- Department of Neurology, The First Affiliated Hospital of Harbin Medical University, Harbin, China
Background: Progressive ischemic stroke (PIS) poses significant challenges in the management of acute ischemic stroke (AIS), with higher morbidity and mortality rates, especially among patients with vascular risk factors such as hypertension and diabetes. This study evaluates the efficacy of human urinary kallidinogenase (HUK) in reducing the incidence of PIS in patients with AIS, with a particular focus on subgroups based on vascular pathology and thrombolytic treatment.
Methods: This retrospective cohort study included 916 patients with AIS treated at a single tertiary care center between January 2022 and September 2023. The patients were divided into two groups based on whether they received HUK treatment in addition to standard care or standard care alone. The primary outcome was the incidence of PIS. Independent sample t-tests or chi-squared tests were used for univariate analysis between groups to identify potential predictors associated with the occurrence of PIS, with factors achieving a p-value < 0.1 considered for multivariate binary logistic regression analysis. Multivariate analysis adjusted for potential confounders to determine independent predictors significantly associated with PIS. The significance threshold was set at p < 0.05. In addition, subgroup analyses were conducted based on stroke subtype (TOAST classification), thrombolysis treatment, and infarction location.
Results: HUK treatment significantly reduced the incidence of PIS (p < 0.001), with the most notable effects observed in patients with large-artery atherosclerosis and small-artery occlusion, those not undergoing intravenous thrombolysis, and those with anterior circulation infarctions. Conversely, no significant reduction was noted in patients with cardioembolic stroke, other etiologies of infarction, intravenous thrombolysis, posterior circulation infarctions, or both anterior and posterior circulation infarctions. Factors such as low body mass index (BMI) and high activated partial thromboplastin time are associated with an increased risk of PIS.
Conclusion: HUK treatment appears to be an effective strategy for reducing the risk of PIS in patients with AIS, particularly in those at higher risk owing to specific vascular pathologies. These findings support the use of HUK in clinical practice to improve the outcomes of patients with stroke. Future prospective, multicenter, randomized controlled trials are warranted to validate these findings and further elucidate the underlying mechanisms.
1 Introduction
Stroke remains the second leading cause of death worldwide and the third most common cause of disability and mortality. Ischemic strokes, representing 62.4% of all new stroke cases, disproportionately affect populations in low to upper-middle-income countries, accounting for over 80% of stroke-related disability-adjusted life years (DALYs) (1). Notably, approximately 25 to 33% of stroke survivors develop PIS within days following the initial event, presenting with worsening neurological deficits and generally poorer prognostic outcomes (2).
HUK, a serine protease derived from urine, has garnered attention for its therapeutic potential in AIS, primarily through enhancing collateral circulation, stimulating angiogenesis, and improving cerebral perfusion (3–5). A longitudinal study involving 300 patients demonstrated that those receiving HUK exhibited notably lower scores on the modified Rankin Scale (mRS) at a 12-month follow-up compared to their counterparts in the control group (6). Moreover, a meta-analysis incorporating data from 24 studies quantified the neurologic improvement attributable to HUK, indicating a 0.56-fold increase in recovery rates (7). Focusing on patients with large artery atherosclerosis, another retrospective analysis revealed that HUK treatment was associated with significantly reduced National Institutes of Health Stroke Scale (NIHSS) scores (8).
Hypertension and diabetes are prevalent risk factors in the AIS patient demographic, with the National Inpatient Sample highlighting that 79% of these patients suffer from hypertension, and 34% are diabetic (9–11). Endothelial dysfunction, insulin resistance, and impaired vascular reactivity caused by hypertension and diabetes may increase the adverse risks in AIS patients. Previous studies have demonstrated that blood pressure levels in AIS patients are significantly associated with their neurological outcomes. Maintaining appropriate blood pressure levels can significantly improve the prognosis of AIS patients (12, 13). Given the high prevalence of these comorbidities and their significant impact on stroke outcomes, investigating the therapeutic potential in this high-risk subgroup is of great clinical significance. Despite these statistics, research into HUK’s effectiveness specifically for patients with AIS concurrently diagnosed with these conditions remains sparse. One study reported that patients with AIS with stage 3 hypertension undergoing HUK treatment showed substantial improvements in mRS scores and recovery rates 3 months post-treatment (14). Another study contrasting patients with AIS with abnormal glucose metabolism observed a significant reduction in NIHSS scores following HUK treatment, although mRS scores did not differ significantly between the treated and control groups (15).
Thus, the present study aimed to investigate the effects of HUK on the incidence of PIS post-admission in patients with AIS with both hypertension and with diabetes, with a subgroup analysis by site of lesions, TOAST subtypes, and the use of intravenous thrombolysis.
2 Methods
2.1 Study design and participants
This retrospective cohort study included 916 patients who presented with mild (NIHSS ≤ 7) or moderate (NIHSS: 8–16) AIS and were admitted to the Department of Neurology at the First Hospital of Harbin Medical University between January 2022 and September 2023 (16). Eligible participants had a confirmed diagnosis of hypertension and diabetes either prior to or during their current treatment.
The study protocol was approved by the Institutional Review Board of the First Hospital of Harbin Medical University (IRB-2023326) and adhered to the ethical standards of the Declaration of Helsinki. All participants provided written informed consent.
Patients were stratified into two groups based on their treatment regimens. The experimental group received HUK treatment (0.15 PNA units/day intravenously) alongside standard care, which included antiplatelet aggregation therapy, lipid-lowering drugs, potential intravenous thrombolysis, and antifibrinolytic treatment for 6 ± 1 days. The control group received only the standard care regimen.
PIS was defined as an increase in the NIHSS score by two or more points from baseline within 6 h to 7 days post-onset of the initial cerebral infarction (17). In diagnosing PIS, patients with alternative causes of neurological deterioration, such as hemorrhagic transformation, recurrent embolism, systemic infections, or hypotension, were excluded. Nevertheless, it is possible that other potential causes of deterioration, such as seizures or metabolic disturbances, were not entirely excluded in this retrospective study.
2.2 Subgroup definitions
Patients were further categorized into thrombolytic and non-thrombolytic subgroups based on their receipt of intravenous thrombolytic agents (e.g., alteplase, urokinase). Additionally, according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification (18), the causes of AIS are subdivided into large artery atherosclerosis, cardioembolic disease, small vessel occlusion, other determined causes, and undetermined causes. The location of the cerebral lesions was classified as either an anterior or a posterior circulation infarction.
2.3 Inclusion and exclusion criteria
Participants were required to meet several criteria to ensure a uniform study population. Each patient was diagnosed with AIS according to the 2018 Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke (19). Additionally, all participants had a concurrent diagnosis of hypertension and diabetes as outlined in the 2022 Chinese Hypertension Clinical Practice Guidelines and 2023 American Diabetes Diagnosis and Treatment Standards. Eligible patients either experienced their first clinical episode or had a history of cerebral infarction without severe sequelae, as indicated by a mRS score of 0–2. Age criteria were set for participants aged from 18 to 80 years. Further requirements included symptom onset within 3 days prior to hospital admission and a completed hospital stay of approximately 6 ± 1 days during which comprehensive clinical data were collected.
Exclusion criteria were as follows. Certain conditions and scenarios excluded potential participants from the study to control confounding variables and focus on the target population. Patients who underwent mechanical thrombectomy after stroke onset were excluded. Patients with severe complications related to hypertension or diabetes, such as malignant hypertension or diabetic ketoacidosis, which could influence study outcomes, were also excluded, as well as patients presenting with large-area cerebral infarction, coma, or brain herniation on admission. Additionally, significant co-morbid conditions, such as severe cardiovascular, hematological, respiratory, or hepatic-renal impairments, were excluded from the study to maintain participant safety and data integrity.
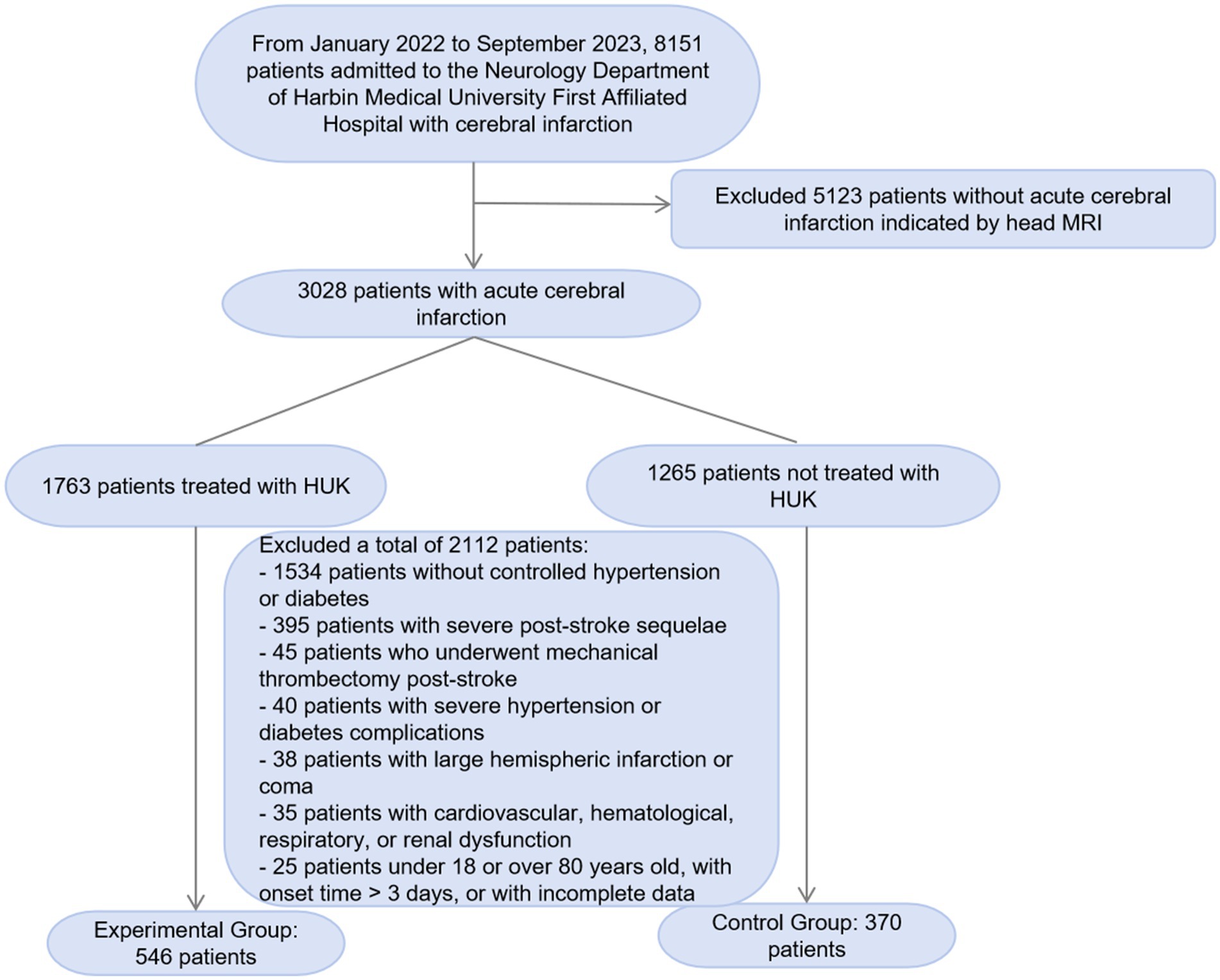
In this study, we screened 8,151 patients with cerebral infarction admitted to the Neurology Department of the First Affiliated Hospital of Harbin Medical University from January 2022 to September 2023. We excluded 5,123 patients whose head MRI did not indicate acute cerebral infarction. Among the remaining 3,028 patients with acute cerebral infarction, we further excluded 2,112 patients who did not meet the study criteria. The exclusion criteria included 1,534 patients without a typical history of hypertension and diabetes, 395 patients with a history of cerebral infarction and severe sequelae, 45 patients who underwent mechanical thrombectomy after the onset of the disease, 40 patients with severe hypertension or diabetes complications, 38 patients with large hemispheric infarction, coma, and cerebral herniation, and 35 patients with severe diseases such as cardiovascular, hematological, respiratory, and renal dysfunction. Additionally, we excluded 25 patients who were outside the age range of 18–80 years, had an onset time exceeding 3 days, or had incomplete data. Ultimately, we included 546 patients in the experimental group and 370 patients in the control group. We have added a flowchart (Figure 1) to the manuscript to illustrate the screening and exclusion process of the participants in detail.
2.4 Collected data and quality control
Personal and clinical data collected included demographics, medical history (e.g., stroke, cerebral hemorrhage, coronary artery disease, and arrhythmias), lifestyle factors (smoking and alcohol consumption), and admission vitals (blood pressure, heart rate, and body temperature). Laboratory data included complete blood count, coagulation profile, fasting glucose level, lipid profile, liver and renal function tests, and other relevant biochemical markers. Imaging assessments included MRI, magnetic resonance angiography, diffusion-weighted imaging, computed tomography angiography, and digital subtraction angiography of head and neck vessels.
The data for this study were sourced from the electronic medical record (EMR) system of the First Hospital of Harbin Medical University, which captures comprehensive clinical information of patients, including demographic data, medical history, laboratory results, imaging findings, and treatment details. To ensure data quality, a multi-step validation process was implemented. Data entry was performed by trained medical staff and verified by a second independent reviewer. Any discrepancies were resolved through consensus discussions. Additionally, a data cleaning process was conducted to identify and correct inconsistencies or missing values. This rigorous approach ensured the accuracy and completeness of the dataset used for analysis.
2.5 Statistical analysis
Continuous variables following a normal distribution were expressed as mean ± standard deviation and analyzed using independent sample t-tests. Categorical variables were presented as frequencies and percentages and analyzed using chi-squared tests. Univariate analysis was conducted to identify potential predictors associated with the occurrence of progressive ischemic stroke (PIS). Factors with a p-value < 0.1 in the univariate analysis were considered to have potential significance and were included in the multivariate binary logistic regression analysis. The multivariate analysis adjusted for potential confounders, including sex, age, body mass index (BMI), history of coronary heart disease, alcohol consumption, stroke location, HUK treatment, and other relevant factors, to determine the independent predictors significantly associated with PIS. The significance threshold was set at p < 0.05. In addition, subgroup analyses were performed to assess the impact of HUK treatment on different patient subgroups. The subgroups were defined based on the following criteria: (1) TOAST classification, including large-artery atherosclerosis, small-artery occlusion, cardioembolic stroke, etc.; (2) thrombolysis treatment, with patients divided into those who received intravenous thrombolysis and those who did not; and (3) infarction location, with patients divided into those with anterior circulation infarction and those with posterior circulation infarction. In the subgroup analyses, both univariate and multivariate analyses were conducted for each subgroup to evaluate the effect of HUK treatment on the incidence of PIS. For each subgroup, odds ratios (OR) and their 95% confidence intervals (CI) were calculated, and logistic regression models were used for significance testing. Significance levels in the tables and figures are indicated by symbols as follows: + for p < 0.1, ∗ for p < 0.05, ∗∗ for p < 0.01, and ∗∗∗ for p < 0.001. Data analysis was conducted using SPSS software version 25.0.
3 Results
This study included 916 patients diagnosed with mild and moderate AIS, of whom 546 received HUK treatment, and the remaining 370 served as the control group and received standard treatment only. Overall, 139 (15.2%) patients developed PIS during the study period. Most participants were male (63.1%), with an average age of 63.50 years, illustrating a typical demographic profile for AIS (Table 1). The average NIHSS score upon patient admission was 3.21 points, with males scoring 3.25 points and females scoring 3.14 points. Among them, there were 875 patients (95.5%) with mild stroke and 41 patients (4.5%) with moderate stroke. Among males, there were 550 patients (95.1%) with mild stroke and 28 patients (4.9%) with moderate stroke. Among females, there were 325 patients (95.8%) with mild stroke and 13 patients (3.8%) with moderate stroke.
3.1 Association of HUK treatment with PIS
Univariate analyses highlighted significant differences in stroke location and outcomes between the PIS and non-PIS groups. Specifically, the PIS group had higher baseline NIHSS scores at admission compared to the non-PIS group (p < 0.05). The rate of HUK treatment was significantly lower in the PIS group than in the non-PIS group (p < 0.001; Supplementary Table 1). Additionally, lipid profile analyses indicated that lipoprotein-associated Lpa levels were significantly elevated in the PIS group (p < 0.05; Supplementary Table 2).
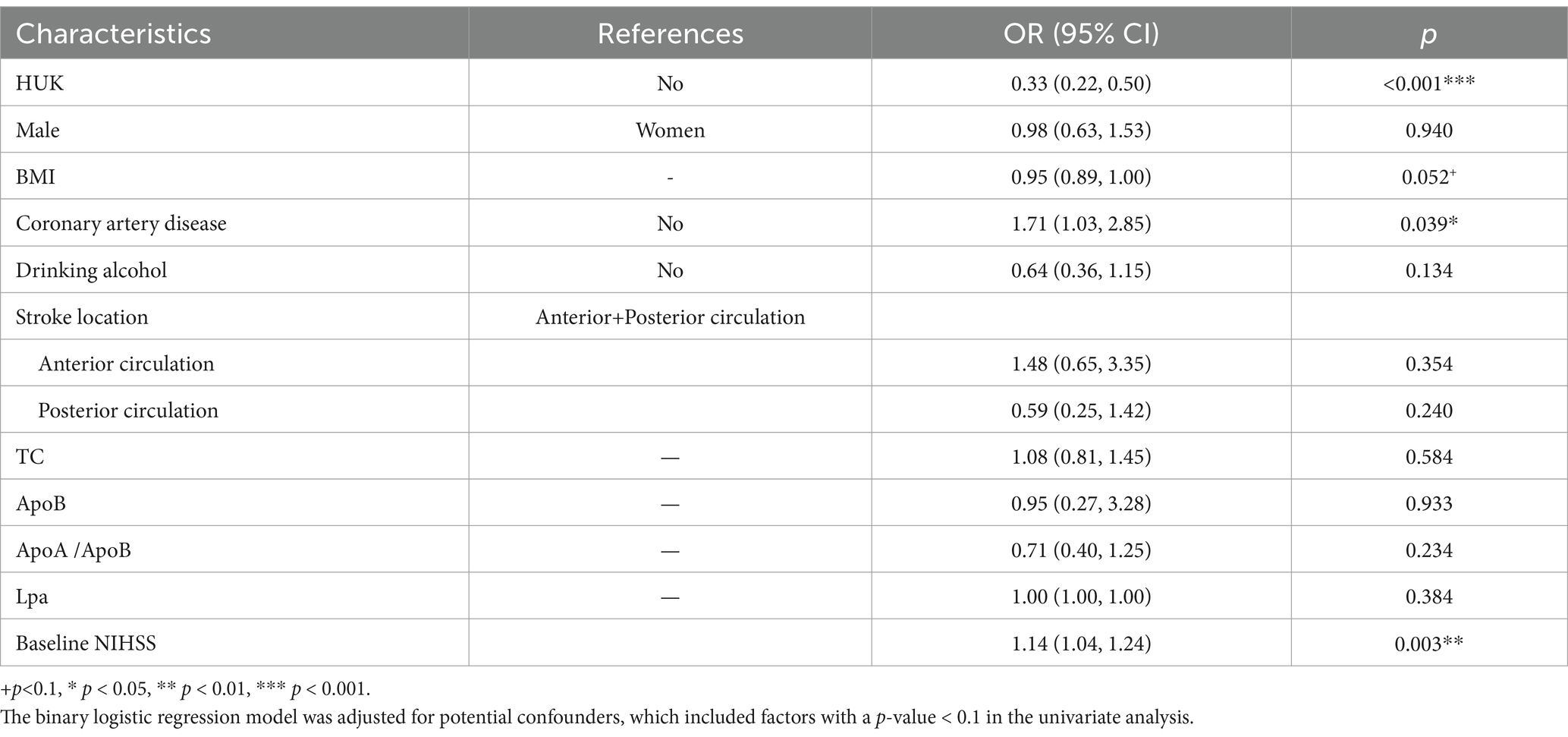
Multivariate adjustment for factors including sex, BMI, coronary heart disease, alcohol consumption, stroke location, HUK treatment, and lipid levels confirmed a robust negative correlation between HUK treatment and the occurrence of PIS, with an odds ratio (OR) of 0.33 (95% confidence interval [CI]: 0.22–0.50, p < 0.001), suggesting that HUK treatment reduced the incidence of PIS by 67%. Additionally, the analysis revealed that coronary heart disease was associated with a 71% increased risk of PIS (OR = 1.71, 95% CI: 1.03–2.85, p = 0.039). Furthermore, each one-unit increase in baseline NIHSS score at admission was associated with a 14% increased risk of PIS (OR = 1.14, 95% CI: 1.04–1.24, p = 0.003) (Table 2).
3.2 Association of HUK treatment with PIS by TOAST classification
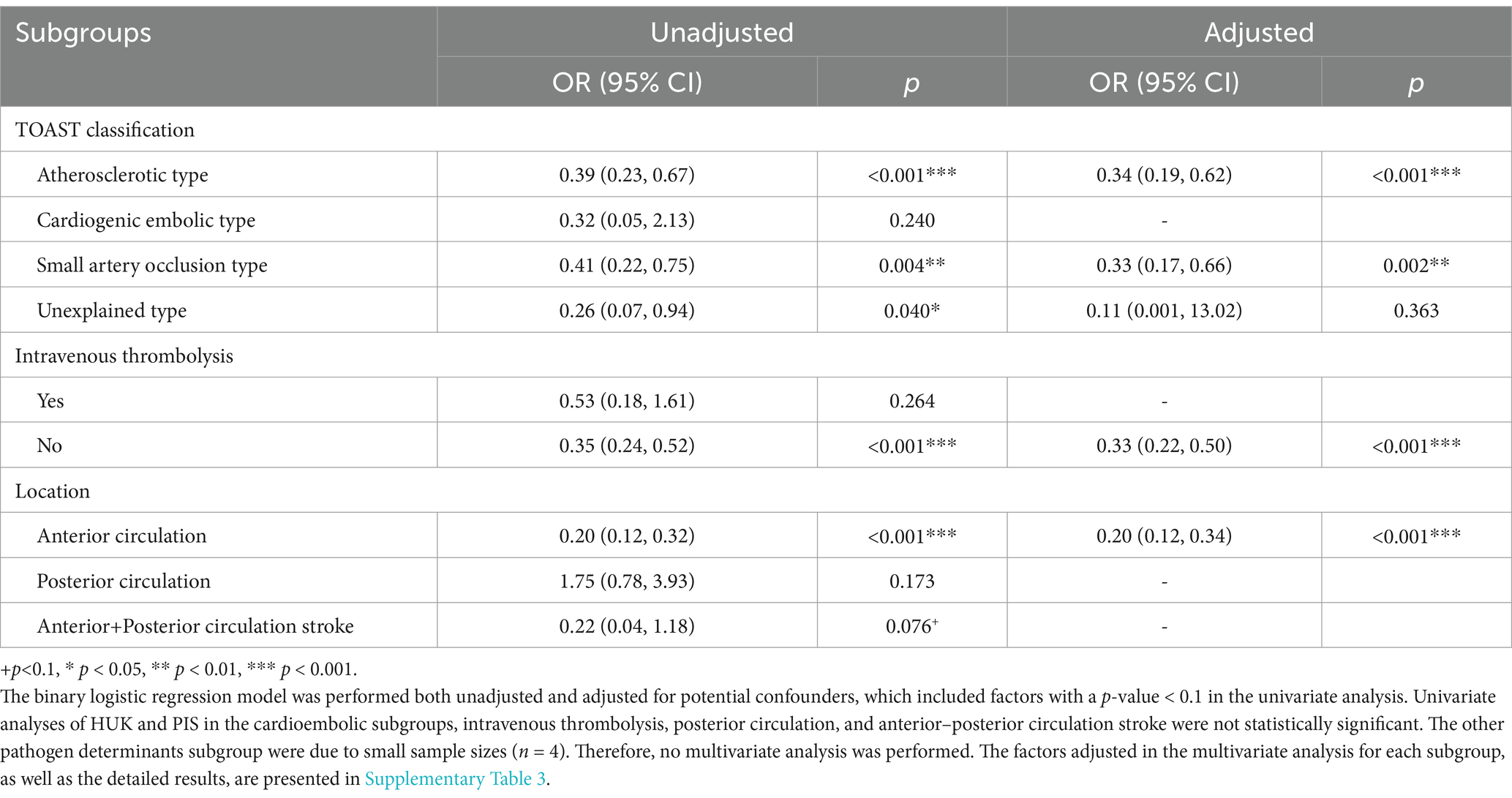
Subgroup analyses revealed variability in stroke outcomes influenced based on the underlying pathologies. Patients with large-artery atherosclerosis who received HUK treatment exhibited a 66% reduction in PIS occurrence (OR = 0.34, 95% CI: 0.19–0.62, p < 0.001). Conversely, in the cardioembolic subgroup, elevated blood sodium levels significantly increased the risk of developing PIS (p < 0.05). For patients with small artery occlusion, significant disparities were observed in CR, and administration of HUK treatment, with patients with PIS showing lower rates of these factors (all p < 0.05; Table 3).
3.3 Association of HUK treatment with PIS by thrombolysis
Differences based on thrombolytic treatment showed that in patients who underwent thrombolysis, smoking prevalence and higher admission body temperatures were more common in the PIS group than in those who did not undergo PIS (p all < 0.05). Multivariate analysis showed that a higher body temperature at admission was associated with an increased risk of developing PIS (p < 0.05). In contrast, among patients who did not receive intravenous thrombolysis, those in the PIS group were less likely to have received HUK treatment than those in the non-PIS group (p < 0.001), and subsequent analyses demonstrated a significant protective effect of HUK against PIS (OR = 0.33, 95% CI: 0.22–0.50, p < 0.001; Table 3).
3.4 Association between HUK treatment and PIS by infarction location
Analysis revealed that patients with anterior circulation stroke who received HUK treatment experienced a substantial reduction in PIS rates (80% reduction, OR = 0.20, 95% CI: 0.12–0.34, p < 0.001). In the posterior circulation subgroup, factors such as the presence of coronary heart disease (OR = 2.61, 95% CI: 1.11–6.17, p = 0.028) and antiplatelet drug treatment (OR = 5.29, 95% CI: 1.46–19.19, p = 0.011) were more prevalent among those who developed PIS, with significant associations found in the multivariate analysis (p < 0.05; Table 3; Supplementary Table 3).
4 Discussion
This study retrospectively examined clinical cases to explore the relationship between HUK and the occurrence of PIS in patients with mild to moderate AIS with hypertension and diabetes. The findings revealed that after adjusting for multiple factors, HUK significantly reduced the risk of PIS in patients with AIS with both hypertension and diabetes. Furthermore, low BMI, elevated blood sodium, and elevated activated partial thromboplastin time were identified as risk factors for increased incidence of PIS in patients with AIS. Subgroup analysis further indicated that HUK notably decreased the incidence of PIS in patients with large-artery atherosclerosis, those with small-artery occlusion, those not receiving intravenous thrombolysis, and those with anterior circulation infarction. Conversely, no statistically significant association was found between HUK and PIS in patients with cardioembolic infarctions, those with other etiologies of infarction, those receiving intravenous thrombolysis, those with posterior circulation infarctions, or those with both anterior and posterior circulation infarctions. The differential effects observed may be partially explained by anatomical and physiological factors. For example, large-artery atherosclerosis and anterior circulation strokes may have better collateral flow and greater vascular responsiveness to HUK’s vasodilatory and angiogenic properties, while posterior circulation and cardioembolic strokes may present less opportunity for perfusion enhancement.
Progressive neurological deterioration is the primary characteristic of PIS (20). Studies indicate that the occurrence of PIS is associated with pathophysiological processes, such as insufficient cerebral blood flow perfusion, thrombosis, and ischemic inflammatory responses (2, 20–22). Events of insufficient cerebral blood perfusion, such as thrombus expansion, poor collateral circulation, and blood pressure decline after AIS, can lead to the enlargement of previously ischemic areas. This results in symptomatic ischemic tissue extending to surrounding asymptomatic ischemic regions (penumbra), thereby causing progressive neurological decline (22–26).
Compared to patients with normal blood pressure, stroke patients with hypertension have smaller salvageable tissue (penumbra) and a larger infarct area, leading to poorer stroke outcomes (27–29). Angiotensin II (Ang II), a key hormone of the renin-angiotensin system (RAS), plays a crucial role in the pathophysiology of hypertension. Ang II increases oxidative stress by activating angiotensin II type 1 receptor, which increases the activity of NADPH oxidase, leading to increased vascular tension and endothelial dysfunction (30–32). During hypertension, increased levels of Ang II result in inward remodeling and increased constriction of cerebral arteries and arterioles, increasing cerebrovascular resistance and exacerbating cerebral blood flow insufficiency during AIS. This further leads to a reduced number of ischemic penumbrae, impaired collateral circulation, and an enlarged infarct area (27, 33, 34).
Hyperglycemia-related superoxides, vasoconstrictive endothelin-1, and matrix metalloproteinases contribute to vascular myogenic responses and remodeling (35). Additionally, diabetes-induced changes such as increased collagen deposition, increased vascular stiffness, and endothelial dysfunction lead to increased ischemic injury-related complications and impaired angiogenesis post-stroke (36, 37).
The relationship between HUK and the reduction in PIS in patients with AIS, particularly in those with hypertension or diabetes, has been reported in previous studies. Several studies have explored the effects of HUK in various subgroups of patients with AIS. Wu et al. investigated the effects of HUK in patients with AIS and grade 3 hypertension and found that the HUK treatment group exhibited significantly lower mRS scores after 3 months than the control group, indicating better recovery outcomes (14). Similarly, Chen et al. studied patients with AIS with abnormal glucose metabolism and reported that HUK significantly reduced the NIH Stroke Scale scores in these patients. However, there was no significant difference in the mRS score reduction between the HUK-treated and control group (15). However, our study uniquely focused on patients with AIS with both hypertension and diabetes comorbidities, a high-risk subgroup that has not been thoroughly studied. In comparison with these studies, our findings also indicate that HUK significantly reduced the incidence of PIS in patients with AIS with both hypertension and diabetes, suggesting that HUK treatment reduces the incidence of PIS by 67%.
Few studies have examined the relationship between the HUK and PIS in patients with different types of AIS. Li et al. conducted a study on the efficacy of HUK in patients with AIS classified according to the TOAST criteria and found that HUK treatment improved the outcomes in patients with large-artery atherosclerosis and small-artery occlusion (38). The RESK study demonstrated that HUK is particularly effective in patients with AIS and anterior circulation infarctions (5). Another study involving 92 patients suggested that a combination of HUK and intravenous thrombolysis enhanced neurological function in patients (39). Our study provides a comprehensive evaluation of the effects of HUK on the incidence of PIS across various AIS subtypes. Specifically, we confirmed the effectiveness of HUK in patients with large-artery atherosclerosis, small-artery occlusion, those who did not receive intravenous thrombolysis, and those with anterior circulation infarctions. Our results indicated that HUK significantly decreased the incidence of PIS in these specific subgroups, highlighting its potential as a targeted therapeutic intervention for these types of AIS.
The mechanisms underlying these results may involve HUK’s ability of HUK to convert kallidinogen to kinins and vasodilators, which selectively dilate arteries in ischemic regions, thereby improving blood perfusion in the penumbra and promoting neurological recovery (40). Additionally, HUK can induce angiogenesis and neurogenesis, inhibit apoptosis in ischemic areas, and promote glucose utilization post-stroke, which may further contribute to its efficacy in reducing PIS incidence (15, 41). The lack of a significant association in some subgroups, such as those with cardioembolic infarctions or those receiving intravenous thrombolysis, may be due to the distinct pathophysiological mechanisms and treatment responses in these conditions.
Another intriguing finding of this study is that elevated blood sodium levels significantly increased the risk of PIS in the cardioembolic subgroup. There are few studies on the association between blood sodium levels and the prognosis of acute ischemic stroke patients, and the specific mechanisms behind this are not yet fully understood. A study on acute ischemic stroke patients undergoing thrombolytic therapy showed that higher blood sodium levels are associated with poor prognosis in acute ischemic stroke patients, which is consistent with our findings (42). Previous studies have clearly demonstrated a significant association between high blood sodium levels and hypertension (43). This suggests that the possible mechanism behind this is that hypertension indirectly promotes the occurrence of poor outcomes in acute ischemic stroke patients with high blood sodium levels through a mediating effect. In addition, elevated blood sodium levels may be associated with underlying comorbidities such as heart failure or renal insufficiency, which are relatively common in patients with cardioembolic stroke (44). These conditions may simultaneously affect electrolyte balance and stroke prognosis, thereby indirectly leading to the observed association. Finally, elevated blood sodium levels may reflect a state of dehydration or electrolyte imbalance, which may exacerbate cerebral ischemia and lead to neurological deterioration. Dehydration can lead to increased blood viscosity and reduced cerebral perfusion, which may exacerbate ischemic injury. However, more studies are still needed in the future to verify the conclusions and pathogenesis of this experiment.
This study also found that low BMI and high APTT are important risk factors for PIS in AIS patients. Numerous previous studies have confirmed that low BMI and abnormal coagulation function are associated with poor prognosis in cardiovascular diseases. A large-scale study showed that being underweight is associated with an increased likelihood of adverse outcomes and in-hospital mortality (45). A meta-analysis including 32 studies also indicated that, in stroke patients, being underweight is associated with increased risks of death, poor functional outcomes, and stroke recurrence (46). This is consistent with our study results, which may be because low BMI is associated with malnutrition, increased stress response, increased frequency of infections, and impaired stroke recovery, as well as exacerbated catabolic activities (47, 48). One study showed that a high international normalized ratio (INR) at admission is independently associated with death or severe disability at discharge in AIS patients (49). Another prospective study also indicated that a high level of PT-INR is associated with an increased risk of all-cause mortality in patients with coronary heart disease (50). This may be because elevated APTT suggests abnormalities in the function or quantity of coagulation factors (such as factors VII, IX, XI, etc.), leading to delayed coagulation processes. In patients with acute ischemic stroke, this delay in coagulation function may weaken the body’s ability to repair vascular damage, disrupt the dynamic balance between microthrombus formation and dissolution, increase the risk of thrombus detachment and new embolus formation, and thereby promote the occurrence of PIS. However, more studies are still needed in the future to explore the specific pathophysiological mechanisms behind this.
This study had several limitations. Firstly, the retrospective cohort design of this study means that the allocation of HUK treatment was non-random. Although we have employed multivariate adjustment methods to minimize the impact of non-random allocation on the results, we acknowledge that this design may have introduced potential selection bias. Additionally, residual confounding from unmeasured variables such as medication adherence, socioeconomic status, or unrecorded clinical characteristics may persist. Moreover, certain etiologies of neurological deterioration, such as post-stroke seizures or metabolic derangements, may not have been fully excluded in our outcome classification. In non-randomized studies, patients who received HUK treatment may differ from those who did not in terms of unmeasured or unrecorded characteristics, which could influence the interpretation of the results. For example, patients who received HUK treatment might have had differences in disease severity, treatment adherence, or comorbidity management compared to the control group. Such selection bias may affect the comparison of PIS incidence rates and thus limit the causal inference of the study results. Future studies should consider a randomized controlled trial (RCT) design to more accurately assess the effects of HUK treatment and reduce the impact of selection bias. Second, the study was conducted at a single center, which may limit the generalizability of the findings to other settings or populations. Patient demographics and clinical practices at this center may differ from those at other institutions, affecting the applicability of the results. Multi-center studies involving diverse populations are necessary to validate these findings across different healthcare environments. Third, the sample sizes for some subgroup analyses were relatively small, which may have affected the robustness and statistical power of the results. The relatively low number of cardioembolic stroke cases in this study may be due to the fact that cardioembolic stroke is primarily associated with cardiac conditions such as atrial fibrillation and myocardial infarction. Patients with these conditions may have already received specialized antithrombotic treatment in cardiology departments, or they may have preferred to seek treatment in other specialized cardiac hospitals at the time of onset. Smaller sample sizes increase the risk of type II errors, where the true effects may not be detected. Future studies should include larger patient cohorts and subgroup analyses to enhance the reliability of our conclusions. Fourth, the observational nature of the study precludes the establishment of a causal relationship between HUK treatment and reduced incidence of PIS. Although the associations observed were compelling, they could not definitively prove causality. Randomized controlled trials are needed to establish causative links and determine the efficacy of HUK treatment. Additionally, potential confounding factors such as variations in treatment protocols, patient adherence, and the presence of comorbid conditions were not fully controlled. These factors could influence outcomes and should be carefully monitored and adjusted for in future studies. Lastly, due to data limitations, key variables such as glycemic and blood pressure control during hospitalization and the timing of HUK initiation were not included in the analysis. These factors may have a significant impact on the occurrence of PIS and the effectiveness of HUK treatment. For example, poor glycemic control and uncontrolled blood pressure could exacerbate ischemic injury and influence the progression of stroke. Similarly, the timing of HUK initiation might affect its therapeutic efficacy. Future studies should collect and analyze these variables to provide a more comprehensive understanding of the factors influencing PIS and HUK treatment outcomes.
5 Conclusion
This study provides robust evidence that HUK significantly reduces the incidence of PIS in patients with AIS with concurrent hypertension and diabetes. Our findings indicate that HUK treatment is particularly effective in patients with large-artery atherosclerosis, small-artery occlusion, those not undergoing intravenous thrombolysis, and those with anterior circulation infarction. This treatment provides a promising strategy for reducing the risk of PIS development in patients with AIS, reducing the incidence of disability in patients with AIS, and improving the likelihood of regaining functional independence after stroke. These findings make it possible for physicians to target patients with AIS with specific risk profiles to optimize stroke management and reduce PIS incidence. Reducing the incidence of PIS in patients with AIS has broader societal benefits, including decreased healthcare costs associated with long-term care and rehabilitation of stroke survivors. Improved recovery rates and reduced disability can reduce the economic burden on healthcare systems and families.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the First Hospital of Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZZhe: Investigation, Writing – original draft. YLi: Investigation, Writing – original draft. SY: Investigation, Writing – original draft, Formal analysis. YX: Investigation, Writing – original draft. LY: Investigation, Writing – original draft. YLiu: Investigation, Writing – original draft. LZ: Investigation, Writing – original draft. ZZha: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the Key Research and Development Plan Project of Heilongjiang Province (2022ZX06C02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1520309/full#supplementary-material
References
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Pan, L, Tang, WD, Wang, K, Fang, QF, Liu, MR, Wu, ZX, et al. Novel Caspase-1 inhibitor CZL80 improves neurological function in mice after progressive ischemic stroke within a long therapeutic time-window. Acta Pharmacol Sin. (2022) 43:2817–27. doi: 10.1038/s41401-022-00913-7
3. Han, L, Li, J, Chen, Y, Zhang, M, Qian, L, Chen, Y, et al. Human urinary Kallidinogenase promotes angiogenesis and cerebral perfusion in experimental stroke. PLoS One. (2015) 10:e0134543. doi: 10.1371/journal.pone.0134543
4. Li, J, Chen, Y, Zhang, X, Zhang, B, Zhang, M, and Xu, Y. Human urinary Kallidinogenase improves outcome of stroke patients by shortening mean transit time of perfusion magnetic resonance imaging. J Stroke Cerebrovasc Dis. (2015) 24:1730–7. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.032
5. Ni, J, Yao, M, Wang, LH, Yu, M, Li, RH, Zhao, LH, et al. Human urinary kallidinogenase in acute ischemic stroke: a single-arm, multicenter, phase IV study (RESK study). CNS Neurosci Ther. (2021) 27:1493–503. doi: 10.1111/cns.13724
6. Han, D, Chen, X, Li, D, Liu, S, Lyu, Y, and Feng, J. Human urinary Kallidinogenase decreases recurrence risk and promotes good recovery. Brain Behav. (2018) 8:e01033. doi: 10.1002/brb3.1033
7. Zhang, C, Tao, W, Liu, M, and Wang, D. Efficacy and safety of human urinary kallidinogenase injection for acute ischemic stroke: a systematic review. J Evid Based Med. (2012) 5:31–9. doi: 10.1111/j.1756-5391.2012.01167.x
8. Chen, SQ, Mao, DY, Wei, DC, and He, WZ. Human urinary kallindinogenase therapy for acute ischemic stroke according to Chinese ischemic stroke subclassification: clinical efficacy and risk factors. Brain Behav. (2020) 10:e01461. doi: 10.1002/brb3.1461
9. Libruder, C, Ram, A, Hershkovitz, Y, Karolinsky, D, Tanne, D, Bornstein, NM, et al. The contribution of potentially modifiable risk factors to acute ischemic stroke burden - comparing young and older adults. Prev Med. (2022) 155:106933. doi: 10.1016/j.ypmed.2021.106933
10. Otite, FO, Liaw, N, Khandelwal, P, Malik, AM, Romano, JG, Rundek, T, et al. Increasing prevalence of vascular risk factors in patients with stroke: a call to action. Neurology. (2017) 89:1985–94. doi: 10.1212/WNL.0000000000004617
11. Lackland, DT, Roccella, EJ, Deutsch, AF, Fornage, M, George, MG, Howard, G, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. (2014) 45:315–53. doi: 10.1161/01.str.0000437068.30550.cf
12. Nam, HS, Kim, YD, Heo, J, Lee, H, Jung, JW, Choi, JK, et al. Intensive vs conventional blood pressure lowering after endovascular thrombectomy in acute ischemic stroke: the OPTIMAL-BP randomized clinical trial. JAMA. (2023) 330:832–42. doi: 10.1001/jama.2023.14590
13. Mistry, EA, Dakay, K, Petersen, NH, Jayaraman, M, McTaggart, R, Furie, K, et al. Pre-endovascular therapy change in blood pressure is associated with outcomes in patients with stroke. J Neurol Neurosurg Psychiatry. (2020) 91:438–9. doi: 10.1136/jnnp-2019-322534
14. Wu, D, Lyu, Y, Zhong, P, Liu, F, and Liu, X. Human urinary kallidinogenase promotes good recovery in ischemic stroke patients with level 3 hypertension. Brain Behav. (2017) 7:e00752. doi: 10.1002/brb3.752
15. Chen, L, Geng, L, Chen, J, Yan, Y, Yang, L, Zhao, J, et al. Effects of urinary Kallidinogenase on NIHSS score, mRS score, and fasting glucose levels in acute ischemic stroke patients with abnormal glucose metabolism: a prospective cohort study. Medicine. (2019) 98:e17008. doi: 10.1097/MD.0000000000017008
16. Bamford, J, Sandercock, P, Dennis, M, Burn, J, and Warlow, C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-o
17. Jung, HJ, Ryu, JC, Joon Kim, B, Kang, DW, Kwon, SU, Kim, JS, et al. Time window for induced hypertension in acute small vessel occlusive stroke with early neurological deterioration. Stroke. (2024) 55:14–21. doi: 10.1161/STROKEAHA.123.044334
18. Adams, HP Jr, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
19. ElSayed, NA, Aleppo, G, Aroda, VR, Bannuru, RR, Brown, FM, Bruemmer, D, et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes-2023. Diabetes Care. (2023) 46:S19–s40. doi: 10.2337/dc23-S002
20. Birschel, P, Ellul, J, and Barer, D. Progressing stroke: towards an internationally agreed definition. Cerebrovasc Dis. (2004) 17:242–52. doi: 10.1159/000076161
21. Weimar, C, Mieck, T, Buchthal, J, Ehrenfeld, CE, Schmid, E, and Diener, HC. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. (2005) 62:393–7. doi: 10.1001/archneur.62.3.393
22. Seners, P, and Baron, JC. Revisiting 'progressive stroke': incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke. J Neurol. (2018) 265:216–25. doi: 10.1007/s00415-017-8490-3
23. Seners, P, Turc, G, Oppenheim, C, and Baron, JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. (2015) 86:87–94. doi: 10.1136/jnnp-2014-308327
24. Tisserand, M, Seners, P, Turc, G, Legrand, L, Labeyrie, MA, Charron, S, et al. Mechanisms of unexplained neurological deterioration after intravenous thrombolysis. Stroke. (2014) 45:3527–34. doi: 10.1161/STROKEAHA.114.006745
25. Alawneh, JA, Moustafa, RR, and Baron, JC. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke. (2009) 40:e443–50. doi: 10.1161/STROKEAHA.108.532465
26. Liebeskind, DS, Kim, D, Starkman, S, Changizi, K, Ohanian, AG, Jahan, R, et al. Collateral failure? Late mechanical thrombectomy after failed intravenous thrombolysis. J Neuroimaging. (2010) 20:78–82. doi: 10.1111/j.1552-6569.2008.00295.x
27. Cipolla, MJ, Liebeskind, DS, and Chan, SL. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. (2018) 38:2129–49. doi: 10.1177/0271678X18800589
28. Letourneur, A, Roussel, S, Toutain, J, Bernaudin, M, and Touzani, O. Impact of genetic and renovascular chronic arterial hypertension on the acute spatiotemporal evolution of the ischemic penumbra: a sequential study with MRI in the rat. J Cereb Blood Flow Metab. (2011) 31:504–13. doi: 10.1038/jcbfm.2010.118
29. McCabe, C, Gallagher, L, Gsell, W, Graham, D, Dominiczak, AF, and Macrae, IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. (2009) 40:3864–8. doi: 10.1161/STROKEAHA.109.559021
30. Rajagopalan, S, Kurz, S, Münzel, T, Tarpey, M, Freeman, BA, Griendling, KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. (1996) 97:1916–23. doi: 10.1172/JCI118623
31. Didion, SP, and Faraci, FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. (2003) 34:2038–42. doi: 10.1161/01.STR.0000081225.46324.AA
32. Griendling, KK, Sorescu, D, and Ushio-Fukai, M. NAD (P) H oxidase: role in cardiovascular biology and disease. Circ Res. (2000) 86:494–501. doi: 10.1161/01.res.86.5.494
33. Mulvany, MJ, Baumbach, GL, Aalkjaer, C, Heagerty, AM, Korsgaard, N, Schiffrin, EL, et al. Vascular remodeling. Hypertension. (1996) 28:505–6.
34. Baumbach, GL, and Heistad, DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. (1989) 13:968–72. doi: 10.1161/01.HYP.13.6.968
35. Guo, Y, Wang, S, Liu, Y, Fan, L, Booz, GW, Roman, RJ, et al. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience. (2020) 42:547–61. doi: 10.1007/s11357-020-00179-z
36. Wei, LM, Zhu, YQ, Bao, YQ, Lu, HT, Zhang, PL, Zhao, YW, et al. Atherosclerosis in intracranial or extracranial vessels in diabetic patients and the association with stroke subtype. Quant Imaging Med Surg. (2019) 9:960–7. doi: 10.21037/qims.2019.04.17
37. Bradley, SA, Spring, KJ, Beran, RG, Chatzis, D, Killingsworth, MC, and Bhaskar, SMM. Role of diabetes in stroke: recent advances in pathophysiology and clinical management. Diabetes Metab Res Rev. (2022) 38:e3495. doi: 10.1002/dmrr.3495
38. Li, C, Zhao, GF, He, QY, Wu, YZ, Wang, TS, and Teng, JF. Study on the clinical efficacy of human urinary kalllikrein in the treatment of acute cerebral infarction according to TOAST classification. Pak J Pharm Sci. (2015) 28:1505–10.
39. Li, X, Zhang, X, Yang, Y, Wang, H, and Zhang, J. Efficacy of urinary Kallidinogenase plus intravenous recombinant tissue plasminogen activator for stroke patients with extended window: a retrospective analysis. Neurologist. (2023) 28:373–8. doi: 10.1097/NRL.0000000000000499
40. Madeddu, P, Emanueli, C, and El-Dahr, S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol. (2007) 3:208–21. doi: 10.1038/ncpneph0444
41. Ling, L, Hou, Q, Xing, S, Yu, J, Pei, Z, and Zeng, J. Exogenous kallikrein enhances neurogenesis and angiogenesis in the subventricular zone and the peri-infarction region and improves neurological function after focal cortical infarction in hypertensive rats. Brain Res. (2008) 1206:89–97. doi: 10.1016/j.brainres.2008.01.099
42. Pan, S, Yu, B, Chen, Y, Gao, Y, Xie, W, Jin, Y, et al. Electrolyte levels in poor prognosis and early neurological deterioration in patients with acute ischemic stroke. J Clin Hypertens. (2025) 27:e70037. doi: 10.1111/jch.70037
43. Hu, H, Eguchi, M, Miki, T, Kochi, T, Kabe, I, Nanri, A, et al. Serum sodium and risk of hypertension: a cohort study. Hypertens Res. (2022) 45:354–9. doi: 10.1038/s41440-021-00797-w
44. Rabinowitz, J, Darawshi, M, Burak, N, Boehm, M, and Dmitrieva, NI. Risk for hypertension and heart failure linked to high normal serum sodium and tonicity in general healthcare electronic medical records. Eur J Prev Cardiol. (2025) 11:zwaf232. doi: 10.1093/eurjpc/zwaf232
45. Miwa, K, Nakai, M, Yoshimura, S, Sasahara, Y, Wada, S, Koge, J, et al. Clinical impact of body mass index on outcomes of ischemic and hemorrhagic strokes. Int J Stroke. (2024) 19:907–15. doi: 10.1177/17474930241249370
46. Qin, J, Zhang, T, Chen, Y, Wei, X, Yang, Y, Yuan, Y, et al. The effect of body mass index on stroke prognosis: a systematic review and meta-analysis of 32 cohort studies with 330, 353 patients. Int J Stroke. (2024) 19:1093–101. doi: 10.1177/17474930241255031
47. Dávalos, A, Ricart, W, Gonzalez-Huix, F, Soler, S, Marrugat, J, Molins, A, et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke. (1996) 27:1028–32. doi: 10.1161/01.STR.27.6.1028
48. Jönsson, AC, Lindgren, I, Norrving, B, and Lindgren, A. Weight loss after stroke: a population-based study from the Lund stroke register. Stroke. (2008) 39:918–23. doi: 10.1161/STROKEAHA.107.497602
49. You, S, Han, Q, Dong, X, Zhong, C, Du, H, Sun, Y, et al. Prognostic significance of international normalised ratio and prothrombin time in Chinese acute ischaemic stroke patients. Postgrad Med J. (2023) 99:333–9. doi: 10.1136/postgradmedj-2021-141204
50. Liu, L, Ying, M, Chen, S, Li, Q, Chen, G, Li, H, et al. The association between prothrombin time-international normalized ratio and long-term mortality in patients with coronary artery disease: a large cohort retrospective study with 44,662 patients. BMC Cardiovasc Disord. (2022) 22:297. doi: 10.1186/s12872-022-02619-4
Keywords: acute ischemic stroke, progressive ischemic stroke, human urinary kallikrein, stroke management, vascular pathology
Citation: Zheng Z, Li Y, Yang S, Xu Y, Yi L, Liu Y, Zhang L and Zhang Z (2025) Role of human urinary kallikrein in reducing progressive ischemic stroke among acute ischemic stroke patients with concurrent hypertension and diabetes: a hospital-based retrospective cohort study. Front. Neurol. 16:1520309. doi: 10.3389/fneur.2025.1520309
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Ozge Altintas Kadirhan, Kırklareli University, TürkiyeDingkang Xu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Xiaoyan Lan, Affiliated Central Hospital of Dalian University of Technology, China
Copyright © 2025 Zheng, Li, Yang, Xu, Yi, Liu, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongling Zhang, emhhbmd6aG9uZ2xpbmc3QDEyNi5jb20=
†These authors have contributed equally to this work
 Zeyang Zheng†
Zeyang Zheng† Shanshan Yang
Shanshan Yang Zhongling Zhang
Zhongling Zhang