- 1Department of Radiology, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2Jiangxi Provincial Key Laboratory of Intelligent Medical Imaging, Nanchang, China
Background: Alzheimer’s disease (AD) is a neurodegenerative disease that imposes a heavy burden on patients and their families. Hypertension is an important risk factor for AD, but the specific mechanism of its impact is still unclear. This study thus aimed to analyze the relationship and trend changes between AD and hypertension through bibliometric methods.
Methods: Literature on AD and hypertension was retrieved from the Web of Science Core Collection (WoSCC) database between 2004 and 2023. Data regarding countries, institutions, authors and journals were sourced from WoSCC. CiteSpace and VOSviewer were used for data visualization, including author collaboration, timelines view of references, reference bursts and overlay visualization maps of keywords. Excel 2018 software was used for the statistical analysis.
Results: A total of 1,833 publications were ultimately included. From 2004 to 2023, the number of publications per year basically showed an increasing trend. The United States (United States) not only had the largest output of publications and the highest H-index but also had the seven highest frequencies of publication institutions. Kehoe, Patrick ranked first with the most articles among 9,330 authors. The journal with the most published articles was the Journal of Alzheimer’s Disease. Reference analysis revealed a hotspot in the exploration of the pathophysiological association between AD and hypertension. Second, the treatment effects and potential risks of antihypertensive drugs (AHDs) on AD are also the focus of research. Researchers have carried out a series of studies ranging from basic research to clinical research on AHDs for the treatment of AD. Finally, personalized treatment strategies will also become one of the hotspots of future research. Controlling hypertension through lifestyle changes and medication interventions in AD patients is a promising strategy. The analysis of keywords revealed that “amyloid deposition,” “preeclampsia,” “Corona Virus Disease 2019 (COVID-19)” and “biomarkers” have been research hotspots in recent years.
Conclusion: By analyzing the references and keywords, we summarized the hot topics and research trends in this field. These findings provide useful information for researchers to explore the relationship between hypertension and AD further, with the hope of providing more effective treatments for AD patients to delay disease progression and improve quality of life.
1 Introduction
Alzheimer’s disease (AD) is an insidious progressive form of neurodegenerative dementia that is typically characterized by memory impairment, cognitive impairment, and limitations in daily activities (1). It is one of the leading age-related brain diseases in older adults, and the fifth leading cause of death in the adult population, which has a strong impact on the physical and mental health and quality of life of patients and their families (2). Its specific pathogenic mechanism is not completely clear. The primary pathological hallmarks of AD include the deposition of β-amyloid (Aβ) within the brain, the development of neurofibrillary tangles resulting from the abnormal phosphorylation and aggregation of tau protein in nerve cells, and the subsequent damage to and death of neurons (3). Cholinesterase inhibitors and antagonists of N-methyl-D-aspartate receptors (4, 5) have been approved for managing the symptoms of AD. According to reports, hypertension, particularly in midlife, is an important risk factor for the development of AD (6, 7). Hypertension accelerates microvascular injury in Aβ-induced AD, ranging from cerebral microhemorrhages to blood–brain barrier (BBB) disruption and subsequent neuroinflammation (8, 9). Clinical trials have indicated that the use of antihypertensive drugs (AHDs) to lower blood pressure can generally enhance cognitive function (10). In addition, researchers have reported that AHDs have significant effects on neuroprotective therapy (11). However, the specific mechanism by which hypertension affects AD is still unclear.
Understanding the relationship between hypertension and AD can increase our understanding of the pathophysiological mechanisms underlying AD, which is highly important for improving and delaying AD progression by controlling blood pressure. Although many studies on hypertension and AD have been conducted, the research trends and dynamic changes in this field are still elusive. Bibliometrics offers statistical analysis and evaluation of literature, including a range of indicators such as document count, citation frequency, author collaboration networks, and journal impact. It is capable of analyzing development and research outcomes within specific disciplines or fields (12). Scholars have employed bibliometric methods to clarify the relationship between AD and other conditions, including epilepsy, sleep and vitamins (13–15), but none of these studies have explored the relationship between AD and hypertension.
Therefore, we aimed to reveal the research trends and dynamic changes in this field for relevant scholars via bibliometric analysis of hypertension and AD, which might promote research on the mechanism and treatment of AD.
2 Methods
2.1 Search strategy
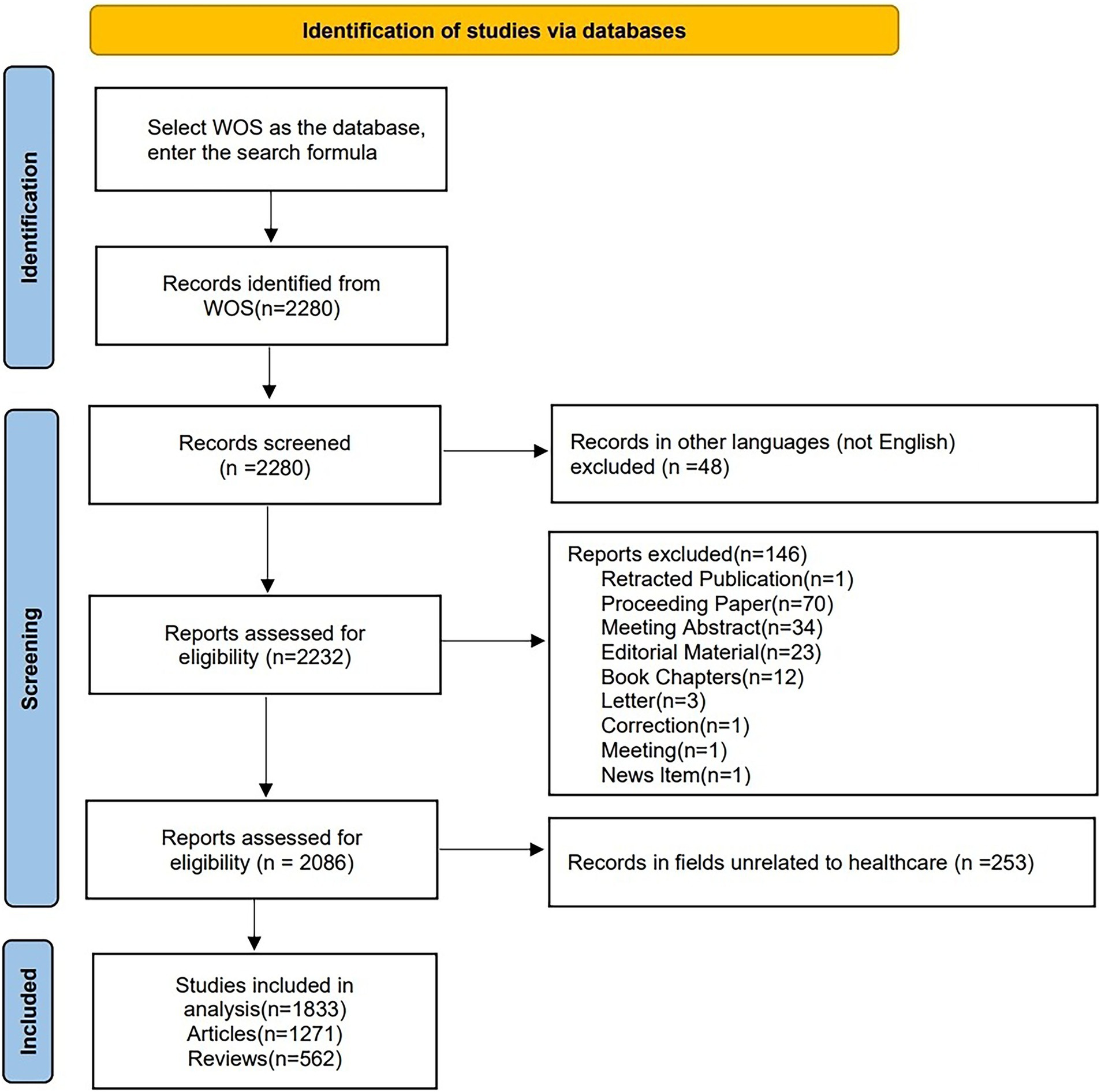
On July 5, 2024, we retrieved all the literature related to hypertension and AD from the Web of Science Core Collection (WoSCC). The search strategy was as follows: TS = ((“Alzheimer’s” OR “Alzheimer’s disease”) AND (“hypertension” OR “high blood pressure” OR “hypertensive” OR “hypertension”)) AND FPY = (2004–2023). To reduce bias further, we limited the search to articles and reviews in English, with Figure 1 illustrating the specific flow chart. Ultimately, 1,833 studies were retrieved and exported in plain text format, which included complete records and references.
2.2 Data analysis and visualization
Excel 2018 was used to display the trends in the number of articles published by year. Visualization analysis of the references was completed via CiteSpace (v.6.3.R4). VOSviewer (v.1.6.20) was used to analyze the number of articles published by each author and the keyword co-occurrence. Additionally, in an overlay visualization map of keywords, the size of the nodes represents the number of keyword occurrences. The H-index is used to evaluate the amount and level of academic output of researchers and journals (16).
3 Results
3.1 Publication trends
Figure 2 shows the growth trend of the number of annual publications and the annual cumulative number of publications from 2004 to 2023. The number of annual publications has shown an increasing trend, with over 150 publications consistently published from 2021 to 2023.
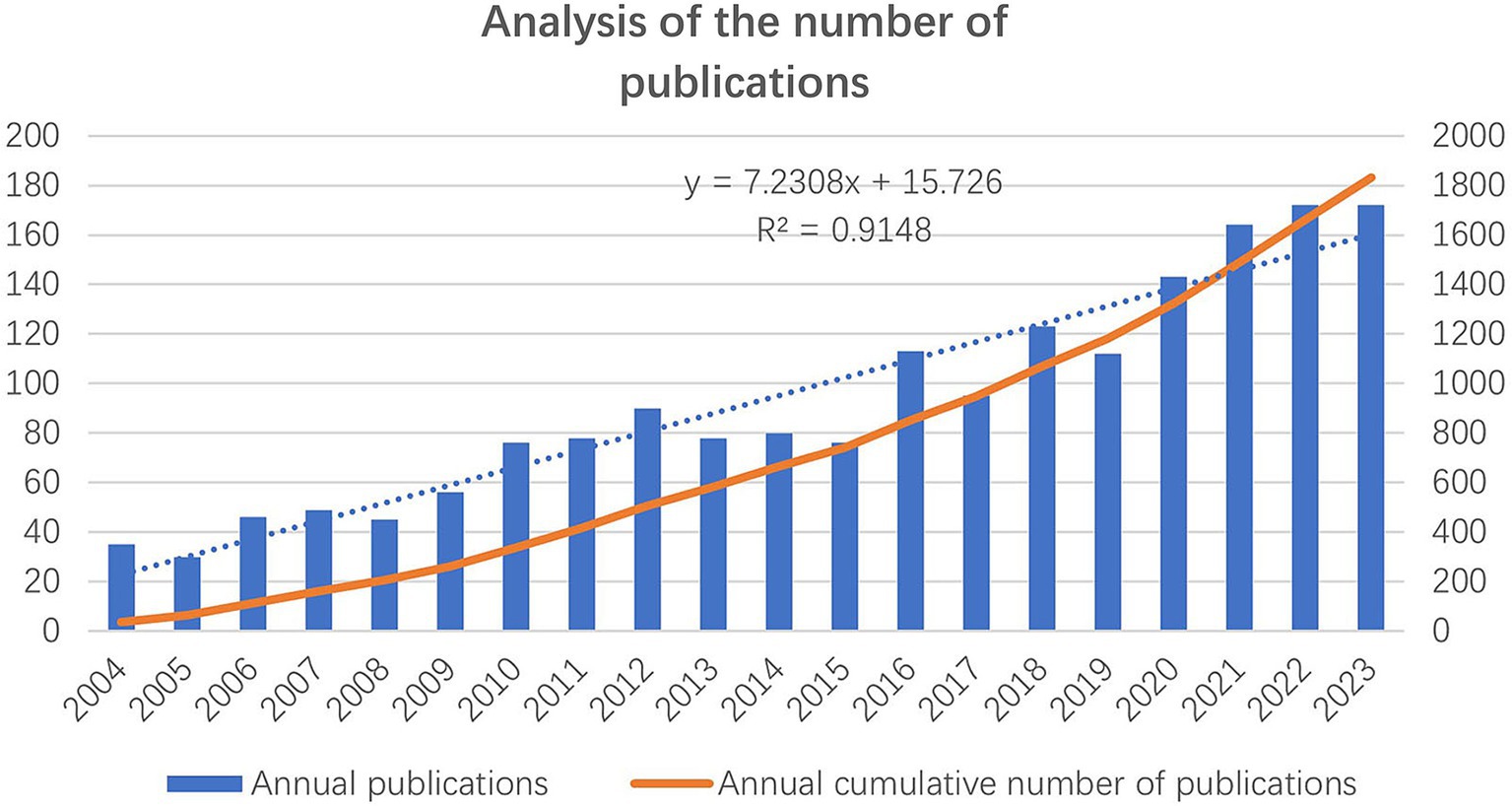
Figure 2. The number of annual publications and the annual cumulative number of publications from 2004 to 2023.
3.2 Analysis of countries and institutions
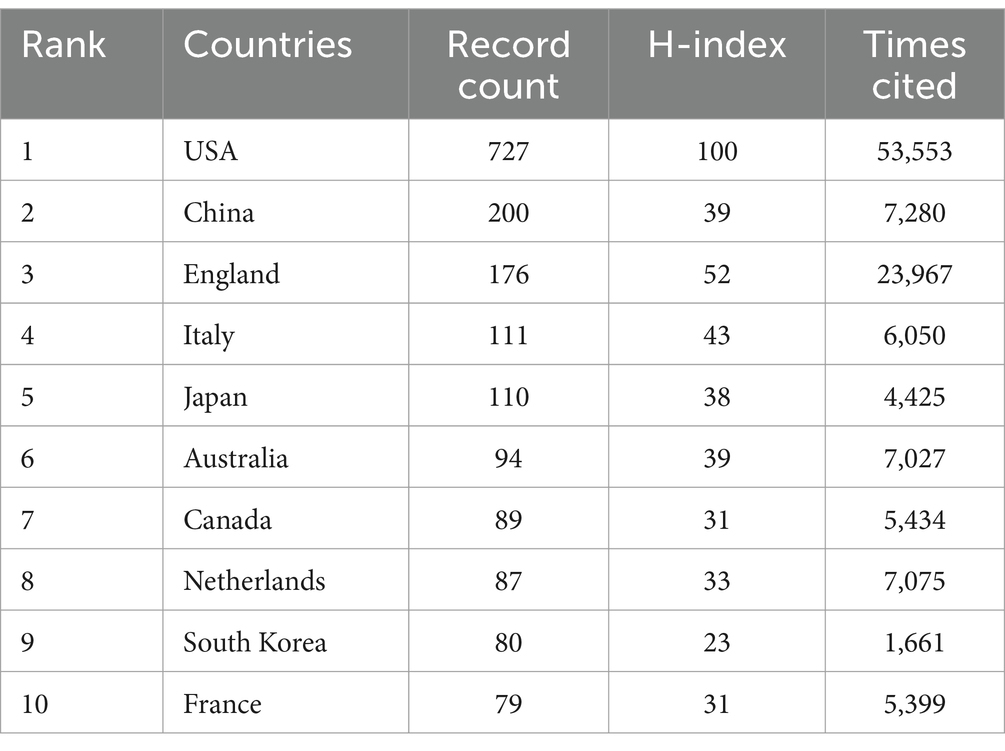
Out of 90 countries, 56 have satisfied the criterion of a minimum document count of 3. In accordance with Table 1, among all the countries where the literature was published, the top three in terms of the number of publications were the United States of America (USA) (727), China (200) and England (176). Among these countries, the top three in terms of citations to the literature were the USA (53,553), England (23,967) and China (7,280). The top three countries in terms of the H-index were the USA (100), England (52), and Italy (43). An analysis of publication, citations to the literature and the H-index indicated that the USA, England, China and Italy were the main research powers in this field.
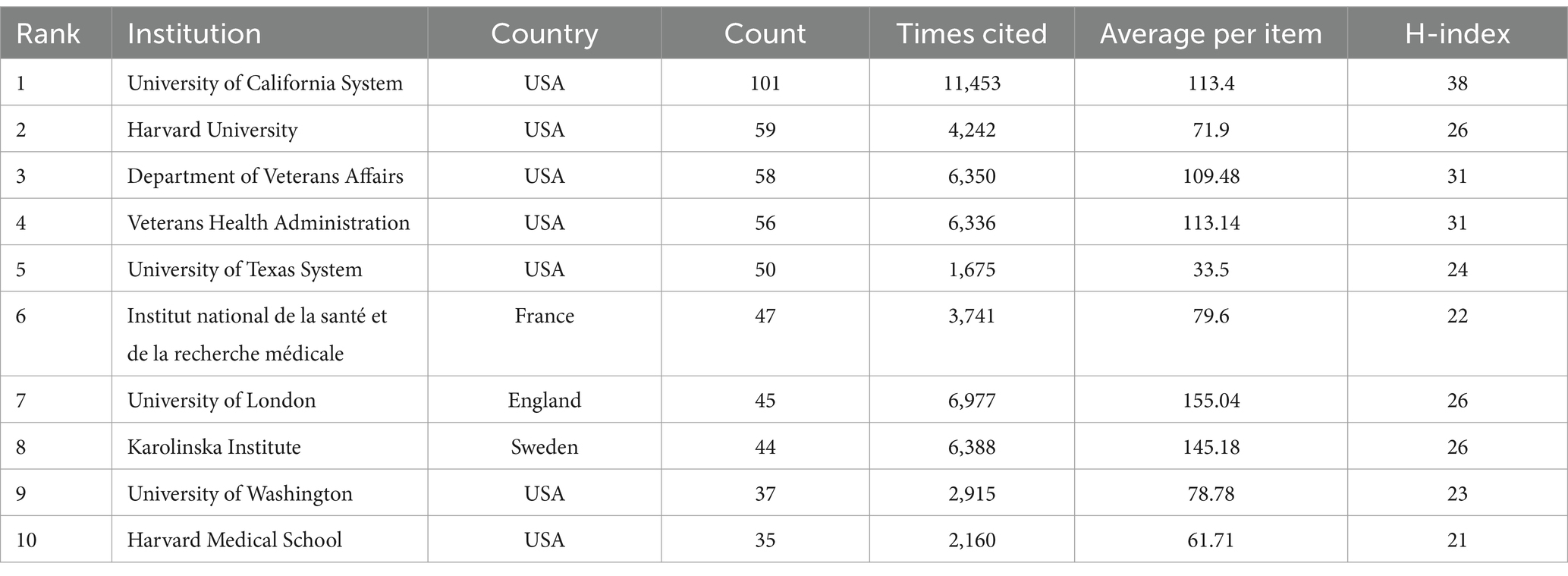
Among the 2,646 institutions, 468 satisfied the criterion of a minimum document count of 3. Table 2 lists the top 10 most productive institutions. The top three in terms of the number of publications were University of California system (101), Harvard University (59) and the Department of Veterans Affairs (n = 58). The top three in terms of citations to the literature were the University of California system (11,453), the University of London (6,977) and the Karolinska Institute (6,388). The top three institutions in terms of the H-index were the University of California system (38), the Department of Veterans Affairs (31) and the Veterans Health Administration (31).
3.3 Analysis of authors and cited authors
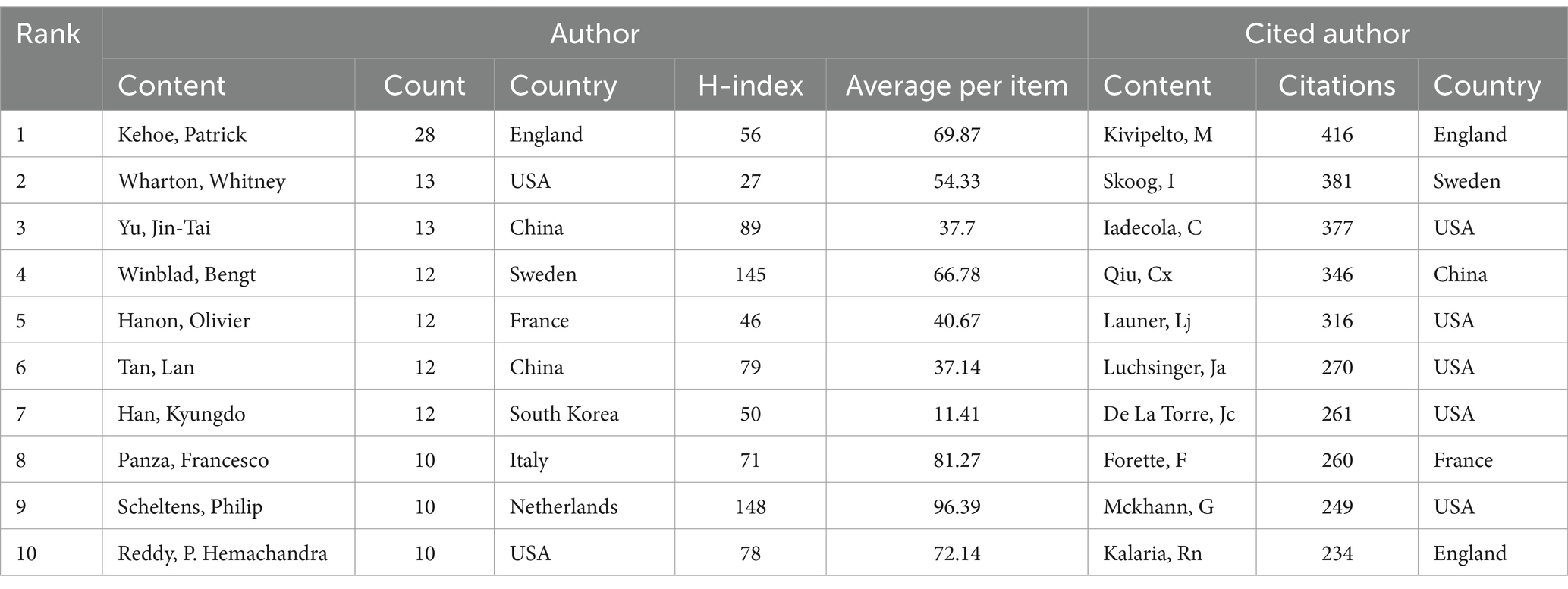
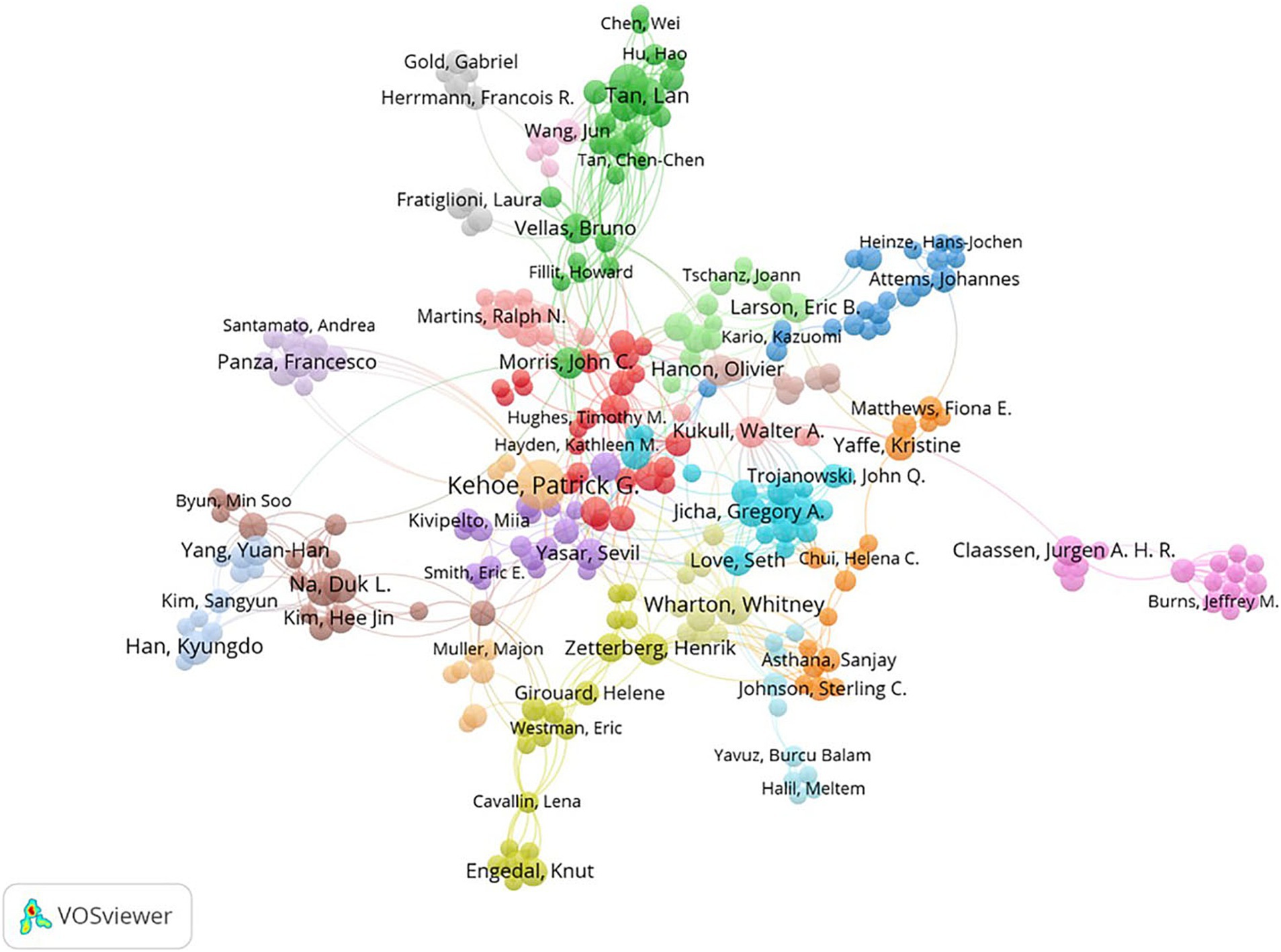
Among 9,330 authors, 416 satisfied the criterion of a minimum document count of 3. The top 10 most productive authors and the most cited authors are listed in Table 3. In terms of publication volume, Kehoe, Patrick (28 publications, H-index = 56) from England ranked first, followed by Wharton, Whitney (13 publications, H-index = 27) from the USA and Yu, Jin-Tai (13 publications, H-index = 89) from China. Kivipelto, M was the author with the highest number of citations and was cited 416 times in total. Figure 3 shows the visualization of cooperation among authors. The cooperation between these authors was not very close.
3.4 Analysis of published journals
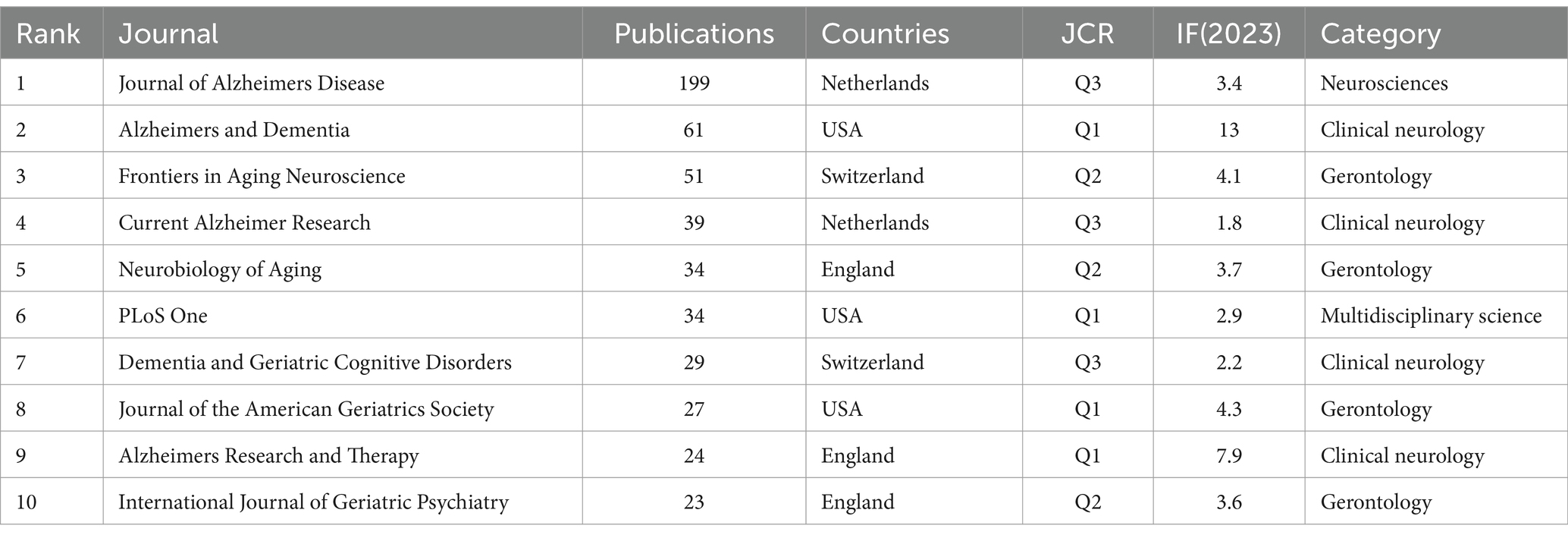
The top 10 journals in terms of publications are listed in Table 4. The top three in terms of the number of publications were the Journal of Alzheimers Disease (n = 199), Alzheimers & Dementia (n = 61) and Frontiers in Aging Neuroscience (n = 51). The IFs (2023) of the top 10 most productive journals ranged from 1.8 to 13, which indicated a significant difference in their influence. In addition, the categories of these journals were mainly clinical neurology and gerontology.
3.5 Analysis of references
A total of 17 major clusters were generated from the reference citation network diagram after cluster analysis. The clustering module value (Q) was 0.7935 > 0.3, indicating that the cluster structure was significant, and the average clustering contour value (S) was 0.9131 > 0.7, indicating that clustering was reliable and satisfactory. As shown in Figure 4, the clusters arranged from top to bottom are #0 high blood pressure, #1 diabetes mellitus, #2 metabolic syndrome, #3 blood pressure, #4 receptor blocker, #5 vascular risk factor, #6 current evidence, #7 public health, #8 targeting renin-angiotensin system, #9 subcortical small-vessel disease, #10 angiotensin receptor blocker, #11 non-ad dementia, #12 microvascular injury, #13 dietary pattern, #14 cell, #15 subcortical ischemic vascular dementia and #16 non-genetic factor. The earliest cluster focused on diabetes mellitus, receptor blockers and subcortical ischemic vascular dementia. Clusters focused on high blood pressure and angiotensin receptor blockers have become increasingly popular in recent years. According to the timeline, it can be speculated that the current frontiers in this field are the clinical application of antihypertensive drugs in AD, with the research focus gradually shifting from the pathophysiological mechanisms of AD and hypertension to specific clinical applications.
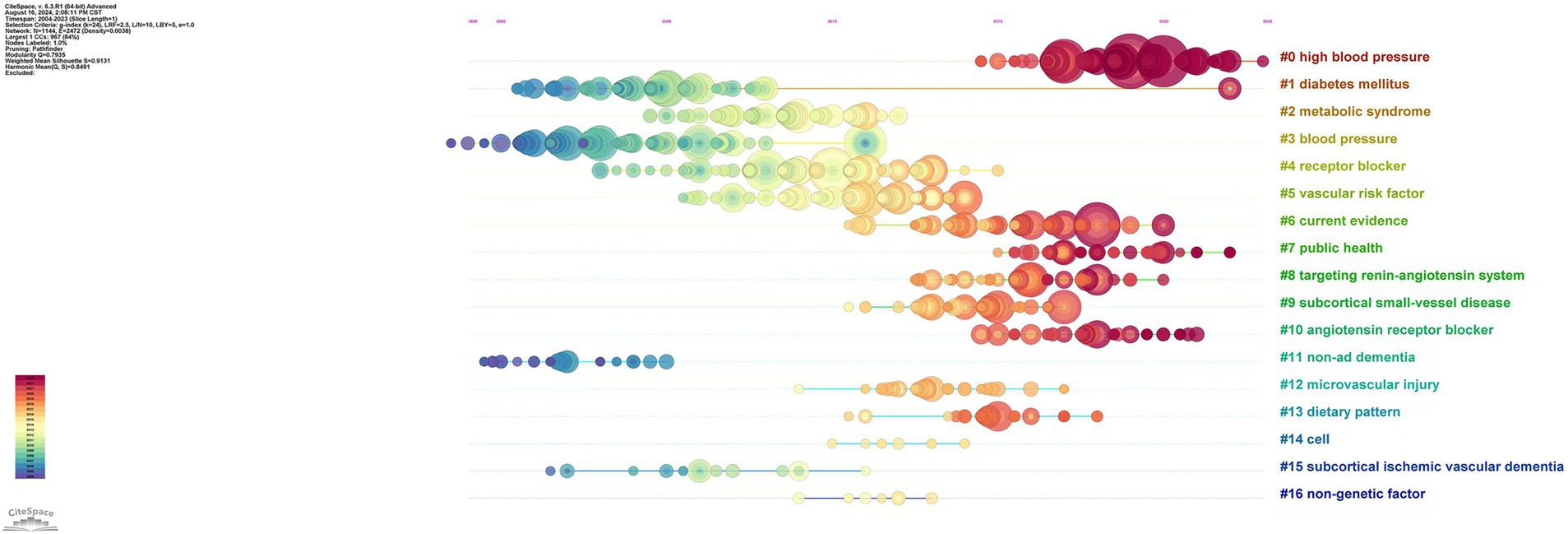
Figure 4. Timeline of reference citations on the relationship between hypertension and AD. Clusters are numbered from #0.
For nearly 20 years, scholars have carried out a series of studies ranging from experimental to clinical applications concerning the relationship between hypertension and AD. As shown in Supplementary Table 1, the main research content of the top 25 papers on outbreak intensity has been collated for better understanding. The strength of the citation bursts of the top 25 papers ranged from 11.68 to 28.22.
3.6 Analysis of keywords
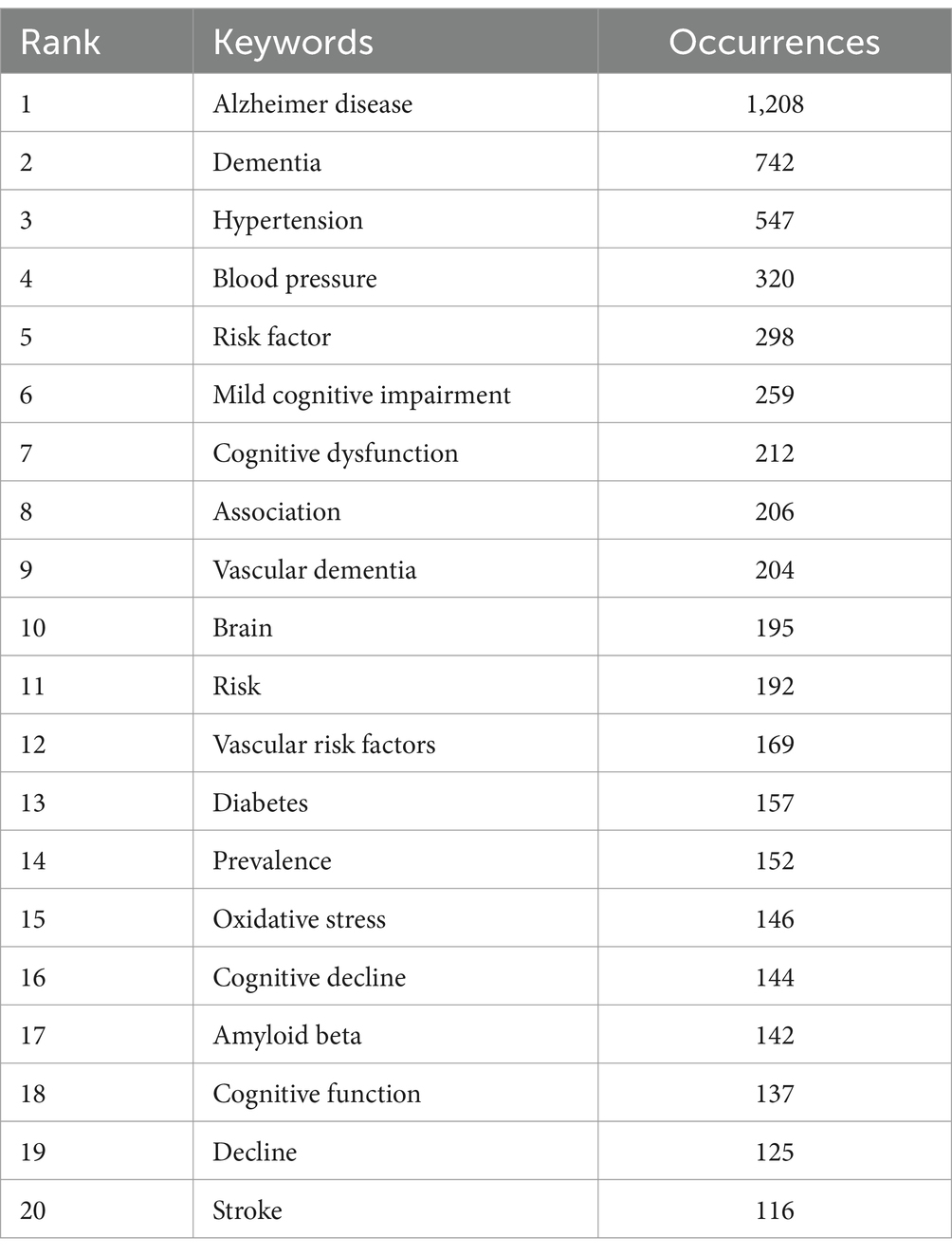
Among the 7,414 keywords, 445 satisfied the criterion of a minimum document count of 7. As shown in Table 5, the top five keywords for frequency and TLS were Alzheimer’s disease (1,208 times), dementia (742 times), hypertension (547 times), blood pressure (320 times), and risk factor (298 times). “Amyloid deposition,” “preeclampsia” and “COVID-19” have appeared in recent years, as shown in the overlay visualization map of keywords in Figure 5.
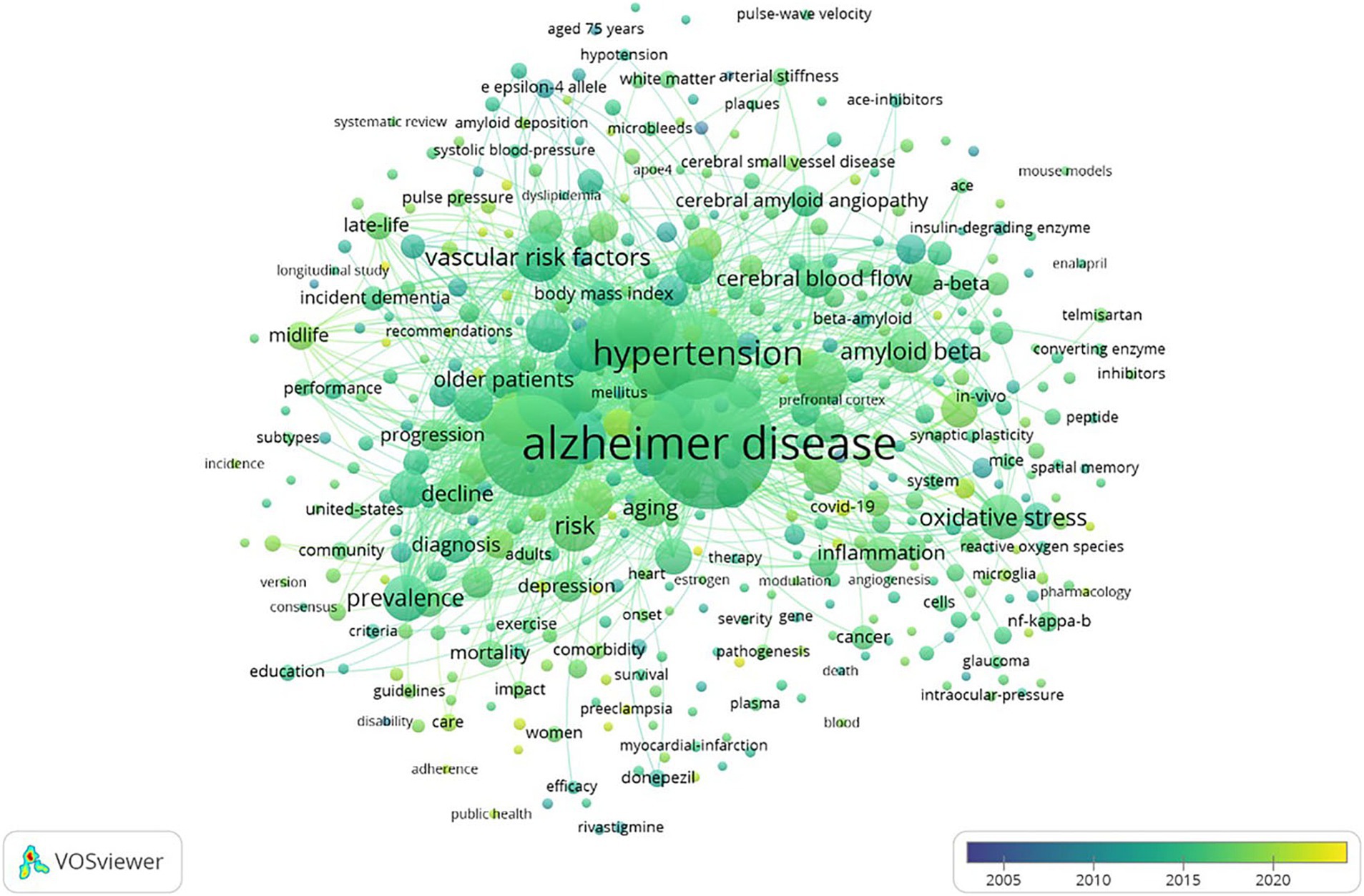
Figure 5. Overlay visualization map of keywords on VOSviewer. The color represents the average time of occurrence, and a color closer to yellow indicates a time closer to the present.
4 Discussion
This study collected 1833 studies on hypertension and AD over the past two decades and evaluated them via bibliometrics method. According to this study, publications related to AD and hypertension research have shown an increasing trend over the past two decades. Additionally, the number of articles published in the past 3 years has remained above 150, which suggests that interest in the research field remains strong. This phenomenon may be related to the increases in hypertension and AD incidence rates with increasing age (17). Among the top 10 most productive countries that published the most publications, the USA not only had the largest output in publications but also had the first H-index ranking. Moreover, seven of the top 10 institutions in terms of productivity were from the USA, indicating that this country has made significant contributions in this field. Although China ranked second in terms of publication frequency, it lacked influential institutions. China should focus on developing influential research institutions and strengthening international cooperation in the future. Author analysis revealed a network of core author collaborations in the field of hypertension and AD research. The cooperation among authors presented regionalization, with relatively less international cooperation. Therefore, it is necessary to strengthen the international cooperation of authors and seek better academic cooperation. By analyzing the journals about hypertension and AD, we determined that researchers have concentrated on the fields of neuroscience and geriatrics. Among these journals, the Journal of Alzheimers Disease (n = 199) was the most productive journal, and Alzheimers & Dementia (n = 61) was the second most productive journal in this field, with an impact factor of 13 from Q1 journals, which indicated that the field is currently a research hotspot.
The strongest citation burst was from an article published by Livingston et al. (18) in the journal Lancet entitled “Dementia prevention, intervention, and care: 2020 report of the Lancet Commission.” This paper provides sufficient new evidence to support the addition of three additional modifiable risk factors for dementia to the 2017 Commission model (excessive alcohol, traumatic brain injury, and air pollution). They added the latest evidence on the nine risk factors implicated in the 2017 Commission (education, hypertension, hearing impairment, smoking, obesity, depression, inactivity, diabetes, and social contact). The updated 12-risk factor life-course model of dementia prevention provides comprehensive prevention, intervention, and care guidance for dementia patients, which will vastly improve living and dying for them and their families. Reports have indicated that midlife hypertension, defined as hypertension at 40 years of age, is associated with reduced brain volume and increased white matter hyperintensity volume but not amyloid deposition (19). Blood pressure decreases later in life, which is related to the development of dementia and may be caused by the development of dementia (20, 21). Four meta-analyses of blood pressure medications used to lower high blood pressure suggested reduced dementia in those in the intervention group for outcomes of any dementia as well as clinically diagnosed AD (22–25). Additionally, amyloid imaging detected amyloid in the brain with high sensitivity and specificity in people with AD when the gold-standard comparison was either neuropathology or clinical diagnosis, distinguishing AD from other neurodegenerative conditions (26).
Dementia is a progressive and typically irreversible deterioration of cognitive function that is most often observed in older adults. Vascular dementia is the second most common cause of dementia after AD and the most common cause of nonneurodegenerative dementia (27). Among vascular risk factors, chronic arterial hypertension is a major contributor to cognitive impairment (28). Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg (29), which is the most important risk factor for cerebrovascular disease. The American Heart Association has discussed the specific role of vascular risk factors in cognitive impairment in previous statements (28). Unlike vascular dementia, which involves mainly cognitive impairment caused by cerebrovascular disease, brain tissue ischemia and hypoxia, and neuronal damage (30), hypertension may exacerbate the potential mechanisms of AD progression, including damage to the structure and function of the brain microcirculation, disruption of the BBB, increased neuroinflammation and oxidative stress, and impaired clearance of Aβ (31).
On the basis of the reference citation timeline and analysis of the top 25 references, we summarized the research trends in the relationship between hypertension and AD, with a focus on the following three aspects:
First, studies have focused on the pathophysiological association between AD and hypertension. Research on the vascular hypothesis and amyloid cascade hypothesis of AD pathogenesis has revealed that hypertension plays an important role in the pathogenesis of AD. As age increases, the functional and structural adaptation of cerebral arteries to hypertension can be impaired, which may lead to a significant decrease in fluid dynamic resistance in the proximal arteries, placing a heavy burden on the downstream part of the cerebral microcirculation and causing microvascular damage (32). In addition, the oxidative stress induced by stress intensifies. Higher pressure results in increased wall tension-related cellular stretch, which promotes oxidative stress in endothelial cells and vascular smooth muscle cells by inducing and upregulating nicotinamide adenine dinucleotide phosphate hydrogen oxidants (33) and increasing the mitochondrial production of reactive oxygen species (34). This may lead to BBB destruction, neuroinflammation, and white matter damage, promoting neuronal damage, amyloid plaques, and cerebral amyloid angiopathy (28). The resulting oxidative stress leads to structural damage to endothelial cells, pericyte injury and increased activation of matrix metalloproteinases (35, 36). Increased matrix metalloproteinase activity leads to disruption of tight junctions and breakdown of the extracellular matrix, resulting in damage to the BBB. The BBB is a functional part of the neurovascular unit that separates the central nervous system from the circulation. The damaged BBB allows IgG, hemoglobin, fibrinogen, and highly inflammatory pathogen-associated molecular patterns in the plasma to enter the brain parenchyma, activating microglia and promoting neuroinflammation, leading to neuronal apoptosis and impaired synaptic function (37, 38). Hypertension is associated with transverse aortic constriction and enhances Aβ deposition. Aβ deposition can be detected in the mouse brain 4 weeks after hypertension is induced (39). In transgenic mice infused with angiotensin II, angiotensin II administration increased the activity of β-secretase and the cleavage rate of the Aβ protein precursor, leading to the accumulation of Aβ in blood vessels (40). These findings validate the key role of hypertension in the pathogenesis of AD. However, the mechanism by which hypertension may lead to AD remains to be studied.
Second, studies have focused on AHDs, particularly calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and β-blockers (BBs), for their role in mitigating AD-related pathology and their preventive effects. Studies indicate that reducing blood pressure, influencing the renin–angiotensin system, and/or modulating intracellular calcium homeostasis may offer protection against neural damage resulting from vascular changes, increased permeability of the BBB and inflammation (41, 42). Law CSW et al. summarized the latest AD-related observations of four groups of 11 AHDs in preclinical studies and proposed that angiotensin II receptor blockers may have the best potential for treating AD (41). Angiotensin II receptor blockers can control AD by reducing neuroinflammation, increasing the clearance rate of Aβ, and/or reducing the level of hyperphosphorylated tau protein (43–45). Calcium channel blockers have mostly failed to demonstrate good efficacy in trials (46, 47), whereas angiotensin-converting enzyme inhibitors might prevent the conversion of neurotoxic Aβ42 to Aβ40, increasing the risk of IQ deterioration (48). BBs have lower potency than other AHDs do, in addition to poor penetration across the BBB (49). Although there is strong and concrete evidence that AHDs are useful in the management of AD, the efficacy of AHDs seems to be limited by the age of patients. Vazirinejad et al. suggested that only AD patients aged 40 years and above presented better cognitive performance (50). However, this may not be a handicap, as AD mostly affects people aged over 65 years. In addition, another potential area to explore is the use of AHDs in conjunction with the currently approved AD drugs, which is expected to lead to improved treatment and prognosis for AD patients.
Finally, the pathogenesis of AD is multifactorial and involves genetic susceptibility and environmental interactions, among which hypertension is the most decisive factor among all modifiable risk factors mediating the development of AD (51). Controlling hypertension through lifestyle changes and medication interventions in AD patients provides promising strategies to alleviate these effects.
Keywords analysis revealed that “amyloid deposition” “preeclampsia” “COVID-19” and “biomarkers” have emerged in recent years and can be considered new research directions in the fields of hypertension and AD. Postmortem studies have revealed that hypertension is related to several features of AD including increased Aβ plaque burden and neurofibrillary tangles (52). In animal models of preeclampsia intracranial cerebral arteries and hippocampal arterioles do not remodel and have impaired myogenic tone in response to pregnancy which may lead to hyperperfusion of the brain increase permeability of the BBB and cause neuroinflammation (53). Studies have shown that a history of preeclampsia is associated with an increase in white matter volume and a decrease in gray matter volume which is usually related to cognitive decline (54–57). Research has also shown that women with preeclampsia have a greater risk of stroke which increases their risk of developing mixed dementia caused by AD and vascular factors (58, 59). AD and COVID-19 have common risk factors including age hypertension diabetes cardiovascular disease and the presence of the apolipoprotein E ε 4 allele (60, 61). A study comparing 5,128 AD-positive patients with 431,695 AD-negative patients revealed that a pre-existing diagnosis of AD predicts a higher risk of COVID-19 and mortality among elderly adults. Moreover the prescription of angiotensin II receptor blockers was significantly associated with a lower risk of COVID-19 occurrence among AD patients (62). George et al. reported that early adult hypertension was associated with differences in late-life neuroimaging biomarkers such as smaller cerebral cerebral gray matter hippocampal frontal cortex and parietal cortex volumes; larger lateral ventricles third ventricles and free water volumes; and lower fractional anisotropy (63). These biomarkers are associated with cognitive decline and dementia. The above research results indicate that there are still many new directions worth exploring in the fields of AD and hypertension, which may provide new ideas for exploring the pathogenesis and treatment of AD.
5 Limitations
There were several limitations in this study. First, all English articles and reviews were retrieved and downloaded from the WoSCC, so they may not represent the complete research field of the relationship between hypertension and AD. Second, this study analyzed only the overall situation over the past 20 years. Finally, factors such as self-citation may have led to research bias.
6 Conclusion
To our knowledge, this is the first bibliometric analysis of hypertension and AD. This study revealed that countries in America and Europe, especially the USA, have advantages in terms of publication and research cooperation in terms of the relationship between hypertension and AD. Asian countries, such as China and Japan, need to actively seek international cooperation to increase their global influence on further development in this field. Analysis of references and keywords indicated that research in this field has shifted from the pathophysiological relationship between AD and hypertension to clinical research. The therapeutic effects and potential risks of AHDs on AD have also been research hotspots in recent years. Additionally, new research areas such as “amyloid deposition,” “preeclampsia,” “COVID-19,” and “biomarkers” may offer new insights into exploring the pathogenesis and treatment of AD. We believe that these findings will provide useful information for researchers to further explore the role of hypertension in AD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WL: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. YZ: Visualization, Writing – original draft, Writing – review & editing. YR: Data curation, Writing – original draft, Writing – review & editing. ZW: Data curation, Writing – original draft, Writing – review & editing. YP: Supervision, Writing – original draft, Writing – review & editing. LG: Conceptualization, Formal analysis, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1514054/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; WoSCC, Web of Science Core Collection; USA, The United States; AHDs, Antihypertensive drugs; COVID-19, Corona Virus Disease 2019; Aβ, β-amyloid; BBB, Blood-brain barrier; BBs, β-blockers.
References
1. Musa, G, Slachevsky, A, Muñoz-Neira, C, Méndez-Orellana, C, Villagra, R, González-Billault, C, et al. Alzheimer's disease or behavioral variant frontotemporal dementia? Review of key points toward an accurate clinical and neuropsychological diagnosis. J Alzheimers Dis. (2020) 73:833–48. doi: 10.3233/JAD-190924
2. Gustavsson, A, Norton, N, Fast, T, Frölich, L, Georges, J, Holzapfel, D, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. (2023) 19:658–70. doi: 10.1002/alz.12694
3. Duyckaerts, C, Delatour, B, and Potier, MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. (2009) 118:5–36. doi: 10.1007/s00401-009-0532-1
4. Eldufani, J, and Blaise, G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: a review of recent clinical applications. Alzheimers Dement (NY). (2019) 5:175–83. doi: 10.1016/j.trci.2019.03.004
5. Breijyeh, Z, and Karaman, R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. (2020) 25:5789. doi: 10.3390/molecules25245789
6. Yu, JT, Xu, W, Tan, CC, Andrieu, S, Suckling, J, Evangelou, E, et al. Evidence-based prevention of Alzheimer's disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. (2020) 91:1201–9. doi: 10.1136/jnnp-2019-321913
7. Valverde, A, and Mitrofanis, J. Photobiomodulation for hypertension and Alzheimer's disease. J Alzheimers Dis. (2022) 90:1045–55. doi: 10.3233/JAD-220632
8. Bowman, GL, Dayon, L, Kirkland, R, Wojcik, J, Peyratout, G, Severin, IC, et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. (2018) 14:1640–50. doi: 10.1016/j.jalz.2018.06.2857
9. Park, L, Zhou, P, Koizumi, K, El Jamal, S, Previti, ML, Van Nostrand, WE, et al. Brain and circulating levels of Aβ1-40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke. (2013) 44:198–204. doi: 10.1161/STROKEAHA.112.670976
10. de Jong, DLK, de Heus, RAA, Rijpma, A, Donders, R, Olde Rikkert, MGM, Günther, M, et al. Effects of Nilvadipine on cerebral blood flow in patients with Alzheimer disease. Hypertension. (2019) 74:413–20. doi: 10.1161/HYPERTENSIONAHA.119.12892
11. Hernandorena, I, Duron, E, Vidal, JS, and Hanon, O. Treatment options and considerations for hypertensive patients to prevent dementia. Expert Opin Pharmacother. (2017) 18:989–1000. doi: 10.1080/14656566.2017.1333599
12. Ninkov, A, Frank, JR, and Maggio, LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. (2022) 11:173–6. doi: 10.1007/s40037-021-00695-4
13. Zhang, GF, Gong, WX, Xu, ZY, and Guo, Y. Alzheimer's disease and epilepsy: the top 100 cited papers. Front Aging Neurosci. (2022) 14:926982. doi: 10.3389/fnagi.2022.926982
14. Tang, M, Wu, L, Shen, Z, Chen, J, Yang, Y, Zhang, M, et al. Association between sleep and Alzheimer's disease: a bibliometric analysis from 2003 to 2022. Neuroepidemiology. (2023) 57:377–90. doi: 10.1159/000533700
15. Sun, X, Xu, H, Qu, H, and Dong, W. A bibliometric review on vitamins and Alzheimer's disease between 1996 and 2023. Front Aging Neurosci. (2023) 15:1144804. doi: 10.3389/fnagi.2023.1144804
16. Joshi, MA. Bibliometric indicators for evaluating the quality of scientifc publications. J Contemp Dent Pract. (2014) 15:258–62. doi: 10.5005/jp-journals-10024-1525
17. Yao, Q, Jiang, K, Lin, F, Zhu, T, Khan, NH, and Jiang, E. Pathophysiological Association of Alzheimer's disease and hypertension: a clinical concern for elderly population. Clin Interv Aging. (2023) 18:713–28. doi: 10.2147/CIA.S400527
18. Livingston, G, Huntley, J, Sommerlad, A, Ames, D, Ballard, C, Banerjee, S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
19. Lane, CA, Barnes, J, Nicholas, JM, Sudre, CH, Cash, DM, Parker, TD, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (insight 46): an epidemiological study. Lancet Neurol. (2019) 18:942–52. doi: 10.1016/S1474-4422(19)30228-5
20. McGrath, ER, Beiser, AS, DeCarli, C, Plourde, KL, Vasan, RS, Greenberg, SM, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology. (2017) 89:2447–54. doi: 10.1212/WNL.0000000000004741
21. Walker, KA, Sharrett, AR, Wu, A, Schneider, ALC, Albert, M, Lutsey, PL, et al. Association of Midlife to late-life blood pressure patterns with incident dementia. JAMA. (2019) 322:535–45. doi: 10.1001/jama.2019.10575
22. Peters, R, Warwick, J, Anstey, KJ, and Anderson, CS. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. (2019) 92:1017–8. doi: 10.1212/WNL.0000000000007543
23. Tully, PJ, Hanon, O, Cosh, S, and Tzourio, C. Diuretic antihypertensive drugs and incident dementia risk: a systematic review, meta-analysis and meta-regression of prospective studies. J Hypertens. (2016) 34:1027–35. doi: 10.1097/HJH.0000000000000868
24. Ding, J, Davis-Plourde, KL, Sedaghat, S, Tully, PJ, Wang, W, Phillips, C, et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. (2020) 19:61–70. doi: 10.1016/S1474-4422(19)30393-X
25. Hussain, S, Singh, A, Rahman, SO, Habib, A, and Najmi, AK. Calcium channel blocker use reduces incident dementia risk in elderly hypertensive patients: a meta-analysis of prospective studies. Neurosci Lett. (2018) 671:120–7. doi: 10.1016/j.neulet.2018.02.027
26. Rice, L, and Bisdas, S. The diagnostic value of FDG and amyloid PET in Alzheimer's disease-a systematic review. Eur J Radiol. (2017) 94:16–24. doi: 10.1016/j.ejrad.2017.07.014
27. Linh, TTD, Hsieh, YC, Huang, LK, and Hu, CJ. Clinical trials of new drugs for vascular cognitive impairment and vascular dementia. Int J Mol Sci. (2022) 23:11067. doi: 10.3390/ijms231911067
28. Gorelick, PB, Scuteri, A, Black, SE, Decarli, C, Greenberg, SM, Iadecola, C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2011) 42:2672–713. doi: 10.1161/STR.0b013e3182299496
29. James, PA, Oparil, S, Carter, BL, Cushman, WC, Dennison-Himmelfarb, C, Handler, J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint National Committee (JNC 8). JAMA. (2014) 311:507–20. doi: 10.1001/jama.2013.284427
30. Canavan, M, and O'Donnell, MJ. Hypertension and cognitive impairment: a review of mechanisms and key concepts. Front Neurol. (2022) 13:821135. doi: 10.3389/fneur.2022.821135
31. Ungvari, Z, Toth, P, Tarantini, S, Prodan, CI, Sorond, F, Merkely, B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. (2021) 17:639–54. doi: 10.1038/s41581-021-00430-6
32. Springo, Z, Toth, P, Tarantini, S, Ashpole, NM, Tucsek, Z, Sonntag, WE, et al. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. (2015) 35:527–30. doi: 10.1038/jcbfm.2014.256
33. Park, L, Anrather, J, Girouard, H, Zhou, P, and Iadecola, C. Nox 2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. (2007) 27:1908–18. doi: 10.1038/sj.jcbfm.9600491
34. Springo, Z, Tarantini, S, Toth, P, Tucsek, Z, Koller, A, Sonntag, WE, et al. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. (2015) 70:1355–9. doi: 10.1093/gerona/glu244
35. Toth, P, Tucsek, Z, Sosnowska, D, Gautam, T, Mitschelen, M, Tarantini, S, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. (2013) 33:1732–42. doi: 10.1038/jcbfm.2013.143
36. Toth, P, Tarantini, S, Springo, Z, Tucsek, Z, Gautam, T, Giles, CB, et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. (2015) 14:400–8. doi: 10.1111/acel.12315
37. Tucsek, Z, Noa Valcarcel-Ares, M, Tarantini, S, Yabluchanskiy, A, Fülöp, G, Gautam, T, et al. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. (2017) 39:385–406. doi: 10.1007/s11357-017-9981-y
38. Davalos, D, Ryu, JK, Merlini, M, Baeten, KM, Le Moan, N, Petersen, MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. (2012) 3:1227. doi: 10.1038/ncomms2230
39. Carnevale, D, Mascio, G, D'Andrea, I, Fardella, V, Bell, RD, Branchi, I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. (2012) 60:188–97. doi: 10.1161/HYPERTENSIONAHA.112.195511
40. Faraco, G, Park, L, Zhou, P, Luo, W, Paul, SM, Anrather, J, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. (2016) 36:241–52. doi: 10.1038/jcbfm.2015.79
41. Law, CSW, and Yeong, KY. Repurposing antihypertensive drugs for the Management of Alzheimer's disease. Curr Med Chem. (2021) 28:1716–30. doi: 10.2174/0929867327666200312114223
42. Lebouvier, T, Chen, Y, Duriez, P, Pasquier, F, and Bordet, R. Antihypertensive agents in Alzheimer's disease: beyond vascular protection. Expert Rev Neurother. (2020) 20:175–87. doi: 10.1080/14737175.2020.1708195
43. Trigiani, LJ, Royea, J, Lacalle-Aurioles, M, Tong, XK, and Hamel, E. Pleiotropic benefits of the angiotensin receptor blocker candesartan in a mouse model of Alzheimer disease. Hypertension. (2018) 72:1217–26. doi: 10.1161/HYPERTENSIONAHA.118.11775
44. Singh, B, Sharma, B, Jaggi, AS, and Singh, N. Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer's disease type: possible involvement of PPAR-γ agonistic property. J Renin-Angiotensin-Aldosterone Syst. (2013) 14:124–36. doi: 10.1177/1470320312459977
45. Gao, Y, Li, W, Liu, Y, Wang, Y, Zhang, J, Li, M, et al. Effect of Telmisartan on preventing learning and memory deficits via peroxisome proliferator-activated receptor-γ in vascular dementia spontaneously hypertensive rats. J Stroke Cerebrovasc Dis. (2018) 27:277–85. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.025
46. Cummings, J, Lee, G, Ritter, A, and Zhong, K. Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement (NY). (2018) 4:195–214. doi: 10.1016/j.trci.2018.03.009
47. Lawlor, B, Segurado, R, Kennelly, S, Olde Rikkert, MGM, Howard, R, Pasquier, F, et al. Nilvadipine in mild to moderate Alzheimer disease: a randomised controlled trial. PLoS Med. (2018) 15:e1002660. doi: 10.1371/journal.pmed.1002660
48. Liu, S, Ando, F, Fujita, Y, Liu, J, Maeda, T, Shen, X, et al. A clinical dose of angiotensin-converting enzyme (ACE) inhibitor and heterozygous ACE deletion exacerbate Alzheimer's disease pathology in mice. J Biol Chem. (2019) 294:9760–70. doi: 10.1074/jbc.RA118.006420
49. Wang, J, Ono, K, Dickstein, DL, Arrieta-Cruz, I, Zhao, W, Qian, X, et al. Carvedilol as a potential novel agent for the treatment of Alzheimer's disease. Neurobiol Aging. (2011) 32:2321.e1–2321.e12. doi: 10.1016/j.neurobiolaging.2010.05.004
50. Vazirinejad, R, Mirmotalebi, M, Bageri, M, Kounis, NG, Koniari, I, Lilley, JM, et al. Age-related effect of antihypertensive treatment on cognitive performance: is it better preventing dementia in older age? Am J Alzheimers Dis Other Dement. (2019) 34:486–91. doi: 10.1177/1533317519859197
51. Lennon, MJ, Koncz, R, and Sachdev, PS. Hypertension and Alzheimer's disease: is the picture any clearer? Curr Opin Psychiatry. (2021) 34:142–8. doi: 10.1097/YCO.0000000000000684
52. Abdulrahman, H, van Dalen, JW, den Brok, M, Latimer, CS, Larson, EB, and Richard, E. Hypertension and Alzheimer's disease pathology at autopsy: a systematic review. Alzheimers Dement. (2022) 18:2308–26. doi: 10.1002/alz.12707
53. Miller, KB, Miller, VM, and Barnes, JN. Pregnancy history, hypertension, and cognitive impairment in postmenopausal women. Curr Hypertens Rep. (2019) 21:93. doi: 10.1007/s11906-019-0997-9
54. Yoshita, M, Fletcher, E, Harvey, D, Ortega, M, Martinez, O, Mungas, DM, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. (2006) 67:2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f
55. Silbert, LC, Nelson, C, Howieson, DB, Moore, MM, and Kaye, JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. (2008) 71:108–13. doi: 10.1212/01.wnl.0000316799.86917.37
56. Wiegman, MJ, Zeeman, GG, Aukes, AM, Bolte, AC, Faas, MM, Aarnoudse, JG, et al. Regional distribution of cerebral white matter lesions years after preeclampsia and eclampsia. Obstet Gynecol. (2014) 123:790–5. doi: 10.1097/AOG.0000000000000162
57. Mielke, MM, Milic, NM, Weissgerber, TL, White, WM, Kantarci, K, Mosley, TH, et al. Impaired cognition and brain atrophy decades after hypertensive pregnancy disorders. Circ Cardiovasc Qual Outcomes. (2016) 9:S70–6. doi: 10.1161/CIRCOUTCOMES.115.002461
58. Inoue, Y, Shue, F, Bu, G, and Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer's disease. Mol Neurodegener. (2023) 18:46. doi: 10.1186/s13024-023-00640-5
59. O'Neal, MA. Women and the risk of Alzheimer's disease. Front Glob Womens Health. (2023) 4:1324522. doi: 10.3389/fgwh.2023.1324522
60. Jordan, RE, Adab, P, and Cheng, KK. Covid-19: risk factors for severe disease and death. BMJ. (2020) 368:m 1198. doi: 10.1136/bmj.m1198
61. Kuo, CL, Pilling, LC, Atkins, JL, Masoli, JAH, Delgado, J, Kuchel, GA, et al. Apo E e4e4 genotype and mortality with COVID-19 in UK biobank. J Gerontol A Biol Sci Med Sci. (2020) 75:1801–3. doi: 10.1093/gerona/glaa169
62. Wang, Y, Li, M, Kazis, LE, and Xia, W. Clinical outcomes of COVID-19 infection among patients with Alzheimer's disease or mild cognitive impairment. Alzheimers Dement. (2022) 18:911–23. doi: 10.1002/alz.12665
Keywords: Alzheimer’s disease, hypertension, bibliometrics, Web of Science, CiteSpace, VOSviewer
Citation: Liu W, Zhao Y, Rao Y, Wu Z, Peng Y and Gong L (2025) Frontiers and hotspot evolution in research on Alzheimer’s disease and hypertension: a bibliometric analysis from 2004 to 2023. Front. Neurol. 16:1514054. doi: 10.3389/fneur.2025.1514054
Edited by:
Anna Maria Lavezzi, University of Milan, ItalyReviewed by:
Ciro Gaona, Alzheimer’s Foundation of Venezuela, VenezuelaSuzana Uzun, Josip Juraj Strossmayer University of Osijek, Croatia
Copyright © 2025 Liu, Zhao, Rao, Wu, Peng and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianggeng Gong, Z29uZzExMTk5OUAxMjYuY29t
 Wenying Liu
Wenying Liu Yaya Zhao1,2
Yaya Zhao1,2 Zijing Wu
Zijing Wu Yun Peng
Yun Peng Lianggeng Gong
Lianggeng Gong