- 1Department of Geriatrics, Lianyungang Hospital Affiliated to Jiangsu University, Lianyungang, China
- 2Department of Geriatrics, Lianyungang Second People’s Hospital, Lianyungang, China
- 3Department of Neurology, Lianyungang Second People’s Hospital, Lianyungang, China
- 4Department of Neurosurgery, Lianyungang First People's Hospital, Lianyungang, China
- 5Department of Neurology, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China
- 6Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
Objective: Ischemic stroke caused by large artery atherosclerosis (LAA) is a major subtype of ischemic stroke and poses a heavy public health burden. Plasma atherogenic index (AIP) reflects the balance between pro- and anti-atherogenic lipid components and has emerged as a potential biomarker of cardiovascular disease. The aim of this study was to investigate the role of AIP in predicting ischemic stroke caused by LAA.
Methods: This retrospective, cross-sectional study involved 2,382 ischemic stroke patients. AIP values were measured, and subjects were further stratified according to AIP levels. Univariate and multivariate logistic regression analyses were conducted to explore the relationship between AIP and the risk of LAA. Restricted cubic spline (RCS) analysis was used to detect the potential non-linear relationship, and receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive ability of AIP. Subgroup analyses were carried out to identify specific populations with a higher risk of LAA.
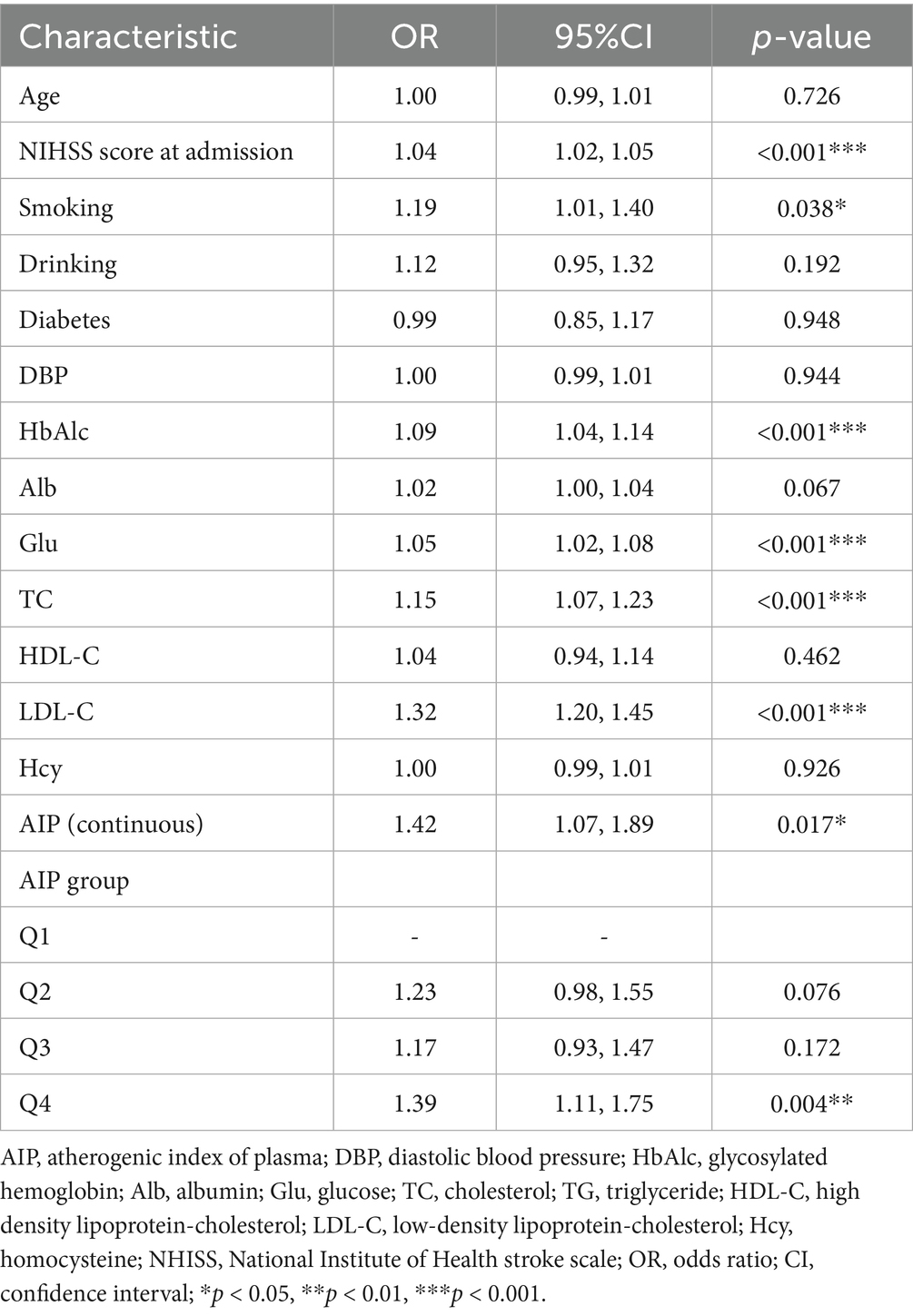
Results: Individuals with consistently high levels of AIP were at increased risk of developing LAA, and this risk increased progressively with increasing levels of AIP. RCS analyses showed a threshold of 0.10 for the AIP index, a significant increase in the probability of LAA above this threshold, and a non-linear relationship between AIP and LAA. Univariate and multivariate logistic regression analyses showed that, as a continuous variable, each unit increase in AIP was significantly associated with an elevated risk of LAA. When divided into quartiles, the risk of LAA was higher in Q4 compared with the lowest quartile (Q1), and ROC curve analyses confirmed that AIP had moderate sensitivity and specificity in predicting LAA. Subgroup analyses showed that among individuals with consistently high AIP levels, those aged ≥60 years with a history of diabetes and low-density lipoprotein cholesterol (LDL-C) < 3.4 mmol/L were at higher risk of developing LAA.
Conclusion: Herein, we found that elevated AIP levels are significantly associated with increased LAA risk and are an important biomarker to help identify patients at high risk for LAA.
Introduction
Atherosclerotic ischemic stroke (AIS) remains a leading cause of morbidity and mortality worldwide, with its rising prevalence posing a significant public health challenge (1, 2). Atherosclerosis—the primary pathological basis of AIS—is characterized by lipid accumulation and chronic inflammation within arterial walls, resulting in plaque formation, luminal narrowing, and thromboembolic complications (3). Among various risk factors, dyslipidemia plays a pivotal role in the pathogenesis of AIS (4, 5). While reducing low-density lipoprotein cholesterol (LDL-C) has been associated with a decreased risk of ischemic events (6, 7), increasing evidence suggests that small dense LDL-C (sdLDL-C) possesses greater atherogenic potential (8).
Nevertheless, the complexity and cost-intensiveness of measuring sdLDL-C have limited its routine clinical use. Consequently, Burns et al. (9) proposed AIP, a novel lipid parameter calculated as the logarithm of the Triglycerides (TG)/High-density lipoprotein cholesterol (HDL-C) ratio, that can be easily determined using routine blood tests, as an alternative biomarker. In addition to reflecting sdLDL-C levels, AIP also evaluates cardiovascular risk. AIP has been recognized as a practical surrogate for sdLDL-C, effectively capturing the balance between atherogenic and anti-atherogenic lipid components (10–12). Elevated AIP has been associated with subclinical atherosclerosis, metabolic syndrome, and increased risk of cardiovascular and cerebrovascular diseases (13–15). Unlike conventional lipid indicators, AIP integrates two key lipid components and may offer superior predictive value in assessing atherosclerotic risk (16–18).
Despite growing interest, the predictive role of AIP in ischemic stroke remains underexplored—particularly in the context of large-artery atherosclerosis (LAA), the most common and debilitating AIS subtype. Most previous studies have evaluated general stroke populations without distinguishing between etiologic subtypes (19, 20), limiting the clinical applicability of AIP in risk stratification for specific stroke mechanisms.
To address this gap, the present study investigates the association between AIP levels and the occurrence of LAA-type AIS, as classified by the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria (21). A clearer understanding of this relationship may improve early identification of high-risk individuals and inform more targeted, lipid-focused strategies for stroke prevention.
Materials and methods
Study design and population
This retrospective study included adult patients diagnosed with AIS who were hospitalized in three tertiary medical centers in China: (1) the First Affiliated Hospital of Soochow University between March 2018 and January 2021, (2) the First People’s Hospital of Lianyungang between October 2019 and December 2021, and (3) the Second People’s Hospital of Lianyungang between January 2022 and July 2024. All participating hospitals used standardized diagnostic protocols and electronic health record systems to ensure data consistency. AIS diagnoses were confirmed by neurologists using clinical criteria in accordance with the World Health Organization definition and supported by neuroimaging (Computed Tomography or Magnetic Resonance Imaging). After admission, fasting blood samples were collected from all patients within 24 h by trained nursing staff for laboratory analysis, including lipid profiles and metabolic markers. Additional demographic and clinical data were extracted from hospital records by two independent investigators using a structured data collection form.
The inclusion criteria were: (1) Patients aged ≥ 18 years; (2) Patients who met the diagnostic criteria for AIS. On the other hand, the exclusion criteria were: (1) Patients with Intracranial Hemorrhage or mass bleeding; (2) Patients with severe infection or septic shock; (3) Patients with liver or kidney failure; (4) Patients with incomplete laboratory or clinical data; (5) Stroke of undetermined etiology.
This study was approved by the Ethics Committees of the First Affiliated Hospital of Soochow University (Approval No. 2020272, 2,019,057), the Second People’s Hospital of Lianyungang (Approval No. 2020050), and the First People’s Hospital of Lianyungang (Approval No. KY-20210917001-01).
Baseline data collection
Experienced clinicians collected general patient information, including age, gender, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), history of hypertension, history of diabetes, history of atrial fibrillation, smoking history, and alcohol consumption history. Within 24 h of admission, professional medical staff collected hematological samples for laboratory testing, including Total Cholesterol (TC), TG, LDL-C, HDL-C, Glucose (Glu), Glycated Hemoglobin (HbA1c), Albumin (Alb), Creatinine (Cr), and Homocysteine (Hcy).
Neurological status was assessed by two experienced neurologists using the National Institutes of Health Stroke Scale (NIHSS) at admission and discharge. Patients were categorized into large-artery atherosclerosis (LAA) or non-LAA subtypes based on the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria (21). Stroke severity was defined as mild (NIHSS < 4) or severe (NIHSS ≥ 4).
AIP assessment
Herein, AIP values [log(TG/HDL-c)] were determined and grouped into quartiles (22): Q1:<−0.0904; Q2: −0.0904-0.0814; Q3:0.0814–0.255; and Q4: ≥ 0.255.
Statistical analysis
All statistical analyses were conducted using R software (version 4.3.2). The Kolmogorov–Smirnov test was applied to assess the normality of continuous variables. Variables with normal distribution were summarized using mean ± standard deviation (SD), and comparisons between groups were made using independent samples t-tests. For non-normally distributed variables, data were presented as medians and interquartile ranges (IQRs), and group comparisons were performed using Mann–Whitney U tests. Categorical variables were expressed as counts (percentages), and intergroup differences were evaluated using the chi-square test or Fisher’s exact test when expected frequencies were <5.
Logistic regression analyses were used to examine the association between AIP and LAA, allowing adjustment for potential confounders. Multiple covariance tests were performed prior to the logistic regression analysis. Restricted cubic spline (RCS) analysis was conducted to explore the potential non-linear association between AIP and LAA risk. ROC curve analysis was performed to evaluate the discriminative power of AIP (23), with Youden’s index used to determine the optimal cutoff point.
Missing data were handled according to the percentage of incompleteness: variables with >10% missing data were excluded from analysis, while variables with <10% missing values were imputed using the Random Forest method via the mice package in R. All tests were two-tailed, and p-values <0.05 were considered statistically significant.
Results
Characteristics of study participants based on AIP quartiles
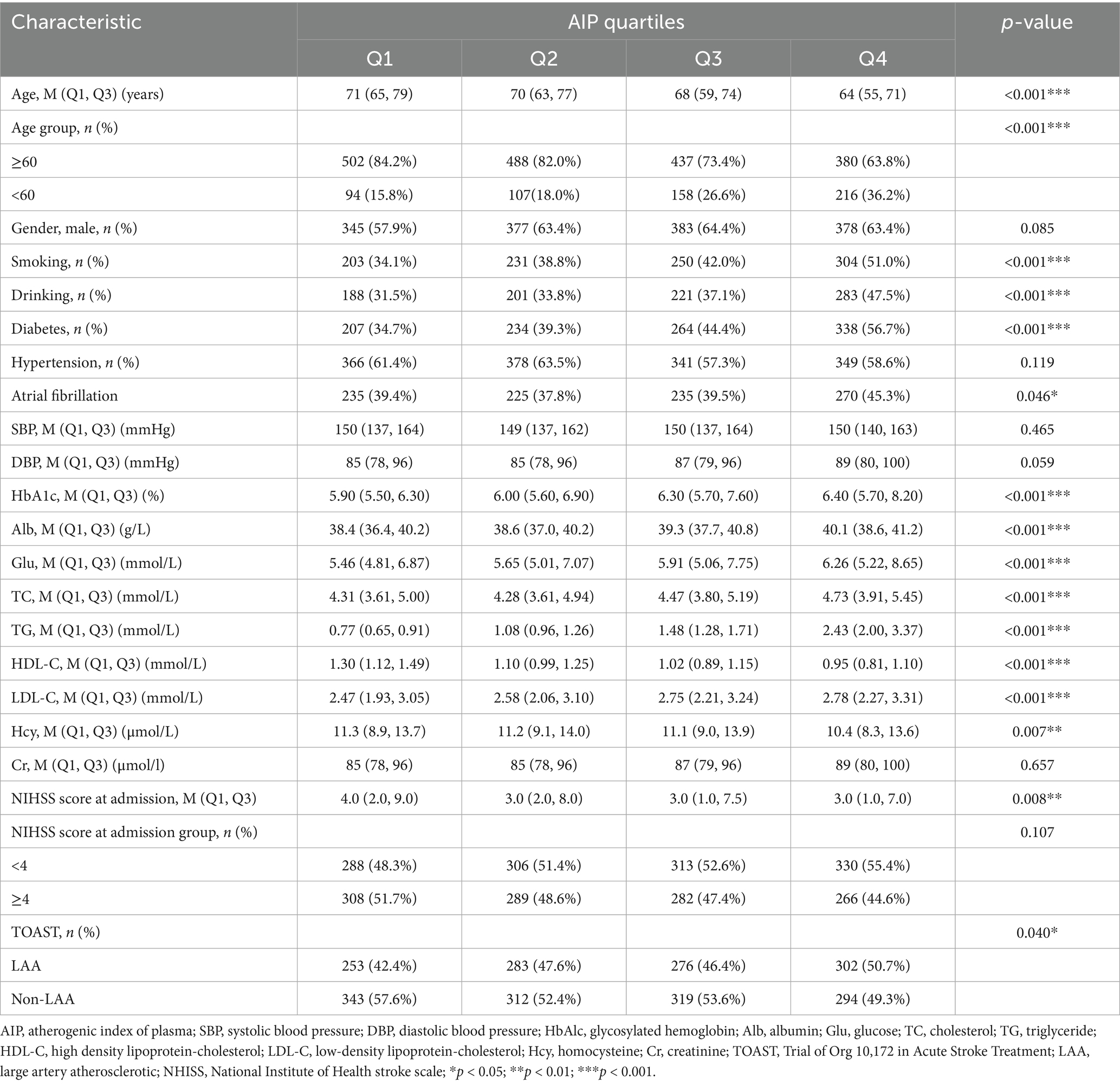
Herein, as shown in Table 1, the baseline characteristics of participants were categorized by AIP quartiles, revealing significant differences across various demographic and clinical parameters. Among the total of 2,382 patients included in the study, 1,483 were male (62.3%) and 899 were female (37.7%). There was a notable trend in age distribution (p < 0.001), with median ages decreasing from Q1 (71 years) to Q4 (64 years). Furthermore, there was a decreasing trend in the proportion of participants aged ≥ 60 years across the quartiles, with Q1 and Q4 having the highest (84.2%) and lowest (63.8%) proportions, respectively. On the other hand, gender distribution was relatively balanced across the quartiles, although each group had more males, but with no significant intergroup difference (p = 0.085). Smoking and drinking habits increased considerably across the quartiles (p < 0.001 for both), with Q4 showing the highest proportions (47.5 and 56.7% for smoking and drinking, respectively). The prevalence of diabetes and atrial fibrillation also increased significantly (p < 0.001), with Q4 showing the highest prevalence (56.7 and 45.3%, respectively). On the other hand, the prevalence of hypertension remained consistent across groups (p = 0.119). Regarding metabolic markers, the quartiles showed significant differences in HbA1c, Alb, Glu, TC, TG, HDL-C, and LDL-C levels (all p < 0.001), with an upward trend in glucose and lipid indices from Q1 to Q4. Conversely, the quartiles showed no significant differences in SBP and Cr levels. Moreover, the median NIHSS scores at admission decreased slightly across quartiles (p = 0.008), with Q4 showing a larger proportion of mild cases (NIHSS < 4). As shown in Figure 1, according to the TOAST classification system, the groups exhibited an increase in LAA prevalence, with Q4 accounting for most cases (50.7%) (p = 0.040).
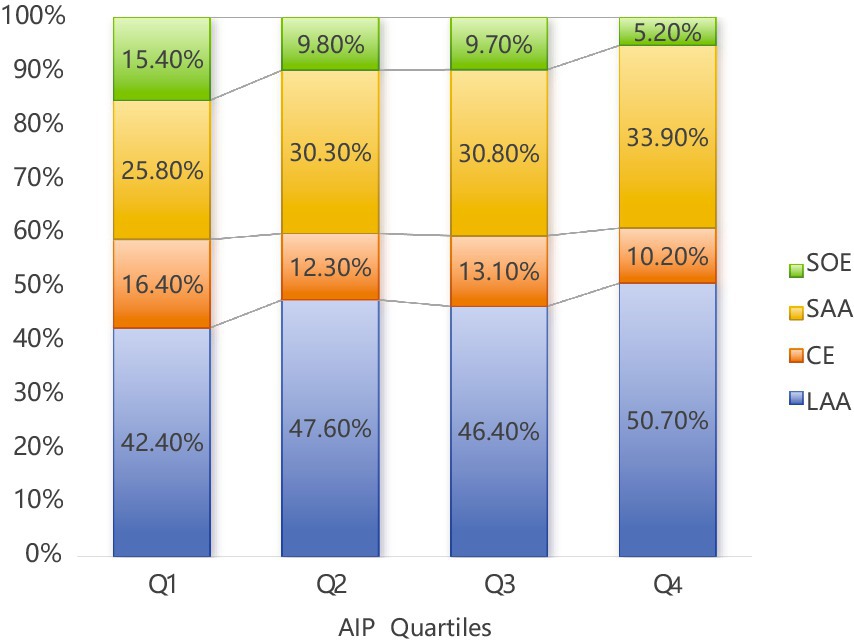
Figure 1. Percentage of AIP in the four quarters (Q1–Q4) according to Toast typing. LAA (Large Atherosclerotic Stroke) had the highest percentage in all quarters and amounted to 50.70% in Q4; LAA, large artery atherosclerotic; CE, cardiogenic; SAA, small artery occlusion; SOE, stroke of other etiology; AIP, atherogenic index of plasma.
Univariate and multivariate logistic analyses of factors associated with LAA
Figure 2 shows a Restricted Cubic Spline (RCS) curve depicting the relationship between AIP and LAA. Notably, AIP correlated significantly positively with LAA (p = 0.011), with an inflection point observed at an AIP value of 0.10, after which the odds ratio (OR) increased significantly. Furthermore, the relationship between AIP and LAA did not deviate significantly from a linear trend (p for non-linearity = 0.233).
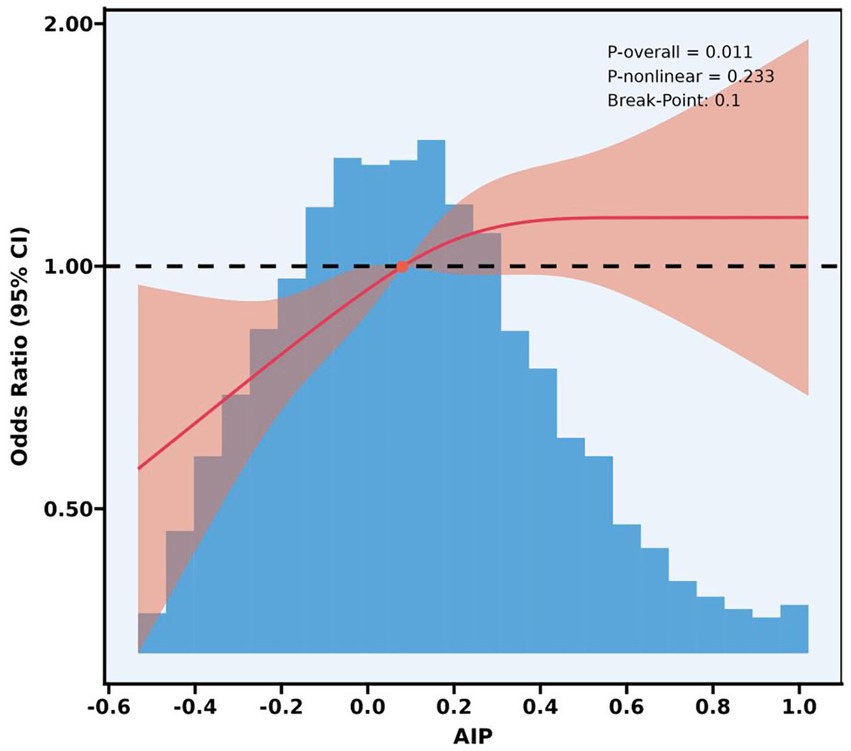
Figure 2. A restricted cubic spline analysis of the relationship between the AIP and LAA. AIP, atherogenic index of plasma; LAA, large artery atherosclerotic; CI, confidence interval.
We assessed collinearity before analysis (Supplementary Table 1). We also assessed the association between various characteristics and LAA, as shown in Table 2, where NIHSS score at admission, smoking, HbAlc, Glu, TC, LDL-C, and AIP were independent risk factors for LAA. The risk of LAA occurrence increased by approximately 42% for each 1-unit increase in AIP when using AIP as a continuous variable [OR = 1.42, 95% confidence interval (CI): 1.07–1.89]. Categorical analysis of AIP showed that only Q4 was significantly associated with risk of LAA (OR = 1.39, 95% CI: 1.11–1.75).
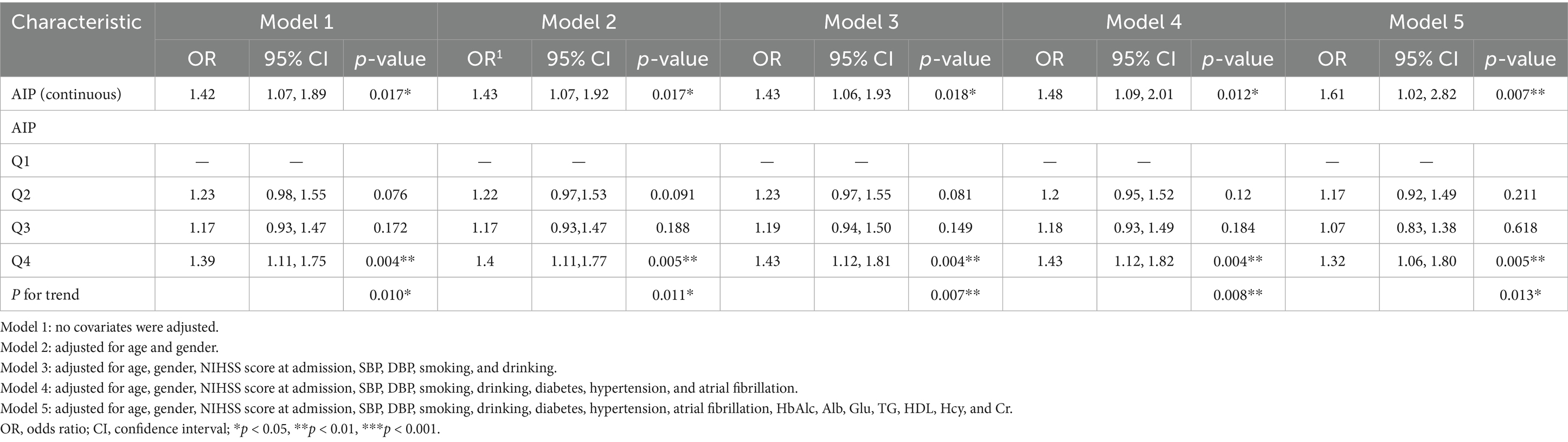
This logistic regression analysis assessed the association between various characteristics and the likelihood of LAA, adjusting for different sets of covariates across five models, as shown in Table 3. When the AIP was treated as a continuous variable, each unit increase was associated with a higher risk of LAA, though the OR increased as more covariates were included: from OR = 1.42 in Model 1 to OR = 1.61 in Model 5. On the other hand, when analyzed categorically, Q4 of AIP showed a consistent and significant association with higher LAA odds across all models, with ORs ranging from 1.39 (95% CI:1.11–1.75, p = 0.004) in Model 1 to 1.32 (95% CI: 1.06–1.80, p = 0.005) in Model 5. Trend tests indicated a significant trend association between AIP groups and LAA across all models. These findings collectively suggest that higher AIP levels correlate with an increased risk of LAA.
ROC analysis of the predictive value of AIP for LAA
Herein, an ROC plot was generated depicting the predictive ability of the five models in Figure 3. According to the plot, the area under curve (AUC) values increased gradually as covariates were progressively added from models 1 to 5, indicating an improvement in the models’ predictive value. The AUC value for Model 1 was 0.532, the optimal threshold value for the AIP index was 0.008, and the Yoden index, sensitivity and specificity values were 0.061, 63.9 and 42.2%, respectively. In Model 5, the AUC value was 0.646, the optimal threshold was 0.514, and the Youden’s index, sensitivity, and specificity values were 0.219, 45.5, and 76.4%, respectively.
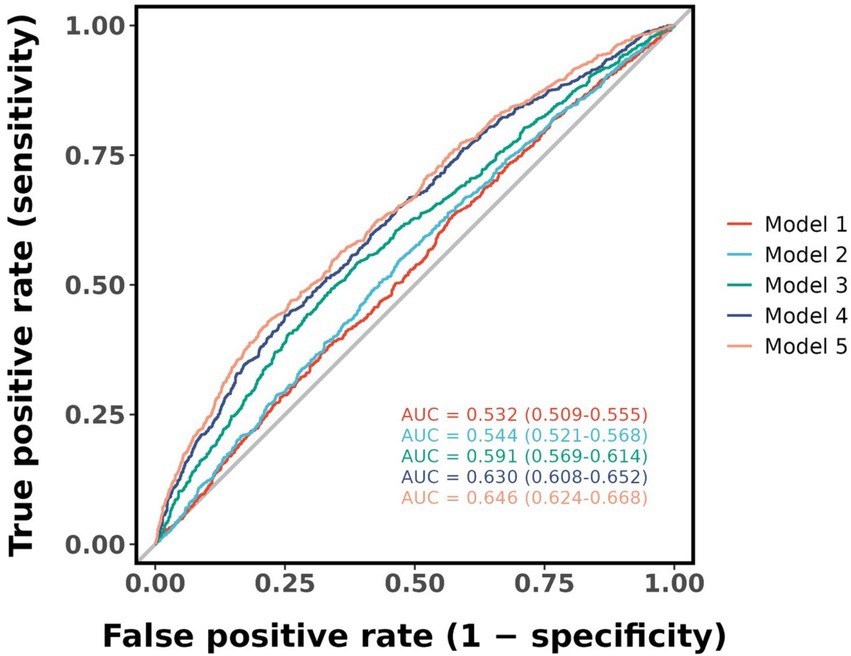
Figure 3. Receiver operating characteristic plot was generated depicting the predictive ability of the five models. AUC, area under curve.
Subgroup analysis of LAA-related factors
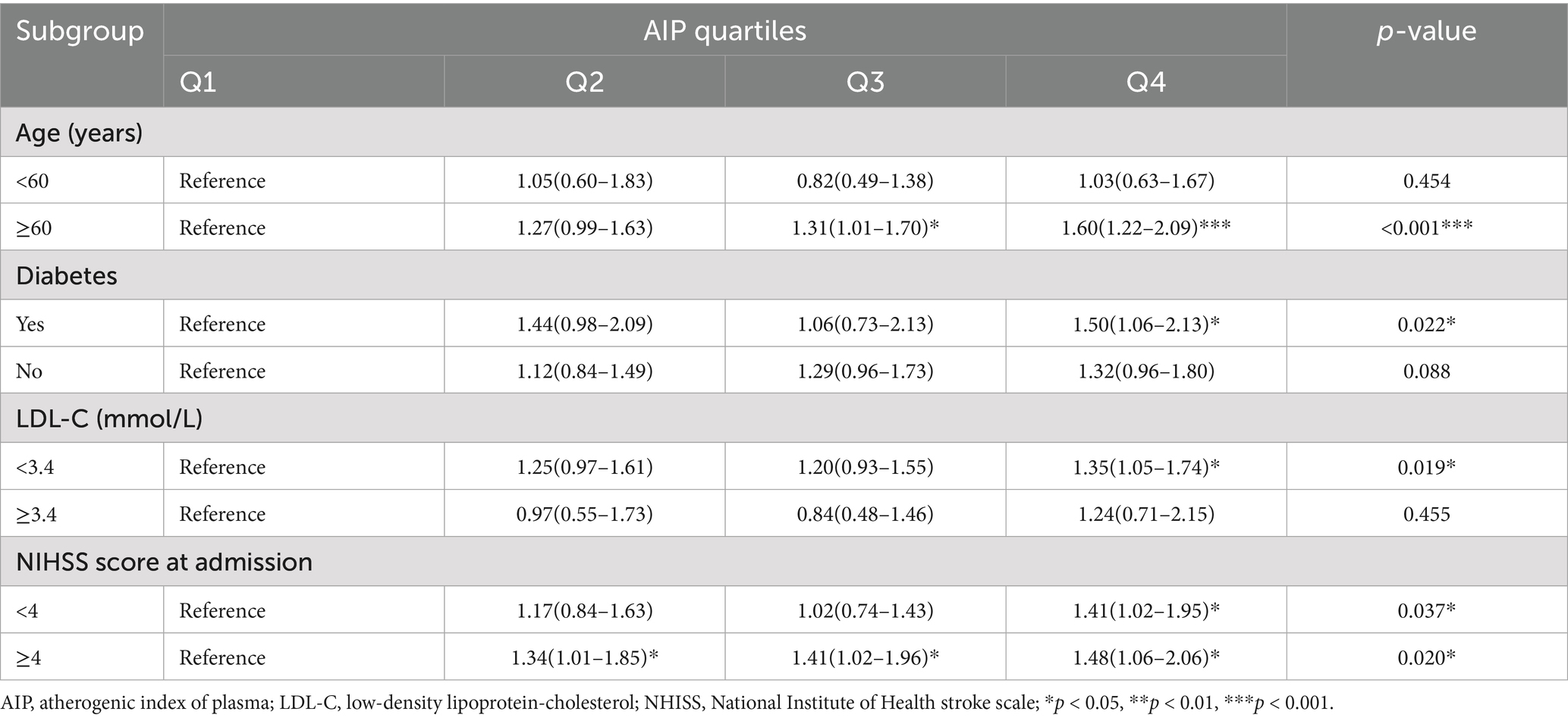
According to the stratified analysis results, among individuals with consistently high AIP levels, the risk of developing LAA was higher in those aged ≥ 60 years compared to those aged < 60 years, and patients with diabetes had a higher risk of LAA than patients without diabetes, as well as in those with LDL-C < 3.4 mmol/L compared to those with LDL-C ≥ 3.4 mmol/L (both p < 0.05; Table 4). Furthermore, in the ≥ 60 years group, compared to Q1, the risk of LAA was higher in Q3 (OR = 1.31, 95% CI: 1.01–1.70) and Q4 (OR = 1.60, 95% CI: 1.22–2.09). In the LDL-C < 3.4 mmol/L group, only Q4 was significantly associated with LAA compared with Q1 (OR = 1.35, 95% CI: 1.05–1.74). On the other hand, in the LDL-C ≥ 3.4 mmol/L group, the risk of developing LAA in Q2, Q3, and Q4 did not change significantly relative to Q1 (all p > 0.05). In addition, in the group with an NIHSS score of ≥4 on admission, compared to Q1, compared with Q1, the risks of LAA in Q2, Q3, and Q4 increased by 34% (OR = 1.34, 95% CI: 1.01–1.85), 41% (OR = 1.41, 95% CI: 1.02–1.96), and 48% (OR = 1.48, 95% CI: 1.06–2.06). In contrast, only Q4 was associated with LAA in the NIHSS score at admission <4 group (OR = 1.41, 95% CI: 1.02–1.95).
Discussion
This retrospective cross-sectional study identified a significant association between elevated AIP and increased risk of LAA in AIS patients, with a threshold effect at an AIP of 0.10. Univariate and multivariate logistic regression confirmed that each unit increase in AIP was associated with a 42% higher LAA risk (OR = 1.42, 95% CI: 1.07–1.89), with the highest quartile (Q4) showing a 39% increased risk (OR = 1.39, 95% CI: 1.11–1.75). These findings align with Zheng et al. prospective cohort study, where 6-year cumulative AIP exposure was linked to a 31% higher AIS risk, reinforcing AIP’s role as a key (but not exclusive) risk factor (19).
The AIP reflects the balance of pro-atherogenic triglycerides and protective HDL-C, driving formation of sdLDL-C particles that penetrate arterial walls and undergo oxidation (24). Concurrently, low HDL-C impairs reverse cholesterol transport, exacerbating lipid accumulation in atherosclerotic plaques (25). Mechanistically, AIP also correlates with oxidative stress and inflammation: elevated AIP triggers reactive oxygen species (ROS) production, damaging endothelial cells and promoting monocyte-derived macrophage activation—key steps in plaque instability (26, 27). Additionally, AIP-related lipid abnormalities may inhibit endothelial nitric oxide synthase (eNOS), reducing nitric oxide (NO) bioavailability—a critical factor in vasodilation and anti-thrombotic defense (28). These mechanisms collectively promote endothelial dysfunction, plaque instability, and heightened LAA risk.
Our subgroup analysis showed that among individuals with persistently high AIP levels, those with LDL-C < 3.4 mmol/L had a higher risk of developing LAA compared to those with LDL-C ≥ 3.4 mmol/L. This seemingly paradoxical result may be explained by the role of AIP in reflecting the formation of sdLDL-C particles, which are more atherogenic despite low total LDL-C levels (8, 29). Dobiásová and Frohlich (30) found that AIP is inversely correlated with LDL-C particle size and increases with the proportion of small-to-medium dense LDL-C. Thus, individuals with low LDL-C but elevated AIP may still carry a significant atherosclerotic burden due to sdLDL-C dominance. In contrast, in individuals with high LDL-C (≥3.4 mmol/L), the strong atherogenic potential of LDL-C may overshadow the added risk from AIP (30, 31). This is consistent with studies showing an inverse relationship between LDL particle size and atherosclerosis risk. While LDL-C are recognized risk factors for AIS (27), residual cardiovascular risk persists even when LDL-C is reduced to guideline-recommended levels (32–34).
The AIP, as a surrogate marker for sdLDL-C, offers a practical alternative. AIP correlates inversely with LDL-C particle size and could indirectly reflect sdLDL-C. Zhou et al. (35) found a positive correlation between sdLDL-C and IS risk, indicating that sdLDL-C could influence stroke progression and patient prognosis. Besides its long plasma half-life, SdLDL-C is resistant to hepatic clearance and prone to oxidation, potentially leading to foam cell formation. Furthermore, due to its small size, sdLDL-C is more likely to adhere to the vascular endothelium, leading to AS. It is also noteworthy that Wang et al. (36) reported a positive correlation between AIP and the risk of Early Neurological Deterioration (END) in AIS patients, with an optimal AIP threshold of 0.115 for predicting END. They also found that AIP had a higher predictive value for END than traditional lipid profiles (36). Additionally, several other studies have shown that AIP correlates with acute-phase neurological impairment and deterioration (37). Kokubo et al. (29) demonstrated that sdLDL-C is more predictive of cardiovascular events than total LDL-C or lipoprotein(a), though its routine measurement is limited by cost and technical complexity. As a cost-effective surrogate for sdLDL-C, AIP’s ease of measurement from routine blood tests supports its clinical utility.
In the ROC analysis, the Youden index was used to determine the optimal AIP threshold for identifying LAA. While the AIP cutoff in the unadjusted model (Model 1) demonstrated moderate sensitivity (63.9%), the specificity was relatively low (42.2%). With progressive covariate adjustment, specificity improved to 76.4% in Model 5, though sensitivity decreased to 45.5%. These findings indicate that although AIP may help identify individuals at higher risk of LAA, its limited specificity in certain models suggests it may be insufficient for use as an independent screening tool. Therefore, AIP may be more suitable as part of a multimodal risk assessment strategy that incorporates clinical features and other laboratory indicators to improve predictive accuracy.
Despite its strengths, this study has several limitations. First, as a cross-sectional study, it cannot establish causality between AIP and LAA. Second, some patients were on lipid-lowering medications, which may have influenced AIP levels and study outcomes. However, due to the retrospective nature of this study and the limitations of available medical records, detailed information regarding the type, dosage, and duration of lipid-lowering medications was not uniformly documented. As a result, potential confounding from lipid-lowering therapy could not be fully assessed. Third, the lack of follow-up precluded the evaluation of IS progression and long-term adverse outcomes. Future studies should incorporate longitudinal follow-up to assess the impact of AIP on stroke prognosis over time. Additionally, further research should explore the combined use of AIP with emerging biomarkers to improve stroke risk prediction and diagnostic accuracy. Investigating the impact of lifestyle modifications on AIP levels and subsequent stroke risk reduction could also provide valuable insights into preventive strategies.
Conclusion
Our study demonstrates that elevated AIP levels are significantly associated with an increased risk of LAA, highlighting AIP as a crucial biomarker for early risk stratification. AIP reflects the balance between pro- and anti-atherogenic lipid components, with higher levels indicating a greater predisposition to atherosclerosis and stroke.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Xiaozhu Shen, NDk5MDYyNzk2QHFxLmNvbQ==.
Ethics statement
The studies involving humans were approved by the Ethics Committees of Lianyungang Second People’s Hospital (Approval number: 2020050), the First Affiliated Hospital of Soochow University (Approval Numbers: 2020272 and 2019057) and Lianyungang First People’s Hospital (Approval number: KY-20210917001-01). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were primarily isolated as part of our previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WZ: Conceptualization, Methodology, Visualization, Writing – original draft. NZ: Conceptualization, Data curation, Visualization, Writing – original draft. XS: Funding acquisition, Resources, Supervision, Writing – review & editing. ZG: Resources, Writing – review & editing. XL: Conceptualization, Data curation, Writing – review & editing. GZ: Conceptualization, Methodology, Writing – review & editing. QF: Conceptualization, Supervision, Writing – review & editing. JL: Investigation, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Open-end Funds of Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (HY202203), Lianyungang Ageing Health Research Project (L202304), Lianyungang Traditional Chinese Medicine Science and Technology Development Program Project (ZD202310).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1529628/full#supplementary-material
References
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Koutsaliaris, IK, Moschonas, IC, Pechlivani, LM, Tsouka, AN, and Tselepis, AD. Inflammation, oxidative stress, vascular aging and atherosclerotic ischemic stroke. Curr Med Chem. (2022) 29:5496–509. doi: 10.2174/0929867328666210921161711
3. Feng, X, Chan, KL, Lan, L, Abrigo, J, Liu, J, Fang, H, et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: classification and clinical implications. Stroke. (2019) 50:2692–9. doi: 10.1161/STROKEAHA.119.025732
4. Rohr, J, Kittner, S, Feeser, B, Hebel, JR, Whyte, MG, Weinstein, A, et al. Traditional risk factors and ischemic stroke in young adults: the Baltimore-Washington cooperative young stroke study. Arch Neurol. (1996) 53:603–7. doi: 10.1001/archneur.1996.00550070041010
5. Nasr, N, Ruidavets, JB, Farghali, A, Guidolin, B, Perret, B, and Larrue, V. Lipoprotein (a) and carotid atherosclerosis in young patients with stroke. Stroke. (2011) 42:3616–8. doi: 10.1161/STROKEAHA.111.624684
6. Banerjee, C, and Chimowitz, MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res. (2017) 120:502–13. doi: 10.1161/CIRCRESAHA.116.308441
7. Guo, Q, Zhou, S, Feng, X, Yang, J, Qiao, J, Zhao, Y, et al. The sensibility of the new blood lipid indicator--atherogenic index of plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis. (2020) 19:27. doi: 10.1186/s12944-020-01208-8
8. Lui, DT, and Tan, KC. Low-density lipoprotein cholesterol and stroke: how low should we go? J Diabetes Investig. (2020) 11:1379–81. doi: 10.1111/jdi.13310
9. Burns, SF, Lee, SJ, and Arslanian, SA. Surrogate lipid markers for small dense low-density lipoprotein particles in overweight youth. J Pediatr. (2012) 161:991–6. doi: 10.1016/j.jpeds.2012.06.013
10. Sharma, P, Purohit, P, and Gupta, R. Cardiac risk factors in descendants of parents with history of coronary artery disease (CAD): an evaluation focusing on small dense low density lipoprotein cholesterol (sdLDLc) and high density lipoprotein cholesterol (HDLc). Indian J Biochem Biophys. (2013) 50:453–61.
11. Sumino, H, Nakajima, K, and Murakami, M. Possibility of new circulating atherosclerosis-related lipid markers measurement in medical and complete medical checkups: small dense low-density lipoprotein cholesterol and lipoprotein lipase. Rinsho Byori. (2016) 64:298–307.
12. Nishikura, T, Koba, S, Yokota, Y, Hirano, T, Tsunoda, F, Shoji, M, et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb. (2014) 21:755–67. doi: 10.5551/jat.23465
13. Frohlich, J, and Dobiásová, M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. (2003) 49:1873–80. doi: 10.1373/clinchem.2003.022558
14. Yildiz, G, Duman, A, Aydin, H, Yilmaz, A, Hür, E, Mağden, K, et al. Evaluation of association between atherogenic index of plasma and intima-media thickness of the carotid artery for subclinic atherosclerosis in patients on maintenance hemodialysis. Hemodial Int. (2013) 17:397–405. doi: 10.1111/hdi.12041
15. S, M, Dr, D, A, N, and Krishnan, M. Atherogenic index of plasma, lipid accumulation and visceral adiposity in metabolic syndrome patients. Bioinformation. (2022) 18:1109–13.
16. Linton, MF, Yancey, PG, Davies, SS, Jerome, WG, Linton, EF, Song, WL, et al. The role of lipids and lipoproteins in atherosclerosis In: KR Feingold, B Anawalt, MR Blackman, A Boyce, G Chrousos, and E Corpas, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. (2000)
17. Generoso, G, Janovsky, C, and Bittencourt, MS. Triglycerides and triglyceride-rich lipoproteins in the development and progression of atherosclerosis. Curr Opin Endocrinol Diabetes Obes. (2019) 26:109–16. doi: 10.1097/MED.0000000000000468
18. Lütjohann, D, Klör, HU, and Stellaard, F. Measurement of serum low density lipoprotein cholesterol and triglyceride-rich remnant cholesterol as independent predictors of atherosclerotic cardiovascular disease: possibilities and limitations. Nutrients. (2023) 15:2202. doi: 10.3390/nu15092202
19. Zheng, H, Wu, K, Wu, W, Chen, G, Chen, Z, Cai, Z, et al. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: a retrospective cohort study. Cardiovasc Diabetol. (2023) 22:313. doi: 10.1186/s12933-023-02044-7
20. Zhang, Y, Chen, S, Tian, X, Xu, Q, Xia, X, Zhang, X, et al. Elevated atherogenic index of plasma associated with stroke risk in general Chinese. Endocrine. (2024) 84:934–42. doi: 10.1007/s12020-023-03677-0
21. Adams, HP, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
22. Zhai, L, Huo, RR, and Zuo, YL. Atherogenic index of plasma and obesity-related risk of stroke in middle-aged and older Chinese adults: a national prospective cohort study. Diabetol Metab Syndr. (2024) 16:245. doi: 10.1186/s13098-024-01481-y
23. Wang, Y, Chen, Y, Chen, R, Xu, Y, Zheng, H, Xu, J, et al. Development and validation of a nomogram model for prediction of stroke-associated pneumonia associated with intracerebral hemorrhage. BMC Geriatr. (2023) 23:633. doi: 10.1186/s12877-023-04310-5
24. Suri, S, Chiesa, ST, Zsoldos, E, Mackay, CE, Filippini, N, Griffanti, L, et al. Associations between arterial stiffening and brain structure, perfusion, and cognition in the Whitehall II imaging sub-study: A retrospective cohort study. PLoS Med. (2020) 17:e1003467. doi: 10.1371/journal.pmed.1003467
25. de Melo, LGP, Nunes, SOV, Anderson, G, Vargas, HO, Barbosa, DS, Galecki, P, et al. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. (2017) 78:34–50. doi: 10.1016/j.pnpbp.2017.04.027
26. Khosravi, A, Sadeghi, M, Farsani, ES, Danesh, M, Heshmat-Ghahdarijani, K, Roohafza, H, et al. Atherogenic index of plasma: a valuable novel index to distinguish patients with unstable atherogenic plaques. J Res Med Sci. (2022) 27:45. doi: 10.4103/jrms.jrms_590_21
27. Amarenco, P, Hobeanu, C, Labreuche, J, Charles, H, Giroud, M, Meseguer, E, et al. Carotid atherosclerosis evolution when targeting a low-density lipoprotein cholesterol concentration <70 mg/dL after an ischemic stroke of atherosclerotic origin. Circulation. (2020) 142:748–57. doi: 10.1161/CIRCULATIONAHA.120.046774
28. Bornfeldt, KE, and Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. (2011) 14:575–85. doi: 10.1016/j.cmet.2011.07.015
29. Kokubo, Y, Watanabe, M, Higashiyama, A, and Honda-Kohmo, K. Small-dense low-density lipoprotein cholesterol: A subclinical marker for the primary prevention of coronary heart disease. J Atheroscler Thromb. (2020) 27:641–3. doi: 10.5551/jat.ED134
30. Dobiásová, M, and Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. (2001) 34:583–8. doi: 10.1016/s0009-9120(01)00263-6
31. Koch, KM, Sherafati, M, Arpinar, VE, Bhave, S, Ausman, R, Nencka, AS, et al. Analysis and evaluation of a deep learning reconstruction approach with denoising for orthopedic MRI. Radiolog. (2021) 3:e200278. doi: 10.1148/ryai.2021200278
32. Knoll, F, Hammernik, K, Zhang, C, Moeller, S, Pock, T, Sodickson, DK, et al. Deep-learning methods for parallel magnetic resonance imaging reconstruction: A survey of the current approaches, trends, and issues. IEEE Signal Process Mag. (2020) 37:128–40. doi: 10.1109/MSP.2019.2950640
33. Arnett, DK, Blumenthal, RS, Albert, MA, Buroker, AB, Goldberger, ZD, Hahn, EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
34. Di Fusco, SA, Maggioni, AP, Scicchitano, P, Zuin, M, D'Elia, E, and Colivicchi, F. Lipoprotein (a), inflammation, and atherosclerosis. J Clin Med. (2023) 12:2529. doi: 10.3390/jcm12072529
35. Zhou, P, Liu, J, Wang, L, Feng, W, Cao, Z, Wang, P, et al. Association of small dense low-density lipoprotein cholesterol with stroke risk, severity and prognosis. J Atheroscler Thromb. (2020) 27:1310–24. doi: 10.5551/jat.53132
36. Wang, Q, Jiang, G, Yan, L, Chen, R, Liu, Y, Liu, L, et al. Association of atherogenic index of plasma with early neurological deterioration in patients with acute ischemic stroke. Clin Neurol Neurosurg. (2023) 234:108014. doi: 10.1016/j.clineuro.2023.108014
37. Seners, P, Turc, G, Oppenheim, C, and Baron, JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. (2015) 86:87–94. doi: 10.1136/jnnp-2014-308327
Glossary
Alb - Albumin
AIP - Atherogenic Index of Plasma
AIS - Atherosclerotic Ischemic Stroke
AUC - Area Under Curve
CE - Cardiogenic (TOAST subtype)
CI - Confidence Interval
Cr - Creatinine
cumAIP - Cumulative Exposure Index of AIP
DBP - Diastolic Blood Pressure
Glu - Glucose
HbA1c - Glycosylated Hemoglobin
HDL-C - High-Density Lipoprotein Cholesterol
Hcy - Homocysteine
IQRs - Interquartile Ranges
LDL-C - Low-Density Lipoprotein Cholesterol
LAA - Large Artery Atherosclerosis
NO - Nitric Oxide
NIHSS - National Institute of Health Stroke Scale
OS - Oxidative Stress
OR - Odds Ratio
RCS - Restricted Cubic Spline
ROS - Reactive Oxygen Species
ROC - Receiver Operating Characteristic
SAA - Small Artery Occlusion
SBP - Systolic Blood Pressure
SD - Standard Deviation
sdLDL-C - Small Dense Low-Density Lipoprotein Cholesterol
SOE - Stroke of Other Etiology
TC - Total Cholesterol
TG - Triglycerides
TOAST - Trial of Org 10,172 in Acute Stroke Treatment
eNOS - Endothelial Nitric Oxide Synthase
END - Early Neurological Deterioration
Keywords: atherogenic index of plasma (AIP), large artery atherosclerosis (LAA), atherosclerotic ischemic stroke (AIS), risk prediction, supplementary lipid metabolism
Citation: Zhong W, Zhu N, Shen X, Ge Z, Liu X, Zhang G, Fang Q and Liao J (2025) Association between the atherogenic index of plasma and risk of large-artery atherosclerotic ischemic stroke. Front. Neurol. 16:1529628. doi: 10.3389/fneur.2025.1529628
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Hiroya Ohta, Hokkaido University of Science, JapanHitesh Singh Chaouhan, National Institute of Neurological Disorders and Stroke (NIH), United States
Ibiene Kalio, Rivers State College of Health Science and Management Technology, Nigeria
Copyright © 2025 Zhong, Zhu, Shen, Ge, Liu, Zhang, Fang and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhu Shen, NDk5MDYyNzk2QHFxLmNvbQ==; Jingxian Liao, c3VwZXJqaW5neGlhbkAxNjMuY29t
†These authors have contributed equally to this work
 Wen Zhong
Wen Zhong Nini Zhu
Nini Zhu Xiaozhu Shen
Xiaozhu Shen Zhonglin Ge3
Zhonglin Ge3 Qi Fang
Qi Fang