- 1Department of Traditional Chinese Medicine, Taizhou Hospital of Zhejiang Province Affiliated with Wenzhou Medical University, Linhai, Zhejiang, China
- 2Department of Nephrology, Ruian City Traditional Chinese Medicine Hospital, Ruian, Zhejiang, China
- 3Department of Urology, Taizhou Cancer Hospital, Wenling, Zhejiang, China
- 4Department of Urology, Taizhou Hospital of Zhejiang Province Affiliated with Wenzhou Medical University, Linhai, Zhejiang, China
Objective: The association between sleep duration and overactive bladder (OAB) risk remains underexplored. The aim of this study was to assess this relationship in U.S. adults using National Health and Nutrition Examination Survey (NHANES) data.
Methods: This cross-sectional study included 24,360 participants aged 20–80 years with OAB who completed the NHANES (2005–2018). NHANES Kidney Conditions-Urology Questionnaire data and the Overactive Bladder Symptom Score (OABSS) were collected. Sleep duration was self-reported by the participants. Propensity score matching (PSM) and multivariate logistic regression were employed to control for confounding variables, whereas a generalized additive model (GAM) was utilized to explore the nonlinear relationship between sleep duration and OAB.
Results: Short sleep (<6 h) significantly increased OAB risk (OR 1.37; 95% CI, 1.21–1.55; p < 0.01). A U-shaped relationship between sleep duration and OAB was observed, suggesting that approximately 6 h of sleep is optimal for minimizing OAB risk. Additional risk factors for OAB included female sex, older age, higher BMI, lower education and income levels, hypertension, diabetes, and smoking.
Conclusion: Both insufficient and excessive sleep durations were independently linked to increased OAB risk, and the optimal sleep duration to minimize OAB risk was approximately 6 h. These findings emphasize the importance of targeted prevention and intervention strategies focused on sleep and other modifiable risk factors for OAB. Further research is needed to better understand the biological mechanisms linking sleep duration and OAB.
1 Introduction
Overactive bladder syndrome (OAB) is characterized by lower urinary tract symptoms (LUTS), such as urgency, and often accompanied by urinary frequency and nocturia, with or without urgency urinary incontinence (1). As a functional bladder disorder, OAB imposes a substantial burden on patients, negatively affecting daily activities and psychological well-being. Epidemiological studies highlight the detrimental impact of OAB symptoms, which restrict routine activities and reduce overall health-related quality of life (2). Notably, OAB often leads to nocturnal disturbances, including urinary urgency and incontinence and nocturia, all of which are closely linked to sleep disruptions. For individuals with OAB, nocturnal bladder symptoms are commonly considered a primary factor in impaired sleep quality and disrupted sleep continuity (3).
Recent research has focused on the role of circadian rhythms in the development of LUTS (4). It is speculated that circadian core clock genes, such as Per2, Bmal1, Cry1/2, and Clock, may be involved in the pathophysiological changes observed in the development of OAB (5). In addition to its role in circadian regulation, sleep is widely recognized as a fundamental component of general health. Sleep hygiene — referring to behaviors that promote healthy sleep — plays a vital role in maintaining physical and mental well-being. Current guidelines recommend that adults aged 18–60 years should obtain at least 7 h of sleep per night to ensure adequate rest and recovery (6). Nonetheless, contemporary lifestyles, marked by shift work and recreational activities, often lead to insufficient sleep durations. In the United States, more than one-third of adults report routinely getting less than 7 h of sleep (7). Importantly, the negative effects of inadequate sleep extend beyond the urinary system, affecting multiple physiological processes, including immune regulation, nervous system stability, and metabolic homeostasis. For example, insufficient sleep has been linked to temporomandibular disorders (TMD), nervous system hyperstimulation, and disruptions in vitamin D metabolism (8). In this context, sleep deprivation has well-documented negative effects on health, particularly regarding nocturia within the spectrum of LUTS (2). This relationship may be bidirectional: frequent nocturia may cause sleep disturbances due to recurrent nocturnal awakening, and sleep disturbances may increase nocturia. Both conditions are linked to daytime fatigue, decreased quality of life, and increased risks of comorbidity (including depression, cardiovascular disease, and diabetes) and mortality (2, 9).
However, the relationship between sleep duration and the onset of OAB remains insufficiently understood, hindering the development of effective preventive measures. To explore this relationship, we analyzed data collected from the National Health and Nutrition Examination Survey (NHANES) collected from 2005 to 2018, aiming to better understand the interplay between sleep duration and OAB.
2 Materials and methods
2.1 Study population
In our study, we used data from seven two-year cycles of the National Health and Nutrition Examination Survey (NHANES), which was administered between 2005 and 2018. These years were selected on the basis of the availability of datasets containing relevant questions on sleep duration and urinary symptoms. The National Health and Nutrition Examination Survey (NHANES), managed by the Centers for Disease Control and Prevention (CDC), is a cross-sectional survey designed to collect nationally representative data through physical examinations, laboratory tests, and structured questionnaires.
Further details are available at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Ethical approval for the use of NHANES data was granted by the Institutional Review Board (IRB) of the National Center for Health Statistics, and informed consent was obtained from all participants.
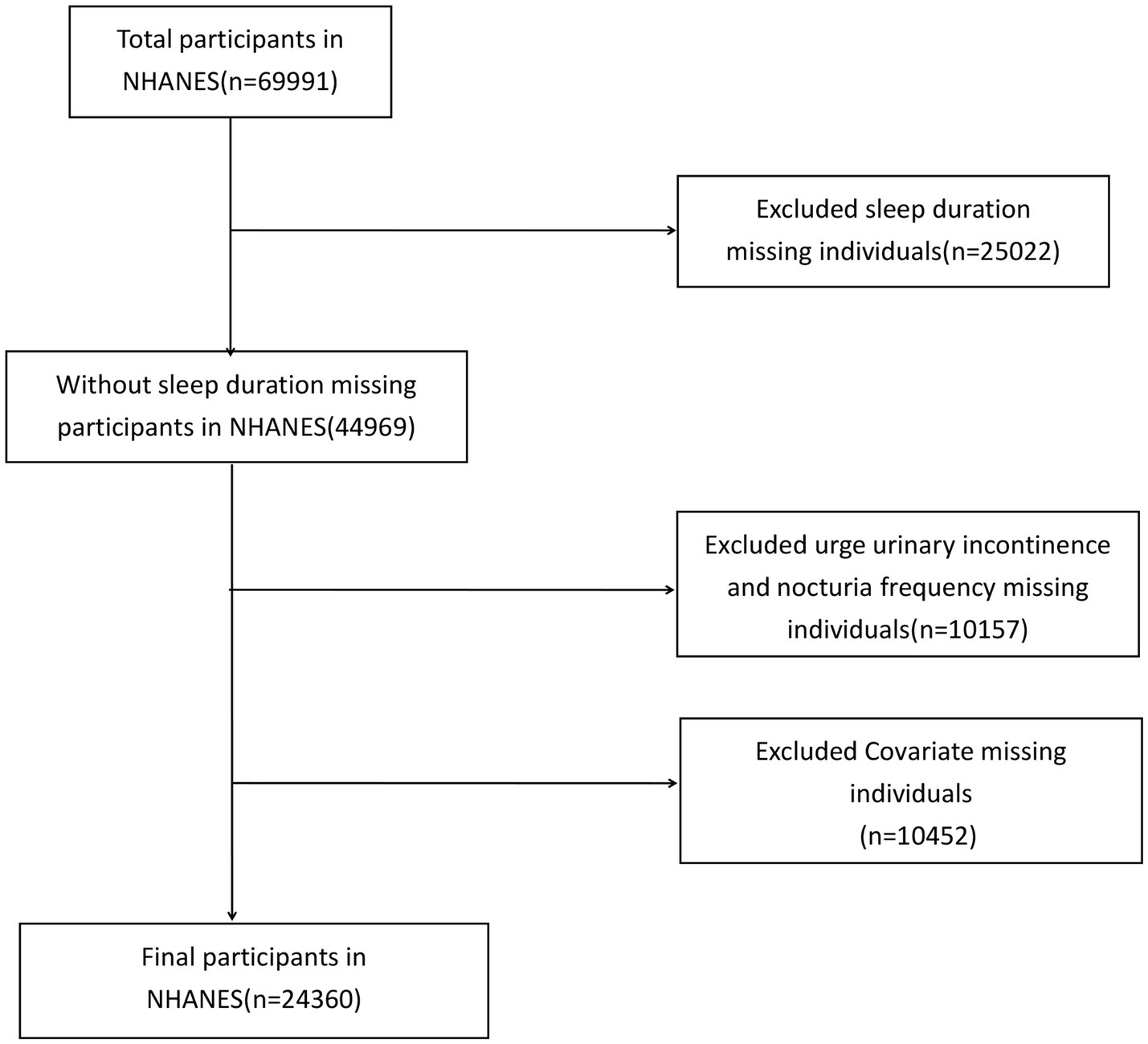
Since our study relies on existing NHANES data and does not generate any new data, additional ethical approval from external institutions is unnecessary. Participants aged 20–80 years who responded to the Kidney Conditions Questionnaire and reported sleep disturbances were included.
Individuals without an OAB diagnosis or with missing sleep duration and covariate information were excluded. A total of 24,360 participants met the inclusion criteria (Figure 1).
2.2 Variable measurement: sleep duration and OAB assessment
Information on OAB symptoms, such as urgency urinary incontinence and nocturia, was gathered from the NHANES Kidney Conditions-Urology Questionnaire.
2.3 The severity of urgency urinary incontinence was assessed with two questions
“In the past 12 months, have you ever leaked or lost control of even a small amount of urine because you could not get to the toilet quickly enough owing to a sudden urge to urinate?”
“How often has this occurred?”
2.4 The severity of nocturia was evaluated via the following question
“In the past 30 days, on average, how many times per night do you wake up to urinate from the time you go to bed at night until the time you get up in the morning?”
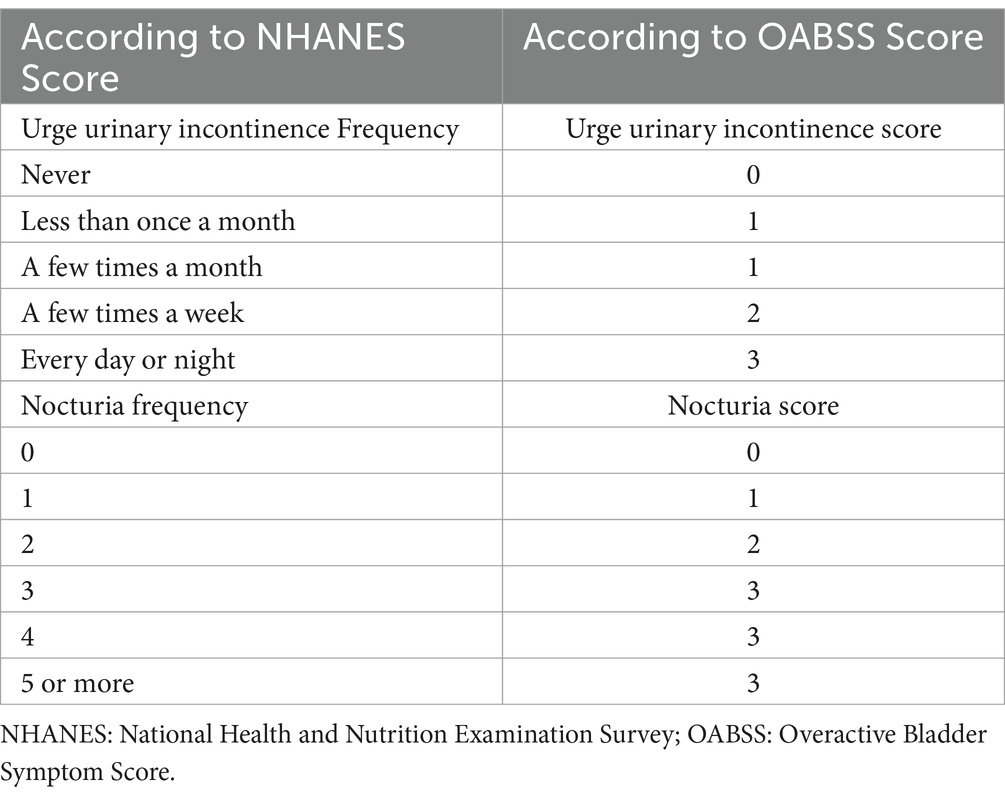
To further quantify the symptoms of OAB, we employed the Overactive Bladder Symptom Score (OABSS) developed by Blaivas et al. (10). The detailed scoring criteria are provided in Table 1. The final OABSS for each NHANES participant was calculated by summing the scores for urgency urinary incontinence and nocturia. A total score ≥3 was considered indicative of OAB.
The participants underwent face–to-face interviews conducted by two well-trained researchers. They self-reported their typical sleep duration on workdays or weekdays and responded to the following question: “On a typical workday or weekday night, how many hours do you usually sleep?” Responses were recorded by the interviewers.
2.5 Other covariates
Demographic variables: Age, sex, race, annual household income, educational level, and marital status at the time of the interview were self-reported and obtained from NHANES demographic files.
Body mass index (BMI) data for participants were obtained from NHANES body measurements, which were conducted by trained health technicians at the Mobile Examination Center (MEC).
Tobacco Use: Smoking status was ascertained on the basis of two questions from the questionnaire section: (1) “Have you ever smoked at least 100 cigarettes in your entire life?” and (2) “Do you currently smoke?” Individuals who reported smoking every day or some days and had smoked more than 100 cigarettes were classified as “current smokers.” Those who reported that they were not currently smoking but had smoked more than 100 cigarettes in the past were classified as “former smokers,” whereas individuals who had smoked fewer than 100 cigarettes were classified as “nonsmokers” (4).
Other covariates, including hypertension, diabetes, and hypercholesterolemia, were selected on the basis of prior research and clinical experience.
2.6 Statistical analysis
Data are shown as medians (interquartile ranges) for continuous variables. Statistical differences between groups were assessed using chi-square tests for categorical variables and t tests for continuous variables. Logistic regression models were used to create propensity scores (PSs) on the basis of potential confounders. The confounding factors included sex (male/female), age, BMI (kg/m2), race (Mexican American/other Hispanic/non-Hispanic White/non-Hispanic Black/other race), education (less than high school/high school graduate/GED or equivalent/more than high school), marital status (married/widowed/divorced/separated/never married/living with partner), annual family income (under $20,000/$20,000 and above), and current smoking and drinking status (never/former smoker/current smoker; yes/no for current drinking).
A generalized additive model (GAM) was employed to explore the nonlinear relationship between sleep duration and OAB following propensity score matching (PSM) analysis. A two-piecewise linear regression model was applied to assess the potential threshold effect of sleep duration on OAB. The inflection point was determined automatically through a recursive method when a clear inflection point in the smoothed curve of OAB and sleep duration was observed.
All analyses were performed using R version 4.1.3, with two-tailed tests and a p < 0.05 indicating statistical significance. Logistic regression results were visualized using the R package “forestplot,” and related charts were created with the R packages “ggplot2” and “gtsummary.”
3 Results
3.1 Baseline characteristics of the study population
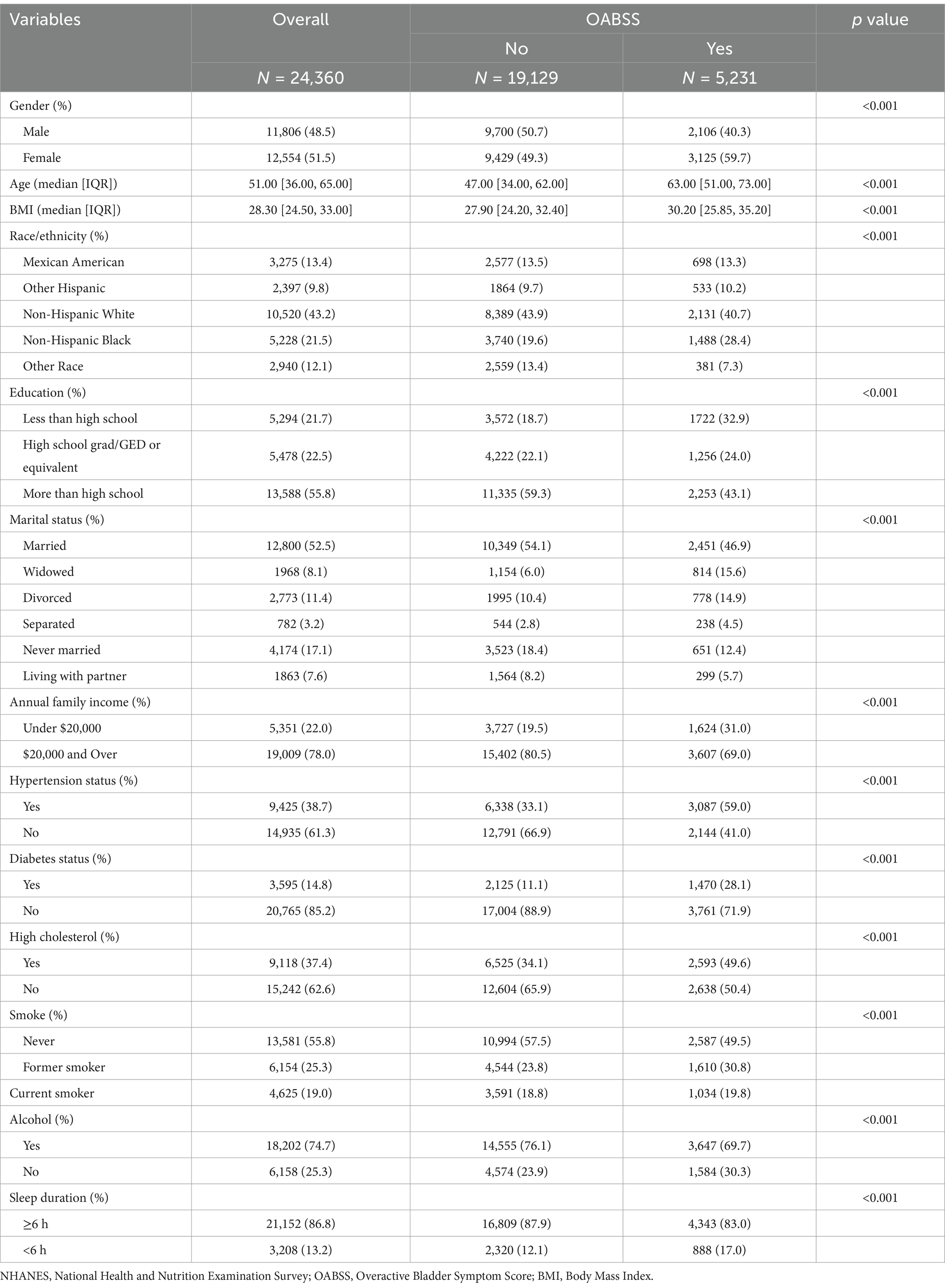
This study enrolled 24,360 participants for the baseline assessment (median age: 51 years [36, 65]; mean age: 49.8 ± 17.6 years; 51.5% female). More than 27.35% of the participants reporting OAB had a BMI over 30 kg/m2. Participants with OAB were more likely to have hypertension, diabetes, hypercholesterolemia, a history of smoking, lower educational levels, and lower annual family income. OAB was more prevalent among participants with shorter sleep durations. Table 2 provides a more detailed summary of participant characteristics.
3.2 Gender-stratified analysis of the association between sleep duration and OAB
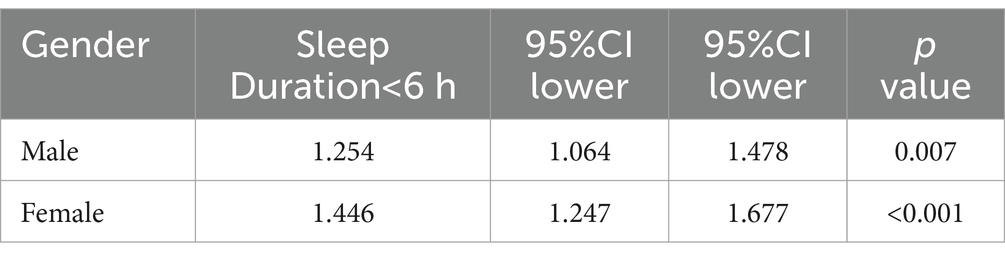
The baseline characteristics revealed a significantly higher prevalence of OAB in females (59.7%) compared to males (40.3%) (p < 0.001). To further investigate this, we performed gender-stratified analyses to explore the relationship between sleep duration and OAB risk. As shown in Table 3, short sleep duration (<6 h) was significantly associated with a higher risk of OAB in both genders, with a stronger association in females. Specifically, females with short sleep duration had a 1.446-fold increased risk of OAB (OR = 1.446; 95% CI: 1.247–1.677; p < 0.001), while the corresponding OR in males was 1.254 (95% CI: 1.064–1.478; p = 0.007).
3.3 Comparison between the low- and long-sleep duration groups before and after PSM
Given that factors such as sex, BMI, education, annual family income, hypertension status, diabetes status, high cholesterol, smoking, and alcohol consumption are closely associated with OAB and significantly differ between the low sleep duration (< 6 h) and long sleep duration (≥ 6 h) groups, we employed propensity score matching (PSM) to reduce these disparities. After PSM, we identified 3,208 matched pairs with comparable sex, BMI, education, annual family income, hypertension status, diabetes status, high cholesterol, smoking, and alcohol consumption (Table 4).
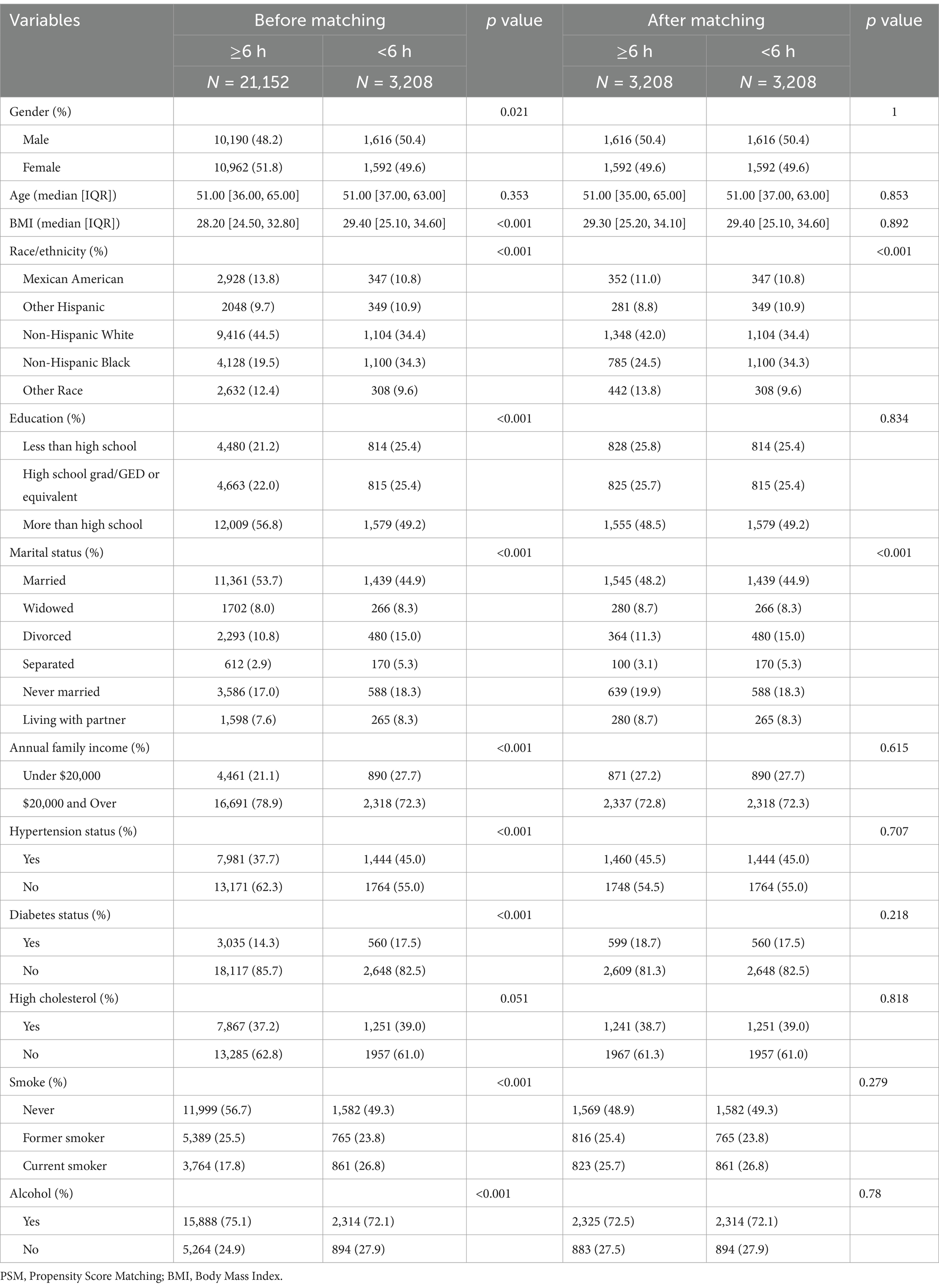
Table 4. Comparison between low sleep duration group and long sleep duration group before and after propensity score matching (PSM).
3.4 Multivariate analysis
After PSM, multivariable logistic regression identified sex, age, BMI, education level, marital status, annual family income, hypertension status, diabetes status, smoking, and sleep duration as independent risk factors for OAB. A sleep duration < 6 h was a significant risk factor for OAB. Patients with <6 h of sleep had a 1.325-fold greater risk of OAB than those with ≥ 6 h of sleep (OR 1.325; 95% CI, 1.169–1.501; p < 0.001; Figure 2). In the forest plot analysis, several significant associations were identified: females had a 1.727-fold greater risk of OAB than males did, and each additional year of age increased OAB risk by 4.2%. Higher education levels were associated with a decreased risk of OAB. Participants with an annual income > $20,000 had a 75.5% lower risk of OAB than those with an annual income < $20,000. Compared with hypertension, the absence of hypertension was associated with an 80.8% lower risk of OAB. Participants without diabetes had a 58.0% lower risk of OAB than those with diabetes did. Compared with nonsmokers, current smokers had a 46.5% increased risk of OAB (Figure 2).
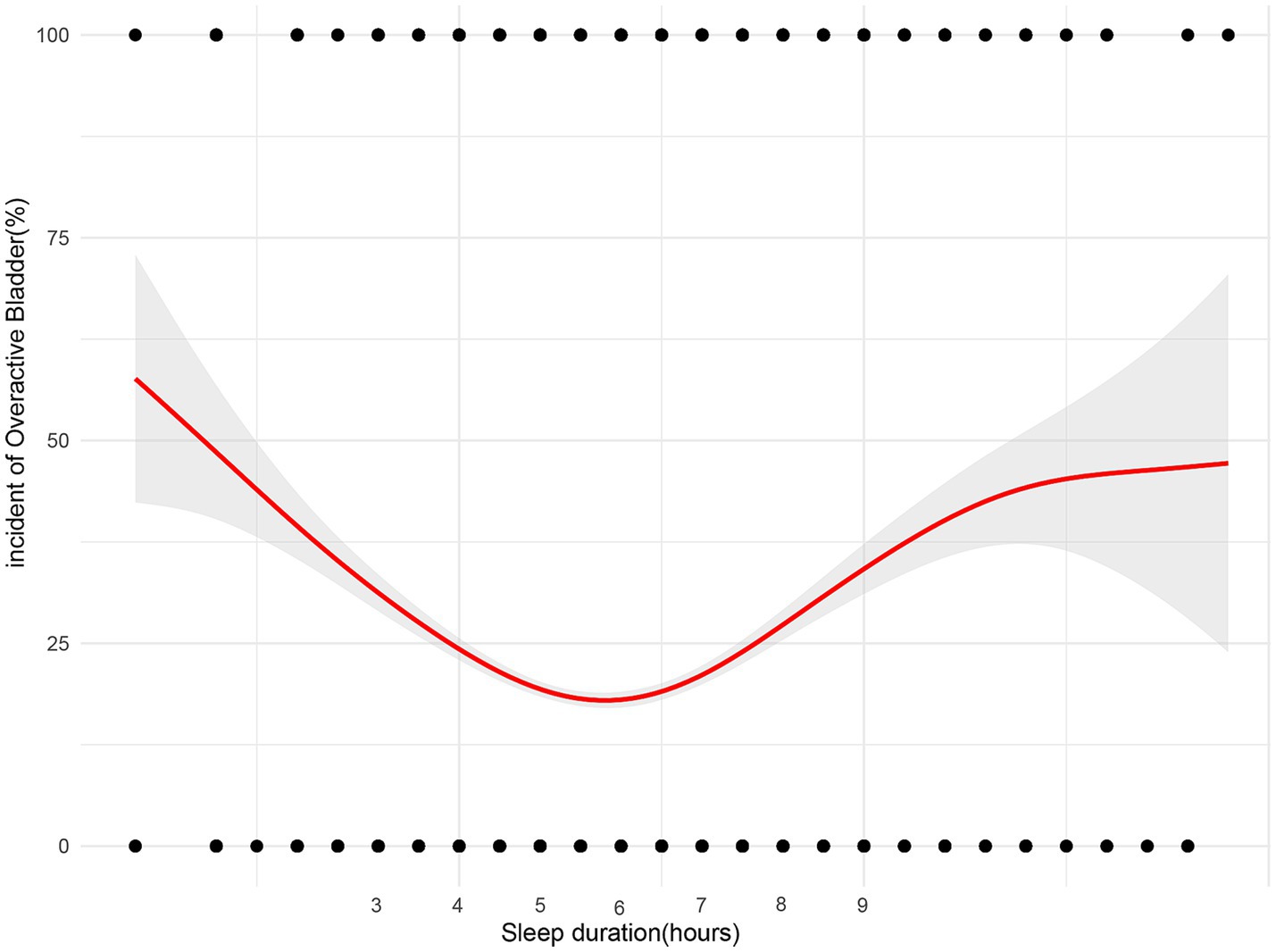
Figure 2. Forest plots depicting the associations between various demographic, socioeconomic, and health-related factors with sleep duration and the risk of Overactive Bladder (OAB). Each plot presents the odds ratios (OR) and corresponding 95% confidence intervals (CI) for individual variables. Statistically significant associations are marked by p-values < 0.05. The horizontal line at OR = 1 represents the reference point, indicating no effect, with factors whose confidence intervals do not cross this line being significantly associated with OAB risk.
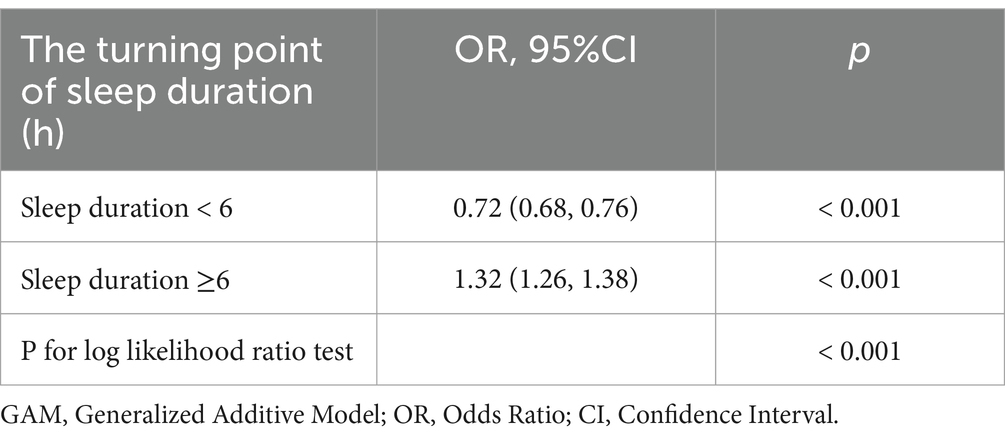
In further analyses, a GAM was utilized to investigate the relationship between sleep duration and the incidence of OAB (Figure 3), revealing a nonlinear relationship between the two variables. Piecewise linear regression (Table 5) revealed that a sleep duration < 6 h was inversely related to the development of OAB [OR = 0.72 (0.68, 0.76), p < 0.001]. However, a sleep duration ≥ 6 h was associated with an increased incidence of OAB [OR = 1.32 (1.26, 1.38), p < 0.001]. On the basis of this analysis, a sleep duration of 6 h appears to be optimal for minimizing the risk of developing OAB.
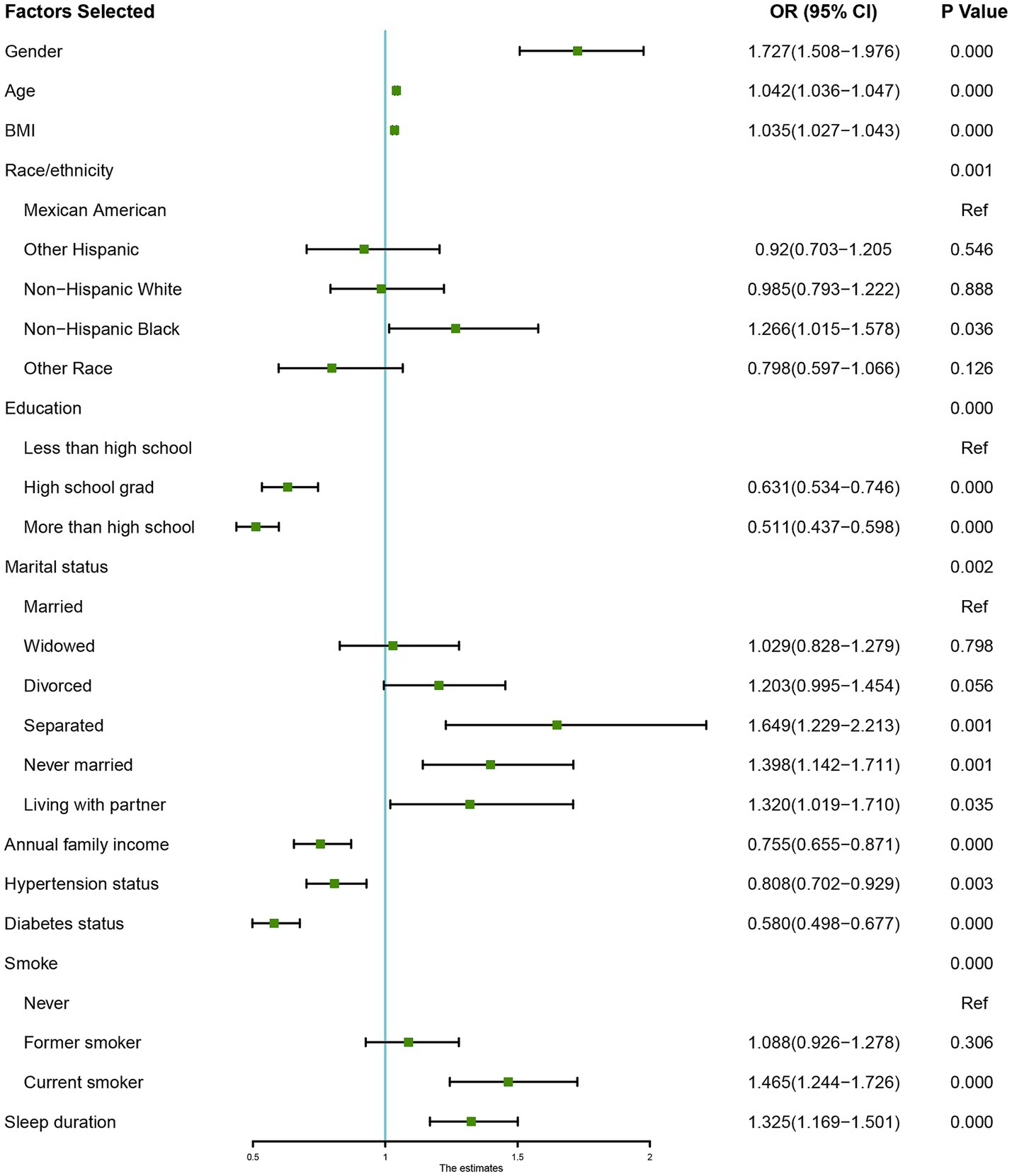
Figure 3. Non-linear relationship between sleep duration and the risk of Overactive Bladder (OAB). The plot illustrates the U-shaped association, with both short (<6 h) and long (>9 h) sleep durations showing increased OAB risk. The optimal sleep duration for minimizing OAB risk is approximately 6 h, as indicated by the curve’s lowest point.
4 Discussion
Our research demonstrated that sleep duration independently influences the development of OAB, revealing a U-shaped association after adjusting for confounding factors via PSM. The optimal sleep duration for minimizing OAB risk is approximately 6 h. Both short and long sleep durations were linked to an increased risk of OAB. Additional risk factors included sex, age, BMI, education, income, hypertension, diabetes, and smoking.
Our gender-stratified analysis found that short sleep duration is linked to a higher risk of OAB, with a stronger association in females. Several factors may explain this difference. Estrogen fluctuations during perimenopause and postmenopause can impair bladder function, increasing OAB risk in women (11). Additionally, females are more likely to experience sleep disturbances, which worsen nocturnal bladder symptoms (12). Anatomically, a shorter urethra and weaker pelvic support in females may also contribute to OAB (13). Furthermore, higher levels of stress, anxiety, and depression in women are known to exacerbate both sleep issues and OAB (14). These findings emphasize the need for gender-specific approaches in addressing sleep and OAB management. In addition, recent research has suggested that central sensitization-characterized by enhanced responsiveness of the central nervous system-may contribute to the presentation of symptoms in individuals with sleep disorders, including those with OAB (15). This mechanism may amplify the perception of bladder discomfort or urgency, particularly in those with chronic sleep disturbances.
OAB is a common urological condition that significantly disrupts daily activities due to its complex and multifactorial pathophysiology. A growing body of evidence suggests that factors such as age, diabetes, obesity, psychological and behavioral changes, and sleep disturbances contribute to OAB onset and progression (12, 16, 17). Sleep disturbances are associated with a greater risk of daytime urinary symptoms, which impact both the storage and micturition phases (18). Many OAB patients also experience sleep disturbances (12, 19). In women with OAB, nocturnal bladder symptoms are often considered the primary cause of poor sleep quality or sleep disruption (20). Women with insomnia may be more sensitive to bladder filling due to hyperarousal, resulting in more frequent nocturnal awakenings for urination. Conversely, they may awaken for other reasons and subsequently experience the urge to urinate, prompting bathroom visits (21). Furthermore, adequate sleep is essential for immune system homeostasis and for stabilizing the detrusor muscle of the bladder. Obstructive sleep apnea triggers cell survival signaling pathways, leading to oxidative stress in the bladder, as evidenced by increased malondialdehyde and advanced oxidation protein products. This oxidative stress contributes to detrusor muscle instability and exacerbates OAB symptoms (22). This study highlights the significant role of sleep duration in OAB risk. A U-shaped curve was observed, suggesting that both excessive and insufficient sleep duration increase OAB risk. This relationship remains robust even after controlling for potential confounding variables through PSM.
Despite the well-established association between sleep duration and OAB, the underlying biological mechanisms that explain why approximately 6 h of sleep may be optimal for reducing OAB risk remain unclear. One possible explanation involves the regulation of circadian rhythms, which are known to govern various physiological processes, including bladder function. Bladder muscle cells possess an intrinsic circadian clock regulating connexin 43 (Cx43), which enhances intercellular electrical and chemical transmission, decreases bladder capacity and increases urinary frequency, mimicking OAB symptoms (23). Sleep plays a key role in regulating circadian rhythms, with the transcription factor BMAL1/Mop3 being a pivotal regulator of the sleep–wake cycle in mammals (24). Disruptions in BMAL1/Mop3 can result in sleep fragmentation, increased electroencephalogram (EEG) delta wave activity, and reduced compensation for acute sleep loss (25). Deficiencies in the circadian rhythm gene BMAL1/Mop3 may alter the excitability of histaminergic neurons, affecting the brain’s biological clock and bladder function (26). This alteration could lead to abnormal bladder activity, potentially contributing to OAB development (27). Notably, sleep duration is a crucial component in maintaining circadian rhythm stability. Both insufficient and excessive sleep can disturb this homeostasis, thereby affecting bladder function. In addition, inflammatory and oxidative stress pathways may serve as important mediators linking abnormal sleep duration to bladder dysfunction. Both short and long sleep durations have been associated with immune dysregulation, elevated inflammatory responses, oxidative stress, hormonal imbalance, and psychological disturbances, all of which may contribute to the development and progression of OAB (28, 29). Sleep disturbances and acute sleep deprivation are linked to elevated levels of proinflammatory cytokines, such as prostaglandin E2 (PGE2), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), increasing susceptibility to infections and inflammation (30–32). The pathophysiology of OAB may involve dysregulated production of inflammatory mediators such as PGE2, leukotrienes, and CRP, indicating a potential inflammatory component in OAB development (33–35).
Moreover, individuals with sleep disorders frequently present with metabolic abnormalities (36). Previous studies have shown that males with metabolic syndrome are more likely to develop nocturia and impaired bladder emptying (37). Evidence indicates that obesity is an independent risk factor for OAB (38, 39). Endoplasmic reticulum stress in the bladder may lead to insulin resistance, potentially contributing to OAB development (40). Therefore, metabolic processes may mediate the link between sleep disturbances and OAB. The link between sleep duration and OAB is complex and requires further investigation.
In addition to sleep duration’s impact on OAB risk, the bidirectional nature of the relationship should be considered. Nocturia, characterized by the need to wake at night to urinate, is a prevalent symptom of OAB can lead to sleep deprivation and poor sleep quality (41). This sleep disruption can result in daytime fatigue, mood changes, and impaired cognitive function. Conversely, inadequate sleep may exacerbate OAB symptoms, creating a cyclical pattern where each condition perpetuates the other (12, 42). Therefore, both the quality and duration of sleep should be considered in future studies exploring OAB and its management. Among various aspects of sleep quality, sleep fragmentation — referring to repeated interruptions or arousals during sleep — has become an increasingly common issue in modern society. Fragmented sleep not only impairs sleep continuity and reduces restorative sleep stages but also adversely affects immune function, metabolic balance, and emotional regulation (43). These negative consequences may, in turn, increase the susceptibility to OAB and other chronic conditions.
Our multivariate analysis revealed that high income, higher education and abstaining from alcohol and smoking are protective factors against OAB. Consistent with previous studies, our research revealed a positive correlation between lower socioeconomic status (lower levels of education and income) and higher OAB incidence (44, 45). Individuals with higher levels of education are more likely to adopt health-promoting behaviors, whereas those with limited education are prone to poor dietary habits and toxin exposure (46). Smoking is a major public health challenge that can be prevented. Previous studies have consistently indicated that smoking is a risk factor for OAB (47, 48). Smoking is thought to affect OAB by disrupting nicotinic acetylcholine receptors in the nervous system (49). Furthermore, the antiestrogenic effects of nicotine on the bladder and urethra may lead to OAB and incontinence, suggesting a direct link between smoking and urological dysfunction (50). Alcohol consumption is associated with a higher risk of OAB (47), although some studies suggest a potential protective role of beer (51). In the context of alcohol consumption, both acute and chronic exposure are associated with dysregulation of serum hormone levels, including increased estrogen and decreased androgen levels (46). These hormonal changes may contribute to OAB development through various biological mechanisms, as elucidated by recent research (52). Experimental evidence suggests that testosterone may alleviate fibrotic changes in bladder tissue (53) and affect urethral mediator release, indicating that androgen deficiency could contribute to OAB (54).
This study is the first comprehensive cross-sectional investigation leveraging the NHANES database to examine the relationship between sleep duration and OAB. However, several limitations should be acknowledged. First, the cross-sectional design prevents the determination of causality regarding sleep duration and OAB. Second, the assessment of OAB relies on self-reported data and historical records, with the onset time of OAB being unknown. Third, sleep duration was based on a single self-reported question, limiting our ability to evaluate sleep patterns, quality, or specific sleep disorders. This approach may also introduce recall and social desirability bias, as participants could overreport what they perceive as ideal sleep duration. The absence of objective measures, such as actigraphy or wearable trackers, further limits accuracy. Additionally, sleep quality indicators such as fragmentation, efficiency, or deep sleep duration, were not included but may also affect OAB risk. Finally, environmental factors such as job stress, shift work, social pressure, noise, and climate—known to influence sleep and possibly worsen OAB—were not captured in the dataset and could not be controlled for. Future studies should incorporate objective sleep measures and account for these environmental and psychosocial variables to clarify the complex link between sleep and OAB.
5 Conclusion
Our cross-sectional study using the NHANES database revealed a U-shaped correlation between sleep duration and OAB, with an optimal duration of approximately 6 h. Moreover, our findings highlight the multifactorial nature of OAB, involving factors such as sex, age, BMI, education, income, hypertension, diabetes, and smoking. Future research should investigate the complex interactions between these factors and sleep duration to develop more precise prevention and treatment strategies for OAB.
These findings provide new evidence for integrating sleep health management into public health policies and chronic disease prevention strategies. Public health measures that emphasize appropriate sleep duration, combined with established lifestyle interventions, may help reduce the burden of OAB, particularly among high-risk groups. Future research should prioritize designing and assessing targeted sleep interventions to optimize bladder health and enhance population outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by participants received approval from the NCHS Research Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because In line with national legislation and institutional guidelines, written informed consent was not required for participation in this study.
Author contributions
J-JW: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. A-HJ: Data curation, Investigation, Methodology, Writing – original draft. L-MH: Conceptualization, Data curation, Investigation, Writing – original draft. X-WT: Supervision, Validation, Writing – review & editing. X-PH: Conceptualization, Methodology, Supervision, Writing – review & editing. L-CM: Formal analysis, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was partially funded by grants from the Zhejiang Province Traditional Chinese Medicine Science and Technology Program (2022ZB388 to L-CM).
Acknowledgments
We gratefully acknowledge AJE (American Journal Experts) for their editorial assistance, particularly in refining the grammar, phrasing, punctuation, and overall logical coherence of the manuscript. Our sincere thanks also go to the contributors of NHANES data used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. We acknowledge the use of AI language tools in the preparation of this manuscript to enhance linguistic fluency and clarity. We use ChatGPT-3.5 (OpenAI) for text editing and grammar improvement. However, the content and conclusions of this study are based on independent work by our research team, and all AI-assisted text has been thoroughly reviewed to ensue academic rigor and originality. We take full responsibility for the accuracy and completeness of this paper.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1537796/full#supplementary-material
References
1. Redmond, EJ, O'Kelly, T, and Flood, HD. The effect of bladder filling rate on the voiding behavior of patients with overactive bladder. J Urol. (2019) 202:326–32. doi: 10.1097/JU.0000000000000199
2. Farag, F, Sakalis, VI, Arteaga, SM, Sihra, N, Karavitakis, M, Arlandis, S, et al. What are the short-term benefits and potential harms of therapeutic modalities for the Management of Overactive Bladder Syndrome in women? A review of evidence under the auspices of the European Association of Urology, female non-neurogenic lower urinary tract symptoms guidelines panel. Eur Urol. (2023) 84:302–12. doi: 10.1016/j.eururo.2023.05.014
3. Warsi, QA, Huang, AJ, Hess, R, Arya, LA, Richter, HE, Bradley, CS, et al. Association of Pharmacologic Treatment of urgency urinary incontinence with sleep quality and daytime sleepiness. Obstet Gynecol. (2018) 131:204–11. doi: 10.1097/AOG.0000000000002443
4. Al Harthi, S, Natto, ZS, Midle, JB, Gyurko, R, Oneill, R, and Steffensen, B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and nutrition examination surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90:16–25. doi: 10.1002/JPER.18-0183
5. Negoro, H, Kanematsu, A, Yoshimura, K, and Ogawa, O. Chronobiology of micturition: putative role of the circadian clock. J Urol. (2013) 190:843–9. doi: 10.1016/j.juro.2013.02.024
6. Watson, NF, Badr, MS, Belenky, G, Bliwise, DL, Buxton, OM, Buysse, D, et al. Joint consensus statement of the American Academy of sleep medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. (2015) 38:1161–83. doi: 10.5665/sleep.4886
7. Liu, Y, Wheaton, AG, Chapman, DP, Cunningham, TJ, Lu, H, and Croft, JB. Prevalence of healthy sleep duration among adults--United States, 2014. MMWR Morb Mortal Wkly Rep. (2016) 65:137–41. doi: 10.15585/mmwr.mm6506a1
8. Seweryn, P, Orzeszek, SM, Waliszewska-Prosół, M, Jenča, A, Osiewicz, M, Paradowska-Stolarz, A, et al. Relationship between pain severity, satisfaction with life and the quality of sleep in polish adults with temporomandibular disorders. Dent Med Probl. (2023) 60:609–17. doi: 10.17219/dmp/171894
9. Sakalis, VI, Karavitakis, M, Bedretdinova, D, Bach, T, Bosch, J, Gacci, M, et al. Medical treatment of Nocturia in men with lower urinary tract symptoms: systematic review by the European Association of Urology guidelines panel for male lower urinary tract symptoms. Eur Urol. (2017) 72:757–69. doi: 10.1016/j.eururo.2017.06.010
10. Blaivas, JG, Panagopoulos, G, Weiss, JP, and Somaroo, C. Validation of the overactive bladder symptom score. J Urol. (2007) 178:543–7. doi: 10.1016/j.juro.2007.03.133
11. Clark, AL. Overactive bladder, stress urinary incontinence, and menopause-what are the associations. Menopause. (2022) 29:125–6. doi: 10.1097/GME.0000000000001929
12. Savoie, MB, Lee, KA, Subak, LL, Hernandez, C, Schembri, M, Fung, CH, et al. Beyond the bladder: poor sleep in women with overactive bladder syndrome. Am J Obstet Gynecol. (2020) 222:600.e1–600.e13. doi: 10.1016/j.ajog.2019.12.005
13. van Geelen, H, and Sand, PK. The female urethra: urethral function throughout a woman's lifetime. Int Urogynecol J. (2023) 34:1175–86. doi: 10.1007/s00192-023-05469-6
14. Mehr, AA, Kreder, KJ, Lutgendorf, SK, Ten Eyck, P, Greimann, ES, and Bradley, CS. Daily symptom associations for urinary urgency and anxiety, depression and stress in women with overactive bladder. Int Urogynecol J. (2022) 33:841–50. doi: 10.1007/s00192-021-05033-0
15. Seweryn, P, Waliszewska-Prosol, M, Straburzynski, M, Smardz, J, Orzeszek, S, Bombala, W, et al. Prevalence of central sensitization and somatization in adults with temporomandibular disorders-a prospective observational study. J Oral Facial Pain Headache. (2024) 38:33–44. doi: 10.22514/jofph.2024.037
16. Kido, K, Hatakeyama, S, Imai, A, Yamamoto, H, Tobisawa, Y, Yoneyama, T, et al. Sleep disturbance has a higher impact on general and mental quality of life reduction than Nocturia: results from the community health survey in Japan. Eur Urol Focus. (2019) 5:1120–6. doi: 10.1016/j.euf.2018.04.017
17. Tam, CA, Helfand, BT, and Erickson, BA. The relationship between diabetes, diabetes severity, diabetes biomarkers, and the presence of lower urinary tract symptoms: findings from the National Health and nutrition examination survey. Urology. (2017) 105:141–8. doi: 10.1016/j.urology.2017.03.040
18. Helfand, BT, McVary, KT, Meleth, S, Sharp, V, Foster, H, Naslund, M, et al. The relationship between lower urinary tract symptom severity and sleep disturbance in the CAMUS trial. J Urol. (2011) 185:2223–8. doi: 10.1016/j.juro.2011.02.012
19. Lu, Z, Zhang, J, Lin, S, Fan, Z, He, Z, and Tang, F. Associations between overactive bladder and sleep patterns: a cross-sectional study based on 2007-2014 NHANES. BMC Urol. (2023) 23:184. doi: 10.1186/s12894-023-01329-z
20. Winkelman, WD, Warsi, A, Huang, AJ, Schembri, M, Rogers, RG, Richter, HE, et al. Sleep quality and daytime sleepiness among women with urgency predominant urinary incontinence. Female Pelvic Med Reconstr Surg. (2018) 24:76–81. doi: 10.1097/SPV.0000000000000547
21. Monaghan, TF, Weiss, JP, Wein, AJ, Rahman, SN, Lazar, JM, Bliwise, DL, et al. Sleep disorders, comorbidities, actions, lower urinary tract dysfunction, and medications ("sleep C.A.L.M.") in the evaluation and management of nocturia: A simple approach to a complex diagnosis. Neurourol Urodyn. (2023) 42:562–72. doi: 10.1002/nau.25128
22. Witthaus, MW, Nipa, F, Yang, JH, Li, Y, Lerner, LB, and Azadzoi, KM. Bladder oxidative stress in sleep apnea contributes to detrusor instability and nocturia. J Urol. (2015) 193:1692–9. doi: 10.1016/j.juro.2014.11.055
23. Negoro, H, Kanematsu, A, Doi, M, Suadicani, SO, Matsuo, M, Imamura, M, et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat Commun. (2012) 3:809. doi: 10.1038/ncomms1812
24. Jagannath, A, Taylor, L, Wakaf, Z, Vasudevan, SR, and Foster, RG. The genetics of circadian rhythms, sleep and health. Hum Mol Genet. (2017) 26:R128–128R138. doi: 10.1093/hmg/ddx240
25. Laposky, A, Easton, A, Dugovic, C, Walisser, J, Bradfield, C, and Turek, F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. (2005) 28:395–410. doi: 10.1093/sleep/28.4.395
26. Yu, X, Zecharia, A, Zhang, Z, Yang, Q, Yustos, R, Jager, P, et al. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr Biol. (2014) 24:2838–44. doi: 10.1016/j.cub.2014.10.019
27. Liao, KK, Chen, JT, Lai, KL, Liu, CY, Lin, CY, Lin, YY, et al. Effect of sacral-root stimulation on the motor cortex in patients with idiopathic overactive bladder syndrome. Neurophysiol Clin. (2008) 38:39–43. doi: 10.1016/j.neucli.2007.09.004
28. Besedovsky, L, Lange, T, and Haack, M. The sleep-immune crosstalk in health and disease. Physiol Rev. (2019) 99:1325–80. doi: 10.1152/physrev.00010.2018
29. van Kerrebroeck, P, Hashim, H, Holm-Larsen, T, Robinson, D, and Stanley, N. Thinking beyond the bladder: antidiuretic treatment of nocturia. Int J Clin Pract. (2010) 64:807–16. doi: 10.1111/j.1742-1241.2010.02336.x
30. Haack, M, Lee, E, Cohen, DA, and Mullington, JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. (2009) 145:136–41. doi: 10.1016/j.pain.2009.05.029
31. Haack, M, Sanchez, E, and Mullington, JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. (2007) 30:1145–52. doi: 10.1093/sleep/30.9.1145
32. Meier-Ewert, HK, Ridker, PM, Rifai, N, Regan, MM, Price, NJ, Dinges, DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. (2004) 43:678–83. doi: 10.1016/j.jacc.2003.07.050
33. Cartwright, R, Afshan, I, Derpapas, A, Vijaya, G, and Khullar, V. Novel biomarkers for overactive bladder. Nat Rev Urol. (2011) 8:139–45. doi: 10.1038/nrurol.2011.7
34. Wang, H, Zhao, M, Liu, J, Liu, L, Liu, H, Ding, N, et al. H2O2 enhances the spontaneous phasic contractions of isolated human-bladder strips via activation of TRPA1 channels on sensory nerves and the release of substance P and PGE2. Free Radic Biol Med. (2023) 209:1–8. doi: 10.1016/j.freeradbiomed.2023.10.001
35. Kupelian, V, Rosen, RC, Roehrborn, CG, Tyagi, P, Chancellor, MB, and McKinlay, JB. Association of overactive bladder and C-reactive protein levels. Results from the Boston area community health (BACH) survey. BJU Int. (2012) 110:401–7. doi: 10.1111/j.1464-410X.2011.10769.x
36. Umbro, I, Fabiani, V, Fabiani, M, Angelico, F, and Del Ben, M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J Gastroenterol. (2020) 26:2669–81. doi: 10.3748/wjg.v26.i20.2669
37. Rohrmann, S, Smit, E, Giovannucci, E, and Platz, EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the third National Health and nutrition examination survey (NHANES III). Int J Obes. (2005) 29:310–6. doi: 10.1038/sj.ijo.0802881
38. Zacche, MM, Giarenis, I, Thiagamoorthy, G, Robinson, D, and Cardozo, L. Is there an association between aspects of the metabolic syndrome and overactive bladder? A prospective cohort study in women with lower urinary tract symptoms. Eur J Obstet Gynecol Reprod Biol. (2017) 217:1–5. doi: 10.1016/j.ejogrb.2017.08.002
39. Lai, HH, Helmuth, ME, Smith, AR, Wiseman, JB, Gillespie, BW, Kirkali, Z, et al. Relationship between central obesity, general obesity, overactive bladder syndrome and urinary incontinence among male and female patients seeking Care for Their Lower Urinary Tract Symptoms. Urology. (2019) 123:34–43. doi: 10.1016/j.urology.2018.09.012
40. Leiria, LO, Sollon, C, Báu, FR, Mónica, FZ, D'Ancona, CL, De Nucci, G, et al. Insulin relaxes bladder via PI3K/AKT/eNOS pathway activation in mucosa: unfolded protein response-dependent insulin resistance as a cause of obesity-associated overactive bladder. J Physiol. (2013) 591:2259–73. doi: 10.1113/jphysiol.2013.251843
41. Verbakel, I, Boukheir, G, Bliwise, D, Vogelaers, D, Hervé, F, Weiss, J, et al. From nocturnal awakenings to nocturnal voiding: the relationship between insomnia and nocturia - a systematic review. Minerva Urol Nephrol. (2023) 77:181–91. doi: 10.23736/S2724-6051.23.05384-3
42. Finamore, P, Scarlata, S, Laudisio, A, Galdi, F, Pipita, ME, Chiarella, I, et al. Occurrence of nocturia is not mediated by nocturnal hypoxia length and severity in patients with sleep-disordered breathing. Sleep Med. (2018) 45:69–73. doi: 10.1016/j.sleep.2018.01.009
43. Martynowicz, H, Wichniak, A, and Więckiewicz, M. Sleep disorders and cardiovascular risk: focusing on sleep fragmentation. Dent Med Probl. (2024) 61:475–7. doi: 10.17219/dmp/185395
44. Coyne, KS, Sexton, CC, Kopp, ZS, Ebel-Bitoun, C, Milsom, I, and Chapple, C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. (2011) 108:1459–71. doi: 10.1111/j.1464-410X.2010.10013.x
45. Kim, SY, Bang, W, and Choi, HG. Analysis of the prevalence of and factors associated with overactive bladder in adult Korean women. PLoS One. (2017) 12:e0185592. doi: 10.1371/journal.pone.0185592
46. Zhu, J, Hu, X, Dong, X, and Li, L. Associations between risk factors and overactive bladder: A Meta-analysis. Female Pelvic Med Reconstr Surg. (2019) 25:238–46. doi: 10.1097/SPV.0000000000000531
47. Joseph, MA, Harlow, SD, Wei, JT, Sarma, AV, Dunn, RL, Taylor, JM, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. (2003) 157:906–14. doi: 10.1093/aje/kwg051
48. de Boer, TA, Slieker-ten Hove, MC, Burger, CW, and Vierhout, ME. The prevalence and risk factors of overactive bladder symptoms and its relation to pelvic organ prolapse symptoms in a general female population. Int Urogynecol J. (2011) 22:569–75. doi: 10.1007/s00192-010-1323-x
49. Maserejian, NN, Kupelian, V, Miyasato, G, McVary, KT, and McKinlay, JB. Are physical activity, smoking and alcohol consumption associated with lower urinary tract symptoms in men or women? Results from a population based observational study. J Urol. (2012) 188:490–5. doi: 10.1016/j.juro.2012.03.128
50. Madhu, C, Enki, D, Drake, MJ, and Hashim, H. The functional effects of cigarette smoking in women on the lower urinary tract. Urol Int. (2015) 95:478–82. doi: 10.1159/000438928
51. Dallosso, HM, Matthews, RJ, CW, MG, Donaldson, MM, and Shaw, CLeicestershire MRC Incontinence Study Group. The association of diet and other lifestyle factors with the onset of overactive bladder: a longitudinal study in men. Public Health Nutr. (2004) 7:885–91. doi: 10.1079/phn2004627
52. Peyronnet, B, Mironska, E, Chapple, C, Cardozo, L, Oelke, M, Dmochowski, R, et al. A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur Urol. (2019) 75:988–1000. doi: 10.1016/j.eururo.2019.02.038
53. de Barros, CA, Lorenzetti, F, Ortiz, V, and Dambros, M. Testosterone supplementation's effects on age-related bladder remodeling - experimental study in rats. Aging Male. (2013) 16:102–7. doi: 10.3109/13685538.2013.807426
Keywords: sleep duration, overactive bladder, cross-sectional study, NHANES, propensity score matching
Citation: Wang J-J, Jin A-H, Hu L-M, Tu X-W, Huang X-P and Mo L-C (2025) Sleep duration and overactive bladder syndrome in US adults: a propensity score matching cohort study using NHANES 2005–2018. Front. Neurol. 16:1537796. doi: 10.3389/fneur.2025.1537796
Edited by:
Jad A. Degheili, Ibn Sina Hospital, KuwaitReviewed by:
Anna Paradowska-Stolarz, Wroclaw Medical University, PolandYaxin Zhang, Xiamen Hongai Hospital, China
Copyright © 2025 Wang, Jin, Hu, Tu, Huang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Peng Huang, aHhwMTM5NTg2NzcyMDFAMTYzLmNvbQ==; Li-Cai Mo, bW9sYzg5MTJAZW56ZW1lZC5jb20=
†These authors have contributed equally to this work
 Jia-Jia Wang
Jia-Jia Wang Ai-Hong Jin1†
Ai-Hong Jin1† Xiao-Peng Huang
Xiao-Peng Huang Li-Cai Mo
Li-Cai Mo