- Department of Neurology, Taizhou First People's Hospital, Zhejiang, China
Background: Bone morphogenetic protein 6 (BMP6) has been implicated in the pathogenesis of Alzheimer's disease (AD), and its levels have been reported to be associated with cognitive performance. However, few studies have examined the association between plasma BMP6 levels and brain atrophy in older adults.
Methods: A total of 340 older adults without dementia were included in the current study. Study participants had baseline plasma BMP6 data available and at least two structural MRI scans. Volumes of six brain regions were measured, including the hippocampus, entorhinal cortex, middle temporal gyrus, fusiform gyrus, ventricles, and whole brain. A series of linear mixed-effects models were built to examine the associations of plasma BMP6 levels with brain atrophy over time.
Results: Our study revealed that higher plasma BMP6 levels were associated with a reduced rate of volume loss in the hippocampus, entorhinal cortex, middle temporal gyrus, and whole brain. However, there was no significant link between plasma BMP6 levels and changes in the volume of the fusiform gyrus or ventricles.
Conclusion: Our results may provide novel insights into the mechanisms of neurodegeneration in AD, contributing to new avenues for timely intervention and potentially slowing disease progression.
Introduction
Alzheimer's disease (AD) is characterized by progressive cognitive decline and neurodegeneration and is the most common cause of dementia (1, 2). AD is associated with substantial brain atrophy, particularly in the hippocampus and entorhinal cortex, regions crucial for memory and learning (3, 4). Recently, the search for blood biomarkers that can predict or monitor disease progression and neurodegeneration has gained increasing interest (5, 6).
Bone morphogenetic protein 6 (BMP6), a member of the transforming growth factor-β superfamily, has been implicated in various biological processes, including neuronal differentiation and axonal growth (7). A previous study has shown that BMP6 levels are significantly increased in the brains of AD patients and APP transgenic mice (8). This elevation in BMP6 is accompanied by impaired hippocampal neurogenesis (8). A recent longitudinal study has examined the association between blood BMP6 levels and cognitive performance in older people (9). This study suggested that higher levels of blood BMP6 are associated with better cognitive function, highlighting the potential of BMP6 not only as a therapeutic target but also as a biomarker for early detection and monitoring of AD progression (9). However, the relationship between plasma BMP6 levels and brain atrophy, as measured by MRI, has not been previously explored.
To fill this gap, we aimed to investigate the association between plasma BMP6 levels and six MRI-based regional brain atrophy, including hippocampus, entorhinal cortex, fusiform gyrus, middle temporal gyrus, ventricles, and whole brain. Our findings could facilitate early detection of neurodegeneration in AD, allowing for timely intervention and potentially slowing disease progression.
Methods
Alzheimer's Disease Neuroimaging Initiative (ADNI) study
Data of the current study were downloaded from the ADNI database (adni.loni.usc.edu). The ADNI study was initiated in 2003 with the primary goal of examining whether demographic, clinical, neuropsychological, neuroimaging, and biological markers can be integrated to track the progression of mild cognitive impairment (MCI) and mild AD. The ADNI study was approved by the Institutional Review Boards of each participating center, and written informed consent was provided by each study participant. More detailed information can be found on the ADNI website (adni.loni.usc.edu) and has been described previously (10).
Study sample
We selected study participants who had baseline plasma BMP6 data available and at least two structural MRI scans. In the current study, we included a total of 340 older adults without dementia, including 52 participants with normal cognition (NC) and 288 participants with MCI. The criteria for NC included a Mini-Mental State Examination (MMSE) (11) score between 24 and 30 and a Clinical Dementia Rating (CDR) (12) score of 0. The criteria for MCI included an MMSE score between 24 and 30, a CDR of 0.5, a subjective memory complaint, and objective memory loss as measured by education-adjusted scores on the Wechsler Memory Scale Logical Memory II, with no significant interference with daily life activities.
Measurement of plasma BMP6 levels
Plasma BMP6 levels were examined using a multiplex-based immunoassay panel based on Luminex immunoassay technology and the detailed procedures of plasma collection and measurement have been described elsewhere (https://adni.loni.usc.edu/wp-content/uploads/2010/11/BC_Plasma_Proteomics_Data_Primer.pdf). Briefly, plasma proteins including BMP6 levels were examined using a subset of plasma samples from the ADNI cohort by a 190-analyte multiplex immunoassay panel. The panel was developed on the Luminex xMAP platform by Rules-Based Medicine (RBM) and contains multiple proteins (13). We used the quality-controlled values of plasma BMP6 in all statistical analyses. Plasma BMP6 values were expressed in ng/mL. Values were log transformed before statistical analyses.
Measurement of MRI neuroimaging markers
The ADNI imaging procedures can be found on the website (https://adni.loni.usc.edu/data-samples/adni-data/neuroimaging/mri/) and have been described previously (14). Volumetric segmentation of T1-weighted sagittal 3D MPRAGE sequences from MRI scans were processed using FreeSurfer image analysis software (http://surfer.nmr.mgh.harvard.edu/) by the ADNI investigators at the University of California, San Francisco (15). We extracted volumes of the hippocampus, entorhinal cortex, fusiform gyrus, middle temporal gyrus, ventricles, and whole brain from the ADNIMERGE dataset. To account for brain volume differences related to head size, we calculated adjusted volumes using the following equation: Adjusted volumes = raw regional brain volume/total intracranial volume ×1,000.
Statistical analysis
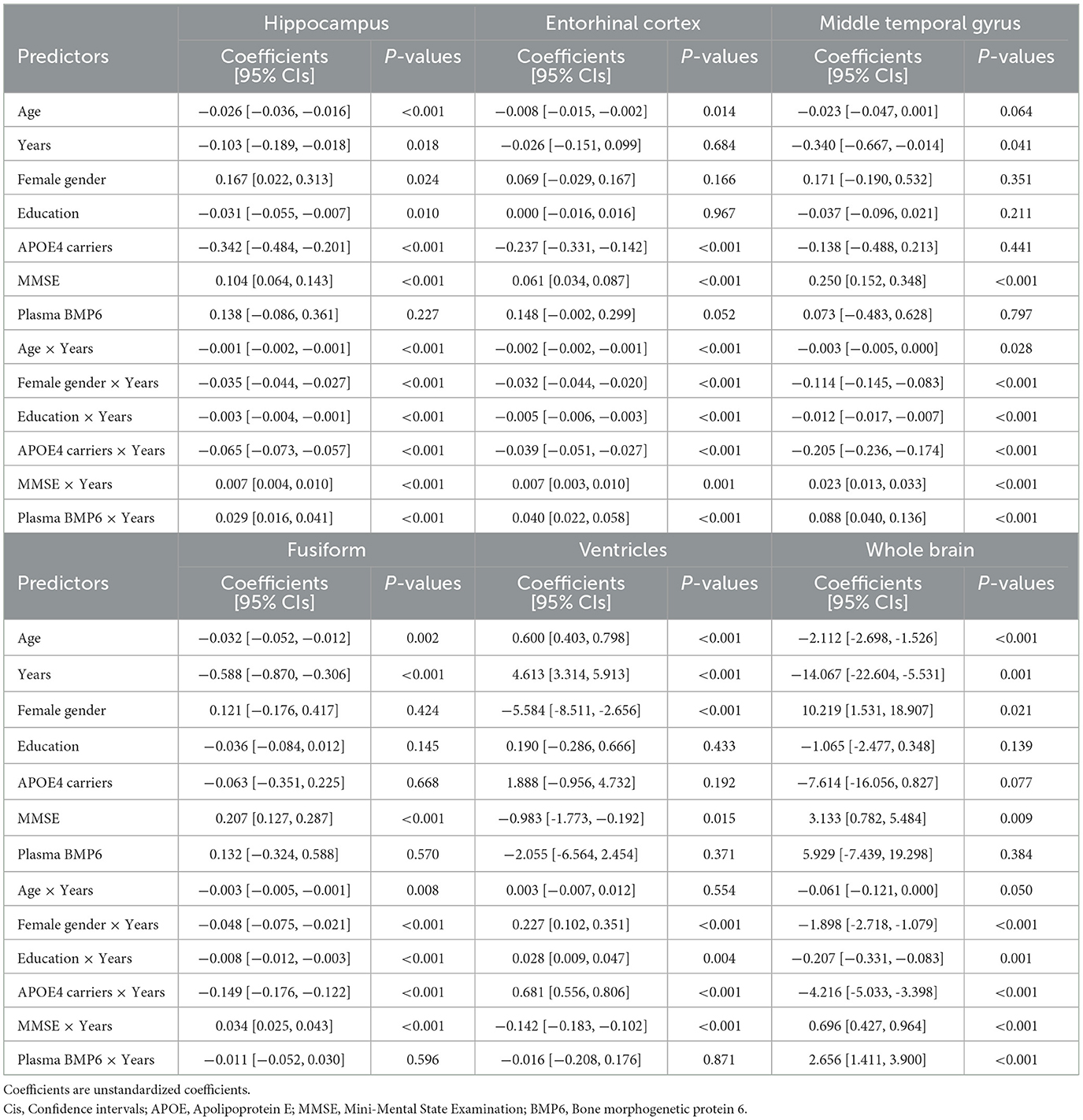
We used Pearson's correlation tests to assess the relationships between baseline plasma BMP6 levels and structural regional brain volumes of hippocampus, entorhinal cortex, middle temporal gyrus, fusiform, ventricles, and whole brain. To investigate the associations of plasma BMP6 levels with the rates of regional brain atrophy among older people without dementia, a series of linear mixed-effects models were performed, adjusting for potential covariates. A total of 6 linear mixed-effects models were built for regional brain volumes (hippocampus, entorhinal cortex, middle temporal cortex, fusiform, ventricles, and whole brain), which were treated as the dependent variables. Models included main effects of age, gender, education, APOE4 status, MMSE score, plasma BMP6, and their interactions with follow-up time (years). Each model included a random intercept for each subject. All statistical analyses were conducted using R statistical software. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
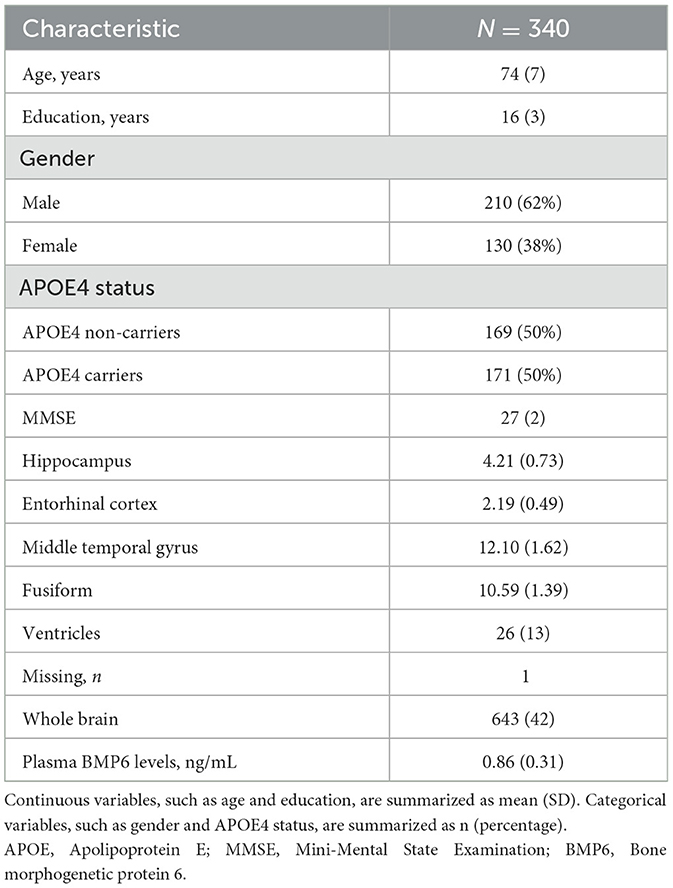
In the present study, we included a total of 340 older adults without dementia [mean age, 74 [SD = 7]; 130 women [38%]; mean education, 16 [SD = 3]; 171 APOE4 carriers [50%], mean MMSE score, 27 (2)]. Table 1 summarizes demographics and imaging data of the study participants. The adjusted volumes of hippocampus, entorhinal cortex, middle temporal gyrus, fusiform, ventricles, and whole brain were 4.21 (SD = 0.73), 2.19 (SD = 0.49), 12.1 (SD = 1.62), 10.59 (SD = 1.39), 26 (SD = 13), and 643 (SD = 42), respectively. The mean of baseline plasma BMP6 was 0.86 ng/mL (SD = 0.31).
Cross-sectional relationships between plasma BMP6 and demographic and cognitive variables
Spearman's correlation and two-sample t-tests were performed to examine the relationships between plasma BMP6 levels and demographic and cognitive variables. Plasma BMP6 levels were not associated with age (rho = 0.04, p = 0.43) or years of education (rho = 0.07, p = 0.19). No significant differences in plasma BMP6 levels were observed between males and females (t = 0.83, p = 0.41), or between APOE4 carriers and non-carriers (t = −0.04, p = 0.97). However, plasma BMP6 levels were positively associated with MMSE scores (rho = 0.11, p = 0.035).
Cross-sectional relationships between plasma BMP6 and MRI neuroimaging markers
Pearson's correlation tests were conducted to examine the relationship between plasma BMP6 levels and 6 MRI neuroimaging markers, and Figure 1 visualizes the relationships. As displayed in Figure 1A, plasma BMP6 levels were not correlated with volumes of hippocampus among older adults without dementia (r = 0.06, p = 0.25). As shown in Figure 1B, plasma BMP6 levels were positively correlated with volumes of entorhinal cortex among older adults without dementia (r = 0.12, p = 0.026). As demonstrated in Figure 1C, plasma BMP6 levels were not associated with volumes of middle temporal gyrus among older adults without dementia (r = 0.03, p = 0.59). As displayed in Figure 1D, plasma BMP6 levels were not correlated with volumes of fusiform among older adults without dementia (r = 0.015, p = 0.78). As displayed in Figure 1E, plasma BMP6 levels were not correlated with the enlargement of ventricles among older adults without dementia (r = −0.002, p = 0.97). As displayed in Figure 1F, plasma BMP6 levels were not correlated with volumes of whole brain among older adults without dementia (r = 0.03, p = 0.57).
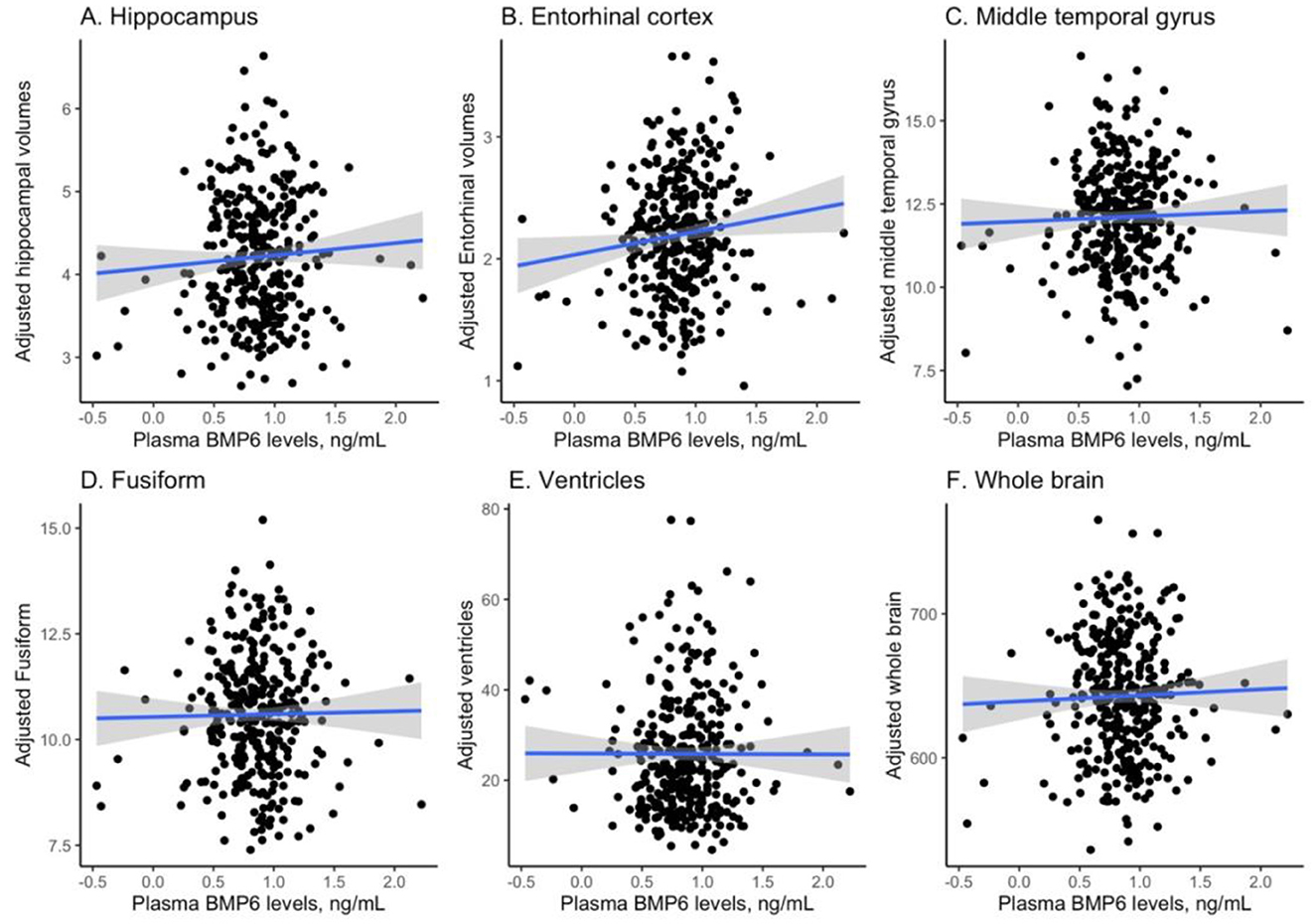
Figure 1. Cross-sectional relationships between plasma BMP6 levels and regional brain volumes. BMP6, Bone morphogenetic protein 6. (A) Hippocampus. (B) Entorhinal cortex. (C) Middle temporal gyrus. (D) Fusiform. (E) Ventricles. (F) Whole brain.
Associations of plasma BMP6 levels with the rates of brain atrophy
We built six linear mixed-effects models for each MRI neuroimaging marker, which was treated as the dependent variable in each model. Regression terms of six linear mixed-effects models were demonstrated in Table 2. In the model with hippocampus as the dependent variable, the interaction term between plasma BMP6 and years (follow-up time) was significant, indicating that higher baseline plasma BMP6 levels were associated with a slower rate of atrophy of hippocampus (coefficient [95% CIs] = 0.029 [0.016, 0.041], p < 0.001; Figure 2A). Likewise, in the model with entorhinal cortex as the dependent variable, the interaction term between plasma BMP6 and years was significant, indicating that higher baseline plasma BMP6 levels were associated with a slower rate of atrophy of entorhinal cortex (coefficient [95% CIs] = 0.040 [0.022, 0.058], p < 0.001; Figure 2B). In the model with middle temporal cortex as the dependent variable, the interaction term between plasma BMP6 and years was significant, indicating that higher baseline plasma BMP6 levels were associated with a slower rate of atrophy of middle temporal gyrus (coefficient [95% CIs] = 0.088 [0.040, 0.136], p < 0.001; Figure 2C). However, plasma BMP6 levels were not associated with the rate of atrophy of fusiform (coefficient [95% CIs] = −0.011 [−0.052, 0.030], p = 0.596; Figure 2D) or the enlargement of ventricles (coefficient [95% CIs] = −0.016 [−0.208, 0.176], p = 0.871; Figure 2E). In the model with whole brain as the dependent variable, the interaction term between plasma BMP6 and years was significant, indicating that higher baseline plasma BMP6 levels were associated with a slower rate of atrophy of whole brain (coefficient [95% CIs] = 2.656 [1.411, 3.900], p < 0.001; Figure 2F).
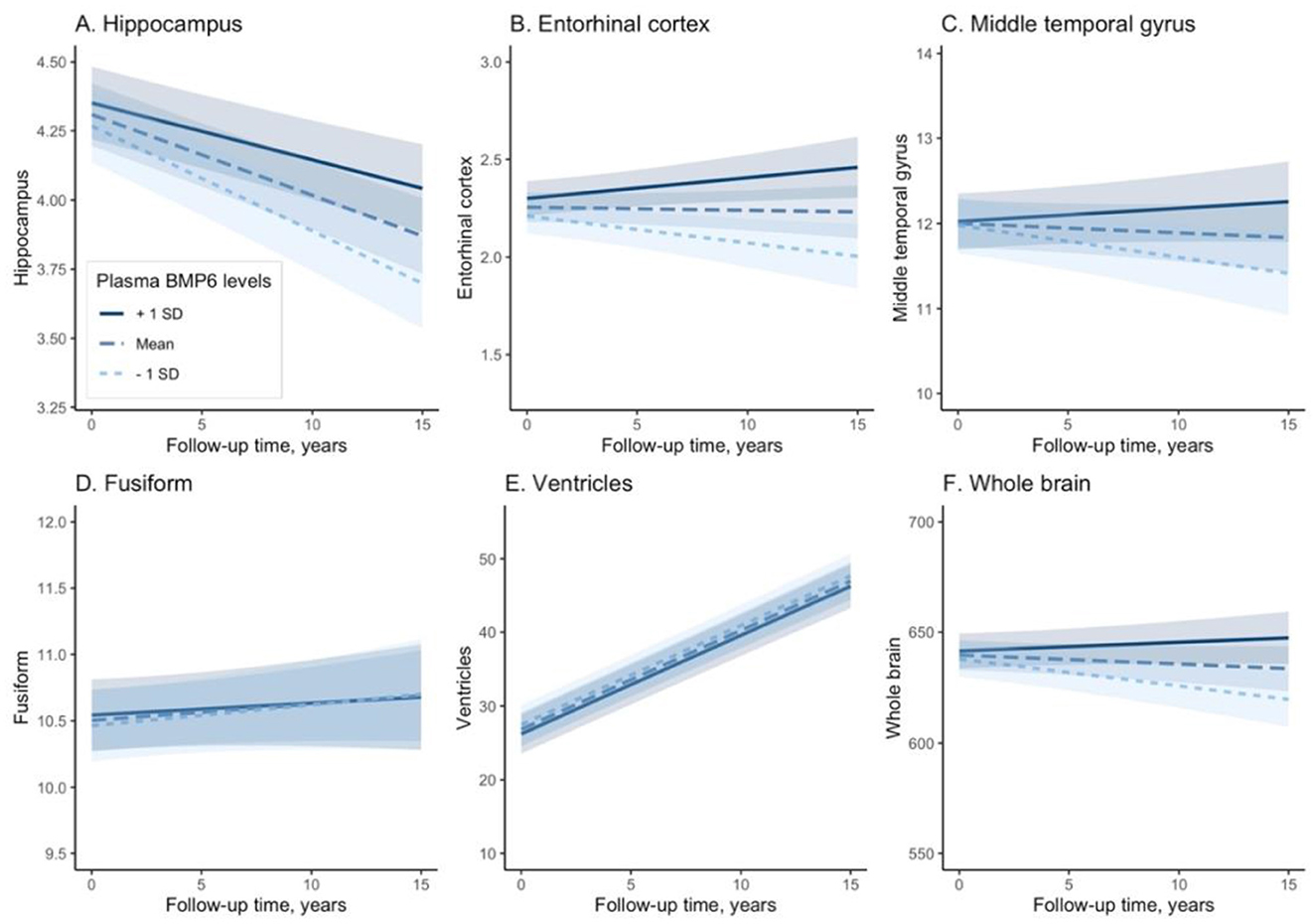
Figure 2. Associations of plasma BMP6 with the rates of regional brain atrophy. BMP6, Bone morphogenetic protein 6. (A) Hippocampus. (B) Entorhinal cortex. (C) Middle temporal gyrus. (D) Fusiform. (E) Ventricles. (F) Whole brain.
Supplementary analyses
First, apolipoprotein C-I (Apo C-I), which is also included in the multiplex-based immunoassay panel, is not a typical biomarker for cognitive decline or AD. Apo C-I, instead of BMP6, was used as the predictor of interest in the linear mixed-effects models. All other model specifications were kept the same as described in the Statistical analysis section. The Apo C-I × Years interaction term was not significant for all MRI features: hippocampus (coefficient [95% CIs] = −0.013 [−0.046, 0.020], p = 0.447), entorhinal cortex (coefficient [95% CIs] = −0.007 [−0.056, 0.042], p = 0.77), middle temporal cortex (coefficient [95% CIs] = −0.047 [−0.174, 0.081], p = 0.473), fusiform (coefficient [95% CIs] = 0.028 [−0.082, 0.137], p = 0.622), ventricles (coefficient [95% CIs] = 0.444 [−0.064, 0.952], p = 0.086), and whole brain (coefficient [95% CIs] = 2.403 [−0.902, 5.708], p = 0.154).
Second, we further examined the associations between plasma BMP6 levels and changes in the MMSE and Clinical Dementia Rating-Sum of Boxes (CDR-SB) scores over time. We found that higher baseline plasma BMP6 levels were associated with slower rates of changes in MMSE (BMP6 × Years term: coefficient = 0.472, 95%CI = [0.294, 0.651], p < 0.001; Supplementary Table S1) and CDR-SB (BMP6 × Years term: coefficient = −0.307, 95%CI = [−0.415, −0.199], p < 0.001; Supplementary Table S2) over time.
Third, the relationships between plasma BMP6 levels and changes in six MRI features over times were investigated solely among MCI individuals. The overall pattern of the findings did not change. The results of the linear mixed-effects models for the hippocampus, entorhinal cortex, fusiform gyrus, middle temporal gyrus, ventricles, and whole brain are summarized in Supplementary Tables S3– S8, respectively.
Fourth, Spearman's correlation tests were performed to examine the cross-sectional relationships between plasma BMP6 and CSF biomarkers, including Aβ42, t-tau, and p-tau, in the overall sample. No significant relationship between plasma BMP6 and CSF Aβ42 was observed (rho = 0.07, p = 0.3). However, there were significant negative associations between plasma BMP6 and both t-tau (rho = −0.15, p = 0.038) and p-tau (rho = −0.16, p = 0.027) levels.
Fifth, MRI images from two timepoints (baseline and 5-year follow-up) for two MCI subjects with differing baseline plasma BMP6 levels are shown in Supplementary Figures S1 and S2.
Discussion
This study examined the association between plasma BMP6 levels and the rates of regional brain atrophy among older adults without dementia. We found that higher levels of plasma BMP6 were associated with a slower rate of volume reduction in the hippocampus, entorhinal cortex, middle temporal gyrus, and whole brain. However, plasma BMP6 levels were not associated with changes in the volumes of the fusiform gyrus or ventricles. Our findings may provide novel insights into the mechanisms of neurodegeneration in AD, contributing to new avenues for timely intervention and potentially slowing disease progression.
The hippocampus, entorhinal cortex, and middle temporal gyrus are among the earliest and most severely affected regions in AD, with atrophy in these areas being a hallmark of the disease (16, 17). Our finding that higher plasma BMP6 levels are associated with slower atrophy in these brain regions is novel and suggests a potential neuroprotective effect of BMP6 (7). This finding is consistent with previous studies showing that BMP6 may play a crucial role in neuronal survival and synaptic plasticity (7), which are critical for maintaining cognitive performance. The supplementary analyses suggested that higher plasma BMP6 levels were associated with lower levels of CSF tau proteins. This finding may indicate that the potential neuroprotective effect of BMP6 on these brain regions is due to its ability to reduce tau pathologies, as tau pathology typically begins in these areas. Additionally, according to a recent study that tracked participants over time, higher blood BMP6 levels in older adults are associated with greater cognitive performance during the study's follow-up (9). However, few studies have investigated the relationship between plasma BMP6 levels and brain atrophy in living humans. Our study extends these findings by suggesting a potentially protective effect of BMP6 in specific brain regions associated with AD in older adults without dementia. Identifying blood biomarkers that predict the rate of brain atrophy is crucial for timely intervention and disease management. If confirmed in larger cohorts, plasma BMP6 levels could serve as a non-invasive tool to monitor disease progression and assess the efficacy of potential therapeutics. Additionally, therapeutic strategies targeting BMP6 signaling pathways may offer new avenues for the treatment of AD. For instance, pharmacological agents that increase BMP6 activity or mimic its effects could potentially slow down the rate of brain atrophy and neurodegeneration. Further studies are needed to explore the feasibility and safety of such approaches.
The lack of correlation between plasma BMP6 levels and alterations in the volumes of the fusiform gyrus or ventricles is intriguing. The fusiform gyrus is implicated in a variety of cognitive functions, including object naming performance (18). This region is typically not one of the earliest brain regions affected in AD (17). The enlargement of ventricles is a well-documented consequence of brain atrophy (19). However, the lack of association between BMP6 levels and ventricle size suggests that the influence of BMP6 is likely targeted to specific regions rather than being a broad indicator of brain atrophy.
Several limitations of this study should be considered. First, the sample size was relatively small, which may limit the generalizability of our findings. Future studies with larger and more diverse populations are needed to validate our findings. Second, association of CSF BMP6 levels and brain atrophy remains to be fully elucidated. Future studies are warranted to explore this research question. Finally, the observational nature of our study precludes causal inferences; further interventional studies are necessary to establish a causal relationship between BMP6 levels and brain atrophy.
In conclusion, our study provides evidence that higher plasma BMP6 levels are associated with a slower rate of volume reduction in the hippocampus, entorhinal cortex, middle temporal cortex, and whole brain among older adults without dementia. These findings offer novel insights into the mechanisms of neurodegeneration in AD and highlight the potential of BMP6 as a biomarker and therapeutic target.
Data availability statement
Publicly available datasets were analyzed in this study. The data used in the present study has been made publicly available by the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).
Ethics statement
The studies involving humans were approved by the Institutional Review Boards of each ADNI participating center. More detailed information can be found on the ADNI website (adni.loni.usc.edu). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. PF: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing – review & editing. YC: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1559219/full#supplementary-material
References
1. Jack Jr CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. (2010) 9:119–28. doi: 10.1016/S1474-4422(09)70299-6
2. Querfurth HW, Laferla FM. Alzheimer's disease. N Engl J Med. (2010) 362:329–44. doi: 10.1056/NEJMra0909142
3. De Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. (2000) 911:240–53. doi: 10.1111/j.1749-6632.2000.tb06730.x
4. Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal MH, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. (2007) 68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7
5. Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. (2018) 284:643–63. doi: 10.1111/joim.12816
6. Peretti DE, Boccalini C, Ribaldi F, Scheffler M, Marizzoni M, Ashton NJ, et al. Association of glial fibrillary acid protein, Alzheimer's disease pathology and cognitive decline. Brain. (2024) 147:4094–04. doi: 10.1093/brain/awae211
7. Sun L, Guo C, Wang T, Li X, Li G, Luo Y, et al. LIMK1 is involved in the protective effects of bone morphogenetic protein 6 against amyloid-β-induced neurotoxicity in rat hippocampal neurons. J Alzheimers Dis. (2014) 42:543–54. doi: 10.3233/JAD-140231
8. Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, et al. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci. (2010) 30:12252–62. doi: 10.1523/JNEUROSCI.1305-10.2010
9. Sun L, Guo C, Song Y, Sheng J, Xiao S. Blood BMP6 Associated with Cognitive Performance and Alzheimer's Disease Diagnosis: A Longitudinal Study of Elders. J Alzheimers Dis. (2022) 88:641–51. doi: 10.3233/JAD-220279
10. Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. (2010) 74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25
11. Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
12. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
13. Nazeri A, Ganjgahi H, Roostaei T, Nichols T, Zarei M. Imaging proteomics for diagnosis, monitoring and prediction of Alzheimer's disease. Neuroimage 102 Pt 2. (2014) 657–65. doi: 10.1016/j.neuroimage.2014.08.041
14. Arani A, Borowski B, Felmlee J, Reid RI, Thomas DL, Gunter JL, et al. Design and validation of the ADNI MR protocol. Alzheimers Dement. (2024) 20:6615–21. doi: 10.1002/alz.14162
15. Fischl BR, Salat DH, Busa E, Albert MS, Dieterich M, Haselgrove C, et al. Whole brain segmentation automated labeling of neuroanatomical structures in the human brain. Neuron. (2002) 33:341–55. doi: 10.1016/S0896-6273(02)00569-X
16. Jack Jr CR, Petersen RC, O'brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. (1992) 42:183–8. doi: 10.1212/WNL.42.1.183
17. Planche V, Manjon JV, Mansencal B, Lanuza E, Tourdias T, Catheline G, et al. Structural progression of Alzheimer's disease over decades: the MRI staging scheme. Brain Commun. (2022) 4:fcac109. doi: 10.1093/braincomms/fcac109
18. Hays Weeks CC, Zlatar ZZ, Meloy MJ, Shin DD, Thomas L, Wierenga CE, et al. APOE genotype modifies the association of fusiform gyrus cerebral metabolic rate of oxygen consumption and object naming performance. J Alzheimers Dis. (2023) 91:1371–83. doi: 10.3233/JAD-220749
Keywords: Alzheimer's disease, BMP6, MRI, brain atrophy, hippocampus
Citation: Zhang X, Fu P and Cai Y (2025) Association of plasma BMP6 levels with the rates of brain atrophy in older people without dementia. Front. Neurol. 16:1559219. doi: 10.3389/fneur.2025.1559219
Received: 12 January 2025; Accepted: 20 June 2025;
Published: 15 July 2025.
Edited by:
Paul Desmond Slowey, Oasis Diagnostics, United StatesReviewed by:
Zhen Yuan, University of Macau, ChinaEkaterina Rudnitsky, Ben-Gurion University of the Negev, Israel
Copyright © 2025 Zhang, Fu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Cai, MTU5ODg5Nzg5MjlAMTYzLmNvbQ==
†Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
 Xin Zhang
Xin Zhang Pan Fu
Pan Fu Yan Cai
Yan Cai