- 1IMMUNOe Research Center, Centennial, CO, United States
- 2Department of Neurology and Neurological Sciences, Stanford University, Stanford, CA, United States
Background: Many patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection experience neurologic changes post-infection, which has been hypothesized to be due to dysregulation in the infectious-immune axis that leads to a neuro-immune response. This immune dysfunction has been termed “Alzheimer’s of the Immune System” or AIS and there are several immune factors that may play a key role. These include, among others, complement activation due to low levels of C1-esterase inhibitor (C1-INH) and function, and a decrease in signaling of Toll-like receptor (TLR)-3. We propose that C1-INH replacement may upregulate the immune dysfunction, thereby improving neurological symptoms.
Methods: In this randomized, double-blind, placebo-controlled, crossover, proof-of-concept study, adults experiencing SARS-CoV-2 post-viral fatigue syndrome for >4 weeks post-recovery from coronavirus disease 2019 (COVID-19) infection were randomized 1:1 to two arms: Arm 1 (C1-INH for 8 weeks, then placebo for 8 weeks) or to Arm 2 (placebo for 8 weeks, then C1-INH for 8 weeks). Patients were assessed for adult executive function, abnormal cognitive decline, depression [Beck Depression Inventory-II (BDI-II)], migraine, fatigue [Fatigue Severity Scale (FSS)] and pain (Short-form McGill Pain Questionnaire). Percent change in TLR signaling in response to zymosan was compared with controls at baseline, Week 8 and Week 16. Safety was assessed throughout.
Results: At this interim analysis, 36 patients with SARS-CoV-2 post-viral fatigue syndrome had completed the two 8-week treatment periods. In Arm 1, trends toward improvements from baseline at Week 8 of C1-INH therapy were observed in BDI-II score (−8.7 points), mean FSS score (0.6 points), and mean McGill Pain Questionnaire score (−0.4 points). These improvements were either sustained or worsened at Week 16, following crossover to placebo. The outcomes in Arm 2 were compatible with those in Arm 1. Patients with SARS-CoV-2 post-viral fatigue syndrome had low levels of TLR-related signaling biomarkers compared with healthy controls.
Conclusion: This proof-of-concept study demonstrates sustained dysregulation of the immune system after COVID-19 infection. Improvements in depression, fatigue, and pain were observed with C1-INH treatment in patients with SARS-CoV-2 post-viral fatigue syndrome, indicating C1-INH may be a potential therapeutic target.
Clinical trial registration: https://clinicaltrials.gov/study/NCT04705831, NCT04705831.
1 Introduction
Findings suggest that neurological symptoms (memory issues, cognitive changes, tremors, etc.) after an infection may be related to a form of post-infectious autoimmunity. Various disorders are associated with neurological and cognitive changes, which may occur post-infection, including chronic fatigue syndrome (1), pediatric acute-onset neuropsychiatric syndrome (PANS) (2), Lyme disease (3, 4), and autism (5). Notably, approximately 30–80% of patients with persistent coronavirus disease 2019 (COVID-19) symptoms (long COVID) develop fatigue and cognitive deficits lasting 1–6 months, including reduced executive functions, memory, processing speed, and attention (6–9).
The key mechanism for brain autoimmunity may be dysfunctional neuroimmune responses to various infectious pathogens. In 2016, we coined the term “Alzheimer’s of the Immune System” (AIS) to identify this syndrome (10). In certain patients, a memory defect of the immune system may result in failure to recognize infectious pathogens that cause the neurological diseases (10). This memory defect may create a neurological storm that likely includes various factors, including low levels of C1-esterase inhibitor (C1-INH) resulting in complement activation, reduction in Toll-like receptor (TLR)-3 signaling, and low response to T-cell antigens (10, 11).
The complement system may be crucial in AIS. As well as directly interacting with pathogens, the complement system forms a bridge between the innate and adaptive immune responses (12–15). For the adaptive immune response, complement components are involved in the regulation of T-cell and B-cell activation (14). For the innate immune response, complement engages in signaling crosstalk with TLRs to coordinate immune and inflammatory responses. Complement component C1 triggers the classical pathway for complement activation; as such, C1-INH plays an important role as a check against uncontrolled complement activation (13, 16). We hypothesize that the dysregulation of the complement and TLR signaling pathways may lead to a dampening of the response to infection and, therefore, persistent post-infectious neuroinflammation.
Dysregulation of the complement system has been linked with various neurodevelopmental disorders, including schizophrenia, autism spectrum disorder, anxiety and mood disorders (17). Several non-clinical studies suggest that targeting this system through treatment with C1-INH can improve neurological function, potentially through anti-inflammatory effects (18–21). In order to further understand the immune mechanisms that lead to post-infectious neuroinflammation, we report an ongoing study investigating post-viral fatigue in patients experiencing long COVID. We investigated whether recombinant human C1-INH (RUCONEST®, Pharming Group N. V.) therapy may upregulate the immune dysfunction and therefore improve neurological symptoms, compared with placebo.
2 Materials and methods
2.1 Study design and oversight
This ongoing Phase 1, randomized, double-blind, placebo-controlled, crossover, proof-of-concept study (ClinicalTrials.gov number, NCT04705831) comprises a 2-week screening period, 8-week initial treatment period, and 8-week crossover treatment period. Study visits occurred during screening (Weeks −2 or −1; when baseline assessments were conducted) and once per week in both treatment periods.
Patients were randomized 1:1 to two arms. C1-INH or placebo were administered once a week, from the first day of Week 0. In Arm 1, patients were treated initially with C1-INH (last dose at Week 7) followed by crossover to placebo (last dose at Week 15), and in Arm 2 with placebo (last dose at Week 7) followed by crossover to C1-INH (last dose at Week 15). Each dose of C1-INH (4,200 U once a week) and placebo was administered intravenously for approximately 5 min.
Randomization was conducted by pharmacy staff using a 10-block method. Pharmacy staff were not blinded and maintained drug accountability records. All other study staff were blinded, including investigators. Should an adverse drug reaction or serious adverse event (SAE) occur, investigators could request unblinding. In the event of unblinding, study participation would cease.
C1-INH (RUCONEST®, Pharming Group N. V.) was supplied in single-use 25 mL glass vials, each containing 2,100 U C1-INH lyophilized powder for reconstitution in 14 mL of sterile water. The reconstituted solution contained 150 IU/mL C1-INH and was clear and colorless. Placebo was sterile saline, administered at the same volume as the study medication, using the same pumps and infusion rates.
A local ethics committee provided unconditional written approval for the study. The study was conducted according to local regulatory requirements and International Conference for Harmonisation Good Clinical Practice guidelines.
2.2 Patients
Patient eligibility criteria are shown in Supplementary Table 1. In brief, eligible patients were adults ≥18 years of age experiencing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) post-viral fatigue syndrome for more than 4 weeks after recovering from COVID-19 infection, documented by polymerase chain reaction (PCR) or spike antibody testing, who provided written informed consent before any study procedures were conducted. There were no cases of severe COVID or hospitalizations in the patient population studied.
2.3 Endpoints
Neuropsychological outcomes were assessed using the following scales: Beck Depression Inventory-II (BDI-II), Behavior Rating Inventory of Executive Function-Adult (BRIEF-A), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), and Montreal Cognitive Assessment (MoCA). BDI-II scores indicate no depression (0–9) or depression that is mild–moderate (10–18), moderate–severe (19–29), and severe (30–63) (22). BRIEF-A captures executive functions across two domains [Behavioral Regulation Index (BRI) and Metacognition Index (MI)], resulting in the Global Executive Composite (GEC) score (23). Lower values represent less impairment. RBANS captures cognitive function (immediate memory, visuospatial/constructional, language, attention, and delayed memory), with scores ≥70, 55–69, and <54 indicating mild, moderate, and severe impairment, respectively (24). MoCA also assesses cognitive function, with a normal score considered to be ≥27.4 (25).
Patient-reported pain, fatigue, and migraine outcomes were assessed using the following questionnaires: Short-form (SF) McGill Pain Questionnaire, Fatigue Severity Scale (FSS), Migraine Disability Assessment (MIDAS), and six-item Headache Impact Scale (HIT-6). The SF McGill Pain scoring scale ranges from 0 (no pain) to 10 (worst pain imaginable) (26). FSS scoring ranges from 1 to 7, with higher scores indicating worse fatigue (27). MIDAS measures both the number of days in the last 3 months that the patient had a headache and uses a scoring scale for pain, ranging from 0 (no pain) to 10 (pain as bad as it can be) (28). HIT-6 (score range, 36–78) was designed as an instrument to measure the impact headaches have on the ability to function at work, in school, and in social situations, with reductions showing improvement (29).
TLR activity was evaluated by measuring three inflammatory markers [tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, and IL-6] in patients before treatment and comparing these to healthy controls. Blood (10 mL) was collected and analyzed by ARUP laboratories. TLR signaling was tested independently by stimulation with TLR6-TLR2 ligand using zymosan cell wall particles from Saccharomyces cerevisiae in a peripheral blood mononuclear cell (PBMC) culture. PBMC production of TNFα, IL-1β, and IL-6 was determined by multiplex bead assay.
Efficacy and safety outcomes were assessed in the initial treatment period (up to Week 8) and crossover treatment period (up to Week 16).
2.4 Statistical analysis
This ongoing, exploratory, proof-of-concept study used descriptive statistics. No sample size calculation was conducted. Analysis was performed for participants with available data. For responder analyses, patients with any improvement from baseline were classified as responders and patients with missing values were classified as non-responders. Statistical analyses were performed in GraphPad Prism (10.2.3). Shapiro–Wilk normality tests were performed prior to the Mann–Whitney U Test for TLR activity between patients and healthy controls, and the Wilcoxon matched-pairs signed rank test for depression, fatigue, and pain scores (between baseline and Week 8, and baseline and Week 16). p < 0.05 were considered statistically significant.
3 Results
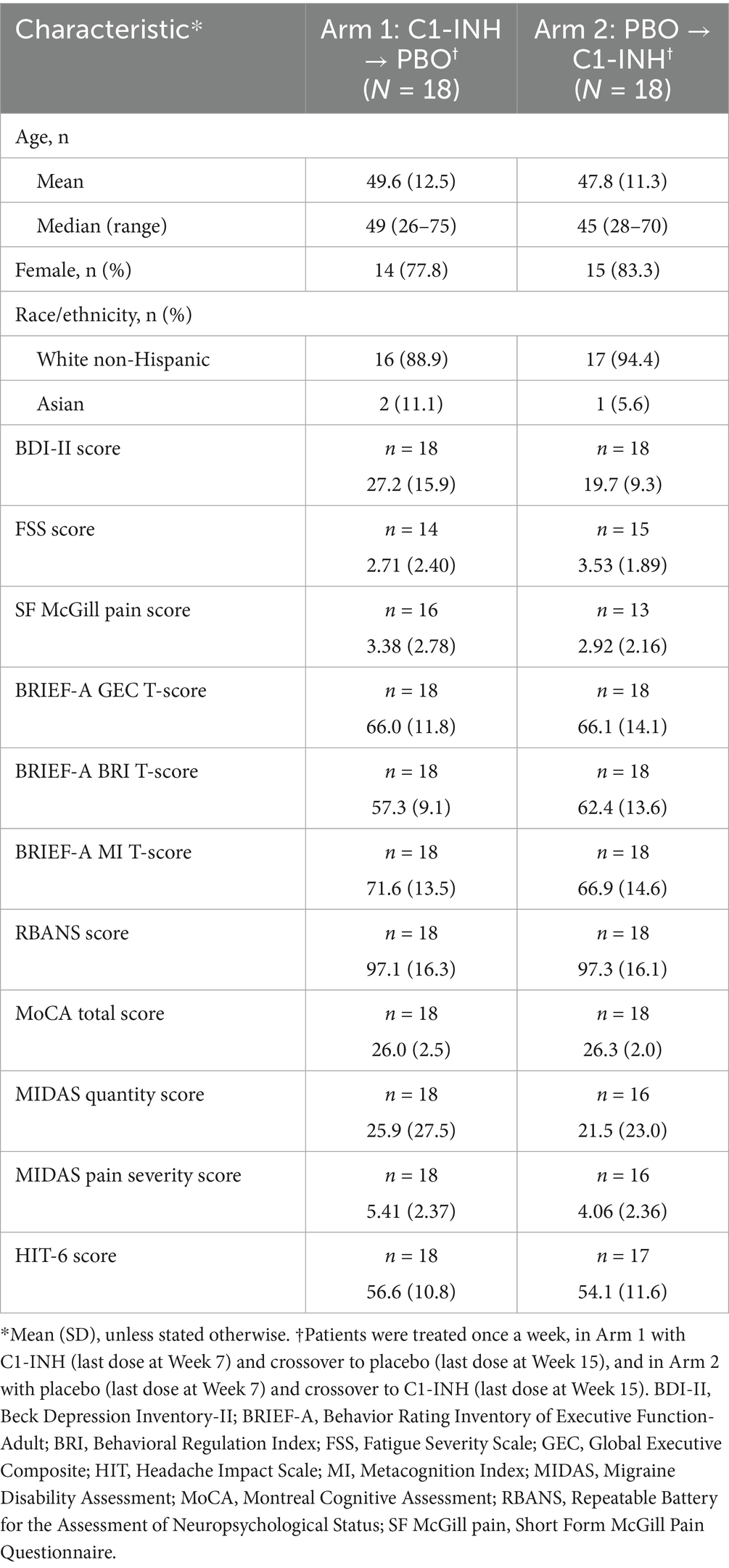
3.1 Baseline characteristics and patient disposition
This ongoing study commenced in December 2020, during which time the predominant strain of SARS-CoV-2 was the Alpha variant (B.1.1.7). The time from infection to enrollment was between 4 weeks and 3 months for all patients and was not related to the acute phase of COVID infection. Overall, 36 participants with SARS-CoV-2 post-viral fatigue syndrome were randomized 1:1 to Arms 1 and 2 (Figure 1). All 36 patients completed initial 8-week and crossover 8-week treatment periods.
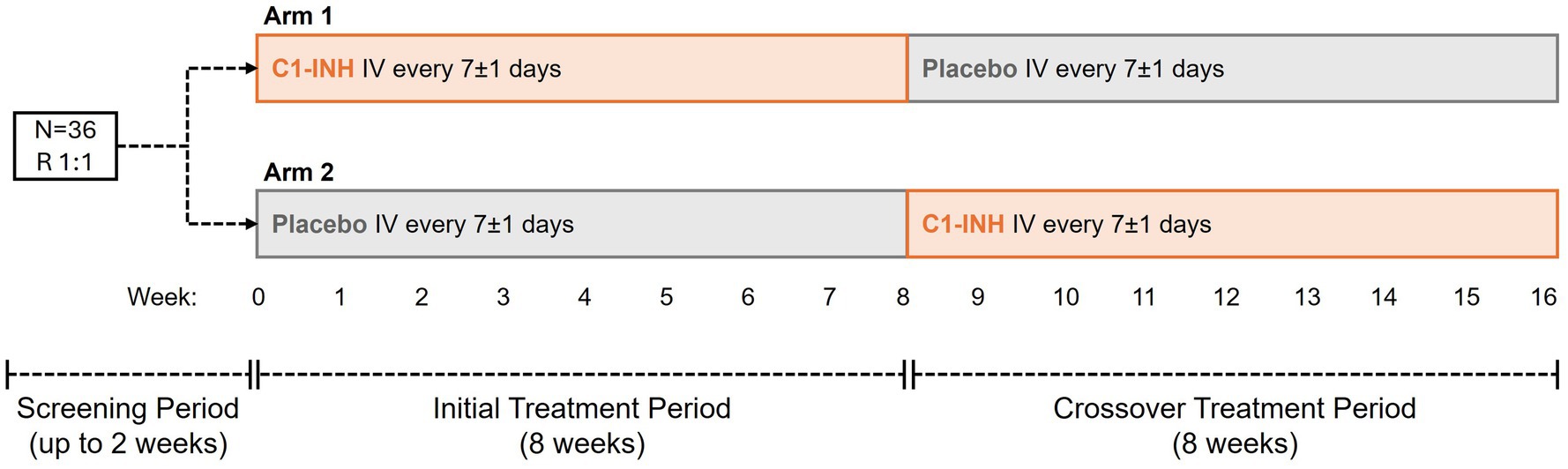
Figure 1. Study design. Thirty-six patients with SARS-CoV-2 post-viral fatigue syndrome were randomized 1:1 to Arms 1 and 2. In Arm 1, patients received C1-INH from Week 0, crossing over to placebo from Week 8. In Arm 2, patients received placebo from Week 0, crossing over to C1-INH from Week 8. C1-INH was dosed at 4,200 U once a week. C1-INH, C1 esterase inhibitor; IV, intravenous; R, randomized.
At baseline, patient demographics were comparable between the treatment arms (Table 1). Nineteen patients were vaccinated against SARS-CoV-2 at study enrollment. Preexisting conditions included attention deficit hyperactivity disorder (ADHD; n = 7), post-traumatic stress disorder (n = 1), and documented insomnia (n = 3). At enrollment, 12 patients began taking antidepressive agents and 15 began taking anti-anxiety medication post-COVID-19 infection. Four patients had been taking sleep medication prior to infection, and two were treated for atypical seizure after infection. Mean age was 48.7 years (standard deviation 11.9 years). Most patients were female (80.5%) and White non-Hispanic (91.7%).
Neuropsychological measures were comparable in Arms 1 and 2 at baseline, with some imbalances. In Arms 1 and 2, mean BDI-II scores were 27.2 and 19.7 (based on a 0–63 point scale), mean FSS scores were 2.7 and 3.5 (based on a 1–7 point scale), and SF McGill Pain scores were 3.4 and 2.9 (based on a 0–10-point scale), respectively (Table 1). Both mean BDI-II scores indicated moderate–severe depression.
3.2 Immunological biomarkers
When assessed at baseline, patients with SARS-CoV-2 post-viral fatigue syndrome (n = 36) had significantly lower mean levels of TLR-related signaling biomarkers, compared with healthy controls (n = 36) (Figure 2). Patients with SARS-CoV-2 had a 37.7% reduction in TNF-α signaling (p = 0.0002), 75.0% reduction in IL-1β signaling (p < 0.0001), and 70.0% reduction in IL-6 signaling (p < 0.0001) as compared with healthy controls.
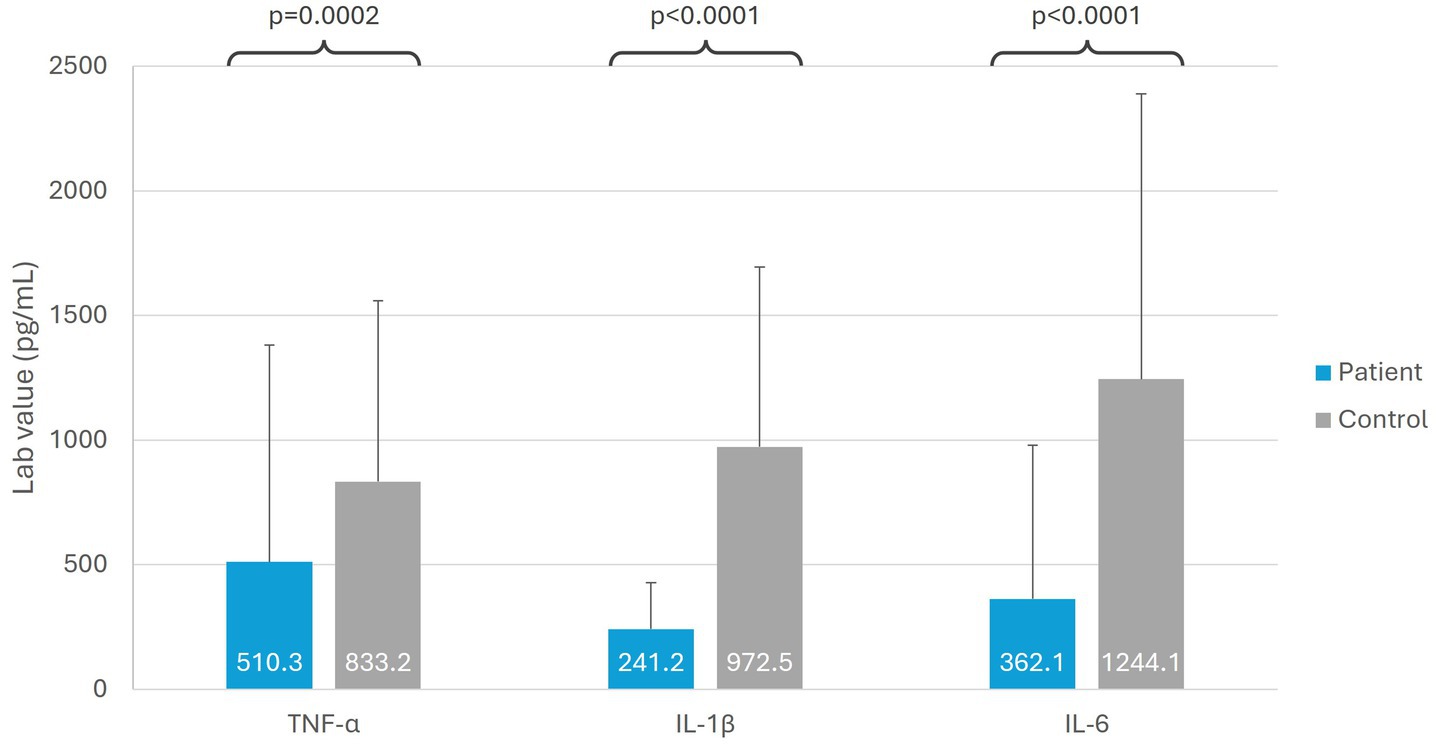
Figure 2. Immunological biomarkers in patients with SARS-CoV-2 post-viral fatigue syndrome versus healthy controls. IL, interleukin; TNF-α, tumor necrosis factor alpha.
3.3 Efficacy outcomes
3.3.1 Cognitive changes
Trends toward improvements were observed in depressed patients based on BDI-II score during treatment with C1-INH (Figure 3A). In Arm 1, mean BDI-II score improved at Week 8 during treatment with C1-INH [decreasing by 32.0% (8.7 points) from baseline; p = 0.0010] and was maintained after crossover to placebo (p = 0.0003). In Arm 2, mean BDI-II score improved slightly at Week 8 with placebo [decreasing by 22.8% (4.5 points) from baseline; p = 0.0132] and, notably, improved further at Week 16 during treatment with C1-INH [by 19.1% (7.4 points) from baseline; p = 0.0036].
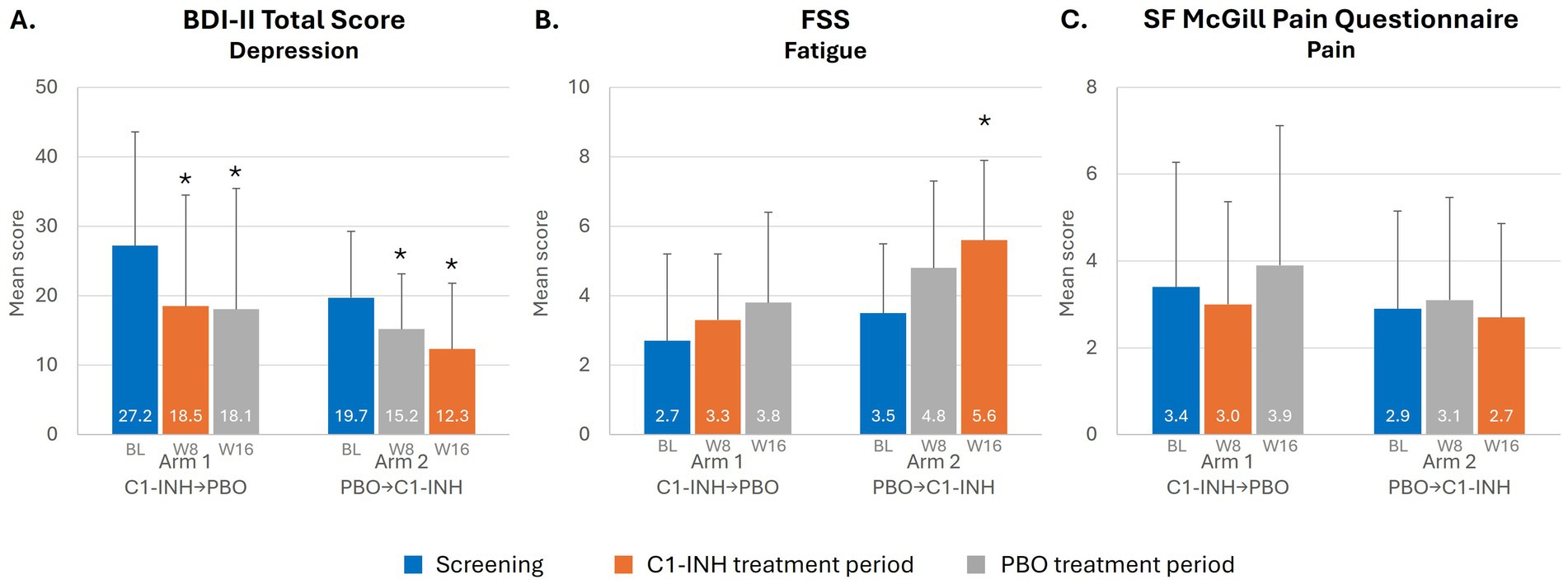
Figure 3. Depression, pain and fatigue in patients with SARS-CoV-2 post-viral fatigue syndrome. *Indicates p < 0.05. BL, baseline (value during the screening period); BDI-II, Beck Depression Inventory-II; C1-INH, C1-esterase inhibitor; FSS, Fatigue Severity Scale; PBO, placebo; SF, short-form; W, week.
No notable improvements with C1-INH treatment were observed in other rating scales, including in executive function (BRIEF-A) and cognitive function (RBANS and MoCA) (Supplementary Table 2). For Arm 1 starting on C1-INH treatment, the mean percent change in RBANS showed an overall improvement in cognition with an increase of 1.9% and a reduction of 4.6% after moving to placebo. In Arm 2, mean RBANS score increased by 2.6% while on placebo followed by a slight decrease of 0.4% while on C1-INH.
In an analysis of RBANS score by patient, seven patients in Arm 1 and three patients in Arm 2 were observed to have improved RBANS score after C1-INH treatment (Figure 4). In general, patients with no other underlying neurological symptoms before SARS-CoV-2 infection had better RBANS responses, and those with conditions such as ADHD and depression had a worse response.
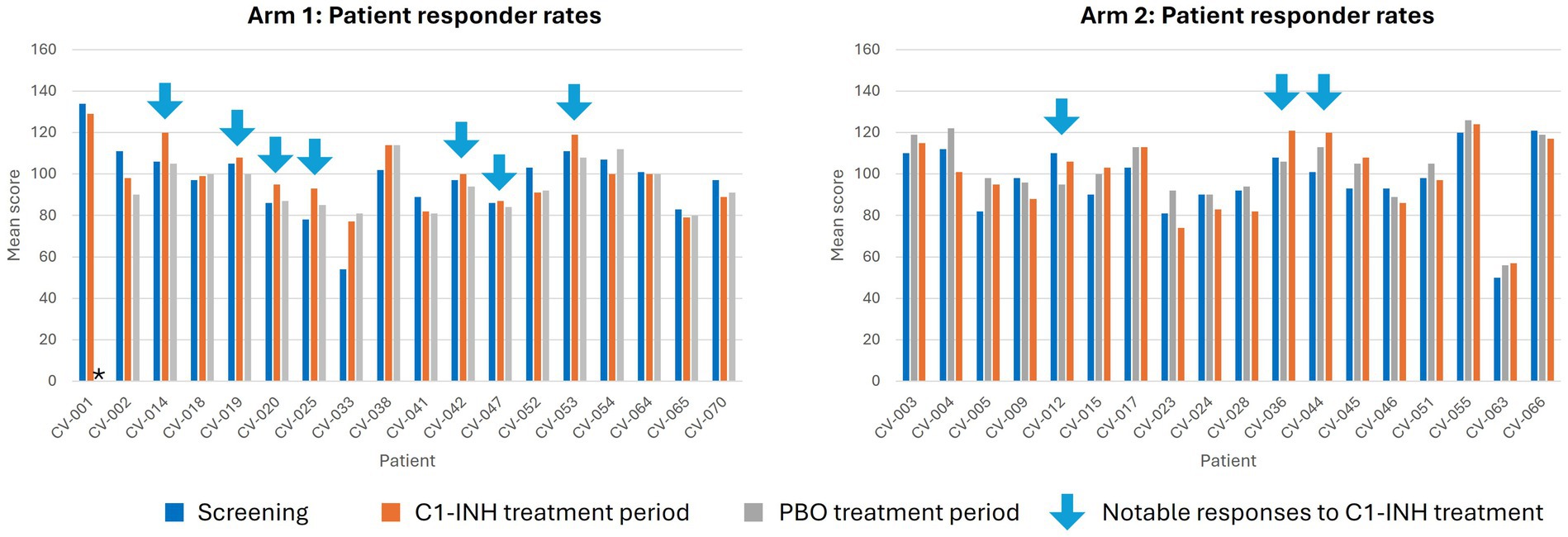
Figure 4. Responder analysis for RBANS score at baseline, Week 8, and Week 16. *Indicates measure not reported. C1-INH, C1-esterase inhibitor; CV, clinical volunteer; PBO, placebo.
3.3.2 Fatigue, migraine, and headache
Mean FSS score improved at Week 8 during treatment with C1-INH (increasing by 0.6 points from baseline; p = 0.5337) in Arm 1, with continued improvement at Week 16 following crossover to placebo (increased by 1.1 points from baseline; p = 0.5137) (Figure 3B). In Arm 2, a similar trend was observed, with FSS score increasing by 1.3 points from baseline at Week 8 with placebo (p = 0.0664), and then further improvement after crossover to C1-INH treatment, increasing by 2.1 points from baseline at Week 16 (p = 0.0078).
Outcomes assessing headache (HIT-6) or migraine (MIDAS) did not observe any notable improvements with C1-INH treatment in Arm 1 or Arm 2 (Supplementary Table 2).
3.3.3 Pain
Mean SF McGill Pain score in Arm 1 improved at Week 8 during treatment with C1-INH (decreasing by 0.4 points from baseline; p = 0.6270) and then worsened at Week 16 following crossover to placebo (increasing by 0.5 points from baseline; p = 0.2031) (Figure 3C). In Arm 2, SF McGill Pain score increased slightly at Week 8 of placebo (by 0.2 points from baseline; p = 0.3750) and decreased slightly at Week 16 after crossover to C1-INH (by 0.2 points from baseline; p = 0.1719).
3.4 Safety
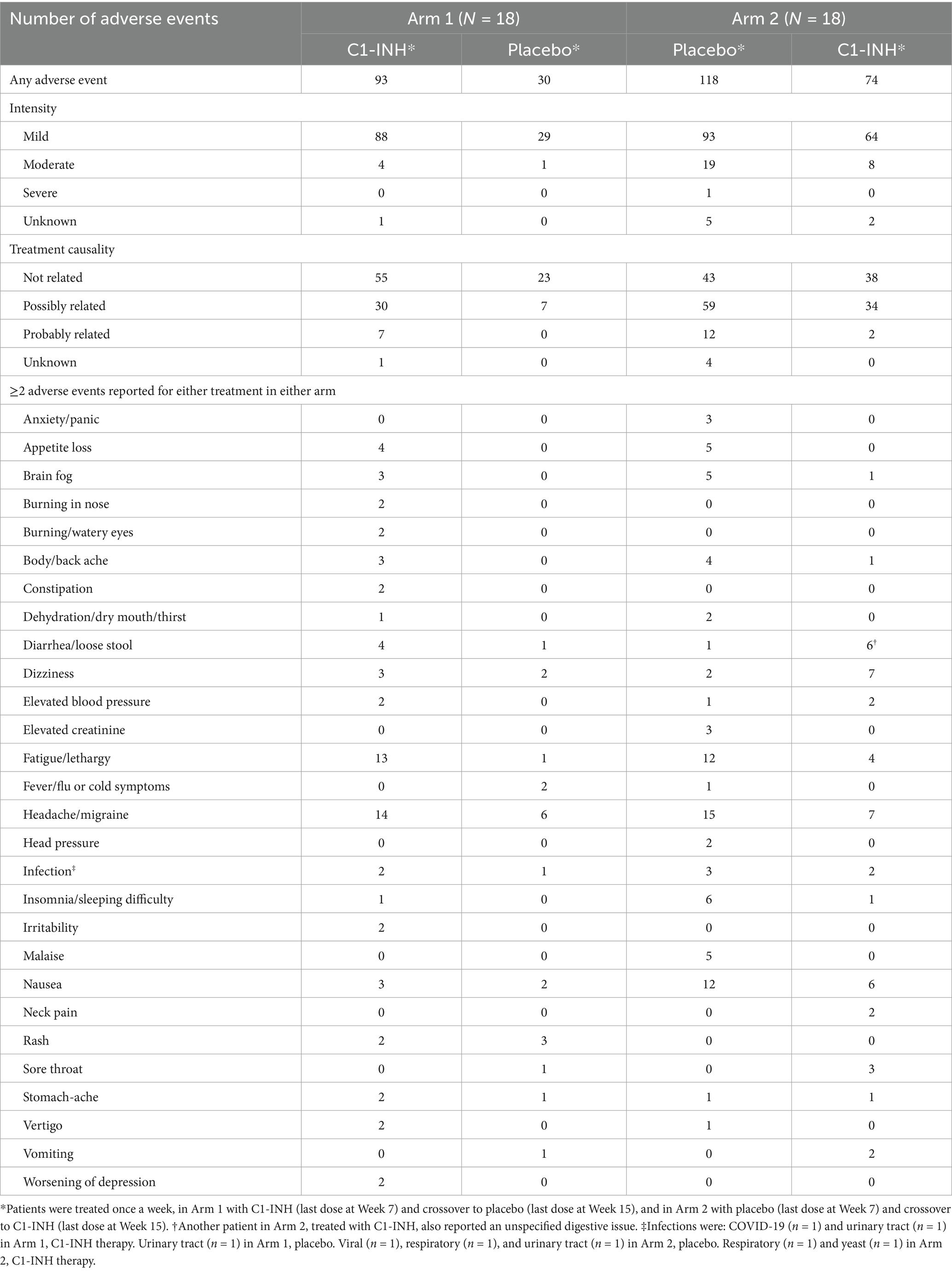
No new safety signals were identified (Table 2). No SAEs were observed. Most adverse events were mild in intensity in each treatment group. One SAE (fatigue) was observed in the Arm 2 placebo group.
4 Discussion
In this ongoing proof-of-concept study, we observed trends toward improvement in depression, fatigue, and pain during 8 weeks of C1-INH therapy in patients with SARS-CoV-2 post-viral fatigue syndrome. Furthermore, we demonstrated reduced TLR signaling components upon stimulation in patients with SARS-CoV-2 post-viral fatigue syndrome, in comparison with healthy controls. Although not statistically significant, we found that patients with no underlying neurological symptoms such as ADHD or depression did not respond as well as those with no underlying conditions.
In this study, a dysfunction in TLR signaling response (TNFα, IL-1β, and IL-6) was demonstrated in patients with SARS-CoV-2 post-viral fatigue syndrome, suggesting a possible dysregulation of innate immunity in these patients. Dysregulation of the innate immune system has been reported in patients with SARS-CoV-2 post-viral fatigue syndrome in several other studies (30–32) and are compatible with our previous reports of reduced TLR-3 expression following other infections (10). Innate immune cells have been shown to play a key role in neuropathic pain, being the first line of immunosurveillance and activation of neurogenic inflammation (33). Inflammatory processes, involving TLR-related molecules, have also previously been implicated in their pathogenesis of depression and fatigue (34–36). The dysfunction observed in TLR signaling in patients with SARS-CoV-2 post-viral fatigue syndrome may therefore play a role in the neurological symptoms of pain, depression and fatigue.
Complement has been previously shown to engage in signaling crosstalk with the TLR and acts as a bridge between the innate and adaptive immune responses to coordinate immune responses (12–15). Therefore, it is notable we observed not only dysfunction in the innate immune response but trends toward improvements in depression, fatigue, and pain during treatment with C1-INH. C1-INH plays a major role in controlling complement activation and has been previously reported to improve neurological functions by exerting an anti-inflammatory effect (18, 19). Complement may therefore play a contributory role in the persistent post-infection inflammation we observed through crosstalk between the innate and adaptive immune systems.
Dysregulation of the immune system and neurological changes have been described previously following other infections, such as Epstein Barr virus in MS (10), PANS (2), Lyme disease (37) and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (38) among others (10). Lyme disease, caused by Borrelia bacteria, is associated with various neurological symptoms such as headache, fatigue, myalgia and arthralgia, with emerging evidence that attributes clinical manifestation to abnormalities in the host immune response (37). Further, SARS-CoV-2 post-viral fatigue syndrome shares similar symptoms to ME/CFS, another chronic condition characterized by neurological symptoms that often occurs following an “infectious-like” illness (38). Like SARS-CoV-2 post-viral fatigue syndrome and Lyme disease, immunological and metabolic abnormalities have also been described in ME/CFS (38) underlying the hypothesis that immune dysregulation may alter the relationship of infectious immune and lead to the neuro-immune response observed.
This proof-of-concept study has notable limitations and strengths. This exploratory analysis was limited by sample size, with some imbalance observed at baseline in disease characteristics and medication use that may have influenced treatment outcomes. Most patients at baseline had very mild RBANS and MoCA scores, indicating no cognitive impairment and, therefore, a possible ceiling effect may explain the lack of improvement in RBANS and MoCA. As some patients had neurological symptoms before they had COVID-19, which was not factored into the randomization, this could also confound the interpretation of the results. However, we hypothesize that some of the population with pre-existing neurological conditions may not have had post-SARS-CoV-2 fatigue syndrome, therefore affecting treatment outcomes with C1-INH. This is further supported through the responder analysis of RBANS score, in which improved cognitive was noted in many patients. Most patients were White non-Hispanic, thus limiting generalizability. Additionally, although the central hypothesis at the heart of this study involves both innate and adaptive immunity, linked through complement system crosstalk, the absence of direct complement activation markers and adaptive immunity data limits our ability to confirm these mechanistic pathways. As a result, further studies are warranted to confirm this. Regarding strengths, the study benefitted from a randomized, double-blind, crossover design, with patients serving as their own placebo controls, theoretically reducing some confounders and variability.
This study provides further evidence to support our hypothesis of AIS, by aiding our understanding of the role of the innate and adaptive immune response in SARS-CoV-2 post-viral fatigue syndrome. Furthering the understanding of the infectious-immune axis is important to provide the tools to identify treatment and management of neurologic changes that occur after infection. Future work will seek to better comprehend the role of C1-INH across other AIS conditions, and to explore further the role of the innate immune response and TLR signaling in SARS-CoV-2 post-viral fatigue syndrome. An open-label, Phase 2 study has been planned to further evaluate the role of C1-INH in patients with SARS-CoV-2 post-viral fatigue syndrome.
In conclusion, this proof-of-concept study demonstrates sustained dysregulation of the immune system in patients with SARS-CoV-2 post-viral fatigue syndrome and that treatment with C1-INH can improve associated symptoms of depression, fatigue, and pain. The results suggest that the complement system may play a key contributory role in this immune deficiency and could be a potential therapeutic target in patients with SARS-CoV-2 post-viral fatigue syndrome, though further studies are needed to confirm this.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Advarra. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IM: Writing – original draft, Writing – review & editing. CB: Writing – review & editing. MB: Writing – review & editing. JW: Writing – review & editing. NO-G: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by an educational grant from Pharming Healthcare Inc. The funder had no influence on study design, conduct, manuscript writing, or the decision to submit the manuscript for publication.
Acknowledgments
Medical writing services were provided by Sarah Good of the Bioscript Group (Macclesfield, UK) in accordance with Good Publication Practice guidelines, which was funded by the educational grant from Pharming Healthcare Inc.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1523814/full#supplementary-material
References
1. Bierle, DM, Aakre, CA, Grach, SL, Salonen, BR, Croghan, IT, Hurt, RT, et al. Central sensitization phenotypes in post acute sequelae of SARS-CoV-2 infection (PASC): defining the post COVID syndrome. J Prim Care Community Health. (2021) 12:21501327211030826. doi: 10.1177/21501327211030826
2. Gagliano, A, Carta, A, Tanca, MG, and Sotgiu, S. Pediatric acute-onset neuropsychiatric syndrome: current perspectives. Neuropsychiatr Dis Treat. (2023) 19:1221–50. doi: 10.2147/ndt.S362202
3. Schwenkenbecher, P, Pul, R, Wurster, U, Conzen, J, Pars, K, Hartmann, H, et al. Common and uncommon neurological manifestations of neuroborreliosis leading to hospitalization. BMC Infect Dis. (2017) 17:90. doi: 10.1186/s12879-016-2112-z
4. Touradji, P, Aucott, JN, Yang, T, Rebman, AW, and Bechtold, KT. Cognitive decline in post-treatment Lyme disease syndrome. Arch Clin Neuropsychol. (2019) 34:455–65. doi: 10.1093/arclin/acy051
5. Al-Beltagi, M, Saeed, NK, Elbeltagi, R, Bediwy, AS, Aftab, SAS, and Alhawamdeh, R. Viruses and autism: a bi-mutual cause and effect. World J Virol. (2023) 12:172–92. doi: 10.5501/wjv.v12.i3.172
6. Chou, SH, Beghi, E, Helbok, R, Moro, E, Sampson, J, Altamirano, V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. (2021) 4:e2112131. doi: 10.1001/jamanetworkopen.2021.12131
7. Liguori, C, Pierantozzi, M, Spanetta, M, Sarmati, L, Cesta, N, Iannetta, M, et al. Depressive and anxiety symptoms in patients with SARS-CoV2 infection. J Affect Disord. (2021) 278:339–40. doi: 10.1016/j.jad.2020.09.042
8. Sobrino-Relaño, S, Balboa-Bandeira, Y, Peña, J, Ibarretxe-Bilbao, N, Zubiaurre-Elorza, L, and Ojeda, N. Neuropsychological deficits in patients with persistent COVID-19 symptoms: a systematic review and meta-analysis. Sci Rep. (2023) 13:10309. doi: 10.1038/s41598-023-37420-6
9. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
10. Melamed, I. Alzheimer’s disease of the immune system: a new variant of immune deficiency. Immunother Open Acc. (2016) 2:115. doi: 10.4172/2471-9552.1000115
11. Melamed, IR, Heffron, M, McGee, S, Ulltate Sanz, L, and Testori, A. A new subset of common variable immune deficiency characterized by reduced C1 esterase inhibitor levels. Ann Allergy Asthma Immunol. (2015) 115:83–4. doi: 10.1016/j.anai.2015.04.023
12. Dunkelberger, JR, and Song, WC. Complement and its role in innate and adaptive immune responses. Cell Res. (2010) 20:34–50. doi: 10.1038/cr.2009.139
13. Song, WC. Crosstalk between complement and toll-like receptors. Toxicol Pathol. (2012) 40:174–82. doi: 10.1177/0192623311428478
14. Fang, C, Zhang, X, Miwa, T, and Song, WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with toll-like receptor signaling and interleukin-6 production. Blood. (2009) 114:1005–15. doi: 10.1182/blood-2009-01-198283
15. Hajishengallis, G, and Lambris, JD. More than complementing tolls: complement-toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol Rev. (2016) 274:233–44. doi: 10.1111/imr.12467
16. Levy, M, and Mealy, MA. Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. (2014) 1:e5. doi: 10.1212/nxi.0000000000000005
17. Sierra, DP, Tripathi, A, and Pillai, A. Dysregulation of complement system in neuropsychiatric disorders: a mini review. Biomark Neuropsychiatry. (2022) 7:100056. doi: 10.1016/j.bionps.2022.100056
18. Heydenreich, N, Nolte, MW, Göb, E, Langhauser, F, Hofmeister, M, Kraft, P, et al. C1-inhibitor protects from brain ischemia-reperfusion injury by combined antiinflammatory and antithrombotic mechanisms. Stroke. (2012) 43:2457–67. doi: 10.1161/strokeaha.112.660340
19. Gesuete, R, Storini, C, Fantin, A, Stravalaci, M, Zanier, ER, Orsini, F, et al. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol. (2009) 66:332–42. doi: 10.1002/ana.21740
20. Longhi, L, Perego, C, Zanier, ER, Ortolano, F, Bianchi, P, Stocchetti, N, et al. Neuroprotective effect of C1-inhibitor following traumatic brain injury in mice. Acta Neurochir Suppl. (2008) 102:381–4. doi: 10.1007/978-3-211-85578-2_73
21. Chen, M, Tieng, QM, Du, J, Edwards, SR, Maskey, D, Peshtenski, E, et al. Effects of C1-INH treatment on neurobehavioral sequelae and late seizures after traumatic brain injury in a mouse model of controlled cortical impact. Neurotrauma Rep. (2023) 4:124–36. doi: 10.1089/neur.2022.0011
22. Beck, AT, Steer, RA, Ball, R, and Ranieri, W. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
23. Roth, RM, Lance, CE, Isquith, PK, Fischer, AS, and Giancola, PR. Confirmatory factor analysis of the behavior rating inventory of executive function-adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. (2013) 28:425–34. doi: 10.1093/arclin/act031
24. Randolph, C, Tierney, MC, Mohr, E, and Chase, TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. (1998) 20:310–9. doi: 10.1076/jcen.20.3.310.823
25. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
26. Dworkin, RH, Turk, DC, Revicki, DA, Harding, G, Coyne, KS, Peirce-Sandner, S, et al. Development and initial validation of an expanded and revised version of the short-form McGill pain questionnaire (SF-MPQ-2). Pain. (2009) 144:35–42. doi: 10.1016/j.pain.2009.02.007
27. Krupp, LB, LaRocca, NG, Muir-Nash, J, and Steinberg, AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. doi: 10.1001/archneur.1989.00520460115022
28. Stewart, WF, Lipton, RB, Dowson, AJ, and Sawyer, J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. (2001) 56:S20–8. doi: 10.1212/wnl.56.suppl_1.s20
29. Yang, M, Rendas-Baum, R, Varon, SF, and Kosinski, M. Validation of the headache impact test (HIT-6™) across episodic and chronic migraine. Cephalalgia. (2011) 31:357–67. doi: 10.1177/0333102410379890
30. Domingo, JC, Battistini, F, Cordobilla, B, Zaragozá, MC, Sanmartin-Sentañes, R, Alegre-Martin, J, et al. Association of circulating biomarkers with illness severity measures differentiates myalgic encephalomyelitis/chronic fatigue syndrome and post-COVID-19 condition: a prospective pilot cohort study. J Transl Med. (2024) 22:343. doi: 10.1186/s12967-024-05148-0
31. Saito, S, Shahbaz, S, Luo, X, Osman, M, Redmond, D, Cohen Tervaert, JW, et al. Metabolomic and immune alterations in long COVID patients with chronic fatigue syndrome. Front Immunol. (2024) 15:1341843. doi: 10.3389/fimmu.2024.1341843
32. Saito, S, Shahbaz, S, Osman, M, Redmond, D, Bozorgmehr, N, Rosychuk, RJ, et al. Diverse immunological dysregulation, chronic inflammation, and impaired erythropoiesis in long COVID patients with chronic fatigue syndrome. J Autoimmun. (2024) 147:103267. doi: 10.1016/j.jaut.2024.103267
33. Dalakas, MC. Post-COVID small Fiber neuropathy, implications of innate immunity, and challenges on IVIG therapy. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200248. doi: 10.1212/nxi.0000000000200248
34. Conti, P, D'Ovidio, C, Conti, C, Gallenga, CE, Lauritano, D, Caraffa, A, et al. Progression in migraine: role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur J Pharmacol. (2019) 844:87–94. doi: 10.1016/j.ejphar.2018.12.004
35. Figueroa-Hall, LK, Paulus, MP, and Savitz, J. Toll-like receptor signaling in depression. Psychoneuroendocrinology. (2020) 121:104843. doi: 10.1016/j.psyneuen.2020.104843
36. Morris, G, Berk, M, Walder, K, and Maes, M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. (2015) 13:28. doi: 10.1186/s12916-014-0259-2
37. Hernández, SA, Ogrinc, K, Korva, M, Kastrin, A, Bogovič, P, Rojko, T, et al. Association of Persistent Symptoms after Lyme Neuroborreliosis and increased levels of interferon-α in blood. Emerg Infect Dis. (2023) 29:1091–101. doi: 10.3201/eid2906.221685
38. Komaroff, AL, and Lipkin, WI. ME/CFS and long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med. (2023) 10:1187163. doi: 10.3389/fmed.2023.1187163
Glossary
AIS - Alzheimer’s of the Immune System
BDI-II - Beck Depression Inventory-II
BRI - Behavioral Regulation Index
BRIEF-A - Behavior Rating Inventory of Executive Function-Adult
C1-INH - C1-esterase inhibitor
COVID-19 - coronavirus disease 2019
FSS - Fatigue Severity Scale
GEC - Global Executive Composite
HIT-6 - six-item Headache Impact Scale
IL - interleukin
ME/CFS - myalgic encephalomyelitis/chronic fatigue syndrome
MI - Metacognition Index
MIDAS - Migraine Disability Assessment
MoCA - Montreal Cognitive Assessment
PANS - pediatric acute-onset neuropsychiatric syndrome
PBMC - peripheral blood mononuclear cell
RBANS - Repeatable Battery for the Assessment of Neuropsychological Status
SAE - serious adverse event
SARS-CoV-2 - severe acute respiratory syndrome coronavirus 2
SF - Short-form
TLR - Toll-like receptor
TNFα - tumor necrosis factor alpha
Keywords: C1-INH, cognitive dysfunction, complement activation, fatigue, immune system, neurological symptoms, SARS-CoV-2
Citation: Melamed I, Buckley C, Bayko ME, Williams JL and Or-Geva N (2025) Does C1 esterase inhibitor play a role in post COVID-19 neurological symptoms? A randomized, double-blind, placebo-controlled, crossover, proof-of-concept study. Front. Neurol. 16:1523814. doi: 10.3389/fneur.2025.1523814
Edited by:
Robert Weissert, University of Regensburg, GermanyReviewed by:
Alberto Spalice, Sapienza University of Rome, ItalyMateus Vidigal de Castro, University of São Paulo, Brazil
Copyright © 2025 Melamed, Buckley, Bayko, Williams and Or-Geva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isaac Melamed, bWVsYW1lZGlAaW1tdW5vZS5jb20=
 Isaac Melamed
Isaac Melamed Caley Buckley2
Caley Buckley2 Noga Or-Geva
Noga Or-Geva