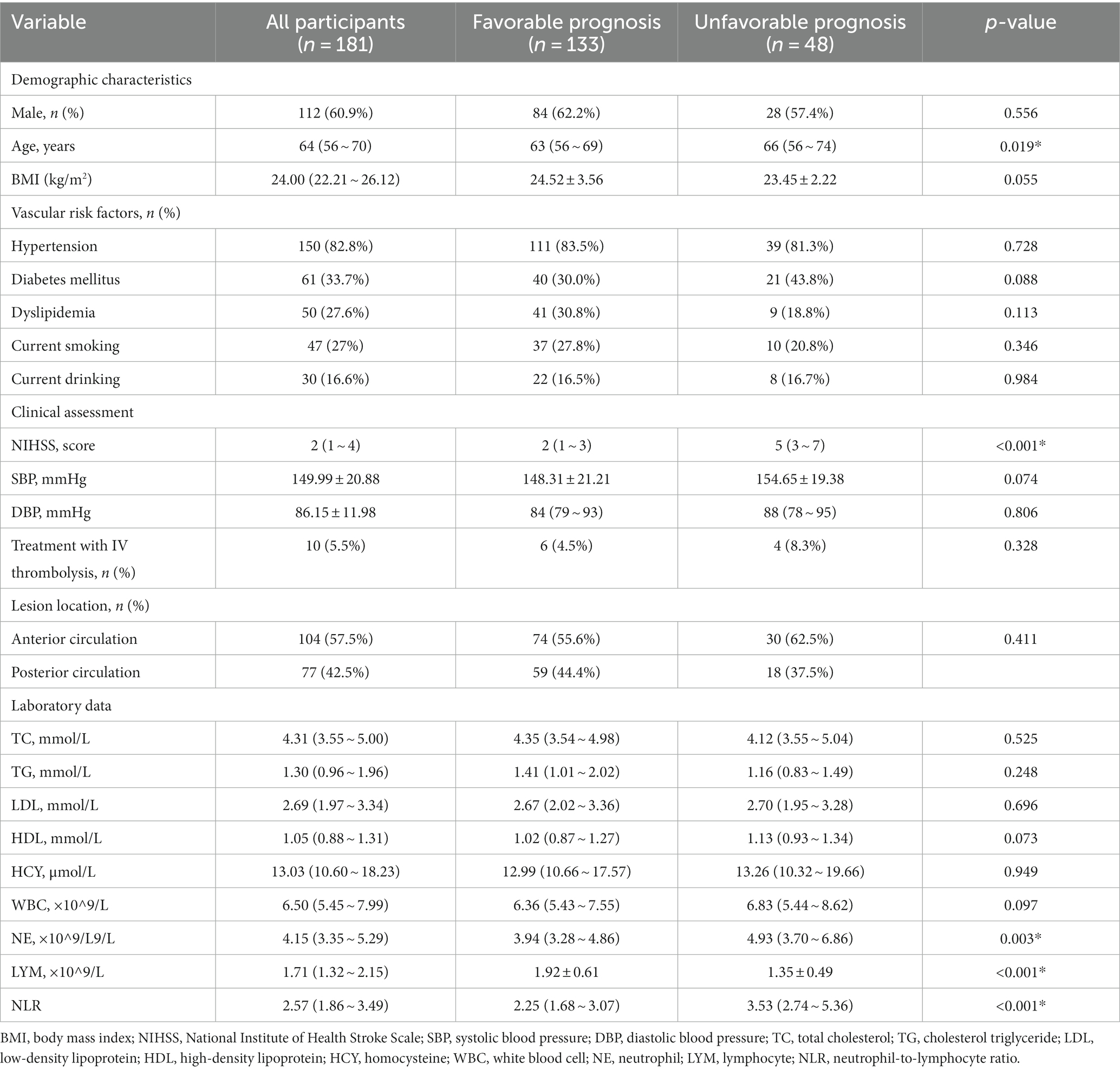
A total of 181 patients with PAD were chosen according to the inclusion and exclusion criteria, comprising 112 male patients and 69 female patients, of which 133 patients were in the favorable prognosis group and 48 patients were in the unfavorable prognosis group. The demographics and clinical characteristics of the two groups are shown in Table 1. Univariate logistic analysis showed that patients in the unfavorable prognosis group were older (median 66 versus 63, p = 0.019) and NIHSS scores at admission (median 5 versus 2, p < 0.001), baseline NE (median 4.93 versus 3.94, p = 0.003), and NLR were higher (median 3.53 versus 2.25, p < 0.001) than those in the favorable prognosis group, whereas the baseline LYM was lower (mean 1.35 versus 1.92, p < 0.001) in the unfavorable prognosis group. There were no statistical differences between the two groups in terms of other demographic characteristics, vascular risk factors, clinical assessment, lesion location, and laboratory data (p > 0.050). Figure 2 shows the boxplots of inflammatory indices between the two groups.
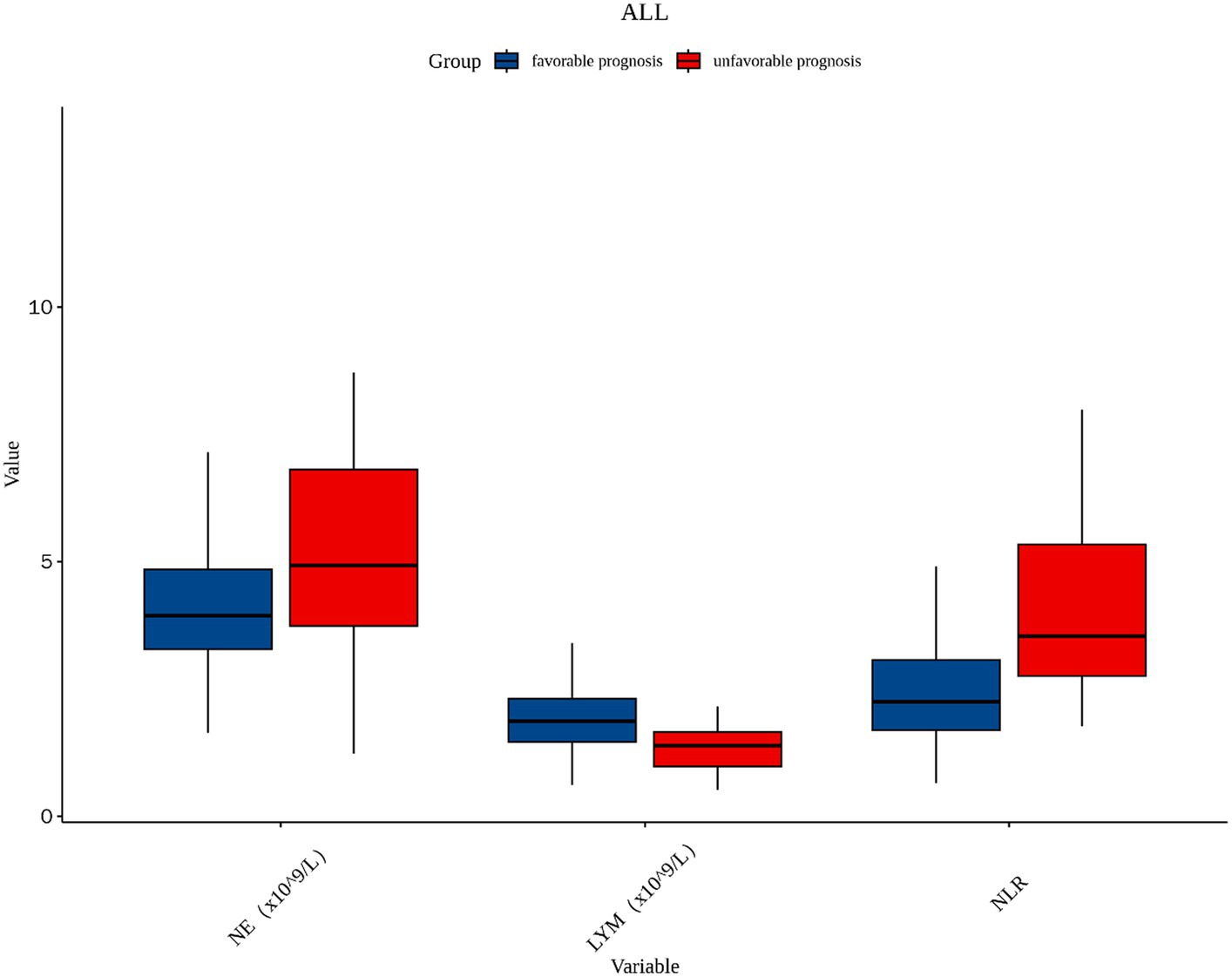
Figure 2. The boxplot in the distribution of inflammatory indices between two different prognosis outcome groups. NE, neutrophil; LYM, lymphocyte; NLR, neutrophil-to-lymphocyte ratio.
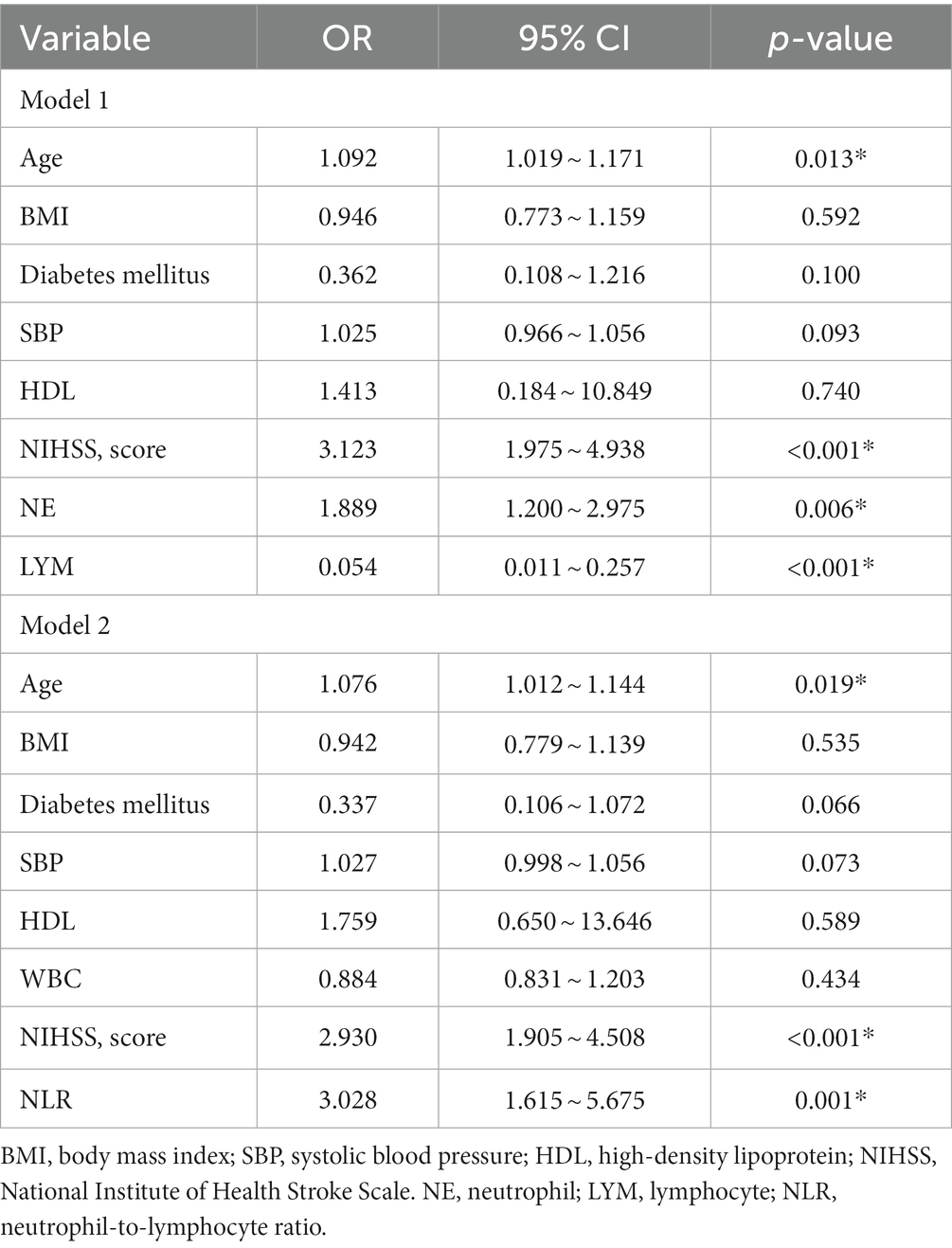
Table 2 displays the results of the multiple logistic regression models for subgroups according to prognosis. Due to the correlation between NE, LYM, and NLR, we divided into two models for analysis. Furthermore, we incorporated potential risk factors of BMI, diabetes mellitus, HDL, SBP, and WBC (p < 0.1). In addition, due to the correlation between WBC NE and LYM, in Model 1, we removed the confounder WBC. After adjustment for all potential confounders, Model 1 showed that age (odds ratio [OR], 1.092; 95% confidence interval [CI] 1.019 ~ 1.171, p = 0.013), NIHSS (OR, 3.123; 95% CI 1.975 ~ 4.938, p < 0.001), NE (OR, 1.889; 95% CI 1.200 ~ 2.975, p = 0.006), and LYM (OR, 0.054; 95% CI 0.011 ~ 0.257, p < 0.001) were identified as independent factors for unfavorable prognosis for patients with PAD, while Model 2 also showed that age (OR, 1.076; 95% CI 1.012 ~ 1.144, p = 0.019), NIHSS (OR, 2.930; 95% CI 1.905 ~ 4.508, p < 0.001), and NLR (OR, 3.028; 95% CI 1.6154 ~ 5.675, p = 0.001) were identified as independent factors for unfavorable prognosis for patients with PAD.
The ROC curves, which were depicted in Figure 3, were used to test the overall discriminative ability of risk factors for unfavorable prognosis for patients with PAD. We observed in Model 1 that the areas under the curve (AUC) of NE and LYM were 0.636 (95% CI, 0.538–0.734) and 0.768 (95% CI, 0.693–0.843) and the composite diagnostic model was 0.960 (95% CI, 0.935–0.984), and in Model 2, the area under the curve (AUC) of NLR was 0.798 (95% CI, 0.727–0.869) and the composite diagnostic model was 0.956 (95% CI, 0.930–0.982). In the performance of predicting prognosis of PAD, the AUC of NLR was superior to NE (0.798 versus 0.636, p < 0.001) and LYM (0.798 versus 0.768, p < 0.001), which means that NLR may be a better biological indicator to predict the prognosis of stroke with PAD. We also established optimal cutoff values at which the Youden Index was highest. The details are described in Supplementary Table S1.
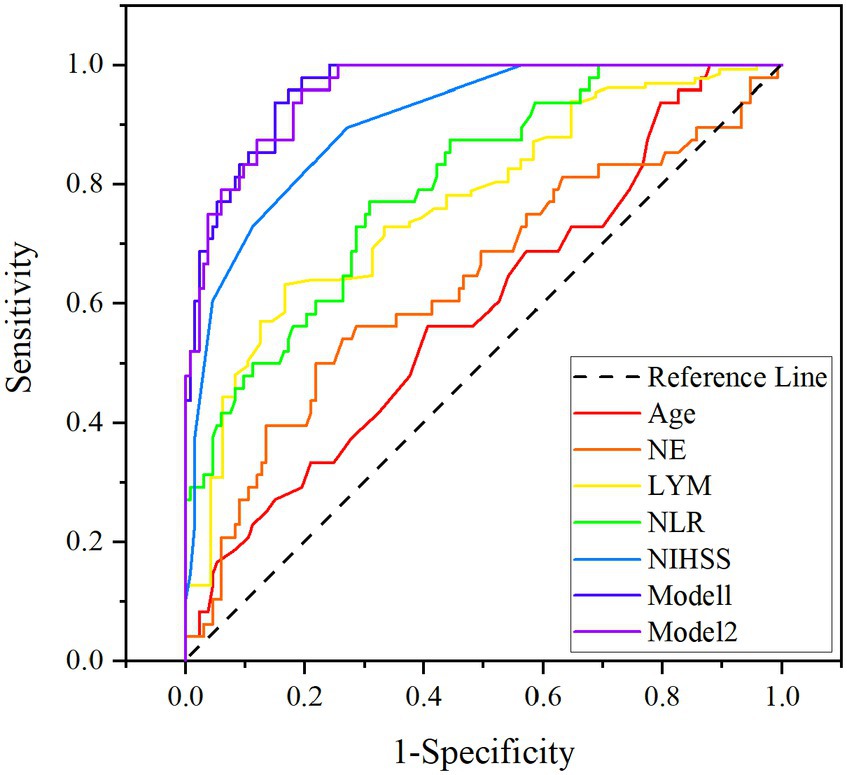
Figure 3. ROC curves for age, NIHSS on admission, NE, LYM, NLR, Model 1 (age, NIHSS, NE, and LYM), and Model 2 (age, NIHSS, and NLR). NIHSS, National Institute of Health Stroke Scale; NE, neutrophil; LYM, lymphocyte; NLR, neutrophil-to-lymphocyte ratio.
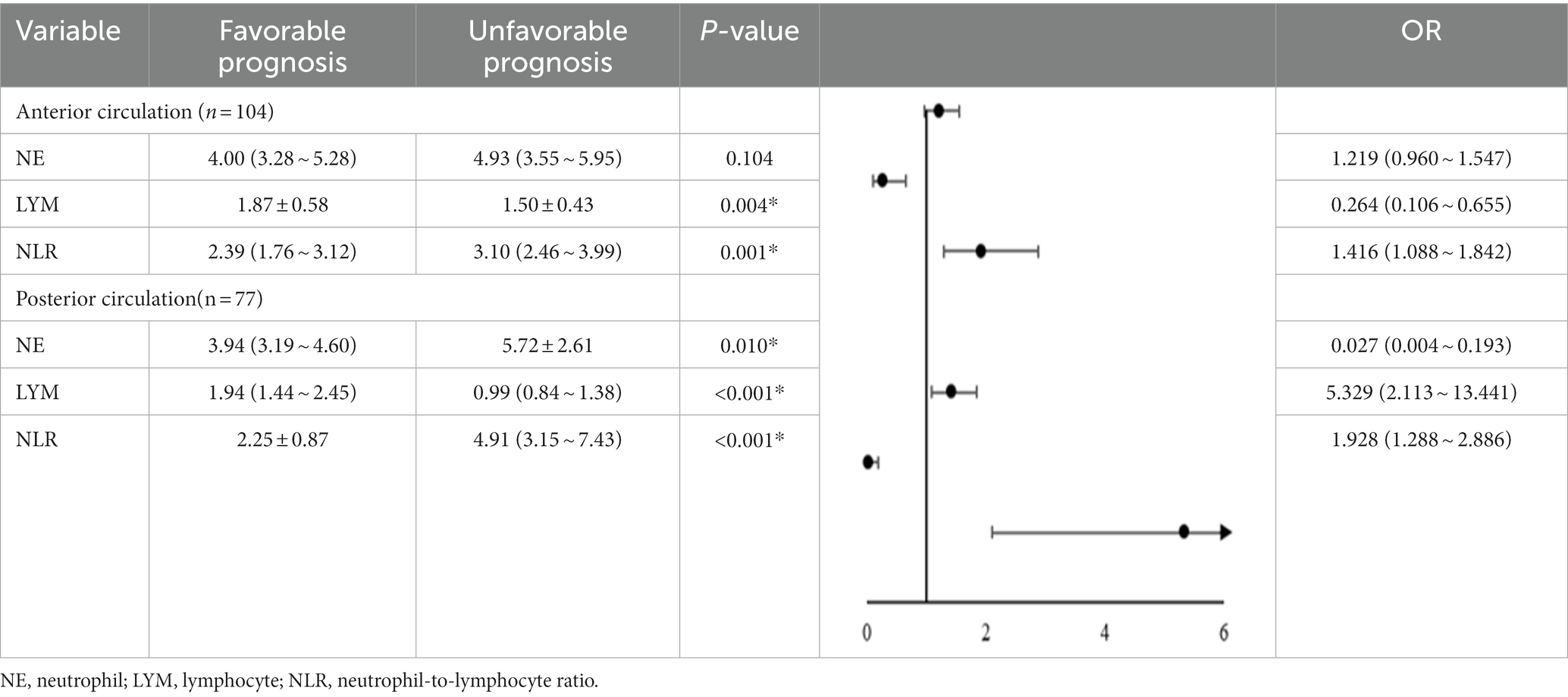
Furthermore, patients with PAD were divided into the anterior circulation group (n = 104) and posterior circulation group (n = 77) according to the lesion site shown on the images, and the changes of inflammatory indices NE, LYM, and NLR were analyzed to see whether there was any difference between the two groups. The results shown in Table 3 suggested that the decrease in LYM and the increase in NLR in the anterior circulation group were significantly correlated with an unfavorable prognosis and that the increase in NE and NLR as well as the decrease in LYM in the posterior circulation group were all significantly correlated with an unfavorable prognosis (p > 0 05).
Discussion
Our study showed that increasing age, higher NIHSS score at admission, decreased LYM, and elevated NE and NLR were significantly associated with unfavorable prognosis in patients with PAD. Further multifactorial analysis showed that elevated NLR was independently associated with an unfavorable prognosis. Further subgroup analysis showed that NLR correlated with unfavorable prognosis in both anterior and posterior groups, which suggests that inflammation and immune factors may play an essential role in the clinical regression process of PAD and the use of NLR to predict prognosis in PAD deserves further investigation.
Aging exacerbates the damage and dysfunction of different components of the non-injurious brain unit, affecting the integrity of the neurovascular unit and the vulnerability to neurodegeneration, thus accelerating the progression and deterioration of brain injury (12). The National Institute of Health Stroke Scale (NIHSS) is currently the most used clinical scale for assessing neurologic deficits. Studies have demonstrated that the more severe a patient’s neurological deficit, the higher the risk of poor prognosis (13). This study further confirms that aging and elevated baseline NIHSS are risk factors for unfavorable prognosis of PAD.
Acute ischemic stroke triggers an inflammatory response at an early stage, leading to the disruption of the blood–brain barrier and the deterioration of neurological function (14, 15). Studies have shown that neutrophils promote atherogenesis and the progression of atherosclerosis by mediating a non-specific inflammatory response (16, 17), and the infiltration of neutrophils can cause an increase in cerebral infarct volume and worsening of ischemic brain damage (18). Whereas lymphocytes have a role in maintaining immune homeostasis and immune tolerance, they can repair damage through inflammatory responses (19, 20). Tregs in lymphocytes are key cerebroprotective immunomodulators in acute stroke (21), which act as endogenous neuroprotective modulators in maintaining blood–brain barrier permeability, delaying leukocyte infiltration, reducing tissue edema, decreasing brain damage, and playing a protective role in improving prognostic recovery (22–24). Lack of lymphocytes can accelerate the process of atherosclerosis progression (25, 26). Previous studies have found that there is a significant tendency for lymphocytes to decrease at the onset of cerebral infarction (27, 28), and although immunosuppression may reduce brain damage, it may impede neuronal cell repair and increase the risk of systemic bacterial infections during the acute phase of the disease (29, 30). Elevated levels of NLR have been observed in diabetic patients who develop renal arterial vitreous degeneration (31, 32), while lipohyalinotic degeneration is the main pathogenesis of PAD, so we hypothesized that elevated NLR plays a role in the development of PAD and may be involved in the progression of penetrating arterial lesions. In the present study, we observed that elevated NLR was independently associated with unfavorable functional outcomes in PAD, suggesting that mediating the inflammatory response could be a new target for the prevention and treatment of PAD.
As a retrospective study, our study has some limitations. First, this study only collected data on baseline inflammatory markers after emergency or admission and did not assess the dynamic changes in NLR during hospitalization, which may provide information for discovering the pathophysiological mechanisms of the inflammatory response after the onset of PAD (33). Second, the single-center study was not fully representative of the total patients with PAD. Moreover, the sample size is small, and there is a possibility of bias in some clinical characteristics. Therefore, more prospective, large-sample, multicenter studies are needed for further validation. Despite these limitations mentioned above, it is the first time the relationship between inflammatory indices and neurological outcomes of PAD was explored, and this can be validated in the future research.
Conclusion
In summary, our study showed that elevated baseline NLR and NIHSS scores and aging may be independent risk factors for unfavorable prognosis in patients with PAD. NLR can be used as a potential biological indicator for predicting the prognosis of PAD, suggesting that intervening in the inflammatory response is expected to be a new target for preventing and treating stroke.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Suzhou Dushu Lake Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LS: Writing – original draft, Data curation, Formal analysis, Methodology, Software. XL: Data curation, Software, Writing – original draft. WH: Formal analysis, Software, Writing – original draft. LY: Data curation, Software, Writing – review & editing. SG: Data curation, Methodology, Writing – review & editing. YJ: Supervision, Visualization, Writing – review & editing. HP: Conceptualization, Supervision, Writing – review & editing. YH: Conceptualization, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Suzhou science and technology plan project (SZM2023017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1340085/full#supplementary-material
References
2. Gao, S, Wang, YJ, Xu, AD, Li, YS, and Wang, DZ. Chinese ischemic stroke subclassification. Front Neurol. (2011) 2:6. doi: 10.3389/fneur.2011.00006
3. Chen, P-H, Gao, S, Wang, Y-J, Xu, A-D, Li, Y-S, and Wang, D. Classifying ischemic stroke, from TOAST to CISS. CNS Neurosci Ther. (2012) 18:452–6. doi: 10.1111/j.1755-5949.2011.00292.x
4. Shi, Y, Guo, L, Chen, Y, Xie, Q, Yan, Z, Liu, Y, et al. Risk factors for ischemic stroke: differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol. (2021) 59:378–85. doi: 10.5114/fn.2021.112007
5. Miyamoto, N, Tanaka, Y, Ueno, Y, Kawamura, M, Shimada, Y, Tanaka, R, et al. Demographic, clinical, and radiologic predictors of neurologic deterioration in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:205–10. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.018
6. Yamamoto, Y, Ohara, T, Hamanaka, M, Hosomi, A, Tamura, A, Akiguchi, I, et al. Predictive factors for progressive motor deficits in penetrating artery infarctions in two different arterial territories. J Neurol Sci. (2010) 288:170–4. doi: 10.1016/j.jns.2009.08.065
7. Gong, P, Liu, Y, Gong, Y, Chen, G, Zhang, X, Wang, S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
8. Sharma, D, Spring, KJ, and Bhaskar, SMM. Neutrophil-lymphocyte ratio in acute ischemic stroke: immunopathology, management, and prognosis. Acta Neurol Scand. (2021) 144:486–99. doi: 10.1111/ane.13493
9. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
10. Broderick, JP, Adeoye, O, and Elm, J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. (2017) 48:2007–12. doi: 10.1161/STROKEAHA.117.017866
11. Tatu, L, Moulin, T, Vuillier, F, and Bogousslavsky, J. Arterial territories of the human brain. Front Neurol Neurosci. (2012):30. doi: 10.1159/000333602
12. Cai, W, Zhang, K, Li, P, Zhu, L, Xu, J, Yang, B, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. (2017) 34:77–87. doi: 10.1016/j.arr.2016.09.006
13. Mistry, EA, Yeatts, SD, Khatri, P, Mistry, AM, Detry, M, Viele, K, et al. National Institutes of Health stroke scale as an outcome in stroke research: value of ANCOVA over Analyzing change from baseline. Stroke. (2022) 53:e150–5. doi: 10.1161/STROKEAHA.121.034859
14. Shi, K, Tian, D-C, Li, Z-G, Ducruet, AF, Lawton, MT, and Shi, F-D. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
15. Jayaraj, RL, Azimullah, S, Beiram, R, Jalal, FY, and Rosenberg, GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. (2019) 16:142. doi: 10.1186/s12974-019-1516-2
16. Moschonas, IC, and Tselepis, AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. (2019) 288:9–16. doi: 10.1016/j.atherosclerosis.2019.06.919
17. Denorme, F, Portier, I, Rustad, JL, Cody, MJ, de Araujo, CV, Hoki, C, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. (2022) 132:e154225. doi: 10.1172/JCI154225
18. Schäbitz, W-R, and Minnerup, J. Neutrophils in acute stroke pathophysiology. Stroke. (2019) 50:e44–5. doi: 10.1161/STROKEAHA.118.024300
19. Iadecola, C, and Anrather, J. The immunology of stroke: from mechanisms to translation. Nat Med. (2011) 17:796–808. doi: 10.1038/nm.2399
20. Zhang, D, Ren, J, Luo, Y, He, Q, Zhao, R, Chang, J, et al. T cell response in ischemic stroke: from mechanisms to translational insights. Front Immunol. (2021) 12:707972. doi: 10.3389/fimmu.2021.707972
21. Liesz, A, Suri-Payer, E, Veltkamp, C, Doerr, H, Sommer, C, Rivest, S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. (2009) 15:192–9. doi: 10.1038/nm.1927
22. Wang, H, Wang, Z, Wu, Q, Yuan, Y, Cao, W, and Zhang, X. Regulatory T cells in ischemic stroke. CNS Neurosci Ther. (2021) 27:643–51. doi: 10.1111/cns.13611
23. Sharma, M, Schlegel, MP, Afonso, MS, Brown, EJ, Rahman, K, Weinstock, A, et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ Res. (2020) 127:335–53. doi: 10.1161/CIRCRESAHA.119.316461
24. Cai, W, Shi, L, Zhao, J, Xu, F, Dufort, C, Ye, Q, et al. Neuroprotection against ischemic stroke requires a specific class of early responder T cells in mice. J Clin Invest. (2022) 132:e157678. doi: 10.1172/JCI157678
25. Huang, Y-L, Chung, H-T, Chang, C-J, Yeh, K-W, Chen, L-C, and Huang, J-L. Lymphopenia is a risk factor in the progression of carotid intima-media thickness in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. (2009) 60:3766–75. doi: 10.1002/art.25019
26. Ammirati, E, Moroni, F, Magnoni, M, and Camici, PG. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin Exp Immunol. (2015) 179:173–87. doi: 10.1111/cei.12477
27. Buck, BH, Liebeskind, DS, Saver, JL, Bang, OY, Yun, SW, Starkman, S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
28. Schulze, J, Gellrich, J, Kirsch, M, Dressel, A, and Vogelgesang, A. Central nervous system-infiltrating T lymphocytes in stroke are activated via their TCR (T-cell receptor) but lack CD25 expression. Stroke. (2021) 52:2939–47. doi: 10.1161/STROKEAHA.120.032763
29. Zhu, L, Huang, L, Le, A, Wang, TJ, Zhang, J, Chen, X, et al. Interactions between the autonomic nervous system and the immune system after stroke. Compr Physiol. (2022) 12:3665–704. doi: 10.1002/cphy.c210047
30. Liu, D-D, Chu, S-F, Chen, C, Yang, P-F, Chen, N-H, and He, X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem Int. (2018) 114:42–54. doi: 10.1016/j.neuint.2018.01.002
31. Zhang, J, Zhang, R, Wang, Y, Wu, Y, Li, H, Han, Q, et al. Effects of neutrophil-lymphocyte ratio on renal function and histologic lesions in patients with diabetic nephropathy. Nephrology (Carlton). (2019) 24:1115–21. doi: 10.1111/nep.13517
32. Ciray, H, Aksoy, AH, Ulu, N, Cizmecioglu, A, Gaipov, A, and Solak, Y. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. (2015) 123:267–71. doi: 10.1055/s-0035-1547257
Keywords: perforator artery disease, neutrophil-to-lymphocyte ratio, unfavorable prognosis, Chinese ischemic stroke subclassification (CISS), modified Rankin Scale
Citation: Song L, Lou X, Han W, Yang L, Guo S, Jiang Y, Peng H and Hao Y (2024) Predictors for unfavorable prognosis after stroke with perforator artery disease. Front. Neurol. 15:1340085. doi: 10.3389/fneur.2024.1340085
Edited by:
Raffael Ornello, University of L'Aquila, ItalyReviewed by:
Yun Luo, Gannan Medical University, ChinaJohn-Ih Lee, Heinrich Heine University of Düsseldorf, Germany
Copyright © 2024 Song, Lou, Han, Yang, Guo, Jiang, Peng and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Hao, aHlnMzYyNUAxMjYuY29t; Hao Peng, cGVuZ2hhb0BzdWRhLmVkdS5jbg==
 Linghua Song
Linghua Song Xiaoli Lou
Xiaoli Lou Wenhao Han1
Wenhao Han1 Hao Peng
Hao Peng Yonggang Hao
Yonggang Hao