- Department of Anesthesiology, Hebei General Hospital, Shijiazhuang, Hebei, China
Background: Precise forecasting of delirium in intensive care unit (ICU) may propel effective early prevention strategies and stratification of ICU patients through delirium risks, avoiding waste of medical resources. However, there are few optimal models of delirium in critically ill older patients. This study aimed to propose and verify a nomogram for predicting the incidence of delirium in elderly patients admitted to ICU.
Methods: We performed a retrospective study using data from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. It included data on 13,175 older patients in total. The patients were randomly divided into a training group (n = 9,223) and an internal verification group (n = 3,452). Risk factors were screened using the least absolute shrinkage and selection operator regression. We successfully constructed a multivariate logistic regression model along with a nomogram. We conducted internal verification using 1,000 bootstrap specimens. Performance assessment was conducted using a receiver operating characteristic (ROC) curve, calibration curve, decision curve analysis (DCA), and clinical impact curve (CIC).
Results: The risk factors included in the nomogram were sepsis, Sequential Organ Failure Assessment (SOFA) score, cerebrovascular disease, mechanical ventilation, sedation, severe hypothermia, and serum calcium levels. The area under the ROC curve (AUC) for the nomogram, incorporating the above-mentioned predictors for the training set was 0.762 (95% confidence interval [CI] 0.749–0.776), whereas that for the verification set was 0.756 (95% CI 0.736–0.776). Based on the calibration curve, the model forecast outcomes matched well with the actual results, and the nomogram’s Brier score was 0.12 in the training set and 0.128 in the verification set. DCA and CIC showed that our model had a good net clinical benefit.
Conclusion: We developed a forecast nomogram for delirium in the critically ill elderly patients that enhances clinical decision-making. However, further verification is required.
Introduction
In view of the progressive degradation of physiological and cognitive functions, elderly patients are vulnerable to various complications following intensive care unit (ICU) admission, of which delirium is one of the most frequent (1). Delirium is characterized by inattention, acute fluctuations or variations in cognitive functions, confusion, and changing consciousness. It is considered as one of the most prevalent neuropsychological complications in elderly patients during their ICU stay. According to previous reports, ICU delirium incidence rates in elderly patients are between 24 and 66%, depending on the screening instrument and patient population (2–4). This leads to increased morbidity and mortality, increased admission rates to long-time care facilities, prolonged hospital stays, increased postoperative complications, and poorer functional outcomes (5, 6). Given these adverse consequences, prevention of delirium is essential.
Many studies have confirmed that the occurrence of delirium may be significantly decreased by timely preventive interventions aimed at various delirium risk factors (7, 8). However, it has been documented that non-pharmacological approaches are effective in preventing delirium, whereas controversy remains regarding whether medications can prevent and treat delirium (9). Therefore, non-pharmacological interventions remain the foundation for the treatment of delirium. Notably, bundle administration strategies for critically ill patients at risk of delirium have attracted increasing attention. The ABCDEF bundle (Assess, prevent, and manage pain; Both spontaneous awakening and spontaneous breathing trials; Choice of analgesia and sedation; Delirium: assess, prevent, and manage; Early mobility and exercise; and Family engagement and empowerment), which is recommended by the Society of Critical Care Medicine (SCCM) can considerably enhance the prevention of delirium (10). However, due to workload and documentation burden, overall bundle adherence is poor in clinical practice with implementation rates of 12–57% (11–13).
In the case of limited resources, a precise and functional assessment of delirium risk in ICU patients is the basis for efficient and effective interventions. A reliable forecast model for delirium will help clinical therapists identify patients at high delirium risk and provide them with timely interventions. Machine learning models have shown effectiveness in predicting delirium, however, the lack of transparency in prediction processes reduced the interpretability and limited their utility in guiding clinical interventions. Additionally, the complexity of model development and the requirement for large datasets hinder their widespread clinical application. Nomograms, as simpler predictive tools, allow for intuitive visualization of variable contributions to outcomes, offering better interpretability and clinical utility. Although numerous studies have developed nomogram models for delirium prediction, the foundation for such models is either findings from patients with wide age ranges (14, 15) or from solely specific ICU units (14, 16), failing to account for generalizability to older ICU patients. This study aimed to develop and verify a predictive model for delirium in elderly ICU patients (over 65-year-old) using a large clinical database. The findings of this study will assist in the early identification of ICU patients with the highest risk of delirium, thus allowing clinical therapists to conduct timely preventive interventions.
Materials and methods
Study design
This retrospective cohort study was conducted using the Medical Information Mart for Intensive Care IV (MIMIC-IV) database (version 2.0.). This database includes information on ICU patients admitted to Beth Israel Deacon-ess Medical Centre in Boston, Massachusetts between 2008 and 2019 (17). One author (LJ) completed the Collaborative Institutional Training Initiative examination and obtained access to the database (certification number: 9771318). The institutional review boards of Beth Israel Deaconess Medical Centre and the Massachusetts Institute of Technology Affiliates approved the application for the MIMIC-IV database. Personal data in this database were processed; therefore, informed consent was not required. We conducted the study according to the Transparent Reporting of a Multivariable forecast Model for Individual Prognosis or Diagnosis (TRIPOD) statement (18).
Research population and data extraction
Figure 1 shows the enrollment procedure of the study patients. We included patients who completed delirium evaluation during their initial ICU admission in the MIMIC-IV database. Patients who stayed in the ICU for less than 24 h or who were under 65 years were excluded from the study. Patients diagnosed with dementia were also excluded because such patients are often misdiagnosed as having cognitive impairment. To make patients less likely to develop delirium prior to ICU admission, patients with a positive delirium test within 24 h after admission were also excluded (19, 20). Therefore, our study included 13,175 patients totally.
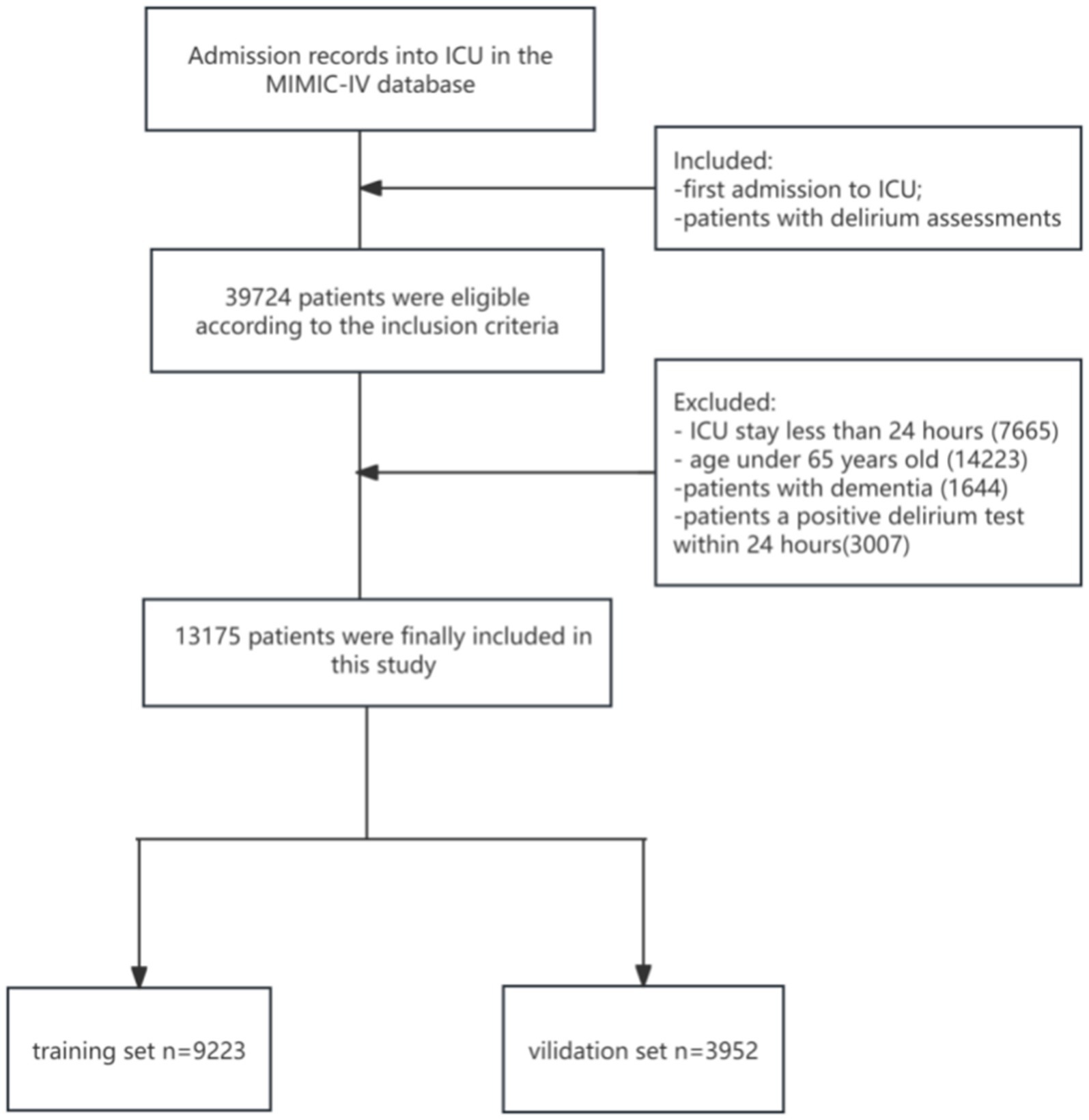
Figure 1. The flow diagram of enrollment procedure. ICU, intensive care unit; MIMIC-IV, Medical Information Mart for Intensive Care-IV.
We extracted all research data from MIMIC-IV using structured query language (SQL) with Navicat Premium. The data included (1) patient demographics; (2) first-day vital signs and laboratory indicators including respiratory rate (RR), mean blood pressure (MBP), temperature: severe hypothermia (<35.0°C), mild hypothermia (35.0°C to <36.0°C), and normothermia (≥36.0°C) (21), heart rate (HR), SpO2, hemoglobin, platelet, white blood cell (WBC), chloride, calcium, sodium, potassium, bicarbonate, blood urea nitrogen (BUN), creatinine, prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), and glucose; (3) relevant comorbidities and scores that reflect patient disease severity, such as cerebrovascular disease (stroke, cerebral infarction, cerebral hemorrhage, stenosis and transient ischemic attack), sepsis (organ dysfunction, hypoperfusion, or hypotension caused by systemic infection based upon the sepsis 3.0 Definition) and Sequential Organ Failure Assessment (SOFA) score; (4) mechanical ventilation (invasive ventilation only) and sedation (use lorazepam, diazepam, midazolam and propofol) within 24 h of ICU admission; and (5) details of admission. For variable data with several measurements, we included the minimum value within the first 24 h of SpO2 and hemoglobin levels in analysis, whereas we included the maximum values of other variables for analysis. We excluded variables with over 20% missing data and the remaining variables with missing values underwent multiple imputation using “MICE” package in R (22). See Supplementary Table S1 for detailed data.
Delirium assessment
In the MIMIC database, delirium screening was conducted using the Confusion Assessment Method in ICU. It is a verified ICU bedside device for routine delirium monitoring with high reliability, sensitivity, and specificity (23). We defined patients scoring positive for delirium as having one or more positive delirium screenings at any ICU stay, whereas we defined those scoring negative for delirium as possessing all negative outcomes in delirium screening.
Statistical analysis
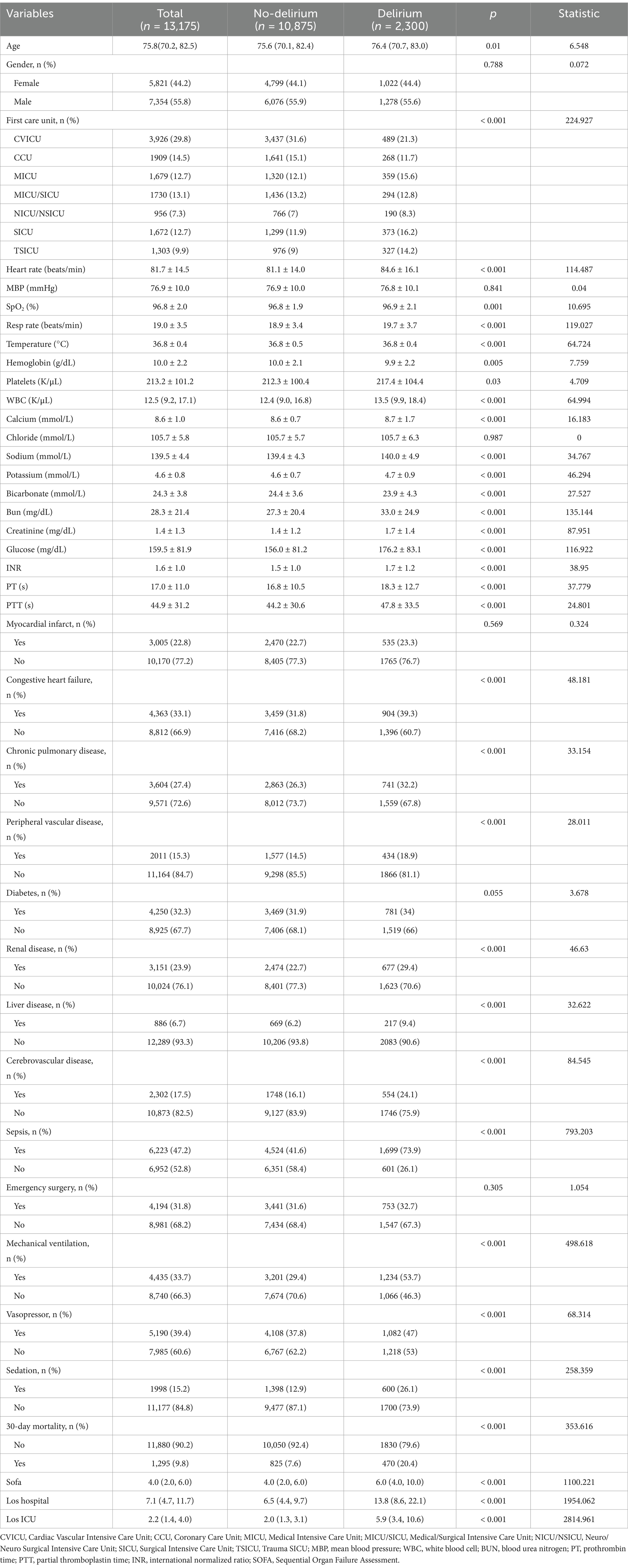
The study population was divided into two groups according to their delirium assessment. The distribution of data was analyzed by the Shapiro–Wilk test. Continuous variables with a normal distribution were expressed as the mean ± standard deviation (SD) and were compared using the independent-sample t-test. The Mann–Whitney U test presented continuous variables with a non-normal distribution. Categorical data were presented as a number (%) and were analyzed using the chi-square test or Fisher’s exact probability test.
To develop the forecast nomogram, we randomly divided patients into a training or verification set in a 7:3 ratio. Variable selection and model development included all patients in the training set. Delirium predictors were screened using the least absolute shrinkage and selection operator (LASSO) expression with 95% confidence intervals (95% CIs) and odds ratios (ORs) computed (24). Variables included in the model were determined via the largest λ value with a mean error within a single standard deviation chosen as per cross-verification outcomes (bootstrap resampling 1,000 times). Model performance was assessed in the training and verification sets using the area under the receiver operating characteristic curve (AUC). The degree of consistency of the actual and forecast results was assessed using the calibration curve, Brier score, and Hosmer–Lemeshow goodness of fit test (HL test). The clinical application of the nomogram was assessed using a clinical impact curve (CIC) and decision curve analysis (DCA).
All statistical analyses were performed using Free Statistics software (version 2.0) and R software packages (The R Foundation)1 (25). Statistical significance was set at p < 0.05.
Results
Patient clinical and demographic data
Finally, our research included 13,175 older ICU patients. See Figure 1 for the elaborate selection process. The enrolled patients included 2,300 patients with delirium (17.5%). Table 1 summarizes the baseline characteristics and details within 24 h of ICU patient admission. Compared with the non-delirium group, patients with delirium were older, and their RR and HR were faster. In addition, these patients had higher creatinine, platelet, BUN, WBC, INR, PT, PTT, and glucose levels and a greater possibility of sepsis; peripheral vascular, chronic pulmonary, and cerebrovascular diseases; congestive heart failure; and renal and liver illnesses. Moreover, patients in the delirium group received more medical treatment and had significantly higher SOFA scores. Patients with delirium had a more unfavorable prognosis. Their hospital and ICU stay periods were much longer and their 30-day mortality rates were higher too.
Nomogram characteristics selection and development
The variables already incorporated into the SOFA scoring system, including MBP, platelet count, creatinine level, and vasopressor use, were not used for LASSO regression, analysis, and cross-verification. Of the 30 variables, we recognized 7 variables as independent delirium predictors (Figure 2). The performance of risk factors was as follows: sepsis (OR: 2.50; 95% CI 2.19–2.86), SOFA score (OR: 1.18; 95%CI 1.16–1.21), sedation (OR: 1.31; 95%CI 1.12–1.52), mechanical ventilation (OR: 1.03; 95% CI 1.17–1.53), severe hypothermia (OR: 1.59; 95% CI 1.41–1.80), calcium levels (OR: 1.22; 95% CI 1.14–1.32), and cerebrovascular disease (OR: 2.03; 95% CI 1.75–2.35) (Table 2). Based on these outcomes, a nomogram was constructed to predict delirium in older patients admitted to the ICU (Figure 3).
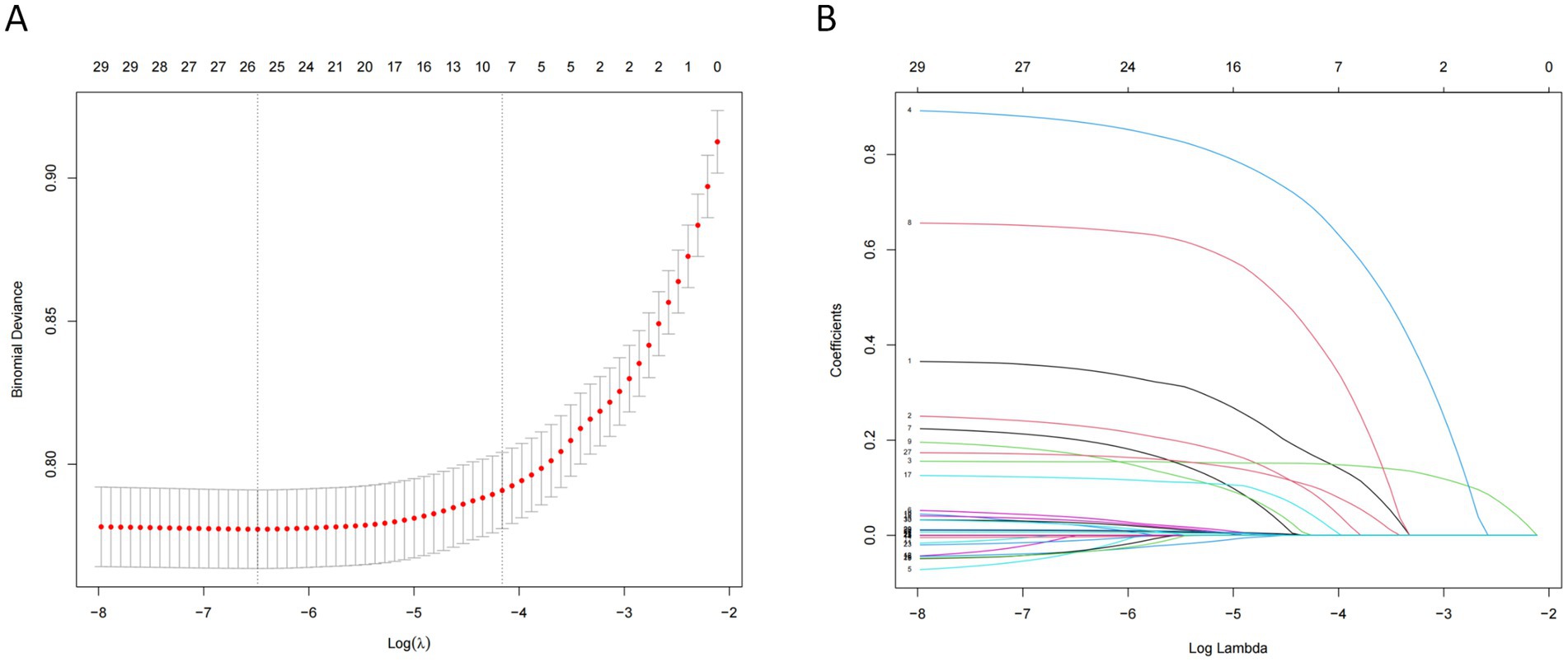
Figure 2. Features selection using the LASSO regression model. (A) The largest λ value with mean error within single standard deviation is determined through cross-validation. (B) Seven coefficients with non-zero values are selected.
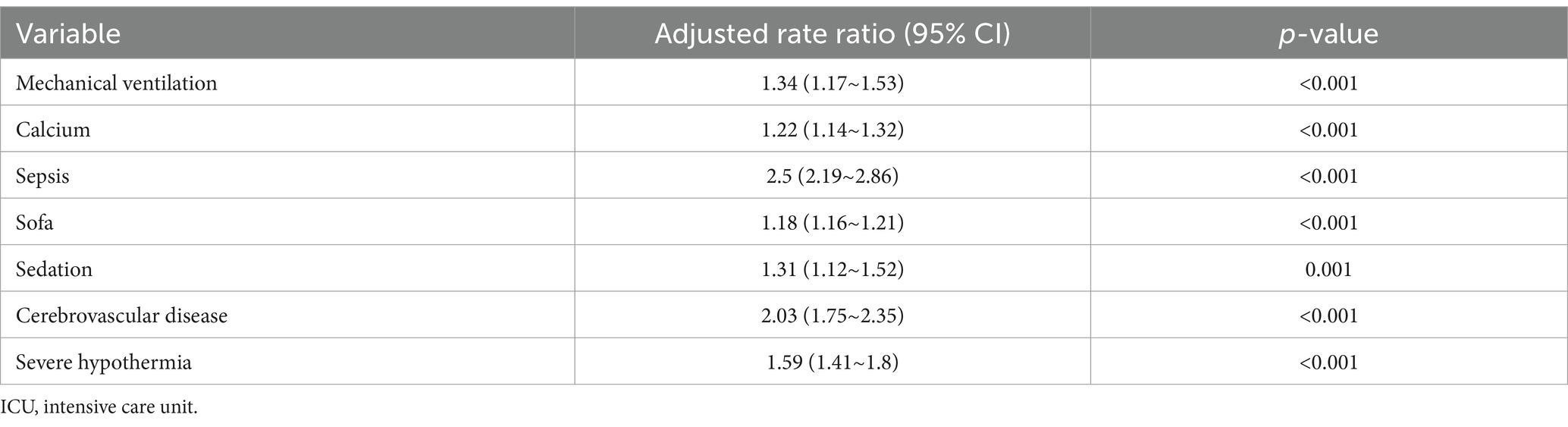
Table 2. Multivariable logistic regression model for predicting the delirium in elderly patients admitted to ICU.
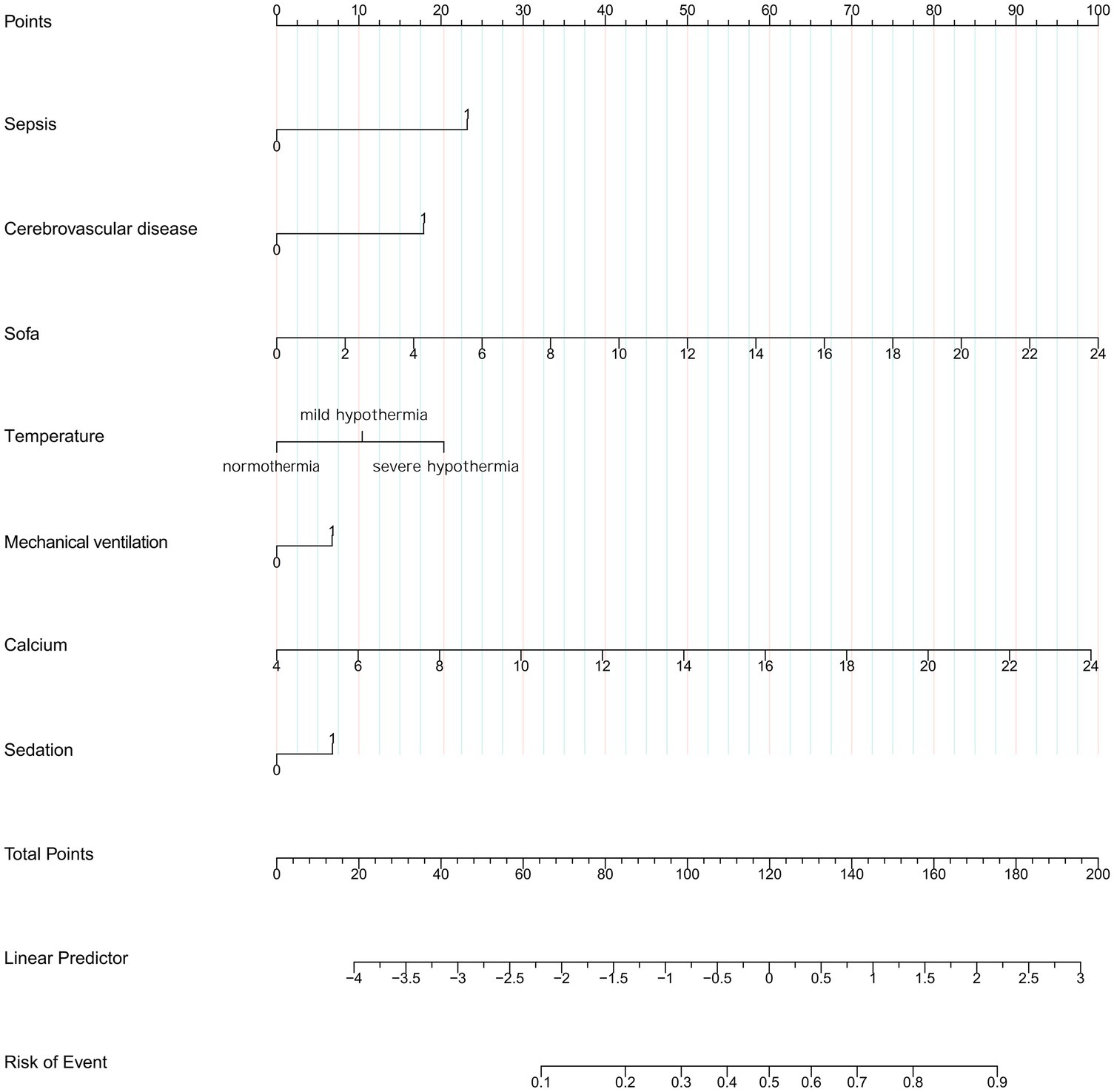
Figure 3. Nomogram to predict the risk to develop delirium in elder admission to ICU. SOFA, Sequential Organ Failure Assessment.
Nomogram performance
The constructed model generated an AUC value of 0.762 (95% CI 0.749–0.776) in the training set and 0.756 (95% CI 0.736–0.776) in the verification set (Figure 4). We plotted the calibration curves for the two sets and formed a bias-corrected line using the bootstrap method. Calibration curves were fairly close to the standard diagonal line of 45° (Figure 5), while the Brier score of the nomogram was 0.12 in the training set and 0.128 in verification set, suggesting favorable consistency between the forecast and actual values. Meanwhile, non-significant p-values of 0.386 and 0.686 in the two sets were observed in the HL test. Furthermore, the clinical utility of the nomogram was assessed using the CIC and DCA. When the threshold probability was between 0.1 and 0.65, a greater net benefit could be generated from such a forecast model, as indicated by the DCA curve (Figure 6A). The high-risk patient number (number of forecast delirium cases via nomogram) matched well with the high-event-risk patient number (number of true-positive delirium cases), whereas the threshold probability exceeded 0.7, as depicted in the CIC findings (Figure 6B). In the verification set, the DCA and CIC curve also demonstrated the benefit of applying the nomogram in clinical practice (Figures 6C,D). In summary, by combining various evaluation parameters, our model showed an excellent predictive value for delirium in elderly patients.
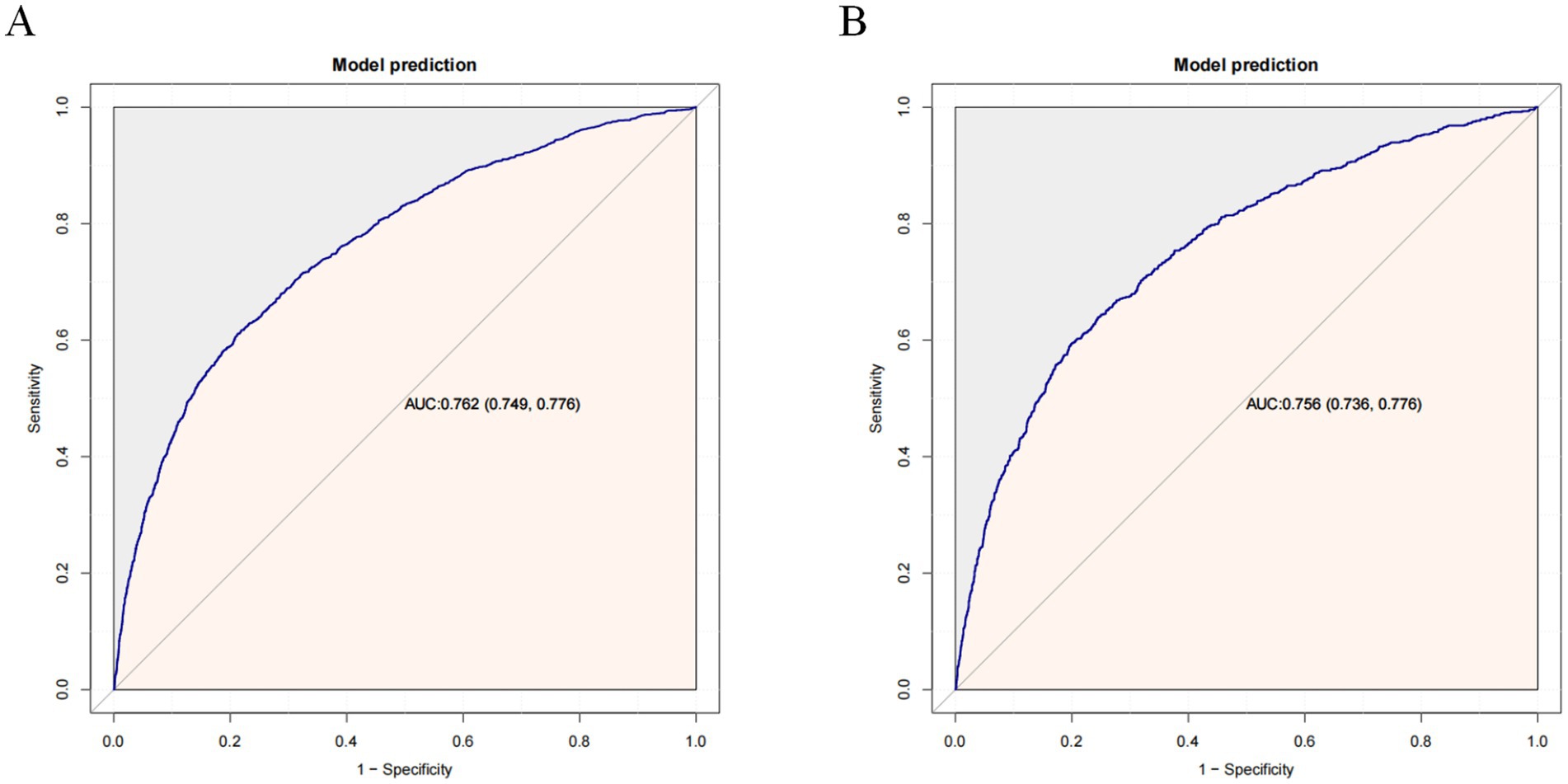
Figure 4. Receiver operating characteristic (ROC) curves in the training set (A) and validation set (B). AUC, area under the ROC curve.
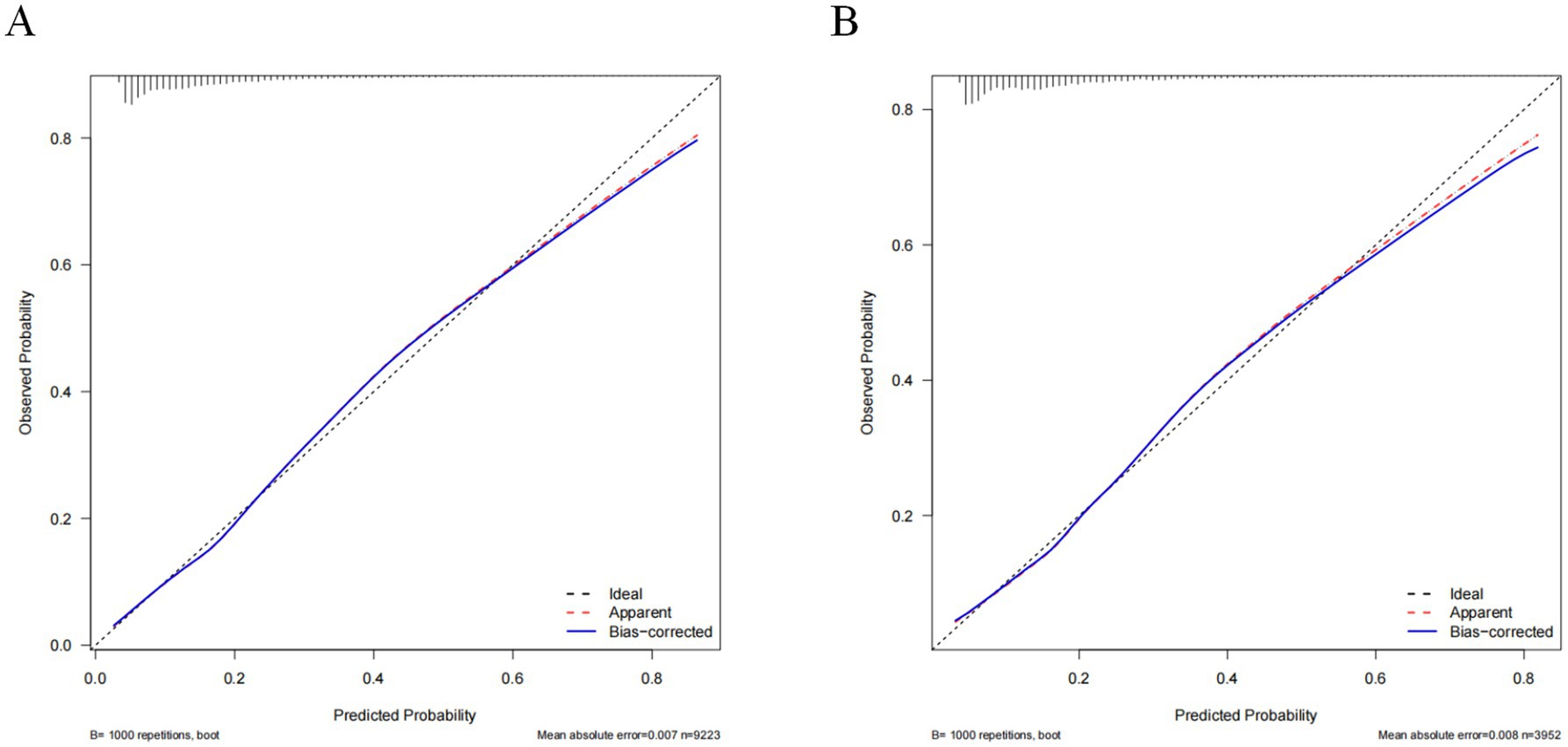
Figure 5. Calibration curves of the nomogram in the training set (A) and validation set (B). The horizontal axis depicts the anticipated likelihood of delirium, and the vertical axis illustrates the actual occurrence of diagnosed delirium relative to the total cases. The diagonal dashed line represents the perfect prediction of the ideal model. The red line represents the prediction of the nomogram; the bule line represents the result after bias correction by bootstrapping (1,000 repetitions).
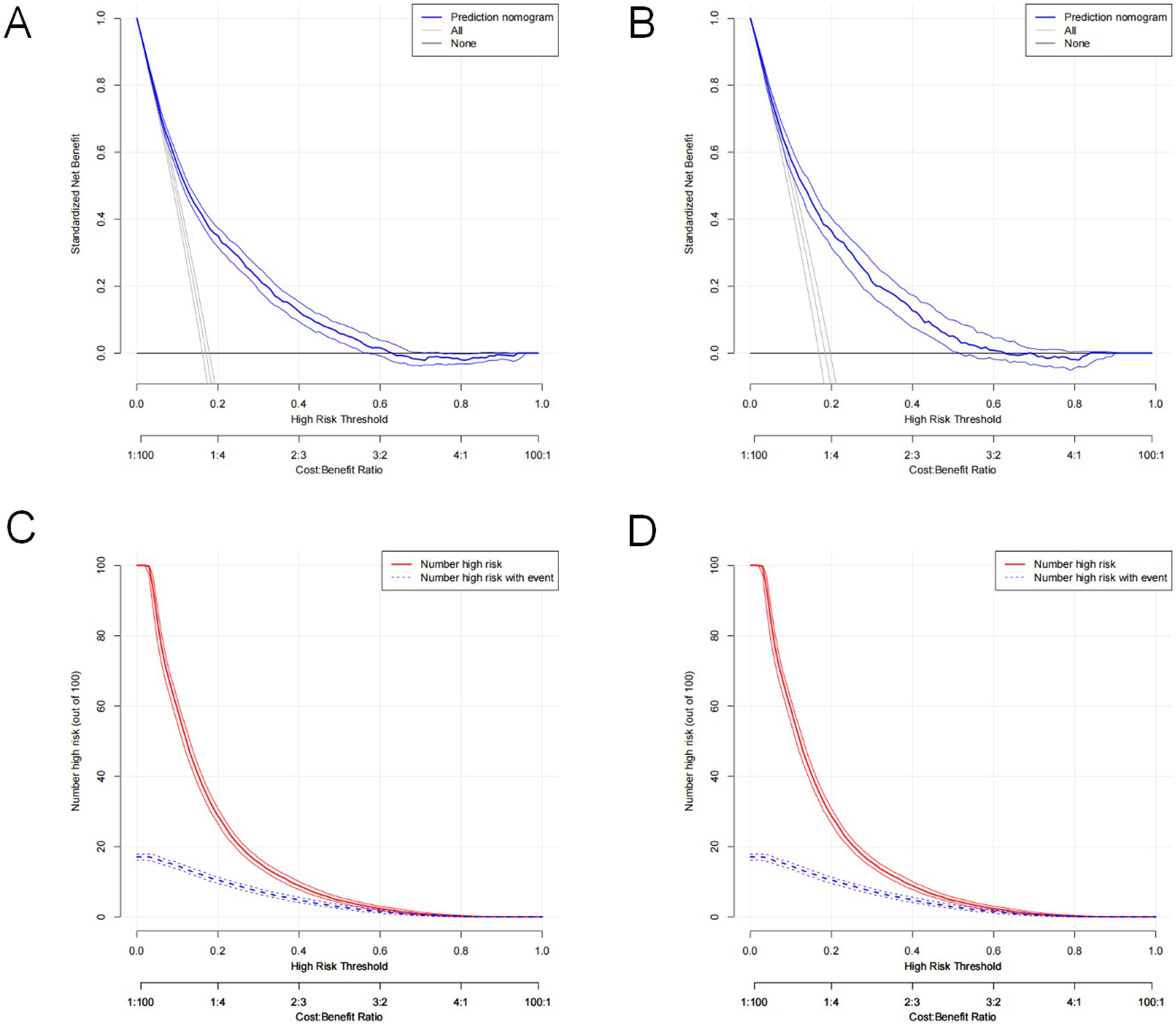
Figure 6. Decision curve analysis of the nomogram in the training set (A) and validation set (C). Clinical impact curve analysis of the nomogram in the training set (B) and validation set (D).
Discussion
Here, we established and verified a novel nomogram model for delirium in elderly critically ill patients. We constructed the nomogram using seven variables: sepsis, SOFA score, cerebrovascular disease, sedation, mechanical ventilation, severe hypothermia, and serum calcium levels. We could easily obtain all the predictors in an objective and reliable manner. Our findings implied that this nomogram has good discrimination and satisfactory calibration, indicating potential clinical applicability.
Delirium subtypes were designated as hypoactive, hyperactive, or mixed based on observed behavior. Significantly, hypoactive delirium is often underrated and is associated with a worse prognosis (26). Medical workers, especially in ICUs, are usually drawn to hyperactive and mixed delirium but often overlook hypoactive delirium (27). A delirium forecasting model in daily practice would facilitate clinicians to screen/detect patients who might benefit from delirium prevention. With limited resources, this could reduce waste and misdiagnosis chances effectively, and might accordingly enhance critical patient outcomes. This is an original finding and the clinical significance of our study. Compared with previous delirium prediction models in the ICU setting, our model performed well. A previous study showed that the AUC was significantly lower for early prediction model for delirium in ICU patients (0.68 (95% confidence interval 0.66–0.71)) (28) compared to our model (0.762 (95% confidence interval 0.749–0.776)) in the training set. The DCA curves further illustrated our model’s favorable clinical utility.
Sepsis had the greatest weight in the nomogram, implying that it serves as the most significant predictor and is the strongest predictor of delirium in older patients in our study. Patients with sepsis complicated by delirium are actually not rare. Literature reports that the incidence of sepsis-associated delirium (SAD) varies from 9 to 71% in severe sepsis patients (29). There is still much to learn about the underlying mechanisms of SAD, but currently, it is believed to be an integration of neuroinflammation and disturbances in cerebral perfusion, the blood–brain barrier, and neurotransmitters (30). A systemic inflammatory response to infection causes brain activation, resulting in an appropriate anti-inflammatory response. However, excessive pro-inflammatory mediators entering the brain may impair the blood–brain barrier and cause abnormal cerebral perfusion (31, 32). Various neuroendocrine dysfunctions have been observed in sepsis, including hypothalamic–pituitary–adrenal axis impairment, autonomic dysfunction, and vasopressin deficiency (33, 34). These dysfunctions may alter immunity in a vicious spiral, leading to metabolic derangement and neurological decline (29).
Cerebrovascular disease is a common comorbidity that can lead to delirium in ICU patients. As mentioned previously, cerebrovascular disease is an independent risk factor of ICU-associated delirium (35, 36). Cerebrovascular disease can lead to neurocognitive deficits, which are postulated to have a higher occurrence rate of delirium, likely via altered cerebral networks and a reduced capability to combine sensory inputs (37). Long-term susceptibility to delirium should be considered an integrative aspect of the overall cerebrovascular disease burden (38), which deserves attention.
The SOFA score was originally designed to successively evaluate the severity of organ dysfunction in critical patients with sepsis, which included an assessment of neurological function and the Glasgow Coma score (39). The SOFA scores are widely used in ICUs and are readily available. Previous studies have reported that higher SOFA scores are indicative of delirium (40–42). One delirium forecast model included the SOFA score as one of the six predictors (40), whereas in our forecast model, the SOFA score emerged as one of the most significant predictors.
Temperature fluctuation is a common phenomenon in critical patients that might reflect an inflammatory response (43). Additionally, it may be an indicator of altered hypothalamic thermoregulatory center function in brain injury (44). It is associated with adverse outcomes (45), including an unfavorable decline in neurocognitive function (46). In a recent study of 27,674 patients undergoing major noncardiac surgery, severe hypothermia was found to be a predictor of postoperative delirium (21). However, the correlation between severe hypothermia and delirium in elderly ICU patients should warrant further investigation.
Serum calcium is an important bivalent cation involved in cognitive decline pathophysiology. High serum calcium levels pertain to an exaggerated decline in global cognitive function over the age of 75 years, regardless of sex or education level (47).Serum calcium level is a potential presymptomatic biomarker for cognitive impairment and can predict longitudinal cognitive decline and conversion (48). In our study, serum calcium levels were correlated with delirium development. Our results were consistent with those of previous studies.
Mechanical ventilation and sedation are critical interventions in the ICU, particularly in patients requiring prolonged mechanical ventilation. Previous studies have revealed that delirium occurred in 38 to 80% of patients who underwent mechanical ventilation in the ICU (49, 50). Mesa et al. demonstrated that a mechanical ventilation duration of 7 days or longer markedly increases the risk of both delirium and mortality (51). Mechanical ventilation appears to be a risk factor for delirium as it could contribute to immobilization, inflammation, physiological stress, sedation, and sleep rhythm disturbance (52). Furthermore, sedation is considered a significant risk factor for delirium, particularly in elderly patients (53). It is crucial to determine optimal sedation strategies and minimize ventilation time to prevent delirium.
Our study had some limitations. First, this was a single-center retrospective study spanning 2009–2018; therefore, a potential selection bias was inevitable. Second, due to database limitations, we missed certain social attributes of older patients, such as income and subjective social status. Third, because > 20% of the data in the dataset were missing, we lacked some key clinical parameters. Fourth, this was a large-sample study, and the robustness of the model was confirmed through internal validation. However, it is crucial to validate its effectiveness in different medical institutions and ensure its applicability in broader scenarios.
Conclusion
In such a large cohort of critically ill patients, we provided a visual and personalized nomogram that enables clinicians to identify and detect delirium in older patients in a timely manner. By precisely assessing an individual’s risk of delirium, physicians can implement effective interventions to decrease the incidence of delirium and thus improve prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
LJ: Writing – original draft. DY: Conceptualization, Writing – review & editing. GY: Data curation, Writing – review & editing. XW: Writing – review & editing. DZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the 2023 Hebei Province Medical Science Research Key Project Plan (grant no. 20230325).
Acknowledgments
We would like to express our gratitude to the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center for the MIMIC Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1580125/full#supplementary-material
Footnotes
References
1. Liu, SB, Wu, HY, Duan, ML, Yang, RL, Ji, CH, Liu, JJ, et al. Delirium in the Icu: how much do we know? A narrative review. Ann Med. (2024) 56:2405072. doi: 10.1080/07853890.2024.2405072
2. Erel, S, Macit Aydin, E, Nazliel, B, and Karabiyik, L. Evaluation of delirium risk factors in intensive care patients. Turk J Anaesthesiol Reanim. (2024) 52:213–22. doi: 10.4274/TJAR.2024.241526
3. Li, X, Zhang, L, Gong, F, and Ai, Y. Incidence and risk factors for delirium in older patients following intensive care unit admission: a prospective observational study. J Nurs Res. (2020) 28:e101. doi: 10.1097/jnr.0000000000000384
4. Liu, H, Zhao, Q, Liu, X, Hu, X, Wang, L, and Zhou, F. Incidence and interaction factors of delirium as an independent risk of mortality in elderly patients in the intensive units: a retrospective analysis from Mimic-iv database. Aging Clin Exp Res. (2022) 34:2865–72. doi: 10.1007/s40520-022-02215-8
5. Stollings, JL, Kotfis, K, Chanques, G, Pun, BT, Pandharipande, PP, and Ely, EW. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. (2021) 47:1089–103. doi: 10.1007/s00134-021-06503-1
6. Yan, E, Veitch, M, Saripella, A, Alhamdah, Y, Butris, N, Tang-Wai, DF, et al. Association between postoperative delirium and adverse outcomes in older surgical patients: a systematic review and meta-analysis. J Clin Anesth. (2023) 90:111221. doi: 10.1016/j.jclinane.2023.111221
7. Hayhurst, CJ, Marra, A, Han, JH, Patel, MB, Brummel, NE, Thompson, JL, et al. Association of Hypoactive and Hyperactive Delirium with Cognitive Function after Critical Illness. Crit Care Med. (2020) 48:e480–8. doi: 10.1097/CCM.0000000000004313
8. Smith, CD, and Grami, P. Feasibility and effectiveness of a delirium prevention bundle in critically ill patients. Am J Crit Care. (2016) 26:19–27. doi: 10.4037/ajcc2017374
9. Oh, ES, Fong, TG, Hshieh, TT, and Inouye, SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. (2017) 318:1161–74. doi: 10.1001/jama.2017.12067
10. Devlin, JW, Skrobik, Y, Gélinas, C, Needham, DM, Slooter, AJC, Pandharipande, PP, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the Icu. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
11. Balas, MC, Pun, BT, Pasero, C, Engel, HJ, Perme, C, Esbrook, CL, et al. Common challenges to effective Abcdef bundle implementation: the Icu liberation campaign experience. Crit Care Nurse. (2019) 39:46–60. doi: 10.4037/ccn2019927
12. Boehm, LM, Dietrich, MS, Vasilevskis, EE, Wells, N, Pandharipande, P, Ely, EW, et al. Perceptions of workload burden and adherence to Abcde bundle among intensive care providers. Am J Crit Care. (2017) 26:e38–47. doi: 10.4037/ajcc2017544
13. Morandi, A, Piva, S, Ely, EW, Myatra, SN, Salluh, JIF, Amare, D, et al. Worldwide survey of the "assessing pain, both spontaneous awakening and breathing trials, choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment" (Abcdef) bundle. Crit Care Med. (2017) 45:e1111–22. doi: 10.1097/CCM.0000000000002640
14. Guo, R, Zhang, S, Yu, S, Li, X, Liu, X, Shen, Y, et al. Inclusion of frailty improved performance of delirium prediction for elderly patients in the cardiac intensive care unit (D-frail): a prospective derivation and external validation study. Int J Nurs Stud. (2023) 147:104582. doi: 10.1016/j.ijnurstu.2023.104582
15. Van Den Boogaard, M, Pickkers, P, Slooter, AJ, Kuiper, MA, Spronk, PE, Van Der Voort, PH, et al. Development and validation of pre-Deliric (prediction of delirium in Icu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ. (2012) 344:e420. doi: 10.1136/bmj.e420
16. De La Varga-Martínez, O, Gómez-Pesquera, E, Muñoz-Moreno, MF, Marcos-Vidal, JM, López-Gómez, A, Rodenas-Gómez, F, et al. Development and validation of a delirium risk prediction preoperative model for cardiac surgery patients (Deliprecas): an observational multicentre study. J Clin Anesth. (2021) 69:110158. doi: 10.1016/j.jclinane.2020.110158
17. Wu, WT, Li, YJ, Feng, AZ, Li, L, Huang, T, Xu, AD, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. (2021) 8:44. doi: 10.1186/s40779-021-00338-z
18. Collins, GS, Reitsma, JB, Altman, DG, and Moons, KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (Tripod): the Tripod statement. BMJ. (2015) 350:g7594. doi: 10.1136/bmj.g7594
19. Girard, TD, Pandharipande, PP, and Ely, EW. Delirium in the intensive care unit. Crit Care. (2008) 12:S3. doi: 10.1186/cc6149
20. Gong, KD, Lu, R, Bergamaschi, TS, Sanyal, A, Guo, J, Kim, HB, et al. Predicting intensive care delirium with machine learning: model development and external validation. Anesthesiology. (2023) 138:299–311. doi: 10.1097/ALN.0000000000004478
21. Ju, JW, Nam, K, Sohn, JY, Joo, S, Lee, J, Lee, S, et al. Association between intraoperative body temperature and postoperative delirium: a retrospective observational study. J Clin Anesth. (2023) 87:111107. doi: 10.1016/j.jclinane.2023.111107
22. Faquih, T, Van Smeden, M, Luo, J, Le Cessie, S, Kastenmüller, G, Krumsiek, J, et al. A workflow for missing values imputation of untargeted metabolomics data. Meta. (2020) 10:486. doi: 10.3390/metabo10120486
23. Ely, EW, Margolin, R, Francis, J, May, L, Truman, B, Dittus, R, et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (cam-Icu). Crit Care Med. (2001) 29:1370–9. doi: 10.1097/00003246-200107000-00012
24. Vasquez, MM, Hu, C, Roe, DJ, Chen, Z, Halonen, M, and Guerra, S. Least absolute shrinkage and selection operator type methods for the identification of serum biomarkers of overweight and obesity: simulation and application. BMC Med Res Methodol. (2016) 16:154. doi: 10.1186/s12874-016-0254-8
25. Xia, J, Hu, C, Wang, L, and Zhang, Y. Association between statin use on delirium and 30-day mortality in patients with chronic obstructive pulmonary disease in the intensive care unit. Eur J Med Res. (2023) 28:572. doi: 10.1186/s40001-023-01551-3
26. Diouf, F, Hoang, B, Bammatter, L, Eshmawey, M, and Unschuld, PG. Hypoactive delirium. Rev Med Suisse. (2023) 19:1282–5. doi: 10.53738/REVMED.2023.19.833.1282
27. Faeder, M, Hale, E, Hedayati, D, Israel, A, Moschenross, D, Peterson, M, et al. Preventing and treating delirium in clinical settings for older adults. Ther Adv Psychopharmacol. (2023) 13:20451253231198462. doi: 10.1177/20451253231198462
28. Wassenaar, A, Schoonhoven, L, Devlin, JW, Van Haren, FMP, Slooter, AJC, Jorens, PG, et al. Delirium prediction in the intensive care unit: comparison of two delirium prediction models. Crit Care. (2018) 22:114. doi: 10.1186/s13054-018-2037-6
29. Ebersoldt, M, Sharshar, T, and Annane, D. Sepsis-associated delirium. Intensive Care Med. (2007) 33:941–50. doi: 10.1007/s00134-007-0622-2
30. Atterton, B, Paulino, MC, Povoa, P, and Martin-Loeches, I. Sepsis associated delirium. Medicina. (2020) 56:40. doi: 10.3390/medicina56050240
31. Hughes, CG, Morandi, A, Girard, TD, Riedel, B, Thompson, JL, Shintani, AK, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. (2013) 118:631–9. doi: 10.1097/ALN.0b013e31827bd193
32. Tsuruta, R, and Oda, Y. A clinical perspective of sepsis-associated delirium. J Intensive Care. (2016) 4:18. doi: 10.1186/s40560-016-0145-4
33. Fowler, C, Raoof, N, and Pastores, SM. Sepsis and adrenal insufficiency. J Intensive Care Med. (2023) 38:987–96. doi: 10.1177/08850666231183396
34. Sharshar, T, Gray, F, Lorin De La Grandmaison, G, Hopkinson, NS, Ross, E, Dorandeu, A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. (2003) 362:1799–805. doi: 10.1016/S0140-6736(03)14899-4
35. Cheng, H, Ling, Y, Li, Q, Li, X, Tang, Y, Guo, J, et al. Association between modified frailty index and postoperative delirium in patients after cardiac surgery: a cohort study of 2080 older adults. CNS Neurosci Ther. (2024) 30:e14762. doi: 10.1111/cns.14762
36. Zhang, Y, Xie, LJ, Wu, RJ, Zhang, CL, Zhuang, Q, Dai, WT, et al. Predicting the risk of postoperative delirium in elderly patients undergoing hip arthroplasty: development and assessment of a novel nomogram. J Investig Surg. (2024) 37:2381733. doi: 10.1080/08941939.2024.2381733
37. Gold, BT, Brown, CA, Hakun, JG, Shaw, LM, Trojanowski, JQ, and Smith, CD. Clinically silent Alzheimer's and vascular pathologies influence brain networks supporting executive function in healthy older adults. Neurobiol Aging. (2017) 58:102–11. doi: 10.1016/j.neurobiolaging.2017.06.012
38. Pendlebury, ST, Thomson, RJ, Welch, SJV, and Rothwell, PM. Cognitive predictors of delirium on long-term follow-up after Tia and Stroke: population-based cohort study. Cerebrovasc Dis. (2022) 51:288–95. doi: 10.1159/000519900
39. Vincent, JL, De Mendonça, A, Cantraine, F, Moreno, R, Takala, J, Suter, PM, et al. Use of the Sofa score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. (1998) 26:1793–800. doi: 10.1097/00003246-199811000-00016
40. Chaiwat, O, Chanidnuan, M, Pancharoen, W, Vijitmala, K, Danpornprasert, P, Toadithep, P, et al. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiol. (2019) 19:39. doi: 10.1186/s12871-019-0694-x
41. Ma, R, Zhao, J, Wen, Z, Qin, Y, Yu, Z, Yuan, J, et al. Machine learning for the prediction of delirium in elderly intensive care unit patients. Eur Geriatr Med. (2024) 15:1393–403. doi: 10.1007/s41999-024-01012-y
42. Tang, D, Ma, C, and Xu, Y. Interpretable machine learning model for early prediction of delirium in elderly patients following intensive care unit admission: a derivation and validation study. Front Med. (2024) 11:1399848. doi: 10.3389/fmed.2024.1399848
43. Papaioannou, VE, Chouvarda, IG, Maglaveras, NK, and Pneumatikos, IA. Temperature variability analysis using wavelets and multiscale entropy in patients with systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care. (2012) 16:R51. doi: 10.1186/cc11255
44. Van Der Kooi, AW, Kappen, TH, Raijmakers, RJ, Zaal, IJ, and Slooter, AJ. Temperature variability during delirium in Icu patients: an observational study. PLoS One. (2013) 8:e78923. doi: 10.1371/journal.pone.0078923
45. Varela, M, Calvo, M, Chana, M, Gomez-Mestre, I, Asensio, R, and Galdos, P. Clinical implications of temperature curve complexity in critically ill patients. Crit Care Med. (2005) 33:2764–71. doi: 10.1097/01.CCM.0000190157.64486.03
46. Grocott, HP, Mackensen, GB, Grigore, AM, Mathew, J, Reves, JG, Phillips-Bute, B, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. (2002) 33:537–41. doi: 10.1161/hs0202.102600
47. Schram, MT, Trompet, S, Kamper, AM, De Craen, AJ, Hofman, A, Euser, SM, et al. Serum calcium and cognitive function in old age. J Am Geriatr Soc. (2007) 55:1786–92. doi: 10.1111/j.1532-5415.2007.01418.x
48. Ma, LZ, Wang, ZX, Wang, ZT, Hou, XH, Shen, XN, Ou, YN, et al. Serum calcium predicts cognitive decline and clinical progression of Alzheimer's disease. Neurotox Res. (2021) 39:609–17. doi: 10.1007/s12640-020-00312-y
49. Ely, EW, Shintani, A, Truman, B, Speroff, T, Gordon, SM, Harrell, FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. (2004) 291:1753–62. doi: 10.1001/jama.291.14.1753
50. Ko, RE, Lee, J, Kim, S, Ahn, JH, Na, SJ, and Yang, JH. Machine learning methods for developing a predictive model of the incidence of delirium in cardiac intensive care units. Rev Esp Cardiol. (2024) 77:547–55. doi: 10.1016/j.recesp.2023.12.015
51. Mesa, P, Previgliano, IJ, Altez, S, Favretto, S, Orellano, M, Lecor, C, et al. Delirium in a Latin American intensive care unit. A prospective cohort study of mechanically ventilated patients. Rev Bras Ter Intensiva. (2017) 29:337–45. doi: 10.5935/0103-507X.20170058
52. Reade, MC, and Finfer, S. Sedation and delirium in the intensive care unit. N Engl J Med. (2014) 370:444–54. doi: 10.1056/NEJMra1208705
Keywords: delirium, prediction model, nomogram, elderly, intensive care unit
Citation: Jiang L, Yu D, Yang G, Wu X and Zhang D (2025) Development and internal verification of nomogram for forecasting delirium in the elderly admitted to intensive care units: an analysis of MIMIC-IV database. Front. Neurol. 16:1580125. doi: 10.3389/fneur.2025.1580125
Edited by:
Chao Luo, Shanghai Mental Health Center, ChinaReviewed by:
Sen Li, Beijing University of Chinese Medicine, ChinaGhada Abd El-Raheem, Soba University Hospital, Sudan
Copyright © 2025 Jiang, Yu, Yang, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Jiang, MjU4MzM3MDQ2QHFxLmNvbQ==
 Li Jiang
Li Jiang Dongdong Yu
Dongdong Yu Dong Zhang
Dong Zhang