- 1Center for Evidence-Based Medicine, Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 2Department of Critical Care Medicine, Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
Background: The findings from studies exploring the prognostic relevance of the lymphocyte-to-monocyte ratio (LMR) in individuals with acute ischemic stroke (AIS) have shown variability. We aimed to conduct a meta-analysis to determine the prognostic significance of LMR in this patient population.
Methods: We carried out a meta-analysis utilizing information from major databases, including PubMed, Embase, and Web of Science until October 26, 2024. Effect sizes, represented as odds ratios (ORs) along with their corresponding 95% confidence intervals (CI), were synthesized employing a random-effects model in Review Manager Version 5.4. To investigate possible sources of variability, we conducted subgroup analyses. Additionally, publication bias was assessed through the use of a funnel plot. Poor functional outcome at 3 months, as indicated via a modified Rankin Scale score of ≥3, was the main outcome. A moderate to severe stroke, determined by a National Institutes of Health Stroke Scale score of ≥6, was the secondary outcome.
Results: Six trials totaling 1,225 individuals were included in our analysis. In AIS patients, we discovered a significant correlation between lower LMR and poorer functional outcome at 3 months, with an OR of 0.63, 95% CI of 0.49 to 0.80, and a p-value of 0.0002. Additionally, lower LMR may be associated with developing moderate to severe stroke, with an OR of 0.89 (95% CI: 0.82–0.97; p = 0.008). In subgroup analyses with an LMR cutoff, a significant association was observed between lower LMR and greater functional impairment in AIS patients, with an odds ratio of 0.74 (95% CI: 0.62–0.88; p = 0.0005) for LMR ≥ 3 and 0.54 (95% CI: 0.47–0.61; p < 0.00001) for LMR < 3. Additionally, when country-stratified, Asian continued to have a significant correlation between worse functional outcome and lower LMR (OR 0.62, 95% CI: 0.50–0.77, p < 0.0001).
Conclusion: This meta-analysis indicated that LMR was a prognostic factor for clinical outcomes in AIS patients.
Introduction
A stroke is characterized by its abrupt onset, representing a sudden neurological disruption resulting from the abrupt cessation of cerebral blood flow, which leads to a wide spectrum of neurological deficits (1). Strokes are generally classified into two main categories: ischemic and hemorrhagic. Of these, ischemic strokes account for approximately 80% of all cases, with an alarmingly high global incidence of over 13.7 million cases annually, leading to around 5.5 million deaths each year (2). Although strokes can affect individuals across all age groups, they primarily affect older adults. However, there has been a concerning rise in the incidence among younger populations (2, 3). Among the different types of stroke, acute ischemic stroke (AIS) is the most common, placing a significant strain on healthcare systems due to its high morbidity and mortality rates, as well as the considerable risks of fatal outcomes and long-term disabling effects (4). Thus, identifying reliable prognostic markers is crucial for informed clinical decision-making and personalized patient care.
Increasing evidence indicates that AIS triggers an inflammatory response (5–7). There is growing interest in the part inflammatory indicators play in the pathophysiology and outcome of AIS. A cost-effective and easily accessible composite indicator of inflammation in cerebrovascular disease, the lymphocyte-to-monocyte ratio (LMR), which was computed by dividing the lymphocyte count by the monocyte count, integrates the prognostic significance of individual lymphocyte and monocyte levels in relation to AIS (8). Previous research has indicated an association between LMR and the severity as well as outcomes of inflammation-related maladies such as myocardial infarction, chronic autoimmune diseases, and peripheral ischemia (9–11).
The link between LMR and adverse outcomes in AIS patients continues to be a subject of debate. Although a number of studies have shown a correlation between AIS patients’ inferior prognosis and lower LMR levels (12–15), others have failed to establish a significant link between LMR and negative clinical outcomes (16). In light of these discrepant results, we carried out this meta-analysis to delve deeper into the relationship between LMR and adverse outcomes among AIS patients.
Methods
In accordance with the PRISMA statement, this systematic review and meta-analysis has been registered in the PROSPERO database under the registration number CRD42025637708 (17).
Data sources and search strategy
A thorough systematic review was performed utilizing various databases, such as PubMed, Web of Science, and Embase. Additionally, we hand-searched the bibliographies of all selected studies to locate further pertinent articles. Literature from the inception of these databases to October 26, 2024, was included in the search. The full search strategies are presented in Supplementary Table S1.
Inclusion and exclusion criteria
The selection process for studies was influenced by these criteria: (1) Patients must be clinically confirmed to have ischemic stroke; (2) The LMR was assessed either upon admission or during the hospital stay for ischemic stroke; (3) Outcome measures included poor functional outcome, or moderate to severe stroke; (4) Participants had to be adults aged 18 years or older. Any studies that met the following criteria were left out of the meta-analysis: (1) They exclusively included patients with hemorrhagic stroke; (2) They were conference abstracts; (3) They were experimental or interventional studies, review articles, preprint studies, or case reports; (4) They lacked sufficient data; (5) They involved non-human subjects.
Outcomes
The main result at 3 months was poor functional outcome, which was characterized as a Modified Rankin Scale (MRS) score of three or above. A stroke score of 6 or higher on the National Institutes of Health Stroke Scale (NIHSS) was considered moderate to severe, and this was the secondary outcome.
Data extraction
Data extraction was done separately by two researchers, RW and CT, from the chosen studies. They collected specific details, including the lead author’s name, publication date, verification methodology, mean participant age, geographical location of the study, total number of participants, gender breakdown, measurement outcomes, the LMR Youden Index, LMR threshold levels, Area Under the Curve (AUC), odds ratio (OR) for adverse functional outcome and moderate to severe stroke cases, and their associated 95% confidence interval (CI). When studies provided both univariate and multivariate regression analyses, the latter was utilized to derive the cumulative OR. To ensure data accuracy, a third researcher (YL) conducted an independent verification. Within the collaboration, disagreements between the original reviewers were settled via cooperative discussion.
Assessment of quality
Cohort study quality was rated by two reviewers (RW and CT) adopting the Newcastle–Ottawa Quality Assessment Scale (NOS), with scores ranging from 0 to 9. Studies scoring 7 or higher were deemed high quality (18). Each study was independently evaluated by the reviewers in line with the designated scale. Any differences were reconciled by means of discussions with an additional author (YL).
Statistical analysis
RevMan 5.4.1 from The Cochrane Collaboration was used for statistical analysis. For categorical data, the OR and associating 95% CI were determined. The degree of variability was quantified by using the I2 statistic to calculate the percentage of variability attributable to study variability, with a p-value of less than 0.05 being deemed statistically significant. Because of the diversity shown in the trials, a random-effects model was used to synthesize the findings. To determine the degree of heterogeneity, I2 statistics and Chi-square tests were applied, with a study considered significantly heterogeneous when p < 0.05 and I2 exceeded 50%. Forest plots were created to visualize the estimated effect sizes along with their 95% CI. By employing these rigorous statistical techniques, our goal was to deliver a thorough and comprehensive analysis of the data. We visually inspected the funnel plot to qualitatively assess the potential effects of small studies. Additionally, we performed a sensitivity analysis by removing studies one at a time in order to examine how each study affected the overall impact size. Moreover, the egger test (19) was applied for quantitative analysis, facilitating a meticulous assessment of any bias that might arise from small-study effects.
Subgroup analysis
We conducted a subgroup analysis of the primary outcome according to age, country, LMR cutoff values, and the specific characteristics of the study subjects.
Results
Study inclusion
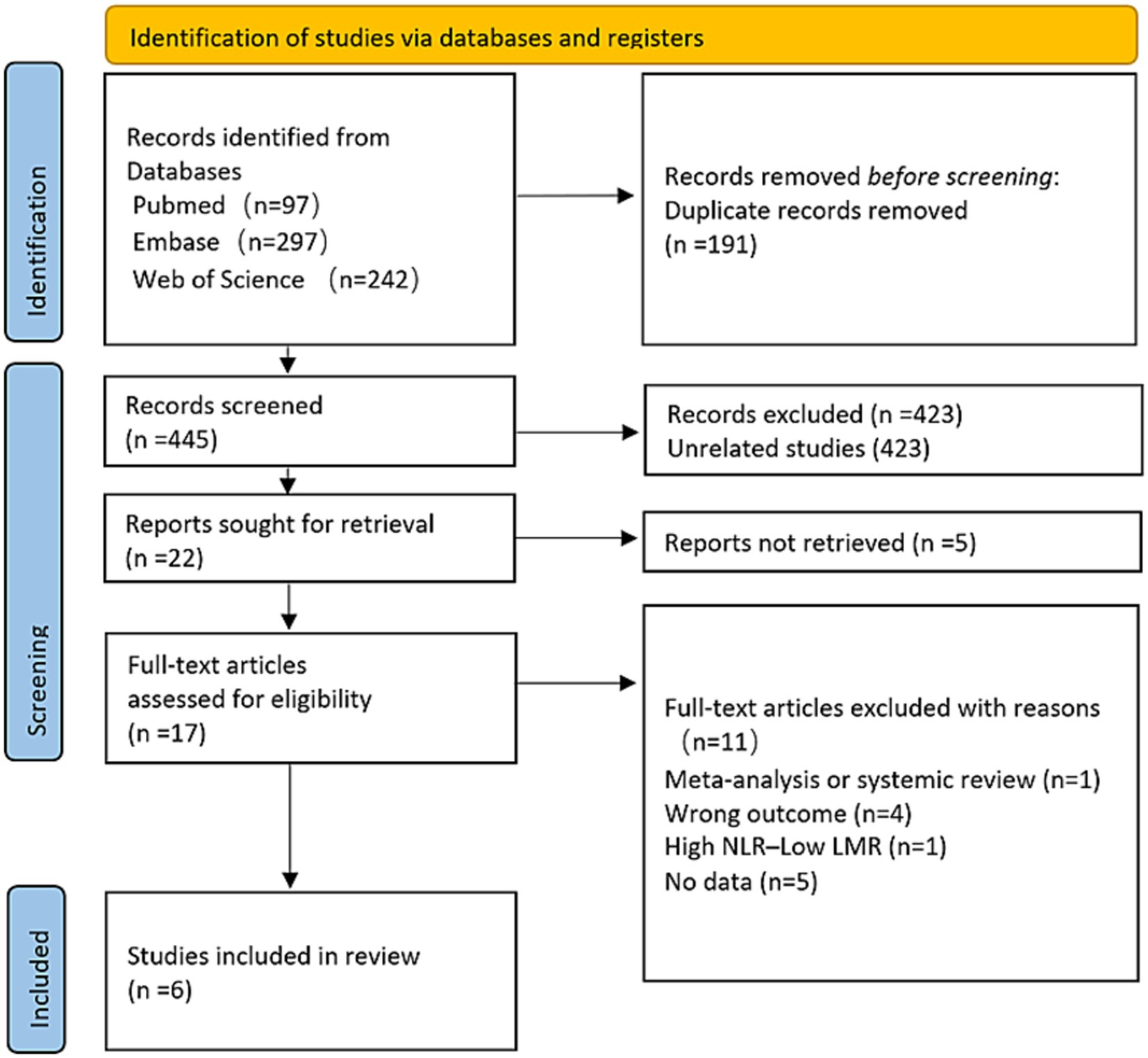
Figure 1 illustrates the flowchart detailing the research design. A comprehensive literature search, employing specific keywords, initially identified 636 articles. After eliminating duplicates, 191 studies were excluded. Subsequently, 423 irrelevant articles were excluded. Among the remaining 22, 5 were excluded for inaccessible full-texts. Following a thorough review, 11 additional articles were disqualified for not fulfilling the predetermined inclusion requirements. In the end, the meta-analysis had six publications (12–15, 20, 21).
Summary of studies
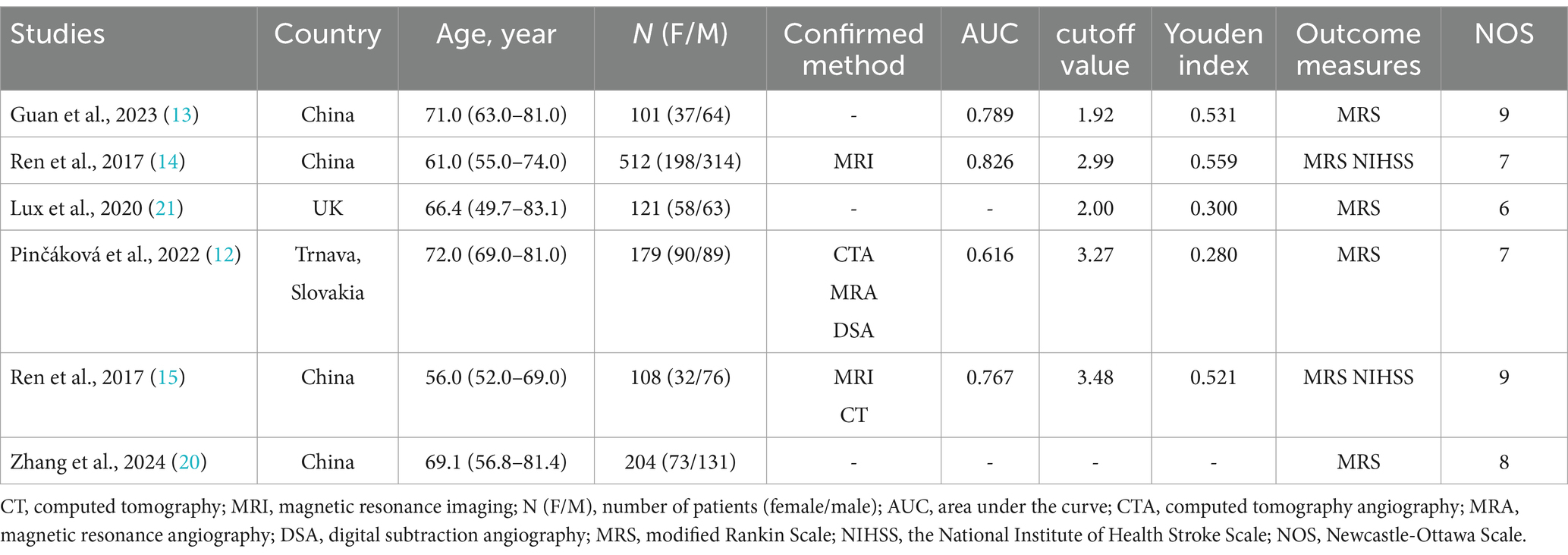
Table 1 offers a comprehensive summary of details of the included research. In total, 1,225 individuals diagnosed with AIS were documented across six retrospective studies. All included studies adjusted for potential confounders through multivariate models. The initial studies predominantly focused on populations from China, the UK (21), and Trnava, Slovakia (12). The primary source of data was the Department of Neurology at Chengde Medical University’s affiliated hospital (14, 15). The analysis revealed a higher prevalence of male patients with AIS, with the mean age exceeding 50 years. Stroke diagnoses were confirmed through clinical evaluations, imaging studies, and comprehensive diagnostic assessments.
Overall assessment of evidence quality
The evaluation of the cohort investigations employing the NOS This led to scores of 9 in two studies (13, 20), 8 in one study (12), 7 in two studies (14, 15), and 6 in another one (21). The differences in scores primarily depended on the extent to which confounders were controlled and the rigor of participant follow-up (Supplementary Table S2).
Overall efficacy
The incidence of poor functional outcome in AIS patients at 3 months was the main outcome that was measured. This was determined by combining effect sizes from six studies, which collectively included 1,225 participants. The pooled OR for these studies was 0.63 (95% CI: 0.49–0.80, p = 0.0002), as shown in Figure 2. According to this finding, AIS patients who have a lower LMR are more likely to experience poorer functional results at 3 months. Notably, there was considerable variation among the trials (I2 = 77%, p = 0.0005).
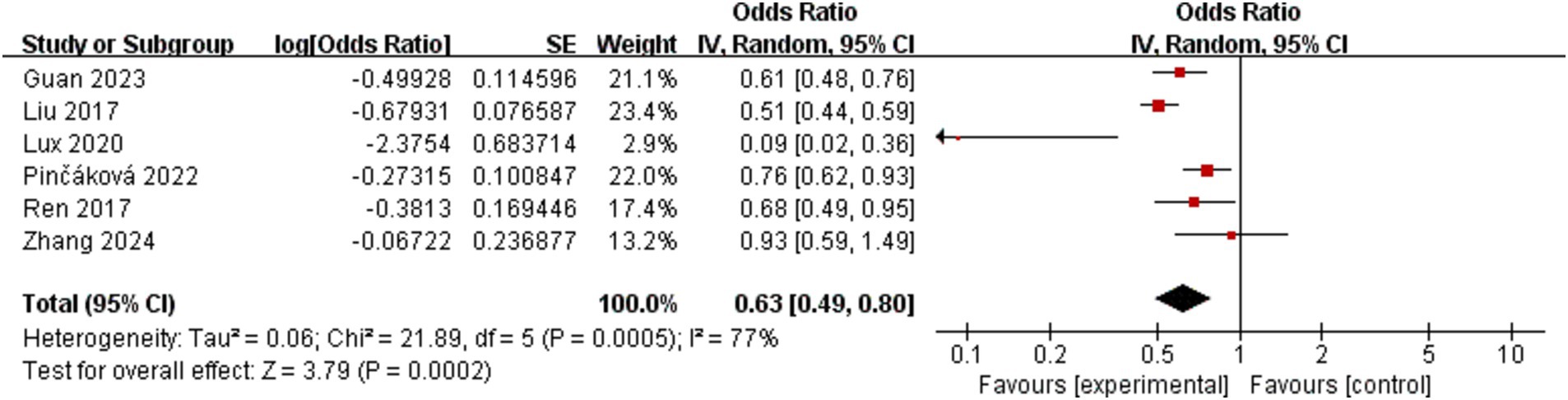
Figure 2. Forest plot of the association between lymphocyte-to-monocyte ratio and poor functional outcome at 3 months.
The secondary outcome was the occurrence of moderate to severe stroke in patients with AIS. Two studies evaluated moderate to severe stroke as an outcome, resulting in a pooled OR of 0.89 (95% CI: 0.82–0.97, p = 0.008), according to Figure 3. There was no difference between the investigations (I2 = 0, p = 0.85).
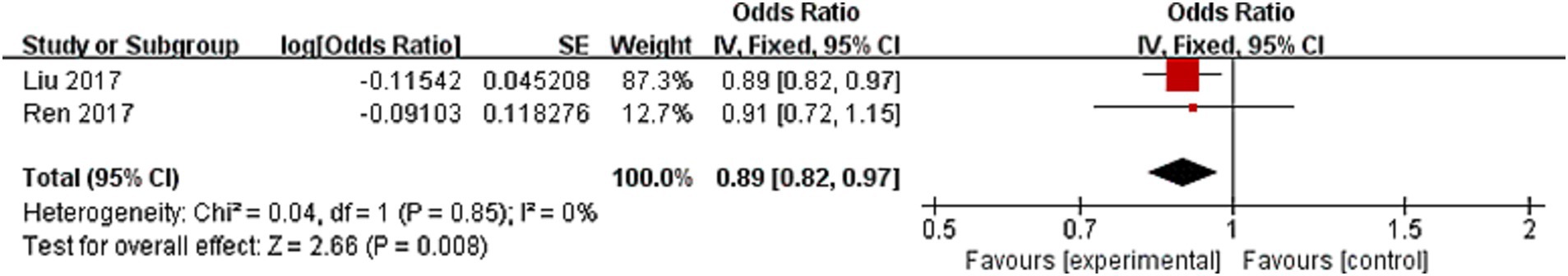
Figure 3. Forest plot of the association between lymphocyte-to-monocyte ratio and moderate to severe stroke.
Subgroup analyses
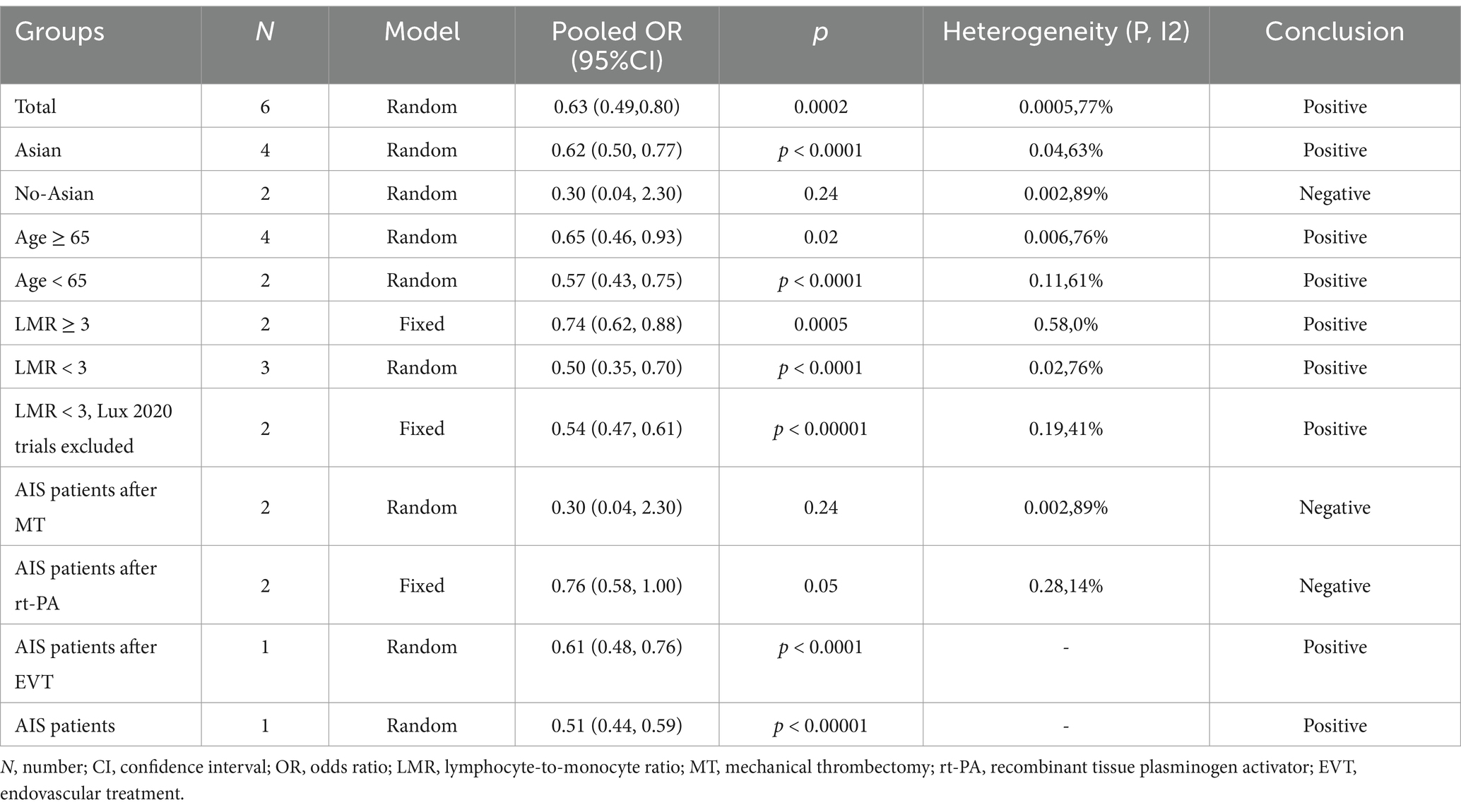
We performed a subgroup analysis focusing on studies that used poor functional outcome at 3 months as the primary outcome measure. The results, shown in Supplementary Figure S1, reveal that the aggregated OR for poor functional outcome was 0.62 (95% CI: 0.50–0.77; p < 0.0001) for studies conducted in Asian, and 0.30 (95% CI: 0.04–2.30; p = 0.24) for studies conducted in non-Asian. Among all included studies, two reported a LMR cutoff value ≥ 3, yielding a pooled OR for poor functional outcome of 0.74 (95% CI: 0.62–0.88; p = 0.0005). In contrast, the LMR cutoff values reported in the other three studies ranged between 0 and 3, resulting in a combined OR of 0.50 (95% CI: 0.35–0.70; p < 0.0001). According to sensitivity analysis, the Lux et al. (21) study affected the overall findings, most likely as a function of the timing of the LMR measurements. In their study, LMR was measured 24 h after mechanical thrombectomy (MT), whereas other included studies measured LMR at admission or during the initial hospital stay (13, 14). This variability in timing of LMR measurement may have contributed to the observed heterogeneity. Excluding this study resulted in a pooled OR of 0.54 (95% CI: 0.47–0.61, p < 0.00001), based on Supplementary Figure S2. One study did not specify an LMR cutoff value. Furthermore, a subgroup analysis based on age (Supplementary Figure S3) demonstrated that the pooled OR for poor functional outcome at 3 months was 0.65 (95% CI: 0.46–0.93; p = 0.02) for patients aged 65 and older, and 0.57 (95% CI: 0.43–0.75; p < 0.0001) for those younger than 65. The analysis also included two studies focusing on AIS patients who underwent MT, resulting in an OR of 0.30 (95% CI: 0.04–2.30; p = 0.24). Additionally, two trials including AIS patients evaluated the impact of recombinant tissue plasminogen activator (rt-PA), resulting in an OR of 0.77 (95% CI: 0.57–1.03; p = 0.08). Another study focused on AIS patients who received endovascular therapy (EVT), yielding a pooled OR of 0.61 (95% CI: 0.48–0.76). Lastly, one study addressed AIS patients more generally, reporting a pooled OR of 0.51 (95% CI: 0.44–0.59), as illustrated in Supplementary Figure S4. All pooled results are summarized in Table 2.
Sensitivity analysis and publication bias
One study at a time was eliminated in order to do a sensitivity analysis. The findings of this analysis are presented in Supplementary Table S3. The sensitivity analysis indicated that the pooled OR for studies reporting poor functional outcome remained stable, with no significant changes observed after the exclusion of any individual study. Additionally, publication bias was evaluated and is presented in Supplementary Figures S5, S6. No substantial bias was identified in the meta-analysis.
Discussion
To our knowledge, this meta-analysis represents the pioneering investigation in its field that, by implementing rigorous search protocols, established the absence of prior systematic reviews—even those tangentially examining inflammatory biomarkers in acute ischemic stroke, including information from six distinct investigations. Our findings suggest that a lower LMR is associated with worse functional outcome at 3 months and may also indicate a higher likelihood of moderate to severe stroke occurrence.
The relationship between the LMR and AIS has not yet been the subject of any meta-analysis. Our study uniquely focuses on this relationship within the AIS population, proving that LMR may indicate a useful predictor of poor functional outcome in patients with AIS. Furthermore, our findings indicate that reduced LMR levels are associated with a higher risk of progressing to moderate to severe stroke. Additionally, the place of origin, research parameters, and cutoff values were among the factors that affected the association between LMR and AIS, according to our analysis.
The LMR is a marker that reflects the proportion of lymphocytes relative to monocytes in the peripheral circulation. This ratio serves as an inflammatory biomarker and has been associated with inflammation, disease activity, and prognosis in various clinical conditions (22). In different clinical settings, variations in LMR can aid physicians in assessing a patient’s immune response. For example, in patients with cancer, higher LMR levels are often associated with more favorable prognosis, as elevated LMR may indicate a more robust immune response (23). On the other hand, a decreased LMR may signify heightened inflammation or immune suppression, factors commonly associated with worse treatment outcomes and lower survival rates. Additionally, LMR has been used to assess the risk of cardiovascular diseases, and evaluate the activity of other chronic inflammatory conditions (22, 24, 25). Clinical observation indicated that lymphocyte levels demonstrated neuroprotective capacities, which may facilitate the restoration of compromised neural functions (26). In contrast, monocytes, recognized as critical immune modulators that amplify post-stroke inflammatory cascades, demonstrate the capacity to migrate into ischemic regions and exacerbate cerebral injury (27). Clinical investigations have further established that elevated monocyte concentrations correlate with unfavorable therapeutic outcomes in acute ischemic stroke, as supported by a study (28). Consequently, decreased LMR levels may serve as a valuable prognostic indicator for AIS outcomes. Clinicians can gain important information about a patient’s general health and the course of their sickness by tracking blood indicators like LMR.
Our analysis suggests that the LMR is a valuable prognostic marker for predicting poor functional outcome 3 months following AIS. It is crucial to understand that a variety of factors, such as comprehensive rehabilitation efforts, the initial severity of the stroke, and the specific emergency interventions administered, affect these results (29–34). Additionally, several potential confounding factors warrant consideration. For instance, stroke subtype may differentially influence inflammatory responses and subsequent LMR dynamics (35). Baseline comorbidities such as diabetes, hypertension, or chronic inflammatory conditions may modulate both lymphocyte and monocyte counts, thereby confounding the LMR-outcome association (36, 37). Pre-stroke functional status, as assessed by metrics like the MRS prior to the index event, might independently predict recovery trajectories and interact with LMR’s prognostic utility (38). Furthermore, variations in acute therapies may alter the inflammatory milieu and LMR values, necessitating careful adjustment in future studies (39, 40). Our findings indicate that a lower LMR cutoff value is more predictive of poorer functional outcome at 3 months in AIS patients. Consistent with this, Guan et al. (13) showed that a threshold value of 1.92 was found for the independent correlation between a lower LMR and a higher likelihood of poor functional outcome 3 months after a stroke. Similarly, Lux et al. (21) reported that a lower LMR cutoff of 2, with a sensitivity of 80% and specificity of 50%, was also associated with AIS patients having a higher chance of experiencing poor functional outcome at 3 months.
We conducted the subgroup analyses to identify and minimize factors contributing to heterogeneity. In the Asian group, a lower LMR was associated with worse functional outcomes, whereas this relationship was non-significant in the non-Asian group, potentially due to the smaller sample size and increased risk of Type II error. Biological factors such as genetic differences in inflammation and platelet biology, as well as healthcare system and environmental variations, may also influence these findings. Furthermore, testing of different LMR cutoff values revealed that a cutoff of 3 was most predictive of disease progression and prognosis, with lower cutoff values showing stronger associations. LMR demonstrated greater prognostic utility in younger patients, likely due to preserved immune function, while aging, comorbidities, and polypharmacy in the elderly may diminish its predictive value. Through the subgroup analyses, we examined potential sources of heterogeneity and identified factors such as country of origin, age and LMR cutoff value as plausible contributors.
Despite our adherence to rigorous standards in study selection, data extraction, and quality assessment, several limitations were inherent in our study. A key limitation of this meta-analysis is the retrospective design of most included studies, which may introduce selection bias and confounding factors, potentially affecting the reliability of the pooled effect estimates. First, subgroup analyses, such as those based on ethnicity and LMR cutoff values, should be interpreted with caution due to limited study numbers, particularly in the non-Asian subgroup, which had only two studies, limiting statistical power. Second, variations in LMR cutoff values and measurement timing across studies contribute to heterogeneity, underscoring the need for standardized protocols in future research to improve comparability and reduce variability. Moreover, as most of the studies in this analysis were based in Asia, particularly China, caution was needed when generalizing these findings. Additionally, the random-effects model highlighted significant variability across studies, suggesting potential publication bias.
The use of the LMR as a biomarker in AIS patients offers significant clinical advantages. LMR can help identify patients at higher risk for moderate to severe stroke and poor functional outcomes, enabling personalized management strategies. It also aids in treatment decisions, guiding clinicians to consider more aggressive interventions for poor prognostic cases. Integration of LMR with point-of-care testing devices, particularly in emergency settings, facilitates real-time risk assessment and decision-making during the critical golden hour. Additionally, educating patients on the implications of LMR can improve patient engagement and treatment adherence, ultimately enhancing outcomes and recovery.
Conclusion
Three months after an acute ischemic stroke, our study unequivocally showed a substantial correlation between the LMR and poor functional outcome. Specifically, lower LMR was associated with poorer functional outcome and a higher risk of developing moderate to severe stroke. These findings indicate that LMR may be a valuable prognostic tool for guiding management and identifying high-risk acute ischemic stroke patients who require closer post-treatment monitoring.
Author contributions
CT: Writing – review & editing, Writing – original draft. YY: Writing – review & editing. JW: Project administration, Writing – original draft. RW: Data curation, Formal analysis, Methodology, Writing – original draft. KZ: Data curation, Investigation, Methodology, Writing – original draft. YL: Data curation, Formal analysis, Writing – original draft. WG: Investigation, Writing – original draft. HL: Methodology, Writing – original draft. YZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1567112/full#supplementary-material
References
1. Sacco, RL, Kasner, SE, Broderick, JP, Caplan, LR, Connors, JJ, Culebras, A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
3. NCD Countdown 2030 Collaborators. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet. (2018) 392:1072–88. doi: 10.1016/S0140-6736(18)31992-5
4. Ospel, JM, Holodinsky, JK, and Goyal, M. Management of Acute Ischemic Stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. (2020) 75:1832–43. doi: 10.1016/j.jacc.2019.10.034
5. Maida, CD, Norrito, RL, Daidone, M, Tuttolomondo, A, and Pinto, A. Neuroinflammatory mechanisms in ischemic stroke: focus on Cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. (2020) 21:454. doi: 10.3390/ijms21186454
6. Endres, M, Moro, MA, Nolte, CH, Dames, C, Buckwalter, MS, and Meisel, A. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ Res. (2022) 130:1167–86. doi: 10.1161/CIRCRESAHA.121.319994
7. Alsbrook, DL, Di Napoli, M, Bhatia, K, Biller, J, Andalib, S, Hinduja, A, et al. Neuroinflammation in acute ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep. (2023) 23:407–31. doi: 10.1007/s11910-023-01282-2
8. Wang, J, Zhang, X, Tian, J, Li, H, Tang, H, and Yang, C. Predictive values of systemic inflammatory responses index in early neurological deterioration in patients with acute ischemic stroke. J Integr Neurosci. (2022) 21:94. doi: 10.31083/j.jin2103094
9. Oh, ES, You, Z, Nowak, KL, and Jovanovich, AJ. Association of Monocyte Count and Monocyte/lymphocyte ratio with the risk of cardiovascular outcomes in patients with CKD. Kidney360. (2022) 3:657–65. doi: 10.34067/KID.0007922021
10. Demirbaş, A, Elmas, ÖF, Atasoy, M, Türsen, Ü, and Lotti, T. Can monocyte to HDL cholesterol ratio and monocyte to lymphocyte ratio be markers for inflammation and oxidative stress in patients with vitiligo? A preliminary study. Arch Dermatol Res. (2021) 313:491–8. doi: 10.1007/s00403-020-02129-3
11. Dai, K, Li, Z, Luo, Y, Xiong, Q, Xiong, Y, Song, Z, et al. Neutrophil percentage-to-albumin ratio and monocyte-to-lymphocyte ratio as predictors of free-wall rupture in patients with acute myocardial infarction. J Clin Lab Anal. (2022) 36:e24136. doi: 10.1002/jcla.24136
12. Pinčáková, K, Krastev, G, Haring, J, Mako, M, Mikulášková, V, and Bošák, V. Low lymphocyte-to-monocyte ratio as a possible predictor of an Unfavourable clinical outcome in patients with acute ischemic stroke after mechanical Thrombectomy. Stroke Res Treatment. (2022) 2022:1–9. doi: 10.1155/2022/9243080
13. Guan, J, Wang, Q, and Zhao, Q. Lymphocyte to monocyte ratio is independently associated with futile recanalization in acute ischemic stroke after endovascular therapy. Neuropsychiatr Dis Treat. (2023) 19:2585–96. doi: 10.2147/NDT.S434225
14. Ren, H, Liu, X, Wang, L, and Gao, Y. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:2595–602. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.019
15. Ren, H, Han, L, Liu, H, Wang, L, Liu, X, and Gao, Y. Decreased lymphocyte-to-monocyte ratio predicts poor prognosis of acute ischemic stroke treated with thrombolysis. Med Sci Monit. (2017) 23:5826–33. doi: 10.12659/MSM.907919
16. Sadeghi, F, Sarkady, F, Zsóri, KS, Szegedi, I, Orbán-Kálmándi, R, Székely, EG, et al. High neutrophil-lymphocyte ratio and low lymphocyte-monocyte ratio combination after thrombolysis is a potential predictor of poor functional outcome of acute ischemic stroke. J Pers Med. (2022) 12:1221. doi: 10.3390/jpm12081221
17. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
18. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
19. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
20. Zhang, TR, Fu, S, Cao, XF, Xia, YJY, Hu, MY, Feng, QH, et al. Correlation of peripheral blood inflammatory indicators to prognosis after intravenous thrombolysis in acute ischemic stroke: a retrospective study. Int J Gen Med. (2024) 17:985–96. doi: 10.2147/IJGM.S456144
21. Lux, D, Alakbarzade, V, Bridge, L, Clark, CN, Clarke, B, Zhang, LQ, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. (2020) 17:60. doi: 10.1186/s12974-020-01739-y
22. Quan, XQ, Wang, RC, Zhang, Q, Zhang, CT, and Sun, L. The predictive value of lymphocyte-to-monocyte ratio in the prognosis of acute coronary syndrome patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2020) 20:338. doi: 10.1186/s12872-020-01614-x
23. Mei, P, Feng, W, Zhan, Y, and Guo, X. Prognostic value of lymphocyte-to-monocyte ratio in gastric cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2023) 14:1321584. doi: 10.3389/fimmu.2023.1321584
24. Liu, K, Tang, S, Liu, C, Ma, J, Cao, X, Yang, X, et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front Immunol. (2024) 15:1337241. doi: 10.3389/fimmu.2024.1337241
25. Arefhosseini, S, Aghajani, T, Tutunchi, H, and Ebrahimi-Mameghani, M. Association of systemic inflammatory indices with anthropometric measures, metabolic factors, and liver function in non-alcoholic fatty liver disease. Sci Rep. (2024) 14:12829. doi: 10.1038/s41598-024-63381-5
26. Macrez, R, Ali, C, Toutirais, O, Le Mauff, B, Defer, G, Dirnagl, U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
27. Jin, R, Yang, G, and Li, G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. (2010) 87:779–89. doi: 10.1189/jlb.1109766
28. Liberale, L, Montecucco, F, Bonaventura, A, Casetta, I, Seraceni, S, Trentini, A, et al. Monocyte count at onset predicts poststroke outcomes during a 90-day follow-up. Eur J Clin Investig. (2017) 47:702–10. doi: 10.1111/eci.12795
29. Gittler, M, and Davis, AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. (2018) 319:820–1. doi: 10.1001/jama.2017.22036
30. González, A, Moniche, F, Cayuela, A, García-Lozano, JR, Torrecillas, F, Escudero-Martínez, I, et al. Effect of CYP2C19 polymorphisms on the platelet response to Clopidogrel and influence on the effect of high versus standard dose Clopidogrel in carotid artery stenting. Eur J Vasc Endovasc Surg. (2016) 51:175–86. doi: 10.1016/j.ejvs.2015.09.020
31. Johnston, SC, Easton, JD, Farrant, M, Barsan, W, Conwit, RA, Elm, JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. (2018) 379:215–25. doi: 10.1056/NEJMoa1800410
32. Nishijima, TF, Muss, HB, Shachar, SS, Tamura, K, and Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. (2015) 41:971–8. doi: 10.1016/j.ctrv.2015.10.003
33. Stinear, CM, Smith, MC, and Byblow, WD. Prediction tools for stroke rehabilitation. Stroke. (2019) 50:3314–22. doi: 10.1161/STROKEAHA.119.025696
34. Winstein, CJ, Stein, J, Arena, R, Bates, B, Cherney, LR, Cramer, SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98–e169. doi: 10.1161/STR.0000000000000098
35. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
36. Dziedzic, T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. (2015) 15:523–31. doi: 10.1586/14737175.2015.1035712
37. Libby, P, Ridker, PM, and Hansson, GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. (2009) 54:2129–38. doi: 10.1016/j.jacc.2009.09.009
38. Quinn, TJ, Dawson, J, Walters, MR, and Lees, KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. (2009) 4:200–5. doi: 10.1111/j.1747-4949.2009.00271.x
39. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
Keywords: lymphocyte-to-monocyte, LMR, acute ischemic stroke, AIS, poor functional outcome, stroke, prognosis
Citation: Tian C, Yang Y, Wan J, Wang R, Zhou K, Li Y, Guo W, Li H and Zhang Y (2025) Prognostic value of lymphocyte-to-monocyte ratio in acute ischemic stroke: a systematic review and meta-analysis. Front. Neurol. 16:1567112. doi: 10.3389/fneur.2025.1567112
Edited by:
Haipeng Liu, Coventry University, United KingdomReviewed by:
Mona Asghariahmadabad, University of California, San Francisco, United StatesMohammed Ahmed Akkaif, QingPu Branch of Zhongshan Hospital Affiliated to Fudan University, China
Wu Zhou, The First Affiliated Hospital of Nanchang University, China
Muhana Fawwazy Ilyas, Sebelas Maret University, Indonesia
Dhrumil Shah, Super Metro Specialized Medical Center, Kuwait
Copyright © 2025 Tian, Yang, Wan, Wang, Zhou, Li, Guo, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang, emhhbmd5dTEwNTdAY2R1LmVkdS5jbg==
†These authors have contributed equally to this work
 Chengli Tian1,2†
Chengli Tian1,2† Yu Zhang
Yu Zhang