- 1Department of Neurology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Neurology, Chongqing Medical University, Chongqing, China
- 3Department of Interventional Medicine, Guangzhou First People’s Hospital, Second Affiliated Hospital of South China University of Technology, Guangzhou, China
Background: Although the administration of dexmedetomidine (DEX) in intensive care unit (ICU) is rapidly increasing, its potential impact on critically ill patients with ischemic stroke has not yet been explored.
Methods: Patient data were extracted from the Medical Information Mart for Intensive Care IV (MIMIC-IV 3.0) database to identify patients who received DEX and those who did not. The primary outcome was ICU mortality. Statistical analyses included multivariate Cox proportional hazards regression, propensity score matching (PSM), and inverse probability of treatment weighting (IPTW) to ensure the robustness of the findings.
Results: This study included 646 patients (22.8%) with ischemic stroke who received DEX treatment, and 2,182 patients who did not receive DEX in the ICU. A significant reduction in ICU mortality was observed in the DEX group compared to the non-DEX group, with an adjusted hazard ratio (HR) of 0.52 [95% confidence interval (CI) 0.40–0.68, p < 0.001]. Within the matched cohort, DEX administration did not show a statistically significant increased risk of bradycardia and improvement in 90-day mortality outcomes.
Conclusion: These findings suggest that DEX administration may reduce ICU mortality in patients with IS.
1 Introduction
Stroke remains one of the leading causes of global disability and mortality (1). According to the Global Burden of Disease Study, stroke accounted for approximately 7.3 million deaths worldwide in 2021, representing 10.7% of all mortalities, of which ischemic stroke (IS) accounted for 65.3% of all cases (2). A recent multicenter retrospective study involving 952,400 patients diagnosed with acute IS found that 19.9% of these patients required ICU-level care (3). However, among stroke patients receiving ICU treatment, mortality rate have been reported to reach as high as 70% (4, 5). Therefore, it is imperative to identify effective interventions to reduce ICU mortality among critically ill patients suffering from IS.
Dexmedetomidine (DEX), a high-affinity α2-adrenergic receptor agonist, exhibits sympathetic inhibitory and anti-inflammatory properties (6–8) and is frequently used for sedation, analgesia, delirium reduction in mechanically ventilated patients in the ICU (9, 10). Recent studies have suggested that early administration of DEX is associated with reduced in-hospital mortality among patients with traumatic brain injury (11). Furthermore, DEX administration may improve outcomes in patients with acute myocardial infarction, partly due to its anti-inflammatory effects (12). However, a multicenter randomized controlled trial found that DEX administration in patients with sepsis did not improve mortality or ventilator-free days (13). At present, there is limited evidence on sedation protocols for critically ill patients, and the impact of DEX on outcomes of critically ill IS patients remain unclear. Therefore, this study aims to investigate the association between DEX administration and prognosis in patients with IS in the ICU.
2 Materials and methods
2.1 Data source
The dataset used in this study was obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV 3.0) database, a comprehensive single-center repository containing information on 364,627 critically ill patients from 2008 to 2022. This database includes detailed records on patient demographics, laboratory results, nursing progress notes, intravenous (IV) medications, fluid balance, and a wide range of other clinical variables. The database was approved for research used by the institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center, with a waiver of informed consent. Additionally, one of the authors of this study obtained permission to access the dataset and extract relevant data.
2.2 Study design and population
We recruited a cohort of 9,759 patients diagnosed with ischemic stroke, identified using the search term “cerebral infarction” in accordance with ICD-9 or ICD-10 coding standards. The exclusion criteria were as follows: (1) patients without ICU admission, (2) DEX IV infusion duration of less than 4 h, (3) ICU stay of less than 48 h, (4) age under 18 years, and (5) recurrent ICU admissions, with only data from the first admission being considered. A total of 2,828 patients were ultimately included in the study and divided into two groups: the DEX administration group (DEX group) and the non-DEX administration group (non-DEX group) (Figure 1).
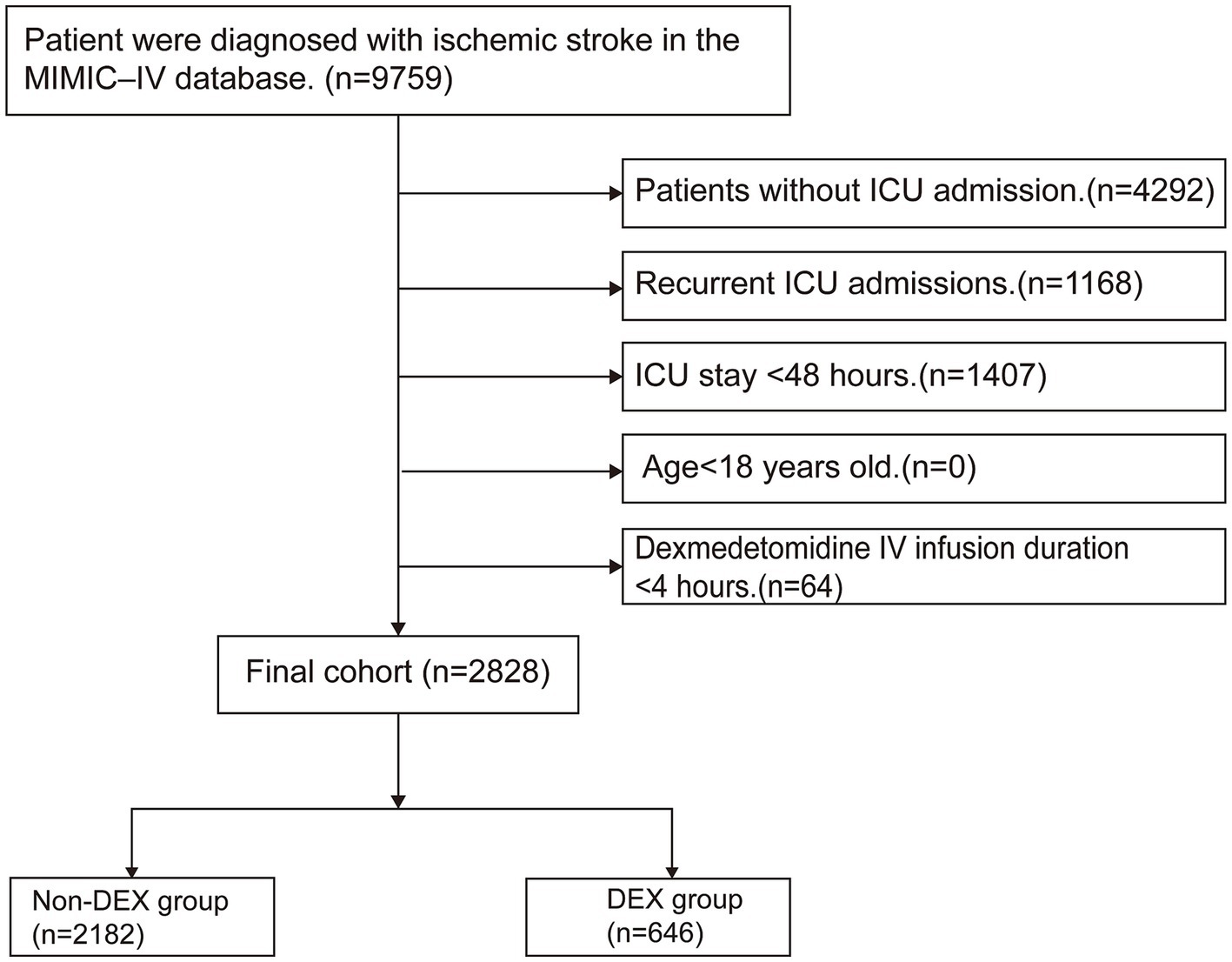
Figure 1. Flowchart for the inclusion of study participants. MIMIC-IV; Medical Information Mart for Intensive Care IV; ICU: intensive care unit; IV: intravenous; DEX: dexmedetomidine.
2.3 Primary outcome and secondary outcomes
The primary outcome of the study was ICU mortality. Secondary outcomes included the duration of mechanical ventilation, reduction in white blood cell (WBC) count, incidence of bradycardia, length of ICU stay, and 90-day mortality. Bradycardia was defined as a heart rate below 50 beats/min following DEX administration. The reduction in WBC count was calculated as following: for the DEX group, it was determined by the difference between the initial WBC count recorded at ICU admission and the WBC count measured on the fourth day after DEX administration. For the non-DEX group, the reduction was defined as the difference between the initial WBC count at ICU admission and the WBC count obtained on the fourth day following the initial measurement.
2.4 Variables
Data extraction was conducted using Navicat Premium (version 16.0.6) by implementing structured query language (SQL). The extracted patient data included the following categories: (1) demographic variables: age, sex, weight and ethnicity; (2) first day vital signs: systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), respiratory rate, temperature and pulse oxygen saturation (Spo2); (3) laboratory results: WBC, red blood cell (RBC), hemoglobin, platelet, creatinine, international normalized ratio (INR), prothrombin time (PT), and partial thromboplastin time (PTT); (4) comorbidities: hypertension, diabetes, atrial fibrillation, respiratory failure, heart failure, acute myocardial infarction, sepsis, chronic renal disease stage V, liver disease and malignancy, ventilator-associated pneumonia (VAP), and delirium; (5) interventions: propofol, midazolam, antiplatelet, norepinephrine, continuous renal replacement therapy (CRRT), and mechanical ventilation; (6) clinical scores: Richmond Agitation-Sedation Scale (RASS), Glasgow coma score (GCS), Charlson comorbidity index (CCI), Sequential Organ Failure Assessment (SOFA), Acute physiology score III (APSIII), Oxford acute severity of illness score (OASIS).
2.5 Statistical analyses
For categorical variables, a chi-square test was conducted, with results expressed as absolute counts and proportions. For continuous data that did not follow a normal distribution, the Kruskal-Wallis test was employed, and outcomes were reported as medians interquartile ranges (IQRs).
To evaluate the relationship between DEX administration and outcomes in critically ill patients with ischemic stroke, a univariate Cox regression analysis was performed. Variables considered clinically relevant and showing a univariate association with outcomes (p < 0.10) were incorporated as covariates in a multivariate Cox regression model. These covariates comprised age, weight, gender, ethnicity, DBP, respiratory rate, temperature, Spo2, WBC, platelets, INR, PT, creatinine, hypertension, atrial fibrillation, chronic renal disease stage V, liver disease, malignancy, propofol, midazolam, antiplatelet, mechanical ventilation, norepinephrine, RASS, CCI, SOFA, APSIII, and OASIS.
To ensure the robustness of our findings, propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) (14, 15) were employed. In the PSM model, the Cox proportional hazards regression model was used to estimate the propensity score for DEX administration. The 1:1 nearest neighbor matching method was selected, and the standardized mean difference (SMD) and p-value were calculated to assess the effectiveness of the PSM approach. In the IPTW model, the distribution of covariate characteristics in each group was balanced with the entire cohort by applying appropriate weighting. The baseline characteristics between the two groups were well-balanced, with SMDs for all variables being less than 0.1 (Supplementary Figure S1). Moreover, a sensitivity analysis was conducted to evaluate the robustness of the findings. In addition, a subgroup analysis was performed to investigate whether the relationship between DEX administration and ICU mortality was influenced by factors such as age, sex, specific comorbidities, the use of other sedatives, and the presence of severe disturbances of consciousness (GCS ≤ 8) upon admission.
Finally, the missing values of all variables were less than 15%, and multiple imputation, regression imputation, and mean value imputation methods were used to address the missing values. Statistical analyses were performed using Stata (version 18.0) and R Studio (version R4.4.1), with p < 0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics of the study individuals
In our study cohort, a total of 2,828 patients admitted to the ICU with IS were enrolled. Among these, 646 patients (22.8%) received DEX following ICU admission, while 2,182 patients did not receive this treatment. Table 1 summarized the characteristics of the DEX and non-DEX groups. Compared to the non-DEX group, the DEX group was younger [65.62 (54.58–73.53) vs. 72.02 (60.92–81.68), p < 0.001], and had a higher proportion of male patients (62.07% vs. 48.12%; p < 0.001). The DEX group also exhibited higher WBC levels [10.40 (7.60–14.30) vs. 9.40 (7.00–12.30), p < 0.001] and a higher heart rate at admission [83.19 (73.89–96.08) vs. 79.72 (70.27–90.96), p < 0.001]. Furthermore, patients in the DEX group were more deeply sedated [−1.00 (−3.00–0.00) vs. 0.00 (−1.00–0.00), p < 0.001] and had significantly higher severity scores upon admission [e.g., SOFA: 4.00 (2.00–6.00) vs. 2.00 (1.00–4.00), p < 0.001]. Additionally, they were more likely to require mechanical ventilation (92.13% vs. 72.32%; p < 0.001) and the administration of additional sedative agents, such as propofol (96.13% vs. 72.32%; p < 0.001) and midazolam (42.57% vs. 17.60%; p < 0.001).
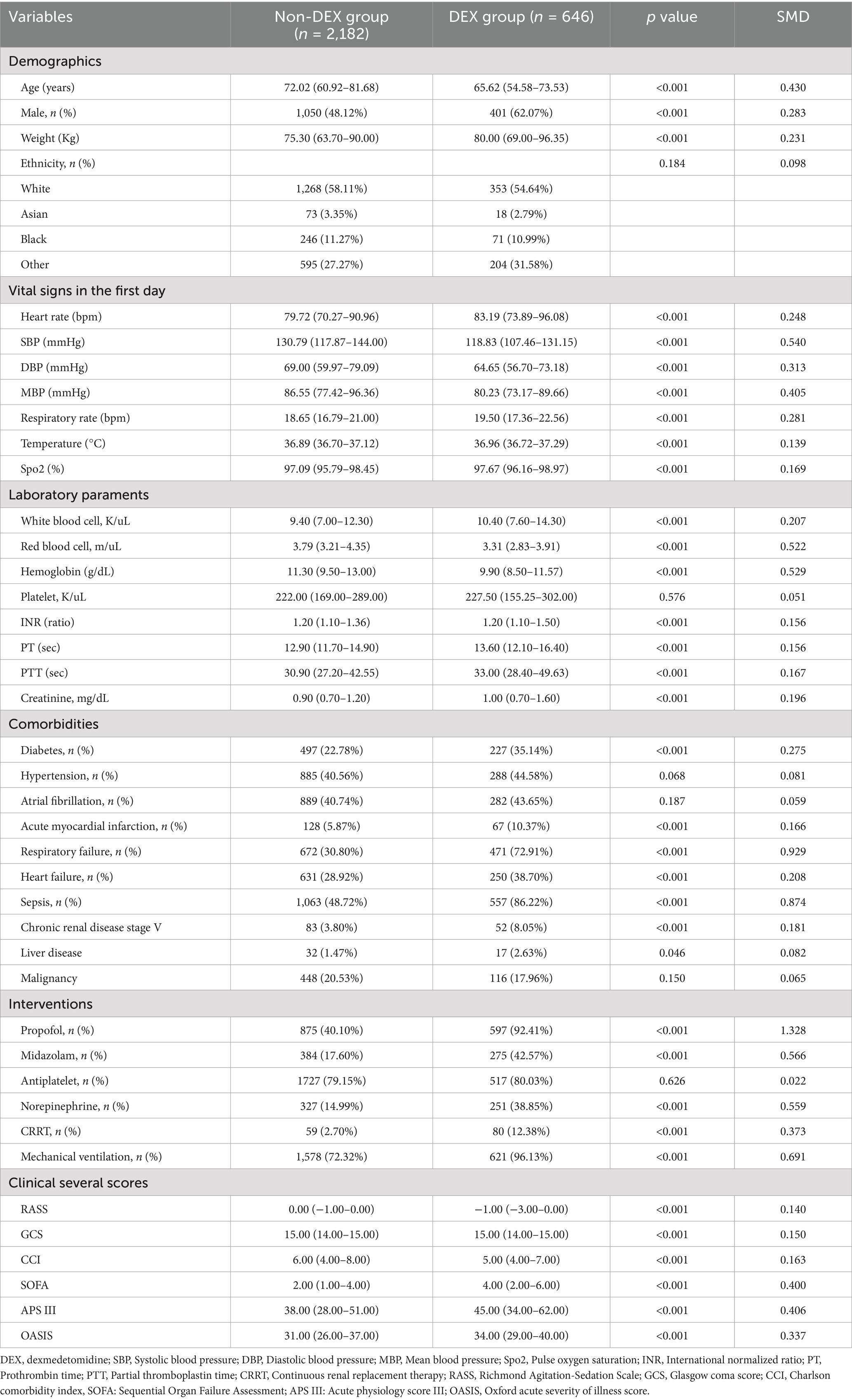
Table 1. Baseline characteristics and comparisons between the DEX group and non-DEX group before propensity score matching.
3.2 Relationship between dexmedetomidine administration and the primary outcome
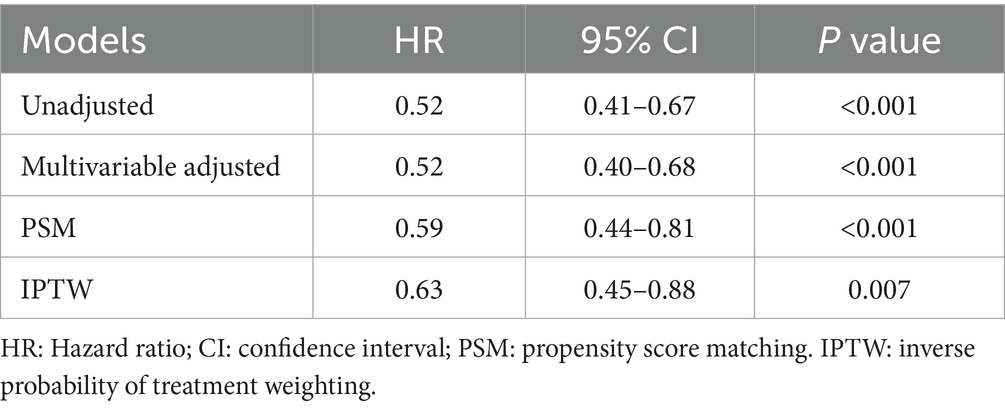
Univariate and multivariate Cox proportional hazards regression models demonstrated that the DEX group was associated with a reduced risk of ICU mortality, with an adjusted hazard ratio (HR) of 0.52 (95% confidence interval (CI) 0.40–0.68, p < 0.001) (Supplementary Tables S1, S2). Following PSM, 503 patients in the DEX group were matched with 503 patients in the non-DEX group using a 1:1 matching algorithm (Supplementary Table S3; Supplementary Figure S1). In the adjusted PSM model, the HR was 0.59 (95% CI: 0.44–0.81, p < 0.001). Furthermore, IPTW analysis further supported this association (Table 2). Details of the IPTW analyses were shown in Supplementary Table S4. Additionally, a sensitivity analysis was also conducted to examine patients with ventilator-associated pneumonia and delirium, which yielded consistent findings (Supplementary Tables S5, S6).
In the subgroup analysis, the relationship between DEX administration and ICU mortality was statistically significant, regardless of whether patients were over 60 years or had severe consciousness impairment at admission, with no significant interactions observed. Notably, it seemed that the DEX administration was more prominent in patients with co-administration of propofol [0.43 (0.33–0.56) vs. 1.27 (0.51–3.14), p for interaction = 0.026] (Figure 2).
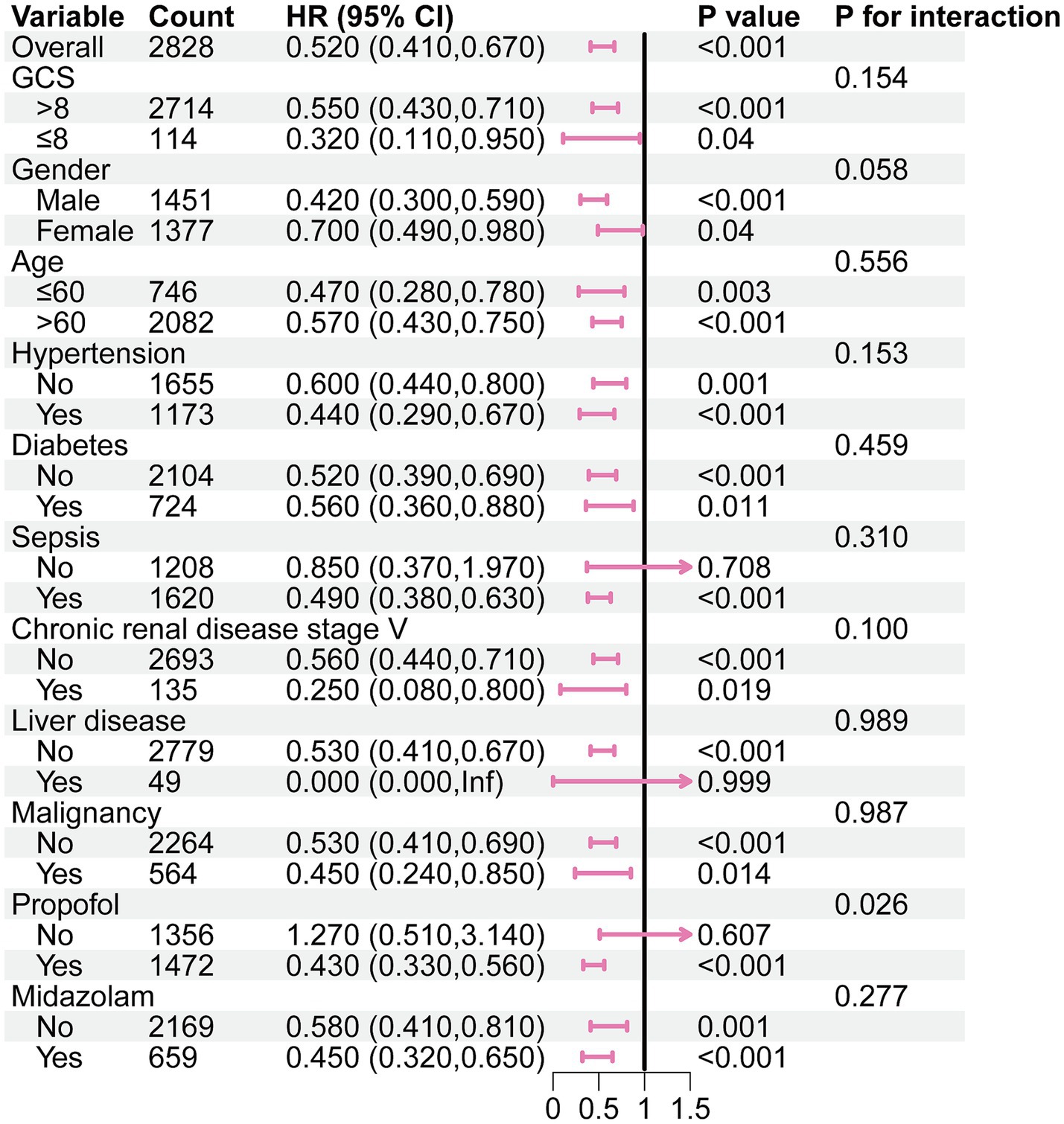
Figure 2. Subgroup analysis of the association between DEX administration and ICU morality in the original cohort. GCS: Glasgow coma scale.
3.3 Relationship between dexmedetomidine administration and the secondary outcomes
We observed that, both in the original cohort and the matched cohort, the reduction in WBC count on the fourth day following DEX administration was significantly greater than in the group that did not receive the drug [original cohort: 1.85 (−0.90–6.00) vs. 0.30 (0.00–2.10), p < 0.001; matched cohort: 2.00 (−0.68–5.90) vs. 0.50 (0.00–3.00), p = 0.006] (Supplementary Table S7; Table 3). Although patients receiving DEX required longer mechanical ventilation [24.88 (10.00–57.00) vs. 18.12 (3.06–51.88), p < 0.001], there was no significant difference between the DEX and the non-DEX group in terms of the incidence of bradycardia, length of stay in the ICU, and even the 90-day mortality.
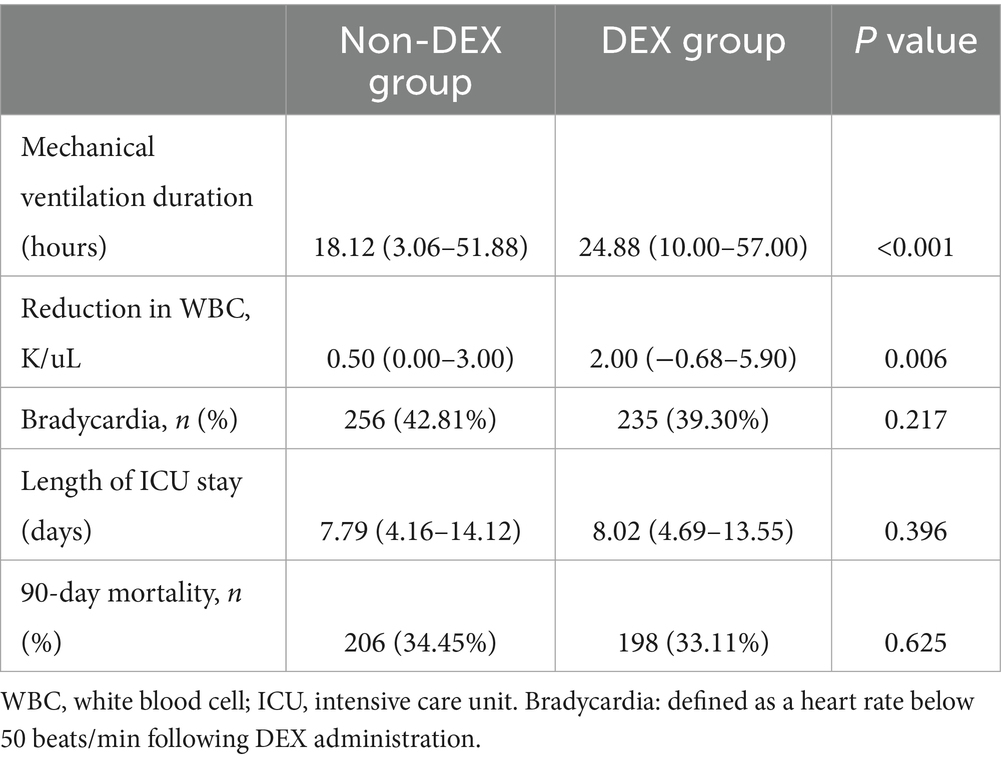
Table 3. Association between dexmedetomidine administration and the secondary outcomes in the matched cohort.
4 Discussion
The results of our study demonstrated that DEX administration was associated with a reduced risk of ICU mortality in patients with IS. And we did not observe an increased risk of bradycardia associated with DEX administration.
Sedatives are essential pharmacological agents that assist clinicians in the management of critically ill patients. Currently, the sedatives employed in clinical practice encompass midazolam, propofol, and DEX, among others. A major concern related to midazolam is its elevated risk of inducing delirium (16, 17). Consequently, DEX and propofol are primarily recommended as sedatives (18). A systematic review and meta-analysis of randomized controlled trials have demonstrated that DEX may provide substantial advantages over propofol, particularly in terms of reducing the duration of mechanical ventilation and the incidence of delirium (19), as well as exhibiting more pronounced neuroprotective effects (20). At present, some studies have also demonstrated that DEX was linked to a decreased risk of new-onset atrial fibrillation in critically ill patients (21) and improved sepsis-associated acute kidney injury (22).
Our results are consistent with those of a previous multicenter, randomized controlled trial study, which indicated that early administration of DEX for sedation in mechanically ventilated ICU patients did not improve 90-day mortality (23). Similarly, another prospective multicenter study also found that early exposure to DEX was not associated with enhanced functional outcomes at 6 months in patients with traumatic brain injury (24). Nonetheless, our research provided additional evidence suggesting that the administration of DEX may significantly reduce the mortality risk within ICU in patients with ischemic stroke, thereby improving short-term adverse outcomes. In the subsequent analysis of secondary outcomes, a significant reduction in WBC count was observed in the DEX group on the fourth day post-administration, in comparison to the non-DEX group. Furthermore, another study demonstrated that DEX significantly decreased serum levels of neuron-specific enolase (NSE), S-100b, and interleukin-6 (IL-6) in patients undergoing surgery for chronic cerebral vascular stenosis (25). These findings suggested that DEX may attenuate the inflammatory response in patients with ischemic stroke, thereby potentially improving their prognosis.
DEX is increasingly being employed as a sedative for patients undergoing mechanical ventilation. In stroke patients, poststroke delirium (PSD) presented within 24 h in 25% of cases and within 72 h in nearly all cases (26). Furthermore, 10 to 15% of stroke patients necessitate mechanical ventilation (27), underscoring the critical role of sedatives in their management. The combination of DEX and propofol not only achieves optimal sedative effects but also significantly reduces the incidence of VAP in mechanically ventilated ICU patients, compared to the administration of DEX alone (28).
Several limitations should be mentioned in the present study. While the incidence of adverse reactions, such as bradycardia, was considered in this study, the evaluation of hypotension incidence remains unfeasible due to insufficient data availability. Furthermore, despite our models adjusted for RASS scores to account for sedation depth, residual confounding related to dynamic changes in sedation levels during ICU stays cannot be ruled out. Future studies should incorporate longitudinal sedation assessments to further elucidate this relationship. Additionally, although we examined the anti-inflammatory properties of DEX by observing the reduction in WBC levels, further research is necessary to comprehensively evaluate its anti-inflammatory characteristics, given the limited presence of inflammation-related indicators in the current database.
5 Conclusion
The administration of DEX is associated with a reduced risk of mortality in the ICU for patients suffering from IS.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by review boards of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SC: Conceptualization, Formal analysis, Methodology, Writing – original draft. SR: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft. XL: Funding acquisition, Supervision, Writing – review & editing. KL: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Science and Technology Projects in Guangzhou (no. 2023A03J0959), Guangdong Basic and Applied Basic Research Foundation (no. 2020A1515110165), and Liu Kewei Scientific Research Start-up Funds (no. BSKY20240024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1571957/full#supplementary-material
References
1. Feigin, VL, Brainin, M, Norrving, B, Martins, SO, Pandian, J, Lindsay, P, et al. World stroke organization: global stroke fact sheet 2025. Int J Stroke. (2025) 20:132–44. doi: 10.1177/17474930241308142
2. He, Q, Wang, W, Zhang, Y, Xiong, Y, Tao, C, Ma, L, et al. Global, regional, and National Burden of stroke, 1990–2021: a systematic analysis for global burden of disease 2021. Stroke. (2024) 55:2815–24. doi: 10.1161/STROKEAHA.124.048033
3. Santos, D, Maillie, L, and Dhamoon, SM. Patterns and outcomes of intensive care on acute ischemic stroke patients in the US. Circ Cardiovasc Qual Outcomes. (2023) 16:e008961. doi: 10.1161/CIRCOUTCOMES.122.008961
4. Sonneville, R, Gimenez, L, Labreuche, J, Smonig, R, Magalhaes, E, Bouadma, L, et al. What is the prognosis of acute stroke patients requiring ICU admission? Intensive Care Med. (2017) 43:271–2. doi: 10.1007/s00134-016-4553-7
5. Alsudayri, MS, Alshammari, KS, Aljameel, OS, Alhuqayl, FS, and Ahmed, HG. Characteristics and outcomes of stroke patients admitted to the medical Ward and the intensive care unit of a tertiary Hospital in Saudi Arabia. AIMDR. (2017) 3:ME17–ME19. doi: 10.21276/aimdr.2017.3.6.me5
6. Fujimoto, M, Higuchi, H, Honda-Wakasugi, Y, Miyake, S, Nishioka, Y, Yabuki-Kawase, A, et al. Dexmedetomidine inhibits LPS-induced inflammatory responses through peroxisome proliferator-activated receptor gamma (PPARγ) activation following binding to α2 adrenoceptors. Eur J Pharmacol. (2021) 892:173733. doi: 10.1016/j.ejphar.2020.173733
7. Wang, C, Chen, Q, Wang, P, Jin, W, Zhong, C, Ge, Z, et al. The effect of Dexmedetomidine as a sedative agent for mechanically ventilated patients with Sepsis: a systematic review and Meta-analysis. Front Med. (2021) 8:776882. doi: 10.3389/fmed.2021.776882
8. Zhang, B, and Shen, J. Dexmedetomidine activates the PKA/CREB pathway and inhibits proinflammatory factor expression through β2 adrenergic receptors. Immun Inflamm Dis. (2024) 12:e1176. doi: 10.1002/iid3.1176
9. Møller, MH, Alhazzani, W, Lewis, K, Belley-Cote, E, Granholm, A, Centofanti, J, et al. Use of dexmedetomidine for sedation in mechanically ventilated adult ICU patients: a rapid practice guideline. Intensive Care Med. (2022) 48:801–10. doi: 10.1007/s00134-022-06660-x
10. Lin, C, Tu, H, Jie, Z, Zhou, X, and Li, C. Effect of Dexmedetomidine on delirium in elderly surgical patients: a Meta-analysis of randomized controlled trials. Ann Pharmacother. (2021) 55:624–36. doi: 10.1177/1060028020951954
11. Liu, SY, Kelly-Hedrick, M, Komisarow, J, Hatfield, J, Ohnuma, T, Treggiari, MM, et al. Association of Early Dexmedetomidine Utilization with Clinical Outcomes after Moderate-Severe Traumatic Brain Injury: a retrospective cohort study. Anesth Analg. (2024) 139:366–74. doi: 10.1213/ANE.0000000000006869
12. Liu, Y, Chen, Q, Hu, T, Deng, C, and Huang, J. Dexmedetomidine administration is associated with improved outcomes in critically ill patients with acute myocardial infarction partly through its anti-inflammatory activity. Front Pharmacol. (2024) 15:1428210. doi: 10.3389/fphar.2024.1428210
13. Kawazoe, Y, Miyamoto, K, Morimoto, T, Yamamoto, T, Fuke, A, Hashimoto, A, et al. Effect of Dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with Sepsis: a randomized clinical trial. JAMA. (2017) 317:1321–8. doi: 10.1001/jama.2017.2088
14. Chesnaye, NC, Stel, VS, Tripepi, G, Dekker, FW, Fu, EL, Zoccali, C, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. (2022) 15:14–20. doi: 10.1093/ckj/sfab158
15. Zhao, Q-Y, Luo, J-C, Su, Y, Zhang, Y-J, Tu, G-W, and Luo, Z. Propensity score matching with R: conventional methods and new features. Ann Transl Med. (2021) 9:812. doi: 10.21037/atm-20-3998
16. Shi, H-J, Yuan, R-X, Zhang, J-Z, Chen, J-H, and Hu, A-M. Effect of midazolam on delirium in critically ill patients: a propensity score analysis. J Int Med Res. (2022) 50:1–10. doi: 10.1177/03000605221088695
17. van Gelder, TG, van Diem-Zaal, IJ, Dijkstra-Kersten, SMA, de Mul, N, Lalmohamed, A, and Slooter, AJC. The risk of delirium after sedation with propofol or midazolam in intensive care unit patients. Br J Clin Pharmacol. (2024) 90:1471–9. doi: 10.1111/bcp.16031
18. Devlin, JW, Skrobik, Y, Gélinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
19. Liu, Z, Zeng, Y, Yang, B, and Liao, P. Efficacy and safety of dexmedetomidine in sepsis patients requiring mechanical ventilation: a systematic review and meta-analysis. J Clin Pharm Ther. (2022) 47:298–305. doi: 10.1111/jcpt.13548
20. Yuan, H-X, Zhang, L-N, Li, G, and Qiao, L. Brain protective effect of dexmedetomidine vs propofol for sedation during prolonged mechanical ventilation in non-brain injured patients. World Journal of Psychiatry. (2024) 14:370–9. doi: 10.5498/wjp.v14.i3.370
21. Song, MJ, Jang, Y, Lee, JH, Yoon, JH, Kim, DJ, Jung, SY, et al. Association of Dexmedetomidine with new-Onset Atrial Fibrillation in patients with critical illness. JAMA Netw Open. (2023) 6:e239955. doi: 10.1001/jamanetworkopen.2023.9955
22. Hu, H, An, S, Sha, T, Wu, F, Jin, Y, Li, L, et al. Association between dexmedetomidine administration and outcomes in critically ill patients with sepsis-associated acute kidney injury. J Clin Anesth. (2022) 83:110960. doi: 10.1016/j.jclinane.2022.110960
23. Shehabi, Y, Howe, BD, Bellomo, R, Arabi, YM, Bailey, M, Bass, FE, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. (2019) 380:2506–17. doi: 10.1056/NEJMoa1904710
24. Liu, SY, Kelly-Hedrick, M, Temkin, N, Barber, J, Komisarow, J, Hatfield, J, et al. Association of Early Dexmedetomidine Utilization with Clinical and Functional Outcomes Following Moderate-Severe Traumatic Brain Injury: a transforming clinical research and knowledge in traumatic brain injury study*. Crit Care Med. (2024) 52:607–17. doi: 10.1097/CCM.0000000000006106
25. Yang, J, Feng, H, Li, J, Jiang, H, Wei, M, Zhao, Y-H, et al. Cerebral protection of intraoperative infusion of Dexmedetomidine in patients with chronic cerebrovascular stenosis undergoing endovascular interventional therapies: a prospective randomized controlled trial. Ann Vasc Surg. (2023) 89:182–9. doi: 10.1016/j.avsg.2022.09.050
26. Fleischmann, R, Warwas, S, Andrasch, T, Kunz, R, Witt, C, Mengel, A, et al. Course and recognition of Poststroke delirium. Stroke. (2021) 52:471–8. doi: 10.1161/STROKEAHA.120.031019
27. Lahiri, S, Mayer, SA, Fink, ME, Lord, AS, Rosengart, A, Mangat, HS, et al. Mechanical ventilation for acute stroke: a multi-state population-based study. Neurocrit Care. (2015) 23:28–32. doi: 10.1007/s12028-014-0082-9
28. Dou, H, Hu, F, Wang, W, Ling, L, Wang, D, and Liu, F. Assessment of the sedative effects of dexmedetomidine and propofol treatment in patients undergoing mechanical ventilation in the ICU and relationship between treatment and occurrence of ventilator-associated pneumonia and detection of pathogenic bacteria. Exp Ther Med. (2020) 20:599–606. doi: 10.3892/etm.2020.8699
Keywords: ischemic stroke, dexmedetomidine, intensive care unit, mortality, sedative
Citation: Chen S, Ren S, Li X and Liu K (2025) Dexmedetomidine administration is associated with a reduced risk of ICU mortality in critically ill patients with ischemic stroke. Front. Neurol. 16:1571957. doi: 10.3389/fneur.2025.1571957
Edited by:
Da Zhou, Capital Medical University, ChinaReviewed by:
José Fernando Oliveira-Costa, Secretaria de Saúde do Estado da Bahia, BrazilKun Fang, Capital Medical University, China
Zhoubin Cao, Capital Medical University, China
Copyright © 2025 Chen, Ren, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kewei Liu, ZXlsaXVrd0BzY3V0LmVkdS5jbg==; Xing Li, ZXlsaXhpbmdAc2N1dC5lZHUuY24=
 Shuiyun Chen1
Shuiyun Chen1 Kewei Liu
Kewei Liu